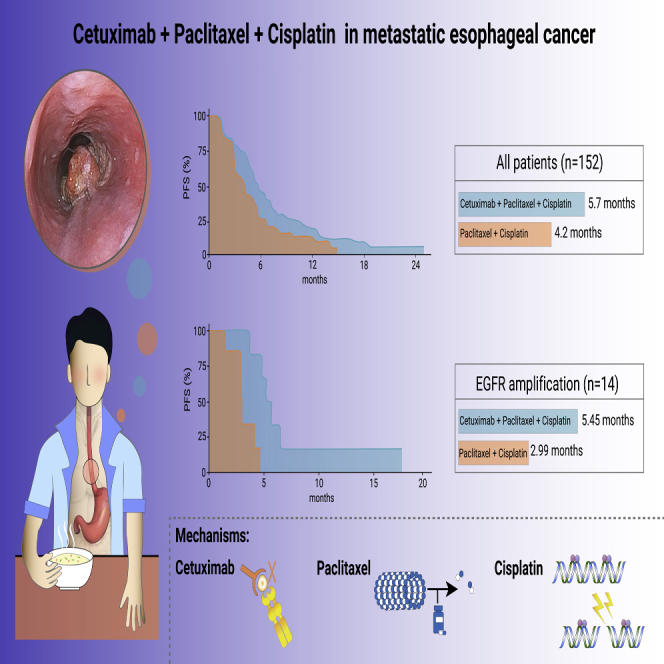
Abstract
Lack of effective targeted therapy in metastatic esophageal squamous cell carcinoma (ESCC) underscores the urgent need for identifying new treatment approaches for this challenging disease. We sought to assess the addition of cetuximab to paclitaxel-cisplatin chemotherapy for first-line treatment in patients with metastatic ESCC. In this randomized, multicenter, open-label, phase II clinical trial, patients were randomized to receive paclitaxel-cisplatin (TP) (paclitaxel [175 mg/m2 intravenously (i.v.) on day 1 of every 3-week cycle] and cisplatin [75 mg/m2 i.v. on day 1 of every 3-week cycle]) and TP plus cetuximab (CTP) (cetuximab, 400 mg/m2 i.v. on day 1 of week 1, followed by 250 mg/m2 weekly), respectively. Targeted next-generation sequencing (NGS) was performed on 89 tumor samples for biomarker exploration. The primary endpoint was progression-free survival (PFS) in the intention-to-treat population. With a median follow-up of 22.6 months, median PFS was 5.7 months (95% confidence interval [CI]: 4.8–7.0) in patients administered CTP versus 4.2 months (95% CI: 3.0–5.3) in the TP group (hazard ratio [HR] = 0.61; 95% CI: 0.40–0.93; p = 0.02). Median overall survival was 11.5 months (95% CI: 7.9–13.1) in the CTP group and 10.5 months (95% CI: 9.0–13.2) in the TP arm (HR = 0.98; 95% CI: 0.67–1.44; p = 0.91). The most common reported greater than or equal to grade 3 adverse events were neutropenia (35.2% versus 22.4%) and leukopenia (25.4% versus 13.2%). In patients with epidermal growth factor receptor (EGFR) amplification tumors (15.7%), PFS was improved with CTP compared with TP treatment (HR = 0.11; 95% CI: 0.01–0.98; p = 0.018). First-line CTP significantly improves PFS, with a manageable safety profile in patients with metastatic ESCC.
Keywords: esophageal squamous cell carcinoma, cetuximab, EGFR, chemotherapy
Graphical abstract
Public summary
-
•
Compare the effect of Cetuximab + chemotherapy with chemotherapy alone in ESCC
-
•
CTP regimen improves progression-free survival with a manageable safety profile
-
•
ESCC patients with EGFR amplification obtain greater therapeutic benefit from CTP
-
•
CTP regimen represents a new treatment option for ESCC
Introduction
Esophageal cancer (EC) is the seventh most common malignancy and the sixth leading cause of cancer deaths worldwide.1 Importantly, more than 50% of global EC cases occur in China, with most of them diagnosed as an advanced stage.2 Platin-based chemotherapy is the most commonly used first-line regimen for advanced esophageal squamous cell carcinoma (ESCC), with a reported median progression-free survival (PFS) of 3.6–6.0 months and a median overall survival (OS) of approximately 10 months.3, 4, 5, 6, 7 These data underscore the need for identifying new treatment approaches for this disease.
It has been reported that epidermal growth factor receptor (EGFR) overexpression is frequently observed in ESCC, with an incidence of 50%–70%,7, 8, 9 suggesting EGFR as a potential therapeutic target in ESCC. Previously, a randomized trial assessing first-line treatments for metastatic ESCC demonstrated that patients administered cetuximab plus 5-fluorouracil (5-FU) and cisplatin have a trend of improved PFS and OS compared with the chemotherapy alone group.7 However, the paclitaxel and cisplatin (TP) regimen is another active treatment option, which is widely used in China.4,10 Whether addition of cetuximab to the TP regimen (CTP) could be applied to first-line treatment in ESCC remains to be elucidated. Therefore, we performed a randomized phase II study to assess the clinical efficacy and safety of cetuximab added to chemotherapy for first-line treatment in metastatic ESCC.
Results
Patient characteristics
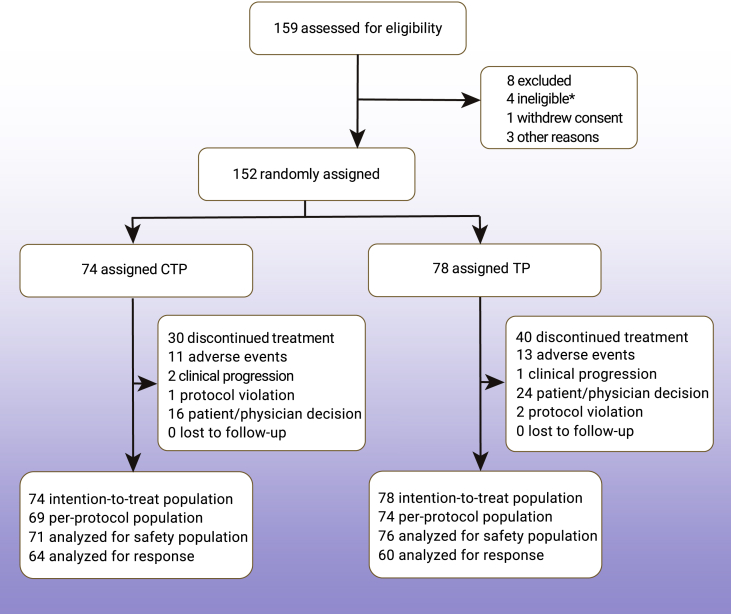
Between April 10, 2017 and Oct 17, 2018, a total of 159 patients with metastatic ESCC in 14 study centers were screened for participation, of whom 152 patients were randomly assigned to the CTP (n = 74) and TP (n = 78) arms (Figure 1). Finally, PFS, OS, time to progression (TTP), objective response rate (ORR), and disease control rate (DCR) were analyzed in 152 patients. Safety profile was assessed in 147 of the 152 patients. Demographic and baseline characteristics are listed in Table 1.
Figure 1.
CONSORT diagram of patient selection
Asterisk indicates a major deviation occurred, because one patient was still randomized.
Table 1.
Baseline characteristics
| Cetuximab + TP (n = 74) | TP (n = 78) | |
|---|---|---|
| Age (years) | ||
| Median (range) | 61 (44–78) | 60.5 (40–76) |
| Sex | ||
| Male | 62 (83.8%) | 69 (88.5%) |
| Female | 12 (16.2%) | 9 (11.5%) |
| ECOG | ||
| 0 | 33 (44.6%) | 33 (42.3%) |
| 1 | 41 (55.4%) | 45 (57.7%) |
| Number of metastases | ||
| 1 | 16 (21.6%) | 3 (28.2%) |
| ≥2 | 58 (78.4%) | 5 (71.8%) |
| Tumor location | ||
| Upper | 9 (12.2%) | 7 (9.0%) |
| Middle | 36 (48.6%) | 31 (39.7%) |
| Lower | 23 (31.1%) | 35 (44.9%) |
| NA | 6 (8.1%) | 5 (6.4%) |
| Previous surgery | ||
| Yes | 27 (36.5%) | 27 (34.6%) |
| No | 47 (63.5%) | 51 (65.4%) |
| Previous radiotherapy | ||
| Yes | 17 (23.0%) | 21 (26.9%) |
| No | 57 (77.0%) | 57 (73.1%) |
At the cutoff date on April 21, 2020, a total of 113 (74%) patients had died, and no patient was lost to follow-up. The median follow-up time was 22.6 months (95% confidence interval [CI]: 21.5–25.6).
Efficacy
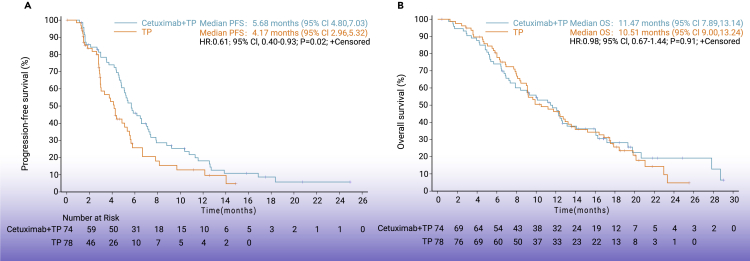
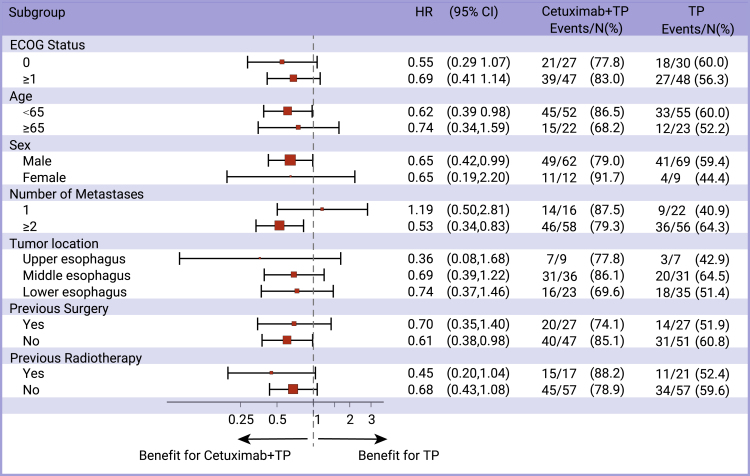
In the intention to treat (ITT) population (n = 152), median PFS was 5.7 months (95% CI: 4.8–7.0) in the CTP arm versus 4.2 months (95% CI: 3.0–5.3) in the TP arm (hazard ratio [HR] = 0.61; 95% CI: 0.40–0.93; p = 0.02; Figure 2A). In most of the predefined subgroups, PFS was improved in the CTP group versus TP-treated patients, particularly in men, patients with more than one metastasis, and below 65 years old (Figure 3).
Figure 2.
Survival outcomes
(A) Kaplan-Meier plot of overall survival.
(B) Kaplan-Meier plot of progression-free survival.
Figure 3.
Forest plot for subgroup analyses of progression-free survival
Median TTP was 6.6 months (95% CI: 5.2–7.9) in patients administered CTP versus 4.3 months (95% CI: 3.0–5.5) in the TP group (HR = 0.58; 95% CI: 0.37–0.91; p = 0.02; Figure S1). Median OS was 11.5 months (95% CI: 7.9–13.1) in patients administered CTP versus 10.5 months (95% CI: 9.0–13.2) in the TP group (HR = 0.98; 95% CI: 0.67–1.44; p = 0.91; Figure 2B).
The ORR data were based on a subset of 64 of the 74 patients in the CTP arm and 60 of the 78 patients administered TP. In terms of overall response, 43 patients (58.1%; 95% CI: 46.9–69.3) showed response in the CTP arm versus 36 patients (46.2%; 95% CI: 35.1–57.3) in the TP arm (Table 2). The DCR was 78.4% (95% CI: 69.0–87.8) in patients administered CTP versus 69.2% in the TP arm (95% CI: 59.0–79.4).
Table 2.
Best overall responses by RECIST
| Cetuximab + TP (n = 74) | TP (n = 78) | |
|---|---|---|
| Best overall response | ||
| Complete response | 0 | 0 |
| Partial response | 43 (58.1%) | 36 (46.2%) |
| Stable disease | 15 (20.3%) | 18 (23.1) |
| Progressive disease | 6 (8.1%) | 6 (7.7%) |
| Not assessable | 10 (13.5%) | 18 (23.1%) |
| Objective response | 43 (58.1%; 95% CI: 46.9–69.3) | 36 (46.2%; 95% CI: 35.1–57.3) |
| Disease controlled | 58 (78.4%; 95% CI: 69.0–87.8) | 54 (69.2%; 95% CI: 59.0–79.4) |
Safety
Adverse events are summarized in Table 3. The incidence rates of all grades adverse events (AEs) were 94.4% (67/71) in the CTP arm and 73.7% (56/76) in the TP arm. The most commonly reported AEs were anemia (52.1% of patients administered CTP versus 51.3% in the TP group), neutropenia (52.1% versus 39.5%) and leukopenia (52.1% versus 40.8%). AEs of grade 3 or worse occurred in 39 (54.9%) of 71 patients in the CTP arm and 24 (31.6%) of 76 cases in the TP arm. The most frequently reported AEs greater than or equal to grade 3 were neutropenia (35.2% versus 22.4%) and leukopenia (25.4% versus 13.2%). In addition, rash occurred in 19/71 (26.8%) patients administered CTP versus 2/76 (2.6%) in the TP arm. No patients experienced greater than or equal to grade 3 rash in either arm. No fatal event related to cetuximab was documented.
Table 3.
Adverse events
| Cetuximab + TP (n = 71) |
TP (n = 76) |
|||
|---|---|---|---|---|
| All grades n (%) | Grades 3–5 n (%) | All grades n (%) | Grades 3–5 n (%) | |
| Any | 67 (94.4) | 39 (54.9) | 56 (73.7) | 24 (31.6) |
| Neutropenia | 37 (52.1) | 25 (35.2) | 30 (39.5) | 17 (22.4) |
| Anemia | 37 (52.1) | 4 (5.6) | 39 (51.3) | 1 (1.3) |
| Leukopenia | 37 (52.1) | 18 (25.4) | 31 (40.8) | 10 (13.2) |
| Thrombocytopenia | 10 (14.1) | 3 (4.2) | 1 (1.3) | 0 |
| Constipation | 4 (5.6) | 0 | 3 (3.9) | 0 |
| Diarrhea | 7 (9.9) | 0 | 2 (2.6) | 0 |
| Mouth ulceration | 5 (7.0) | 0 | 0 | 0 |
| Nausea | 10 (14.1) | 1 (1.4) | 3 (3.9) | 1 (1.3) |
| Vomiting | 9 (12.7) | 4 (5.6) | 3 (3.9) | 0 |
| Alanine aminotransferase increased | 12 (16.9) | 2 (2.8) | 4 (5.3) | 0 |
| Aspartate aminotransferase increased | 7 (9.9) | 1 (1.4) | 3 (3.9) | 0 |
| Hypoesthesia | 8 (11.3) | 0 | 6 (7.9) | 1 (1.3) |
| Rash | 19 (26.8) | 0 | 2 (2.6) | 0 |
Biomarker exploration
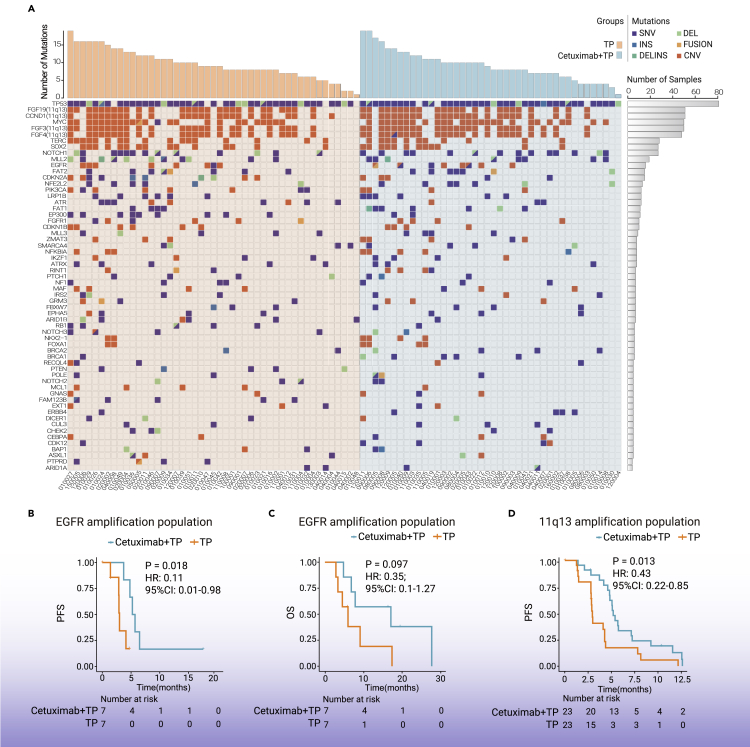
To identify potential biomarkers associated with treatment benefit, a total of 89 baseline tumor samples were profiled by targeted next-generation sequencing. In samples from 42 patients in CTP group and 47 patients from TP group, gene mutations or amplification were successfully detected in at least one of the analyzed exons. The genetic landscape presented in our study was robustly consistent with previous reports,11, 12, 13 demonstrating the high frequency of mutations in TP53, NOTCH1, and amplifications in Myc and CCND1 (Figure 4A; Table S1). Notably, in EGFR amplification patients (n = 14; 15.7%), PFS benefit was observed in patients who received CTP compared with those who received TP (5.45 versus 2.99 months; HR = 0.11; 95% CI: 0.01–0.98; p = 0.018; Figure 4B). A similar trend of improved OS was also associated with CTP treatment in EGFR-amplified cases (17.18 versus 6.01 months; HR = 0.35; 95% CI: 0.1–1.27; p = 0.097; Figure 4C). Response rate was higher in CTP group than TP group for EGFR amplification patients, but difference did not reach the statistical significance (Figure S2).
Figure 4.
Biomarker analysis
(A) Genetic landscape of 89 patients with sufficient pretreatment tumor material for targeted NGS.
(B and C) Kaplan-Meier curves of progression-free survival (B) and overall survival (C) for patients with EGFR amplification.
(D) Kaplan-Meier curves of progression-free survival in patients with 11q13 amplification.
We also identified PFS was improved in 11q13-amplified patients who received CTP compared with those who received TP (5.19 versus 2.96 months; HR = 0.43; 95% CI: 0.22–0.85; p = 0.013; Figure 4D), but OS analysis did not reach significance in this subset of patients (Figure S3). The processed data are displayed in Table S2, and the analysis of the other genetic biomarkers are shown in Figure S4.
Discussion
This is a randomized trial comparing the efficacy and safety of CTP and TP alone as first-line treatment in patients with metastatic ESCC. The results showed that addition of cetuximab significantly improved PFS. Furthermore, this combinational regimen showed an acceptable toxicity profile. Jointly, these findings formed the basis for further phase III trials evaluating the CTP regimen as first-line treatment in metastatic ESCC.
Anti-EGFR treatment has been previously investigated in combination with chemotherapy regimens in metastatic ESCC, but no significant benefit was associated with the addition of cetuximab or panitumumab, as reported in the AIO and POWER studies.6,7 The present study provides the first proof of concept that cetuximab addition to chemotherapy resulted in increased PFS in metastatic ESCC. Two important factors might contribute to this difference. The first parameter is the selected chemotherapy backbone. Lorenzen et al. employed the cisplatin and 5-FU regimen as the chemotherapy backbone,6,7 while this study used the cisplatin and paclitaxel regimen instead. There are strong biological and mechanistic rationales for this combination, because addition of cetuximab to paclitaxel and cisplatin may have highly synergistic activities due to non-overlapping cell-killing mechanisms.14 Specifically, cetuximab inhibits the cell cycle and induces pro-apoptotic molecules,15 paclitaxel promotes mitosis arrest,16 and platinum triggers the formation of DNA adducts,17 all leading to apoptosis. In addition, docetaxel has been previously shown to induce immunogenic cell death in cancer cells and elicit various immunogenic actions in the tumor microenvironment,18, 19, 20, 21 which may exacerbate cetuximab’s immunostimulatory effects.14 Beyond these preclinical observations, several phase III clinical trials have evaluated the added benefit of combining immunotherapy and chemotherapy in the first-line setting for ESCC patients. According to recently released results,22, 23, 24, 25, 26 chemoimmunotherapy combinations using TP regimen appear to confer better survival than using 5-FU and cisplatin regimen, indicating that TP regimen could generate a more favorable tumor microenvironment to maximize the immunotherapy or targeted therapy efficacy and might be more suitable for combination. Notably, evidence from clinical studies of head and neck squamous cell carcinoma also supported the combination of cetuximab and the TP regimen, reporting a slightly longer time to treatment failure (TTF) and improved safety profile with a taxane versus 5-FU in combination with anti-EGFR antibody and platinum.27, 28, 29, 30 Together with available evidence, the present study suggests that cetuximab combined with the cisplatin and paclitaxel regimen may be preferable to the 5-FU and cisplatin regimen. The second potential factor is the heterogeneity in different races. Specifically, the AIO and POWER trial was conducted in Germany alone, whereas this study was based on the Chinese population. ESCC is a highly heterogeneous disease with a distinct molecular basis among races.12,31 Therefore, it is plausible that racial factors may account for the different results of this study versus previous reports.
Although cetuximab added to the TP regimen chemotherapy failed to improve the median OS in patients with metastatic ESCC, we did observe a “tail” on the OS curve in CTP group, suggesting a subset of patients would derive long-term benefit from CTP regimen. Based on the previous preclinical and clinical reports,32, 33, 34, 35, 36 we hypothesized that those patients whose tumors were driven by EGFR signaling would accordingly benefit from anti-EGFR treatment. Notably, our biomarker program demonstrated the patients with EGFR amplification derive greater therapeutic benefit from CTP treatment, further providing insights of EGFR signaling alterations in guiding the selection of ESCC patients treated by EGFR-directed monoclonal antibodies (mAbs).36 This is very similar to the results observed in lung squamous cell carcinoma,37,38 which also found that EGFR-directed mAbs in combination with chemotherapy are associated with greater clinical benefits in selected patients with high EGFR expression and/or increased EGFR gene copy number. It provided an important basis for future large-scale study accessing EGFR amplification as a biomarker to select advanced ESCC patients, who would benefit from CTP treatment. In addition, we also found patients with 11q13 amplification obtain more PFS benefit when treated with CTP, indicating that ESCC with aberrations in cell cycle pathway may also be susceptible to EGFR-directed mAbs. Previous studies found that 11q13 amplification is associated with poor prognosis in ESCC.11 Our results suggest that CTP treatment strategy may represent an alternative treatment for this subset patients, which should be validated in the future.
Recently, programmed cell death-1 (PD-1) blockade has emerged as a standard second-line treatment option for metastatic ESCC.39, 40, 41 Moreover, the recently reported results of the KEYNOTE-590 study (pembrolizumab combined with chemotherapy versus chemotherapy alone) in the first-line setting are really promising and could revolutionize the treatment algorithm for metastatic ESCC.24 However, the survival benefit of immunotherapy is correlated with PD-L1 expression status, with only a subset of patients possibly deriving long-term survival benefit from these immunotherapeutic treatment options.24,41 Notably, it was observed that PD-L1 expression either on tumor-infiltrating immune cells or tumor cells is negatively associated with EGFR expression in ESCC.42 Meanwhile, EGFR is considered a target of anti-EGFR antibodies.34,35 Therefore, the patients who could not benefit form PD-1 and PD-L1 blockade treatment would probably be suitable for anti-EGFR antibodies. Moreover, pembrolizumab combined with cetuximab has yielded a promising efficacy in recurrent and metastatic head and neck squamous cell carcinoma.43 It has also been reported that EGFR is a potential drug target for combinatorial immunotherapy with strong scientific rationale.44 These findings provided a novel implication that targeting EGFR in combination with immune checkpoint inhibitor may bring more survival benefit in patients with ESCC.
In this study, the safety profile showed that grade 3–5 AEs seem higher in the CTP arm than TP arm (54.9% versus 31.6%), which was similar with previous reports, including ESCC and colorectal cancer.7,45 Specifically, dermatologic toxicity, such as rash, was the most frequently observed AE after addition of cetuximab, with 38% of patients experiencing grade 1/2 and no grade 3–5 dermatologic AEs. However, 28 (40%) patients in CTP group and 27 (36%) patients in TP group have taken the administration with the maximum six courses, suggesting the toxicities of CTP did not delay the course of treatment and the CTP treatment had manageable safety profiles. Compared with the 5-FU and cisplatin chemotherapy backbone,6,7 the paclitaxel-based combination had low rates of blood system and gastrointestinal disorders. This was generally consistent with our historical data, which might be associated with pretreatment with glucocorticoids while using paclitaxel and our highly experienced management of adverse events.10,46 These findings further support the favorable safety profile of paclitaxel-based regimens for combination with cetuximab.
This study had some limitations. First, the sample size was relatively small, and the study was not powered enough to detect a difference in OS. A large, randomized phase III trial is warranted to further confirm these results. Secondly, although our post hoc analysis identified a fraction of patients with EGFR or 11q13 amplification may derive more PFS benefit from CTP than TP, the observation needs to be further validated in the future prospective study with a large subset of this specific population.
In conclusion, the combination of cetuximab with the TP regimen is safe and effective, with significantly improved PFS, as first-line treatment in metastatic ESCC. A randomized, biomarker-driven, phase III study is warranted for further confirming the efficacy of this combination in ESCC patients.
Materials and methods
Study design and patients
This was an open-label, randomized, multicenter phase II trial (NCT03126708) evaluating the efficacy and safety of CTP versus TP alone for the first-line treatment of Chinese patients with metastatic ESCC.
Inclusion criteria were histologically confirmed squamous cell carcinoma of the esophagus, 18 years of age or older, metastatic ESCC not suitable for local-regional treatment, no (neo)adjuvant chemotherapy within 6 months of study entry or prior chemotherapy for metastatic disease, at least one measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and adequate organ function. Patients with prior EGFR-targeted therapy were excluded.
This study was approved by the medical ethics committee of Peking University Cancer Hospital (2016YJZ47-ZY01). The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent before enrollment.
Randomization and treatment
Randomization was performed by the stratified block randomization method, according to previous treatment (previous surgery or radiotherapy versus no previous treatment), ECOG performance status (0 versus 1), and the number of metastatic sites (one versus greater than or equal to two affected organs). Patients were randomly assigned (1:1) to the TP (paclitaxel [175 mg/m2 i.v. on day 1 of every 3-week cycle] and cisplatin [75 mg/m2 i.v. on day 1 of every 3-week cycle]) and CTP (Erbitux; Merck KGaA, Darmstadt, Germany) arms. Cetuximab was administered at a dose of 400 mg/m2 (i.v. on day 1 of week 1), followed by 250 mg/m2 weekly. Cisplatin was replaced by carboplatin (area under the plasma concentration-time curve [AUC] 5) in case of intolerance. All patients received a maximum of six cycles of chemotherapy. After six cycles of treatment, the patients in the CTP arm who had clinical benefits continued treatment with cetuximab as monotherapy. Tumor assessment was performed by computed tomography (CT) or magnetic resonance imaging (MRI) at baseline and every 6 weeks. Treatment was continued until disease progression (defined according to RECIST version 1.1), unacceptable toxicity, patient withdrawal, or investigator decision, whichever occurred first. Further details regarding study design, procedures, and assessment are summarized in the supplement.
Outcomes
The primary endpoint was PFS, defined as the time from randomization to radiological disease progression or death from any cause. The secondary endpoints were OS, TTP, ORR, DCR, and safety profile. OS was defined as the time from randomization to death from any cause. TTP was defined from the date of randomization until the first confirmed evidence of disease progression, death due to progressive disease, or censoring. Tumor response was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to RECIST version 1.1. ORR cases were defined as patients with PR or CR as the best overall response. The DCR was defined as the number of patients whose best response was CR, PR, or SD, divided by the number of patients belonging to the trial set of interest. Adverse events were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Samples collection and targeted DNA sequencing
Baseline formalin-fixed, paraffin-embedded (FFPE) tissue samples and matched peripheral blood mononuclear cells (PBMCs) were collected retrospectively. All patients provided written informed consent for the biomarker analysis of their tissue specimens.
Genomic DNA (gDNA) from FFPE and paired PBMC (germline) samples were isolated by using the Maxwell 16 FFPE Plus Lev DNA Purification Kit according to the manufacturer’s instructions. Before library construction, gDNA was sheared to 200- to 250-bp fragments with a Covaris LE220 ultrasonicator. Libraries were prepared using the KAPA Library Preparation Kit (Kapa Biosystems, Wilmington, MA, USA). A 1,021-gene panel with potential clinical relevance was used to capture target regions.47 DNA sequencing was carried out with paired-end reads on the DNBSEQ-T7 sequencing system.
Sequencing data processing and mutation calling
Terminal adaptor sequences and low-quality reads were removed separately from raw data of paired samples using realSeq (version 3.1.0; in house) and NCfilter (version 2.0.0; in house). Burrows-Wheeler Aligner (BWA) (version 0.7.15-r1140) tool was used to align clean reads to the reference human genome (hg19). Duplicate reads of cancer sample derived from PCR amplification were marked using realSeq, which was designed to retain reads containing rare events by treating unique molecular indices, and the normal sample was marked using Picard tools (version 2.6.0).
Single-nucleotide variants (SNV) and indels were detected by comparing tumor-normal pairs using TNSCOPE (version 201,808) and RealDcaller (version 1.8.1; in house), a software developed in house to review hotspot variants, and the results of these analyses were merged using NChot (version 2.7.2; in house) and then annotated to multiple public databases using NCanno (version 1.14; in house). For somatic copy-number alteration, an in-house software CNVKIT (version 0.9.2) was performed, and the matched peripheral blood cell samples served as matched controls. Significant copy number variations were calculated as the ratio of adjusted depth between case gDNA and control gDNA. An in-house algorithm NCSV (0.2.3; in house) was used to identify split-read and discordant read-pair to identify structural variants (SVs). All candidate variants were manually verified with the integrative genomics viewer browser.48
The WES-FASTQ files data were deposited at Genome Sequence Archive, https://ngdc.cncb.ac.cn/gsa-human/browse/HRA001904 (BioProject: PRJCA007995; accession GSA: HRA001904). The raw sequence data will be available via controlled access by reasonable request.
Statistical analysis
This study was designed to have an 80% power with a two-sided type I error rate of 0.2 to detect a median PFS HR of 0.66 in favor of CTP. Concurrent with the randomization ratio (1:1) and a predicted dropout rate of 1% per month, the required number of patients was 150.
Survival and efficacy analyses were performed in the ITT population. All patients who received one or more doses of treatment were included in safety analyses. Baseline characteristics were presented as mean and standard deviation (SD) for continuous variables or number and percentage for categorical variables. Differences between study groups in baseline characteristics were assessed by two-sample t test for continuous variables and the Fisher exact test for categorical ones. PFS, OS, and TTP were estimated by the Kaplan-Meier method. Comparisons between groups in PFS, OS, and TTP were assessed by two-sided stratified log rank test. HRs and their associated 95% CIs were calculated using Cox proportional hazards models, adjusted for stratification factors. The corresponding 95% CIs of ORRs and DCRs were calculated by the Clopper-Pearson method. Statistical analyses were carried out with SAS 9.4. Two-sided p < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by the Merck KGaA (Darmstadt, Germany), the Clinical Medicine Plus X - Young Scholars Project of Peking University (PKU2020LCXQ008), the Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (no. XXT19), and Beijing Hospitals Authority Youth Programme (QML20191102).
Author contributions
Z.L., Y.Z., and Q.F. contributed equally to this work. L.S. designed the study. Y.P., D.J., P.L., J.Z., X.Y., J.F., S.Y., W.Y., L.Z., Y.X., and J.L. provided study material or patients. Z.L., Y.Z., Q.F., and L.S. contributed to the data collection, analysis, and interpretation. All authors contributed to editing the text and discussed the scientific questions.
Declaration of interests
The authors declare no competing interests.
Published Online: April 4, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xinn.2022.100239.
Lead contact website
Supplemental information
References
- 1.Bray F., Ferlay J., Soerjomataram I., et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.He Y., Li D., Shan B., et al. Incidence and mortality of esophagus cancer in China, 2008-2012. Chin. J. Cancer Res. 2019;31:426–434. doi: 10.21147/j.issn.1000-9604.2019.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin T.J., An G.L., Zhao X.H., et al. Combined treatment of oxaliplatin and capecitabine in patients with metastatic esophageal squamous cell cancer. World J. Gastroenterol. 2009;15:871–876. doi: 10.3748/wjg.15.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao W., Xu C., Lou G., et al. A phase II study of paclitaxel and nedaplatin as first-line chemotherapy in patients with advanced esophageal cancer. Jpn. J. Clin. Oncol. 2009;39:582–587. doi: 10.1093/jjco/hyp058. [DOI] [PubMed] [Google Scholar]
- 5.Wang F.H., Wang Y., Sun G.P., et al. Efficacy and safety of recombinant human lymphotoxin-α derivative with cisplatin and fluorouracil in patients with metastatic esophageal squamous cell carcinoma: a randomized, multicenter, open-label, controlled, phase 2b trial. Cancer. 2017;123:3986–3994. doi: 10.1002/cncr.30845. [DOI] [PubMed] [Google Scholar]
- 6.Moehler M., Maderer A., Thuss-Patience P.C., et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER) Ann. Oncol. 2020;31:228–235. doi: 10.1016/j.annonc.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzen S., Schuster T., Porschen R., et al. Cetuximab plus cisplatin-5-fluorouracil versus cisplatin-5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann. Oncol. 2009;20:1667–1673. doi: 10.1093/annonc/mdp069. [DOI] [PubMed] [Google Scholar]
- 8.Gibault L., Metges J.P., Conan-Charlet V., et al. Diffuse EGFR staining is associated with reduced overall survival in locally advanced oesophageal squamous cell cancer. Br. J. Cancer. 2005;93:107–115. doi: 10.1038/sj.bjc.6602625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanawa M., Suzuki S., Dobashi Y., et al. EGFR protein overexpression and gene amplification in squamous cell carcinomas of the esophagus. Int. J. Cancer. 2006;118:1173–1180. doi: 10.1002/ijc.21454. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X., Shen L., Li J., et al. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am. J. Clin. Oncol. 2008;31:29–33. doi: 10.1097/COC.0b013e3181131ca9. [DOI] [PubMed] [Google Scholar]
- 11.Cui Y., Chen H., Xi R., et al. Whole-genome sequencing of 508 patients identifies key molecular features associated with poor prognosis in esophageal squamous cell carcinoma. Cell Res. 2020;30:902–913. doi: 10.1038/s41422-020-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research, Analysis Working Group. Asan U., Agency B.C.C., et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin D.C., Hao J.J., Nagata Y., et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat. Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guigay J., Tahara M., Licitra L., et al. The evolving role of taxanes in combination with cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: evidence, advantages, and future directions. Front. Oncol. 2019;9:668. doi: 10.3389/fonc.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotz B., Keilholz U., Fusi A., et al. In vitro and in vivo antitumor activity of cetuximab in human gastric cancer cell lines in relation to epidermal growth factor receptor (EGFR) expression and mutational phenotype. Gastric Cancer. 2012;15:252–264. doi: 10.1007/s10120-011-0102-9. [DOI] [PubMed] [Google Scholar]
- 16.Abal M., Andreu J.M., Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr. Cancer Drug Targets. 2003;3:193–203. doi: 10.2174/1568009033481967. [DOI] [PubMed] [Google Scholar]
- 17.Siddik Z.H. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 18.Galluzzi L., Humeau J., Buqué A., et al. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020;17:725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 19.Tsavaris N., Kosmas C., Vadiaka M., et al. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br. J. Cancer. 2002;87:21–27. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnett C.T., Schlom J., Hodge J.W. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin. Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfannenstiel L.W., Lam S.S., Emens L.A., et al. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol. 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen L., Lu Z., Wang J., et al. LBA52 Sintilimab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced or metastatic esophageal squamous cell cancer: first results of the phase III ORIENT-15 study. Ann. Oncol. 2021;32:S1330. [Google Scholar]
- 23.Wang Z., Cui C., Yao J., et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40:277–288e3. doi: 10.1016/j.ccell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 25.Doki Y., Ajani J.A., Kato K., et al. Nivolumab combination therapy in advanced esophageal squamous cell carcinoma. N. Engl. J. Med. 2022;386:449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 26.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vermorken J.B., Mesia R., Rivera F., et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 28.Bossi P., Miceli R., Locati L.D., et al. A randomized, phase 2 study of cetuximab plus cisplatin with or without paclitaxel for the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Ann. Oncol. 2017;28:2820–2826. doi: 10.1093/annonc/mdx439. [DOI] [PubMed] [Google Scholar]
- 29.Tahara M., Kiyota N., Yokota T., et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02) Ann. Oncol. 2018;29:1004–1009. doi: 10.1093/annonc/mdy040. [DOI] [PubMed] [Google Scholar]
- 30.Tsakonas G., Specht L., Kristensen C.A., et al. Randomized phase II study with cetuximab in combination with 5-FU and cisplatin or carboplatin vs. cetuximab in combination with paclitaxel and carboplatin for treatment of aatients with relapsed or eetastatic squamous cell carcinoma of the head and neck (CETMET Trial) Cancers (Basel) 2020;12:3110. doi: 10.3390/cancers12113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawada G., Niida A., Uchi R., et al. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Petty R.D., Dahle-Smith A., Stevenson D.A.J., et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J. Clin. Oncol. 2017;35:2279–2287. doi: 10.1200/JCO.2016.70.3934. [DOI] [PubMed] [Google Scholar]
- 33.Janmaat M.L., Gallegos-Ruiz M.I., Rodriguez J.A., et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J. Clin. Oncol. 2006;24:1612–1619. doi: 10.1200/JCO.2005.03.4900. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Suzarte M., Lorenzo-Luaces P., Lazo N.G., et al. Treatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol. Ther. 2012;13:600–605. doi: 10.4161/cbt.19849. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y., Wu X., Bu S., et al. Promising outcomes of definitive chemoradiation and cetuximab for patients with esophageal squamous cell carcinoma. Cancer Sci. 2012;103:1979–1984. doi: 10.1111/j.1349-7006.2012.02393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H., Wang C., Wang J., et al. A subset of esophageal squamous cell carcinoma patient-derived xenografts respond to cetuximab, which is predicted by high EGFR expression and amplification. J. Thorac. Dis. 2018;10:5328–5338. doi: 10.21037/jtd.2018.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbst R.S., Redman M.W., Kim E.S., et al. Cetuximab plus carboplatin and paclitaxel with or without bevacizumab versus carboplatin and paclitaxel with or without bevacizumab in advanced NSCLC (SWOG S0819): a randomised, phase 3 study. Lancet Oncol. 2018;19:101–114. doi: 10.1016/S1470-2045(17)30694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonomi P.D., Gandara D., Hirsch F.R., et al. Predictive biomarkers for response to EGFR-directed monoclonal antibodies for advanced squamous cell lung cancer. Ann. Oncol. 2018;29:1701–1709. doi: 10.1093/annonc/mdy196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21:832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 40.Kato K., Cho B.C., Takahashi M., et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–1517. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 41.Kojima T., Shah M.A., Muro K., et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 2020;38:4138–4148. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W., Pang Q., Zhang X., et al. Programmed death-ligand 1 is prognostic factor in esophageal squamous cell carcinoma and is associated with epidermal growth factor receptor. Cancer Sci. 2017;108:590–597. doi: 10.1111/cas.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacco A.G., Chen R., Worden F.P., et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22:883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PubMed] [Google Scholar]
- 44.Feng B., Shen Y., Pastor H.X., et al. Integrative analysis of multi-omics data identified EGFR and PTGS2 as key nodes in a gene regulatory network related to immune phenotypes in head and neck cancer. Clin. Cancer Res. 2020;26:3616–3628. doi: 10.1158/1078-0432.CCR-19-3997. [DOI] [PubMed] [Google Scholar]
- 45.Maughan T.S., Adams R.A., Smith C.G., et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomized phase 3 MRC COIN trial. Lancet. 2011;377:2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu M., Wang X., Shen L., et al. Nimotuzumab plus paclitaxel and cisplatin as the first line treatment for advanced esophageal squamous cell cancer: a single centre prospective phase II trial. Cancer Sci. 2016;107:486–490. doi: 10.1111/cas.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ai X., Cui J., Zhang J., et al. Clonal architecture of EGFR mutation predicts the efficacy of EGFR-tyrosine kinase inhibitors in advanced NSCLC: a prospective multicenter study ( NCT03059641) Clin. Cancer Res. 2021;27:704–712. doi: 10.1158/1078-0432.CCR-20-3063. [DOI] [PubMed] [Google Scholar]
- 48.Thorvaldsdóttir H., Robinson J.T., Mesirov J.P. Integrative genomics viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.