Abstract
Objectives
To describe the baseline characteristics and to evaluate the risk factors for in-hospital mortality in patients admitted to hospitals with coronavirus disease (COVID-19) in Kuwait.
Subjects and Methods
This retrospective cohort study analyzed data of patients admitted to two hospitals in Kuwait with COVID-19. The outcome was assessed by using multivariable analysis of factors affecting survival and mortality.
Results
In 962 patients, the case fatality ratio was 9.04%. The mean age of nonsurvivors was 63.5 ± 14.8 years, and most deaths occurred in males (80.5%). For the whole sample, the source of transmission was significantly related to mortality and the median duration of in-hospital stay was 15 days (interquartile range: 2–52 days). In patients with high oxygen requirements, the case fatality rate was 96.6%. Multivariable analysis identified age, hypertension, cardiovascular disease (CVD), and dyspnea on presentation as independent risk factors for COVID-19 mortality.
Conclusions
The mortality rate was higher in older patients with comorbidities such as hypertension and CVD. Early recognition of high-risk patients may help to improve care and reduce mortality.
Keywords: COVID-19, Severe acute respiratory syndrome coronavirus-2, Mortality, Survival
Highlights of the Study
Risk factors for coronavirus disease-related mortality among patients in Kuwait were evaluated.
Older age, male sex, and hypertension were significantly linked to mortality.
Cardiovascular disease and presentation with dyspnea were also risk factors.
Introduction
Coronavirus disease (COVID-19) is an infection caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which is a novel enveloped, single-stranded, RNA betacoronavirus. The virus is coated by spike S, envelope E, and membrane M proteins. The S protein on the virus envelope binds to the host cell via the angiotensin-converting enzyme II (ACE II) receptor [1]. ACE II is expressed in multiple tissues of the human body, including the lungs, small intestines, kidneys, heart, thyroid, and adipose tissue. This may explain the multiorgan involvement, and hence presentations observed in COVID-19 range from asymptomatic infections to severe life-threatening disease with multiorgan involvement and death [2].
In December 2019, the first case of COVID-19 was identified in the city of Wuhan, China. The virus then started to spread and infected about 85,000 patients in China. In the early 2020, the virus spread to many countries, and in March 2020, COVID-19 was declared a pandemic by the World Health Organization [3], affecting around 252.2 million people and resulting in 5.1 million deaths worldwide [4]. In Kuwait, as of November 11, 2021, 412,936 cases of COVID-19 had been reported, including 2,462 COVID-19 deaths (State of Kuwait, Ministry of Health Data) [5].
Multiple studies have evaluated the risk factors associated with COVID-19-related mortality [6, 7, 8]. A retrospective cohort study that was conducted in New York City in the USA showed an increased risk of mortality from COVID-19 in patients with one or more of the following factors: older age, male sex, tachypnea, hypoxia, impaired renal function, elevated troponin, and elevated D-dimer levels [9]. A similar retrospective cohort study was conducted in Kuwait on 1,096 patients admitted to a single center; the study concluded that a more severe infection was associated with age above 50 years, smoking, presence of asthma, a high quick Sequential Organ Failure Assessment score, and elevated levels of inflammatory markers [10].
The purpose of the present study was to identify the underexplored baseline clinical characteristics of patients on presentation that put them at high risk of COVID-19-related death [11, 12, 13, 14, 15, 16]. Multiple factors were considered in patients admitted to two hospitals with SARS-CoV-2 infections in Kuwait.
Subjects and Methods
Study Design and Participants
A total of 962 patients diagnosed with COVID-19, above the age of 18 years, were enrolled in this retrospective cohort study. The study was conducted between February 26, 2020 and September 8, 2020. The data were collected from the electronic medical records of two hospitals in Kuwait: Jaber Al-Ahmed Hospital and Al Adan General Hospital [17, 18, 19]. During the data entry stage, an electronic case report form was used. The diagnoses of the patients were based on positive results on reverse transcription-polymerase chain reaction assays of nasopharyngeal swabs. The patients received standard care according to the protocols of the Ministry of Health of Kuwait. The Standing Committee for the Coordination of Health and Medical Research at the Ministry of Health in Kuwait approved the protocol of this study and waived the requirement for informed consent (Institutional Review Board Number 2020/1422).
Definitions
The intended outcome was COVID-19-related death, based on ICD 10 code U07.1. The following variables were assessed: sociodemographic characteristics, body mass index, smoking status, source of infection, underlying comorbidities, clinical presentations, use of certain medications before admission, oxygen requirement, duration of intensive care unit (ICU), and in-hospital stay. Patients who were on immunosuppressive therapy were included in the immunosuppressed group. Patients with underlying obstructive or restrictive lung disease were grouped under chronic lung disease. The patients' oxygen requirement was classified into three groups: high, low, and none. The high requirement group included patients on extracorporeal membrane oxygenation, invasive ventilation, noninvasive ventilation, or high-flow oxygen; whereas the low requirement group included patients who required oxygen via a nasal canula or a nonrebreather mask.
Statistical Analysis
The data collected were analyzed using descriptive statistical methods (mean and standard deviation) and the independent samples t tests. The χ2 test or Fisher's exact test was used for comparisons of the categorical data, as appropriate. Cox regression and Kaplan-Meier method were performed to analyze mortality risk by sex; hazard ratio values of >1 represented an increased risk of death. The log-rank test was used to compare the survival curves. Logistic regression was used to estimate odd ratios and was employed in the multivariable model. For identifying predictors of mortality, the multivariable model was constructed using the stepwise backward elimination approach. Statistical analyses were performed using SPSS Version 26 (IBM Corp., Armonk, NY, USA) and R statistics version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Sociodemographic and Baseline Characteristics
Data from 962 patients who were admitted to the Jaber Al-Ahmed Hospital and Al Adan General Hospital in Kuwait were included in the analysis. Table 1 shows the sociodemographic characteristics of the study participants. The case fatality ratio, defined as the incidence proportion of death in patients, was 9.04%. The mean age of the nonsurvivors was 63.5 years and was significantly higher than that of the survivors. Of the fatal cases, 80.5% of the patients were male and 47.7% reported community transmission. The proportion of hospital-acquired COVID-19 was higher in this group (5.81% vs. 0.78%). The median duration of in-hospital stay for nonsurvivors was 23.0 days (interquartile range: 5.0–73.5 days). Coexisting hypertension, diabetes mellitus (DM), cardiovascular disease (CVD), chronic kidney disease, or immunosuppression were significant risk factors for mortality.
Table 1.
Baseline characteristics of the study participants
| All (N = 962) | Survivor (N = 875) | Nonsurvivor (N = 87) | p value | N | |
|---|---|---|---|---|---|
| Age, mean ± SD, years | 50.2 (15.9) | 48.9 (15.4) | 63.5 (14.8) | <0.001 | 962 |
| BMI, mean ± SD, kg/m2 | 29.0 (6.18) | 29.1 (6.28) | 28.2 (5.31) | 0.221 | 606 |
| Sex | |||||
| Female | 344 (35.9) | 327 (37.5) | 17 (19.5) | 0.001 | 959 |
| Male | 615 (64.1) | 545 (62.5) | 70 (80.5) | ||
| Smoking | |||||
| Current smoker | 38 (14.1) | 32 (13.8) | 6 (15.8) | 0.694 | 270 |
| Ex-smoker | 28 (10.4) | 23 (9.91) | 5 (13.2) | ||
| Never smoked | 204 (75.6) | 177 (76.3) | 27 (71.1) | ||
| Source of transmission | |||||
| Community | 346 (40.2) | 305 (39.4) | 41 (47.7) | <0.001 | 860 |
| Contact | 386 (44.9) | 349 (45.1) | 37 (43.0) | ||
| Healthcare worker | 22 (2.56) | 22 (2.84) | 0 (0.00) | ||
| Hospital acquired | 11 (1.28) | 6 (0.78) | 5 (5.81) | ||
| Imported | 95 (11.0) | 92 (11.9) | 3 (3.49) | ||
| Admission to discharge, days [IQR] | 15.0 [2.00; 52.0] | 14.0 [2.00; 47.0] | 23.0 [5.00; 73.5] | <0.001 | 950 |
| Hypertension | 324 (33.7) | 263 (30.1) | 61 (70.1) | <0.001 | 962 |
| Diabetes mellitus | 335 (34.8) | 288 (32.9) | 47 (54.0) | <0.001 | 962 |
| Cardiovascular disease | 79 (8.21) | 60 (6.86) | 19 (21.8) | <0.001 | 962 |
| Chronic lung disease | 87 (9.04) | 82 (9.37) | 5 (5.75) | 0.353 | 962 |
| Chronic kidney disease | 43 (4.47) | 32 (3.66) | 11 (12.6) | 0.001 | 962 |
| Immunosuppression | 16 (1.66) | 11 (1.26) | 5 (5.75) | 0.011 | 962 |
The values are N (%) unless specified otherwise. BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Clinical Presentations
The association between mortality due to COVID-19 and clinical presentation is shown in Table 2. Among patients who died with COVID-19, 2.3% were asymptomatic (p < 0.001). History of fever was the most commonly reported symptom among nonsurvivors (71.3%) and was significantly associated with mortality in patients with COVID-19. Dyspnea at the time of presentation was also associated with increased mortality (p < 0.001).
Table 2.
Associations between COVID-19-related mortality and multiple clinical presentations
| All (N = 962) | Survivor (N = 875) | Nonsurvivor (N = 87) | p value | N | |
|---|---|---|---|---|---|
| Asymptomatic | 155 (16.1) | 153 (17.5) | 2 (2.30) | <0.001 | 962 |
| Headache | 100 (10.4) | 92 (10.5) | 8 (9.20) | 0.841 | 962 |
| Sore throat | 93 (9.67) | 86 (9.83) | 7 (8.05) | 0.729 | 962 |
| Fever | 547 (56.9) | 485 (55.4) | 62 (71.3) | 0.006 | 962 |
| Dry cough | 459 (47.7) | 416 (47.5) | 43 (49.4) | 0.824 | 962 |
| Productive cough | 68 (7.07) | 64 (7.31) | 4 (4.60) | 0.469 | 962 |
| SOB | 309 (32.1) | 250 (28.6) | 59 (67.8) | <0.001 | 962 |
| Fatigue or myalgia | 216 (22.5) | 198 (22.6) | 18 (20.7) | 0.781 | 962 |
| Diarrhea | 113 (11.7) | 104 (11.9) | 9 (10.3) | 0.802 | 962 |
| Nausea | 60 (6.24) | 56 (6.40) | 4 (4.60) | 0.667 | 962 |
| Vomiting | 59 (6.13) | 57 (6.51) | 2 (2.30) | 0.184 | 962 |
| Change of taste or smell | 34 (3.53) | 30 (3.43) | 4 (4.60) | 0.540 | 962 |
The values are N (%) unless specified otherwise. COVID-19, coronavirus disease; SOB, shortness of breath.
Treatment Characteristics
Table 3 summarizes the associations between multiple treatment modalities and mortality. Sixteen (25.4%) patients in the mortality group reported current use of ACE inhibitors compared to 71 (9.31%) in the survival group (p < 0.001). Among patients admitted with COVID-19 who were using statins, 182 (23.2%) survived and 37 (52.1%) died (p < 0.001). However, current use of angiotensin II receptor blockers was not associated with mortality (p = 0.293). Patients with higher oxygen requirements had a higher mortality (p < 0.001): the mortality rate among patients in the high oxygen requirement group was 96.6%, compared with 2.3% among those in the low oxygen requirement group, and 1.15% among those who did not need oxygen. The mortality rate was also significantly higher in patients with longer ICU stays (p = 0.011).
Table 3.
Association of medications and COVID-19 treatment modalities with mortality due to COVID-19
| All (N = 887) | Survivor (N = 800) | Nonsurvivor (N = 87) | p value | N | |
|---|---|---|---|---|---|
| Receiving ACE inhibitors | 87 (10.5) | 71 (9.31) | 16 (25.4) | <0.001 | 826 |
| Receiving ARBs | 110 (13.3) | 99 (12.9) | 11 (18.6) | 0.293 | 826 |
| Receiving statin | 219 (25.6) | 182 (23.2) | 37 (52.1) | <0.001 | 855 |
| Oxygen requirements | |||||
| High oxygen requirement | 139 (15.7) | 55 (6.88) | 84 (96.6) | <0.001 | 887 |
| Low oxygen requirement | 249 (28.1) | 247 (30.9) | 2 (2.3) | ||
| None | 499 (56.3) | 498 (62.3) | 1 (1.15) | ||
| ICU length of stay, mean ± SD, days | 18.0 (17.4) | 14.1 (16.1) | 21.2 (17.9) | 0.011 | 151 |
The values are N (%) unless specified otherwise. ACE, angiotensin-converting enzyme; ARBs, angiotensin II receptor blockers; COVID-19, coronavirus disease; ICU, intensive care unit; SD, standard deviation.
Sex and Mortality
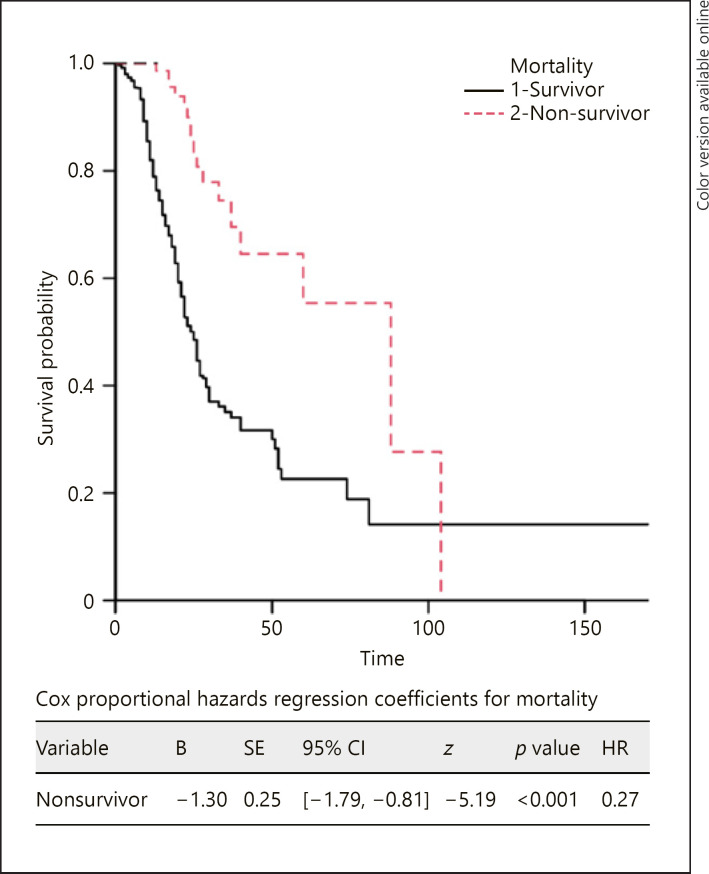
The risk of death was lower in females in the Cox proportional hazards regression (hazard ratio: 0.27, p < 0.001) and the Kaplan-Meier survival analysis (Fig. 1).
Fig. 1.
Kaplan-Meier survival probability plot for mortality. Female sex was used to represent a hazard event and male sex was used to indicate survival. x-axis: days since admission.
Multivariable Logistic Regression Model
Risk factors for death were assessed using a multivariable logistic regression model that included age, hypertension, DM, CVD, dyspnea at time of presentation, current ACE inhibitor use, and current statin use (Table 4). The data showed that age (adjusted odds ratio [aOR]: 1.05, 95% confidence interval [CI]: 1.03–1.08, p < 0.001), hypertension (aOR: 2.25, 95% CI: 1.10–4.67, p = 0.028), CVD (aOR: 2.45, 95% CI: 1.10–5.43, p = 0.027), and dyspnea (aOR: 4.07, 95% CI: 2.27–7.52, p < 0.001) were significantly associated with mortality. However, DM (p = 0.697), ACE inhibitor use (p = 0.121), and statin use (p = 0.142) had no significant effect on mortality.
Table 4.
Multivariable logistic regression model between certain variables and in-hospital mortality due to COVID-19
| Survivor | Nonsurvivor | Crude OR (95% CI, p) | Adjusted OR (95% CI, p) | ||
|---|---|---|---|---|---|
| Age | Mean (SD) | 48.9 (15.4) | 63.5 (14.8) | 1.06 (1.05–1.08, p < 0.001) | 1.05 (1.03–1.08, p < 0.001) |
| Hypertension | Yes | 263 (81.2) | 61 (18.8) | 5.46 (3.41–8.97, p < 0.001) | 2.25 (1.10–4.67, p = 0.028) |
| DM | Yes | 288 (86.0) | 47 (14.0) | 2.39 (1.54–3.75, p < 0.001) | 1.14 (0.59–2.23, p = 0.697) |
| CVD | Yes | 60 (75.9) | 19 (24.1) | 3.80 (2.10–6.63, p < 0.001) | 2.45 (1.10–5.43, p = 0.027) |
| SOB | Yes | 250 (80.9) | 59 (19.1) | 5.27 (3.31–8.56, p < 0.001) | 4.07 (2.27–7.52, p < 0.001) |
| ACE inhibitors | Yes | 71 (81.6) | 16 (18.4) | 3.32 (1.74–6.05, p < 0.001) | 1.84 (0.84–3.93, p = 0.121) |
| Statin | Yes | 182 (83.1) | 37 (16.9) | 3.60 (2.20–5.92, p < 0.001) | 0.56 (0.25–1.20, p = 0.142) |
The values are N (%) unless specified otherwise. ACE, angiotensin-converting enzyme; CI, confidence interval; COVID-19, coronavirus disease; CVD, cardiovascular disease; DM, diabetes mellitus; OR, odds ratio; SD, standard deviation; SOB, shortness of breath.
Discussion
In this study, multiple risk factors for mortality were considered in 962 patients with COVID-19. The data showed that patients who died due to COVID-19 were likely to be older. This finding is consistent with the results of previous studies that showed a higher mortality rate in older patients with COVID-19, particularly in those aged ≥60 years [20, 21]. There was also a significant relationship between sex and mortality due to COVID-19. A recent study showed that males had a higher risk of severe acute respiratory distress syndrome than females, with the mortality rates ranging from 59% to 75%. This may be attributed to the higher expression of ACE II in males, which is the main receptor for the binding of SARS-CoV-2 to host cells [22].
We also investigated the relationship between existing comorbidities and mortality due to COVID-19. A higher mortality rate was observed in patients with hypertension; this finding is similar to the results of a recent prospective cohort study that was conducted in Spain, in which hypertension was the most reported previous comorbidity in the nonsurvivors' group (61.10%, p < 0.0001) [23]. Furthermore, in our study, a significant relationship was also observed between DM and mortality. A study conducted in Mexico showed a significant relationship between mortality and diabetes in patients hospitalized with COVID-19 (aOR: 1.28, 95% CI: 1.23–1.34, p < 0.0001) [24]. Additionally, comorbid CVD, chronic kidney disease, and immunosuppression were also associated with an increased mortality in COVID-19; however, chronic lung disease was not significantly associated with an increased risk of death. This finding was contrary to a previous study that showed patients who were hospitalized with COVID-19 and had comorbid chronic obstructive pulmonary disease had a higher mortality (aOR: 1.26, 95% CI: 1.15–1.38, p < 0.0001) [24].
In the present study, in-hospital oxygen therapy was also associated with higher mortality. This result was in accordance with a recent study conducted in China showing that nonsurvivors were more likely to have received oxygen therapy (high-flow nasal cannula [89%, p < 0.001], noninvasive mechanical ventilation [57%, p < 0.001], invasive mechanical ventilation [35%, p < 0.001], extracorporeal membrane oxygenation [1%, p = 0.005]) [25]. Mortality was also significantly associated with the duration of ICU stay. This finding was also observed in a cohort study conducted in Brazil, which showed a mortality rate of 18.9% in patients admitted to the ICU (p < 0.001), with a median interval between ICU admission and death/end of the survey of 12.5 days (p < 0.001) [26]. An explanation for this finding may be the late presentation of patients with complicated COVID-19 to the hospital, which necessitated earlier ICU admission.
This study has several limitations. The data were obtained from electronic medical records, which are prone to underdocumentation of history and presentation. This was especially evident for smoking; data on smoking history were missing for more than 50% of patients. Another limitation is that despite a significant association between DM and COVID-19-related mortality in the univariable analysis, the sample size was too small to confirm an effect in the multivariable analysis. Our study was performed retrospectively because a prospective study design would require a strict timeframe and follow-up, which was not possible for several reasons, including the burden of the outbreak on the health system.
Conclusions
This study highlighted certain baseline characteristics as predictors of COVID-19 mortality in Kuwait. Older age, male sex, hypertension, CVD, and presentation with dyspnea were significantly associated with increased risk of death in hospitalized COVID-19 patients. Healthcare authorities should identify these factors at the time of diagnosis to improve patient care and outcomes.
Statement of Ethics
The study protocol was reviewed and approved by the Standing Committee for the Coordination of Health and Medical Research of the Ministry of Health, Kuwait (Institutional Review Board Number: 2020/1422). The requirement of written informed consent was covered by the same committee.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
M.A. designed the study. M.A.S., N.A., K.S., F.A., and M.A. wrote the manuscript. A.A. and J.P. analyzed the data. The remaining authors collected the data and took responsibility for the integrity and accuracy of the data. All authors have read and approved the manuscript.
Data Availability Statement
The data that support the results of the study are available upon request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgment
The authors thank Dr Danah Alothman for her support in manuscript review.
References
- 1.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020 May;17((5)):259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020 Oct 23;371:m3862. doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Coronavirus disease (COVID-19) Situation Report: 52 [cited 2020 Dec 30] Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_4.
- 4.Johns Hopkins University and Medicine COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU) [cited 2021 Nov 11] Available from: https://coronavirus.jhu.edu/map.html.
- 5.State of Kuwait, Ministry of Health COVID-19 updates [cited 2021 Nov 11] Available from: https://corona.e.gov.kw.
- 6.Albitar O, Ballouze R, Ooi JP, Sheikh Ghadzi SM. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract. 2020 Aug 1;166:108293. doi: 10.1016/j.diabres.2020.108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalised COVID−19 patients: a systematic review and meta-analysis. J Med Virol. 2020 Oct;92((10)):1875–83. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escobedo-de la Peña J, Rascón-Pacheco RA, de Jesús Ascencio-Montiel I, González-Figueroa E, Fernández-Gárate JE, Medina-Gómez OS, et al. Hypertension, diabetes and obesity, major risk factors for death in patients with COVID-19 in Mexico. Arch Med Res. 2021 May 1;52((4)):443–9. doi: 10.1016/j.arcmed.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2021;36:17–26. doi: 10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almazeedi S, Al-Youha S, Jamal MH, Al-Haddad M, Al-Muhaini A, Al-Ghimlas F, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi: 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alshukry A, Ali H, Ali Y, Al-Taweel T, Abu-Farha M, AbuBaker J, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) patients in Kuwait. PLoS One. 2020 Nov 20;15((11)):e0242768. doi: 10.1371/journal.pone.0242768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamal MH, Doi SA, AlYouha S, Almazeedi S, Al-Haddad M, Al-Muhaini A, et al. A biomarker based severity progression indicator for COVID-19: the Kuwait prognosis indicator score. Biomarkers. 2020 Nov 16;25((8)):641–8. doi: 10.1080/1354750X.2020.1841296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayed M, Borahmah AA, Yazdani A, Sultan A, Mossad A, Rawdhan H. Assessment of clinical characteristics and mortality-associated factors in COVID-19 critical cases in Kuwait. Med Princ Pract. 2021;30((2)):185–92. doi: 10.1159/000513047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alshukry A, Abbas MB, Ali Y, Alahmad B, Al-Shammari AA, Alhamar G, et al. Clinical characteristics and outcomes of COVID-19 patients with diabetes mellitus in Kuwait. Heliyon. 2021 Apr 1;7((4)):e06706. doi: 10.1016/j.heliyon.2021.e06706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sabah S, Al-Haddad M, Al-Youha S, Jamal M, Almazeedi S. COVID-19: impact of obesity and diabetes on disease severity. Clin Obes. 2020 Dec;10((6)):e12414. doi: 10.1111/cob.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ElAbd R, AlTarrah D, AlYouha S, Bastaki H, Almazeedi S, Al-Haddad M, et al. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) are protective against ICU admission and mortality for patients with COVID-19 disease. Front Med. 2021 Mar 4;8:600385. doi: 10.3389/fmed.2021.600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Jarallah M, Rajan R, Saber AA, Pan J, Al-Sultan AT, Abdelnaby H, et al. In-hospital mortality in SARS-CoV-2 stratified by hemoglobin levels: a retrospective study. EJHaem. 2021 doi: 10.1002/jha2.195. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Jarallah M, Rajan R, Saber AA, Pan J, Al-Sultan AT, Abdelnaby H, et al. In-hospital Mortality in SARS-CoV-2 stratified by serum 25-hydroxy-vitamin D levels: a retrospective study. J Med Virol. 2021 Oct;93((10)):5880–5. doi: 10.1002/jmv.27133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alroomi M, Rajan R, Omar AA, Alsaber A, Pan J, Fatemi M, et al. Ferritin level: a predictor of severity and mortality in hospitalized COVID-19 patients. Immun Inflamm Dis. 2021 Dec;9((4)):1648–55. doi: 10.1002/iid3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395((10229)):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) − United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020 Mar 27;69((12)):343–6. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020 Dec 1;116((14)):2197–206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. IL-6–based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020 Oct;146((4)):799–807. doi: 10.1016/j.jaci.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020 Dec;52:93–8.e2. doi: 10.1016/j.annepidem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Yu J, He W, Chen L, Yuan G, Dong F, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020 Aug;34((8)):2173–83. doi: 10.1038/s41375-020-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sousa GJB, Garces TS, Cestari VRF, Florêncio RS, Moreira TMM, Pereira MLD. Mortality and survival of COVID-19. Epidemiol Infect. 2020 Jun 25;148:e123. doi: 10.1017/S0950268820001405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the results of the study are available upon request from the corresponding author. The data are not publicly available due to ethical restrictions.