Abstract
This study evaluated the effects of vitamin E (Vit E) and selenium (Se) supplementation on mRNA abundance of antioxidant, immune response, and tight junction genes, as well as taxonomic and functional profiles of ileal microbiota of broilers exposed to daily 4-h elevated temperature during d 28 to 35. A total of 640-day-old Cobb male broiler chicks were randomly allocated to 32 floor pens in a 2 × 2 factorial arrangement that included ambient temperature (thermoneutral [TN] or heat stress [HS]) and dietary treatments (basal diet or Vit E + Se). Vit E and organic Se were added to the basal diet at the rate of 250 mg/kg and 1 mg/kg, respectively. Liver and jejunum tissue samples were taken on d 27 (1 bird/pen), d 28 and d 35 (2 birds/pen) from birds for qPCR analysis. Data were subjected to a 2-way ANOVA using the GLM procedure of JMP. Ileal contents were taken on d 27 and d 35 for microbial profiling. Microbiota data were analyzed in QIIME 2 and significance between treatments identified linear discriminant analysis effect size (LEfSe, P < 0.05). Dietary Vit E/Se significantly downregulated the mRNA levels of HSPs in liver and jejunal tissues of the HS-challenged birds both on d 28 and d 35. Moreover, mRNA abundance of TLR2, TNFα, IFNγ, IL-1β, IL-10, and iNOS in the liver were significantly downregulated in birds fed the Vit E/Se diet on d 35. However, dietary treatment had no significant impact on oxidative stress, immunity, and gut integrity related genes analyzed in jejunal tissues on d 28 and d 35, except downregulation of IFNγ on d 35 (P = 0.052). LEfSe analysis revealed that Lachnospiraceae FE2018 and Ruminococcaceae NK4A214 groups was enriched in the Vit E/Se birds on d 35. Moreover, PICRUSt analysis predicted significant functional differences among the treatment groups. In conclusion, dietary supplementation of Vit E/Se mitigated the negative effects of HS potentially via improving antioxidant status, regulating cytokine responses and modifying ileal microbiota and its function.
Key words: antioxidant, broiler, heat stress, immunity, microbiota
INTRODUCTION
Current environmental challenges including prolonged durations of high heat in certain regions have presented a threat to plant and animal production worldwide (Chowdhury et al., 2020). Further, the need for agricultural products, especially protein sources, continues to rise with the growth rate of the world's population (Bruinsma, 2009). Modern broilers are more efficient than other terrestrial food producing animals in converting plant-based nutrients into healthy protein (Macelline et al., 2021), making poultry a key source for supplying the increasing demand of animal-based protein in the future. However, these animals are susceptible to elevated temperatures due to their anatomic features (e.g., lack of sweat glands), physiological state, and greater metabolic activity (Sahin et al., 2009; Lara and Rostagno, 2013). Therefore, urgent management and nutritional strategies are needed to mitigate the negative effects of heat stress (HS) on poultry (Vandana et al., 2021).
High ambient temperature is one of the most common stressors in commercial broiler production resulting in reduced feed intake and weight gain, and increased mortality leading to significant economic losses (Lara and Rostagno, 2013). Besides its adverse effect on poultry production, HS also causes important pathophysiological changes at the cellular level including excessive production of reactive oxygen species (ROS), disturbed gut permeability, reduced energy metabolism, altered immune function and gut microbiota which further leads to increased susceptibility to pathogens and compromised overall health (Liu et al., 2016; Shi et al., 2019; Lian et al., 2020).
Birds respond to HS conditions by reprograming several defense mechanisms including antioxidant enzymes, heat-shock proteins (HSPs), and cytokines to alleviate or reduce the negative effects of HS (Tan et al., 2010; Slimen et al., 2016; Emami et al., 2020). HSPs are important molecular chaperones that play a crucial role in folding/unfolding of polypeptides to achieve a functional structure and the repair of denatured proteins, or assist in their degradation after stress challenge (Whitley et al., 1999). Expression of HSPs, especially HSP70 and HSP90, significantly increased in response to elevated ambient temperature to protect and repair cells (Varasteh et al., 2015; Rajaei-Sharifabadi et al., 2017). HS-induced signaling events lead to upregulation of HSP70 through the HS-related increase in ROS level (Banerjee Mustafi et al., 2009). Along with the protective roles, HSPs also have regulatory functions during cellular stress as they inhibit the production of inflammatory cytokines (Ferat-Osorio et al., 2014) and modulate tight junction proteins (Dokladny et al., 2008).
Further, HS can induce alterations in cellular functions and disturbances in intestinal microbiome that need to be managed with several nutritional interventions to alleviate the negative impact of HS (Quinteiro-Filho et al., 2017; Rajaei-Sharifabadi et al., 2017; Siddiqui et al., 2020). Therefore, several nutritional strategies, especially non-drug-based feed additives, are being tested to maintain growth performance and overall health of the birds (Flees et al., 2021; Greene et al., 2021). Vit E and Se are important nutrients with antioxidant function. The combined dietary use of Vit E and Se is a more effective nutritional strategy than individual use as these feed additives act synergistically to modulate antioxidant activity (Harsini et al., 2012; Kumbhar et al., 2018). Even though these nutrients are present in the diets at certain levels, dietary addition of Vit E/Se above the recommended levels might have beneficial effects on performance, oxidative status, and immune response during a stress challenge (Harsini et al., 2012; Habibian et al., 2014; Surai, 2018).
As metabolically active organs, the liver and intestinal tissues, especially the jejunum, are highly responsive to HS conditions, thus such disturbances directly lead to reduced performance and increased susceptibility to disease. The impact of HS on oxidative balance, immune response and gut barrier function of broilers has been well documented; however, little is known about the combined use of additional Vit E/Se on the mechanisms of molecular regulation in the small intestine and liver along with the changes in the intestinal microbiota. Therefore, the current study aimed to investigate the effects of dietary Vit E/Se supplementation on mRNA abundance of antioxidant, immune response, and tight junction genes, as well as on the ileum microbial profile of broiler chickens subjected to cyclic acute HS.
MATERIALS AND METHODS
Experimental Diets, Birds and Management
This project was approved and conducted under the guidelines of the Virginia Tech Institutional Animal Care and Use Committee. A total of 640 one-day old Cobb male chicks were randomly allocated to 32 floor pens in a 2 × 2 factorial arrangement that included ambient temperature (thermoneutral [TN] or heat stress [HS]) and dietary treatments (basal diet or Vit E/Se) as main factors. Four rooms were used (2 TN and 2 HS) each housing half of the 8 replicate pens per group. For the Vit E/Se diet, the basal diet was supplemented with 250 mg/kg Vit E (Rovimix E50, DSM Nutritional Products, Basel, Switzerland) and 1 mg/kg organic Se (Sel-Plex, Alltech Inc., Nicholasville, KY). Birds were raised in a controlled environment for 35 d. Starter and grower diets were based on corn-soybean meal formulations and provided to the birds from 0 to 21 d and 21 to 35 d of age, respectively. All diets were formulated to meet or exceed the NRC (1994) nutrient recommendations. Each pen was equipped with a bucket feeder and an automatic nipple drinker. Water and experimental diets (in mash form) were provided ad libitum throughout the study period.
Heat Stress Protocol
Heat stress was applied during the last week of the experiment (d 28–d 35). For the HS groups, room temperature was raised and maintained at 35 ± 1°C from 10 AM to 2 PM, then reduced again to 25°C ± 1 for the remainder of the day. Relative humidity was monitored and maintained at <50%. Birds in the TN groups were kept under recommended temperatures (25°C ± 1).
Total RNA Extraction and Reverse Transcription
On d 27 (1 bird/pen), d 28 and d 35 (2 birds/pen) birds were selected, euthanized, and jejunal sections (1 cm) and liver tissues were excised and snap-frozen in liquid nitrogen to later assess the mRNA abundance of oxidative stress, immunity, and gut integrity related genes. A 20-30 mg aliquot of jejunal and liver tissues was weighed into a 2 mL microcentrifuge tube and homogenized in 600 μL TRI Reagent (Zymo Research, Irvine, CA) by a TissueLyser II (Qiagen, Valencia, CA). Total RNA was extracted from the homogenate using the Direct-zol RNA Miniprep Plus Kit (Zymo Research, Irvine, CA) according to the manufacturer's recommendations. Total RNA concentration was determined at optical density (OD) of 260 using a NanoDrop-1000 (Thermo Fisher Scientific, Waltham, MA), and RNA purity was verified by evaluating the 260/280 OD ratios. RNA integrity was evaluated by gel electrophoresis on 1.5% agarose gel in 0.5 × TAE buffer. After extraction, 2 µg total RNA were used to synthesize first-strand cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's recommendation and the cDNA was stored at −20°C.
Quantitative Real-Time PCR
The mRNA abundance of oxidative stress related genes [superoxide dismutase (SOD), glutathione peroxidase (GPx), heat shock protein (HSP)70 and HSP90], cytokines [interleukin (IL)-1β, IL-4, IL-10, IL-17A, IL-22, tumor necrosis factor alpha (TNFα), inducible nitric oxide synthase (iNOS), interferon gamma (IFNγ)], Toll-like receptor (TLR)2 and TLR4, tight junction proteins [occludin, zona occludens (ZO)-1], mechanistic target of rapamycin (mTOR) were determined by quantitative real-time PCR (ABI 7500 Fast Real-Time PCR System, Applied Biosystems, Foster City, CA) using Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA). Details of primer sets are provided in Table 1. Product specificity was confirmed by analysis of the melting curves produced by the ABI 7500 software (version 2.0.3). mRNA abundance was analyzed using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an endogenous control. Average mRNA abundance relative to GAPDH for each sample was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001). The calibrator for each gene was the average ΔCt value from the negative control group for each sampling day.
Table 1.
Sequences of primer pairs used for amplification of target and reference genes.
| Gene1 | Primer sequence | Size | Acc (reference) | |
|---|---|---|---|---|
| SOD | GTGACCTCGGCAATGTGACT GCAGTGTGGTCCGGTAAGAG |
86 | NM_205064.1 | |
| GPx | AAGAACTTCGGGCACCAGGA TGTACTGCGGGTTGGTCATC |
214 | NM_001277853.2 | |
| HSP70 | CGTCAGTGCTGTGGACAAGAGTA CCTATCTCTGTTGGCTTCATCCT |
145 | NM_001006685 | |
| HSP90 | GATCCCCAGACACATGCCAA CTCGGTAACTGCAGGACTGG |
105 | NM_001109785 | |
| TLR2 | CGGTCATCTCAGCTACACCA GCATCGCATGAAAGACAGGC |
150 |
NM_204278 |
|
| TLR4 | CCACACACCTGCCTACATGAA GGATGGCAAGAGGACATATCAAA |
63 |
NM_001030693 |
|
| IL-1β | CCCGCCTTCCGCTACA CACGAAGCACTTCTGGTTGATG |
66 |
NM_204524.1 |
|
| IL-4 | GCTCTCAGTGCCGCTGATG GAAACCTCTCCCTGGATGTCAT |
60 | NM_001007079.1 | |
| IL-10 | CGCTGTCACCGCTTCTTCA CGTCTCCTTGATCTGCTTGATG |
63 | NM_001004414.2 | |
| IL-17A | AGCTGGACCACAGCGTCAAC GGCGGAGGACGAGGATCT |
57 | NM_204460.1 | |
| IL-22 | CTTCTCACCAGCCCCCTACCTCCA GCTGACCGATGAGTCTGTTGTCAGTG |
175 | NM_001199614.1 | |
| TNFα | CCCATCCCTGGTCCGTAAC ATACGAAGTAAAGGCCGTCCC |
77 | MF000729.1 | |
| iNOS | CCTGTACTGAAGGTGGCTATTGG AGGCCTGTGAGAGTGTGCAA |
66 | NM_204961.1 | |
| IFNγ | AGTCAAAGCCGCACATCAAAC TTCACCTTCTTCACGCCATCA |
82 | NM_205149.1 | |
| Occludin | CCGTAACCCCGAGTTGGAT ATTGAGGCGGTCGTTGATG |
214 | NM_205128.1 | |
| ZO-1 | GGAGTACGAGCAGTCAACATAC GAGGCGCACGATCTTCATAA |
101 | XM_413773 | |
| mTOR | CATGTCAGGCACTGTGTCTATTCTC CTTTCGCCCTTGTTTCTTCACT |
77 | XM_417614.6 | |
| GAPDH | CCTAGGATACACAGAGGACCAGGTT GGTGGAGGAATGGCTGTCA |
64 | NM_204305 | |
GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; GPx: Glutathione peroxidase; HSP: Heat shock protein; IL: Interleukin; iNOS: Inducible nitric oxide synthase; IFNγ: Interferon gamma; SOD: Superoxide dismutase; TNFα: Tumor necrosis factor alpha; TLR: Toll Like Receptor; mTOR: Mechanistic target of rapamycin; ZO: Zonula occluden. For each gene, the primer sequence for forward (F) and reverse (R) (5ʹ-3ʹ) primers, the amplicon size (bp) and the NCBI Accession number (Acc) used for the primer design are listed.
16S rRNA Gene Sequencing
On d 27 and d 35, one bird/pen was euthanized, ileal digesta were collected and snap-frozen in liquid nitrogen, and then stored at −80°C until further analysis. Bacterial DNA was extracted from ileal digesta using the Qiagen DNeasy PowerSoil Pro Kit (Qiagen GmbH, Hilden, Germany) based on the supplier's instructions. Quality and quantity of extracted DNA were measured using gel electrophoresis and spectrophotometer. All DNA samples were diluted to 300 ng/μL and 50 μL of each for sequencing. The V4 region of the 16S rRNA gene was amplified using the Illumina MiSeq kit V3 (600-cycle format, 25 million reads, read length 2 × 300). Earth Microbiome Project primer sets 515F (Parada et al., 2016) and 806R (Apprill et al., 2015) generating 390 bp amplicons were used.
To assess the association between microbial community and dietary treatment within each sampling day, pairwise PERMANOVA analysis implemented in QIIME 2 was performed on an unweighted UniFrac distance matrix. The significance of PERMANOVA was obtained by 999 permutation tests. Relative abundance of taxa for each sampling day and treatment were calculated at the phylum and genus levels. The distinctive taxa between treatment groups were identified with linear discriminant analysis effect size (LEfSe), which was performed through Galaxy/Hutlab workflow platform (Segata et al., 2011). In order to predict the function of ileal microbiota, data analysis was performed through the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) pipeline (Weber et al., 2018). Then, PICRUSt outputs for the level 3 of the Kyoto Encyclopedia of Genes and Genomes (KEGG) were analyzed and illustrated with statistical analysis of the taxonomic and functional profiles (STAMP) software version 2.1.3 (Parks et al., 2014).
Statistical Analysis
Data were subjected to a 2-way ANOVA using the GLM procedure of JMP (Pro13). The models included ambient temperature (TN and HS) and dietary treatments (basal diet or VitE/Se) as the main factors, and the 2-way interactions Tukey's multiple range test was only carried out for significant interactions and was performed using simple effect analysis. The probability P < 0.05 was considered significant unless otherwise noted.
RESULTS
mRNA Abundance of Oxidative Stress, Immunity, and Gut Integrity Related Genes in the Jejunum and Liver on d 27
Jejunum: The effects of dietary Vit E/Se administration on the mRNA abundance of oxidative stress, immunity, and gut integrity related genes in the jejunum of broilers are shown in Table 2. mRNA abundance of genes involved in oxidative stress, immunity, and gut integrity were not influenced by Vit E/Se supplementation under standard commercial conditions.
Table 2.
Effect of dietary supplementation of Vit E/Se on relative mRNA abundance of several genes in the jejunum and liver tissues of broiler chickens on d 27.1
| Item | Treatment groups2 |
Statistics |
||
|---|---|---|---|---|
| Control | Vit E/Se | SEM | P-value | |
| Jejunum | ||||
| HSP70 | 1.04 | 0.88 | 0.05 | 0.099 |
| HSP90 | 1.03 | 0.96 | 0.06 | 0.539 |
| TLR2 | 1.05 | 1.23 | 0.09 | 0.300 |
| TLR4 | 1.16 | 1.31 | 0.12 | 0.511 |
| IL-1β | 1.10 | 1.10 | 0.08 | 0.988 |
| IL-4 | 1.14 | 1.08 | 0.10 | 0.781 |
| IL-10 | 1.24 | 1.13 | 0.17 | 0.761 |
| TNFα | 1.11 | 1.19 | 0.09 | 0.637 |
| iNOS | 1.10 | 1.11 | 0.06 | 0.900 |
| IFNγ | 1.07 | 1.20 | 0.09 | 0.454 |
| Occludin | 1.04 | 1.17 | 0.06 | 0.261 |
| ZO-1 | 1.11 | 1.31 | 0.11 | 0.397 |
| Liver | ||||
| HSP70 | 1.17 | 0.88 | 0.11 | 0.204 |
| HSP90 | 1.04 | 0.85 | 0.06 | 0.118 |
| SOD | 1.05 | 1.04 | 0.05 | 0.934 |
| GPx | 1.09 | 0.84 | 0.07 | 0.079 |
| mTOR | 1.17 | 1.01 | 0.14 | 0.594 |
| TLR2 | 1.07 | 0.79 | 0.06 | 0.023 |
| TLR4 | 1.71 | 1.10 | 0.28 | 0.290 |
| IL-1β | 1.09 | 0.80 | 0.08 | 0.106 |
| IL-4 | 1.37 | 0.86 | 0.16 | 0.110 |
| IL-10 | 1.19 | 1.08 | 0.17 | 0.773 |
| TNFα | 1.17 | 0.89 | 0.11 | 0.218 |
| iNOS | 1.07 | 0.77 | 0.07 | 0.031 |
| IFNγ | 1.14 | 0.97 | 0.11 | 0.477 |
Data represent mean values of 16 replicates per treatment.
Control: birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg vitamin E and 1 mg/kg selenium.
Liver: Dietary Vit E/Se supplementation significantly downregulated mRNA levels of TLR2 and iNOS as compared to the basal diet group (Table 2). However, no differences were observed between control and treatment groups for the other targets examined.
mRNA Abundance of Oxidative Stress, Immunity, and Gut Integrity Related Genes in the Jejunum and Liver on d 28
Jejunum: A significant interaction was observed in mRNA abundance of HSP70 and HSP90 between dietary treatment and ambient temperature in jejunal tissues (Table 3). Dietary treatment significantly downregulated (P < 0.05) mRNA levels of HSP70, HSP90, and IL-4, and upregulated (P = 0.017) that of IL-17A on d 28. Heat stress significantly upregulated HSP70, HSP90, IL-4, IL-22, IFNγ, and ZO-1 levels and downregulated those of TLR2, TNFα, and occludin.
Table 3.
Effect of dietary supplementation of Vit E/Se on relative mRNA abundance of oxidative stress, immunity, and gut integrity related genes in the jejunum of heat-stressed broiler chickens on d 28.1
| Treatments2 |
Diet |
Ambient temperature |
Statistics3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Vit E/Se |
P-value |
||||||||||
| Item | TN | HS | TN | HS | Control | Vit E/Se | TN | HS | RMSE | Diet | AT | D × AT |
| HSP70 | 1.13c | 13.31a | 1.34c | 4.31b | 7.22 | 2.82 | 1.23 | 8.81 | 3.82 | <0.001 | <0.001 | <0.001 |
| HSP90 | 1.11c | 5.42a | 1.36c | 2.66b | 3.26 | 2.01 | 1.23 | 4.04 | 1.43 | 0.002 | <0.001 | <0.001 |
| TLR2 | 1.09 | 0.80 | 1.20 | 0.87 | 0.94 | 1.04 | 1.14 | 0.84 | 0.49 | 0.453 | 0.016 | 0.848 |
| TLR4 | 1.14 | 1.15 | 1.39 | 1.17 | 1.14 | 1.28 | 1.26 | 1.16 | 0.66 | 0.414 | 0.529 | 0.472 |
| IL-1β | 1.14 | 1.03 | 1.20 | 1.00 | 1.09 | 1.10 | 1.17 | 1.02 | 0.60 | 0.930 | 0.306 | 0.749 |
| IL-4 | 1.39 | 3.57 | 1.10 | 2.56 | 2.48 | 1.83 | 1.25 | 3.06 | 1.18 | 0.032 | <0.001 | 0.228 |
| IL-10 | 1.34 | 1.92 | 1.78 | 2.14 | 1.63 | 1.96 | 1.56 | 2.03 | 1.34 | 0.337 | 0.174 | 0.741 |
| IL-17A | 1.11 | 0.83 | 1.60 | 1.33 | 0.97 | 1.47 | 1.36 | 1.09 | 0.79 | 0.017 | 0.186 | 0.974 |
| IL-22 | 1.28 | 3.92 | 1.62 | 4.45 | 2.60 | 3.03 | 1.45 | 4.18 | 2.07 | 0.447 | <0.001 | 0.860 |
| TNFα | 1.27 | 0.82 | 1.48 | 0.73 | 1.05 | 1.10 | 1.37 | 0.77 | 0.83 | 0.759 | 0.002 | 0.406 |
| iNOS | 1.23 | 1.91 | 1.48 | 1.59 | 1.57 | 1.54 | 1.36 | 1.75 | 0.92 | 0.872 | 0.090 | 0.217 |
| IFNγ | 1.23 | 1.88 | 1.14 | 1.64 | 1.55 | 1.39 | 1.18 | 1.76 | 0.85 | 0.435 | 0.009 | 0.719 |
| Occludin | 1.05 | 0.85 | 1.11 | 0.88 | 0.95 | 1.00 | 1.08 | 0.87 | 0.33 | 0.579 | 0.015 | 0.843 |
| ZO-1 | 1.32 | 2.40 | 1.61 | 2.42 | 1.86 | 2.01 | 1.46 | 2.41 | 0.92 | 0.500 | <0.001 | 0.557 |
Means with different superscripts in the same row are significantly different (P < 0.05).
Data represent mean values of 16 replicates per treatment.
Control: birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg vitamin E and 1 mg/kg selenium; TN: thermoneutral; HS: heat stress
AT: Ambient Temperature; D: Diet.
Liver: A significant interaction was observed in abundance of HSP90 between dietary treatment and ambient temperature in liver tissues (Table 4). Supplementation of Vit E/Se reduced (P < 0.05) mRNA abundance of HSP90 and GPx. Heat stress significantly upregulated SOD, GPx, HSP70, HSP90, TLR4, and downregulated that of mTOR, TNFα, and IFNγ on d 28.
Table 4.
Effect of dietary supplementation of Vit E/Se on relative mRNA abundance of oxidative stress and immunity related genes in the liver of heat-stressed broiler chickens on d 28.1
| Treatments2 |
Diet |
Ambient Temperature |
Statistics3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Vit E/Se |
P-value |
||||||||||
| Item | TN | HS | TN | HS | Control | Vit E/Se | TN | HS | RMSE | Diet | AT | D × AT |
| HSP70 | 1.09 | 8.28 | 1.15 | 6.14 | 4.68 | 3.64 | 1.12 | 7.21 | 5.33 | 0.463 | <0.001 | 0.436 |
| HSP90 | 1.16a | 4.86a | 1.25b | 2.00b | 3.01 | 1.62 | 1.20 | 3.43 | 2.42 | 0.026 | 0.001 | 0.018 |
| SOD | 1.05 | 1.31 | 1.01 | 1.30 | 1.18 | 1.15 | 1.03 | 1.30 | 0.42 | 0.814 | 0.013 | 0.864 |
| GPx | 1.10 | 2.53 | 0.83 | 1.64 | 1.82 | 1.22 | 0.97 | 2.10 | 1.04 | 0.032 | <0.001 | 0.246 |
| mTOR | 1.05 | 0.73 | 1.07 | 0.79 | 0.89 | 0.93 | 1.06 | 0.76 | 0.33 | 0.665 | 0.001 | 0.818 |
| TLR2 | 1.08 | 1.09 | 0.87 | 1.07 | 1.09 | 0.97 | 0.98 | 1.08 | 0.45 | 0.293 | 0.363 | 0.418 |
| TLR4 | 1.17 | 2.31 | 1.30 | 2.08 | 1.74 | 1.69 | 1.24 | 2.19 | 0.80 | 0.792 | <0.001 | 0.361 |
| IL-1β | 1.12 | 1.01 | 1.16 | 1.10 | 1.06 | 1.13 | 1.14 | 1.06 | 0.53 | 0.611 | 0.553 | 0.850 |
| IL-4 | 1.13 | 1.02 | 1.38 | 1.01 | 1.07 | 1.19 | 1.25 | 1.02 | 0.54 | 0.386 | 0.090 | 0.341 |
| IL-10 | 1.14 | 0.89 | 0.96 | 0.80 | 1.01 | 0.88 | 1.04 | 0.84 | 0.57 | 0.355 | 0.164 | 0.757 |
| TNFα | 1.11 | 0.66 | 1.01 | 0.63 | 0.87 | 0.82 | 1.05 | 0.64 | 0.44 | 0.579 | <0.001 | 0.748 |
| iNOS | 1.15 | 1.31 | 1.40 | 1.19 | 1.23 | 1.30 | 1.28 | 1.25 | 0.78 | 0.728 | 0.904 | 0.336 |
| IFNγ | 1.14 | 0.82 | 1.29 | 0.76 | 0.97 | 1.03 | 1.21 | 0.79 | 0.69 | 0.747 | 0.007 | 0.495 |
Means with different superscripts in the same row are significantly different (P < 0.05).
Data represent mean values of 16 replicates per treatment.
Control: birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg vitamin E and 1 mg/kg selenium; TN: thermoneutral; HS: heat stress
AT: Ambient Temperature; D: Diet.
mRNA Abundance of Oxidative Stress, Immunity, and Gut Integrity Related Genes in the Jejunum and Liver on d 35
Jejunum: No significant interaction effect on oxidative stress, immunity and gut integrity related markers was observed between diet and ambient temperature (Table 5). Dietary treatment had no significant impact on oxidative stress, immunity, and gut integrity related genes analyzed in jejunum on d 35. However, mRNA abundance of IFNγ decreased (P = 0.052) in Vit E/Se treatment. Heat stress caused an increase in mRNA levels of HSP70, HP90, TLR2 IL-4, IL-10, IL-22, occludin, ZO-1, and a decrease in IL-17A and IFNγ.
Table 5.
Effect of dietary supplementation of Vit E/Se on relative mRNA abundance of oxidative stress, immunity, and gut integrity related genes in the jejunum of heat-stressed broiler chickens on d 35.1
| Treatments2 |
Diet |
Ambient Temperature |
Statistics3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Vit E/Se |
P-value |
||||||||||
| Item | TN | HS | TN | HS | Control | Vit E/Se | TN | HS | RMSE | Diet | AT | D × AT |
| HSP70 | 1.05 | 8.08 | 1.03 | 6.54 | 4.20 | 3.28 | 1.04 | 7.38 | 4.06 | 0.482 | <0.001 | 0.494 |
| HSP90 | 1.09 | 4.52 | 1.10 | 3.86 | 2.75 | 2.39 | 1.10 | 4.20 | 1.69 | 0.454 | <0.001 | 0.451 |
| TLR2 | 1.05 | 1.88 | 0.89 | 1.72 | 1.46 | 1.31 | 0.97 | 1.80 | 0.98 | 0.518 | 0.001 | 0.983 |
| TLR4 | 1.07 | 0.93 | 0.84 | 1.20 | 1.00 | 1.02 | 0.96 | 1.07 | 0.53 | 0.871 | 0.416 | 0.064 |
| IL-1β | 1.09 | 1.23 | 0.88 | 1.08 | 1.16 | 0.98 | 0.99 | 1.15 | 0.53 | 0.187 | 0.214 | 0.824 |
| IL-4 | 1.08 | 2.02 | 0.96 | 1.75 | 1.55 | 1.35 | 1.02 | 1.88 | 1.13 | 0.488 | 0.003 | 0.780 |
| IL-10 | 1.14 | 1.68 | 0.74 | 1.42 | 1.41 | 1.08 | 0.94 | 1.55 | 0.76 | 0.087 | 0.002 | 0.737 |
| IL-17A | 1.12 | 0.47 | 1.33 | 0.42 | 0.79 | 0.87 | 1.22 | 0.44 | 0.55 | 0.564 | <0.001 | 0.346 |
| IL-22 | 1.07 | 2.17 | 0.88 | 1.47 | 1.62 | 1.18 | 0.98 | 1.82 | 1.09 | 0.121 | 0.004 | 0.355 |
| TNFα | 1.04 | 1.09 | 0.95 | 1.09 | 1.06 | 1.02 | 1.00 | 1.09 | 0.46 | 0.709 | 0.420 | 0.701 |
| iNOS | 1.05 | 1.15 | 0.90 | 1.15 | 1.10 | 1.02 | 0.97 | 1.15 | 0.48 | 0.500 | 0.141 | 0.537 |
| IFNγ | 1.13 | 0.72 | 0.84 | 0.53 | 0.93 | 0.68 | 0.98 | 0.63 | 0.49 | 0.052 | 0.005 | 0.696 |
| Occludin | 1.06 | 2.16 | 0.96 | 2.41 | 1.61 | 1.68 | 1.01 | 2.28 | 0.91 | 0.753 | <0.001 | 0.450 |
| ZO-1 | 1.06 | 2.10 | 0.94 | 2.23 | 1.58 | 1.58 | 1.00 | 2.17 | 0.86 | 0.976 | <0.001 | 0.566 |
Data represent mean values of 16 replicates per treatment.
Control: birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg vitamin E and 1 mg/kg selenium; TN: thermoneutral; HS: heat stress
AT: Ambient Temperature; D: Diet.
Liver: There was significant interaction between dietary and ambient temperature treatments in terms of liver HSP70 and HSP90 mRNA levels (Table 6). Dietary Vit E/Se treatment significantly downregulated mRNA levels of HSP70, HSP90, mTOR, TLR2, IL-1β, IL-10, TNFα, iNOS, and IFNγ on d 35. Heat stress significantly upregulated GPx, HSP70, and HSP90, and downregulated mTOR, IL-1β and IFNγ.
Table 6.
Effect of dietary supplementation of Vit E/Se on relative mRNA abundance of oxidative stress and immunity related genes in the liver of heat-stressed broiler chickens on d 35.1
| Treatments2 |
Diet |
Ambient Temperature |
Statistics3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control |
Vit E/Se |
P-value |
||||||||||
| Item | TN | HS | TN | HS | Control | Vit E/Se | TN | HS | RMSE | Diet | AT | D × AT |
| HSP70 | 1.14c | 13.96a | 1.02c | 4.97b | 7.55 | 3.00 | 1.08 | 9.47 | 4.49 | 0.001 | <0.001 | 0.001 |
| HSP90 | 1.24c | 5.97a | 1.25c | 3.65b | 3.60 | 2.45 | 1.25 | 4.81 | 1.74 | 0.015 | <0.001 | 0.014 |
| SOD | 1.06 | 1.19 | 1.13 | 1.06 | 1.13 | 1.10 | 1.10 | 1.13 | 0.37 | 0.755 | 0.750 | 0.299 |
| GPx | 1.13 | 2.31 | 1.16 | 2.39 | 1.72 | 1.77 | 1.14 | 2.35 | 1.26 | 0.866 | <0.001 | 0.941 |
| mTOR | 1.09 | 0.85 | 0.82 | 0.70 | 0.97 | 0.76 | 0.96 | 0.78 | 0.35 | 0.018 | 0.046 | 0.515 |
| TLR2 | 1.20 | 1.00 | 0.85 | 0.60 | 1.10 | 0.73 | 1.02 | 0.80 | 0.53 | 0.006 | 0.096 | 0.851 |
| TLR4 | 1.22 | 1.00 | 0.84 | 0.77 | 1.11 | 0.81 | 1.03 | 0.88 | 0.65 | 0.070 | 0.370 | 0.641 |
| IL-1β | 1.09 | 0.37 | 0.82 | 0.34 | 0.73 | 0.58 | 0.96 | 0.36 | 0.29 | 0.047 | <0.001 | 0.101 |
| IL-4 | 1.05 | 0.88 | 0.95 | 0.99 | 0.96 | 0.97 | 1.00 | 0.93 | 0.46 | 0.944 | 0.553 | 0.352 |
| IL-10 | 1.21 | 1.46 | 0.70 | 0.68 | 1.33 | 0.69 | 0.95 | 1.07 | 0.78 | 0.002 | 0.552 | 0.497 |
| TNFα | 1.11 | 0.93 | 0.82 | 0.61 | 1.02 | 0.72 | 0.97 | 0.77 | 0.52 | 0.025 | 0.141 | 0.935 |
| iNOS | 1.06 | 1.02 | 0.65 | 0.98 | 1.04 | 0.82 | 0.85 | 1.00 | 0.43 | 0.050 | 0.185 | 0.092 |
| IFNγ | 1.26 | 0.85 | 0.74 | 0.40 | 1.05 | 0.57 | 1.00 | 0.62 | 0.56 | 0.001 | 0.009 | 0.778 |
Means with different superscripts in the same row are significantly different (P < 0.05).
Data represent mean values of 16 replicates per treatment.
Control: birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg vitamin E and 1 mg/kg selenium; TN: thermoneutral; HS: heat stress
AT: Ambient Temperature; D: Diet.
Composition of Ileal Microbiota
The relative abundance of amplicon sequence variants of ileal microbiota was analyzed at different ranking levels from phylum to genus. The dominant phyla across the groups were Firmicutes, Bacteroidetes, Tenericutes, and Proteobacteria together contributing more than 99% of the whole phyla on d 27 (Supplementary Figure 1) and d 35 (Supplementary Figure 2). The LeFSe analysis revealed shifts in ileal microbiota communities in response to dietary Vit E/Se supplementation or heat challenge. On d 27, relative abundance of Anaeroplasmataceae, Christensenellaceae, Sellimonas, Eubacterium rectale group, Ruminococcaceae UCG_005, Ruminococcus gauvreauii group, and Anaeroplasma was lower in Vit E/Se group compared to control. However, Lachnospiraceae FE2018 and Ruminococcaceae NK4A214 groups were higher in Vit E/Se group on d 35 (Supplementary Table 1). Moreover, TN birds had greater abundance of Porphyromonadaceae, Alcaligenaceae, Enterococcaceae, Parabacteroides, Parasutterella, and Enterococcus compared to HS birds while relative abundance of Clostridiales vadinBB60 group and Erysipelatoclostridium was higher in the HS group on d 35 (Supplementary Table 2).
Predicted Functions of Ileal Microbiota
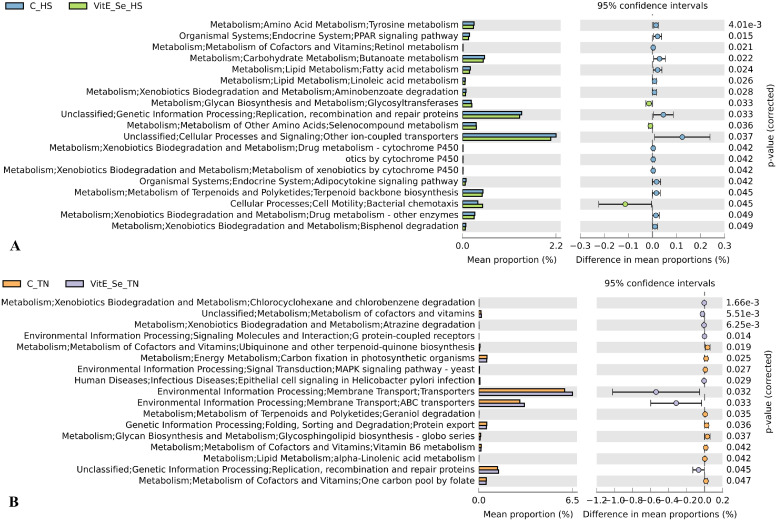
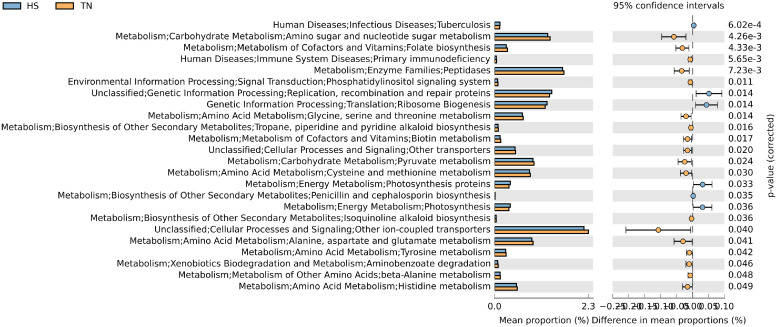
There were 8 pathways at KEGG level 3 with distinctive enrichment between control and Vit E/Se groups on d 27 (Figure 1). Among these, the Vit E/Se group microbiota had lower numbers of functional genes associated with genetic information including mismatch repair, nucleotide excision repair, DNA replication, and DNA replication proteins compared to the control group. There were 19 pathways at KEGG level 3 with distinctive enrichment between control and Vit E/Se groups under HS conditions on d 35 (Figure 2A). In the HS group, ileal microbiota of the control group was enriched in functions involved in tyrosine metabolism, PPAR signaling pathway, retinol metabolism, butanoate metabolism, fatty acid and linoleic acid metabolism, replication, recombination and repair proteins, adipocytokine signaling pathway and bisphenol degradation compared to Vit E/Se group. Conversely, ileal microbiota of the Vit E/Se group subjected to HS were enriched in functions associated with the glycosyltransferases, seleno-compound metabolism, and bacterial chemotaxis compared to control group. There were 17 pathways at KEGG level 3 with distinctive enrichment between control and Vit E/Se groups under TN conditions on d 35 (Figure 2B). Among these, the Vit E/Se group had higher number of functional genes involved in cofactor and vitamin metabolism, membrane transports, and replication, recombination and repair proteins compared to control group. At third level of KEGG hierarchy, there were 24 differentially enriched pathways (Figure 3) between TN and HS groups (regardless of dietary treatments). Among them, metabolic pathways associated with carbohydrates (sugar and pyruvate), amino acids metabolism (glycine, serine and threonine, cysteine and methionine, alanine, aspartate and glutamate, tyrosine, and histidine), peptidases, folate biosynthesis, and biotin were enriched in the TN group compared to HS. Pathways involved in ribosome biogenesis and replication, recombination, and repair proteins were enriched in the HS group as compared to TN birds.
Figure 1.
Predicted functions of ileal microbiota in broiler chickens at KEGG level 3 (n = 8/treatment). Differentially regulated metabolic pathways in C vs. Vit E/Se group on d 27. Control (C): birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg Vit E and 1 mg/kg selenium.
Figure 2.
Predicted functions of ileal microbiota in broiler chickens at KEGG level 3 (n = 8/treatment). Differentially regulated metabolic pathways in C vs. Vit E/Se group during TN (2A) and HS (2B) conditions on d 35. Control (C): birds fed a basal diet; Vit E/Se: birds fed a basal diet supplemented with 250 mg/kg Vit E and 1 mg/kg selenium. TN: thermoneutral; HS: heat stress.
Figure 3.
Predicted functions of ileal microbiota in broiler chickens at KEGG level 3 (n = 16/treatment). Differentially regulated metabolic pathways in TN vs. HS condition on d 35. TN: thermoneutral; HS: heat stress.
DISCUSSION
Environmental challenges cause several changes in the birds’ physiological and immunological responses. Under HS conditions, the immune system, intestinal barrier function, and oxidative stability are compromised in broilers, resulting in reduced performance and welfare as well as increased mortality and susceptibility to pathogens (Lara and Rostagno, 2013). Therefore, several management and nutritional strategies are applied in modern poultry production systems to reduce or eliminate the effects of such challenges in order to maintain bird's growth and health (Emami et al., 2020). Dietary Vit E and/or Se supplementation is a well-known nutritional strategy that improves the antioxidant status of the host through eliminating free radicals to alleviate oxidative stress (Singh et al., 2006). In this context, the central hypothesis of the present study was that the adverse effects of heat stress on the immune response, oxidative stability, gut integrity, and intestinal microbiota can be mitigated by incorporating Vit E and organic Se into poultry diets.
Dietary Vit E/Se supplementation did not affect mRNA abundance of oxidative stress, immunity and gut integrity related genes in the jejunum on d 27, one day before the HS challenge. Similarly, mRNA abundance of oxidative stress and immunity related genes in the liver were not influenced by dietary treatments, except for a downregulation of TLR2 and iNOS on d 27. This outcome was not unexpected as under standard production practices, dietary supplementation of Vit E/Se above the recommended levels is not needed as these additives exert more pronounced effects during a challenge (Surai, 2018; Surai et al., 2019a).
Generally, protein synthesis is reduced under HS conditions except for a group of highly conserved proteins known as HSPs (Gu et al., 2012). These proteins, also called molecular chaperones, are responsible for the correct folding/unfolding of polypeptides to achieve a functional shape and to repair denatured proteins or assist in their degradation after stress (Whitley et al., 1999). The mRNA abundance of HSPs, along with the oxidative stress markers such as SOD, CAT, and GPx, help to evaluate the effectiveness of dietary antioxidants under thermal stress conditions. Heat stress-associated increase in the mRNA and protein abundance of HSP70 and HSP90 were recently reported (Rimoldi et al., 2015; Liu et al., 2016; Santos et al., 2019). As expected, we observed a significant upregulation in HSP70 and HSP90 mRNA abundance in jejunal and liver tissues both at the start (d 28) and end (d 35) of the HS challenge. Consistent with our hypothesis, the mRNA levels of HSP70 and HSP90 were regulated by the dietary Vit E/Se treatment, where a significant downregulation was observed in HS birds. Similarly, other studies reported that dietary Vit E (Jang et al., 2014) and Vit E/Se (Kumbhar et al., 2018) significantly decreased the mRNA abundance of HSPs in several tissues of heat stressed broilers. Elevated environmental temperatures induce oxidative stress, which further leads to an increase in ROS level and an imbalance in redox state of cells (Gorman et al., 1999; Banerjee Mustafi et al., 2009). Hyperthermia-related overexpression of HSP70 is dependent on the activation of p38 Mitogen-activated protein kinase (p38MAPK), which is stimulated by the presence of ROS (Banerjee Mustafi et al., 2009). It is known that unfavorable effects of ROS are eliminated by enzymatic and nonenzymatic antioxidant defense mechanisms and free radical scavenging antioxidants, such as Vit E, Vit C, and Coenzyme Q10 (CoQ10) (Surai et al., 2019b). Such nutrients support the first level of antioxidant defense mechanisms and thereby reduce the need for the HSP response (Surai et al., 2019b). Therefore, downregulation of HSP70 and HSP90 in heat-stressed broilers fed Vit E/Se supplemented diet are likely due to the well-known antioxidant properties of these additives.
SOD and GPx are the first line of antioxidant defense system and play an important role in maintaining the oxidative stability of the host and cooperate to eliminate superoxide anions and hydrogen peroxide in cells (Gu et al., 2012). The expression of these antioxidant enzymes is regulated by mutable factors including cellular oxidative state and several antioxidant substances (Rodriguez et al., 2004) and also dietary nonenzymatic antioxidants (Surai et al., 2019a). Heat stress, being a promoter of oxidative stress (Emami et al., 2020), increased liver mRNA abundance of GPx (d 28 and d 35) and SOD (d 28). Such upregulations strongly correlate with the increase of HSPs mRNA abundance in the liver (Gu et al., 2012), as a strategy to eliminate excessive ROS caused by HS and to protect cells from oxidation. However, regardless of dietary Vit E/Se treatment, high temperature did not influence mRNA abundance of SOD on d 35. Even though increased SOD synthesis against oxidative stress has been evidenced under HS, extreme stress conditions cause a decrease in SOD activity (Surai et al., 2019b). Similarly, no noticeable change in SOD activity was observed in birds (Lin et al., 2006; Willemsen et al., 2011; Rimoldi et al., 2015) and rats (Morrison et al., 2005) exposed to HS. This observation is most likely an adaptation strategy against prolonged heat challenge as birds show differential expression patterns during acute and chronic HS conditions (Xie et al., 2015). Unexpectedly, there were no interaction or diet main effect on SOD and GPx mRNA levels except a downregulation of GPx in HS birds fed Vit E/Se supplemented diets on d 28. Unlike previous reports (Harsini et al., 2012; Kumbhar et al., 2018), additional Se, a component of the GPx, did not upregulate mRNA abundance of this enzyme in the current study. This outcome could be attributed to the ROS scavenging activity of Vit E (Harsij et al., 2020). Another potential explanation for the observed decrease in GPx mRNA abundance is that other antioxidant enzymes such as CAT might compensate the redox status (Ajuwon et al., 2015). Both GPx and CAT neutralize H2O2 to water and molecular oxygen, consequently completing the dismutation process initiated by SOD (Ajuwon et al., 2015; Ighodaro and Akinloye, 2018).
Cytokines are regulatory small proteins involved in immune cell development, inflammation, and immune responses (Hedger and Meinhardt, 2003). Hyperthermia influences the immune response and alters cytokine expression and consequently leads to changes in cell energy metabolism and reduced performance (Quinteiro-Filho et al., 2010; Siddiqui et al., 2020). Heat stress-related suppression of proinflammatory immune response and induction of anti-inflammatory cytokine production were evidenced in previous studies (Siddiqui et al., 2020; Yan et al., 2020; Emami et al., 2021; Greene et al., 2021). Similarly, HS significantly suppressed the pro-inflammatory cytokines TNFα, IFNγ and IL-17A in the jejunum, and IL-1β, TNFα and IFNγ in the liver. However, upregulation of the anti-inflammatory cytokines IL-4 and IL-10 was only noted in the jejunum of HS birds. Although IL-4 and IL-10 exert an anti-inflammatory role, the absence of IL-4 and IL-10 upregulation in the liver could have been dampened by elevated levels of other anti-inflammatory elements, such as transforming growth factor (TGF)-β and HSPs (Lee and Repasky, 2012). Chronic HS at 31 ± 1°C for 6 d upregulated TGF-β in cecal tonsils, while IL-10 expression remained unaltered in both spleen and cecal tonsils (Quinteiro-Filho et al., 2017). Further, elevated HSP70 during HS was accompanied with downregulated IL-6 and TNFα levels and upregulated IL-10 mRNA abundance in the small intestine of broilers (Siddiqui et al., 2020). Similarly, our data corroborate with the fact that HSP70 diminishes the TNFα, a pro-inflammatory cytokine, through the recruitment of heat shock transcription factor 1 (HSF-1) to the cytokine promoter (Ferat-Osorio et al., 2014). Suppressed inflammatory response during HS, possibly caused by increased HSF-1, exerts a competitive inhibition on nuclear factor kappa B (NF-κB) a regulator of proinflammatory cytokine production (Song et al., 2008). Therefore, modulated cytokine response in the jejunum and the liver of the HS broilers could be related to HSP upregulation as a regulatory function to protect cells (Ferat-Osorio et al., 2014) and prevent tissue damage (Lee and Repasky, 2012). Along with the HS induced rapid change in cytokine expression, dietary Vit E/Se showed additional modulation of the immune response by decreasing the mRNA levels of TLR2, IL-1β, IL-10, TNFα, iNOS, and IFNγ especially in the liver on d 35. Dietary Vit E and Se can act synergistically and influence antioxidant and immune responses during oxidative stress (Habibian et al., 2014; Dalia et al., 2018). In agreement with these findings, Khan et al. (2019) reported that dietary Se-enriched probiotics (containing viable Lactobacillus acidophilus and Saccharomyces cerevisiae) reduced liver IL-1β, TNFα, and IFNγ mRNA abundance through the inhibition of NF-κB in birds exposed to natural high temperature (ranges 30 to 39°C during daytime). Both pro- and anti-inflammatory cytokine responses in intestinal tissues were downregulated with increasing level of dietary Vit E under normal conditions (Pitargue et al., 2019). Vit E and Se, alone or combined, have anti-inflammatory characteristics, which lower pro-inflammatory cytokine expression through NF-κB (Sen and Packer, 1996; Duntas, 2009) and mitogen activated protein kinase (MAPK) pathways (Zhang et al., 2014). Similar results are also evidenced using several dietary antioxidants such as resveratrol (Sahin et al., 2012) and Vit C (Jang et al., 2014). Therefore, the observed cytokine modulation in the present study appears to be associated with antioxidant characteristics of Vit E/Se. Moreover, it can be speculated that the anti-inflammatory modulatory effect of Vit E/Se is more noticeable under prolonged stress conditions as we observed more pronounced cytokine mRNA modulation on d 35 compared to d 28.
mTOR is a serine/threonine kinase that plays a regulatory role in a variety of cellular functions including cell growth, differentiation, protein synthesis, and autophagy (Sarbassov et al., 2005; Fazolini et al., 2015; Du et al., 2019). We found that mTOR expression in liver tissues was significantly decreased in birds exposed to HS (d 28 and d 35) and birds fed Vit E/Se supplemented diet (only d 35). A recent study also reported that heat exposure suppressed mTOR activity in chickens (He et al., 2019). Potentially, reduction of mTOR is a protective mechanism by slowing down protein synthesis burden during HS (Sun et al., 2015). However, there are limited reports regarding the effects of Vit E/Se combination on mTOR activity in broilers. More recently, CoQ10 treatment significantly upregulated autophagy and suppressed the PI3K/Akt/mTOR pathway to protect primary chicken myocardial cells exposed to HS (Xu et al., 2019). Similarly, decrease in mTOR mRNA abundance was also reported in HS broilers fed Noni- (a plant source rich in a variety of phytochemicals and antioxidants) supplemented diets (Rajaei-Sharifabadi et al., 2017). Therefore, our findings suggest that additional Vit E/Se supplementation might decrease mRNA abundance of mTOR in the liver to mitigate HS-induced oxidative stress.
Heat stress-induced hypoxia disturbs the antioxidant balance and immune response that leads to epithelial damage and loss of barrier integrity (Lian et al., 2020). Tight junction proteins, which seal the paracellular space between adjacent epithelial cells, are highly responsive to HS. We herein, observed a main effect of HS on TJ proteins with a significant modulation during thermal challenge. In conjunction with our results, Varasteh et al. (2015) reported significant increase in E-cadherin, claudin-1, claudin-5, and ZO-1 protein expressions in the small intestine of broilers exposed to HS. Heat stress induced upregulation of HSF-1 increases the occludin protein expression by binding the occludin promoter and improves the participation of occludin in junctional complex (Dokladny et al., 2008). Therefore, it is possible that enhanced abundance of HSPs in this study might be related to the increased mRNA levels of TJ protein genes under HS conditions (Lian et al., 2020). Moreover, observed upregulation in TJs coincide with the IL-22 mRNA upregulation, which is a cytokine critical for maintenance of intestinal barrier function and tissue regeneration (Wang et al., 2017). Intestinal integrity-related effects of Vit E/Se are not broadly studied during a HS challenge. Contrary to our expectation, dietary Vit E/Se supplementation did not influence jejunal TJs mRNA abundance, likely related to the duration and severity of HS applied in the current study.
The intestinal microbiota play an important role in health and disease conditions of animals. Colonization, composition, growth, and diversity of this dynamic environment is influenced by many factors including diet and stress (Shi et al., 2019; Yang et al., 2020). Dietary antioxidants may regulate the microbiota composition and help to alleviate the adverse effects of several stressors on gut microbiota (Yang et al., 2020). LEfSe analysis revealed that, at the genus level, dietary Vit E/Se supplementation significantly enriched Lachnospiraceae FE2018 and Ruminococcaceae NK4A214 groups compared to control birds on d 35. Lachnospiraceae and Ruminococcaceae families are known to produce butyrate by fermenting polysaccharides and degrade resistant starch and non-starch polysaccharides (Yacoubi et al., 2018; Chen and Yu, 2020). Selenium nanoparticles (0.9 mg/kg) improved intestinal health by increasing abundance of Lactobacillus and Faecalibacterium, and butyric acid in broilers (Gangadoo et al., 2018). Under healthy conditions, intestinal lumen is strictly anoxic; however oxidative stress can change the oxygen gradient and shift the equilibrium of microbiota from anaerobic to facultative anaerobic groups (Dam et al., 2019). Such shift may decrease the butyrate producers and anaerobic fermenters of complex carbohydrates possibly leading to improper nutrient absorption and pathogen colonization (Million and Raoult, 2018; Dam et al., 2019). Butyrate is a preferred energy source for enterocytes and could enhance the epithelial regeneration; therefore, it can improve the host energy metabolism and performance (Calik and Ergun, 2015). Accordingly, along with their antioxidant effects on host metabolism, higher relative abundance of these two microbial groups might have contributed to the improved performance in HS birds fed Vit E/Se diets. Heat challenge could alter the composition of ileal and cecal microbiota (Wang et al., 2018; Shi et al., 2019). Our results showed that heat challenge had no significant effect on relative abundance of major phyla of ileal microbiota. However, at the family and genus levels, we identified distinctive features, which coincide with previous findings (Wang et al., 2018). We observed an increase in Clostridiales vadinBB60 group and Erysipelatoclostridium and a decrease in Porphyromonadaceae and Enterococcaceae in HS birds. This result aligns with the general opinion that HS change the gastrointestinal microbiota. In addition to changes in the ileal microbiota composition, the PICRUSt analysis revealed significant functional differences among the treatment groups. In birds subjected to HS, Vit E/Se supplementation downregulated the microbial genes associated with amino acid, retinol, carbohydrate and lipid metabolism along with ion-coupled transporters and replication recombination and repair proteins compared to control. Elevated levels of ROS have DNA-damaging effect which trigger several DNA repair pathways in a cell (Brierley and Martin, 2013). Therefore, the reduction in the replication recombination and repair proteins is likely due to the antioxidant features and ROS scavenger effects of Vit E and Se. This finding also coincides with the enriched selenocompound metabolism in Vit E/Se group. Although we are not certain about the enriched nutrient metabolism pathways in HS-control group compared to HS-Vit E/Se group, this might be related to the stress adaptation strategy to overcome growth limiting conditions (Tran and Bonilla, 2021). Unlike HS conditions, ileal microbiota of the birds fed Vit E/Se supplemented diets and raised under TN conditions showed significantly enriched microbial functions related to the membrane transporters and replication, recombination and repair proteins. Membrane transport is related to the survival of the bacteria in gastrointestinal environment (Lyons et al., 2017). Thus, adding 250 mg/kg Vit E and 1 mg/kg Se can improve the renewal of bacteria and maintain vigorous vitality by influencing functions related to replication and repair and membrane transport (Tian et al., 2020). However, the modes of action of Vit E/Se on predicted functions of ileal microbiota could be different between optimal and suboptimal thermal conditions. When examining the direct physiological effects of HS on predicted microbial functions regardless of dietary treatments (Figure 3), nutrient metabolism pathways such as carbohydrate, amino acid, peptidases, folate biosynthesis, and biotin were adversely affected by heat challenge, suggesting differences in metabolism between broilers in different temperature conditions. Further investigation regarding the use of natural antioxidants on intestinal microbiome and metabolome needed for better understanding of its effect on the host under different stress conditions.
Based on the presented findings, HS challenge significantly upregulated the mRNA abundance of HSPs, antioxidant enzymes, and TJ proteins and downregulated pro-inflammatory cytokines as well as suppressed the nutrient metabolism pathways of the ileal microbiota. Dietary supplementation of Vit E/Se helped to maintain the antioxidant balance especially by modulating mRNA abundance of antioxidant and immunity related genes in jejunal and liver tissues of the HS-challenged broilers. Ileal microbial profiling and functional analysis revealed taxonomic and functional signatures associated with dietary supplementation of Vit E/Se and HS challenge. In conclusion, the present study revealed that dietary supplementation of Vit E/Se mitigated the negative effects of HS by improving antioxidant status, regulating cytokine responses, and modulating ileal microbiota and its function.
ACKNOWLEDGMENTS
This work was supported, in part, by USDA-NIFA Hatch funds to the Virginia Agricultural Experiment Station, Virginia Tech.
DISCLOSURES
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101858.
Appendix. Supplementary materials
REFERENCES
- Ajuwon O.R., Marnewick J.L., Davids L.M. In: Pages 171-217 in Basic Principles and Clinical Significance of Oxidative Stress. Gowder S.J.T., editor. InTech; Rijeka, Croatia: 2015. Rooibos (Aspalathus linearis) and its major flavonoids—potential against oxidative stress-induced conditions. [Google Scholar]
- Apprill A., McNally S., Parsons R., Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 2015;75:129–137. [Google Scholar]
- Banerjee Mustafi S., Chakraborty P.K., Dey R.S., Raha S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ROS), p38MAPK, and Akt. Cell Stress Chaperones. 2009;14:579. doi: 10.1007/s12192-009-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley D.J., Martin S.A. Oxidative stress and the DNA mismatch repair pathway. Antioxid. Redox Signal. 2013;18:2420–2428. doi: 10.1089/ars.2012.4994. [DOI] [PubMed] [Google Scholar]
- Bruinsma J. FAO, 2009 Expert Meeting on How to Feed the World in 2050; Rome, Italy: 2009. The Resource Outlook to 2050: By How Much Do Land, Water and Crop Yields Need to Increase by 2050. 12-13 October 2009. [Google Scholar]
- Calik A., Ergun A. Effect of lactulose supplementation on growth performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2015;94:2173–2182. doi: 10.3382/ps/pev182. [DOI] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury V.S., Han G., Eltahan H.M., Haraguchi S., Gilbert E.R., Cline M.A., Cockrem J.F., Bungo T., Furuse M. Potential role of amino acids in the adaptation of chicks and market-age broilers to heat stress. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.610541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalia A.M., Loh T.C., Sazili A.Q., Jahromi M.F., Samsudin A.A. Effects of vitamin E, inorganic selenium, bacterial organic selenium, and their combinations on immunity response in broiler chickens. BMC Vet. Res. 2018;14:249. doi: 10.1186/s12917-018-1578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam B., Misra A., Banerjee S. In: Pages 43-82 in Oxidative Stress in Microbial Diseases. Chakraborti S., Chakraborti T., Chattopadhyay D., editors. Springer Nature; Singapore: 2019. Role of gut microbiota in combating oxidative stress. eds. [Google Scholar]
- Dokladny K., Ye D., Kennedy J.C., Moseley P.L., Ma T.Y. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am. J. Pathol. 2008;172:659–670. doi: 10.2353/ajpath.2008.070522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Zhang X., Zeng Y., Huang X., Chen H., Wang S., Wu J., Li Q., Zhu W., Li H., Liu T., Yu Q., Wu Y., Jie L. A novel phytochemical, DIM, inhibits proliferation, migration, invasion and TNF-alpha induced inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients by targeting MAPK and AKT/mTOR signal pathway. Front. Immunol. 2019;10:1620. doi: 10.3389/fimmu.2019.01620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duntas L.H. Selenium and inflammation: underlying anti-inflammatory mechanisms. Horm. Metab. Res. 2009;41:443–447. doi: 10.1055/s-0029-1220724. [DOI] [PubMed] [Google Scholar]
- Emami N.K., Greene E.S., Kogut M.H., Dridi S. Heat stress and feed restriction distinctly affect performance, carcass and meat yield, intestinal integrity, and inflammatory (chemo)cytokines in broiler chickens. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.707757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazolini N.P., Cruz A.L., Werneck M.B., Viola J.P., Maya-Monteiro C.M., Bozza P.T. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667–2676. doi: 10.1080/15384101.2015.1041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferat-Osorio E., Sánchez-Anaya A., Gutiérrez-Mendoza M., Boscó-Gárate I., Wong-Baeza I., Pastelin-Palacios R., Pedraza-Alva G., Bonifaz L.C., Cortés-Reynosa P., Pérez-Salazar E. Heat shock protein 70 down-regulates the production of toll-like receptor-induced pro-inflammatory cytokines by a heat shock factor-1/constitutive heat shock element-binding factor-dependent mechanism. J. Inflamm. 2014;11:1–12. doi: 10.1186/1476-9255-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flees J.J., Emami N.K., Greene E., Ganguly B., Dridi S. Phytogenic water additives improve broiler growth performance via modulation of intermediary metabolism-related signaling pathways. Animals. 2021;11:750. doi: 10.3390/ani11030750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadoo S., Dinev I., Chapman J., Hughes R.J., Van T.T.H., Moore R.J., Stanley D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl. Microbiol. Biotechnol. 2018;102:1455–1466. doi: 10.1007/s00253-017-8688-4. [DOI] [PubMed] [Google Scholar]
- Gorman A.M., Heavey B., Creagh E., Cotter T.G., Samali A. Antioxidant-mediated inhibition of the heat shock response leads to apoptosis. FEBS Lett. 1999;445:98–102. doi: 10.1016/s0014-5793(99)00094-0. [DOI] [PubMed] [Google Scholar]
- Greene E.S., Emami N.K., Dridi S. Research Note: Phytobiotics modulate the expression profile of circulating inflammasome and cyto(chemo)kine in whole blood of broilers exposed to cyclic heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.H., Hao Y., Wang X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012;91:790–799. doi: 10.3382/ps.2011-01628. [DOI] [PubMed] [Google Scholar]
- Habibian M., Ghazi S., Moeini M.M., Abdolmohammadi A. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int. J. Biometeorol. 2014;58:741–752. doi: 10.1007/s00484-013-0654-y. [DOI] [PubMed] [Google Scholar]
- Harsij M., Kanani H.G., Adineh H. Effects of antioxidant supplementation (nano‑selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquaculture. 2020;521 [Google Scholar]
- Harsini S.G., Habibiyan M., Moeini M.M., Abdolmohammadi A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012;148:322–330. doi: 10.1007/s12011-012-9374-0. [DOI] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedger M.P., Meinhardt A. Cytokines and the immune-testicular axis. J. Reprod. Immunol. 2003;58:1–26. doi: 10.1016/s0165-0378(02)00060-8. [DOI] [PubMed] [Google Scholar]
- Ighodaro O., Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018;54:287–293. [Google Scholar]
- Jang I.S., Ko Y.H., Moon Y.S., Sohn S.H. Effects of Vitamin C or E on the pro-inflammatory cytokines, heat shock protein 70 and antioxidant status in broiler chicks under summer conditions. Asian-Australas. J. Anim. Sci. 2014;27:749–756. doi: 10.5713/ajas.2013.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A.Z., Khan I.U., Khan S., Afzal S., Hamid M., Tariq M., Haq I.U., Ullah N., Khan M.A., Bilal S. Selenium-enriched probiotics improve hepatic protection by regulating pro-inflammatory cytokines and antioxidant capacity in broilers under heat stress conditions. J. Adv. Vet. 2019;6:355. doi: 10.5455/javar.2019.f354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumbhar S., Khan A.Z., Parveen F., Nizamani Z.A., Siyal F.A., El-Hack M.E.A., Gan F., Liu Y., Hamid M., Nido S.A., Huang K. Impacts of selenium and vitamin E supplementation on mRNA of heat shock proteins, selenoproteins and antioxidants in broilers exposed to high temperature. AMB Express. 2018;8:112. doi: 10.1186/s13568-018-0641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-T., Repasky E. Opposing roles for heat and heat shock proteins in macrophage functions during inflammation: a function of cell activation state? Front. Immunol. 2012;3:1–7. doi: 10.3389/fimmu.2012.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian P., Braber S., Garssen J., Wichers H.J., Folkerts G., Fink-Gremmels J., Varasteh S. Beyond heat stress: intestinal integrity disruption and mechanism-based intervention strategies. Nutrients. 2020;12:734. doi: 10.3390/nu12030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Liu L., Fu C., Yan M., Xie H., Li S., Yu Q., He S., He J. Resveratrol modulates intestinal morphology and HSP70/90, NF-kappaB and EGF expression in the jejunal mucosa of black-boned chickens on exposure to circular heat stress. Food Funct. 2016;7:1329–1338. doi: 10.1039/c5fo01338k. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyons P.P., Turnbull J.F., Dawson K.A., Crumlish M. Phylogenetic and functional characterization of the distal intestinal microbiome of rainbow trout Oncorhynchus mykiss from both farm and aquarium settings. J. Appl. Microbiol. 2017;122:347–363. doi: 10.1111/jam.13347. [DOI] [PubMed] [Google Scholar]
- Macelline S.P., Chrystal P.V., Liu S.Y., Selle P.H. The dynamic conversion of dietary protein and amino acids into chicken-meat protein. Animals. 2021;11:2288. doi: 10.3390/ani11082288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Million M., Raoult D. Linking gut redox to human microbiome. Hum. Microbiome J. 2018;10:27–32. [Google Scholar]
- Morrison J.P., Coleman M.C., Aunan E.S., Walsh S.A., Spitz D.R., Kregel K.C. Thiol supplementation in aged animals alters antioxidant enzyme activity after heat stress. J. Appl. Physiol. 2005;99:2271–2277. doi: 10.1152/japplphysiol.00412.2005. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Parada A.E., Needham D.M., Fuhrman J.A. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016;18:1403–1414. doi: 10.1111/1462-2920.13023. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Tyson G.W., Hugenholtz P., Beiko R.G. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitargue F.M., Kim J.H., Goo D., Delos Reyes J.B., Kil D.Y. Effect of vitamin E sources and inclusion levels in diets on growth performance, meat quality, alpha-tocopherol retention, and intestinal inflammatory cytokine expression in broiler chickens. Poult. Sci. 2019;98:4584–4594. doi: 10.3382/ps/pez149. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W., Ribeiro A., Ferraz-de-Paula V., Pinheiro M., Sakai M., Sá L., Ferreira A., Palermo-Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Calefi A.S., Cruz D.S.G., Aloia T.P.A., Zager A., Astolfi-Ferreira C.S., Piantino Ferreira J.A., Sharif S., Palermo-Neto J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017;186:19–28. doi: 10.1016/j.vetimm.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress- and metabolic-related genes in broilers exposed to acute heat stress. Front. Genet. 2017;8:192. doi: 10.3389/fgene.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S., Lasagna E., Sarti F.M., Marelli S.P., Cozzi M.C., Bernardini G., Terova G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene. 2015;6:17–25. doi: 10.1016/j.mgene.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez C., Mayo J.C., Sainz R.M., Antolin I., Herrera F., Martin V., Reiter R.J. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal. Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Akdemir F., Tuzcu M., Iben C., Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J. Anim. Physiol. Anim. Nutr. (Berl) 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- Sahin K., Sahin N., Kucuk O., Hayirli A., Prasad A.S. Role of dietary zinc in heat-stressed poultry: a review. Poult. Sci. 2009;88:2176–2183. doi: 10.3382/ps.2008-00560. [DOI] [PubMed] [Google Scholar]
- Santos R.R., Awati A., Roubos-van den Hil P.J., van Kempen T.A.T.G., Tersteeg-Zijderveld M.H.G., Koolmees P.A., Smits C., Fink-Gremmels J. Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathol. 2019;48:582–601. doi: 10.1080/03079457.2019.1648750. [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Sabatini D.M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen C.K., Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- Shi D., Bai L., Qu Q., Zhou S., Yang M., Guo S., Li Q., Liu C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019;98:2405–2413. doi: 10.3382/ps/pez026. [DOI] [PubMed] [Google Scholar]
- Siddiqui S.H., Kang D., Park J., Khan M., Shim K. Chronic heat stress regulates the relation between heat shock protein and immunity in broiler small intestine. Sci. Rep. 2020;10:1–11. doi: 10.1038/s41598-020-75885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sodhi S., Kaur R. Effects of dietary supplements of selenium, vitamin E or combinations of the two on antibody responses of broilers. Br. Poult. Sci. 2006;47:714–719. doi: 10.1080/00071660601040079. [DOI] [PubMed] [Google Scholar]
- Slimen I.B., Najar T., Ghram A., Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. (Berl) 2016;100:401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- Song M., Pinsky M.R., Kellum J.A. Heat shock factor 1 inhibits nuclear factor–κB nuclear binding activity during endotoxin tolerance and heat shock. J. Crit. Care. 2008;23:406–415. doi: 10.1016/j.jcrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Sun L., Lamont S.J., Cooksey A.M., McCarthy F., Tudor C.O., Vijay-Shanker K., DeRita R.M., Rothschild M., Ashwell C., Persia M.E., Schmidt C.J. Transcriptome response to heat stress in a chicken hepatocellular carcinoma cell line. Cell Stress Chaperones. 2015;20:939–950. doi: 10.1007/s12192-015-0621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F. Wageningen Academic Publishers; Wageningen, The Netherlands: 2018. Selenium in Poultry Nutrition and Health. [Google Scholar]
- Surai P.F., Kochish, II. Romanov M.N., Griffin D.K. Nutritional modulation of the antioxidant capacities in poultry: the case of vitamin E. Poult. Sci. 2019;98:4030–4041. doi: 10.3382/ps/pez072. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Tian Y., Li G., Chen L., Bu X., Shen J., Tao Z., Zeng T., Du X., Lu L. High-temperature exposure alters the community structure and functional features of the intestinal microbiota in Shaoxing ducks (Anas platyrhynchos) Poult. Sci. 2020;99:2662–2674. doi: 10.1016/j.psj.2019.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H.T., Bonilla C.Y. SigB-regulated antioxidant functions in gram-positive bacteria. World J. Microbiol. Biotechnol. 2021;37:38. doi: 10.1007/s11274-021-03004-7. [DOI] [PubMed] [Google Scholar]
- Vandana G.D., Sejian V., Lees A.M., Pragna P., Silpa M.V., Maloney S.K. Heat stress and poultry production: impact and amelioration. Int. J. Biometeorol. 2021;65:163–179. doi: 10.1007/s00484-020-02023-7. [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J., Feng J.H., Zhang M.H., Li X.M., Ma D.D., Chang S.S. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult. Sci. 2018;97:2153–2158. doi: 10.3382/ps/pey032. [DOI] [PubMed] [Google Scholar]
- Wang Y., Mumm J.B., Herbst R., Kolbeck R., Wang Y. IL-22 increases permeability of intestinal epithelial tight junctions by enhancing Claudin-2 expression. J. Immunol. 2017;199:3316–3325. doi: 10.4049/jimmunol.1700152. [DOI] [PubMed] [Google Scholar]
- Weber N., Liou D., Dommer J., MacMenamin P., Quiñones M., Misner I., Oler A.J., Wan J., Kim L., McCarthy M.Coakley. Nephele: a cloud platform for simplified, standardized and reproducible microbiome data analysis. Bioinformatics. 2018;34:1411–1413. doi: 10.1093/bioinformatics/btx617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley D., Goldberg S.P., Jordan W.D. Heat shock proteins: a review of the molecular chaperones. J. Vasc. Surg. 1999;29:748–751. doi: 10.1016/s0741-5214(99)70329-0. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Swennen Q., Everaert N., Geraert P.A., Mercier Y., Stinckens A., Decuypere E., Buyse J. Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011;90:2311–2320. doi: 10.3382/ps.2011-01353. [DOI] [PubMed] [Google Scholar]
- Xie J., Tang L., Lu L., Zhang L., Lin X., Liu H.-C., Odle J., Luo X. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015;94:1635–1644. doi: 10.3382/ps/pev105. [DOI] [PubMed] [Google Scholar]
- Xu J., Huang B., Tang S., Sun J., Bao E. Co-enzyme Q10 protects primary chicken myocardial cells from heat stress by upregulating autophagy and suppressing the PI3K/AKT/mTOR pathway. Cell Stress Chaperones. 2019;24:1067–1078. doi: 10.1007/s12192-019-01029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoubi N., Saulnier L., Bonnin E., Devillard E., Eeckhaut V., Rhayat L., Ducatelle R., Van Immerseel F. Short-chain arabinoxylans prepared from enzymatically treated wheat grain exert prebiotic effects during the broiler starter period. Poult. Sci. 2018;97:412–424. doi: 10.3382/ps/pex297. [DOI] [PubMed] [Google Scholar]
- Yan F.F., Wang W.C., Cheng H.W. Bacillus subtilis-based probiotic promotes bone growth by inhibition of inflammation in broilers subjected to cyclic heating episodes. Poult. Sci. 2020;99:5252–5260. doi: 10.1016/j.psj.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Liang Q., Balakrishnan B., Belobrajdic D.P., Feng Q.J., Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. doi: 10.3390/nu12020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhang R., Wang T., Jiang H., Guo M., Zhou E., Sun Y., Yang Z., Xu S., Cao Y. Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-κB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation. 2014;37:478–485. doi: 10.1007/s10753-013-9761-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.