Abstract
Objective:
Overfeeding is a strategy for evaluating the effects of excess energy intake. In this secondary analysis we tested the possibility that different levels of dietary protein might differentially modify the response of fatty acyl-carnitines to overfeeding.
Methods:
Twenty-three healthy adult men and women were overfed by 40% for 8 weeks while in-patients with diets containing 5% (LPD), 15% (NPD) or 25% (HPD) protein. Plasma fatty acyl-carnitines were measured by gas chromatography/mass spectrometry (GC/MS) at baseline and after 8 weeks of overfeeding. Measurements included: body composition by DXA, energy expenditure by ventilated hood and doubly-labeled water, fat cell size from subcutaneous fat biopsies, and fat distribution by CT scan.
Results:
Analysis was done on 5 groups of fatty acyl-carnitines identified by principal components analysis and 6 individual short-chain fatty acyl carnitines. Higher protein intake was associated with significantly lower 8 week levels of medium chain fatty acids and C2, C4-OH and C 6:1, but higher values of C3 and C5:1 acyl-carnitines derived from essential amino acids. In contrast energy and fat intake were only weakly related to changes in fatty acyl-carnitines. A decease or smaller rise in 8 week medium chain acyl-carnitines was associated with an increase in sleeping energy expenditure (P = 0.0004), and fat free mass (P < 0.0001) and a decrease in free fatty acid concentrations (FFA) (P = 0.0067). In contrast changes in short-chain fatty acyl-carnitines were related to changes in resting energy expenditure (P = 0.0026), and fat free mass (P = 0.0007), and C4-OH was positively related to FFA (P = 0006).
Conclusion:
Protein intake was the major factor influencing changes in fatty acyl carnitines during overfeeding with higher values of most acyl-fatty acids on the low protein diet. The association of dietary protein and fat intake may explain the changes in energy expenditure and metabolic variables resulting in the observed patterns of fatty acyl carnitines
Keywords: Overfeeding, Body fat, Fat free mass, Fatty acyl-carnitines, Fat oxidation, Fat cell size
1. Introduction
Obesity is a major public health problem affecting more than one-third of the adult American population [1]. The obesity epidemic also has a major impact on health [2,3]. The health problems resulting from obesity are modulated by many environmental and genetic factors [4,5]. Understanding how obesity develops and what can be done to reduce its impact provides an important medical challenge.
Conscious overfeeding has been one strategy used to unravel the effects of a positive energy balance which is the ultimate cause of obesity [6]. Overfeeding increases body weight, body fat, fat free body mass, fat cell size, energy expenditure and has a variety of effects on metabolic phenotypes [7–16]. The level of dietary protein in turn modifies the response to overfeeding [11]. A low protein diet during overfeeding increases the percentage of body fat [13] whereas a high protein diet reduces fat storage and increases protein storage [12]. Body fat increases during overfeeding in direct relation to overfed energy, but overfed protein is not significantly related to the increase in body fat [11]. The size of subcutaneous adipocytes at the onset of overfeeding is related to the increase in visceral adipose tissue and the induction of insulin resistance during overfeeding [13]. Dietary fat is composed of many different fatty acids with multiple functions and overfeeding thus offers an opportunity to examine the effects of differing levels of dietary protein and fat on changes in fatty acyl-carnitines. Since the increase in energy intake predicted the increase in body fat, we hypothesized that the change in dietary fat – the reciprocal of dietary protein – during overfeeding would explain the changes in fatty acyl-carnitines.
2. Methods and Materials
2.1. Participants
Twenty-three healthy men and women between the ages of 18 and 35 years with a BMI of 19.7 to 29.6 kg/m2, who led a sedentary lifestyle (<2 h of moderate to vigorous exercise per week) and who completed the 8-week overfeeding protocol and a hyperinsulemic-euglycemic clamp at both baseline and week 8 were included in this secondary analysis. Participants were informed that they would be randomly assigned to one of three different overfeeding diets, would have to live as in-patients at the Pennington Biomedical Research Center for approximately 12 weeks and would be required to eat all foods and only those foods provided by the metabolic kitchen. All participants provided verbal and written consent prior to study initiation. This trial was approved and monitored by the PBRC Institutional Review Board. This trial was registered at Clinicaltrials.gov (NCT00565149).
2.2. Protocol
Details of this randomized, parallel-arm in-patient study have been previously described [11–13]. Overfeeding was planned at approximately 40% above energy requirements for weight maintenance or about 1000 kcal/day (4180 kJ/d). Diets contained either 5% (low protein diet or LPD), 15% (normal protein diet or NPD) or 25% (high protein diet or HPD) protein. The first 13 to 25 days of the inpatient stay was used to establish energy requirements for weight maintenance. Once weight stability was achieved baseline assessments were performed including a 2-step hyperinsulinemic-euglycemic clamp, measurements of body composition by DXA and abdominal CT scan and collection of tissue biopsies (subcutaneous fat and skeletal muscle) and blood samples for analysis of fatty acyl carnitines. The blood sampling, hyperinsulinemic-euglycemic clamp, DXA, and CT scans were repeated in the 8th week.
2.3. Diets
All food was prepared by the research kitchen at PBRC and provided to the participants in a 5-day rotating menu [11]. All meals were prepared in duplicate and food composites were prepared, frozen and later analyzed for nutrient composition [11]. To assure compliance to the overfeeding diets, all meals were consumed while being supervised by kitchen or in-patient personnel. There were three experimental diets with goals of providing 5% of energy from protein, 15% of energy from protein or 25% of energy from protein. Protein intake was 90 ± 16 g/d at baseline, and 47 ± 4.7, 140 ± 29 and 228 ± 48 g/d in the LPD, NPD and HPD groups respectively; in grams of protein per kilogram of body weight daily the values were 1.83 ± 0.98 g/kg/d at baseline; 0.68 ± 0.069; 1.80 ± 0.25; and 3.01 ± 0.29 in the LPD, NPD and HPD groups respectively. Absolute intake of carbohydrate (grams per day) was maintained constant across groups throughout overfeeding and therefore the difference in calories was made up by increasing the amount of fat used in each of the diets. At baseline, carbohydrate provided 50% of calories declining to 36% of calories in each of the 3 overfeeding diets. Fat, as a percent of calories was 59% in the LPD, 49% in the NPD and 39% in the HPD.
2.4. Energy Expenditure
Resting energy expenditure was measured weekly with a ventilated hood system (Deltatrac II Metabolic Monitor Datex-Ohmeda Helsinki, Finland) [11]. Participants were fasting at least 10 h and remained motionless, but awake under the transparent plastic hood for 30 min. Total daily energy expenditure (TDEE) was measured during baseline and weeks 7–8 by the doubly-labeled water method [11]. Sleeping energy expenditure (Sleep EE) (reported as kcal/d) was measured during the 3 times that each individual lived in the whole body metabolic chamber for 24 h [12]. Sleeping energy expenditure was the energy expenditure measured between 0200 h and 0500 h and extrapolated to 24 h, when there was no physical activity as indicated by the radar motion sensor in the chamber.
2.5. Body Composition
Body composition was determined by dual-energy X-ray absorptiometry (DXA) performed using a Hologics QDR 4500A whole-body scanner. Scans were analyzed with the V11.1 QDR software for Windows [11].
2.6. Computed Tomography for Measurement of Adipose Tissue Volume
Volunteers were supine on the Computed Tomography (CT) scanner table with the arms extended over the head. Eight contiguous images were obtained, one every 5 cm with 5 images obtained above and 2 below a slice centered on L4-L5 inter-vertebral disc. Adipose tissue cross-sectional area was determined using the Analyze PC™ software package (Mayo Clinic, Rochester, MN) and with this data visceral, subcutaneous-deep and subcutaneous-superficial fat areas were calculated [17]. Areas were calculated using a triangulation method.
2.7. Adipose Tissue Biopsy for Fat Cell Size
Approximately 500 mg of adipose tissue was removed from a 1 cm incision on the abdomen, 5 cm from the umbilicus. Approximately 50 mg of tissue was placed in osmium tetroxide for the determination of fat cell size as previously described [17]. Adipocyte cell number (cells per mg wet weight of tissue) was determined from the amount of sample (ml) the quantity of cells (per ml), and the tissue weight (mg).
All data are μmol/L × 10−3 except carnitine which is μmol/L. Baseline values are Mean ± SD; changes from baseline are Mean ± SEM adjusted for age, sex and baseline group value; values are ×10−3 except for carnitine. Statistical comparisons were by Tukey HSD. The bold indicates a significant value as defined under methods.
2.8. Protein and Fat Oxidation
Resting metabolic rate (RMR) was measured with a Deltatrac II metabolic monitor (Datex, Helsinki, Finland), three times during the baseline of the hyperinsulinemic-euglycemic clamp procedure (fasted state) before beginning the insulin infusion, and three times during the last 30 min of each step of the insulin infusion [11]. Each RMR was 30 min in length and the last 20 min were used for analysis of energy expenditure and substrate oxidation. Two blood samples were collected during each RMR period for determination of plasma insulin, glucose and FFA concentration at −15 and −5 min before the insulin infusion during the baseline period, and −15 and −5 min before the second and third RMR periods. Patients voided before the test, and the urine collected during and at the end of the clamp was used to determine urinary nitrogen concentration and glucose loss (if any).
2.9. Laboratory Measurements
Glucose was measured using a glucose oxidase electrode (Beckman Coulter DXC 600 Pro, Brea, CA) and insulin by immunoassay (Siemens Immulite 2000, Los Angeles, CA). Free fatty acids were measured with a high sensitivity kit (Wako Kit). Leptin was measured by immunoassay. Triiodothyronine, thyrotrophin stimulating hormone (TSH); and thyroxine were measured by immunoassay (Siemens Immulite 2000, Los Angeles, CA). Fatty acyl-carnitine species were measured in plasma at baseline (obtained prior to overfeeding) and during the last week of overfeeding. Plasma from the two fasted blood samples collected 30 min apart during the basal period of the hyperinsulinemic-euglycemic clamp were pooled and used to measure fatty acyl-carnitines by gas chromatography/mass spectrometry and tandem mass spectrometry [18–20]. A total of 76 fatty acyl-carnitines were identified and subjected to principal components analysis.
2.10. Statistical Analysis
A principal components analysis was conducted on the baseline samples and groupings with an Eigen value ≥ 4 are shown in Table 1. The same clusters of fatty acyl-carnitines were used at 8 weeks. In addition, several short-chain fatty acyl-carnitines were examined since they reflect input from glucose, ketone bodies and some amino acids to the acyl-carnitine pool. Protein diets were randomly assigned. Baseline data are expressed as mean ± SD, change from baseline as mean ± SE. Effects of treatment on change from baseline on body weight, abdominal fat, energy expenditure, and glucose metabolism were evaluated using simple regression analysis and again after adjusting for age, sex and baseline covariates. Analysis of variance was used to compare treatment effects and where significant differences existed post-hoc comparisons were done with the Tukey-HSD test. Sex and age were included as fixed effects. Because of multiple comparisons, alpha was set at P ≤ 0.005. Analyses were done with the JMP-7 statistical package (SAS Cary, NC).
Table 1.
Groups of fatty acyl-carnitines identified in the principal components analysis.
| Group | Group name | Constituent fatty acyl carnitines | Eigen value |
|---|---|---|---|
| 1 | Fatty acyl carnitines mostly C14 and C16 in length | C8-DC; C14:1; C16:1; C16:2; C16:3; C20:3-OH | 24.8 |
| 2 | Dicarboxyl and hydroxyl fatty acyl carnitines of C12 to C18 length | C14/C8:1-DC; C14:1-OH; C16/C10:1-DC; C16:2-OH; C18/C12:1-DC;C18:1/C12:2-DC; C18:2/C12:3-DC; C18:3 |
9.14 |
| 3 | Mono and di unsaturated fatty acyl carnitines of C12 to C18 length | C12-DC; C12/C6:1-DC; C12:1;C12:2; C14:2; C12:2-OH; C14:3; C18:3-OH/C16:3-DC | 5.60 |
| 4 | Short-chain fatty acyl carnitines of C3 to C-5 length with one dicarboxyl fatty acyl carnitine | C3; C4-OH; C5; C5-OH; C5:1;C20/C14:1-DC | 4.70 |
| 5 | Short and medium chain fatty acyl carnitines of C4 to C 10 length | C4-DC/Ci4-DC; C6; C8; C10; C10:1 | 4.00 |
| Carnitine | Carnitine | Carnitine |
3. Results
3.1. Participants
Twenty-three of the initial 25 participants in the PROtein Overfeeding (PROOF) study [11] had fatty acyl-carnitines measured at baseline and again at 8 weeks and were included in this secondary analysis. At baseline the subjects were 24 ± 4 (mean ± SD) years of age (range 18–35 years), and weighed 74.4 ± 14.0 kg (range 52.8 to 107.4 kg), of which was 56.6 ± 12.4 kg of fat free mass (range 35.8 to 84.4 kg), and 18.5 ± 6.4 kg was fat mass (range 7.2 to 19.1 kg). Fifteen participants were African-American, 6 were Caucasian and 2 were of other race/ethnicity.
3.2. Description of Groups Identified by Principal Components Analysis and Their Relationship to One Another at Baseline
Group 1 (Table 1) consisted of 4 long chain (C14:1 to C16:3) unsaturated fatty acids, one hydroxylated long-chain fatty acid (C20:3-OH) and one dicarboxylated medium chain (C8 DC) fatty acid; group 2 consisted mainly of long-chain mostly unsaturated fatty acids (C14 to C18:3) that were dicarboxylated or hydroxylated; group 3 consisted mostly of unsaturated hydroxyl or dicarboxyl fatty acids (C12-DC to C18:2-OH/C16:3 DC); Group 4 consisted of 5 short chain fatty acids (C3 to C5:1) and 1 long chain fatty acid (C20/C14:1-DC); group 5 had five mostly saturated medium-chain fatty acids (C6 to C10), one mono-unsaturated fatty acid (C10:1) and one dicarboxylic acid ratio. At baseline, the acyl-carnitines clustered in groups 1, 2, and 3 were positively and strongly related to each other (P < 0.001). Group 4 had a regression slope that was generally opposite to groups 1 to 3. Group 5 was positively related to group 3 (P = 0.0001).
3.3. Relationship of Principal Component Groups After Overfeeding
After overfeeding the change acyl-carnitine concentrations clustered in group 1 were strongly related to changes in acyl-carnitine concentrations clustered in groups 2, 3, and 5 (P < 0.001) adjusted for baseline fatty acyl-carnitine levels. The change in group 4 was inversely related to changes in group 2 (P = 0.0050), but only marginally related and inversely related to group 1 (P = 0.014), group 3 (P = 0.010), and group 5 (P = 0.027). Since changes in groups 1, 2 and 3 were strongly and positively related to each other (P < 0.0001), and group 4 was inversely related to these groups several figures that follow only use data for group 2 and group 4.
3.4. Relation of Diet to Change in Fatty Acyl Carnitines
Table 2 shows the overall change from baseline and the change from baseline by diet for the fatty acyl-carnitine groups identified by principal components analysis. Overfeeding produced a significant overall increase in carnitine and the concentrations of acyl-carnitines clustered in group 3 but not the other groups. Two patterns were evident in the changes of fatty acyl-carnitines in response to different doses of dietary protein. The predominant pattern in was a significantly greater increase in the concentration of fatty acyl-carnitines in groups 1, 2, 3 and 5 for those individuals eating the low protein diet (LPD). In contrast, the individuals eating the LPD diet had a significantly lower concentration of fatty acyl-carnitines in cluster 4 at eight weeks than at baseline.
Table 2.
Baseline and change from baseline to week 8 for fatty acyl-carnitines from the principal components analysis.
| Group | Baseline value | Change from baseline | Change from baseline by diet group |
P change from baseline | P for diet effect | ||
|---|---|---|---|---|---|---|---|
| LPD | NPD | HPD | |||||
| 1 | 54.6 ± 14.9 | 21.7 ± 8.62 | 56.2 ± 14.1 | 3.94 ± 14.0 | 2.33 ± 15.2 | 0.020 | L > HP = 0.013 L > NP = 0.0005 |
| 2 | 204.6 ± 48.7 | 31.2 ± 12.6 | 81.8 ± 19.6 | 6.17 ± 19.4 | 1.20 ± 20.7 | 0.022 |
L > HP = 0.0016 L> NP < 0.0001 |
| 3 | 129.5 ± 33.1 | 73.1 ± 22.3 | 177.1 ± 33.7 | 22.3 ± 33.4 | 10.8 ± 36.3 | 0.0034 | L > HP = 0.016 L > NP = 0.0002 |
| 4 | 684.8 ± 211.4 | −48.7 ± 30.2 | −174.9 ± 49.4 | 46.4 ± 49.0 | −23.4 ± 53.2 | 0.12 | L < NP = 0.0008 |
| 5 | 363.9 ± 95.2 | 209.9 ± 80.6 | 550.8 ± 138.1 | 50.9 ± 136.8 | −6.20 ± 148.7 | 0.016 | L > NP = 0.0008 |
| Carnitine | 45.4 ± 10.9 | −4.52 ± 1.13 | −4.78 ± 2.25 | −1.98 ± 2.23 | −8.01 ± 2.42 | 0.0006 | NS |
All data are μmol/L × 10−3 except carnitine which is μmol/L. Baseline values are Mean ± SD; changes from baseline are Mean ± SEM adjusted for age, sex and baseline group value; values are ×10−3 except for carnitine. Statistical comparisons were by Tukey HSD. The bold indicates a significant value as defined under methods.
3.5. Effect of Diet on the Changes in the Individual Short-chain Fatty Acyl-carnitines
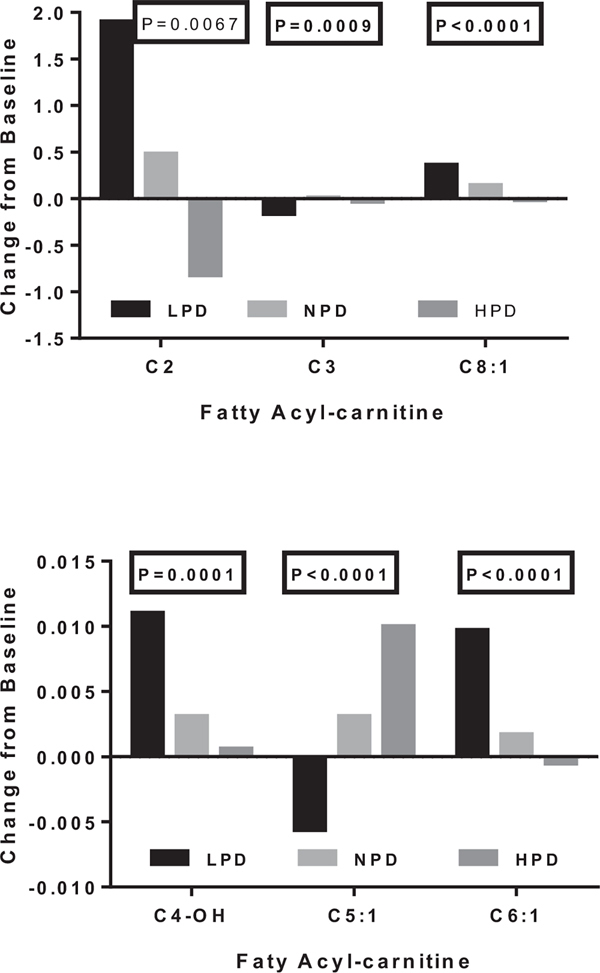
The changes from baseline for the short chain fatty acyl-carnitines with P values < 0.005 are depicted by diet in Fig. 1 which places the three acyl-carnitines with the highest concentration in the top panel and the three with the lowest concentration in the lower panel. There were two patterns of response. C-2, C4-OH, C6:1, and C8:1 acyl-carnitines tended to change in parallel, and showed a decline after 8 weeks of overfeeding as dietary protein increased (or dietary fat decreased). In contrast C3 and C5:1 acyl-carnitines changed together and showed a positive relationship with increasing dietary protein.
Fig. 1.
Effect of dietary protein on change from baseline in several short-chain fatty acyl-carnitines.
3.6. Relationship Between Dietary Intake of Protein, Fat and Energy With Changes in Fatty Acyl-carnitines
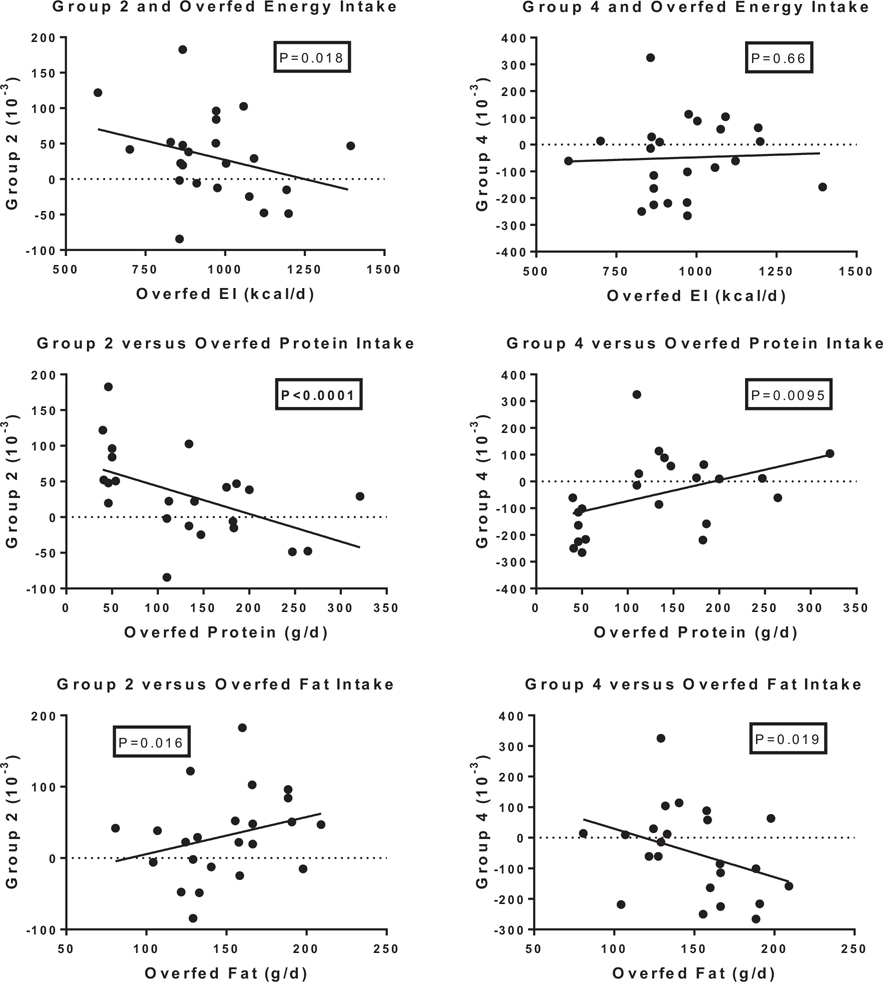
The top portion of Table 3 and Fig. 2 show the association of fatty acyl-carnitines with dietary intake of protein, energy and fat. The “P” values in these figures are adjusted for age, sex, baseline values of the metabolic variables and baseline values for the appropriate grouping from the principal components analysis. Dietary protein intake was strongly and inversely related to changes in all groups of fatty acyl-carnitines (P < 0.005) except the acyl-carnitines in group 4 where the relationship was positive, but marginally significant (P = 0.0095) (Fig. 2). Neither energy intake nor dietary fat intake were significantly related to any of the groups of fatty acyl-carnitines.
Table 3.
Association of changes in fatty acyl-carnitines with changes in dietary, metabolic and body composition variables.
| Variable | Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | R | P | |
| Overfed nutrients | ||||||||||
| Overfed protein g/d | −0.78 | <0.0001 | −0.78 | <0.0001 | −0.78 | <0.0001 | +0.62 | 0.0095 | −0.71 | 0.0006 |
| Overfed EI kcal/d | −0.49 | 0.048 | −0.57 | 0.018 | −0.50 | 0.026 | +0.34 | 0.66 | −0.38 | 0.13 |
| Overfed fat g/d | +0.57 | 0.014 | +0.57 | 0.016 | +0.53 | 0.017 | −0.59 | 0.019 | +0.50 | 0.029 |
| Body composition variables | ||||||||||
| ΔFFM, kg | −0.78 | <0.0001 | −0.81 | <0.0001 | −0.78 | <0.0001 | +0.71 | 0.0007 | −0.74 | 0.0003 |
| ΔFM, kg | 0.88 | 0.81 | 0.71 | 0.76 | 0.54 | |||||
| ΔSAT, kg | 0.51 | 0.99 | 0.65 | 0.24 | 0.75 | |||||
| ΔVAT, kg | 0.17 | 0.26 | 0.14 | 0.57 | 0.070 | |||||
| ΔFCS, μL | 0.34 | 0.31 | 0.29 | −0.55 | 0.042 | 0.23 | ||||
| Energy expenditure variables | ||||||||||
| ΔTDEE, kcal/d | −0.57 | 0.14 | −0.55 | 0.056 | −0.56 | 0.061 | +0.69 | 0.0021 | −0.62 | 0.10 |
| ΔREE, kcal/d | −0.55 | 0.025 | −0.55 | 0.025 | −0.54 | 0.020 | +0.68 | 0.0026 | −0.46 | 0.076 |
| ΔSEE, kcal/d | −0.66 | 0.0038 | −0.75 | 0.0004 | −0.68 | 0.0017 | +0.48 | 0.057 | −0.68 | 0.0027 |
| Metabolic variables | ||||||||||
| ΔGlucose mg/dL | 0.66 | 0.55 | 0.69 | 0.79 | 0.85 | |||||
| ΔInsulin, μU/mL | 0.96 | 0.59 | 0.82 | 0.91 | +0.49 | 0.50 | ||||
| ΔInsulin supp, % | +0.54 | 0.062 | +0.58 | 0.046 | +0.52 | 0.054 | −0.56 | 0.054 | +0.49 | 0.094 |
| ΔHOMA-IR AU | 0.96 | 0.53 | 0.85 | 0.92 | 0.54 | |||||
| ΔFree fatty acids, mmol/L | +0.69 | 0.0013 | +0.62 | 0.0067 | +0.63 | 0.0035 | −0.56 | 0.021 | +0.60 | 0.0072 |
| ΔLeptin, ng/nL | +0.50 | 0.052 | +0.62 | 0.048 | +0.44 | 0.080 | − 0.44 | 0.60 | +0.49 | 0.056 |
| ΔT3, ng/dL | +0.59 | 0.0109 | +0.48 | 0.058 | +0.59 | 0.0065 | −0.38 | 0.16 | +0.60 | 0.0074 |
Values are for change from baseline, adjusted for baseline value, age and sex. The R values are unadjusted for the whole model. Abbreviations: FFM = fat free mass; FM = fat mass; SAT = subcutaneous adipose tissue; VAT = visceral adipose tissue; FCS = fat cell size; TDEE = total daily energy expenditure; REE = resting energy expenditure; SEE = sleeping energy expenditure; Ins Supp = Insulin Suppression; HOMA-IR = Homeostatic model insulin resistance; FFA = free fatty acids; T3 = triiodothyronine; AU = Area underthe curve. The bold indicates a significant value as defined under methods.
Fig. 2.
Relation of overfed energy intake, protein intake and fat intake to changes in fatty acyl-fatty carnitines in group 2 and group 4 from the principal components analysis.
Among the short-chain acyl-carnitines, dietary protein was also strongly and positively related to C3 (r = +0.65; P = 0.0045) and C5:1 (r = +0.85; P < 0.0001), but negatively related to the other 4 [C2 (r = −0.85; P = 0.0005), C4-OH (r = −0.82; P < 0.0001), C6:1 (r = −0.84; P < 0.0001) and C8:1 (r = −0.88; P < 0.0001)]. Energy intake was likewise without effect on the short chain fatty acyl-carnitines, but fat intake showed a significant, but divergent, effect on 3 of the short chain acyl-carnitines (C5:1 r = −0.90 P < 0.0001; C6:1 r = +0.60 P = 0.0008 and C8:1 r = +0.72, P = 0.0005).
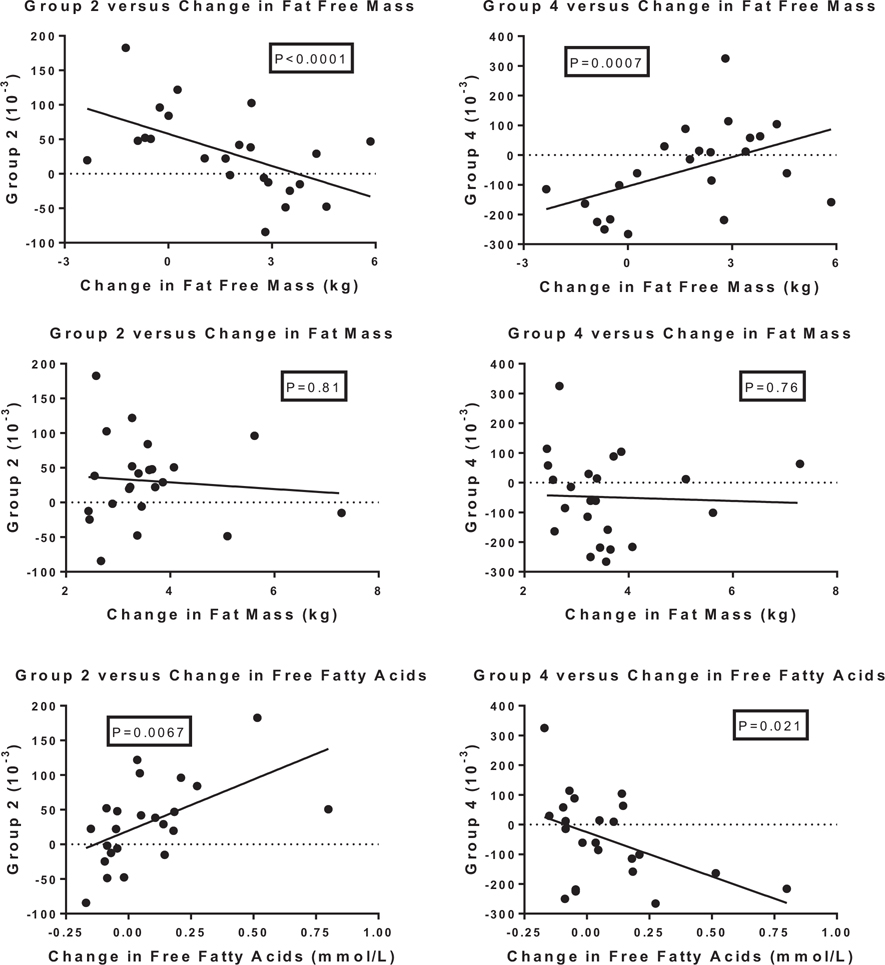
Three other variables, i.e., fat free mass, free fatty acids and energy expenditure also showed striking relationships with changes in the clusters of fatty acyl-carnitines included in the principal components analysis (Table 3 and Fig. 3). Changes in fat free mass (FFM) were strongly and negatively related to the acyl-carnitines clustered in groups 1, 2, 3, and 5 and positively related to group 4 as shown for groups 2 and 4 in Fig. 3. The short chain fatty acyl-carnitines were also strongly related to changes in FFM with C3 and C5:1 showing positive correlations (r = +0.70; P = 0.0007 and C = +0.66 P = 0.0024), and three of the other short chain acyl-carnitines showing significant negative relationships (C4-OH r = −0.75; P(=0.0002); C6:1 r = −0.83 (P < 0.0001) and C8:1 (R = −0.74 P = 0.0002)). Free fatty acids were positively and significantly related to the acyl-carnitines clustered in groups 1, 2, 3 and 5, and among the short chain acyl-carnitines two of them (C4-OH R = +0.72; P = 0.0005 and C6:1 R = +0.68 P = 0.0010) were also positively related to the change in free fatty acids. There were no relations with the change in fat mass.
Fig. 3.
Relation of changes in fat free mass, fat mass, and free fatty acids to changes in acyl-fatty acids in group 2 and group 4 of from the principal components analysis. P value based on regression analysis adjusted for age and sex and the baseline values of group 2 or group 4 and the baseline value of FFM, FM or FFA.
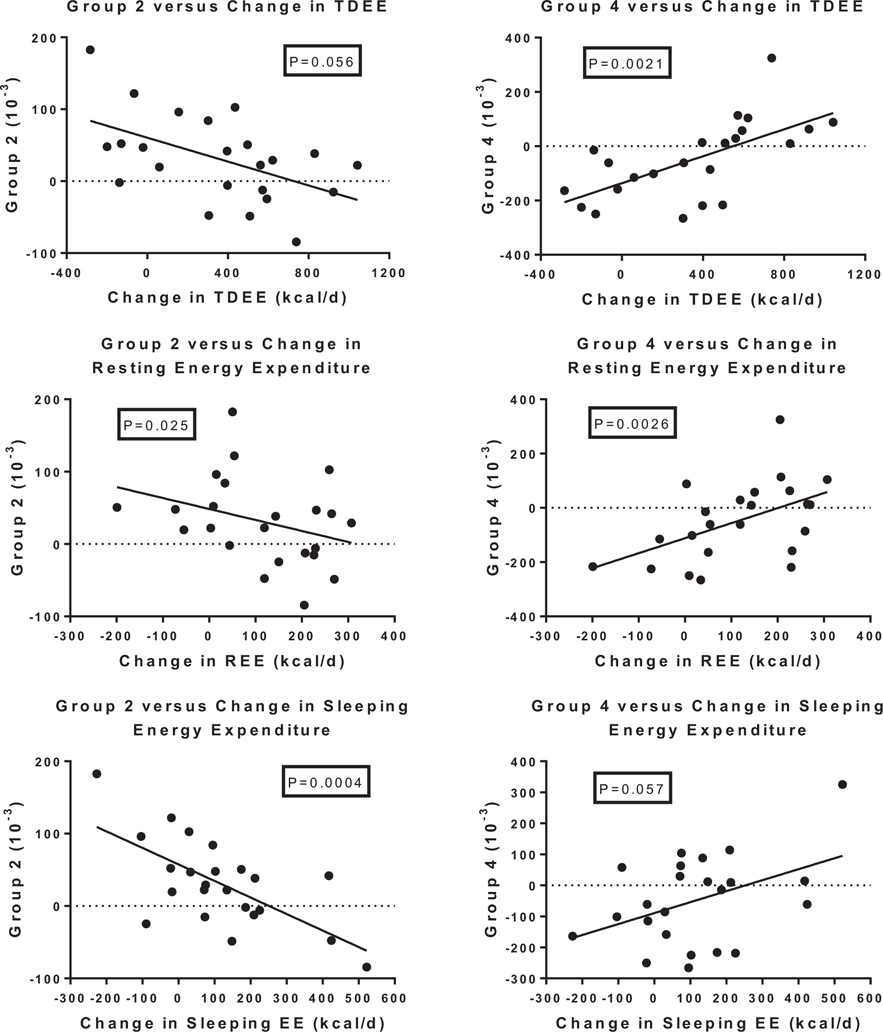
Several measures of energy expenditure were examined for their relationship to changes in fatty acyl-carnitines including total daily energy expenditure (TDEE), resting energy expenditure (REE) and sleeping energy expenditure (SEE). As seen in Table 3 and Fig. 4 SEE was strongly and negatively related to the changes in acyl-carnitines clustered in groups 1, 2, 3 and 5, and positively, but not significantly to the change in group 4. Changes in TDEE and REE were positively related to changes of acyl-carnitines in Group 4 (Table 3). In contrast to the finding with the groups of longer chain fatty acyl-carnitines, the individual short chain fatty acyl-carnitines showed strong relationships with REE which were positive for C3 (R = +70 P = 0.0024) and C5:1 (R = +0.74 P = 0.0002) and negative for C 4-OH (R = −0.60; P = 0.0088), C 6:1 (R = −0.67; P = 0.0021) and D8:1 (R = −0.68; P = 0.0031). SEE was negatively related to only 2 short chain fatty acyl-carnitines (C2 R = −0.65; P = 0.0048 and C6:1 r = −0.64 P = 0.0039).
Fig. 4.
Relation of changes in total daily energy expenditure (TDEE), resting energy expenditure (REE) and sleeping energy expenditure (SEE) to changes in acyl-fatty acids in group 2 and group 4 of from the principal components analysis. P value based on regression analysis adjusted for age and sex and the baseline values of group 2 or group 4 and the baseline value of TDEE, REE or SEE.
One explanation for the relation of changes in fatty acyl-carnitines to energy expenditure and metabolic variables might be their relationship to components of the overfed diet. Table 4 shows the changes in dietary components adjusted for baseline variables and the change in energy expenditure (TDEE, REE and SEE) and three metabolic variables (FFM, FM and FFA). Overfed protein was significantly related to the change in REE and SEE and to the change in FFM and FFA. Change in fat intake was also significantly related to the change in REE and the change in FFM, but not the other variables. There were no relationships between overfed energy and changes in any of these variables.
Table 4.
Relationship of change in energy expenditure, fat free mass, fat mass and free fatty acids to the change in energy intake, fat intake and protein intake adjusted for baseline values.
| Energy or metabolic component | Δ Energy intake, kcal/d |
Δ Protein intake, kcal/d |
Δ Fat intake, kcal/d |
|||
|---|---|---|---|---|---|---|
| R | P | R | P | P | R | |
| Change in energy component | ||||||
| Δ TDEE, kcal/d | +0.57 | 0.15 | +0.56 | 0.16 | −0.51 | 0.46 |
| Δ REE, kcal/d | +0.69 | 0.41 | +0.86 | =0.0001 | −0.82 | 0.0025 |
| Δ SEE, kcal/d | +0.30 | 0.29 | +0.58 | 0.0079 | −0.47 | 0.039 |
| Change in metabolic component | ||||||
| Δ FFM, kg | +0.59 | 0.032 | +0.84 | <0.0001 | −0.67 | 0.0049 |
| Δ FM, kg | +0.40 | 0.11 | −0.24 | 0.61 | +0.34 | 0.23 |
| Δ FFA mmol/d | +0.34 | −0.14 | −0.57 | 0.0070 | +0.43 | 0.061 |
= Adjusted for baseline nutrient and energy intake.
4. Discussion
This planned secondary analysis of the PROtein OverFeeding (PROOF) study has examined changes in fatty acyl-carnitines measured by gas chromatography and tandem mass spectrometry at baseline and after 7–8 weeks of overfeeding in 23 healthy men and women. Individuals were overfed for 8 weeks at 40% above their baseline energy requirements with diets containing 5%, 15% or 25% protein. Five groups or clusters of fatty acyl-carnitines were identified at baseline from a principal components analysis of the 76 species that were measured. Contrary to our hypothesis, this study clearly shows that protein intake, not fat intake or energy intake, was the major driving factor for most of the changes in fatty acyl-carnitines, although this effect was expressed differently in the short chain fatty acyl-carnitines than in the medium and long-chain fatty acyl-carnitines.
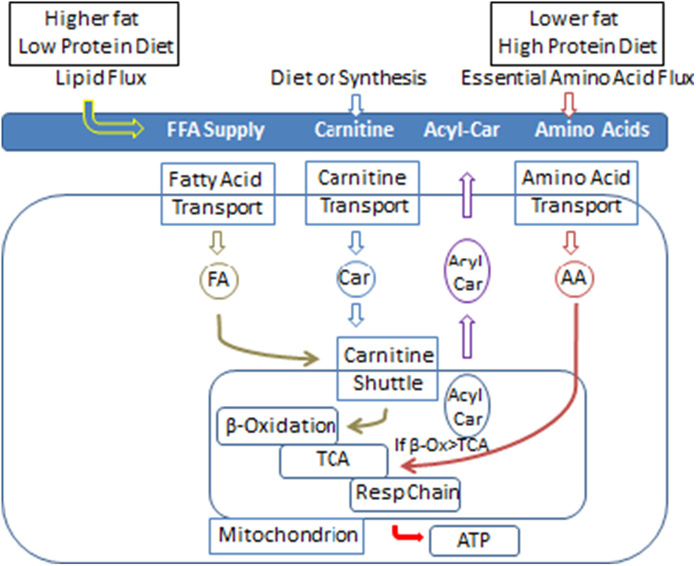
Carnitine, primarily of dietary origin, participates in the transport of fatty acids in both directions across the mitochondrial and cell membrane by the carnitine phosphotransferase (CPT-1/CPT-2) transporters and solute carrier family 22 member 5 transporter (OCTN2 or SLC22A5). A schematic presentation of key steps in this process and the effect of our diets is shown in Fig. 5. The significant increase in carnitine with overfeeding reflects its dietary origin. Most acyl-carnitines are derived during the process of fatty acid oxidation, although glucose funnels acetate into the acyl-carnitine pool as acetyl-carnitine (C2) and ketone bodies can produce acyl-carnitines (C4-OH). Some essential amino acids (leucine, isoleucine and valine) also provide C3 and C5 length acyl-carnitines [21]. Acyl-carnitines are detectable in plasma under physiological conditions and their levels vary with a number of physiological factors. Transmembrane export of acyl-carnitines is one mechanism for preventing the accumulation of acyl-CoA which is crucial for maintain many metabolic pathways.
Fig. 5.
Diagram of fatty acyl-carnitine flux for this study
Protein intake was inversely and strongly related to concentrations of the medium chain and some long chain fatty acyl-carnitines (clusters 1 to 3) and the shorter chain, even numbered acyl fatty acids (cluster 5). In contrast there was little relationship with fat intake or energy intake. Several essential amino acids contribute to the acyl-carnitine pool (Fig. 5). In contrast to the medium chain fatty acyl-carnitines, dietary protein intake was positively and significantly associated with the concentrations of C3 and C5:1 fatty acyl-carnitines. In an earlier paper [22] we found increasing dietary protein increased the concentration of most essential amino acids since there were more of these amino acids in the diet. One consequence of this higher intake of protein is that more acyl-carnitines were derived from their metabolism [23,24]. Thus the higher protein diet would seem the best explanation for the increasing concentration of several short-chain acyl-carnitines
Dietary fat intake was changed in our study to accommodate the changes in dietary protein (Fig. 5). Thus as percent dietary protein increased from 5% to15% to 25%, dietary fat decreased from 59%, to 49% and to 39% respectively. The higher fat intake in participants eating the low protein diet in the face of excess calorie intake provided an “overabundance” of fatty acids for metabolism. Thus, the low protein-high fat diet was significantly associated with increased levels of the medium and longer chain fatty acyl-carnitines in clusters 1, 2, 3. However, the correlation of dietary fat intake with changes in acyl-carnitines clustered in groups 1, 2, 3, and 5, although positive, was not statistically significant by the criterion used in this paper (P < 0.005). The strong inverse association of dietary protein intake with changes in the medium chain acyl-carnitines would appear to provide a more convincing relationship. The lower protein intake with fewer amino acids for catabolism may have allowed more fatty acids to be metabolized and thus be reflected in changes in the circulating levels of fatty acyl-carnitines. The resulting surfeit of substrate might be expected to reduce the removal rate of all fatty acyl-carnitines thus leading to more of them in the circulation. Our data are thus consistent with the speculation that limitations in flux through the tricarboxylic acid (TCA) cycle produces an “anaplerotic” state with increasing fatty acyl-carnitines as a consequence [25].
In this study, energy intake was increased by 40% over that required for weight maintenance [11]. The intake of protein was strongly related to the changes in resting energy expenditure and sleeping energy expenditure as well as fat free mass and free fatty acids across the 8-week overfeeding protocol (Table 4). Change in fat intake was related to two of these variables (REE and FFM), but not to the others. In contrast, energy intake had no relationship to any component of energy expenditure or FFM, FM or FFA. The effects of the medium and longer chain fatty acyl-carnitines were mainly on sleep energy expenditure, suggesting that they may be involved in this adaptation. In contrast, the short-chain fatty acyl carnitines had their effects mainly on resting energy expenditure which include a component related to “arousal”. This may suggest different roles for fatty acid substrates during the process of arousal compared with substrate use during sleep.
Fatty acyl-carnitine concentrations in plasma or serum are modified in many circumstances [25], including exercise [20,26], fasting [27,28], pregnancy [29,30], growth and development [31], diabetes mellitus [32], and obesity [33,34]. Three of these, pregnancy, obesity and overfeeding may be relevant to the current study since they all reflect positive energy balance of variable duration. In pregnant women with obesity those in the second trimester had higher C8:1. In the third trimester there were higher values for C2, C4-OH, C8:1, and C18: than in women who were not obese [29]. In our overfeeding study, C4-OH and C8:1 were significantly increased, but C2 was not. In women with obesity propionylcarnitine (C3), butyryl carnitine (C4), and hexanoylcarnitine (C6) were all elevated [32]. In our overfeeding study the cluster of fatty acyl-carnitines with C6, C8 and C10 were increased as protein intake increased. In adolescents with diabetes, several species of fatty acyl-carnitines were lower, including C2-carnitine, the last product of fatty acid oxidation, as well as C6-carnitine, C10-carnitine, and C10:1-carnitine when compared to adolescents with obesity or those who were of normal weight [32]. Again, these are the same cluster of fatty acyl-carnitines that were affected by overfeeding in our study. In addition, both C3-carnitine and C5-carnitine derived from oxidation of branched chain amino acids, were significantly lower in adolescents with diabetes compared with normal weight adolescents. In our overfeeding study, both C3-carnitine and C5:1-carnitine were significantly higher with higher intake of branched chain amino acids. In the study of adolescents, C4-carnitine, a short chain species that is derived from both the latter stages of fatty acid oxidation and from valine, was lower in patients with diabetes compared to those who were of normal weight [32]. In contrast, our study found that C4-OH-carnitine was lower in those with more dietary branched chain amino acids. In a meta-analysis of 37 studies, BMI was significantly associated with four acyl-carnitines including propionylcarnitine, butyryl carnitine (C4), isovalerylcarnitine, (C5:1) and hexanoylcarnitine (C6) [33]. In large group of children including 353 nonobese and 450 Hispanic children with obesity, branched-chained amino acids (BCAAs) (leucine, isoleucine, and valine) and their catabolites, propionylcarnitine and butyrylcarnitine, were significantly elevated [31].
The response of fatty acyl-carnitines to experimental overfeeding of a high fat diet has been examined in one short term study [35]. Before overfeeding Ribel-Madsen et al. [35] found higher C2 and C4-OH levels in men with low birth weight than in men of normal birth weight when both were eating their normal diet. They also found higher levels of C6-DC, C10-OH/C8-DC, and total hydroxyl-/dicarboxyl-acylcarnitine levels, which may suggest an increased fatty acid omega-oxidation in the liver. In response to overfeeding the men with low birth weight and normal birth weight both decreased several fatty acyl-carnitines which may reflect an upregulation of fatty acid oxidation due to the dietary challenge. The levels of C10-OH/C8-DC and total hydroxyl-/dicarboxyl acyl-carnitine tended to be negatively associated with the serum insulin level, and the total hydroxyl-/dicarboxyl-acyl-carnitine level additionally tended to be negatively associated with the hepatic insulin resistance index [30]. Before and after this high fat diet, plasma short-chain fatty acyl-carnitine species were higher in the subjects with obesity. Following the 5 day high fat diet, skeletal muscle medium-chain acyl carnitines (C6, C8, C10:2, C10:1, C10, and C12:1) were increased in subjects with obesity, but decreased in lean subjects. This group of fatty acyl-carnitines are nearly identical to group or cluster 5 in our study. Plasma content of C10:1 was decreased in the lean subjects, but increased in the subjects with obesity. Lower skeletal muscle amino acid content and accumulation of plasma short-chain acyl-carnitines in subjects with obesity could reflect increased anaplerosis for TCA cycle intermediates, while accumulation of medium chain acyl-carnitines suggest limitations on β-oxidation of fatty acids.
Our study has both strengths and weaknesses. The small number of subjects is a clear limitation. Offsetting this weakness is the tight control of food intake, carefully prescribed study diets and activity during the time the subjects lived in the inpatient setting. Additional strengths are the high caliber methods used to measure the fatty acyl-carnitines and the components of body composition and metabolic assessments. Offsetting the small number of participants is also the inclusion of both men and women which increases the generalizability, even with limited numbers.
In conclusion we have made several important observations. The first is that during overfeeding there are changes in fatty acyl-carnitines in the circulation. Second, dietary intake of protein was the principal factor influencing the changes in fatty acyl-carnitines; neither energy intake nor fat intake had much influence on the metabolic responses to overfeeding. The third observation is that overfeeding was associated with differential changed in short chain versus medium chain fatty acids. In most analyses the medium chain and short chain fatty acids went in opposite directions. Finally, there were strong relationships between energy expenditure, fat free mass and plasma total fatty acids and changes in fatty acyl-carnitines.
Acknowledgements
The authors want to thank each of the volunteers who participated in this study. Without their help and cooperation it would not have been possible. The nursing, dietary and laboratory personnel were also essential for the initiation and completion of this study and we will be forever indebted to them.
The study was designed by GAB, SRS.
Data collection: LdJ, SRS, GAB.
Data analysis: LRM, JR, GAB.
Paper drafted by GAB.
Reviewed by LRM, LdJ, SRS, ES, JR.
Grant Support
Supported in part by a grant from the US Department of Agriculture: 2010-34323-21052.
Grant support: supported in part by a grant from the US Department of Agriculture: 2010-34323-21052.
Registered at Clintrials.gov NCT# NCT00565149.
Footnotes
Conflict of Interest Statement
The authors declare no conflicts relative to this manuscript.
References
- [1].Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].The Global BMI Mortality CollaborationDi Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process: a position paper of world obesity. Obes Rev 2017;18(7):715–23. 10.1111/obr.12551 [DOI] [PubMed] [Google Scholar]
- [4].Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes 2006;30(11):1585–94. [DOI] [PubMed] [Google Scholar]
- [5].Hruby A, Manson JE, Qi L, Malik VS, Rimm EB, Sun Q, et al. Determinants and consequences of obesity. Am J Public Health 2016;106(9):1656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev 2017;38(4):267–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sims EA, Danforth E, Horton ES, Bray GA, Glennon JA, Salans B. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res 1973;29:457–87. [DOI] [PubMed] [Google Scholar]
- [8].Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332(10):621–8. [DOI] [PubMed] [Google Scholar]
- [9].Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, et al. What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications? Int J Obes (Lond) 2017;41(6):853–65. [DOI] [PubMed] [Google Scholar]
- [10].Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord 1999; 23:1105–17. [DOI] [PubMed] [Google Scholar]
- [11].Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 2012;307(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bray GA, Redman LM, de Jonge L, Covington J, Rood J, Brock C, et al. Effect of protein overfeeding on energy expenditure measured in a metabolic chamber. Am J Clin Nutr 2015;101(3):496–505. [DOI] [PubMed] [Google Scholar]
- [13].Bray GA, Redman LM, de Jonge L, Rood J, Smith SR. Effect of three levels of dietary protein on metabolic phenotype of healthy individuals with 8 weeks of overfeeding. J Clin Endocrinol Metab 2016;101(7):2836–43. [DOI] [PubMed] [Google Scholar]
- [14].Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 1999;283:212–4. [DOI] [PubMed] [Google Scholar]
- [15].Johannsen DL, Tchoukalova Y, Tam CS, Covington JD, Xie W, Schwarz JM, et al. Effect of 8 weeks of overfeeding on ectopic fat deposition and insulin sensitivity: testing the “adipose tissue expandability” hypothesis. Diabetes Care 2014;37(10):2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bouchard C, Tremblay A, Després JP, Nadeau A, Lupien PJ, Thériault G, et al. The response to long-term overfeeding in identical twins. N Engl J Med 1990;322(21):1477–82. [DOI] [PubMed] [Google Scholar]
- [17].Smith SR, Lovejoy JC, Greenway F, Ryan D, de Jonge L, de la Bretonne J, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001;50(4):425–35. [DOI] [PubMed] [Google Scholar]
- [18].Redman LM, Huffman KM, Landerman LR, Pieper CF, Bain JR, Muehlbauer MJ, et al. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J Clin Endocrinol Metab 2011;96(2):E312–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 2004;10(3):268–74. [DOI] [PubMed] [Google Scholar]
- [20].Xu G, Hansen JS, Zhao XJ, Chen S, Hoene M, Wang XL, et al. Liver and muscle contribute differently to the plasma acyl-carnitine pool during fasting and exercise in humans. J Clin Endocrinol Metab 2016;101(12):5044–52. [DOI] [PubMed] [Google Scholar]
- [21].Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 2013;62(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bray GA, Redman LM, de Jonge L, Rood J, Sutton EF, Smith SR. Plasma amino acids during 8 weeks of overfeeding: relation to diet, body composition and fat cell size in the PROOF studyo. Obesity (Silver Spring) 2018;26(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Newgard CB. Interplay between Li; pids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Park S, Sadanala KC, Kim E-K. A metabolomic approach to understanding the metabolic link between obesity and diabetes. A mini-review. Mol Cell 2015;38(7):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muhsen Ali A, Burleigh M, Daskalaki E, Zhang T, Easton C, Watson DG. Metabolomic profiling of submaximal exercise at a standardised relative intensity in healthy adults. Metabolites 2016;6(1) (pii: E9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 2010;5:e15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Soeters MR, Sauerwein HP, Duran M, Wanders RJ, Ackermans MT, Fliers E, et al. Muscle acylcarnitines during short-term fasting in lean healthy men. Clin Sci (Lond) 2009;116(7):585–92. [DOI] [PubMed] [Google Scholar]
- [29].Ryckman KK, Donovan BM, Fleener DK, Bedell B, Borowski KS. Pregnancy-related changes of amino acid and acylcarnitine concentrations: the impact of obesity. Am J Perinatol Rep 2016;6:e329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sandler V, Reisetter AC, Bain JR, Muehlbauer MJ, Nodzenski M, Stevens RD, et al. HAPO Study Cooperative Research Group. Associations of maternal BMI and insulin resistance with the maternal metabolome and newborn outcomes. Diabetologia 2017;60(3):518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Butte NF, Liu Y, Zakeri IF, Mohney RP, Mehta N, Voruganti VS, et al. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr 2015;102(2):256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mihalik SJ, Michaliszyn SF, de las Heras J, Bacha F, Lee S, Chace DH, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 2012;35(3):605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics 2014;10:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baker PR II, Boyle KE, Koves TR, Ilkayeva OR, Muoio DM, Houmard JA, et al. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity (Silver Spring) 2015;23(5):981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ribel-Madsen A, Ribel-Madsen R, Brøns C, Newgard CB, Vaag AA, Hellgren LI. Plasma acylcarnitine profiling indicates increased fatty acid oxidation relative to tricarboxylic acid cycle capacity in young, healthy low birth weight men. Physiol Rep 2016;4(19) (pii: e12977). [DOI] [PMC free article] [PubMed] [Google Scholar]