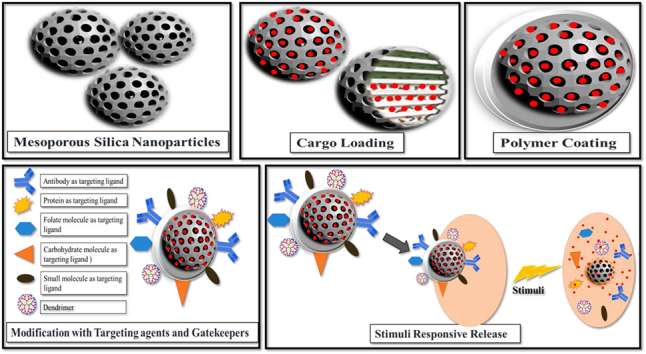
Abstract
Rapid progress in developing multifunctional nanocarriers for drug delivery has been observed in recent years. Inorganic mesoporous silica nanocarriers (MSNs), emerged as an ideal candidate for gene/drug delivery with distinctive morphological features. These ordered carriers of porous nature have gained unique attention due to their distinctive features. Moreover, transformation can be made to these nanocarriers in terms of pores size, pores volume, and particle size by altering specific parameters during synthesis. These ordered porous materials have earned special attention as a drug carrier for treating multiple diseases. Herein, we highlight the strategies employed in synthesizing and functionalizing these versatile nanocarriers. In addition, the various factors that influence their sizes and morphological features were also discussed. The article also summarizes the recent advancements and strategies for drug and gene delivery by rendering smarter MSNs by incorporating functional groups on their surfaces. Averting off-target effects through various capping strategies is a massive milestone for the induction of stimuli-responsive nanocarriers that brings out a great revolution in the biomedical field.
Keywords: Mesoporous silica nanoparticles, Gatekeepers, Capping agents, Targeted drug delivery, Stimuli-responsive, Chemical modification
Graphical abstract
Highlights
-
•
MSNs serve as an ideal candidate for gene/drug delivery with unique and excellent attributes.
-
•
MSNs surface can be functionalized using specific materials to impart unique structural features.
-
•
Functionalization of MSNs with stimuli-responsive molecules can act as gatekeepers by responding to the desired stimulus after uncapping.
-
•
These capping agents act as vital targeting agents in developing MSNs being employed in various biomedical applications.
1. Introduction
Nanomedicine’s advent in the field of nanotechnology has gained much escalation with the provision of promising solutions towards drug delivery for the treatment of several diseases (Bernal et al., 2021; Nagraik et al., 2021). The development of nanocarriers to transport and release a therapeutic agent at the diseased site in a controlled and selective manner has gained significant attention from researchers (Scicluna and Vella-Zarb, 2020). Nanometric agents provide an effective alternative for treating diseases requiring the potent administration of cytotoxic drugs. These agents are mainly categorized as organic or inorganic nanocarriers and brought tremendous achievement in treating various infectious diseases. These organic nanometric agents include different polymeric agents (Begines et al., 2020; Kong et al., 2020; Palanikumar et al., 2020; Siddiqui et al., 2020), lipid-based carriers (Barkat et al., 2020; Plaza-Oliver et al., 2021), dendrimers (Mandal, 2021; Nikzamir et al., 2021) and micelles (Atanase, 2021; Hwang et al., 2020). These agents are widely employed for the treatment of various dreadful diseases. However, in contrast to organic nanometric agents, the carriers composed of inorganic composition have gained remarkable attention owing to their higher mechanical, chemical, and thermal stabilities. These agents mainly include nanocarriers consisting of carbon, metal, or silica (Zhang et al., 2021)..
Nanoporous silica nanoparticles are the inorganic particles having pores in the nano-size of 1-100 nm. These nanopores are categorized as micropores having a diameter of < 2 nm, mesopores of 2-50 nm pore diameter, and macropores with a pore diameter of 50 nm or larger (Zhang et al., 2021). These agents also depict good biocompatibility but a relatively low degradation profile (Shi et al., 2020). Among different substances used for the composition of these agents, mesoporous silica nanocarriers (MSNs) acts as one of the astounding achievements. These carriers shared significant attributes of high loading and encapsulation efficiency, ease of production, biocompatibility, zero premature release, and increased capacity of tunability in terms of their size, pores diameter, and morphology (Alyassin et al., 2020; Manzano and Vallet-Regí, 2020). In the initial phase, they were only employed to increase the solubility of drugs having lower solubility. However, further exploration and advancement in their applications widens their purpose for improving bioavailability, designing controlled drug release, and targeting the active moieties at the desired site. In recent years, receptor-based targeting at the desired site through stimuli-responsive action has gained significant importance (Haddick et al., 2020; Kundu et al., 2020).
The most promising advantages of MSNs as a drug delivery system are their “zero premature controlled release” property (Slowing et al., 2008) by ensuring drugs be delivered without leakage. This property of the MSNs can be achieved by designing them as intelligent drug delivery carriers, which enables them to release the drug in the specified area of interest without any premature release at the off-target sites. Conventional polymeric nanocarriers suffered from the limited drug loading capacity and the abrupt departure of matrix-encapsulated agents due to poor stability profile. In contrast, MSNs are highly stable and can deliver large payloads of drugs with precise temporal control. This kind of release is particularly beneficial for the delivery of cytotoxic agents requiring precise drug control. For this purpose, various gatekeepers play a crucial role in controlling the release of drugs by capping the entrances of the carriers and can only be removed under specific conditions. The internal structure of these agents acts as a safer micro-environmental region that serves as effective loading of the therapeutic agent and at the same time its protection from deactivation or degradation from the external environment. These pores of the MSNs may act as drug reservoir systems and permit controlled diffusion of a drug to the surrounding tissues over specified intervals of time upon the influence of any internal (endogenous molecules, redox potential, pH, or biomolecules) or external triggering stimuli (Light, pH, heat, ultrasound, magnetic field or chemicals) (Chen et al., 2020a; Irshad et al., 2020; Kundu et al., 2020). The fabrication of such an intelligent carrier system demonstrated precise release of drug at a specified area, exhibiting zero premature release of drug in the systemic circulation. However, without capping or surface functionalization, these drug carriers may exhibit release of drug in the systemic circulation, having a premature release of a drug.
These different gatekeepers may include certain materials acting as hard caps or soft caps. The distinction is made based on the templating method which is being used for the fabrication of porous MSNs. Two types of templating materials are commonly employed for their fabrication. The hard templating method, also called exotemplate, utilizes a porous solid agent, in which inorganic precursor is utilized to fill the hollow spaces of MSNs. These are synthesized from nanomaterials of non-silica sources such as polymeric beads, semiconductor NPs, metal or metal oxides (Cadmium sulfide (CdS), iron oxide (Fe3O4)), and gold nanocarriers (AuNPs). While in the soft templating method, also called endotemplate, a surfactant is usually utilized without use of any hard template. Soft capping agents include various biomolecular agents, micelles, organic molecules, microemulsions, or supramolecular assemblies. In comparison to hard MSNs, these agents are produced under mild reaction conditions following an easy method of synthesis (Ghaferi et al., 2021). The capping technique also influences the release pattern of the encapsulated agent. The templating agent affects the structural properties of NPs, which in turn affects the release of encapsulated material from their surface, e.g., shell thickness of the carrier. For example, Lin et al. demonstrated the different release kinetics of ATP, encapsulated in various capped MSNs through real-time imaging. The hard-capped MSNs revealed the faster release of a small payload of the drug while the soft capped MSNs like polamidoamine demonstrated sustained and slow release for larger drug amounts (Gruenhagen et al., 2005).
Moreover, functionalization of MSNs can be provided by decorating their external surfaces with ligands capable of active targeting at the diseased site. Attachment of one or more stimuli-responsive functional groups to the capped MSNs enables them to release drugs on command. For this purpose, modifications of the silanol groups have been made on the surface of MSNs to create highly modified nanocarriers with remarkable and efficient properties. In addition, all these materials can be phagocytosed by physiological cells without posing any cytotoxicity, which makes these multifunctional MSNs a superior candidate for the site-specific-controlled delivery of drug/gene or any other therapeutic moiety (Kankala et al., 2020). Herein, we will discuss the types, synthesis techniques, and the recent advancements in the development of novel stimuli-responsive MSNs by various capping molecules. Moreover, we will also focus on the surface modifications of these agents for release of a therapeutic agent at the target site, upon exposure to internal or external stimuli.
2. Types of MSNs
Synthesis of MSNs dates back to the 1970s, after which in 1992, Mobil Research and Development Corporation synthesized MSNs from gels of alumino-silicate. They utilized a template of liquid crystals and named these materials MCM-41 (Mobil Composition of Matter or Mobil Crystalline Materials). Usually, these MSNs are categorized into various types, such as Santa Bar-bara (SBA including SBA-1, 2, 3, 6, 12, 15, 16, etc.), Mobile Crystalline Materials that further includes (MCM-41, 48 and 50), Michigan State University (MSU), Fudan University (FDU) and Hexagonal Mesoporous Silica (HMS). According to IUPAC, MSNs are materials with an ordered presentation of pores with a 2-50 nm porous diameter. The sizes of the pores can be altered by employing specific surfactants under suitable reaction conditions (Beck et al., 1992). These surfactants can be of four types based on charges present on them, including cationic surfactants (quaternary ammonium salts or cetyltrimethylammonium bromide (CTAB)), anionic type (compounds carrying sulfonic acid, phosphoric acid), non-ionic type polyethylene oxide (PEO), and ampholytic type. Apart from these, certain other carriers of mesoporous nature were also synthesized through variation in the starting templating agents and reaction conditions (Vallet-Regi et al., 2001). These materials have changed geometrical arrangements with variations in pores sizes.
Due to the availability of a wide variety of surfactants, different structures of MSNs have been introduced as shown in Fig. 1.
Fig. 1.
Different types of MSNs.
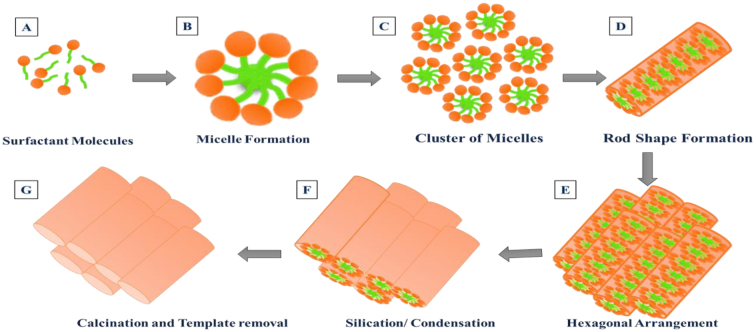
Different members have been fabricated among MCM families by utilizing different reaction conditions. Among these, MCM-41 are widely used carriers employing cationic surfactants as a templating agent. They are composed of the regular two-dimensional hexagonal arrangement having a pore size of 2.5 nm to 6 nm. By considering the importance of the MCM family, MCM-48, another member of the MCM family has also been designed as a drug carrier. MCM-48 depicts a cubical arrangement with a three-dimensional structure. In contrast to MCM-41, these materials displayed bi-continuous channels and offered quick materials transport. MCM-50 is also designed among the MCM family which exhibited lamellar arrangement (Øye et al., 2001). The general mechanism of MSM formation is depicted in Fig. 2. Firstly, the surfactant molecules self-aggregate themselves at alkaline pH above the critical micelle concentration, resulting in the formation of micelles. Afterward, with the addition of tetraethoxysilane (TEOS), the micellar packing started through the electrostatic interaction between positively charged N+(CH3)3 and negatively charged silanes (Si-O-), which leads to the formation of ordered architectures of silica. The silica precursors overlap at the polar head of surfactant micelles by forming a wall around them. Removal of surfactant to expose the channels of mesopores resulted in the formation of MSMs.
Fig. 2.
Schematic representation of MCM-41 synthesis using cationic surfactant template.
Another mesoporous structure of highly ordered nature is termed as SBA or Santa Barbara; due to their synthesis at Santa Barbara, the University of California. These materials possess thicker walls of silica and larger pores of 4.6 to 30 nm (Zhao et al., 1998). The initial templating agent for their synthesis can influence the symmetry of these materials. Non-ionic co-polymer alkyl poly (ethylene oxide) helps to design cubic mesopores named SBA-11 while oligomeric surfactants result in the formation of three-dimensional hexagonal mesostructured SBA-12. In the same way, poly (alkylene oxide) brings out a regular hexagonal mesostructured carrier named SBA-15 or a cubic cage-like structure termed as SBA-16 (Zhao et al., 1998).
FSM-16 is another exciting type of mesoporous material that can be utilized for wide pharmaceutical purposes, besides its use as a catalyst and adsorbent. (Tozuka et al., 2005). Some other types of mesoporous materials are coined as Korea Advanced Institute of Science and Technology (KIT) and Technical Delft University (TUD-1) with different symmetries and pore shapes (Heikkilä et al., 2007; Nandiyanto et al., 2009). Among the above-mentioned MSNs, MCM-41, 48, and SBA-15, 16 are employed widely for drug and gene delivery.
3. Advances in synthesis methods
MSNs can be synthesized through various methods, including soft or hard templating method, fast self-assembly, Stober method, modified aerogel methods, hydrothermal technique, and dissolving reconstruction method. Synthesis of spherical micron-sized silica nanocarriers was pioneered by Stöber, by employing specific chemical reactions, and the method was afterward named as ‘Stöber synthesis’(Stöber et al., 1968). Mostly Stöber’s method is followed for the fabrication of MSNs, which is also known as the sol-gel method. In the sol-gel method, the sol phase is generated by the reactions of hydrolysis and condensation with the production of colloidal particles at alkaline or acidic pH, while the condensation of colloidal particles results in the production of gel phase of three-dimensional structure through cross-linking of siloxane bond (Corbalan et al., 2012b). This general reaction is depicted in Equation 1 and 2 as:
| (1) |
| (2) |
Where R, maybe any alkyl group.
Hydrolysis of alkoxide groups depends upon the molar concentration of Si/H2O and other reaction conditions. The condensation process occurs only after hydrolysis reaction, in which repeated condensation forms a chain-like structure in the colloidal solution, while in gel form, it forms a huge network (Danks et al., 2016). Drying of this phase results in the formation of various biomolecules that were embedded in the silica gel matrix resulting in the formation of MCM-41. Through this technique, the produced MSNs are in the micron range, while the alkyl silicates hydrolysis and condensation control the particles' dispersity in the presence of alcohol. The method utilizes four components; silica source, water, alcohol, and base (Stöber et al., 1968). The expression of MSNs reaction through sol-gel reaction is:
| (3) |
| (4) |
| (5) |
Several other modifications can be made to get the particles of highly ordered nature in a nanometric range of different shapes (Wang et al., 2016). Further modifications were made to this method by utilization of cationic surfactant template to obtained spherical structure of MSN rather than hexagonal one, having same properties as provided by other methods. A lot of research has been conducted to obtain stable monodisperse particulates of MSNs (Grün et al., 1997). This method is advantageous over others in terms of its simplicity and cost-effectiveness, with a uniform structure and controlled properties. Also, the process utilizes fewer excipients and is also time-saving (Bharti et al., 2015; Shahbazi et al., 2012).
The other technique for producing MSNs include soft and hard templating methods. In the soft templating method, MSNs are produced through the use of organic templating agents to create porosity. In the next step, heat is provided to remove the templating material and for the isolation of pure mesoporous carriers (Wu et al., 2013). While, in hard templating method or nano casting technique, the precursors of silica fills the templating mesopores through capillary force, followed by the treatment of chemical or thermal removal of the templating agent, that produces a mirror image of mesoporous silica structure (reverse structure) (Egger et al., 2015).
Hydrothermal technique for the development of MSNs was discovered by Roderick Murchison, a British Geologist. He introduced it as the formation of minerals that were obtained by the hot water solutions from cooling magmas. The process can be described as the reaction occurring in the sealed container where the system's pressure and temperature are kept high (Feng and Li, 2017). The method follows similar steps to that of the sol-gel process, except that the mixture is transferred to an autoclave having a lining of Teflon at a certain temperature, followed by the template removal. MSNs obtained through this method have improved regularity and greater hydrothermal stability (Yu et al., 2012). Table 1 listed the distinguishing features of the Sol-Gel and Hydrothermal process
Table 1.
Distinguishing features of sol-gel and hydrothermal process.
| Distinction | Sol-gel process | Hydrothermal process | References |
|---|---|---|---|
| Process | This process involves two main steps of synthesis, including the formation of particles in solution followed by gel formation due to the 3D polymeric network. | In this process, an inorganic substance is added to the templating agent (acid or alkali), and the fabricated hydrogel is afterward subjected to autoclaving. | (Varshney et al., 2021) |
| Reaction conditions | In this process, the requirement of a sealed container is not required. | The reaction takes place in a sealed container, having maintained temperature and pressure. | (Mohamed Isa et al., 2021; Yu et al., 2012) |
| Requirement of Autoclave | The method doesn’t require autoclaving or any other parameter of high temperature. | The method requires a Teflon-lined autoclave to maintain process parameters. | (Yu et al., 2012) |
| Formation of mesoporous particles | Desired morphology is obtained through simultaneous hydrolysis and condensation of metal oxide. | The powdered solid SiO2 got dispersed during heat treatment, and the formation of mesophase assembly occurs after the removal of heat. | (Galabova, 2021). |
| Morphology of MSNs | Formulation parameters, such as temperature, pH, and reagent concentrations, affect the morphology and size of the particles. | Particle morphology and size have a significant influence on cooling rate after thermal treatment. | (Narayan et al., 2018) |
| Advantages | The main advantages include increased purity and ease of synthesis at moderate reaction conditions | The advantage of this method is to obtain of MSNs having greater hydrothermal stability. | (Bharti et al., 2015; Shahbazi et al., 2012) (Lin et al., 2011). |
| Disadvantages | The method produces particles in micron size and requires further modifications | The process is complex and requires increased time. | (Miller et al., 2014). |
Three important materials are necessary for the formation of MSNs; silica precursor (tetramethylorthosilicate, tetraethylorthosilicate, tetrakis (2-hydroxyethyl) orthosilicate, and sodium metasilicate), a surfactant that may act as a template directing agent (cationic or non-ionic) and a catalyst. Other reagents may include co-solvents that are incorporated for the prevention of aggregation.
4. Factors that affect cellular uptake and drug loading in MSNs
Mesoporous silica nanoparticles (MSNs) possess many attributes that make them beneficial for use in drug delivery due to their unique mesoporous structure. They also offer a high level of chemical stability, easy surface functionality, and biocompatibility that ensures controlled drug release and targeted delivery of various encapsulated agents. MSNs have gained attention in the scientific community due to the aforementioned properties: large pore volume, high surface area, narrow pore size distribution, and tunable pore diameter. Targeted drug delivery can also be achieved by surface modification of the MSNs.
Several factors influence the cellular uptake of MSNs by targeting cells, including particle size, pore size, shape, charge, and surface modification. In order to obtain MSNs to act as an ideal carrier for drug delivery, the particles must be of uniform shape with larger pore volume to maximize the loading capacity of the drug. The key properties of MSNs are affected by the following parameters.
4.1. Size of MSNs
Particle size is one of the most crucial factors that affect the carrier properties and deliver the entrapped agent to the target site. Particles of smaller diameter are usually preferable for effective delivery of the agent with better cellular uptake properties. The particle size of the MSNs is affected by a variety of factors such as pH, temperature, reaction time, the addition of functional organo-silanes.
One of the foremost vital factors that contributes a significant role in controlling the particle size is the pH of the reaction medium. The hydrolysis process is affected by the initial pH that further controls the size of particles. In contrast, the particle's nucleation is determined by the silica condensation that affects the quality of nanoparticles. In a basic medium, the rate of hydrolysis increases with an increase in pH. In contrast, the rate of condensation is not proportional and is higher at pH 8.4 with a further decrease with an alteration of pH. A study demonstrated by Qiao et al. reported an increase in particles size from 30 to 85 nm with a subsequent reduction in pH from 10 to 6.8. These reports depicted that the minimum particle size can be attained at a pH of 9-10, showing the increased effect of condensation rates on the particles size compared to hydrolysis (Qiao et al., 2009). Moller et al. used triethanolamine as a base catalyst to create a basic environment instead of ammonium hydroxide (NH4OH) or sodium hydroxide (NaOH). Triethanolamine (TEA) may also perform its function as a complexing agent and prevent aggregates formation with a production of smaller discrete particles by affecting the pH of the system (Moeller et al., 2007). Another study reported by Bouchoucha et al. also demonstrated the control of particle size of MSNs particles by employing TEA as a base catalyzing additive and as a dispersing agent (Bouchoucha et al., 2016). Chiang et al. demonstrated the control of particle size of MSNs by observing different parameters. They concluded that among the various parameters that influence the size of the particles, pH plays the most important role, and the sequence of the influencing parameters is 57% for the pH, 29% for the reaction time, and 13% for the pH the amount of TEOS. They demonstrated the effect of particle size by changing the pH values to 9.5, 12.16, or 13 and observed the smaller particle size of around 100 nm at pH 13.
The reaction time also has a notable influence on the particle size of MSNs. Chiang et al. also observed the effect of reaction time on particle size. They reported that the size of the particles increases at the start with a subsequent decrease as the reaction period increases from 2-10 h. (Chiang et al., 2011). Wang et al. developed virus-like MSNs having improved cellular uptake characteristics. The structural properties of these carriers changes at different reaction times. On prolonging the total reaction time, the walls of mesopores got thicker. After 18 h, the surface area of particles reduces due to the blocking of MSNs channels with excessive silicates growth. On further increasing the reaction time up to 24 h, the shorter nanotubes of silica adhere to the surface of nanoparticles without a noticeable change in the shape of nanoparticles. With a further increase of reaction time, these nanotubes increase in length up to ~5 nm with an increase in particle size of 130 nm. Finally, after 48 h, the total length of mesopores extends up to ~15 nm with particles size of 160 nm with elongation of nanotubes (Wang et al., 2017a).
Particle size can also be altered by the addition of certain solvents or other compounds. The effect of different co-solvents and mixtures on the sizes of the particles demonstrated a significant reduction of particle size by using ethylene glycol. It was proposed that the use of ethylene glycol decreases the interaction of surfactant with silica precursors and inhibits the growth of particles (Gu et al., 2007). Change in the composition of the alkoxy group (Si (OR) 4) of tetraalkoxysilanes also brings out the change in the particle size. Also, the addition of certain additives like alcohols also influences the hydration rate of the reaction and causes alteration of particle size (Yamada et al., 2013). CTAB concentration also affects the process of hydrolysis and its micellization. Therefore the lower concentration of CTAB brings more homogenous and spherical distribution of particles sizes, while agglomerates were observed with variation in its concentration (Vazquez et al., 2017). The concentration of polymer F127 also plays a critical role in controlling the particle size of MSNs. Studies also reported the effect of tetramethylorthosilicate (TEOS) concentration on the particle size of MSNs. They observed that with the increasing value of TEOS, an increase in the particle size was observed. Although this increase in the particle size is not in proportion to the increase of TEOS, indicating a little effect of TEOS on particle size (Chiang et al., 2011). Also, increased concentration of TEOS is accountable for the change of monodisperse system to a heterogeneous system of particles size distribution. This is due to the presence of an excess quantity of silica precursor that leads to the production of new nuclei among the already formed particles (Zainala et al., 2013).
The introduction of some capping agent or functionalization of MSNs also affects the particle sizes. Capping of MSNs through polyethylene glycol (PEG)-silanes helps in the attenuation of the growth of particles, hence inhibiting their size through the process of steric stabilization. The addition of PEG-silane immediately to MSNs after the addition of tetramethyl orthosilicate (TMOS) limits its size up to 5 nm. Still, an increase in particle size of >13 nm was observed if there was a delay in addition after 50 minutes (Ma et al., 2013). Stirring rate is another crucial factor in determining the particle size; slow stirring resulted in the formation of larger particles while fine particles were formed upon fast stirring (Beltrán-Osuna et al., 2017).
Temperature is another parameter that affects the particles sizes of MSNs. Increasing the temperature from 30°C to 70°C increases the particles size of MSNs from 28 to 113 nm. This may be due to polycondensation of silica precursors due to an increase in reaction rate, which results in the formation of a denser structure of silica with increased particle size (Zainala et al., 2013).
4.2. Pore size
Pore size is one of the essential parameters of MSNs for loading a variety of drug bio-macromolecules. Self-assembly of surfactant molecules with the silica precursor leads to the formation of MSNs with various pore sizes. These pores are formed upon the removal of charged surfactant molecules. In the case of non-ionic surfactants, the formed MSNs will have smaller pores sizes with low surface area. Several attempts have been made to synthesize MSNs with uniform pore sizes with highly ordered hexagonal regular structures. The persistent urge to obtain large pores of MSNs for the accommodation of carrier drug molecules brought the discovery of MSNs in 1992.
Surfactant selection plays a key role in ordering MSNs and affects their pore size. It was postulated that the sizes of the pores could be expanded by employing new templating materials. Pore sizes of the MSNs carriers can be varied by using surfactants of various chain lengths. The surfactants having longer chain lengths will form MSNs of larger pores in comparison to the surfactants of shorter chain lengths. Yano et al. developed monodispersed MSNs having highly ordered regularity by employing n- alkyl trimethylammonium chloride (CnTMACl) as a surfactant, where n =14, 16, and 18. The developed MSNs were aligned from the central point to outwards in a radial manner in the spherical particles (Yano and Fukushima, 2004).
For the generation of MSNs, usually, salts of dodecyl or cetyltrimethylammonium (CTA) were used, such as cetyltrimethylammonium chloride (CTAC) or cetyltrimethylammonium bromide (CTAB). A study reported the formation of MSNs with high porosity by employing a combination of surfactant (CTAB), co-surfactant (dimethyl hexadecyl amine), and oily phase (decane) to introduce new swelling strategies. The obtained carriers also exhibited high mechanical and thermal stability (Egger et al., 2015). In another study, PEG was employed as a stabilizer and added during the reaction process, which resulted in the formation of MSNs having larger cavities with high surface area (Ganguly et al., 2010). In addition, by changing the molar composition of the medium, the arrangement of pores can be changed to cubic (MCM-48) or hexagonal (MCM-41) type.
The ordering of mesostructures in the carriers is dependent on the concentration of TEOS. Increased concentration of TEOS resulted in the formation of a disordered structure of MSNs. However, a lesser concentration of TEOS will not be sufficient that help in the formation of regular mesoporous structures (Chiang et al., 2011). The concentration of surfactant also significantly impacts the ordering of mesostructures. Less concentration of CTAB resulted in poor micellar structures, while its increased concentration may lead to the formation of a highly disordered structure.
Pores size also has a great influence on the release kinetics of drugs. Izquierdo et al. studied the release rate of erythromycin and ibuprofen by observing pore sizes and demonstrated the decreased release rate of the drug with a pore size of the matrix. They also observed a change in the delivery profile of hydrophobic drugs with modification of surface matrix (Izquierdo-Barba et al., 2005).
Further, different strategies were developed in order to change the morphological characteristics of MSNs (small pore size, non-uniformity). In this regard, research was conducted to synthesize monodisperse nanocarriers of silica with dendritic morphology and large porous structure. For this purpose, a partially fluorinated anionic fluorocarbon surfactant (Capstone FS-66) was mixed with CTAB through a sol-gel reaction. The increased concentration of Capstone FS-66 resulted in a larger particle size having a dendritic pore channel (Huang et al., 2017). This strategy was also employed by Yu and his co-workers. They designed MSNs using imidazolium ionic liquid with Pluronic F-127 as particle growth inhibitor and co-surfactants of various alkyl lengths and obtained dendritic MSNs with a particle size of < 200 nm (Yu et al., 2014).
4.3. Shape of MSNs
The MSNs shape plays an important role in the cellular uptake and trafficking of MSNs into cells (Shao et al., 2017). Trewyn et al. showed that tubular MSNs achieve more efficient uptake by normal and cancerous cells than those of spherical shape. This suggests the impact of different shapes on cellular uptake behavior (Trewyn et al., 2008). Huang et al. reported the influence of particle shape on in vivo bio-distribution of nanocarriers; by designing the fabrication of fluorescent MSNs of two different shapes. The distribution of MSNs in various organs has a high influence on shape effects. Rods of shorter MSNs showed more distribution in the liver, while rods of longer MSNs displayed more concentration in the spleen. Also, PEG modification on both types of MSNs makes their distribution higher in the lungs (Huang et al., 2011). Huang and his coworkers; also explained the relationship of shapes of MSNs and the observed cellular responses. They developed MSNs of the same chemical composition, surface charge, and diameter but different aspect ratios and observed the effect of particle shapes of three MSNs on cellular uptake studies. The results demonstrated different internalization behavior exhibited by particles of different shapes. Particles of larger aspect ratios demonstrated increased internalization in human melanoma cells (Huang et al., 2010).
The molar concentration of TEOS, surfactant, base catalyst, and water affects the morphology of MSNs. Non-spherical MSNs can be of cube shape, rod-like, ellipsoid, sheet or film-like materials. However, spherical MSNs have more potential in drug delivery in comparison to non-spherical MSNs. Zhang et al. studied the shape of MSNs on the delivery of Indomethacin. They prepared MSNs of various shapes by using different concentrations of surfactants and alkyl alcohols as co-surfactants. The result depicted different performances of MSNs due to the presence of different shapes (Zhang et al., 2019). Cai et al. synthesize MSNs of various shapes, including silica rods, oblate silica of nanometric size, or in spherical shape by changing the concentration of CTAB, TEOS, NH4OH/NaOH concentration (Cai et al., 2001). Another simple method for the preparation of MSNs was developed by using dodecanol (C12-OH) and CTAB as a soft templating agent. Six different shapes of MSNs were synthesized with modulation of temperature and C12-OH with the development of sphere-like, yolk-shell, hollow and peanut-like structures. The different morphologies were obtained through the use of dodecanol as a soft templating agent (Han et al., 2013). So, changing the micellar concentrations at initial levels may bring out changes in particles morphologies having different aspect ratio values. MSNs of rod-shape act as counterparts of MSNs of spherical shape and can be obtained through modulation of reaction parameters in a typical reaction. By adding the co-solvents in a reaction, i.e., heptane, increasing the concentration of catalyst or by the change of temperature, rod-shaped MSNs can be obtained (Björk et al., 2013; Pang et al., 2005).
Another important shape of MSNs that take part in drug delivery includes ellipsoidal MSNs, but the major challenge associated with this shape is its inability to retain this shape and convert it into a spherical one in order to minimize the surface free energy. This kind of MSNs can be generated by employing ethanol, co-surfactant, and potassium chloride (Shen et al., 2012). Highly porous MSNs of platelet-shaped are designed by employing lower concentrations of heptane, ammonium fluoride, non-ionic block co-polymer P-104, Pluronic 123, sodium dodecyl sulfate, and CTAB (Björk et al., 2013; Chen et al., 2004; Cui et al., 2006). Certain organosilanes can also be used for the purpose of shape transformation. The morphological characteristics mostly depends upon the type and concentration of organoalkoxysilanes precursors. These morphological features of MSNs are generated due to the presence of different bonding and interactions among the chemical groups such as; hydrophobic interactions, hydrogen bonding, etc., between surfactant templates and organoalkoxysilanes (Huh et al., 2003).
5. Surface modification and functionalization of MSNs for drug delivery
The premature release of drugs is a great challenge for the delivery of drugs at the required site. Various modifications have been made on the surfaces of MSNs for imparting desired properties which may include controlling the drug release profile or improving the loading capacity of the drug, together with reduction of toxicity profile in comparison to free drug (Nik et al., 2020). Surface chemical modification in MSNs can significantly increase the targeted delivery of drug with an increase uptake in affected cells. Modification can be performed by attaching different functional groups with characteristic features that alter the environmental interface of nanocarriers, thereby controlling their undesirable biological fluids interactions. These functional groups include amino groups containing polymers (polyethylene-glycol, dendrimers, polyethyleneimine, phospholipids), thiols, organic phosphates, etc. Chemical moieties can also be attached or adsorbed onto the external surface of MSNs, especially to the surface of silica, by either covalent linkage or electrostatic interactions. Positively charged moieties interact with the negatively charged silica surfaces to impart desirable features to the MSNs (Tarn et al., 2013). Modification can be made to their internal surface by grafting (post-synthesis functionalization) or co-condensation (in-situ functionalization), enabling their wider use in various applications. In comparison to the grafting modification, the technique of co-condensation provides improved bonding properties between the porous walls of MSNs with subsequent organic groups. This results in the development of a more homogenous structure of organic functional groups onto the porous structure of MSNs (Kobler et al., 2008).
The presence of silanol groups in the structures of MSNs allows greater modifications in order to make tremendous biomedical applications. Various modifications can be performed onto the hydroxyl groups of MSNs that can be amine, hydroxyl, thiols or other organic chain modifications. These modified surfaces perform an important role in the release of a drug under particular physiological conditions and control the release of the drug at particular stimuli. These modifications also improve various agents loading capacity, particularly siRNA and DNA, promoting targeted delivery of various cytotoxic agents (Natarajan and Selvaraj, 2014). The first capped MSN, developed in 2003, was coumarin-modified MSN, after which various MSNs were developed, decorated by capping agents of diverse properties. These agents can be proteins, polymeric supramolecules, or DNA fragments that respond to specific internal or external stimuli to release loaded cargo/drug. As glutathione (GSH) concentration is remarkably higher inside the cancerous cells (10 mM) as compared to extracellular matrix (2 um), suggesting the presence of reduction potential for triggering drug release (Wang et al., 2015). Reduction potential provides selectivity by the splitation of disulfide bonding due to the presence of a large quantity of thiol groups as compared to the normal cellular environment (Karimi et al., 2016).
Capping of MSNs is mainly involved in two parts. In the first part, the gating mechanism is provided by certain immobilized molecular stalks that are covalently bonded to MSNs covering the pores of MSNs; while in the second part, certain labile molecular groups are non-covalently attached to the stalks. Alterations in the normal physiological conditions results in the reversible alteration of mobile molecular binding, resulting in the cleavage of weaker bonds between movable molecules and stalk that uncaps the mesoporous structures of MSNs, provoking drug release (Pan et al., 2012a, Pan et al., 2012b). Tremendous work has been carried out in order to develop various materials that can be used as gate keepers. About a decade ago, nanoparticles of certain inorganic materials such as FeO and CdS were used to cap the pores through the formation of redox-sensitive disulfide bonds (Giri et al., 2005; Lai et al., 2003). On the addition of a thiol-containing moiety dithiothreitol (DTT), the disulfide bonds break their interaction with the nanoparticles while releasing the encapsulated agents. After that, other gatekeepers were introduced, such as synthetic polymers (Hong et al., 2008), cyclodextrin or its supramolecular systems (Zhou et al., 2014), biomacromolecules (Wen and Oh, 2014), inorganic nanoparticles (Liu et al., 2010), peptides (Li et al., 2014), etc.
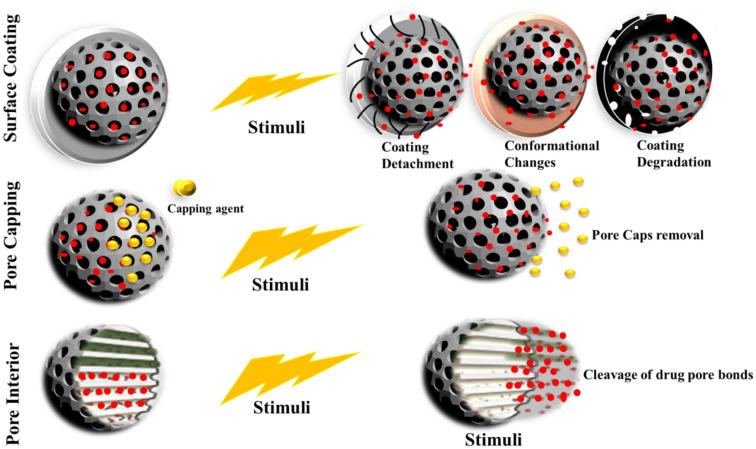
The cargo release from the MSNs carriers can be done through the use of three approaches, as depicted in Fig. 3. Among which, first can be achieved by coating the entire surface of particles (Zhang et al., 2015), in the second approach, pores of MSNs can be sealed by employing certain gatekeepers or molecular gates (Song and Yang, 2015), while the third approach utilizes the coupling of drug cargo with the internal walls of the porous structure (Moreira et al., 2016). All of these approaches will, in turn, undergo any of the two responses, i.e., conformational destruction or any change of the gate keeper covering the pores or the cleavage of the pore covering agent upon required stimulus (Moreira et al., 2016; Peng et al., 2013). In this respect, various approaches have been developed in order to control the release of a drug; that may include applications of different polymeric combinations that may form firm bonds covalently or either form surface adsorption onto the cargo. The carriers releases the drug upon exposure to either external or internal stimuli by either uncoiling or swelling (Corbalan et al., 2012a).
Fig. 3.
Strategies utilized for the creation of stimuli-responsive MSNs and their release mechanism.
Different groups used in the functionalization of MSNs are discussed below.
5.1. Chitosan
MSNs can be modified by using various materials that may act as a gate keeper such as polysaccharides, polymers, or biomolecules to obtain targeted drug release under the influence of specific stimuli. Polysaccharides have gained immense importance to be used as gate keepers due to a variety of attractive features (Wen and Oh, 2014). Among these polysaccharides, Chitosan is one of the remarkable polymer, that has been extensively utilized as gate keeping agent for MSNs. Chitosan, a linear polysaccharide having basic properties, is widely used as a capping agent for MSNs due to its higher biocompatibility and biodegradability (Qindeel et al., 2019). Moreover, the molecular weight and degree of deacetylation of chitosan also greatly affect its properties and functionalities. The molecular weight of chitosan generally ranges from 10000 and 1 million Dalton, with a degree of deacetylation extending from 50 -95 %. As most of the polymer applications are mainly attributed to its properties based on protonation of amine groups molecules in the acidic medium. This protonation degree or pKa is dependent on molecular weight of the polymer and ultimately affect the particle size and release properties of drugs. Increase in the molecular weight of polymer permits faster release of drug (Fernández-Pan et al., 2015; Safdar et al., 2019). Cui et al. proposed the development of unique charge reversal MSNs through hybrid technology by using negatively charged carboxymethyl chitosan and positively charged chitosan. This charge reversal chitosan/carboxymethyl chitosan is designed as a pH stimuli-responsive trigger through the effect of protonation and deprotonation of respective polymer layers. This kind of surface charge reversal improves the delivery and retention time of Doxorubicin for the efficient treatment of breast cancer. He also demonstrated the effect of the molecular weight of chitosan on the particle size of nanocarriers. Increased particle size was observed with a molecular weight of 1000 kDa than with a molecular weight of 200 kDa (Cui et al., 2019). The deacetylation degree of chitosan also affects its properties related to cross-linking, solubility, degradation behavior, and release characteristics. The increased value of polymer’s deacetylation degree decreases its crystallinity with increased degradation rate and higher release profile (Safdar et al., 2019).
Different studies reported the capping of MSNs with chitosan through the attachment of functional groups for targeting the drug at the required site by making it responsive to specific stimuli. Among these, the changing pH is extensively studied on account of the fact that most tumors have an acidic microenvironment. Therefore, chitosan is mostly studied as capping material for MSNs as a pH-responsive gatekeeper. Chitosan-capped MSNs can be activated by an environment of acidic pH and degraded under the action of lysozymes (enzymes of the immune system). As, the amino groups of chitosan became activated in acidic pH and converted into their cationic form, thereby acting as a positively charged polyelectrolyte. Zeiderman et al. designed gold nanorods that were coated with MSNs capped with chitosan for targeted delivery to pancreatic tumors. The pH-sensitive variant was attached with the chitosan to deliver the carriers specifically at the acidic environment of tumors. The encapsulated drug gemcitabine displayed a greater cytotoxic effect when delivered through these carriers than free drugs (Zeiderman et al., 2016).
Chitosan being a cationic polymer, also improves the loading of genes and related molecules. Lin et al. reported the co-delivery of Doxorubicin and p53 gene through MSNs modified with dendronized chitosan that acts as a ‘gate keeping agent’ for the achievement of combination therapy with zero premature drug release. The drug was released from the carriers upon activation of a polymer through redox stimulus in the presence of GSH. Stimulus-induced release of the doxorubicin was reported with efficient gene delivery having increased expression of p-53 proteins in Hela cell culture (Lin et al., 2017).
The polymer also allows grafting of other active ligands for the design of site-specific targeted delivery. Yan et el. developed safe and biodegradable hollow MSNs by capping chitosan polymer on its outer surface that was further linked with glycidoxypropyl-tri-methoxy-silane (GPTMS) through siloxy bonding. Further targeting properties were provided by the folic acid attachment that allows the encapsulated agents, Doxorubicin and Pheophorbide (photosensitizer), to be available at the tumor site. The results demonstrated very efficient antitumor activity through the design of efficient delivery carriers (Yan et al., 2020). Shakeran et al. developed biodegradable MSNs by their modification with 3-triethoxysilylpropylamine (APTES) followed by chitosan modification through glutaraldehyde. This helps to increase the loading capacity of Methotrexate with more efficient uptake as an anti-cancer agent for the treatment of breast cancer (Shakeran et al., 2021).
Functionalization of MSNs through Chitosan is advantageous in terms of its natural origin, increase biocompatibility, and biodegradability with non-toxic profile, which imparts useful characteristics to MSNs in terms of biocompatibility and degradation. Also, the carrier is present with innate properties of anti-inflammatory, anti-bacterial, and mucoadhesive characteristics. Thus it is utilized in different biomedical fields in terms of targeting potential, anti-inflammatory, and wound healing properties (Jhaveri et al., 2021). However, the polymer is associated with a poor solubility profile at physiological pH and can only be dissolved at acidic pH. Moreover, the amino groups of the polymer become protonated at intestinal or basic pH and can be activated in the bloodstream through different enzymes (Heidari et al., 2021).
5.2. Multifunctional polymers
Various polysaccharides can be employed as gatekeepers due to the presence of their tunable surface, ease of synthesis, low toxicity profile, abundance in nature, and excellent biocompatibility. In this kind of approach, multiple layers of the polymers can be capped on the surface of MSNs for the implementation of the desired properties. These multilayered capping strategies are biocompatible and also allow functionalization to the MSNs surface.
Through the development of the stimuli-responsive system, the carriers prevent the release of cargo at the off-target site, as at higher pH values, the polymer remains present in a deprotonated state or in hydrophobic form causing a collapse of polymer onto the surface of silica. After their entry into endosomes or at acidic pH, the drug-loaded in the MSNs got released by switching the carriers into an open state. In a recent study, MSNs were constructed by utilizing the multiple layers of chitosan and alginate through a layer by layer technique for developing the pH-sensitive nanocargoes. Alginate is a polysaccharide of acidic properties that, when combined with the basic chitosan, provides multilayered coatings to the structure of MSNs. These kinds of MSNs displayed excellent bioavailability with low cytotoxicity and hemolytic activity compared to bare MSNs with the provision of the pH-dependent release of cytotoxic drug ‘Doxorubicin’ at the tumor site (Yang et al., 2010). In recent work, 5-fluorouracil was encapsulated in MSNs, and capping was done with the glucuronic acid-chitosan layers, for its delivery to the colorectal tumor environment. The drug was delivered to the targeted area by employing the strategy of the acidic tumor microenvironment, together with overexpression of lectin receptors. These lectins are proteinous in nature and bound specifically with the glycoproteins promoting active targeting of a drug (Narayan et al., 2021). Niedermayer et al. demonstrated multifunctional polymer capped MSNs for targeted delivery to cancer cells, in which a highly stable sequential attachment of specified functionalities to the surface of MSNs was presented. Through this approach, they developed multifunctional delivery carriers, having higher loading capacity through the presence of mesoporous silica core capped with poly-2-vinylpyridine (pH-responsive polymer). The bi-functional polymer was also functionalized with other groups, like PEG (for increasing permeation, retention and circulation time), additional endosomal opening groups (for release of drug in endosomal region), and targeting ligands.
In addition to pH, the introduction of photosensitizer polymer and magnetic nanocarriers were also developed. So, by developing this kind of multifunctional carriers, improved targeting was achieved through cellular uptake at acidic pH, photoactivation, or applying a magnetic field (Niedermayer et al., 2015). Hegazy et al. designed polymeric MSNs by modification with disulfide bonding and photoresponsive polymer, poly (2-nitrobenzyl acrylate) loaded with ibuprofen. Upon treatment with ultraviolet light, o-nitrobenzyl ester cleavage in the polymeric chains releases the loaded drug in a controlled manner (Hegazy et al., 2019). In another study, Zhang et al. developed polymeric MSNs that release the encapsulated drug upon the influence of triple stimuli of light, pH, and reducing agent (Zhang et al., 2015). The MSNs were modified with a capping of poly (2-(diethylamino) ethyl methacrylate, which is sensitive to pH and contains cleavable disulfide bonds and o-nitrobenzyl ester that is cleavable to light. The nanocarriers encapsulate Doxorubicin, demonstrating efficient cytotoxicity against HeLa cells, presented promising potential for cancer treatment (Zhang et al., 2015). Guisasola et al. developed iron oxide magnetic responsive MSNs and coated them with thermoresponsive polymer. Upon application of alternating magnetic field, these magnetic nanocarriers behave as an internal source of heat, increasing the surrounding temperature of a cancerous area with the release of the entrapped drug. This kind of synergic action provides huge tumor treatment innovation (Guisasola et al., 2018).
Functionalizing MSNs with different polymeric agents helps design a more advanced nanocomplex, combining advantages of different polymers. Polycationic complexes with MSNs are more advantageous and versatile with promising biological applications. Alginate, an acidic polysaccharide that combines with the basic chitosan through multilayered coatings, provides increased protection to the loaded cargoes. In this way, this kind of polymeric system is not only advantageous to increase the biocompatibility of the polymer but is also helpful in effective functionalization with a more shielding effect (Feng et al., 2014). The layering of different polymers on the surface of MSNs helps provide enhanced biomedical activities, i.e., a study demonstrated enhanced anti-cancerous activity after surface decoration of MSN with PEG and PEI than a single layer of chitosan (You et al., 2015). However, by implementing this strategy, the particle size and the release characteristics of the carrier can be altered. A detailed study should be performed to evaluate the in vivo fate of these carriers.
5.3. Dextrin
Dextrin is another low molecular weight starch derivative and has been widely used for the capping of MSNs surfaces. It is commonly used as a gate capping agent for the design of pH-sensitive MSNs with more water solubility. In recent years, various strategies have been developed for the design of dextrin capped MSNs. Research conducted by Chen et al. developed MSNs loaded with doxorubicin hydrochloride and capped them with dextrin. Afterward, dextrin was oxidized to obtain dextrin dialdehyde for its coupling with tetraethylenepentamine (pH sensitive Schiff’s base) for ‘closing the gate’ to avoid the premature release of a drug. The aldehyde groups in the dextrin dialdehyde form cross-linking with the Schiff’s base produced from tetraethylenepentamine to ‘close the gate.’ In this way, the modified dextrin capping of MSNs acts as a gatekeeper for the control of the release of drugs, particularly at the acidic tumor microenvironment, by acting as a pH-sensitive delivery system. Under a normal physiological environment, Schiff’s base will remain stable, avoiding the premature release of a drug, while at low pH of tumor region (5-6.8), the Schiff’s base will be hydrolyzed, allowing slow diffusion of the drug illustrating its controlled release behavior (Chen et al., 2016).
Dextrin-coated MSNs sensitive to glutathione and internal enzymes were also fabricated for targeted delivery in tumor cells. Chen et al. developed an on-demand drug delivery system by developing stimuli-responsive MSNs that were end-capped with dialdehyde dextrin that acts as a gate keeper for sealing the drug inside the pores of MSNs. The polymer is further interlinked with GSH sensitive disulfide bonds. The polymer tightly capped the surface of MSNs and responded only under acidic or GSH conditions for the release of the entrapped drug. These Dextrin-modified dual responsive MSN carriers behave as promising nanocarriers for the release of on-demand drug delivery (Chen et al., 2018b). Another research reported that the capping of organic silica nanocarriers with dialdehyde dextrin through Schiff base bonding releases the drug upon stimulation of acidic pH and redox potential (Li et al., 2020). Graphene oxide nanocarriers were also conjugated with dextrin, which was stimulated by α-amylase enzyme and released drug at the tumor site having higher permeability through the endothelial barrier with better penetration profile (Kiew et al., 2017).
Other starch derivatives of hydrolyzed form, i.e., Glucidex 29, Glucidex 37, Glucidex, and 47, are also used for capping purposes having different concentrations of maltose and glucose. These were also degraded by enzymatic action for uncapping the surface of MSNs.
The advantage of dextrin functionalization helps to impart greater bio-compatibility and bio-degradability to the MSNs nanocarriers. Moreover, the polymer is also of lower molecular weight and can be utilized as a superior capping agent for the release of targeted drug delivery. However, the disadvantage associated with the polymers is their low stability profile (Kagami et al., 2003).
5.4. Hyaluronic acid
In 1934, a chemical substance from bovine eyes containing two sugar molecules was isolated by Karl Meyer and John Palmer and was termed as ‘hyaluronic acid’ due to the presence of uronic acid (one sugar molecule) and its isolation from hyaloid (vitreous body) of bovine eyes (Meyer and Palmer, 1934). Further research demonstrated that the molecule is composed of non-sulfated glycosaminoglycan (polysaccharide composed of monomers of N-acetyl-D-glucosamine molecules joined together by -1, 3, and -1, 4 glycosidic linkages. It is considered as one of the vital biopolymers playing an important role in a number of physiological activities.
The polymer is negatively charged, having non-immunogenicity, increased biodegradability, and biocompatibility. Moreover, its increasing ability to target a variety of chemical receptors like CD-44 (Cluster of Differentiation 44), CD-168 (Cluster of Differentiation 168, playing a role in wound healing), RHAMM (receptor for hyaluronan mediated motility), Toll-like receptors, HARE (HA receptor for endocytosis) plays an important function in cellular internalization of nanocarriers at the targeted site. Moreover, the polymer degrades by the action of hyaluronidase-1 (lysosomal enzyme) into fragments of lower molecular weight.
The enveloping of hyaluronic acid to the surface of MSNs help them to act not only as the capping agent but also as a targeting agent to the targeted site. Massive research has been carried out to target hyaluronic acid capped MSNs at the diseased site with increased expression of its receptors (Chen et al., 2013b). Zhou et al. developed hollow MSNs and functionalized them with dopamine-modified hyaluronic acid, which acts both as a gate keeper and a targeting agent. The system delivered the encapsulated drug ‘Doxorubicin hydrochloride’ together with the fluorescent dye ‘indocyanine green’ to the cancerous region. The developed system releases the enveloped agents at the targeted site by acting as a pH-sensitive system (Zhou et al., 2021). Chen et al. fabricated hybrid MSNs by capping with hyaluronic acid through hydrazine bonds. The system releases the drug in response to acidic pH with enhanced targeting towards CD-44 expressed cells in Hela cells. The developed carriers displayed enhanced release of Doxorubicin in response to acidic pH, demonstrating effective treatment against cancer cells (Chen et al., 2018a).
Nairi et al. demonstrated the effect of chain length of hyaluronic acid on cellular internalization to achieve targeted drug delivery. For this purpose, they functionalized MSNs first with amino acids and then with hyaluronic acid of various molecular weights for targeting cancer cells (Nairi et al., 2018). A study was also conducted in which hydrophobic drug camptothecin was loaded in MSNs capped with hyaluronic acid for the treatment of cancer with enhanced cytotoxicity (Ma et al., 2012). A study was also conducted to target drugs through hyaluronic acid capped MSNs to treat human colon cancer by demonstrating their effective cytotoxicity against HCT-116 cell lines (Yu et al., 2013). Gary-Bobo et al. developed MSNs capped with a layer of poly-(L-lysine) together with hyaluronic acid through layer by layer technique for the treatment of colorectal cancerous cells. Polyelectrolyte multilayered system was developed by electrostatic interactions of oppositely charged polyelectrolytes consisting of positively charged poly (L-lysine) and negatively charged hyaluronic acid. These functionalized MSNs were targeted to the colorectal cancer cells having CD-44 overexpression, which is mainly involved in cancer progression with tumor angiogenesis (Gary-Bobo et al., 2012). Similarly, redox responsive drug delivery was developed based on hyaluronic acid capped MSNs, in which drug (6-mercaptopurine) was released in the presence of GSH (Zhao et al., 2014).
The advantage of functionalization of MSNs with hyaluronic acid resulted in the formation of MSNs with high bio-degradability and biocompatibility. Moreover, a study reported the effect of different chains of hyaluronic acid on MSN properties. The smaller diameter of particles was obtained with a high molecular weight of hyaluronic acid, with efficient internalization through the process of endocytosis. This is due to the multipoint attachment of longer hyaluronic acid chains and its entanglement on the MSN surface, which produces particles of smaller size (Chen et al., 2018a). However, the technique requires extensive in vivo studies for the determination of bio-distribution and fate of MSNs.
5.5. Amino groups
Functionalization with amino groups can be done on the matrices of MSNs for the control of drug interaction with the matrix surface that further controls its release rate. Amino-tagged MSNs were developed especially for the better interaction of siRNA. The MSNs shell having a negative charge is being functionalized with the positively charged blocks of amino acid for the development of a highly efficient delivery system.
This method was utilized for modification of MCM-41 nanocarriers encapsulated with enrofloxacin hydrochloride by the grafting of amine carriers to inner channels while thiol groups were introduced onto the outer surface of MSNs through a stepwise approach. Introduction of –SH and –NH2 groups form bonding with negatively charged carboxyl groups avoiding the premature drug release. Moreover, attachment of –NH2 functional group promotes increased attachment of ibuprofen on MSNs facilitating sustained drug release and better relaxivity in MRI (Carniato et al., 2015). However, little control over final functional groups localization brings random distribution of functionalized groups on the surface of MSN (Linares et al., 2011). He et al. developed amino-functionalized MSNs by post grafting method for Doxorubicin delivery. Amino functionalized MSNs resulted in increased loading of drug and releases drug at acidic pH. Doxorubicin-loaded amino-functionalized MSNs displayed efficient cytotoxic potential against non-small cell lung cancerous cells (He et al., 2017). Geng et al. developed amino-functionalized MSNs for delivery of GLP-1AR (Glucagon-like peptide) and FGF-21 (Fibroblast growth factor 21) plasmids for treatment of Diabetes mellitus type 2. Liraglutide is a glucagon-like agonist of peptide-1 receptors, and FGF-21 helps to treat insulin resistance and glucose metabolism. The developed amino-functionalized MSNs carrying both FGF-21 and liraglutide for transfection of Hepa 1-6 cells (Geng et al., 2021). Similarly, co-functionalization of MSNs by chelating it with gadolinium (Gd-DOTA) and amino groups provides better contrast for MRI by binding of amine groups with the terminal carboxylic moieties on gadolinium chelate (Carniato et al., 2015).
Amino functionalized MSNs are also used for improving the release profile of poorly water-soluble drugs. Wang et al. synthesized chiral MSNs and functionalized them with an amino group for enhancing the release profile of poorly water-soluble drug Indomethacin. The resultant 2-D hexagonal carriers with curled channels differed in morphological features from other 2D hexagonal MSNs with straight channels. After loading a drug through hydrogen bonding into silica carriers, the crystalline drug was converted into an amorphous form with improved drug dissolution. These chiral curvature channels hold more drugs in comparison to straight channels carriers. The loaded carriers displayed fast drug release with an improved dissolution profile (Wang et al., 2019).
The amino-functionalized MSNs are more frequently utilized for siRNA delivery. The MSN shell having a negative charge is normally functionalized with the cationic co-polymer with the production of an advanced capped system, having an efficient binding ability. The system helps in the delivery of loaded cargo at the targeted site by uncapping through an endosomal pathway. The presence of multi-amine moieties enhances the affinities of siRNA with the particles. The main disadvantage of this method is the hindrance of the release of siRNA in the cytoplasm due to the formation of strong polyplexes, which ultimately decreases the efficacy of siRNA, also called ‘vector unpacking.’ The second main disadvantage of this method is polycation cytotoxicity, raising the concern about the nanocarrier’s toxicity (Hartono et al., 2014).
5.6. Nucleic acids
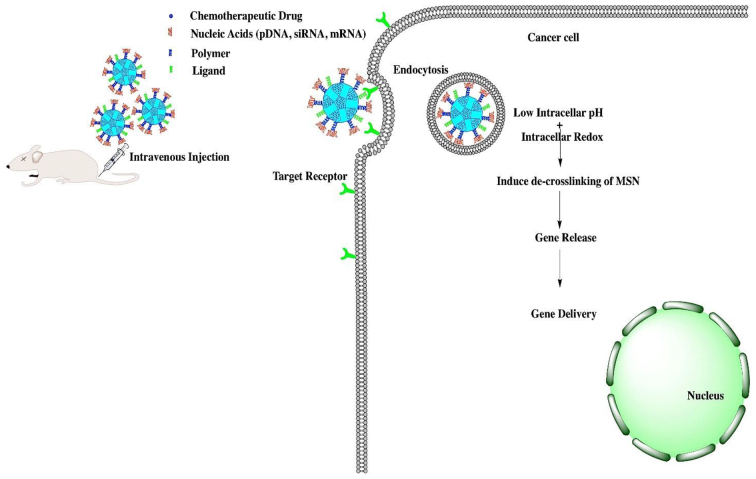
Nucleic acids are recognized as one of the most attractive building molecules due to their specific sequence, polymorphic conformation, and versatile physicochemical attributes. Due to their universal properties, DNA molecules are designed to fabricate stimuli-responsive MSNs with intelligent and on-demand delivery of the required therapeutic agent. Researchers developed various strategies for achieving targeted delivery using DNA molecules by complexing DNA molecules with agents that act as gatekeepers and release drugs at the target site in response to the specific stimuli as depicted in Fig. 4 (Li et al., 2011).
Fig. 4.
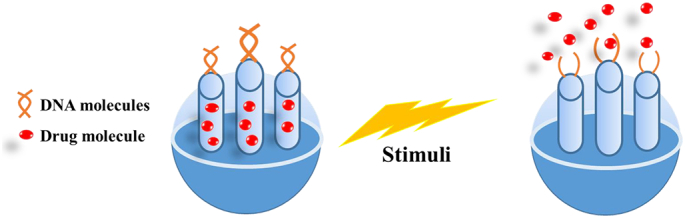
Stimuli-responsive DNA gated release of drug from MSNs.
Controlled mechanisms for the release of encapsulated agents can be made by utilizing the principle of complex binding of metal ions, i.e., silver or mercury with thymine and cytosine residues of DNA complexes. Functionalization of the pores of MSNs with DNA molecules is being made that are rich in cytosine and are further complexed by the binding of the Ag+ ions that closes the outlet and acts as an intelligent molecule gated switch by forming a complex of C-Ag-C. The porous opening is controlled by the competitive reaction by the molecules that displaces the complex of C-Ag-C and converts it into the single-stranded molecule of DNA. This deformation of the DNA complex resulted in the presence of certain thiol molecules that resulted in the opening of the porous structure with site-specific release of the encapsulated drug molecule. So, the reversible opening and closing of the porous structures depend on the external concentrations of Ag linkers and the molecules containing thiol groups (He et al., 2012).
The development of highly selective MSNs carriers that are sensitive to GSH concentration has been developed; by employing thymine-rich DNA molecules coordinated with mercury (Hg+) ions. Hg+ ions form stronger interaction with the thymine bases resulting in the formation of a complex of T-Hg+-T. Upon the detection of specific target GSH, this complex undergoes deformation into single-stranded DNA, with the release of active molecules of methylene blue (Wang et al., 2017b). Another study reported that DNA molecule acts as a very responsive stimuli for delivery of active moiety (Rhodamine) by employing quadruplex DNA with stretches of Cytosine base and act as gate keeper onto the pores of MSNs. It undergoes conformational changes upon a change of pH, providing unique property to function as a gate coupling agent. Under acidic conditions, the cytosine bases will remain present in their protonated form with the perseverance of closed DNA folds in a quadruplex form, while an increase in pH results in the deprotonation of the C+ residues, resulting in the unfolding of DNA structure to single-stranded form. This leads to the release of entrapped moiety into the surrounding area through the inter-conversional opening and closing of the pores upon a change in the pH (Chen et al., 2011a).
Similarly, DNA-controlled molecular gate was also developed by attachment of DNA to the outer pores of MSNs carriers that were further operated by the melting of its strands upon certain temperature with the release of entrapped agent (Schlossbauer et al., 2010). Another study described the DNA-modified hollow MSNs for the treatment of cancer based on chemo-photothermal therapy (Hai et al., 2018). Researchers also reported the development of DNA functionalized MSNs carriers that release the cytotoxic agent upon exposure to visible light. In this context, MSNs based on Ruthenium polypyridyl complexed DNA were developed for the release of cytotoxic moieties including docetaxel and paclitaxel. This hybrid complex of ruthenium cleaved only under the exposure of visible light, releasing active moiety at the targeted site and present as a promising system that can be selectively used for the treatment of breast cancer (Frasconi et al., 2013).
Another study reported that DNA grafted MSNs that are sensitive to increasing the concentration of ATP, particularly in the tumor microenvironment. MSNs were modified with ATP and amine-modified aptamers of mucine that were covalently anchored onto the surface of MSNs. ATP aptamers were immobilized on MSNs surface with hybridization of mucine aptamer and resulting in the formation of a Y-shaped structure of DNA, acting as a gatekeeper on the surface of MSNs. The developed DNA grafted MSN carrier displayed an efficient loading capacity of Doxorubicin and released it at the tumor environment, where the concentration of ATP is high (10mM). This strategy displayed that the release of the drug occurs in the tumor region without its leakage at any undesired area or in the systemic circulation, where the concentration of ATP is less (1uM), and avoiding systemic toxicity of the cytotoxic drug (Bagheri et al., 2021). In this way, modification of MSNs with DNA by utilizing various approaches provides new insights for the treatment of a wide variety of dreadful diseases.
Functionalization of MSNs with nucleic acid helps in more precise manipulation of nanocarriers with the opportunity to develop MSNs in larger quantities. This allows the MSNs to combine with the nucleic acids with the development of a unique and more precise nanostructure, which removes only under the influence of specific stimulus. However, the method requires the determination of more sequences of reactions to identify specific interactions and binding properties of these agents with MSNs (Xu et al., 2021). General scheme of stimuli-responsive DNA gated release of drug from MSNs is shown in Fig. 4.
Surface modification of MSNs in response to specific stimuli are given in Table 2.
Table 2.
Surface modification of MSNs based stimuli-responsive controlled release system of various drugs.
| Chemical modification/functionalization of MSNs | Drug loaded in MSNs | Active stimulus | Effect of functionalization | References |
|---|---|---|---|---|
| Chemical modification of MSNs with Pegylated poly amino acid | Celastrol | pH | Targeted drug delivery to solid cancer by the release of drug at acidic pH, targeted delivery of drug to mitochondria | (Choi et al., 2018) |
| Chemical modification of MSNs with Calcium | siRNA, Chloroquine | pH | Targeted drug delivery to ovarian cancer cells at acidic pH | (Choi et al., 2020) |
| Chemical modification of MSNs with gold nanoparticles- biotin complexes | Doxorubicin | Enzyme | Cytotoxic potential against cells highly expressed with matrix metalloproteinase enzyme | (Eskandari et al., 2019) |
| Chemical modification of MSNs with quadruplex DNA with stretches of the Cytosine base | Rhodamine | pH | Targeted delivery to cancer cells having acidic pH | (Chen et al., 2011a) |
| Chemical modification of MSNs with poly(amidoamine) dendrimer (PAMAM) | Doxorubicin | pH | Increase mucoadhesive properties for bladder cancer therapy, Targeted delivery to cancer cells having acidic pH | (Wang et al., 2020a) |
| Chemical modification of MSNs with cytochrome C and aptamer | Doxorubicin | Redox potential | Targeted delivery to a liver tumor (HCT-116 cell lines) having increased GSH concentration | (Zhang et al., 2014) |
| Phosphonated MSNs | Ruthenium complexes | Hypoxic condition | Detection and quantification of molecular oxygen with targeted release at a reduced oxygen concentration | (Umehara et al., 2021) |
| Chemical modification of MSNs with DNA molecules | Fluorescein | Temperature | Targeted drug delivery upon increased temperature | (Schlossbauer et al., 2010) |
| Chemical modification of MSNs with Thymine rich DNA molecules coordinated with mercury (Hg+) ions | Methylene blue | Redox potential | Stimuli-responsive effect of changing in GSH concentration | (Wang et al., 2017b) |
| MSNs modified with amino groups | Octahedral Organoruthenim complex | pH | Efficient cytotoxic potential against glioblastoma cells having acidic pH, anti-cancerous activity | (Martínez-Carmona et al., 2020) |
| MSNs Ruthenium polypyridyl complexed DNA | Docetaxel, Paclitaxel | Light | Stimuli-responsive effect to light for the treatment of breast cancer, drug release on application to light | (Frasconi et al., 2013) |
| MSNs modified with the amino group | Doxorubicin | pH | Efficient cytotoxic potential against non-small cell lung cancer cells having acidic pH | (He et al., 2017) |
| MSNs capped with gold nanoparticles and linked with short single-stranded DNA as gatekeeping agent | Doxorubicin | Laser stimulation, pH | Stimuli-responsive effect upon acidic pH and laser stimuli, for treatment of cancer | (Zhou et al., 2019) |
| MSNs modified with an amino group | Liraglutide, Fibroblast growth factor 21 | – | Diabetes mellitus type 2 | (Geng et al., 2021) |
| MSNs modified with ruthenium complexes | Safranin O | Light | Photochemical drug delivery system | (Salinas et al., 2020) |
| Y-Shaped DNA grafted MSNs | Doxorubicin | ATP | Stimuli-responsive effect to ATP for treatment of cancer | (Bagheri et al., 2021) |
| Chemical modification of MSNs with Dextrin coupled with Schiff’s base | Doxorubicin hydrochloride | pH | Stimuli-responsive effect to acidic pH for treatment of cancer | (Chen et al., 2016) |
| Chemical modification of MSNs with gold nanoparticles | – | Redox potential | Anti-cancerous activity having increase GSH concentration, by A-549 cells | (Augspurger et al., 2018) |
| Carboxyl functionalized MSNs capped with cerium oxide nanoparticles | Doxorubicin | pH | Targeted delivery to Hela cells at acidic pH | (Singh et al., 2018) |
| Chemical modification of MSNs with dextrin | Doxorubicin | Enzyme | Anti-cancerous activity with increased penetration having increased concentration of α-amylase enzyme | (Kiew et al., 2017) |
| Chemical modification of MSNs with peptide-based amphiphile | Doxorubicin | Redox potential | Targeted drug delivery to tumor cells at increased GSH concentration | (Cheng et al., 2017) |
| Chemical modification of MSNs with gelatin | Doxorubicin | pH | Targeted drug delivery to tumor cells at acidic pH | (Zou et al., 2013) |
| Chemical modification of MSNs with Cytosine rich DNA complexed with Ag+ ions | – | Thiol groups | Targeted drug delivery, stimuli-responsive effect to thiol groups | (He et al., 2012) |
| Chemical modification of MSNs with gelatin | Doxorubicin | Enzyme | Cytotoxic potential against cells, highly expressed with matrix metalloproteinase enzyme | (Xu et al., 2013) |
| Multifunctional polymer capped MSNs (Chitosan and alginate) | Doxorubicin hydrochloride | pH | Targeted delivery to cancer cells at acidic pH | (Yang et al., 2010) |
| Chitosan capped MSNs linked with glycidoxypropyl-tri-methoxy-silane | Doxorubicin and Pheophorbide | pH | Targeted delivery to cancer cells at acidic pH | (Yan et al., 2020) |
| MSNs modified with dendronized chitosan | Doxorubicin and P-53 gene | Redox potential | Stimuli-responsive effect of increasing GSH concentration for treatment of cancer | (Lin et al., 2017) |
| Gold nanorods coated with MSNs capped with chitosan attached with pH-sensitive variant 7 | Gemcitabine | pH | Targeted delivery to pancreatic tumors Targeted delivery to breast carcinoma cells at acidic pH | (Zeiderman et al., 2016) |
| MSNs coated with chitosan | Raloxifene hydrochloride | pH | Targeted delivery to breast carcinoma cells at acidic pH | (Shah and Rajput, 2018) |
| MSNs modification with 3-triethoxysilylpropylamine (APTES) followed by chitosan modification | Methotrexate | pH | Increase the loading capacity of Methotrexate with more efficient uptake as an anti-cancer agent for breast cancer treatment. Targeted delivery to breast carcinoma cells at acidic pH | (Shakeran et al., 2021) |
| MSNs modification with dextrin dialdehyde | Doxorubicin | pH | Targeted delivery to cancer at acidic pH | (Chen et al., 2016) |
| Charge reversal MSNs modified by using carboxymethyl/chitosan | Doxorubicin | pH | Targeted delivery to breast carcinoma cells at acidic pH | (Cui et al., 2019) |
| MSNs modification with Fe3O4 nanoparticles | Camptothecin | Magnetic field | Targeted delivery to cancer cells upon application of magnetic field | (Chen et al., 2011b) |
| MSNs modification with dopamine modified hyaluronic acid | Doxorubicin | pH | Targeted delivery to mammary carcinoma cells at acidic pH | (Zhou et al., 2021) |
| MSNs modification with glucuronic acid-chitosan layer | 5-Fluorouracil | pH | Targeted delivery to colorectal cancer cells at acidic pH | (Narayan et al., 2021) |
| MSNs modification with gelatin | Paclitaxel | External magnetic field | Targeted delivery to tumor cells upon application of magnetic field | (Che et al., 2015) |
| MSNs modification with protamine | Curcumin | Enzyme | Targeted delivery to colorectal cancer cells having protease enzyme | (Radhakrishnan et al., 2014) |
| Hollow MSNs modification with poly (3-acrylamidophenylboronic acid) (PAPBA) | – | Glucose | Potential use in diabetes treatment, the release of a drug on glucose detection | (Wang et al., 2021) |
| MSNs capped with hyaluronic acid | Doxorubicin | pH | Targeted delivery to Hela cells at acidic pH | (Chen et al., 2018a) |
| MSNs capped with hyaluronic acid | 6-mercaptopurine | Redox potential | Targeted delivery to HCT-116 cell lines at increased GSH concentration | (Zhao et al., 2014) |
| MSNs modified with multifunctional polymer | – | pH | Targeted delivery to Hela cells upon acidic pH | (Niedermayer et al., 2015) |
| MSNs modified with dialdehyde dextrin | Doxorubicin | pH and Redox potential | On-demand drug delivery to increase GSH concentration and acidic pH to cancer cells | (Chen et al., 2018b) |
| MSNs modified with polyethyleneimine | Plasmid DNA | Ultrasound | Drug delivery to cancer cells | (Du et al., 2020) |
6. Mesoporous silica nanoparticles (MSNs) for co-delivery of nucleic acids and small-molecular drugs for cancer therapy
The use of nanocarriers for co-delivery of desirable gene/drug to the disease site is an attractive strategy for clinical therapy (Chen et al., 2017b; Shi et al., 2017). MSNs can also be utilized as a promising carrier for the transfection of genes besides conventional delivery of active agents. As naked genetic material is associated with poor penetration properties, these agents play a great role in the delivery of genetic material. Genetic delivery can be through viral or non-viral means. The viral genetic delivery system is a more efficient system in terms of various safety measures, such as non-specificity, gene recombination, and immunogenicity (Zhou et al., 2018b). Different cationic carriers, inorganic or organic molecules, and recombinant proteins have been served as vital carriers for the delivery of genetic carriers. Among various nanocarriers, liposomal agents displayed great attention to providing efficient transfection of genes but associated with greater instability. The inorganic nanocarriers displayed a greater advantage in terms of physicochemical stability, easy preparation, and surface functionalization. In this way, MSNs showed attractive features for gene delivery with efficient cellular uptake and enhanced transfection efficiency (Kesse et al., 2019). However, MSNS carry negative charge on their surfaces mainly due to the ionization of silanol groups, which ultimately reduces their binding efficiency to the molecules with negative surface charges such as DNA. In this way, modification is being made to the surface of MSNs with net positive charge through techniques such as functionalization with a cationic polymer, co-delivery of cationic metals, and through amination modification. These modifications bring out greater interactions of these MSNs with the negatively charged nucleic material. Stimuli responsive gene delivery for cancer therapy
Modification through amidation is one of the simplest and most common techniques for loading the genetic material in MSNs, in which aminopropyl trimethoxy silane (APTMS) and aminopropyltriethoxy silane (APTES) are commonly employed for modification. Studies also demonstrated increased adsorption efficiency of plasmid DNA with increasing amination degree. Modification of MSNs surface can also be modified with metal cations for facilitation of gene delivery. Metal counter ions such as Mg+2, Na+ or Ca+2 demonstrated efficient affinity with genetic material. Moreover, cationic polymers such as poly-L-lysine, polyethyleneimine, poly-L-arginine, and polyamidoamine can bind effectively to the negatively charged nucleic acid. Yu et al. developed MSNs modified with poly-dimethylamino ethyl acrylate as an efficient nanocarrier for gene delivery. The polymer degrades in water to poly-acrylic acid, depicting controlled siRNA delivery (Hartono et al., 2014; Lin et al., 2014). Studies by Zhou and colleagues demonstrated an innovative approach for nucleic acids delivery into cancer cells, which enhanced the transfection efficiency. Their experiments revealed that encapsulation of Nucleic acids (pDNA siRNA) via ammonium salt (A) modified conical pores MSNs (CMSN-A) enabled intracellular trafficking enhanced gene transfection with the efficient release of cargos. The authors suggest this is due to the presence of increased positive charges of ammonium salts present in CMSN, which lead to an increase the gene loading capacity, gene binding stability and cellular uptake efficiency. This system was able to deliver the cargo at the site of action due to acidic pH and redox potential (Zhou et al., 2020).
MSNs have in general small porous diameter (< 3 nm), due to which most of the plasmids and genes were being adsorbed on the surface of MSNs instead of being encapsulated. Also, the adsorbed genes can be degraded by the action of several enzymes such as lysosomes and nucleases. Therefore, the need of a suitable modification is desired in which MSNs can encapsulate the drug in their pores (Hartono et al., 2012; Wu et al., 2015). For this purpose, MSN carriers with large porous diameters have been prepared by use of pore expanding agents such as 1, 3, 5 trimethylbenzene (Hao et al., 2021). This type of nanocarriers displays increased transfection efficiency with enhanced protection from the cellular environment. Kim et al. demonstrated delivery of chloroquine with plasmid DNA after modification of phosphonated MSNs having ultra-large pore size with polyethyleneimine of different molecular weights. The increased transfection efficiency of these MSNs was examined through green fluorescent protein (Kim et al., 2011). After efficient loading of the genetic material, the carriers respond to specific stimuli for the release of encapsulated genetic material. A recent study reported the release of plasmid DNA from MSNs microbubbles for efficient cancer therapy (Du et al., 2020).
In order to enhance the MSN's biocompatibility, bio-distribution, accumulation, binding affinity, and cellular uptake at the tumor site, widely explored strategies have been developed. Mickler et al. targeted epidermal growth factor receptor (EGFR), a protein commonly expressed on tumor cells, by decorated MSNs with natural EGF or artificial EGFR-binding peptide (GE11). The authors were able to successfully prove that this complex effectively accelerates MSNs endocytosis by tumor cells due to ligand-receptor binding. Folate receptor (FR), a glycosylphosphatidylinositol (GPI)-anchored membrane protein, over-expressed in tumor cells and is an attractive target for drug delivery. López et al. (López et al., 2017) developed an asymmetrically decorated MSN particle with targeting moieties [folic acid (FA) and Triphenylphosphine (TPP)]. The authors demonstrated successful MSN particle accumulation inside tumor cells and binding to mitochondria membrane by the action of FA and TPP moieties, respectively (Mickler et al., 2012). Babaei et al. developed PEGylated road-shaped MSNs as a nanocarrier for the co-delivery of an anticancer drug (camptothecin) and surviving shRNA-expressing plasmid (iSur-DNA) to colorectal cancer. Moreover, these MSNs were tagged with AS1411 aptamer, which has high affinity and specificity to nucleolin protein, facilitating increased drug uptake into the nucleolin positive colorectal cancer cells and suppressing tumor growth (Babaei et al., 2020). Wanger et al. demonstrated MSNs loaded anticancer immune-stimulant R-848 (resiquimod), which were rapidly taken up by specialized antigen-presenting cells (APCs), with potent activation of the dendritic immune cells (DCs) (Wagner et al., 2021). This suggests that MSNs hold significant potential as a carrier for cancer vaccine delivery. General scheme for stimuli responsive gene delivery for cancer therapy is shown in Fig. 5.
Fig. 5.
Stimuli responsive gene delivery for cancer therapy.
In Table 3, various molecules decorated on MSNs surface that are able to interact selectively with specific membrane receptors overexpressed in tumor cells are shown.
Table 3.
. Summary of targeting drug delivery system based on MSNs.
| Targeting receptor | Targeting ligand | Target cell type | Refs. |
|---|---|---|---|
| EGFR | Epidermal growth factor | HuH-7 | (Mickler et al., 2012) |
| α-Folate receptor | Folic acid | MDA-MB 435 MDA-MB-231 PANC-1, MiaPaCa-2 MCF-7, Hela Hela |
(Wang et al., 2010) (Tao et al., 2010) (Sarkar et al., 2016) (Lu et al., 2012) (Prasad et al., 2016) |
| α-Folate receptor Mitochondria membrane |
Folic acid Triphenylphosphine |
LnCAP | (López et al., 2017) |
| Mannose receptor | Mannose | MCF-7, HCT-116, MDA-MB-231 | (Brevet et al., 2009) |
| Galactose receptor | Galactose | Hela, A549 | (Niemelä et al., 2015) |
| Mucin-1 glycoprotein | Mucin-1 antibody | MMT, Mtag | (Dréau et al., 2016) |
| CD105 protein | TRC105 antibody | 4T1 | (Wang et al., 2013) |
| CD44 protein | Hyaluronic Acid | MCF-7, MDA-MB-321, 4T1 | (Kang et al., 2019) |
| αvβ3 integrins | cRGD | MDA-MB 435 | (Ferris et al., 2011) |
| Transferrin receptor | Transferrin | HepG2 | (Montalvo-Quiros et al., 2019) |
7. Biomedical applications of MSNs
It is becoming increasingly evident that MSNs hold strong potential as carriers for biomedical applications. Multifunctional MSNs, which typically range from 50 to 3000 nm in diameter, exhibit numerous outstanding properties, including large surface areas and pore volume with high loading efficiency, tunable release mechanisms, and easy surface decoration by attaching ligands for active targeting. These characteristics of MSNs created a vast era of formulation development in the field of nanomedicine. MSNs found their immense importance in different areas of drug targeting (Bagheri et al., 2021; Murugan et al., 2013), tissue engineering (Nekounam et al., 2021; Pouroutzidou et al., 2021), cell tracking (Kim et al., 2021; Umehara et al., 2021), and genes transfection (Hosseinpour et al., 2021) due to their peculiar characteristics of large surface properties, tremendous internal volume and unique channels that facilitate the adsorption of various protein and drugs. Capping agents can control the behavior of pores opening and closing, hence controlling the precise spatial and temporal release of encapsulated material with characteristics of lower degradation kinetics (Manzano and Vallet-Regí, 2020). These controlled gatekeepers capping the pore entrances of MSNs play prominent and crucial roles in achieving specific drug release and avoiding premature leakage in the delivery process before the target is reached. The perfect gatekeepers can only be removed under specific internal or external stimuli, such as pH, redox potential, temperature, biomolecules, light, magnetic field and ultrasound, or a combination of these stimuli, which is significant for precise therapeutic treatments and potential applications in human bodies. Further, their intrinsic ability of functionalization at interior or exterior surfaces, along with biocompatible profile make these delivery vesicles ideal for biomedical applications (Gruenhagen et al., 2005). Fig. 6. shows, some of the targeting ligands used for the functionalization of MSNs.
Fig. 6.
MSNs modifications with targeting ligands.
Attachment of these moieties results in the modification of drug loading, protein adsorption, biocompatibility, protein adsorption, imaging ability, and immune system interactions (Bürglová et al., 2014). For example, surface functionalization of MSNs with amino acids improves the drug's loading capacity and provides sustained delivery of therapeutic moieties (Liu et al., 2015). Similarly, carboxylic group addition to the surface of MSNs improves drug dispersion and release due to the creation of more negative charges. It also aids in the attachment of fluorescent probes for the facilitation of imaging and diagnosis (Liu et al., 2015). Ibuprofen adsorption on MSNs functionalized surface is extensively studied, in which carbonyl groups formed bonding with the silanol groups. However, in the case of non-functionalized surfaces, Ibuprofen dimer formation has taken place through concomitant interaction of drug molecules through intermolecular bonding through hydrogen bonding. A study conducted by Babonneau et al. demonstrated the incompatibility of ibuprofen loaded in MSNs through an NMR study by observing increased drug on the pore walls of MSNs, showing higher drug motility (Babonneau et al., 2003; Babonneau et al., 2004). Researchers have made numerous innovations for delivering various drugs through MSNs in order to make them more efficient while reducing their unwanted effects.
Notably, MSNs possess advantages over traditional nanocarriers, especially for cancer immunotherapies. Small interference RNA (siRNA) has shown great promise in gene therapy because it can efficiently silence the expression of the desired target with high specificity and minimal side effects. Despite the great promise of siRNA in biomedical application, there have been several obstacles that restrict their clinical application, which includes: (i) naked siRNA is easily degradable by endogenous nucleases with rapid clearance by the renal route and (ii) they cannot be internalized efficiently by target cells due to polar nature of phospholipid bilayer membrane (Chen et al., 2015; de Fougerolles et al., 2007; Liu et al., 2016; van den Brand et al., 2018). Nowadays, enormous efforts have been devoted to developing nanoparticles for gene therapeutics. Ideal gene carriers should meet critical clinical criteria for successful implementation in clinical studies. The most important points are encapsulation efficiency, gene loading capacity, gene protection from degradation, stability of nanoparticles, endocytosis by target cells, controlled gene release at the target site, and lesser cytotoxic effect (Descamps and Benihoud, 2009; Duerner et al., 2008; Li and Huang, 2006; Yue and Wu, 2013). Polyethyleneimine-coated MSN nanocarrier has been demonstrated to be a promising intracellular carrier for glucuronidase (GUS)-encoding plasmid DNA or siRNA due to its high loading efficiency. Since, polyethyleneimine create a homogenous coating on MSN particles to adsorb the negatively charged siRNA. (Cha et al., 2017; Pinese et al., 2018). Pinese et al. described a novel approach for the scaffold-mediated sustained release of siRNA/MSN polyethyleneimine complexes. This system's successful uptake of siRNA downregulated the expression of collagen-1 compared to conventional bolus delivery. Together, these studies provided new insights into MSNs mediated nanoparticle-cell communication and intracellular trafficking (Pinese et al., 2018).
Further, MSNs also help in the monitoring of the targeted chemotherapeutic agent in order to avoid systemic toxicity. Different molecules such as carbon dots, FITC, chlorin, Hoechst, and multicolored up-conversion nanocarriers are generally utilized for the fabrication of nanocarriers. These agents transport the chemotherapeutic agent to the target site and are advantageous in monitoring nanocarrier’s bio-distribution (Hu et al., 2016; Wang et al., 2020b; Zhou et al., 2018a). Yu et al. designed an interesting strategy for the real-time tracking of a drug by employing chitosan capped MSNs loaded with fluorescent dye. These novel nanocarriers mainly consisted of three components: the MSNs for encapsulating the drug molecule, chitosan as a gate keeper for the opening and closing of pores of MSNs and the fluorophore 1 8-naphthalimide for a signal of fluorescence. Pores will remain closed under the absence of GSH concentration as the sulfone blocks the intramolecular transfer of charge, leading to no fluorescence emission. However, in the presence of GSH, its nucleophilic attack removes the chitosan with the opening of pores, triggering drug release and emission of green fluorescence. This strategy helps the researchers in expanding the knowledge of gating the nanocarriers for monitoring the real-time release of drugs for various ailments (Chen et al., 2020b).
MSNs are also ideal candidates as bio-catalysts due to their increased adsorption capacity and increased surface area. The enzymes can be encapsulated efficiently with high stability and act as bio-catalysts for intercellular bioanalysis. Luciferin-loaded nanocarriers in MSN pores through disulfide bonding with conjugation of PEGylated luciferase enzyme were developed for identification of self-catalyst luminescence for detection of the tumor. In the presence of redox potential and ATP, the release of luciferase catalyzed luciferin with the emission of luminescence. This system provides a unique platform for tumor development and its therapeutic response to treatment (Sun et al., 2011).
8. Safety, biodistribution, and fate of the MSNs
Despite various biomedical applications of MSNs, the Food and Drug Administration hasn’t approved MSNs for medical applications until their final fate, bio-distribution, and clearance routes have been addressed. In general, any administered agent undergoes the process of absorption, distribution, metabolism, and clearance. Most commonly, the nanocarriers are administered through subcutaneous or intravenous routes. The injection of these nanocarriers into the bloodstream prompts rapid bio-distribution of these agents through the bloodstream. However, the stability of these nanocarriers is an important determinant factor that decides their ultimate biomedical use (Bourquin et al., 2018). These carriers must be robust enough for the protection of loaded carriers in the bloodstream and degrade after delivering the cargo at the target site. As, MSNs are composed of SiO2 matrix, which is susceptible to OH- (nucleophilic attack) by water molecules present in the aqueous media, leading to the production of orthosilicic acid in the dissolution media and can be excreted by the urine. The produced acid is water-soluble and helps in the maintenance of bones integrity. Thus FDA announces silica as a safe biomolecule for over 50 years (Bourquin et al., 2018; Croissant et al., 2018; Croissant et al., 2017a).
The dissolution rate of MSNs mainly depends upon different parameters of particle properties, such as pores size, surface area, particle size, condensation degree, and functionalization molecules. The incorporation of different additives such as drugs, photosensitizer molecules, or other in-organic agents also affects its dissolution characteristics. The addition of the covalent addition of organic agents leads to the production of a new category of MSNs, termed periodic mesoporous organosilica (PMOs). These materials share the same protocols of synthesis but differ in the degradation rates. In this way, the mesoporosity of the silica molecules can be protected for longer periods of time. In these PMOs, different cleavable organic moieties are introduced, such as lysine, oxamide, disulfide, tetrasulfide, etc., to introduce their on-demand degradation (Croissant et al., 2017b). The dissolution also depends on the properties of degradation or dissolution media, such as its temperature, concentration, and pH, etc. In this way, the MSNs dissolution process can range from a few hours up to several weeks, depending upon its characteristics and final application. The in vitro degradation rate of the MSNs molecules revealed their long-term stability long enough to guarantee the release of loaded cargo at the site of action (Braun et al., 2016). While the in vivo biodegradation of MSNs in different animal models demonstrated increase biodegradation behavior of the carriers having polymeric coatings on outer surfaces. This increases their circulation time in the bloodstream with an increased stability profile.
The bio-distribution profile of MSNs demonstrated their accumulation in the reticuloendothelial system, including the liver, lungs, and spleen (Zhang et al., 2016). This is due to the adsorption of proteins on their external surfaces. These proteins are normally derived from serum and sometimes can also enhance the selectivity of targeted MSNs, indicating the effect of proteins corona on the bio-distribution of nanocarriers (Beck et al., 2017). However, in order to avoid this phenomenon, the surfaces of MSNs are normally coated with hydrophilic polymers such as poly (ethylene glycol) (PEG). . Functionalization of MSNs was also observed, demonstrating increased circulation time of MSNs functionalized with PEG molecule (Chen et al., 2017a; Goel et al., 2016). Also, the non-pegylated nanocarriers demonstrated high accumulation in the spleen, liver, and kidneys. In another recent study, Kang et al. developed hydroxyapatite (HAP) hybrid MSNs, loaded with anticancer drug doxorubicin (DOX) to target tumor cells. HAP departs excellent features of biocompatibility and nontoxicity, to MSNs with further coating of hyaluronic acid (HA), which is a ligand that can bind specifically to CD-44 receptor with enhanced cellular uptake. These strategies improve the biodegradability, drug loading, and drug release and eventually lead to better therapeutic outcomes (Kang et al., 2019).
Bio-distribution of MSNs has also been investigated after their radiolabeling with 64Cu through positron emission tomography in BALB/c mice having xenografted breast and glioblastoma tumors. The bio-distribution in the liver is presented in the highest concentration, regardless of the presence or absence of targeting ligands. The lower amount of concentrations was also present in the spleen, lungs, kidney, and intestines (Chen et al., 2013a; Goel et al., 2014). In another bio-distribution study, it was concluded that by an increase in particles from 80 to 160 nm, a higher accumulation of nanocarriers was observed in spleen in comparison to the liver The particle shape of MSNs also has a strong influence on MSNs bio-distribution. The particles having elongated and spherical morphology showed higher accumulation in the spleen in comparison to spherical particles (Huang et al., 2011). Different studies have been performed regarding renal clearance of MSNs from the body. These studies confirmed the increase elimination of these nanocarriers from the renal route. In addition, hepatobiliary excretion and excretion through bile are normally under the influence of proteins adsorption, while excretion through feces is normally governed by aggregation and accumulation of smaller particles (Manzano and Vallet-Regí, 2020).
9. Conclusions and future perspectives
MSNs are one of the most promising approaches in the biomedical field and introduced as superior carriers to cope with the hurdles of limited drug release and premature release of cargo with a better stability profile. Research has been conducted in controlling the structural properties of MSNs, including their morphology, pore size, particle size, and structure ordering. Further, to achieve increased loading of drugs with their precise control of release at a target site, functionalization of MSNs’ surface by an external or internal decoration of functional groups is being explored. The combination of MSNs with other agents and functional groups, including polymeric substances, chemical moieties or nucleic acids, helps to create hybrid MSNs having great potential for various biomedical applications. Surface treatment of MSNs also improves the interaction of nanocarriers with the biological environment and improves the biocompatibility of the carriers. These modified MSNs can be utilized in various biomedical applications for achieving targeted drug delivery and facilitating controlled release kinetics.
However, despite numerous physio-chemical advantages that led the researchers to develop MSNs for various biomedical applications, especially cancer, there are still some prominent challenges that need to be addressed before achieving any clinical translation. First of all, standardizing the protocols of scalability with reproducibility in the production of MSNs is very important. The carriers should be produced on a larger scale with the same colloidal stability, particle size, and pore area. Any functionalization strategy must be standardized with a depiction of an appropriate stability profile and drug loading capacity. Secondly, the bio-distribution studies must be carried out in different animal models in order to predict the final fate of MSNs. A mandatory step is to evaluate the toxicological profile of these nanocarriers in human models. In general, it is quite evident that there is a requirement of proper clinical evaluation of these nanocarriers together with the collaboration of interdisciplinary industrial efforts and other scientists to accelerate the entire process from bench to bed translation.
Credit author statment
Bazla Siddiqui: Writing-Original draft, Designing of diagrams.
Asim Ur Rehman: Designing of diagrams, Language Correction.
Ihsan ul Haq: Editing and Language and scientific correction.
Amal A. Al-Dosary: Write Up of some parts and Proof reading.
Abdelhamid Elaissari: Overall supervision, Corresponding author.
Naveed Ahmed: Conceptualization of idea, Scientific correction, Supervision.
Declaration of Competing Interest
Authors declare no conflict of Interest.
References
- Alyassin Y., Sayed E.G., Mehta P., Ruparelia K., Arshad M.S., Rasekh M., Shepherd J., Kucuk I., Wilson P.B., Singh N. Application of mesoporous silica nanoparticles as drug delivery carriers for chemotherapeutic agents. Drug Discov. Today. 2020;25(8):1513–1520. doi: 10.1016/j.drudis.2020.06.006. [DOI] [PubMed] [Google Scholar]
- Atanase L.I. Micellar drug delivery systems based on natural biopolymers. Polymers. 2021;13:477. doi: 10.3390/polym13030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augspurger A.E., Sun X., Trewyn B.G., Fang N., Stender A.S. Monitoring the stimulated uncapping process of gold-capped mesoporous silica nanoparticles. Anal. Chem. 2018;90:3183–3188. doi: 10.1021/acs.analchem.7b04532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei M., Abnous K., Taghdisi S.M., Taghavi S., Sh Saljooghi A., Ramezani M., Alibolandi M. Targeted rod-shaped mesoporous silica nanoparticles for the co-delivery of camptothecin and survivin shRNA in to colon adenocarcinoma in vitro and in vivo. Eur. J. Pharm. Biopharm. 2020;156:84–96. doi: 10.1016/j.ejpb.2020.08.026. [DOI] [PubMed] [Google Scholar]
- Babonneau F., Camus L., Steunou N., Ramila A., Vallet-Regi Maria. Encapsulation of ibuprofen in mesoporous silica: solid state NMR characterization. MRS Online Proc. Library. 2003;775:3261–3266. [Google Scholar]
- Babonneau F., Yeung L., Steunou N., Gervais C., Ramila A., Vallet-Regi M. Solid state NMR characterisation of encapsulated molecules in mesoporous silica. J. Sol-Gel Sci. Technol. 2004;31:219–223. [Google Scholar]
- Bagheri E., Alibolandi M., Abnous K., Taghdisi S.M., Ramezani M. Targeted delivery and controlled release of doxorubicin to cancer cells by smart ATP-responsive Y-shaped DNA structure-capped mesoporous silica nanoparticles. J. Mater. Chem. B. 2021;9:1351–1363. doi: 10.1039/d0tb01960g. [DOI] [PubMed] [Google Scholar]
- Barkat M.A., Das S.S., Pottoo F.H., Beg S., Rahman Z. Lipid-based nanosystem as intelligent carriers for versatile drug delivery applications. Curr. Pharm. Des. 2020;26:1167–1180. doi: 10.2174/1381612826666200206094529. [DOI] [PubMed] [Google Scholar]
- Beck J.S., Vartuli J., Roth W.J., Leonowicz M., Kresge C., Schmitt K., Chu C., Olson D.H., Sheppard E., McCullen S. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992;114:10834–10843. [Google Scholar]
- Beck M., Mandal T., Buske C., Linden M. Serum protein adsorption enhances active leukemia stem cell targeting of mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces. 2017;9:18566–18574. doi: 10.1021/acsami.7b04742. [DOI] [PubMed] [Google Scholar]
- Begines B., Ortiz T., Pérez-Aranda M., Martínez G., Merinero M., Argüelles-Arias F., Alcudia A. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials. 2020;10:1403. doi: 10.3390/nano10071403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Osuna Á.A., Ribelles J.L.G., Perilla J.E. A study of some fundamental physicochemical variables on the morphology of mesoporous silica nanoparticles MCM-41 type. J. Nanopart. Res. 2017;19:1–14. [Google Scholar]
- Bernal A., Calcagno C., Mulder W.J., Pérez-Medina C. Imaging-guided nanomedicine development. Curr. Opin. Chem. Biol. 2021;63:78–85. doi: 10.1016/j.cbpa.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti C., Nagaich U., Pal A.K., Gulati N. Mesoporous silica nanoparticles in target drug delivery system: a review. Int. J. Pharm. Iinvest. 2015;5:124. doi: 10.4103/2230-973X.160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk E.M., Söderlind F., Odén M. Tuning the shape of mesoporous silica particles by alterations in parameter space: from rods to platelets. Langmuir. 2013;29:13551–13561. doi: 10.1021/la403201v. [DOI] [PubMed] [Google Scholar]
- Bouchoucha M., Cote M.-F., Gaudreault R., Fortin M.-A., Kleitz F. Size-controlled functionalized mesoporous silica nanoparticles for tunable drug release and enhanced anti-tumoral activity. Chem. Mater. 2016;28:4243–4258. [Google Scholar]
- Bourquin J., Milosevic A., Hauser D., Lehner R., Blank F., Petri-Fink A., Rothen-Rutishauser B. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv. Mater. 2018;30:1704307. doi: 10.1002/adma.201704307. [DOI] [PubMed] [Google Scholar]
- van den Brand D., Mertens V., Massuger L.F., Brock R. siRNA in ovarian cancer–delivery strategies and targets for therapy. J. Control. Release. 2018;283:45–58. doi: 10.1016/j.jconrel.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Braun K., Pochert A., Beck M., Fiedler R., Gruber J., Lindén M. Dissolution kinetics of mesoporous silica nanoparticles in different simulated body fluids. J. Sol-Gel Sci. Technol. 2016;79:319–327. [Google Scholar]
- Brevet D., Gary-Bobo M., Raehm L., Richeter S., Hocine O., Amro K., Loock B., Couleaud P., Frochot C., Morère A., Maillard P., Garcia M., Durand J.O. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. (Camb.) 2009:1475–1477. doi: 10.1039/b900427k. [DOI] [PubMed] [Google Scholar]
- Bürglová K., Noureddine A., Hodačová J., Toquer G., Cattoën X., Wong Chi Man M. A general method for preparing bridged organosilanes with pendant functional groups and functional mesoporous organosilicas. Chemistry. 2014;20(33):10371–10382. doi: 10.1002/chem.201403136. [DOI] [PubMed] [Google Scholar]
- Cai Q., Luo Z.-S., Pang W.-Q., Fan Y.-W., Chen X.-H., Cui F.-Z. Dilute solution routes to various controllable morphologies of MCM-41 silica with a basic medium. Chem. Mater. 2001;13:258–263. [Google Scholar]
- Carniato F., Muñoz-Úbeda M., Tei L., Botta M. Selective functionalization of mesoporous silica nanoparticles with ibuprofen and Gd (III) chelates: a new probe for potential theranostic applications. Dalton Trans. 2015;44:17927–17931. doi: 10.1039/c5dt03144c. [DOI] [PubMed] [Google Scholar]
- Cha W., Fan R., Miao Y., Zhou Y., Qin C., Shan X., Wan X., Li J. Mesoporous silica nanoparticles as carriers for intracellular delivery of nucleic acids and subsequent therapeutic applications. Molecules. 2017;22 doi: 10.3390/molecules22050782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che E., Gao Y., Wan L., Zhang Y., Han N., Bai J., Li J., Sha Z., Wang S. Paclitaxel/gelatin coated magnetic mesoporous silica nanoparticles: Preparation and antitumor efficacy in vivo. Microporous Mesoporous Mater. 2015;204:226–234. [Google Scholar]
- Chen B.C., Lin H.P., Chao M.C., Mou C.Y., Tang C.Y. Mesoporous silica platelets with perpendicular nanochannels via a ternary surfactant system. Adv. Mater. 2004;16:1657–1661. [Google Scholar]
- Chen C., Pu F., Huang Z., Liu Z., Ren J., Qu X. Stimuli-responsive controlled-release system using quadruplex DNA-capped silica nanocontainers. Nucleic Acids Res. 2011;39:1638–1644. doi: 10.1093/nar/gkq893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.-J., Hu S.-H., Hsiao C.-S., Chen Y.-Y., Liu D.-M., Chen S.-Y. Multifunctional magnetically removable nanogated lids of Fe 3 O 4–capped mesoporous silica nanoparticles for intracellular controlled release and MR imaging. J. Mater. Chem. 2011;21:2535–2543. [Google Scholar]
- Chen F., Hong H., Zhang Y., Valdovinos H.F., Shi S., Kwon G.S., Theuer C.P., Barnhart T.E., Cai W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radiolabeled mesoporous silica nanoparticles. ACS Nano. 2013;7:9027–9039. doi: 10.1021/nn403617j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Li Z., Lin Y., Yin M., Ren J., Qu X. Bioresponsive hyaluronic acid-capped mesoporous silica nanoparticles for targeted drug delivery. Chem. A Eur. J. 2013;19:1778–1783. doi: 10.1002/chem.201202038. [DOI] [PubMed] [Google Scholar]
- Chen J., Dong X., Feng T., Lin L., Guo Z., Xia J., Tian H., Chen X. Charge-conversional zwitterionic copolymer as pH-sensitive shielding system for effective tumor treatment. Acta Biomater. 2015;26:45–53. doi: 10.1016/j.actbio.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Chen H., Zheng D., Liu J., Kuang Y., Li Q., Zhang M., Ye H., Qin H., Xu Y., Li C. pH-Sensitive drug delivery system based on modified dextrin coated mesoporous silica nanoparticles. Int. J. Biol. Macromol. 2016;85:596–603. doi: 10.1016/j.ijbiomac.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Chen F., Valdovinos H.F., Hernandez R., Goel S., Barnhart T.E., Cai W.J.A.P.S. vol. 38. 2017. Intrinsic Radiolabeling of Titanium-45 Using Mesoporous Silica Nanoparticles; pp. 907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Zhang W., Zhu G., Xie J., Chen X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017;2 doi: 10.1038/natrevmats.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Sun W., Wang X., Wang Y., Wang P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018;111:1106–1115. doi: 10.1016/j.ijbiomac.2018.01.093. [DOI] [PubMed] [Google Scholar]
- Chen C., Sun W., Yao W., Wang Y., Ying H., Wang P. Functional polymeric dialdehyde dextrin network capped mesoporous silica nanoparticles for pH/GSH dual-controlled drug release. RSC Adv. 2018;8:20862–20871. doi: 10.1039/c8ra03163k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Chang C., Liu Z., Zhou Y., Xu Q., Li C., Huang Z., Xu H., Xu P., Lu B. Hyaluronic acid targeted and pH-responsive nanocarriers based on hollow mesoporous silica nanoparticles for chemo-photodynamic combination therapy. Colloids Surf. B: Biointerfaces. 2020;194 doi: 10.1016/j.colsurfb.2020.111166. [DOI] [PubMed] [Google Scholar]
- Chen Y., Lu W., Guo Y., Zhu Y., Song Y. Chitosan-gated fluorescent mesoporous silica nanocarriers for the real-time monitoring of drug release. Langmuir. 2020;36:6749–6756. doi: 10.1021/acs.langmuir.0c00832. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-J., Zhang A.-Q., Hu J.-J., He F., Zeng X., Zhang X.-Z. Multifunctional peptide-amphiphile end-capped mesoporous silica nanoparticles for tumor targeting drug delivery. ACS Appl. Mater. Interfaces. 2017;9:2093–2103. doi: 10.1021/acsami.6b12647. [DOI] [PubMed] [Google Scholar]
- Chiang Y.-D., Lian H.-Y., Leo S.-Y., Wang S.-G., Yamauchi Y., Wu K.C.-W. Controlling particle size and structural properties of mesoporous silica nanoparticles using the Taguchi method. J. Phys. Chem. C. 2011;115:13158–13165. [Google Scholar]
- Choi J.Y., Gupta B., Ramasamy T., Jeong J.-H., Jin S.G., Choi H.-G., Yong C.S., Kim J.O. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surf. B: Biointerfaces. 2018;165:56–66. doi: 10.1016/j.colsurfb.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Choi E., Lim D.-K., Kim S. Calcium-doped mesoporous silica nanoparticles as a lysosomolytic nanocarrier for amine-free loading and cytosolic delivery of siRNA. J. Ind. Eng. Chem. 2020;81:71–80. [Google Scholar]
- Corbalan J.J., Medina C., Jacoby A., Malinski T., Radomski M.W. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int. J. Nanomedicine. 2012;7:631. doi: 10.2147/IJN.S28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalan J.J., Medina C., Jacoby A., Malinski T., Radomski M.W. Amorphous silica nanoparticles aggregate human platelets: potential implications for vascular homeostasis. Int. J. Nanomedicine. 2012;7:631. doi: 10.2147/IJN.S28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croissant J.G., Fatieiev Y., Khashab N.M. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv. Mater. 2017;29:1604634. doi: 10.1002/adma.201604634. [DOI] [PubMed] [Google Scholar]
- Croissant J.G., Fatieiev Y., Khashab N.M. 2017. Functional Nanoparticles: Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles Advanced Materials; p. 29. [DOI] [PubMed] [Google Scholar]
- Croissant J.G., Fatieiev Y., Almalik A., Khashab N.M. Mesoporous silica and organosilica nanoparticles: physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthc. Mater. 2018;7:1700831. doi: 10.1002/adhm.201700831. [DOI] [PubMed] [Google Scholar]
- Cui X., Moon S.-W., Zin W.-C. High-yield synthesis of monodispersed SBA-15 equilateral hexagonal platelet with thick wall. Mater. Lett. 2006;60:3857–3860. [Google Scholar]
- Cui L., Liu W., Liu H., Qin Q., Wu S., He S., Pang X., Zhu C., Shen P. pH-triggered charge-reversal mesoporous silica nanoparticles stabilized by chitosan oligosaccharide/carboxymethyl chitosan hybrids for effective intracellular delivery of doxorubicin. ACS Appl. Bio Mater. 2019;2:1907–1919. doi: 10.1021/acsabm.8b00830. [DOI] [PubMed] [Google Scholar]
- Danks A.E., Hall S.R., Schnepp Z. The evolution of ‘sol–gel’chemistry as a technique for materials synthesis. Mater. Horiz. 2016;3:91–112. [Google Scholar]
- Descamps D., Benihoud K. Two key challenges for effective adenovirus-mediated liver gene therapy: innate immune responses and hepatocyte-specific transduction. Curr. Gene Ther. 2009;9:115–127. doi: 10.2174/156652309787909544. [DOI] [PubMed] [Google Scholar]
- Dréau D., Moore L.J., Alvarez-Berrios M.P., Tarannum M., Mukherjee P., Vivero-Escoto J.L. Mucin-1-antibody-conjugated mesoporous silica nanoparticles for selective breast cancer detection in a mucin-1 transgenic murine mouse model. J. Biomed. Nanotechnol. 2016;12:2172–2184. doi: 10.1166/jbn.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M., Chen Y., Tu J., Liufu C., Yu J., Yuan Z., Gong X., Chen Z. Ultrasound responsive magnetic mesoporous silica nanoparticle-loaded microbubbles for efficient gene delivery. ACS Biomater. Sci. Eng. 2020;6:2904–2912. doi: 10.1021/acsbiomaterials.0c00014. [DOI] [PubMed] [Google Scholar]
- Duerner L.J., Schwantes A., Schneider I.C., Cichutek K., Buchholz C.J. Cell entry targeting restricts biodistribution of replication-competent retroviruses to tumour tissue. Gene Ther. 2008;15:1500–1510. doi: 10.1038/gt.2008.92. [DOI] [PubMed] [Google Scholar]
- Egger S.M., Hurley K.R., Datt A., Swindlehurst G., Haynes C.L. Ultraporous mesostructured silica nanoparticles. Chem. Mater. 2015;27:3193–3196. [Google Scholar]
- Eskandari P., Bigdeli B., Porgham Daryasari M., Baharifar H., Bazri B., Shourian M., Amani A., Sadighi A., Goliaei B., Khoobi M. Gold-capped mesoporous silica nanoparticles as an excellent enzyme-responsive nanocarrier for controlled doxorubicin delivery. J. Drug Target. 2019;27:1084–1093. doi: 10.1080/1061186X.2019.1599379. [DOI] [PubMed] [Google Scholar]
- Feng S.-H., Li G.-H. Hydrothermal and solvothermal syntheses. Modern Inorgan. Synth. Chem. 2017:73–104. Elsevier. [Google Scholar]
- Feng W., Nie W., He C., Zhou X., Chen L., Qiu K., Wang W., Yin Z.J.A.A.M. Effect of pH-responsive alginate/chitosan multilayers coating on delivery efficiency, cellular uptake and biodistribution of mesoporous silica nanoparticles based nanocarriers. ACS Appl. Mater. Interfaces. 2014;6:8447–8460. doi: 10.1021/am501337s. [DOI] [PubMed] [Google Scholar]
- Fernández-Pan I., Maté J.I., Gardrat C., Coma V. Effect of chitosan molecular weight on the antimicrobial activity and release rate of carvacrol-enriched films. Food Hydrocoll. 2015;51:60–68. [Google Scholar]
- Ferris D.P., Lu J., Gothard C., Yanes R., Thomas C.R., Olsen J.C., Stoddart J.F., Tamanoi F., Zink J.I. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small. 2011;7:1816–1826. doi: 10.1002/smll.201002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles A., Vornlocher H.P., Maraganore J., Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007;6:443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasconi M., Liu Z., Lei J., Wu Y., Strekalova E., Malin D., Ambrogio M.W., Chen X., Botros Y.Y., Cryns V.L. Photoexpulsion of surface-grafted ruthenium complexes and subsequent release of cytotoxic cargos to cancer cells from mesoporous silica nanoparticles. J. Am. Chem. Soc. 2013;135:11603–11613. doi: 10.1021/ja405058y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galabova B.B. Mesoporous silica nanoparticles: synthesis, functionalization, drug loading and release-A review. Trop. J. Pharm. Res. 2021;20 [Google Scholar]
- Ganguly A., Ahmad T., Ganguli A.K. Silica mesostructures: control of pore size and surface area using a surfactant-templated hydrothermal process. Langmuir. 2010;26:14901–14908. doi: 10.1021/la102510c. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo M., Brevet D., Benkirane-Jessel N., Raehm L., Maillard P., Garcia M., Durand J.-O. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiagn. Photodyn. Ther. 2012;9:256–260. doi: 10.1016/j.pdpdt.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Geng S., Qin L., He Y., Li X., Yang M., Li L., Liu D., Li Y., Niu D., Yang G. Effective and safe delivery of GLP-1AR and FGF-21 plasmids using amino-functionalized dual-mesoporous silica nanoparticles in vitro and in vivo. Biomaterials. 2021;271 doi: 10.1016/j.biomaterials.2021.120763. [DOI] [PubMed] [Google Scholar]
- Ghaferi M., Koohi Moftakhari Esfahani M., Raza A., Al Harthi S., Ebrahimi Shahmabadi H., Alavi S.E. Mesoporous silica nanoparticles: synthesis methods and their therapeutic use-recent advances. J. Drug Target. 2021;29:131–154. doi: 10.1080/1061186X.2020.1812614. [DOI] [PubMed] [Google Scholar]
- Giri S., Trewyn B.G., Stellmaker M.P., Lin V.S.Y. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew. Chem. 2005;117:5166–5172. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- Goel S., Chen F., Hong H., Valdovinos H.F., Hernandez R., Shi S., Barnhart T.E., Cai W. VEGF121-conjugated mesoporous silica nanoparticle: a tumor targeted drug delivery system. ACS Appl. Mater. Interfaces. 2014;6:21677–21685. doi: 10.1021/am506849p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S., Chen F., Luan S., Valdovinos H.F., Shi S., Graves S.A., Ai F., Barnhart T.E., Theuer C.P., Cai W. Engineering intrinsically zirconium-89 radiolabeled self-destructing mesoporous silica nanostructures for in vivo biodistribution and tumor targeting studies. Adv. Sci. 2016;3:1600122. doi: 10.1002/advs.201600122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenhagen J.A., Lai C.-Y., Radu D.R., Lin V.S.-Y., Yeung E.S. Real-time imaging of tunable adenosine 5-triphosphate release from an MCM-41-type mesoporous silica nanosphere-based delivery system. Appl. Spectrosc. 2005;59:424–431. doi: 10.1366/0003702053641513. [DOI] [PubMed] [Google Scholar]
- Grün M., Lauer I., Unger K.K. The synthesis of micrometer-and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv. Mater. 1997;9:254–257. [Google Scholar]
- Gu J., Fan W., Shimojima A., Okubo T. Organic–inorganic mesoporous nanocarriers integrated with biogenic ligands. Small. 2007;3:1740–1744. doi: 10.1002/smll.200700311. [DOI] [PubMed] [Google Scholar]
- Guisasola E., Asín L., Beola L., de la Fuente J.M., Baeza A., Vallet-Regí M. Beyond traditional hyperthermia: in vivo cancer treatment with magnetic-responsive mesoporous silica nanocarriers. ACS Appl. Mater. Interfaces. 2018;10:12518–12525. doi: 10.1021/acsami.8b02398. [DOI] [PubMed] [Google Scholar]
- Haddick L., Zhang W., Reinhard S., Möller K., Engelke H., Wagner E., Bein T. Particle-size-dependent delivery of antitumoral miRNA using targeted mesoporous silica nanoparticles. Pharmaceutics. 2020;12:505. doi: 10.3390/pharmaceutics12060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai L., Jia X., He D., Zhang A., Wang T., Cheng H., He X., Wang K. DNA-functionalized hollow mesoporous silica nanoparticles with dual cargo loading for near-infrared-responsive synergistic chemo-photothermal treatment of cancer cells. ACS Appl. Nano Mater. 2018;1:3486–3497. [Google Scholar]
- Han L., Zhou Y., He T., Song G., Wu F., Jiang F., Hu J. One-pot morphology-controlled synthesis of various shaped mesoporous silica nanoparticles. J. Mater. Sci. 2013;48:5718–5726. [Google Scholar]
- Hao Y., Tian R., Lv K., Liu Z., Ni J., Yuan P., Bai Y., Chen X. Stimuli responsive co-delivery of celecoxib and BMP2 from micro-scaffold for periodontal disease treatment. J. Mater. Sci. Technol. 2021;75:216–224. [Google Scholar]
- Hartono S.B., Gu W., Kleitz F., Liu J., He L., Middelberg A.P., Yu C., Lu G.Q., Qiao S.Z. Poly-L-lysine functionalized large pore cubic mesostructured silica nanoparticles as biocompatible carriers for gene delivery. ACS Nano. 2012;6:2104–2117. doi: 10.1021/nn2039643. [DOI] [PubMed] [Google Scholar]
- Hartono S.B., Phuoc N.T., Yu M., Jia Z., Monteiro M.J., Qiao S., Yu C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J. Mater. Chem. B. 2014;2:718–726. doi: 10.1039/c3tb21015d. [DOI] [PubMed] [Google Scholar]
- He D., He X., Wang K., Chen M., Cao J., Zhao Y. Reversible stimuli-responsive controlled release using mesoporous silica nanoparticles functionalized with a smart DNA molecule-gated switch. J. Mater. Chem. B. 2012;22:14715–14721. [Google Scholar]
- He Y., Luo L., Liang S., Long M., Xu H. Amino-functionalized mesoporous silica nanoparticles as efficient carriers for anticancer drug delivery. J. Biomater. Appl. 2017;32:524–532. doi: 10.1177/0885328217724638. [DOI] [PubMed] [Google Scholar]
- Hegazy M., Zhou P., Rahoui N., Wu G., Taloub N., Lin Y., Huang X., Huang Y.J.C. A facile design of smart silica nanocarriers via surface-initiated RAFT polymerization as a dual-stimuli drug release platform. Colloids Surf. A Physicochem. Eng. Asp. 2019;581 [Google Scholar]
- Heidari R., Khosravian P., Mirzaei S.A., Elahian F.J.S.R. siRNA delivery using intelligent chitosan-capped mesoporous silica nanoparticles for overcoming multidrug resistance in malignant carcinoma cells. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-00085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä T., Salonen J., Tuura J., Hamdy M., Mul G., Kumar N., Salmi T., Murzin D.Y., Laitinen L., Kaukonen A.M. Mesoporous silica material TUD-1 as a drug delivery system. Int. J. Pharm. 2007;331:133–138. doi: 10.1016/j.ijpharm.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Hong C.-Y., Li X., Pan C.-Y. Smart core− shell nanostructure with a mesoporous core and a stimuli-responsive nanoshell synthesized via surface reversible addition− fragmentation chain transfer polymerization. J. Phys. Chem. C. 2008;112:15320–15324. [Google Scholar]
- Hosseinpour S., Cao Y., Liu J., Xu C., Walsh L.J. Efficient transfection and long-term stability of rno-miRNA-26a-5p for osteogenic differentiation by large pore sized mesoporous silica nanoparticles. J. Mater. Chem. B. 2021;9:2275–2284. doi: 10.1039/d0tb02756a. [DOI] [PubMed] [Google Scholar]
- Hu J.-J., Liu L.-H., Li Z.-Y., Zhuo R.-X., Zhang X.-Z. MMP-responsive theranostic nanoplatform based on mesoporous silica nanoparticles for tumor imaging and targeted drug delivery. J. Mater. Chem. B. 2016;4:1932–1940. doi: 10.1039/c5tb02490k. [DOI] [PubMed] [Google Scholar]
- Huang X., Teng X., Chen D., Tang F., He J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31:438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- Huang X., Li L., Liu T., Hao N., Liu H., Chen D., Tang F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano. 2011;5:5390–5399. doi: 10.1021/nn200365a. [DOI] [PubMed] [Google Scholar]
- Huang M., Liu L., Wang S., Zhu H., Wu D., Yu Z., Zhou S. Dendritic mesoporous silica nanospheres synthesized by a novel dual-templating micelle system for the preparation of functional nanomaterials. Langmuir. 2017;33:519–526. doi: 10.1021/acs.langmuir.6b03282. [DOI] [PubMed] [Google Scholar]
- Huh S., Wiench J.W., Yoo J.-C., Pruski M., Lin V.S.-Y. Organic functionalization and morphology control of mesoporous silicas via a co-condensation synthesis method. Chem. Mater. 2003;15:4247–4256. [Google Scholar]
- Hwang D., Ramsey J.D., Kabanov A.V. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020;156:80–118. doi: 10.1016/j.addr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad S., Siddiqui B., Rehman A., Farooq R.K., Ahmed N. Recent trends and development in targeted delivery of therapeutics through enzyme responsive intelligent nanoplatform. Int. J. Polym. Mater. Polym. Biomater. 2020:1–11. [Google Scholar]
- Izquierdo-Barba I., Martinez Á., Doadrio A.L., Pérez-Pariente J., Vallet-Regí M. Release evaluation of drugs from ordered three-dimensional silica structures. Eur. J. Pharm. Sci. 2005;26:365–373. doi: 10.1016/j.ejps.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Jhaveri J., Raichura Z., Khan T., Momin M., Omri A.J.M. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules. 2021;26:272. doi: 10.3390/molecules26020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami Y., Sugimura S., Fujishima N., Matsuda K., Kometani T., Matsumura Y. Oxidative stability, structure, and physical characteristics of microcapsules formed by spray drying of fish oil with protein and dextrin wall materials. J. Food Sci. 2003;68:2248–2255. [Google Scholar]
- Kang Y., Sun W., Li S., Li M., Fan J., Du J., Liang X.J., Peng X. Oligo hyaluronan-coated silica/hydroxyapatite degradable nanoparticles for targeted cancer treatment. Adv. Sci. (Weinh.) 2019;6:1900716. doi: 10.1002/advs.201900716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankala R.K., Han Y.H., Na J., Lee C.H., Sun Z., Wang S.B., Kimura T., Ok Y.S., Yamauchi Y., Chen A.Z.J.A.M. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020;32:1907035. doi: 10.1002/adma.201907035. [DOI] [PubMed] [Google Scholar]
- Karimi M., Sahandi Zangabad P., Ghasemi A., Amiri M., Bahrami M., Malekzad H., Ghahramanzadeh Asl H., Mahdieh Z., Bozorgomid M., Ghasemi A. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl. Mater. Interfaces. 2016;8:21107–21133. doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse S., Boakye-Yiadom K.O., Ochete B.O., Opoku-Damoah Y., Akhtar F., Filli M.S., Asim Farooq M., Aquib M., Maviah Mily B.J., Murtaza G. Mesoporous silica nanomaterials: Versatile nanocarriers for cancer theranostics and drug and gene delivery. Pharmaceutics. 2019;11:77. doi: 10.3390/pharmaceutics11020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiew S.F., Ho Y.T., Kiew L.V., Kah J.C.Y., Lee H.B., Imae T., Chung L.Y. Preparation and characterization of an amylase-triggered dextrin-linked graphene oxide anticancer drug nanocarrier and its vascular permeability. Int. J. Pharm. 2017;534:297–307. doi: 10.1016/j.ijpharm.2017.10.045. [DOI] [PubMed] [Google Scholar]
- Kim M.-H., Na H.-K., Kim Y.-K., Ryoo S.-R., Cho H.S., Lee K.E., Jeon H., Ryoo R., Min D.-H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery. ACS Nano. 2011;5:3568–3576. doi: 10.1021/nn103130q. [DOI] [PubMed] [Google Scholar]
- Kim J.-S., Lee S.K., Doh H., Kim M.Y., Kim D.K. Real-time tracking of highly luminescent mesoporous silica particles modified with europium β-diketone chelates in living cells. Nanomaterials. 2021;11:343. doi: 10.3390/nano11020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobler J., Möller K., Bein T. Colloidal suspensions of functionalized mesoporous silica nanoparticles. ACS Nano. 2008;2:791–799. doi: 10.1021/nn700008s. [DOI] [PubMed] [Google Scholar]
- Kong M., Peng X., Cui H., Liu P., Pang B., Zhang K. pH-responsive polymeric nanoparticles with tunable sizes for targeted drug delivery. RSC Adv. 2020;10:4860–4868. doi: 10.1039/c9ra10280a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu M., Chatterjee S., Ghosh N., Manna P., Das J., Sil P.C. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater. Sci. Eng. C. 2020;116 doi: 10.1016/j.msec.2020.111239. [DOI] [PubMed] [Google Scholar]
- Lai C.-Y., Trewyn B.G., Jeftinija D.M., Jeftinija K., Xu S., Jeftinija S., Lin V.S.-Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003;125:4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- Li S.D., Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13:1313–1319. doi: 10.1038/sj.gt.3302838. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang J., Gu H. Adsorption and desorption behaviors of DNA with magnetic mesoporous silica nanoparticles. Langmuir. 2011;27:6099–6106. doi: 10.1021/la104653s. [DOI] [PubMed] [Google Scholar]
- Li Z.-Y., Liu Y., Hu J.-J., Xu Q., Liu L.-H., Jia H.-Z., Chen W.-H., Lei Q., Rong L., Zhang X.-Z. Stepwise-acid-active multifunctional mesoporous silica nanoparticles for tumor-specific nucleus-targeted drug delivery. ACS Appl. Mater. Interfaces. 2014;6:14568–14575. doi: 10.1021/am503846p. [DOI] [PubMed] [Google Scholar]
- Li X., Chen Y., Zhang X., Zhao Y. Fabrication of biodegradable auto-fluorescent organosilica nanoparticles with dendritic mesoporous structures for pH/redox-responsive drug release. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110914. [DOI] [PubMed] [Google Scholar]
- Lin Y.-S., Abadeer N., Hurley K.R., Haynes C.L. Ultrastable, redispersible, small, and highly organomodified mesoporous silica nanotherapeutics. J. Am. Chem. Soc. 2011;133:20444–20457. doi: 10.1021/ja208567v. [DOI] [PubMed] [Google Scholar]
- Lin J.-T., Wang C., Zhao Y., Wang G.-H. Mesoporous silica nanoparticles with controlled loading of cationic dendrimer for gene delivery. Mater. Res. Express. 2014;1 [Google Scholar]
- Lin J.-T., Liu Z.-K., Zhu Q.-L., Rong X.-H., Liang C.-L., Wang J., Ma D., Sun J., Wang G.-H. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf. B: Biointerfaces. 2017;155:41–50. doi: 10.1016/j.colsurfb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Linares N., Serrano E., Rico M., Balu A.M., Losada E., Luque R., García-Martínez J. Incorporation of chemical functionalities in the framework of mesoporous silica. Chem. Commun. 2011;47:9024–9035. doi: 10.1039/c1cc11016k. [DOI] [PubMed] [Google Scholar]
- Liu R., Zhang Y., Zhao X., Agarwal A., Mueller L.J., Feng P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010;132:1500–1501. doi: 10.1021/ja907838s. [DOI] [PubMed] [Google Scholar]
- Liu F., Wang J., Huang P., Zhang Q., Deng J., Cao Q., Jia J., Cheng J., Fang Y., Deng D.Y. Outside-in stepwise functionalization of mesoporous silica nanocarriers for matrix type sustained release of fluoroquinolone drugs. J. Mater. Chem. B. 2015;3:2206–2214. doi: 10.1039/c4tb02073a. [DOI] [PubMed] [Google Scholar]
- Liu Y., Du J., Choi J.S., Chen K.J., Hou S., Yan M., Lin W.Y., Chen K.S., Ro T., Lipshutz G.S., Wu L., Shi L., Lu Y., Tseng H.R., Wang H. A high-throughput platform for formulating and screening multifunctional nanoparticles capable of simultaneous delivery of genes and transcription factors. Angew. Chem. Int. Ed. Eng. 2016;55:169–173. doi: 10.1002/anie.201507546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López V., Villegas M.R., Rodríguez V., Villaverde G., Lozano D., Baeza A., Vallet-Regí M. Janus mesoporous silica nanoparticles for dual targeting of tumor cells and mitochondria. ACS Appl. Mater. Interfaces. 2017;9:26697–26706. doi: 10.1021/acsami.7b06906. [DOI] [PubMed] [Google Scholar]
- Lu J., Li Z., Zink J.I., Tamanoi F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: enhanced efficacy by folate modification. Nanomedicine. 2012;8:212–220. doi: 10.1016/j.nano.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Chen H., Chen Y., Zhang K., Wang X., Cui X., Shi J. Hyaluronic acid-conjugated mesoporous silica nanoparticles: excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 2012;22:5615–5621. [Google Scholar]
- Ma K., Werner-Zwanziger U., Zwanziger J., Wiesner U. Controlling growth of ultrasmall sub-10 nm fluorescent mesoporous silica nanoparticles. Chem. Mater. 2013;25:677–691. [Google Scholar]
- Mandal K.A. Dendrimers in targeted drug delivery applications: a review of diseases and cancer. Int. J. Polym. Mater. 2021;70:287–297. doi: 10.1080/00914037.2020.1713780. [DOI] [Google Scholar]
- Manzano M., Vallet-Regí M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020;30:1902634. [Google Scholar]
- Martínez-Carmona M., Ho Q.P., Morand J., García A., Ortega E., Erthal L.C., Ruiz-Hernandez E., Santana M.D., Ruiz J., Vallet-Regí M. Amino-functionalized mesoporous silica nanoparticle-encapsulated octahedral organoruthenium complex as an efficient platform for combatting cancer. Inorg. Chem. 2020;59:10275–10284. doi: 10.1021/acs.inorgchem.0c01436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K., Palmer J.W. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934;107:629–634. [Google Scholar]
- Mickler F.M., Möckl L., Ruthardt N., Ogris M., Wagner E., Bräuchle C. Tuning nanoparticle uptake: live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012;12:3417–3423. doi: 10.1021/nl300395q. [DOI] [PubMed] [Google Scholar]
- Miller L., Winter G., Baur B., Witulla B., Solbach C., Reske S., Lindén M. Synthesis, characterization, and biodistribution of multiple 89 Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale. 2014;6:4928–4935. doi: 10.1039/c3nr06800e. [DOI] [PubMed] [Google Scholar]
- Moeller K., Kobler J., Bein T. Colloidal suspensions of nanometer-sized mesoporous silica. Adv. Funct. Mater. 2007;17:605–612. [Google Scholar]
- Mohamed Isa E.D., Ahmad H., Abdul Rahman M.B., Gill M.R. Progress in mesoporous silica nanoparticles as drug delivery agents for cancer treatment. Pharmaceutics. 2021;13:152. doi: 10.3390/pharmaceutics13020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo-Quiros S., Aragoneses-Cazorla G., Garcia-Alcalde L., Vallet-Regí M., González B., Luque-Garcia J.L. Cancer cell targeting and therapeutic delivery of silver nanoparticles by mesoporous silica nanocarriers: insights into the action mechanisms using quantitative proteomics. Nanoscale. 2019;11:4531–4545. doi: 10.1039/C8NR07667G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A.F., Dias D.R., Correia I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous Mesoporous Mater. 2016;236:141–157. [Google Scholar]
- Murugan B., Ramana L.N., Gandhi S., Sethuraman S., Krishnan U.M. Engineered chemoswitchable mesoporous silica for tumor-specific cytotoxicity. J. Mater. Chem. B. 2013;1:3494–3505. doi: 10.1039/c3tb20415d. [DOI] [PubMed] [Google Scholar]
- Nagraik R., Sharma A., Kumar D., Mukherjee S., Sen F., Kumar A.P. Amalgamation of biosensors and nanotechnology in disease diagnosis: mini-review. Sens. Int. 2021;100089 [Google Scholar]
- Nairi V., Magnolia S., Piludu M., Nieddu M., Caria C.A., Sogos V., Vallet-Regì M., Monduzzi M., Salis A. Mesoporous silica nanoparticles functionalized with hyaluronic acid. Effect of the biopolymer chain length on cell internalization. Colloids Surf. B: Biointerfaces. 2018;168:50–59. doi: 10.1016/j.colsurfb.2018.02.019. [DOI] [PubMed] [Google Scholar]
- Nandiyanto A.B.D., Kim S.-G., Iskandar F., Okuyama K. Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters. Microporous Mesoporous Mater. 2009;120:447–453. [Google Scholar]
- Narayan R., Nayak U.Y., Raichur A.M., Garg S. Mesoporous silica nanoparticles: a comprehensive review on synthesis and recent advances. Pharmaceutics. 2018;10:118. doi: 10.3390/pharmaceutics10030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan R., Gadag S., Mudakavi R.J., Garg S., Raichur A.M., Nayak Y., Kini S.G., Pai K.S.R., Nayak U.Y. Mesoporous silica nanoparticles capped with chitosan-glucuronic acid conjugate for pH-responsive targeted delivery of 5-fluorouracil. J. Drug Deliv. Sci. Technol. 2021;63 [Google Scholar]
- Natarajan S.K., Selvaraj S. Mesoporous silica nanoparticles: importance of surface modifications and its role in drug delivery. RSC Adv. 2014;4:14328–14334. [Google Scholar]
- Nekounam H., Kandi M.R., Shaterabadi D., Samadian H., Mahmoodi N., Hasanzadeh E., Faridi-Majidi R. Silica nanoparticles-incorporated carbon nanofibers as bioactive biomaterial for bone tissue engineering. Diam. Relat. Mater. 2021;108320 [Google Scholar]
- Niedermayer S., Weiss V., Herrmann A., Schmidt A., Datz S., Müller K., Wagner E., Bein T., Bräuchle C. Multifunctional polymer-capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. Nanoscale. 2015;7:7953–7964. doi: 10.1039/c4nr07245f. [DOI] [PubMed] [Google Scholar]
- Niemelä E., Desai D., Nkizinkiko Y., Eriksson J.E., Rosenholm J.M. Sugar-decorated mesoporous silica nanoparticles as delivery vehicles for the poorly soluble drug celastrol enables targeted induction of apoptosis in cancer cells. Eur. J. Pharm. Biopharm. 2015;96:11–21. doi: 10.1016/j.ejpb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Nik A.B., Zare H., Razavi S., Mohammadi H., Ahmadi P.T., Yazdani N., Bayandori M., Rabiee N., Mobarakeh J.I. Smart drug delivery: Capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020;299 [Google Scholar]
- Nikzamir M., Hanifehpour Y., Akbarzadeh A., Panahi Y. Applications of dendrimers in nanomedicine and drug delivery: a review. J. Inorg. Organomet. Polym. Mater. 2021:1–16. [Google Scholar]
- Øye G., Sjöblom J., Stöcker M.J.A.I.C., Science I. Vol. 89. 2001. Synthesis, Characterization and Potential Applications of New Materials in the Mesoporous Range; pp. 439–466. [DOI] [PubMed] [Google Scholar]
- Palanikumar L., Al-Hosani S., Kalmouni M., Nguyen V.P., Ali L., Pasricha R., Barrera F.N., Magzoub M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020;3:1–17. doi: 10.1038/s42003-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L., He Q., Liu J., Chen Y., Ma M., Zhang L., Shi J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012;134:5722–5725. doi: 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- Pan L., He Q., Liu J., Chen Y., Ma M., Zhang L., Shi J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012;134:5722–5725. doi: 10.1021/ja211035w. [DOI] [PubMed] [Google Scholar]
- Pang X., Gao J., Tang F. Controlled preparation of rod-and top-like MCM-41 mesoporous silica through one-step route. J. Non-Cryst. Solids. 2005;351:1705–1709. [Google Scholar]
- Peng H., Dong R., Wang S., Zhang Z., Luo M., Bai C., Zhao Q., Li J., Chen L., Xiong H. A pH-responsive nano-carrier with mesoporous silica nanoparticles cores and poly (acrylic acid) shell-layers: Fabrication, characterization and properties for controlled release of salidroside. Int. J. Pharm. 2013;446:153–159. doi: 10.1016/j.ijpharm.2013.01.071. [DOI] [PubMed] [Google Scholar]
- Pinese C., Lin J., Milbreta U., Li M., Wang Y., Leong K.W., Chew S.Y. Sustained delivery of siRNA/mesoporous silica nanoparticle complexes from nanofiber scaffolds for long-term gene silencing. Acta Biomater. 2018;76:164–177. doi: 10.1016/j.actbio.2018.05.054. [DOI] [PubMed] [Google Scholar]
- Plaza-Oliver M., Santander-Ortega M.J., Lozano M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021:1–27. doi: 10.1007/s13346-021-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouroutzidou G.K., Liverani L., Theocharidou A., Tsamesidis I., Lazaridou M., Christodoulou E., Beketova A., Pappa C., Triantafyllidis K.S., Anastasiou A.D. Synthesis and characterization of mesoporous Mg-and Sr-doped nanoparticles for moxifloxacin drug delivery in promising TISSUE engineering applications. Int. J. Mol. Sci. 2021;22:577. doi: 10.3390/ijms22020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R., Aiyer S., Chauhan D.S., Srivastava R., Selvaraj K. Bioresponsive carbon nano-gated multifunctional mesoporous silica for cancer theranostics. Nanoscale. 2016;8:4537–4546. doi: 10.1039/c5nr06756a. [DOI] [PubMed] [Google Scholar]
- Qiao Z.-A., Zhang L., Guo M., Liu Y., Huo Q. Synthesis of mesoporous silica nanoparticles via controlled hydrolysis and condensation of silicon alkoxide. Chem. Mater. 2009;21:3823–3829. [Google Scholar]
- Qindeel M., Ahmed N., Khan G.M., Rehman A.U. Ligand decorated chitosan as an advanced nanocarrier for targeted delivery: a critical review. Nanomedicine. 2019;14:1623–1642. doi: 10.2217/nnm-2018-0490. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K., Gupta S., Gnanadhas D.P., Ramamurthy P.C., Chakravortty D., Raichur A.M.J.P. Protamine-capped mesoporous silica nanoparticles for biologically triggered drug release. Part. Part. Syst. Charact. 2014;31:449–458. [Google Scholar]
- Safdar R., Omar A.A., Arunagiri A., Regupathi I., Thanabalan M. Potential of Chitosan and its derivatives for controlled drug release applications–a review. J. Drug Deliv. Sci. Technol. 2019;49:642–659. [Google Scholar]
- Salinas Y., Brüggemann O., Monkowius U., Teasdale I. Visible light photocleavable ruthenium-based molecular gates to reversibly control release from mesoporous silica nanoparticles. Nanomaterials. 2020;10:1030. doi: 10.3390/nano10061030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Ghosh S., Chowdhury S., Pandey B., Sil P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim. Biophys. Acta. 2016;1860:2065–2075. doi: 10.1016/j.bbagen.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Schlossbauer A., Warncke S., Gramlich P.M., Kecht J., Manetto A., Carell T., Bein T. A programmable DNA-based molecular valve for colloidal mesoporous silica. J. German Chem. Soc. 2010;49:4734–4737. doi: 10.1002/anie.201000827. [DOI] [PubMed] [Google Scholar]
- Scicluna M.C., Vella-Zarb L. Evolution of nanocarrier drug-delivery systems and recent advancements in covalent organic framework–drug systems. ACS Appl. Nano Mater. 2020;3:3097–3115. [Google Scholar]
- Shah P.V., Rajput S.J.J.A.P. Vol. 19. 2018. Facile Synthesis of Chitosan Capped Mesoporous Silica Nanoparticles: A pH Responsive Smart Delivery Platform for Raloxifene Hydrochloride; pp. 1344–1357. [DOI] [PubMed] [Google Scholar]
- Shahbazi M.-A., Herranz B., Santos H.A. Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomatter. 2012;2:296–312. doi: 10.4161/biom.22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeran Z., Keyhanfar M., Varshosaz J., Sutherland D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111526. [DOI] [PubMed] [Google Scholar]
- Shao D., Lu M.-M., Zhao Y.-W., Zhang F., Tan Y.-F., Zheng X., Pan Y., Xiao X.-A., Wang Z., Dong W.-F. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017;49:531–540. doi: 10.1016/j.actbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- Shen S., Gu T., Mao D., Xiao X., Yuan P., Yu M., Xia L., Ji Q., Meng L., Song W. Synthesis of nonspherical mesoporous silica ellipsoids with tunable aspect ratios for magnetic assisted assembly and gene delivery. Chem. Mater. 2012;24:230–235. [Google Scholar]
- Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Zhou Y., Fan T., Lin Y., Zhang H., Mei L. Inorganic nano-carriers based smart drug delivery systems for tumor therapy. Smart Mater. Med. 2020;1:32–47. [Google Scholar]
- Siddiqui B., Rehman A.U., Haq I.-U., Ahmad N.M., Ahmed N. Development, optimisation, and evaluation of nanoencapsulated diacerein emulgel for potential use in osteoarthritis. J. Microencapsul. 2020;37:595–608. doi: 10.1080/02652048.2020.1829140. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Patel K.D., Mahapatra C., Parthiban S.P., Kim T.-H., Kim H.-W. Combinatory cancer therapeutics with nanoceria-capped mesoporous silica nanocarriers through pH-triggered drug release and redox activity. ACS Appl. Mater. Interfaces. 2018;11:288–299. doi: 10.1021/acsami.8b17958. [DOI] [PubMed] [Google Scholar]
- Slowing I.I., Vivero-Escoto J.L., Wu C.W., Lin V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Song N., Yang Y.-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015;44:3474–3504. doi: 10.1039/c5cs00243e. [DOI] [PubMed] [Google Scholar]
- Stöber W., Fink A., Bohn E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968;26:62–69. [Google Scholar]
- Sun X., Zhao Y., Lin V.S.-Y., Slowing I.I., Trewyn B.G. Luciferase and luciferin co-immobilized mesoporous silica nanoparticle materials for intracellular biocatalysis. J. Am. Chem. Soc. 2011;133(46):18554–18557. doi: 10.1021/ja2080168. [DOI] [PubMed] [Google Scholar]
- Tao Z., Toms B., Goodisman J., Asefa T. Mesoporous silica microparticles enhance the cytotoxicity of anticancer platinum drugs. ACS Nano. 2010;4:789–794. doi: 10.1021/nn9015345. [DOI] [PubMed] [Google Scholar]
- Tarn D., Ashley C.E., Xue M., Carnes E.C., Zink J.I., Brinker C.J. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc. Chem. Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozuka Y., Wongmekiat A., Kimura K., Moribe K., Yamamura S., Yamamoto K. Effect of pore size of FSM-16 on the entrapment of flurbiprofen in mesoporous structures. Chem. Pharm. Bull. 2005;53:974–977. doi: 10.1248/cpb.53.974. [DOI] [PubMed] [Google Scholar]
- Trewyn Brian G., Zhao Yannan, Lin Victor S.-Y. Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chem. Eng. J. 2008;137:23–29. [Google Scholar]
- Umehara Y., Kimura Y., Kleitz F., Nishihara T., Kondo T., Tanabe K. Phosphonated mesoporous silica nanoparticles bearing ruthenium complexes used as molecular probes for tracking oxygen levels in cells and tissues. RSC Adv. 2021;11:5865–5873. doi: 10.1039/d0ra08771h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet-Regi M., Rámila A., Del Real R., Pérez-Pariente J. A new property of MCM-41: drug delivery system. Chem. Mater. 2001;13:308–311. [Google Scholar]
- Varshney S., Nigam A., Pawar S.J., Mishra N. Phosphorus, Sulfur, Silicon and the Related Elements A. 2021. An overview on biomedical applications of versatile silica nanoparticles, synthesized via several chemical and biological routes: a review; pp. 1–17. [Google Scholar]
- Vazquez N.I., Gonzalez Z., Ferrari B., Castro Y. Synthesis of mesoporous silica nanoparticles by sol–gel as nanocontainer for future drug delivery applications. Boletín Soc. Española Cerámica y Vidrio. 2017;56:139–145. [Google Scholar]
- Wagner J., Gößl D., Ustyanovska N., Xiong M., Hauser D., Zhuzhgova O., Hočevar S., Taskoparan B., Poller L., Datz S., Engelke H., Daali Y., Bein T., Bourquin C. Mesoporous silica nanoparticles as pH-responsive carrier for the immune-activating drug resiquimod enhance the local immune response in mice. ACS Nano. 2021;15:4450–4466. doi: 10.1021/acsnano.0c08384. [DOI] [PubMed] [Google Scholar]
- Wang L.S., Wu L.C., Lu S.Y., Chang L.L., Teng I.T., Yang C.M., Ho J.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: improved water suspensibility and decreased nonspecific protein binding. ACS Nano. 2010;4:4371–4379. doi: 10.1021/nn901376h. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu Y., Zhang W., Chen X., Yang T., Ma G. Microspheres and microcapsules for protein delivery: strategies of drug activity retention. Curr. Pharm. Des. 2013;19:6340–6352. doi: 10.2174/1381612811319350010. [DOI] [PubMed] [Google Scholar]
- Wang Y., Cui Y., Huang J., Di D., Dong Y., Zhang X., Zhao Q., Han N., Gao Y., Jiang T. Redox and pH dual-responsive mesoporous silica nanoparticles for site-specific drug delivery. Appl. Surf. Sci. 2015;356:1282–1288. [Google Scholar]
- Wang X., Zhang Y., Luo W., Elzatahry A.A., Cheng X., Alghamdi A., Abdullah A.M., Deng Y., Zhao D. Synthesis of ordered mesoporous silica with tunable morphologies and pore sizes via a nonpolar solvent-assisted stober method. Chem. Mater. 2016;28:2356–2362. [Google Scholar]
- Wang W., Wang P., Tang X., Elzatahry A.A., Wang S., Al-Dahyan D., Zhao M., Yao C., Hung C.-T., Zhu X. Facile synthesis of uniform virus-like mesoporous silica nanoparticles for enhanced cellular internalization. ACS Cent. Sci. 2017;3:839–846. doi: 10.1021/acscentsci.7b00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jiang L., Chu L., Liu W., Wu S., Wu Y., He X., Wang K. Electrochemical detection of glutathione by using thymine-rich DNA-gated switch functionalized mesoporous silica nanoparticles. Biosens. Bioelectron. 2017;87:459–465. doi: 10.1016/j.bios.2016.08.102. [DOI] [PubMed] [Google Scholar]
- Wang X., Li C., Fan N., Li J., Zhang H., Shang L., He Z., Sun J. Amino functionalized chiral mesoporous silica nanoparticles for improved loading and release of poorly water-soluble drug. Asian J. Pharm. Sci. 2019;14:405–412. doi: 10.1016/j.ajps.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhang K., Wang J., Zhao R., Zhang Q., Kong X. Poly (amidoamine)-modified mesoporous silica nanoparticles as a mucoadhesive drug delivery system for potential bladder cancer therapy. Colloids Surf. B: Biointerfaces. 2020;189 doi: 10.1016/j.colsurfb.2020.110832. [DOI] [PubMed] [Google Scholar]
- Wang J., Li Z., Yin Y., Liu H., Tang G., Ma Y., Feng X., Mei H., Bi J., Wang K. Mesoporous silica nanoparticles combined with MoS2 and FITC for fluorescence imaging and photothermal therapy of cancer cells. J. Mater. Sci. 2020;55:15263–15274. [Google Scholar]
- Wang Y., Cheng S., Hu W., Lin X., Cao C., Zou S., Tong Z., Jiang G., Kong X. Polymer-grafted hollow mesoporous silica nanoparticles integrated with microneedle patches for glucose-responsive drug delivery. Front. Mater. Sci. 2021;15:98–112. [Google Scholar]
- Wen Y., Oh J.K. Recent strategies to develop polysaccharide-based nanomaterials for biomedical applications. Macromol. Rapid Commun. 2014;35:1819–1832. doi: 10.1002/marc.201400406. [DOI] [PubMed] [Google Scholar]
- Wu S.-H., Mou C.-Y., Lin H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013;42:3862–3875. doi: 10.1039/c3cs35405a. [DOI] [PubMed] [Google Scholar]
- Wu M., Meng Q., Chen Y., Du Y., Zhang L., Li Y., Zhang L., Shi J. Large-pore ultrasmall mesoporous organosilica nanoparticles: micelle/precursor co-templating assembly and nuclear-targeted gene delivery. Adv. Mater. 2015;27:215–222. doi: 10.1002/adma.201404256. [DOI] [PubMed] [Google Scholar]
- Xu J.-H., Gao F.-P., Li L.-L., Ma H.L., Fan Y.-S., Liu W., Guo S.-S., Zhao X.-Z., Wang H. Gelatin–mesoporous silica nanoparticles as matrix metalloproteinases-degradable drug delivery systems in vivo. Microporous Mesoporous Mater. 2013;182:165–172. [Google Scholar]
- Xu W., He W., Du Z., Zhu L., Huang K., Lu Y., Luo Y. Functional nucleic acid nanomaterials: Development, properties, and applications. Angew. Chem. Int. Ed. 2021;60:6890–6918. doi: 10.1002/anie.201909927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Urata C., Ujiie H., Yamauchi Y., Kuroda K. Preparation of aqueous colloidal mesostructured and mesoporous silica nanoparticles with controlled particle size in a very wide range from 20 nm to 700 nm. Nanoscale. 2013;5:6145–6153. doi: 10.1039/c3nr00334e. [DOI] [PubMed] [Google Scholar]
- Yan T., He J., Liu R., Liu Z., Cheng J. Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr. Polym. 2020;231 doi: 10.1016/j.carbpol.2019.115706. [DOI] [PubMed] [Google Scholar]
- Yang Y.-J., Tao X., Hou Q., Ma Y., Chen X.-L., Chen J.-F. Mesoporous silica nanotubes coated with multilayered polyelectrolytes for pH-controlled drug release. Acta Biomater. 2010;6:3092–3100. doi: 10.1016/j.actbio.2010.02.042. [DOI] [PubMed] [Google Scholar]
- Yano K., Fukushima Y. Synthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant. J. Mater. Chem. 2004;14:1579–1584. [Google Scholar]
- You Y., Hu H., He L., Chen T. Differential effects of polymer-surface decoration on drug delivery, cellular retention, and action mechanisms of functionalized mesoporous silica nanoparticles. Chem. Asian J. 2015;10:2744–2754. doi: 10.1002/asia.201500769. [DOI] [PubMed] [Google Scholar]
- Yu Q., Hui J., Wang P., Xu B., Zhuang J., Wang X. Hydrothermal synthesis of mesoporous silica spheres: effect of the cooling process. Nanoscale. 2012;4:7114–7120. doi: 10.1039/c2nr31834b. [DOI] [PubMed] [Google Scholar]
- Yu M., Jambhrunkar S., Thorn P., Chen J., Gu W., Yu C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale. 2013;5:178–183. doi: 10.1039/c2nr32145a. [DOI] [PubMed] [Google Scholar]
- Yu Y.-J., Xing J.-L., Pang J.-L., Jiang S.-H., Lam K.-F., Yang T.-Q., Xue Q.-S., Zhang K., Wu P. Facile synthesis of size controllable dendritic mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces. 2014;6:22655–22665. doi: 10.1021/am506653n. [DOI] [PubMed] [Google Scholar]
- Yue Y., Wu C. Progress and perspectives in developing polymeric vectors for in vitro gene delivery. Biomater. Sci. 2013;1:152–170. doi: 10.1039/c2bm00030j. [DOI] [PubMed] [Google Scholar]
- Zainala N.A., Shukor S., Wabb H.A.A., Razakb K. Study on the effect of synthesis parameters of silica nanoparticles entrapped with rifampicin. Chem. Eng. 2013;32 [Google Scholar]
- Zeiderman M.R., Morgan D.E., Christein J.D., Grizzle W.E., McMasters K.M., McNally L.R. Acidic pH-targeted chitosan-capped mesoporous silica coated gold nanorods facilitate detection of pancreatic tumors via multispectral optoacoustic tomography. ACS Biomater. Sci. Eng. 2016;2(7):1108–1120. doi: 10.1021/acsbiomaterials.6b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Luo Z., Liu J., Ding X., Li J., Cai K. Cytochrome c end-capped mesoporous silica nanoparticles as redox-responsive drug delivery vehicles for liver tumor-targeted triplex therapy in vitro and in vivo. J. Control. Release. 2014;192:192–201. doi: 10.1016/j.jconrel.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Ang C.Y., Li M., Tan S.Y., Qu Q., Luo Z., Zhao Y. Polymer-coated hollow mesoporous silica nanoparticles for triple-responsive drug delivery. ACS Appl. Mater. Interfaces. 2015;7:18179–18187. doi: 10.1021/acsami.5b05893. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-N., Poon W., Tavares A.J., McGilvray I.D., Chan W.C. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Release. 2016;240:332–348. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zheng N., Chen L., Xie L., Cui M., Li S., Xu L. Effect of shape on mesoporous silica nanoparticles for oral delivery of indomethacin. Pharmaceutics. 2019;11:4. doi: 10.3390/pharmaceutics11010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Hua M., Liu H., Li J. How to design nanoporous silica nanoparticles in regulating drug delivery: Surface modification and porous control. Mater. Sci. Eng. B. 2021;263 [Google Scholar]
- Zhao D., Feng J., Huo Q., Melosh N., Fredrickson G.H., Chmelka B.F., Stucky G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science. 1998;279:548–552. doi: 10.1126/science.279.5350.548. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Geng H., Wang Y., Gao Y., Huang J., Wang Y., Zhang J., Wang S. Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces. 2014;6:20290–20299. doi: 10.1021/am505824d. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Tan L.L., Li Q.L., Qiu X.L., Qi A.D., Tao Y., Yang Y.W. Acetylcholine-triggered cargo release from supramolecular nanovalves based on different macrocyclic receptors. Chem Eur J. 2014;20:2998–3004. doi: 10.1002/chem.201304864. [DOI] [PubMed] [Google Scholar]
- Zhou R., Sun S., Li C., Wu L., Hou X., Wu P. Enriching Mn-doped ZnSe quantum dots onto mesoporous silica nanoparticles for enhanced fluorescence/magnetic resonance imaging dual-modal bio-imaging. ACS Appl. Mater. Interfaces. 2018;10:34060–34067. doi: 10.1021/acsami.8b14554. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Quan G., Wu Q., Zhang X., Niu B., Wu B., Huang Y., Pan X., Wu C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B. 2018;8:165–177. doi: 10.1016/j.apsb.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Liu G., Wang Y., Liu J., Zhang Y., Ma Y. AuNP and ssDNA capped mesoporous silica nanoparticles for laser controlled drug release. RSC Adv. 2019;9:34958–34962. doi: 10.1039/c9ra07404j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Ding C., Wang C., Fu J. UV-light cross-linked and pH de-cross-linked coumarin-decorated cationic copolymer grafted mesoporous silica nanoparticles for drug and gene co-delivery in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;108 doi: 10.1016/j.msec.2019.110469. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chang C., Liu Z., Zhao Q., Xu Q., Li C., Chen Y., Zhang Y., Lu B. Hyaluronic acid-functionalized hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for cancer chemo-photodynamic therapy. Langmuir. 2021;37:2619–2628. doi: 10.1021/acs.langmuir.0c03250. [DOI] [PubMed] [Google Scholar]
- Zou Z., He D., He X., Wang K., Yang X., Qing Z., Zhou Q. Natural gelatin capped mesoporous silica nanoparticles for intracellular acid-triggered drug delivery. Langmuir. 2013;29:12804–12810. doi: 10.1021/la4022646. [DOI] [PubMed] [Google Scholar]