Abstract
Background
Recent surveillance studies following nationwide mass vaccination are investigating rare complications such as myocarditis, pericarditis, and thromboembolic events related to mRNA-based Covid-19 vaccines.
Summary
In the current report, we present an overview of the incidence, clinical presentation and management of post-mRNA vaccine myocarditis, and pericarditis in view of the currently available data. Our main focus is directed toward myocarditis.
Key Messages
Myocarditis following mRNA-based Covid-19 vaccines is rare, more frequently affects younger men <30 years and is usually of mild severity with spontaneous recovery. The overall benefit of mRNA vaccines in terms of protecting from severe Covid-19 infection and associated cardiovascular complications outweighs the risk of postvaccination myocarditis. Currently, there are no dedicated guidelines for patients with postvaccination myocarditis or pericarditis in terms of the frequency of follow-up including clinical assessment, repeated echocardiography, and cardiac resonance imaging. However, follow-up studies in terms of long-term consequences are underway.
Keywords: Covid-19, mRNA vaccine, Myocarditis, Pericarditis
Introduction
Covid-19, caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been a global health crisis for almost 2 years, with approximately 266 million confirmed cases and more than 5.2 million deaths worldwide as of December 6, 2021 [1]. Covid-19 has a wide clinical spectrum, ranging from asymptomatic cases to development of bilateral pneumonia, respiratory failure, severe systemic inflammation, hypercoagulability, pulmonary embolism, stroke, and multi-organ injury [2, 3]. The cardiovascular manifestations of Covid-19 include myocardial infarction, right and left heart failure, myocarditis, pericarditis or myopericarditis, thromboembolic events, and arrhythmias [2, 3]. Covid-19-induced acute cardiac injury including myocarditis is associated with higher mortality. Although systematic lockdowns have had some effect on controlling the virus spread, the development of Covid-19 vaccines and their deployment for mass vaccination worldwide has been the most effective strategy for controlling viral transmission and reducing the risk of severe Covid-19 infection, hospitalization, and death. Although this has been successful in developed countries, with the current implementation of a third vaccination dose to further lower the rates of severe Covid-19 infection [4], access to Covid-19 vaccines in some Asian and African countries remains inconsistent. While the goals of the Expanded Program on Immunization are not being achieved [5], understandable ethical and moral dilemmas have emerged that relate to access to equitable healthcare in times of a pandemic.
The implementation of Covid-19 vaccine programs has not been without controversy. The global crisis inevitably led to an unprecedented rate of vaccine development and administration. During this process, a number of adverse events were reported in the scientific literature which led potential recipients of the vaccine to question the published safety data.
Although preapproval (phase 3) clinical trials documented a satisfactory safety profile and effectiveness of Covid-19 vaccines, rare adverse events such as myocarditis and myopericarditis were not reported. Preapproval studies often involve a relatively large sample which may be sufficient for ensuring safety and effectiveness, but insufficient for detecting rare or very rare adverse events. However, recent surveillance studies in a nationwide mass vaccination setting are now investigating rare complications such as myocarditis, pericarditis, and thromboembolic events related to mRNA-based Covid-19 vaccines: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). In the current report, we focus on the incidence, clinical presentation, and management of post-mRNA vaccination myocarditis in view of the currently available data. The report is based upon a systemic search of the bibliographic database PubMed for publications on myocarditis and pericarditis following mRNA-based Covid-19 vaccines, and the information reported on websites of the World Health Organization, Public Health England, and Norwegian Institute of Public Health.
Incidence, Clinical Presentation, and Management of Myocarditis following mRNA Covid-19 Vaccines
In a recent study, Abbas et al. [6] investigated the immediate side effects of Covid-19 vaccine (Sinopharm − Beijing, China) in 205 participants, mean age 33 ± 8 years, 42.9% males. Among these, 19.5% (n = 40) had tested positive and 80.5% (n = 165) negative for Covid-19 infection, and 29.3% (n = 60) experienced symptoms whereas 70.7% (n = 145) did not. Most participants experienced fatigue (45.4%), headache (39.5%), and fever (33.7%), followed by soreness at site in 27.3%, gastrointestinal symptoms in 26.8%, chills/rigors in 20.5%, and flu-like symptoms in 13.7%. The manifestations of side effects had a linear association with the presence of comorbidities. However, smaller studies often detect the most common side effects with mild severity, and may not detect rare and more severe adverse events. By contrast, larger studies often exclude mild adverse events such as fever, malaise, and local injection site reactions in order to detect a wide range of clinically meaningful potential adverse events [7].
Myocarditis and pericarditis following Covid-19 mRNA vaccines are rare. However, myocarditis, in particular, has received much attention in western media and raised a great deal of public concern. The European Medical Association recently concluded that pericarditis and myocarditis were rare complications following coronavirus vaccination, and hence both conditions were included in the Summary of Product Characteristics of BNT162b2 and mRNA-1273 vaccines against Covid-19. Similarly, the World Health Organization Global Advisory Committee on Vaccine Safety (GACVS) also issued its first statement in May 2021 on a causal relationship between myocarditis and Covid-19 mRNA vaccines [8].
The true incidence of mRNA-vaccine-related myocarditis is difficult to ascertain owing to heterogenous reporting in the published literature, and given the fact that most data on the prevalence of myocarditis are based upon public health databases and not from studies systematically assessing myocarditis after Covid-19 vaccination (Table 1). Hence, missed diagnoses of post-Covid-19 mRNA vaccine myocarditis would typically underestimate the true incidence. However, a study from the USA showed that between December 14, 2020, and June 26, 2021, a total of 11,845,128 doses of mRNA vaccines (57% BNT162b2 and 43% mRNA-1273 vaccines) were administered to 6.2 million individuals. There were in total 87 cases of myocarditis and pericarditis, thereof 34 cases in the age category 12–39 years and 53% between 12 and 24 years, and 85% were male [9]. Nearly all were recovered at record review. In another study, also from the USA, among 2,000,287 individuals receiving at least one Covid-19 vaccination, 20 individuals developed myocarditis (1 per 100,000) and 37 developed pericarditis (1.8 per 100,000) [10]. Myocarditis occurred rapidly in younger patients and most frequently after the second dose, while pericarditis occurred in older patients with delay, following their either first or second dose [10].
Table 1.
Incidence of myocarditis and pericarditis following Covid-19 mRNA vaccines
| Country | Period of observation | Administered doses | Demographics | Cases of myocarditis and pericarditis | Groups at risk | ||
|---|---|---|---|---|---|---|---|
| Klein et al. [9] | US | 14 Dec 2020 to 26 June 2021 | 11,845,128 doses in 6.2 million individuals, 57% BNT162b2, 43% mRNA-1273, 6,175,813 first doses and 5,669,315 second doses | Mean age 49, 54% female | 87 cases of myocarditis and pericarditis (131.7 events/million person-years), adjusted rate ratio 1.18 (95% CI, 0.79–1.79) | 34 cases in the age category 12–39 years, 85% male, 29 cases within 7 days of vaccine (321 events/million person-years), adjusted RR 9.83 (95% CI, 3.35–35.77) | |
|
| |||||||
| Diaz et al. [10] | US | Feb to May 2021 | 2,000,287 individuals, 52.6% BNT162b2, 44.1% mRNA-1273 | Median age 57, 59% female | 20 cases of myocarditis (1.0 per 100,000 persons [95% CI, 0.61–1.54]), 37 cases of pericarditis (1.8 per 100,000 [95% CI, 1.30–2.55]) | Myocarditis 75% men, median age 36 years, pericarditis 73% men, median age 59 years | |
|
| |||||||
| Barda et al. [7] | Israel | 20 Dec 2020 to 24 May 2021 | 938,812 doses of BNT162b2 | Median age 38, 48% female | 21 cases of myocarditis, risk ratio 3.24 (95% CI, 1.55–12.44), risk difference 2.7 events per 100,000 (95% CI, 1.0–4.6) | Most cases were males and the median age was 25 years | |
|
| |||||||
| Mevorach et al. [11] | Israel | 20 Dec 2020 to 31 May 2021 | 9,289,765 doses of BNT162b2,5,442,696 individuals received a first vaccine dose and 5,125,635 received two doses | N/A | 136 cases of definite or probable myocarditis, standardized incidence ratio 5.34 (95% CI, 4.48–6.40), rate ratio 2.35 (95% CI, 1.10–5.02) | Mostly males in young age groups, rate ratio of 8.96 (95% CI, 4.50–17.83) for those between 16 and 19 years, 6.13 (95% CI, 3.16–11.88) for those 20–24 | |
|
| |||||||
| Witberg et al. [12] | Israel | 20 Dec 2020 to 24 May 2021 | 2,558,421 individuals with at least one dose of BNT162b2 | Median age 44, 51% female | 54 cases of myocarditis (41 mild, 12 intermediate, and 1 fulminant), estimated incidence of 2.13 per 100,000 persons (95% CI, 1.56–2.70) | Incidence particularly high among male patients between 16–29 years (10.69 cases per 100,000 persons; 95% CI, 6.93–14.46) | |
|
| |||||||
| Data from PHE [13] | UK | Until 28 July 2021 | BNT162b2: 20.46 million first and 13.8 million second doses, ChAdOx1 nCov-19: 24.8 million first and 23.6 million second doses, mRNA-1273:1.3 million first doses | N/A | BNT162b2:4.3 myocarditis cases per million doses and 3.8 pericarditis cases per million doses, ChAdOx1 nCov-19:1.7 myocarditis cases per million doses and 3.0 pericarditis cases per million doses, mRNA-1273:14.7 myocarditis cases per million doses and 13.0 pericarditis cases per million doses | N/A | |
|
| |||||||
| Data from NIPH [14] | Norway | 2 Dec 2020 to 6 Oct 2021 | 7.8 million doses, nearly 4.2 million first doses, thereof 3,490,657 BNT162b2 and 540,248 mRNA-1273, 3.7 million second doses | 51% female | 173 pericarditis cases (59 women and 114 men, 131 with BNT162b2, 36 with mRNA-1273, 3 with ChAdOx1 nCov-19), 95 myocarditis cases (23 women and 72 men, 52 with BNT162b2, 40 with mRNA-1273) | 53.7% of myocarditis cases in individuals <30 years, The pericarditis cases were more evenly divided across the age categories | |
PHE, Public Health England; NIPH, Norwegian Institute of Public Health; BNT 162b2, Comirnaty − Covid-19 vaccine developed by BioNTech; mRNA-1273, Spikevax − Covid-19 vaccine developed by Moderna; ChAdOx1 nCov-19, Vaxzevria − Covid-19 vaccine developed by AstraZeneca.
An initial study from Israel showed an excess risk of myocarditis with 1–5 events per 100,000 persons in people over 16 receiving the BNT162b2 vaccine. Most cases were males, and the median age was 25 years [7]. During a nationwide vaccination campaign in Israel involving more than 5 million people, a total of 136 cases of definite or probable myocarditis were identified following the receipt of two doses of the BNT162b2 mRNA vaccine [11]. The disease course was mild in 95% (n = 129) of the cases and fulminant/fatal in one case. The authors reported a standardized incidence ratio of 5.34 (95% CI, 4.48–6.40), highest after the second dose in males aged 16–19 years (13.60; 95% CI, 9.30–19.20). The definite or probable cases of myocarditis in the overall Israeli population were estimated at a rate of approximately 1 per 26,000 males and 1 per 218,000 females after the second vaccine dose. In the study of Witberg et al. [12], also from Israel, involving more than 2.5 million patients ≥16 years who had received at least one dose of BNT162b2 mRNA vaccine, 54 cases of myocarditis (76% of mild and 22% of intermediate severity, and 1 case with cardiogenic shock) were detected, with an estimated incidence of 2.13 per 100,000 persons (95% CI 1.56–2.70). The incidence was particularly high among male patients between 16 and 29 years (10.69 cases per 100,000 persons; 95% CI, 6.93–14.46).
According to a report by Public Health England, the overall rates of myocarditis and pericarditis after both first and second doses of the BNT162b2 were 4.3 and 3.8 cases per million doses, respectively [13]. For mRNA-1273, the rates of myocarditis were 14.7 cases per million doses and pericarditis 13.0 cases per million doses. Finally, in Norway, by July 9, 2021, over 3 million people had received at least one dose, and 1.6 million people their second dose of Covid-19 vaccines. Overall, 61 cases of acute pericarditis were reported (48 cases with BNT162b2, 10 with mRNA-1273 and 3 with Vaxzevria [AstraZeneca]) and 14 cases of acute myocarditis (10 cases with BNT162b2 and 4 with mRNA-1273) [14]. A new update on October 6, 2021, showed that after the administration of more than 7.8 million doses of Covid-19 vaccines (nearly 4.2 million first doses of 3,490,657 BNT162b2 and 540,248 mRNA-1273, and 3.7 million second doses) between December 2, 2020, and October 6, 2021, a total of 173 pericarditis (59 women and 114 men, 131 with BNT162b2, 36 with mRNA-1273, 3 with Vaxzevria, 2 following vaccination with both BNT162b2 and mRNA-1273 and 1 following both Vaxzevria and BNT162b2) and 95 myocarditis cases (23 women and 72 men, 52 with BNT162b2, 40 with mRNA-1273 and 3 following vaccination with both BNT162b2 and mRNA-1273) were reported by the Norwegian Medicines Agency [15]. Of the 95 myocarditis cases, 51 occurred in individuals <30 years, whereas pericarditis cases were more evenly divided across the age categories.
Discussion
Based on the current literature, postvaccination myocarditis appears to affect younger men <30 years, and more often following the second dose of the Covid-19 mRNA vaccines, typically within 3–5 days of vaccination [16, 17, 18]. Although the exact mechanism of myocarditis following Covid-19 mRNA vaccines is yet to be determined, it may be related to the active component of the vaccine, the nucleoside-modified mRNA that codes for the spike glycoprotein of SARS-CoV-2, or to the immune response that follows vaccination and/or dysregulated cytokine expression [11, 19]. Furthermore, the reasons why younger males more often develop myocarditis following Covid-19 vaccination, as historically shown in clinical and experimental studies, are unknown. However, it may be possibly related to sex hormone differences in the immune response and myocarditis, as well as the fact that women may less often undergo cardiovascular investigations, including imaging modalities [19, 20].
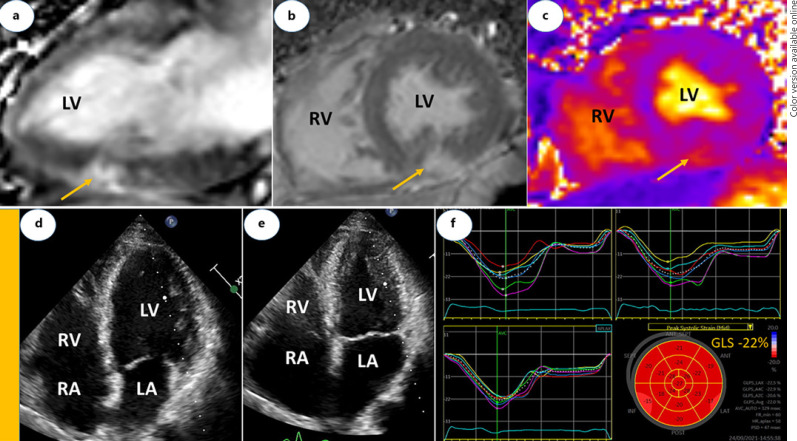
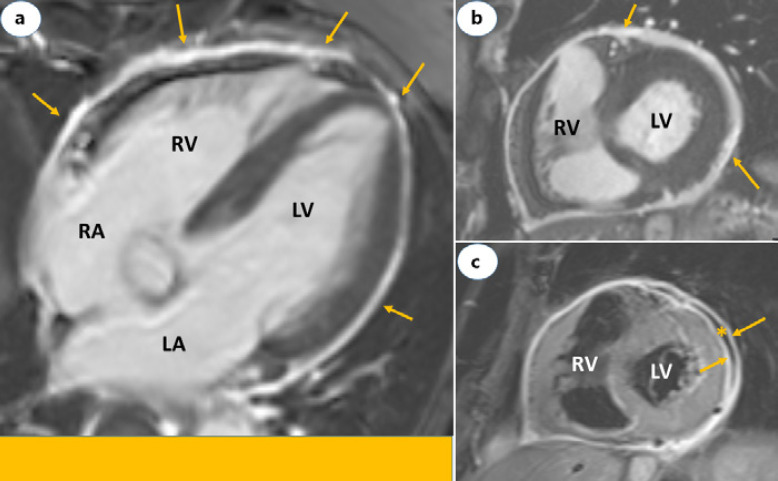
The clinical presentation in most patients involves acute chest pain, shortness of breath or palpitation, and abnormal electrocardiogram findings or evidence of myocardial injury demonstrated by elevated troponin levels in the absence of flow-limiting stenosis or culprit lesion on coronary angiography. Cardiac magnetic resonance imaging shows typical findings consistent with acute myocarditis, perimyocarditis (Fig. 1a-c), or pericarditis (Fig. 2a-c). Echocardiography may show normal left ventricular function (Fig. 1d-f). No alternative etiologies for acute myocarditis can be found. Hence, the clinical similarities in the presentation following recent mRNA-based Covid-19 vaccines strongly suggest an association with myocarditis. Furthermore, two rare cases of histologically confirmed fulminant myocarditis after mRNA Covid-19 vaccines have also been reported [21]. The authors regarded these cases as part of a systemic hyper-inflammatory syndrome, quite different from the mild myocarditis experienced by most others following Covid-19 vaccines. The first case, a 45-year-old woman, received inotropic support, intravenous diuretics, methylprednisolone 1 g daily for 3 days and guideline-directed medical therapy for heart failure. After 7 days at hospital, she recovered, and the ejection fraction increased from 15 to 20% at presentation to 60% on discharge. The second case involved a 42-year-old man who developed cardiogenic shock and died 3 days after presentation. Although no other causes were identified by PCR or serologic examination, the authors acknowledged that a direct causal relationship could not be definitively established as testing for viral genomes or autoantibodies in the tissue specimens were not performed. In most studies [12, 13], the diagnosis of myocarditis was clinically suspected and not validated by myocardial biopsy. Endomyocardial biopsy should be considered in severe cases to confirm the diagnosis and guide further treatment [22].
Fig. 1.
Cardiac magnetic resonance (a–c) and echocardiographic (d–f) images of a 61-year-old male with perimyocarditis. a is vertical long and b is short axis views showing LGE lesions in inferior wall including epicardium (yellow arrows). c is short axis view of T2 mapping showing edema in inferior wall (yellow arrow). d is end-diastolic and e end-systolic frames of apical 4-chamber views. In end-systole, there is normal and homogenous LV contraction, LV diameter reduction (E) and LV ejection fraction of 60% by Simpson's method. f Demonstrates strain curves and Bulls eye plot. GLS is normal (−22%), and remained stable (−23%) at 6-week follow-up. Cardiac troponin T was 6 ng/L at admission with a peak level of 169 ng/L following day before it decline to 122 ng/L on discharge (day 5). LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle; LGE, late gadolinium enhancement; GLS, global longitudinal strain.
Fig. 2.
Cardiac magnetic resonance images of a 73-year-old male with acute pericarditis. Four-chamber (a) and short axis (b) views showing LGE restricted to the pericardium (yellow arrows). c is STIR image short axis view and demonstrates edema of both in the visceral and parietal layers of the pericardium (yellow arrows) separated by a mild pericardial effusion (asterisk). LA, left atrium; RA, right atrium; LV, left ventricle; RV, right ventricle; LGE, late gadolinium enhancement; STIR, short-tau inversion-recovery.
Myocarditis and pericarditis are generally mild, self-limiting, and respond well to conservative treatment (rest, nonsteroidal anti-inflammatory drugs, colchicine, and other supportive measures) with a good short-term prognosis. High-dose steroid (methylprednisolone) therapy for severe myocarditis related to the Covid-19 vaccines should be considered only in the absence of viral genome in the tissues samples. Published case reports indicate that in addition to supportive care and nonsteroidal anti-inflammatory drugs, intravenous immunoglobulins were in few cases used for treating myocarditis after Covid-19 vaccination [19]. In patients who present with left ventricular systolic dysfunction, cardioprotective medications such as β-blocker and angiotensin-converting enzyme inhibitors may be considered, though prospective or randomized studies are lacking. Finally, expert opinion recommends avoiding or deferring high-intensity physical activity and competitive sports until complete recovery in terms of regression of signs and symptoms, achieving hemodynamic stability, and normalization of diagnostic and/or biomarker abnormalities [19]. An evaluation for acute Covid-19 infection by PCR sample and past exposure to the disease through SARS-CoV-2 nucleocapsid and spike protein antibodies is important in the management of post-Covid-19 vaccine myocarditis.
The current advice by Public Health England for patients who have had myocarditis/pericarditis following the first dose of Covid-19 vaccine is that the second dose should be deferred until further information becomes available, including the results of serological testing. This is also in line with the recommendations from the Norwegian Public Health Institute suggesting that if a person has experienced myocarditis or pericarditis following the first Covid-19 mRNA vaccine, he/she should abstain from the second dose [14]. In case of high risk for serious Covid-19 infection, a second dose can be considered following close consultation with a cardiologist. This guidance is likely also to apply to individuals considering a third Covid-19 booster dose.
There are currently no dedicated guidelines for patients with postvaccination myocarditis or pericarditis in terms of the frequency of follow-up including clinical assessment, repeated echocardiography, and cardiac resonance imaging. However, follow-up studies in terms of long-term consequences are underway.
Conclusion
Myocarditis following mRNA-based Covid-19 vaccines is rare. It more frequently affects younger men and is usually of mild severity with spontaneous recovery. The overall benefit of mRNA vaccines in terms of protecting from severe Covid-19 infection and associated cardiovascular complications outweighs the risk of postvaccination myocarditis.
Statement of Ethics
This work is based on the published literature and the presented cardiac images were obtained as part of routine medical care. Ethical approval for the use of these samples for research purposes was not required in accordance with local guidelines. Written informed consent was obtained from the patients for the publication of the accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was not funded.
Author Contributions
S.S. wrote the first draft of the article, which was subsequently revised by R.R. and T.H.L. L.K. did literature search and edited the manuscript. All authors approved the final submission.
References
- 1.WHO Coronarvirus (COVID-19) updates: global situation. 2021 Oct 9. Available from: https://covid19.who.int/
- 2.Saeed S, Rajani R. The cardiovascular complications in COVID-19 infection: focus on acute cardiac injury. Pak J Med Sci. 2021 May–Jun;37((3)):908–12. doi: 10.12669/pjms.37.3.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeed S, Tadic M, Larsen TH, Grassi G, Mancia G. Coronavirus disease 2019 and cardiovascular complications: focused clinical review. J Hypertens. 2021 Jul 1;39((7)):1282–92. doi: 10.1097/HJH.0000000000002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021 Oct 7;385((15)):1393–400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid MAB, Tariq S. Challenges of COVID-19 vaccination delivery in Pakistan. Pak J Med Sci. 2021;37((7)):2036. doi: 10.12669/pjms.37.7.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abbas S, Abbas B, Amir S, Wajahat M. Evaluation of adverse effects with COVID-19 vaccination in Pakistan. Pak J Med Sci. 2021 Nov-Dec;37((7)):1959. doi: 10.12669/pjms.37.7.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021 Sep 16;385((12)):1078–90. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated guidance regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines. Statement. 2021 Jul 9. Available from: www.who.int/news/item/09-07-2021-gacvs-guidance-myocarditis-pericarditis-covid-19-mrna-vaccines.
- 9.Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021 Oct 12;326((14)):1390–9. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. JAMA. 2021 Sep 28;326((12)):1210–2. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021 Dec 2;385((23)):2140–9. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021 Dec 2;385((23)):2132–9. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health England Information for healthcare professionals on myocarditis and pericarditis following COVID-19 vaccination. 2021. Aug 23, Available from: https://www.gov.uk/government/publications/covid-19-vaccination-myocarditis-and-pericarditis-information-for-healthcare-professionals/information-for-healthcare-professionals-on-myocarditis-and-pericarditis-following-covid-19-vaccination.
- 14.Norwegian Institute of Public Health Updated vaccine recommendation for individuals who have suffered pericarditis or myocarditis. 2021. Aug 13, Available from: www.fhi.no/nyheter/2021/oppdatert-vaksinasjonsanbefaling-for-vaksinerte-som-har-fatt-perikarditt-og/
- 15.The Norwegian Medicine Agency Koronavaksiner og betennelse i hjertet. 2021. Oct 6, Available from: https://legemiddelverket.no/nyheter/koronavaksiner-og-betennelse-i-hjertet.
- 16.Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acutemyocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021 Oct 1;6((10)):1196–201. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021 Oct 1;6((10)):1202–6. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021 Sep;148((3)):e2021052478. doi: 10.1542/peds.2021-052478. [DOI] [PubMed] [Google Scholar]
- 19.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021 Aug 10;144((6)):471–84. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairweather D, Cooper LT, Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. doi: 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med. 2021 Sep 30;385((14)):1332–4. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caforio ALP. Receipt of mRNA vaccine against Covid-19 and myocarditis. N Engl J Med. 2021 Dec 2;385((23)):2189–90. doi: 10.1056/NEJMe2116493. [DOI] [PMC free article] [PubMed] [Google Scholar]