Abstract
Background & Aims
Hepatic inflammation is a hallmark of nonalcoholic fatty liver disease (NAFLD). Double negative T (DNT) cells are a unique subset of T lymphocytes that do not express CD4, CD8, or natural killer cell markers, and studies have suggested that DNT cells play critical and diverse roles in the immune system. However, the role of intrahepatic DNT cells in NAFLD is largely unknown.
Methods
The proportions and RNA transcription profiling of intrahepatic DNT cells were compared between C57BL/6 mice fed with control diet or methionine-choline–deficient diet for 5 weeks. The functions of DNT cells were tested in vitro and in vivo.
Results
The proportion of intrahepatic DNT cells was significantly increased in mice with diet-induced NAFLD. In NAFLD mice, the proportion of intrahepatic TCRγδ+ DNT cells was increased along with elevated interleukin (IL) 17A; in contrast, the percentage of TCRαβ+ DNT cells was decreased, accompanied by reduced granzyme B (GZMB). TCRγδ+ DNT cell depletion resulted in lowered liver IL17A levels and significantly alleviated NAFLD. Adoptive transfer of intrahepatic TCRαβ+ DNT cells from control mice increased intrahepatic CD4 and CD8 T cell apoptosis and inhibited NAFLD progression. Furthermore, we revealed that adrenic acid and arachidonic acid, harmful fatty acids that were enriched in the liver of the mice with NAFLD, could induce apoptosis of TCRαβ+ DNT cells and inhibit their immunosuppressive function and nuclear factor kappa B (NF-κB) or AKT signaling pathway activity. However, arachidonic acid facilitated IL17A secretion by TCRγδ+ DNT cells, and the NF-κB signaling pathway was involved. Finally, we also confirmed the variation of intrahepatic TCRαβ+ DNT cells and TCRγδ+ DNT cells in humans.
Conclusions
During NAFLD progression, TCRγδ+ DNT cells enhance IL17A secretion and aggravate liver inflammation, whereas TCRαβ+ DNT cells decrease GZMB production and lead to weakened immunoregulatory function. Shifting of balance from TCRγδ+ DNT cell response to one that favors TCRαβ+ DNT regulation would be beneficial for the prevention and treatment of NAFLD.
Keywords: NAFLD, TCRαβ+ DNT Cells, TCRγδ+ DNT Cells, IL17A, GZMB
Abbreviations used in this paper: AA, arachidonic acid; ADA, adrenic acid; ALN, axillary lymph node; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CDHFD, choline-deficient, high-fat diet; Cxcl16, CXCR6 ligand 16; CXCR6, chemokine (C-X-C motif) receptor 6; DLN, draining lymph node; DNT, double negative T; FA, fatty acid; GO, Gene Ontology; GSEA, gene set enrichment analysis; GZMB, granzyme B; IHC, immunohistochemistry; IL17A, interleukin 17A; ILN, inguinal lymph node; KEGG, Kyoto Encylopedia of Genes and Genomics; MCD, methionine-choline–deficient; MLN, mesenteric lymph node; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NCD, normal control diet; NF-κB, nuclear factor kappa B; PCR, polymerase chain reaction; PI3K-AKT, phosphatidylinositol 3-kinase- protein kinase B; TFBS, transcription factor binding site
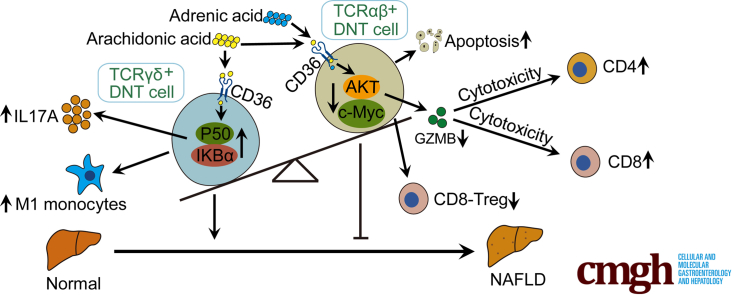
Graphical abstract
Summary.
During NAFLD development, the proportion of TCRαβ+ DNT cells decreased, whereas TCRγδ+ DNT cells increased significantly. TCRγδ+ DNT cells enhance IL17A secretion and aggravate liver inflammation, whereas TCRαβ+ DNT cells decrease GZMB production and lead to weakened immunoregulation in NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is one of the most important causes of liver disease worldwide.1 NAFLD is driven by a combination of genetic and lifestyle factors and involves excessive liver fat accumulation or progression to necroinflammation (nonalcoholic steatohepatitis [NASH]) or fibrosis to cirrhosis and eventually hepatocellular carcinoma.2 Although a wealth of information on the pathogenesis of NAFLD/NASH is available, no specific treatments have been approved by regulatory agencies.2,3
The liver is an important immune organ with many immune cells, and the interaction between innate and adaptive immune cells plays a critical role in the pathogenesis and progression of NAFLD/NASH.4,5 Double negative T (DNT) cells are a unique subset of T lymphocytes that do not express CD4, CD8, or natural killer cell markers. Although DNT cells account for only a small proportion of total T lymphocytes in the peripheral blood and lymph organs of humans and mice, an increasing number of studies have suggested that this unique T-cell population plays critical and diverse roles in the immune system.6, 7, 8
Some reports have indicated that DNT cells highly express perforin, granzyme B (GZMB), and Fas ligand and strongly restrain CD4+ and CD8+ T cells,9, 10, 11, 12 as well as B cells,13,14 dendritic cells,15 and natural killer cells,16 thus regulating antigen-specific T-cell responses and various immune responses.9,17,18 However, several lines of evidence suggest that DNT cells can aggravate the pathogenesis of autoimmune disorders by producing abundant levels of the proinflammatory cytokine interleukin 17 (IL17) in patients with systemic lupus erythematosus and autoimmune disorders such as primary Sjögren’s syndrome.19, 20, 21 Overall, current studies strongly support the notion that DNT cells are composed of a rare but heterogeneous T-lymphocyte subset.
We recently demonstrated that the adoptive transfer of ex vivo-generated CD4+ T cell–converted DNT cells ameliorated the development of NASH by inhibiting liver-infiltrating Th17 cells and proinflammatory M1 macrophages.22 However, the role of intrahepatic natural DNT cells in the development and progression of NAFLD/NASH is still unknown. In this study, we found that DNT cells consist of TCRγδ+ DNT cells and TCRαβ+ DNT cells. The balance of TCRγδ+ and TCRαβ+ DNT cells plays critical roles in maintaining hepatic immune homeostasis. During NAFLD development, TCRγδ+ DNT cells enhance IL17A secretion and aggravate liver inflammation, whereas TCRαβ+ DNT cells decrease GZMB production and lead to weakened immunoregulatory function. Shifting the balance toward hepatic inflammation promotes the progression of NAFLD/NASH.
Results
The Proportion and Number of Hepatic DNT Cells Increased Rapidly in Mice With NAFLD
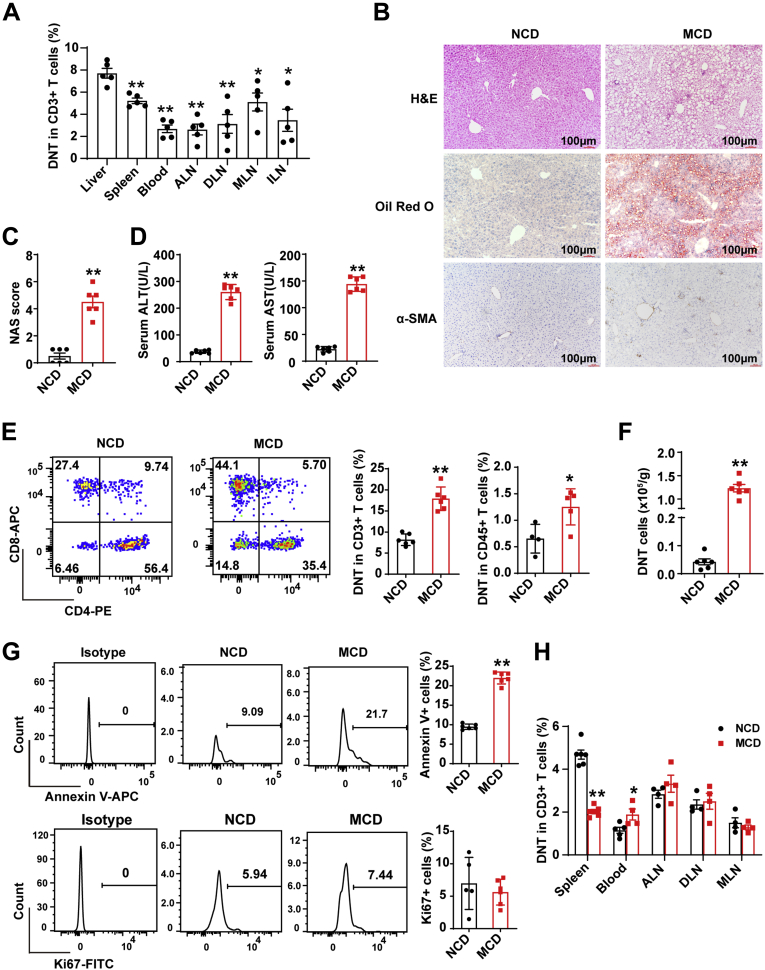
The percentage of DNT cells in total T cells in the liver was markedly higher than that in the spleen, peripheral blood, axillary lymph nodes (ALNs), draining lymph nodes from the liver (DLNs), mesenteric lymph nodes (MLNs), and inguinal lymph nodes (ILNs), suggesting that DNT cells might exert critical roles in the liver immune system (Figure 1A). To study the proportion of DNT cells in NAFLD, we used a mouse model of methionine-choline–deficient (MCD)-induced NAFLD. As shown in Figure 1B and C, H&E staining and Oil Red O and α-SMA staining displayed more severe inflammation, lipid droplet accumulation, and liver fibrosis in the MCD-fed mice than in the normal control diet (NCD)-fed mice. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) significantly increased in the MCD-fed mice (Figure 1D). Interestingly, flow cytometric analysis also showed that the proportion of DNT (CD3+CD4–CD8–NK1.1–) cells in CD3+ T cells and CD45+ cells in the liver increased in the MCD-fed mice compared with the NCD-fed mice (Figure 1E). Although the absolute number of intrahepatic DNT cells in the MCD-fed mice was higher than that in the NCD-fed mice (Figure 1F), flow cytometry showed that the apoptosis of intrahepatic DNT cells increased significantly in the MCD-fed mice compared with the NCD-fed mice, whereas the intrahepatic DNT cell proliferation in the MCD-fed mice did not change significantly (Figure 1G). Moreover, the proportion of DNT cells in CD3+ T cells in the spleen decreased but increased in the blood. The proportions of DNT cells in the ALNs, DLNs, and MLNs in the MCD-fed mice were comparable with those in the NCD-fed mice (Figure 1H).
Figure 1.
Proportion of DNT cells increased in MCD-induced NAFLD for 5 weeks. (A) Percentages of DNT cells in CD3+ T cells among the liver, spleen, blood, ALNs, DLNs, MLNs, and ILNs from normal mice. (B) Representative H&E staining and Oil Red O and α-SMA staining in livers of NCD- and MCD-fed mice. (C) NAFLD activity score (NAS) in livers of NCD and MCD mice. (D) Serum ALT and serum AST were measured in NCD- and MCD-fed mice. (E) Representative flow cytometry plots and statistical analysis of percentages of intrahepatic DNT cells in CD3+ T cells and CD45+ T cells of NCD- and MCD-fed mice. (F) Numbers of intrahepatic DNT cells in NCD- and MCD-fed mice. (G) Representative flow cytometry plots and statistical analysis of annexin V+ and Ki67+ expression in intrahepatic DNT cells in NCD- and MCD-fed mice. (H) Percentages of DNT cells in CD3+ T cells among the spleen, blood, ALNs, DLNs, and MLNs of NCD- and MCD-fed mice. n ≥ 4 mice/group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
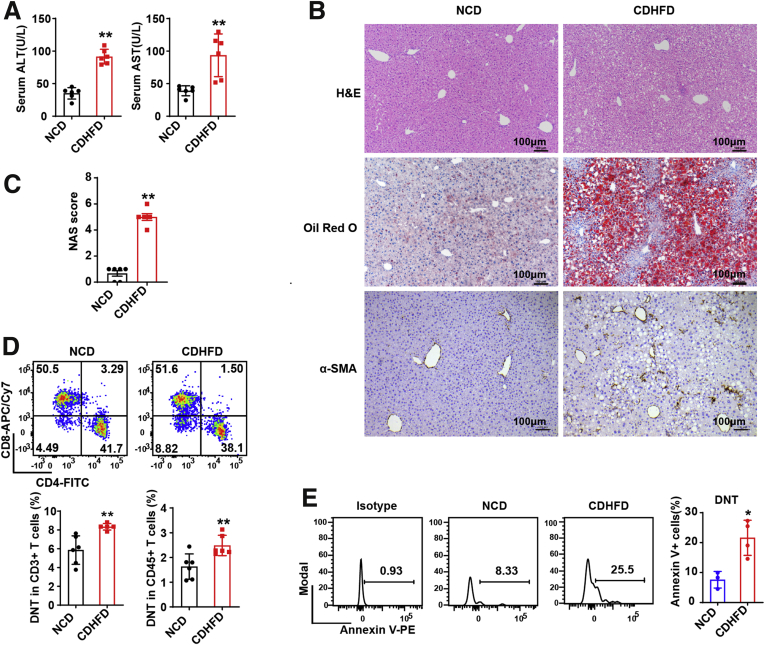
We also established NAFLD mouse models with a choline-deficient high-fat diet (CDHFD) for 18 weeks (Figure 2A–C). The proportions of intrahepatic DNT cells in CD3+ T cells and CD45+ cells were also increased in the CDHFD-fed mice, and annexin V analysis showed significantly increased apoptosis of DNT cells in the CDHFD-fed mice (Figure 2D and E). These observations indicated that the proportions, numbers, and apoptosis rate of intrahepatic DNT cells increased markedly in NAFLD.
Figure 2.
Proportion of DNT cells increased in CDHFD-induced NAFLD. (A) Serum ALT and serum AST were measured in NCD- and CDHFD-fed mice. (B) Representative H&E staining and Oil Red O and α-SMA staining in livers of NCD- and CDHFD-fed mice. (C) NAFLD activity score (NAS) in livers of NCD and CDHFD mice. (D) Representative flow cytometry plots and statistical analysis of percentages of intrahepatic DNT cells in CD3+ T cells and CD45+ T cells of NCD- and CDHFD-fed mice. (E) Representative flow cytometry plots and statistical analysis of annexin V+ expression in intrahepatic DNT cells in NCD- and CDHFD-fed mice. n ≥ 3 mice/group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
Transcriptome Sequencing Analysis of Intrahepatic DNT Cells in the NCD- and MCD-Fed Mice
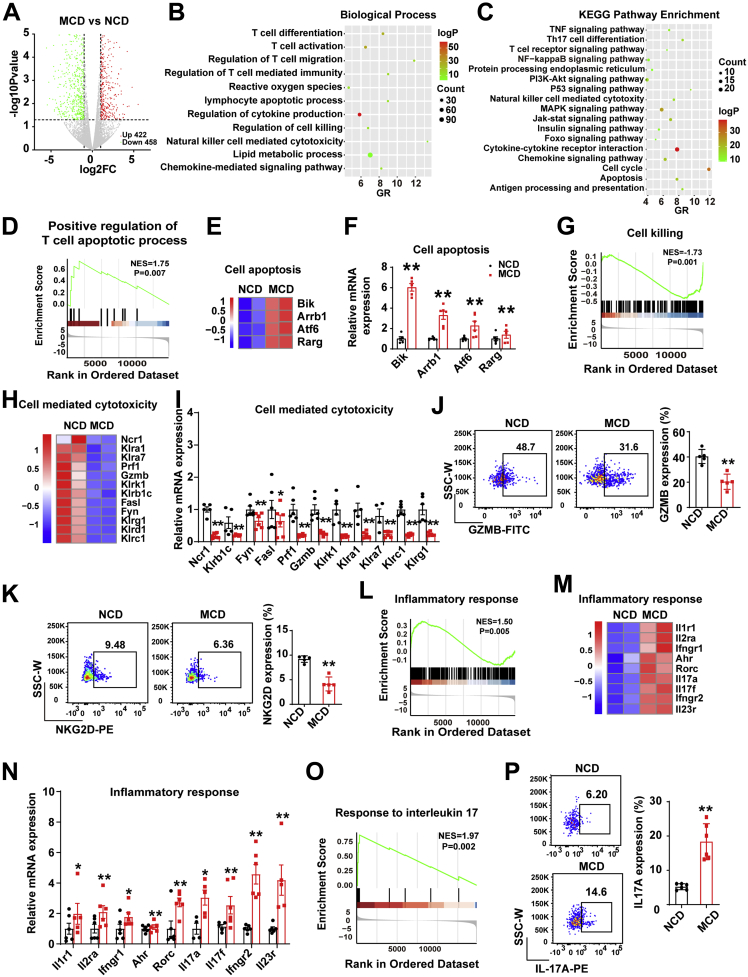
To further elucidate the function of intrahepatic DNT cells in NAFLD, we compared DNT cells from the livers of the MCD- and NCD-fed mice in a transcriptome sequencing study. The volcano study showed that DNT cells in the MCD-fed mice had 880 differentially expressed genes (P value <.05 and fold change ≤0.5 or fold change ≥1.5) compared with the NCD-fed mice (Figure 3A). Gene Ontology (GO) and Kyoto Encylopedia of Genes and Genomics (KEGG) pathway analyses revealed that the differentially expressed genes were involved in T-cell activation, regulation of lymphocyte apoptotic process, cytokine production, cell killing, positive regulation of natural killer cell–mediated cytotoxicity, lipid metabolic process, and apoptosis among others. In addition, various signaling pathways, such as the phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT) and nuclear factor kappa B (NF-κB) signaling pathways, were involved (Figure 3B and C). Analysis of all genes in DNT cells and gene set enrichment analysis (GSEA) of DNT cells showed that positive regulation of the T-cell apoptotic process was increased (NES = 1.75, P = .007) (Figure 3D). Moreover, compared with that in the NCD-fed mice, the expression of apoptosis-related genes (Bik, Arrb1, Atf6, and Rarg) in DNT cells was increased, and the changes in these genes were validated through real-time polymerase chain reaction (PCR) (Figure 3E and F). Together, these data indicated that the apoptosis of intrahepatic DNT cells increased in NAFLD and that the AKT and NF-κB signaling pathways may be involved.
Figure 3.
RNA-sequencing showed the differentially expressed genes and biological function of DNT cells from livers of MCD-fed mice compared with NCD-fed mice. (A) Differences of genes with up-regulated (422) and down-regulated (458) expression in intrahepatic DNT cells from MCD-fed mice compared with NCD-fed mice; fold change ≤ 0.5 or fold change ≥ 1.5; P < .05. (B and C) Biological Process and KEGG pathway analyses were performed on the basis of the genes with significantly up-regulated and down-regulated expression in intrahepatic DNT cells from MCD-fed mice compared with NCD-fed mice. (D) GSEA of positive regulation of the T-cell apoptotic process in intrahepatic DNT cells from MCD-fed mice compared with NCD-fed mice. (E and F) Heatmap showing up-regulated and down-regulated genes related to cell apoptosis in intrahepatic DNT cells from MCD-fed mice compared with NCD-fed mice. The significantly changed genes related to cell apoptosis were confirmed via real-time PCR. (G) GSEA of cell killing in intrahepatic DNT cells of MCD-fed mice. (H) Heatmap showing the genes with down-regulated expression related to cell-mediated cytotoxicity in intrahepatic DNT cells from MCD-fed mice compared with NCD-fed mice. (I) The significantly changed genes related to cell-mediated cytotoxicity were confirmed via real-time PCR. (J and K) Representative flow cytometry plots and statistical analysis of GZMB and NKG2D expression in intrahepatic DNT cells from NCD- and MCD-fed mice. (L) GSEA of the inflammatory response in intrahepatic DNT cells from NCD- and MCD-fed mice. (M) Heatmap showing genes with up-regulated and down-regulated expression related to the inflammatory response in intrahepatic DNT cells from livers of MCD-fed mice compared with NCD-fed mice. (N) The significantly changed genes related to the inflammatory response were confirmed via real-time PCR. (O) GSEA of the response to IL17 in intrahepatic DNT cells from NCD- and MCD-fed mice. (P) Representative flow cytometry plots and statistical analysis of IL17A expression in intrahepatic DNT cells from NCD- and MCD-fed mice. n ≥ 5 mice/group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
Compared With the NCD-Fed Mice, the MCD-Fed Mice Exhibited Decreased Immunosuppressive Functions of Intrahepatic DNT Cells and Enhanced Proinflammatory Response
To explore the function of intrahepatic DNT cells during NAFLD development, we analyzed the cytotoxicity and inflammatory response of DNT cells. GSEA showed that the cell killing of DNT cells in the MCD-fed mice declined significantly compared with that in the NCD-fed mice (Figure 3G). The levels of cell-mediated cytotoxicity genes, such as Ncr1, Klrb1c, Fyn, Fasl, Klrc2, Klra3, Klra9, Prf1, Gzmb, Klrk1, Klra1, Klra7, Klrd1, and Klrc1, in DNT cells of the MCD-fed mice were down-regulated sharply (Figure 3H and I). As expected, flow cytometry also showed that GZMB expression decreased in DNT cells of the MCD-fed mice compared with the NCD-fed mice (Figure 3J), which indicated that the suppressive function of DNT cells decreased. We also observed that the expression of NKG2D (encoded by Klrk1) decreased significantly, which indicated that DNT cells may be involved in inhibitory conditions during NAFLD development (Figure 3K).
GSEA also showed that the inflammatory response of intrahepatic DNT cells was markedly enhanced in the MCD-fed mice compared with NCD-fed mice (NES = 1.50, P = .005) (Figure 3L). Some inflammatory response genes, such as Il1r1, Il2ra, Ifngr1, Ahr, Rorc, Il17a, Il17f, Ifngr2, and Il23r, showed increased expression (Figure 3M and N). Interestingly, GSEA of intrahepatic DNT cells showed that the IL17 pathway was increased (Figure 3O). Flow cytometry also showed that IL17A secretion in intrahepatic DNT cells from the MCD-fed mice was increased dramatically compared with that in the NCD-fed mice (Figure 3P).
TCRαβ+ DNT Cells Mainly Decreased GZMB Production, Whereas TCRγδ+ DNT Cells Mainly Enhanced IL17A Secretion During NAFLD Development
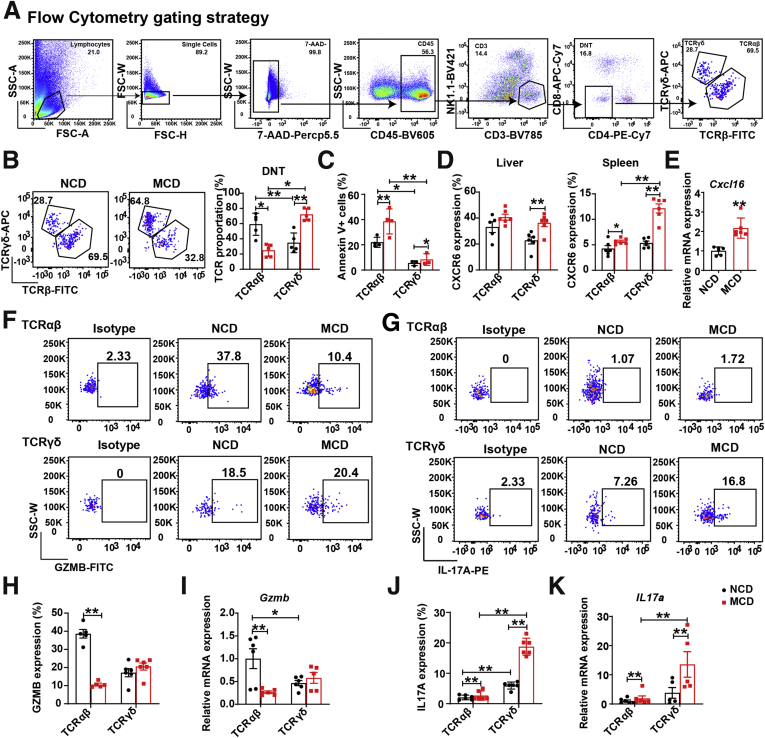
To further determine the function of different DNT cell subsets in NAFLD, we assessed DNT cells with TCRβ chains and TCRγδ chains (Figure 4A). As shown in Figure 4B, the DNT cells included CD4 and CD8 double negative TCRαβ+ and TCRγδ+ cells. The proportion of TCRαβ+ DNT cells decreased significantly, whereas TCRγδ+ DNT cells increased obviously during NAFLD development. Annexin V staining showed that the apoptosis of TCRαβ+ DNT cells was heavily increased compared with that of TCRγδ+ cells in the MCD-fed mice (Figure 4C). Although chemokine (C-X-C motif) receptor 6 (CXCR6) expression increased in TCRγδ+ DNT cells from the spleen and the liver in MCD-fed mice, we also confirmed that the corresponding chemokine C-X-C motif chemokine ligand 16 (Cxcl16) increased markedly in the liver at the mRNA level (Figure 4D and E), so we believe the elevation of TCRγδ+ DNT cells in MCD-fed liver is due to the migration of these cells from the spleen. These differences in TCRαβ+ and TCRγδ+ DNT cell survival and CXCR6 expression may induce changes in the proportions of DNT subsets during NAFLD development.
Figure 4.
Immunosuppressive function of DNT cells was controlled by TCRαβ+ DNT cells, and secretion of inflammatory factors was controlled by TCRγδ+DNT cells. (A) Representative flow cytometry gating strategy of TCR proportions in intrahepatic DNT cells. (B) Representative flow cytometry plots of TCR proportions in intrahepatic DNT cells from MCD- and NCD-fed mice. (C) Statistical analysis in apoptosis of TCR subtypes in intrahepatic DNT cells from MCD- compared with NCD-fed mice by flow cytometry. n = 3 mice/group. (D) Statistical analysis by flow cytometry of CXCR6 expression in liver and spleen TCRγδ+ DNT cells and TCRαβ+ DNT cells among NCD- and MCD-fed mice. (E) The mRNA expression of Cxcl16 in liver among NCD- and MCD-fed mice. (F, H, and I) Representative flow cytometry plots and mRNA of GZMB expression in intrahepatic TCRγδ+ DNT cells and TCRαβ+ DNT cells among NCD- and MCD-fed mice. (G, J, and K) Representative flow cytometry plots and mRNA of IL17A expression in intrahepatic TCRγδ+ DNT cells and TCRαβ+ DNT cells from NCD- and MCD-fed mice. n ≥ 5 mice/group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
Flow cytometry also showed that GZMB expression in TCRαβ+ DNT cells was higher than that in TCRγδ+ DNT cells of the NCD-fed mice. During NAFLD development, GZMB expression in TCRαβ+ DNT cells decreased significantly but did not change in TCRγδ+ DNT cells (Figure 4F, H, and I). These results suggested that the decreased DNT cell suppressive function during NAFLD development was mainly caused by TCRαβ+ DNT cells.
We also found a markedly elevated expression of IL17A produced in TCRγδ+ DNT cells but not in TCRαβ+ DNT cells, which indicated that the increased IL17A secretion from intrahepatic DNT cells in the MCD-fed mice was mainly from TCRγδ+ DNT cells (Figure 4G, J, and K). Taken together, these observations suggest that TCRαβ+ DNT cells decrease GZMB production, commonly leading to weakened immunoregulatory function during NAFLD progression, whereas TCRγδ+ DNTs enhance IL17A secretion and aggravate liver inflammation.
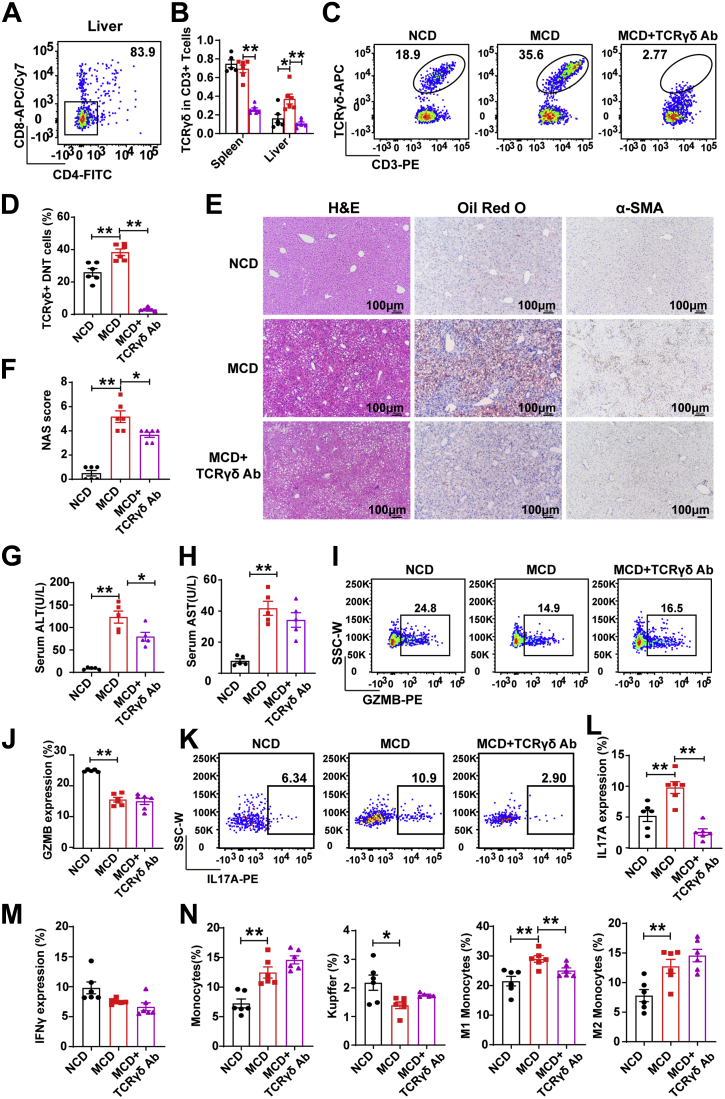
TCRγδ+ DNT Depletion Resulted in Lowered Liver IL17A Levels and Significantly Alleviated NAFLD
CD4–CD8– cells accounted for 83.9% of hepatic TCRγδ+ T cells (Figure 5A). To further confirm the roles of TCRγδ+ DNT cells during NAFLD development, we used a depletion antibody against TCRγδ to eliminate TCRγδ+ DNT cells in the MCD-fed mice. As shown in Figure 5B, TCRγδ T cells in CD3+ T cells were reduced as expected in the spleen and liver in the MCD-fed mice. TCRγδ+ DNT cells were also significantly decreased in the MCD-fed mice after TCRγδ depletion (Figure 5C and D). To further verify the effects of TCRγδ T-cell depletion in the MCD-fed mice, we examined the liver pathology. As shown in Figure 5E and F, TCRγδ depletion decreased liver fat accumulation and lobular inflammation but not α-SMA levels in the MCD-fed mice. We also found that TCRγδ T-cell elimination lowered serum ALT and AST levels in the mice with NAFLD (Figure 5G and H).
Figure 5.
TCRγδ+DNT depletion resulted in lowered liver IL17A levels and significantly alleviated diet-induced NAFLD. (A) Representative flow cytometry plots of DNT proportions in TCRγδ+ T cells. (B) Statistical analysis of TCRγδ+ T cells in CD3+ T cells from the spleen and liver of NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. (C and D) Representative flow cytometry plots and statistical analysis of TCRγδ+ T cells in intrahepatic DNT cells of NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. (E) Representative H&E staining and Oil Red O and α-SMA staining in livers of NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. (F) NAFLD activity score (NAS) in livers of NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. (G and H) Serum ALT and serum AST were measured in livers from NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. (I–L) Representative flow cytometry plots and statistical analysis of GZMB and IL17A expression in intrahepatic DNT cells from NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. (M) Statistical analysis of interferon γ expression in intrahepatic DNT cells from NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice by flow cytometry. (N) Proportions of monocytes (CD45+Ly6G-F4/80intCD11bhi), Kupffer cells (CD45+Ly6G-F4/80hiCD11bint), M1 monocytes (CD45+Ly6G-F4/80intCD11bhiCD11c+CD206-), and M2 monocytes (CD45+Ly6G-F4/80intCD11bhiCD11c-CD206+) among intrahepatic DNT cells from NCD- and MCD-fed mice and TCRγδ+ DNT-depleted mice. n ≥ 5 mice/group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
TCRγδ+ DNT cell clearance had no effects on GZMB expression in all DNT cells (Figure 5I and J) but significantly decreased DNT cell IL17A secretion in the MCD-fed mice (Figure 5K and L). TCRγδ+ DNT cell clearance had no effects on interferon γ expression in all DNT cells (Figure 5M). In addition, we investigated whether TCRγδ+ DNT cell clearance had any effect on the progression of inflammation. As shown in Figure 5N, flow cytometry showed that depleting TCRγδ+ DNT cells reduced M1 monocyte proportions but not total monocytes, Kupffer cells, or M2 monocytes in the MCD-fed mice. These observations indicated that the clearance of TCRγδ+ DNT cells decreased IL17A expression but not GZMB expression, ultimately inhibiting hepatic inflammation and NAFLD development.
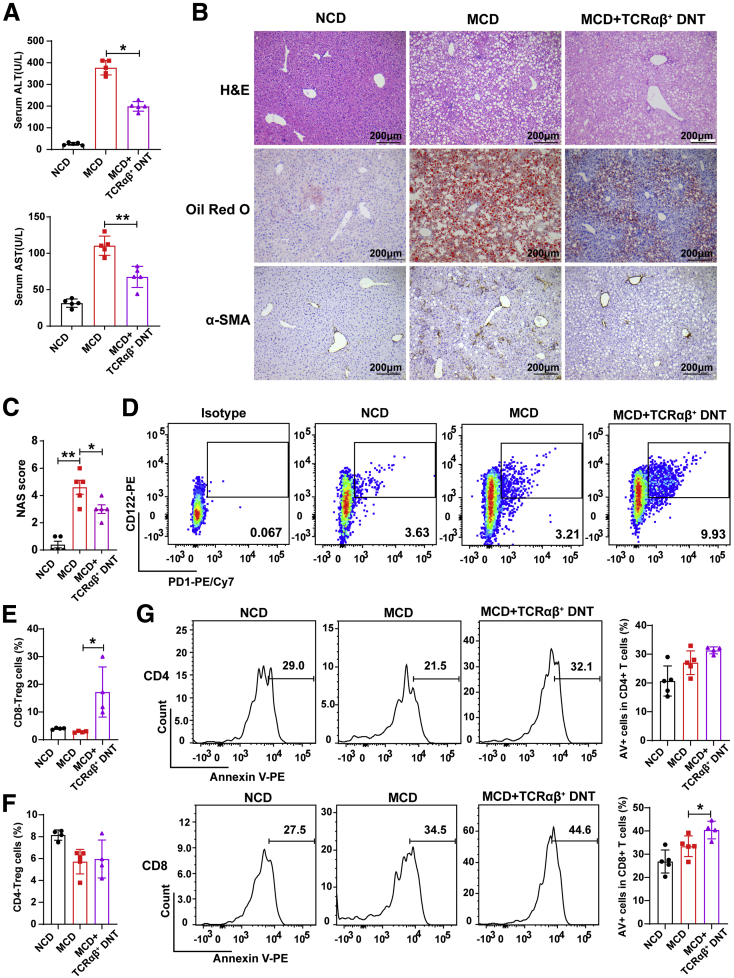
Adoptive Transfer of TCRαβ+ DNT Cells Could Regulate the Intrahepatic Immunologic Environment and Prevent NAFLD Development
To further confirm the roles of TCRαβ+ DNT cells during NAFLD development, we isolated TCRαβ+ DNT cells from normal mouse liver and then adoptively transferred them into recipient mice. After 5 weeks of MCD feeding, as shown in Figure 6A, adoptive transfer of TCRαβ+ DNT cells significantly reduced serum ALT and AST levels in the MCD-fed mice. H&E, Oil Red O, and α-SMA staining also demonstrated that TCRαβ+ DNT cell transfer significantly decreased liver fat accumulation, lobular inflammation, and liver fibrosis (Figure 6B and C). In addition, we found that intrahepatic PD-1+CD122+CD8+ Treg cells but not FoxP3+CD4+ Treg cells increased after administration of TCRαβ+ DNT cells (Figure 6D–F). TCRαβ+ DNT cells induced intrahepatic T-cell apoptosis, especially CD8+ T cells, in the MCD-fed mice (Figure 6G). These observations indicated that TCRαβ+ DNT cells could regulate the liver immunologic environment and inhibit NAFLD development.
Figure 6.
Adoptive transfer of TCRαβ+DNT cells prevented the development and progression of NAFLD. (A) Serum ALT and serum AST levels were measured in NCD- and MCD-fed mice and TCRαβ+ DNT cell-transferred mice. (B) Representative H&E staining and Oil Red O and α-SMA staining in livers from NCD- and MCD-fed mice and TCRαβ+ DNT-transferred mice. (C) NAFLD activity score (NAS) in livers of NCD- and MCD-fed mice and TCRαβ+ DNT-transferred mice. (D–F) Representative flow cytometry plots and statistical analysis of proportion of CD4+ and CD8+ Treg cells among livers of NCD- and MCD-fed mice and TCRαβ+DNT cell-transferred mice. (G) Representative flow cytometry plots and statistical analysis of apoptosis of intrahepatic CD4+ and CD8+ cells of NCD- and MCD-fed mice and TCRαβ+DNT cell-transferred mice. n ≥ 4 mice/group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
Harmful Fatty Acids Inhibited the TCRαβ+ DNT Cell Immunosuppressive Functions but Facilitated TCRγδ+ DNT Cell IL17A Secretion
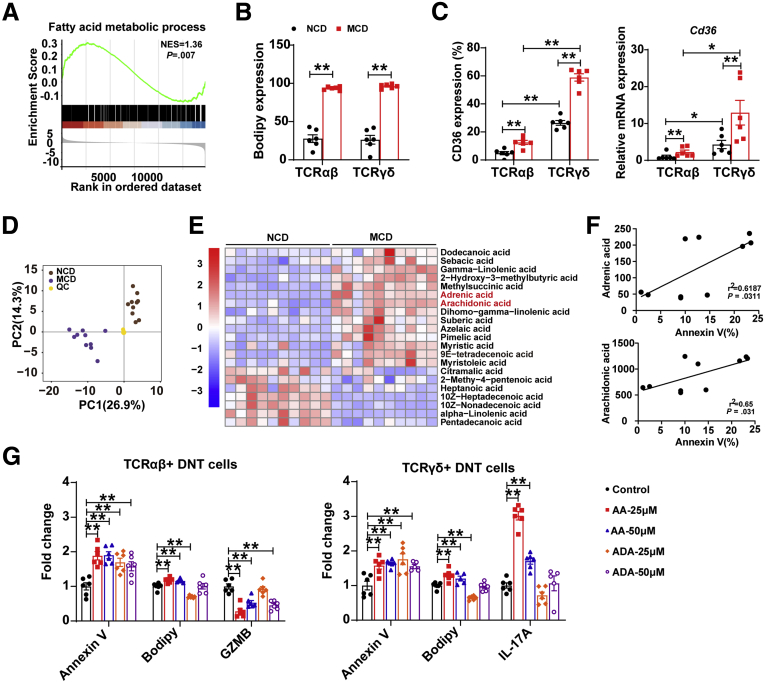
GSEA showed that fatty acid (FA) metabolism was increased in intrahepatic DNT cells of the MCD-fed mice (NES = 1.36, P = .007; Figure 7A). Bodipy staining (Thermo Fisher, Waltham, MA) also showed FA accumulation in DNT cells of the MCD-fed mice (Figure 7B). CD36, a molecule mediating lipid uptake, was markedly increased in intrahepatic DNT cells in the MCD-fed mice compared with the NCD-fed mice, especially in TCRγδ+ DNT cells, which was consistent with the real-time PCR results (Figure 7C). These observations indicated that the FA metabolic process may play important roles in DNT cells during NAFLD development.
Figure 7.
FAs are involved in regulating apoptosis and the function of TCR subtypes of DNT cells. (A) GSEA of the response to FA metabolism. (B) The level of Bodipy in intrahepatic TCRαβ+ DNT cells and TCRγδ+ DNT cells in NCD- and MCD-fed mice. n = 6 mice/group. (C) CD36 expression in intrahepatic DNT cells of NCD- and MCD-fed mice by flow cytometry and real-time PCR. n = 6 mice/group. (D) Principal component analysis of metabonomics in livers of NCD- and MCD-fed mice. (E) FA content in livers of NCD- and MCD-fed mice. n = 6 mice/group. (F) Correlation between the harmful FAs ADA, AA levels, and DNT cell apoptosis. n = 9 mice/group. (G) Apoptosis, Bodipy, GZMB, IL17A expression in TCRγδ+ and TCRαβ+ DNT cells after ADA and AA stimulation. n = 6 biologically independent samples per group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
To further explore which FAs affect DNT cell survival and function, we assessed liver samples from the NCD- and MCD-fed mice by metabonomics. Principal component analysis of metabonomics showed that the different components were distinct from each other (Figure 7D). FA metabonomics analysis showed many FAs changed during NAFLD development, especially the harmful FAs; increased adrenic acid (ADA) and arachidonic acid (AA) (Figure 7E) were correlated with DNT cell apoptosis (Figure 7F), which indicated these FAs might affect DNT cell survival.
To further reveal the potential mechanism of ADA acid and AA in DNT cell survival and function, we stimulated TCRαβ+ and TCRγδ+ DNT cells in vitro (Figure 7G). Compared with the control, treatment with AA induced DNT cell apoptosis and increased Bodipy content in TCRαβ+ DNT cells. GZMB expression was markedly down-regulated after AA stimulation in TCRαβ+ DNT cells. In addition, treatment with AA enhanced DNT cell apoptosis and increased Bodipy content, IL17A expression in TCRγδ+ DNT cells. ADA could also induce cell apoptosis and down-regulate GZMB expression in TCRαβ+ DNT cells but had fewer effects on TCRγδ+ DNT cells. These data revealed that ADA and AA, harmful FAs that were enriched in the liver of the mice with NAFLD, could induce apoptosis and decrease the immunosuppressive function of TCRαβ+ DNT cells. However, AA facilitated IL17A secretion by TCRγδ+ DNT cells.
AA Modulates TCRαβ+ and TCRγδ+ DNT Cell Survival and Functions via Different Regulatory Pathways
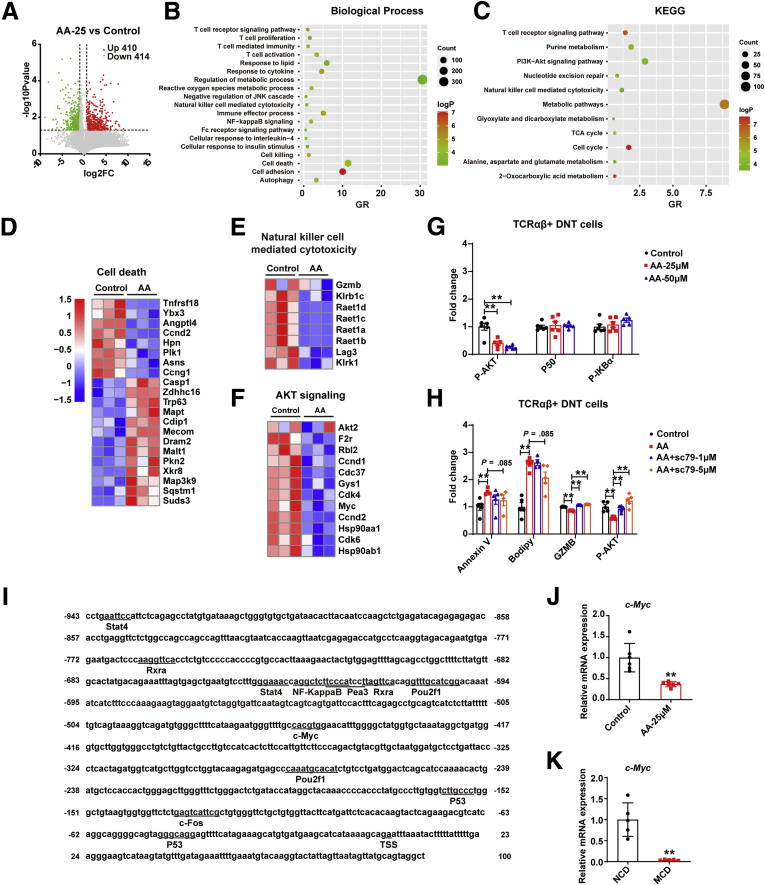
AA affected GZMB and IL17A secretion by TCRαβ+ and TCRγδ+ DNT cells, respectively, and we further focus on the underlying mechanisms. First, transcriptome sequencing was conducted in TCRαβ+ and TCRγδ+ DNT cells after stimulating with or without AA for 48 hours. The volcano plot showed TCRαβ+ DNT cells after AA treatment had 824 differentially expressed genes (P value <.05 and fold change ≤0.5 or fold change ≥1.5) (Figure 8A). GO and KEGG pathway enrichment analysis revealed that the significant differentially expressed genes were involved in T-cell activation, regulation of metabolic process, natural killer cell–mediated cytotoxicity, cell death, and PI3K-Akt signaling pathway, among others (Figure 8B and C). Moreover, compared with control group, the expression of pro-apoptosis genes in TCRαβ+ DNT cells was up-regulated, whereas the expression of anti-apoptosis genes in TCRαβ+ DNT cells was down-regulated (Figure 8D). In addition, the genes related to natural killer cell–mediated cytotoxicity were decreased, such as GzmB, lymphocyte activation gene 3, and others, consistent with those observed in mice (Figure 8E). Further investigation demonstrated that PI3K-AKT signaling pathway was enriched, and related genes were down-regulated (Figure 8F). In vitro stimulation confirmed that AA can down-regulate P-AKT signaling pathway in a dose-dependent manner (Figure 8G). When AKT agonist SC-79 was added, it could partly reverse the effects of AA stimulation, which confirmed the role of AKT signaling pathway in AA regulating TCRαβ+ DNT cells (Figure 8H). To further probe the modulation mechanism of FA AA on GZMB expression in TCRαβ+ DNT cells, transcription factor binding sites (TFBSs) in the GzmB promoter region were also predicted and confirmed by transcription sequencing; the promoter region of GzmB includes some TFBSs, such as retinoid X receptor alpha (Rxra), signal transducer and activator of transcription 4 (Stat4), Myc, Nfkb2, Trp53 (Figure 8I). We further verified that c-Myc was down-regulated after AA treatment and was down-regulated in TCRαβ+ DNT cells of NAFLD mice liver simultaneously by real-time PCR (Figure 8J and K). It is well-known that c-Myc was the downstream of PI3K-AKT signaling pathway,23,24 suggesting that c-Myc may play a critical role in AA regulating GZMB expression.
Figure 8.
AA regulates TCRαβ+DNT cells function by AKT signaling pathway. (A) Distributions of differential genes with up-regulated (410) and down-regulated (414) expression in TCRαβ+ DNT cells with or without AA stimulation; fold change ≤ 0.5 or fold change ≥ 1.5; P < .05. n = 3/group. (B and C) Biological Process and KEGG pathway analysis were performed on basis of differential genes with significantly up-regulated and down-regulated expression in TCRαβ+ DNT cells with or without AA stimulation. (D and E) Heatmap showing the genes related to cell death and natural killer cell mediated cytotoxicity in AA-treated TCRαβ+ DNT cells and control. (F) Heatmap showing expression of AKT signaling pathway in AA-treated TCRαβ+ DNT cells and control. (G) P-AKT, P50, and P-IKBα expression in TCRαβ+ DNT cells after AA stimulation. n = 6 biologically independent samples per group. (H) Apoptosis, Bodipy, GZMB, P-AKT expression in TCRαβ+ DNT cells after treating with AA and AKT agonist SC-79. n = 6 biologically independent samples per group. (I) The TFBSs in the upstream region (2k base pairs upstream from transcription starting site) and downstream region (100 base pairs downstream from transcription starting site) of GzmB were predicted. (J) Real-time PCR verified expression of c-Myc in AA-treated TCRαβ+ DNT cells and control. n = 6 biologically independent samples per group. (K) Real-time PCR verified expression of c-Myc in TCRαβ+ DNT cells in NAFLD and normal mice livers. n = 5 biologically independent samples per group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
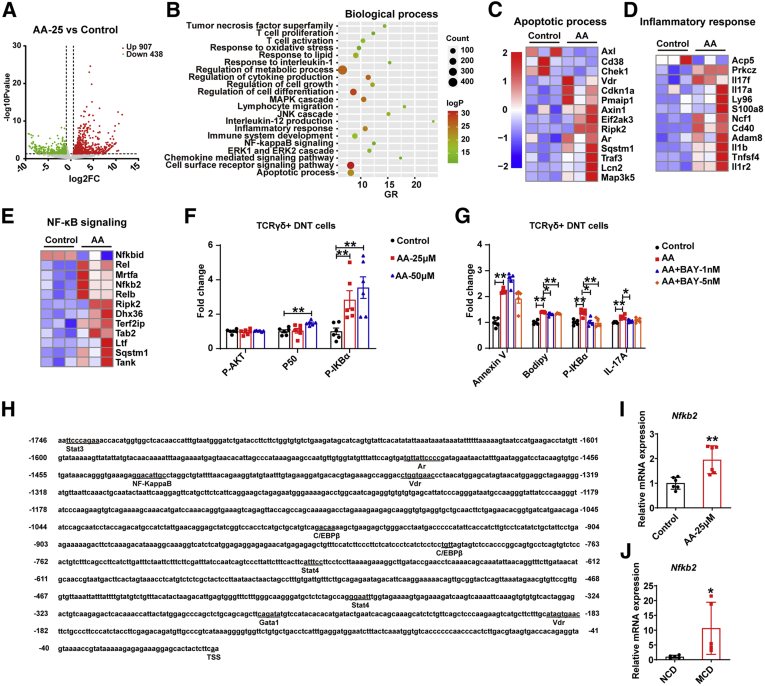
The volcano plot displayed that TCRγδ+ DNT cells after treating with AA had 1345 differentially expressed genes (P value <.05 and fold change ≤0.5 or fold change ≥1.5) compared with control (Figure 9A). GO analysis revealed that the significant differentially expressed genes were involved in T-cell activation, cell death, regulation of metabolic process, and NF-κB signaling pathway, among others (Figure 9B). Compared with control, the expression of pro-apoptosis genes in TCRγδ+ DNT cells was increased, whereas the expression of anti-apoptosis genes in TCRγδ+ DNT cells was decreased (Figure 9C). Meanwhile, the inflammatory response genes, such as IL17a, IL17f, Il1b, and S100a8, were also increased (Figure 9D). Further analysis showed that NF-κB signaling pathway related genes were up-regulated (Figure 9E), consistent with the results in vitro (Figure 9F). When NF-κB inhibitor BAY 11-7082 was added, it could partly reverse the effects of AA stimulation, which confirmed the role of NF-κB signaling pathway in AA regulating TCRγδ+ DNT cells (Figure 9G). TFBSs in the Il17a promoter region were also predicted and confirmed by transcription sequencing; the promoter region of Il17a contains TFBSs such as vitamin D (Vdr), Stat3, Stat4, Nfkb2, androgen receptor (Ar), and Gata binding protein (Gata1) (Figure 9H). We further verified that Nfkb2 was up-regulated after AA treatment and was up-regulated significantly in TCRγδ+ DNT cells of NAFLD mice liver simultaneously by real-time PCR (Figure 9I and J), suggesting NF-κB signaling pathway may play a vital role in AA regulating IL17A expression.
Figure 9.
AA regulatesTCRγδ+DNT cells function via NF-κB signaling pathway. (A) Distributions of differential genes with up-regulated (907) and down-regulated (438) expression in AA-treated TCRγδ+ DNT cells and control; fold change ≤ 0.5 or fold change ≥ 1.5; P < .05. n = 3/group. (B) Biological Process analysis was performed on basis of differential genes with significantly up-regulated and down-regulated expression in AA-treated TCRγδ+ DNT cells and control. (C and D) Heatmap showing the genes related to apoptotic process and inflammatory response in AA-treated TCRγδ+ DNT cells and control. (E) Heatmap showing expression of NF-κB signaling pathway in AA-treated TCRγδ+ DNT cells and control. (F) P-AKT, P50, and P-IKBα expression in TCRγδ+ DNT cells after AA stimulation. n = 6 biologically independent samples per group. (G) Apoptosis, Bodipy, P-IKBα, and IL17A expression in TCRγδ+ DNT cells after treating with AA and NF-κB inhibitor BAY 11-7082. n = 6 biologically independent samples per group. (H) The TFBSs in the upstream region (2k base pairs upstream from the transcription starting site) and downstream region (100 base pairs downstream from the transcription starting site) of Il17a were predicted. (I) Real-time PCR verified the expression of Nfkb2 in AA-treated TCRγδ+ DNT cells and control. n = 6 biologically independent samples per group. (J) Real-time PCR verified the expression of Nfkb2 in TCRγδ+ DNT cells in NAFLD and normal mice livers. n = 5 biologically independent samples per group. Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
The Numbers and Proportions of TCRαβ+ DNT Cells and TCRγδ+ DNT Cells in Healthy and NAFLD Patients
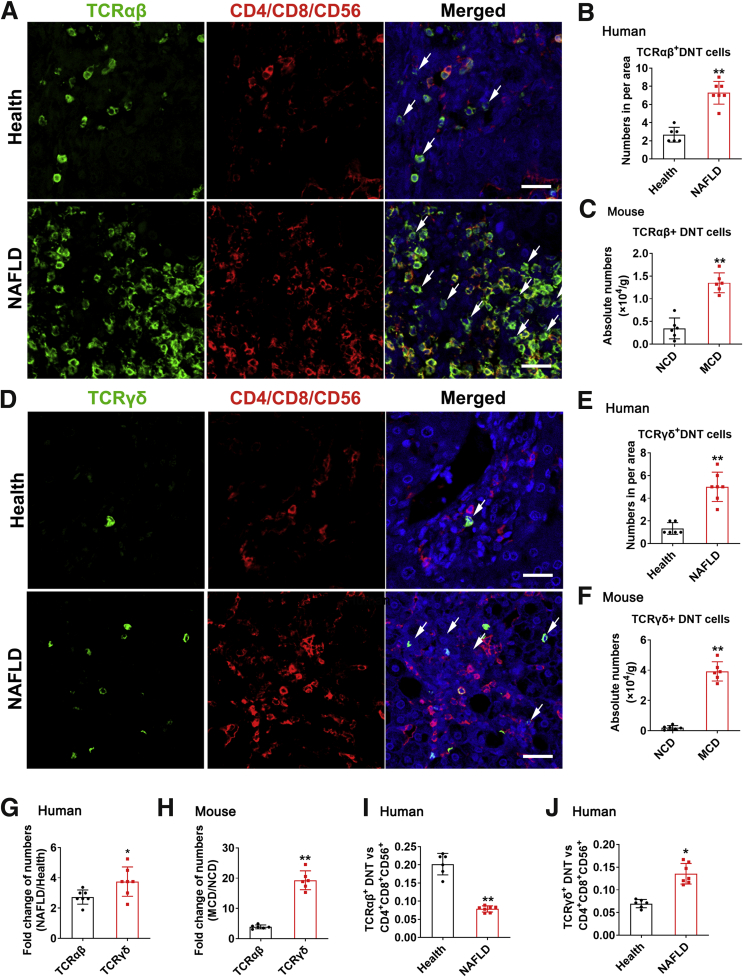
To validate the conclusions in mice, we analyzed the liver biopsy samples of 7 NAFLD patients and 6 healthy controls. CD4, CD8, CD56, TCRαβ, and TCRγδ antibodies were used for multiplex immunofluorescence staining in liver tissues of healthy and NAFLD patients. We found higher number of TCRαβ+CD4–CD8–CD56– cells (Figure 10A and B) and TCRγδ+CD4–CD8–CD56– DNT cells (Figure 10D and E) per area in NAFLD patients’ livers, which further confirmed the results of increased DNT cells in NAFLD mice liver (Figure 10C and F). Further analysis of the fold change of NAFLD/Health, the increase of TCRγδ+ DNT cell numbers in NAFLD patients was significantly higher than that of TCRαβ+ DNT cells, which was consistent with the changes in mice (Figure 10G and H).
Figure 10.
The variation tendency of DNT cells in livers of NAFLD patients was consistent with that in mice. (A) Typical picture of multiplex immunofluorescence staining of human liver sections. TCRαβ (green), CD4/CD8/CD56 (red). White arrowheads indicate DNT cells. Scale bar, 25 μm. (B) Number of TCRαβ+ DNT cells per area in human liver. The number of DNT cells per area (150 μm × 150 μm) was counted. (C) Absolute number of TCRαβ+DNT cells per g mouse liver. (D) Typical picture of multiplex immunofluorescence staining of human liver sections. TCRγδ (green), CD4/CD8/CD56 (red). White arrowheads indicate DNT cells. Scale bar, 25 μm. (E) Number of TCRαβ+ DNT cells per area in human liver. The number of DNT cells per area (150 μm × 150 μm) was counted. (F) Absolute number of TCRγδ+ DNT cells per g mouse liver. (G) The amplitude of number variation of TCRγδ+CD4–CD8–CD56– T cells and TCRαβ+CD4–CD8–CD56– T cells in NAFLD/Health. (H) The amplitude of number variation of TCRαβ+ DNT cells and TCRγδ+ DNT cells in MCD/NCD. (I) Ratio of TCRαβ+DNT cells with CD4+CD8+CD56+ cells (0.02 cm2 per area). (J) Ratio of TCRγδ+ DNT cells with CD4+CD8+CD56+ cells (0.02 cm2 per area). Two-sided P values <.05 were considered significant. ∗∗P < .01; ∗P < .05.
In addition, to further detect the variation of TCRαβ and TCRγδ subgroups in DNT cells in human liver, we counted the ratio of TCRαβ+CD4–CD8–CD56– T cells and TCRγδ+CD4–CD8–CD56– with CD4+CD8+CD56+ cells in healthy and NAFLD patients. During NAFLD development, the ratio of TCRαβ+CD4–CD8–CD56– T cells decreased (Figure 10I), whereas the ratio of TCRγδ+CD4–CD8–CD56– T cells increased (Figure 10J). All these results further certified the decrease of TCRαβ+ DNT cell proportion and the increase of TCRγδ+ DNT cell proportion in NAFLD patients, which were consistent with the conclusion in mice.
Discussion
NAFLD is now the leading cause of chronic liver disease and is increasing worldwide, but there is still a lack of satisfactory treatment. In-depth exploration of the mechanisms during the development and progression of NAFLD and NASH is important to develop strategies to address this important chronic liver disease.
Inflammation in the liver is crucial for the progression from hepatic steatosis to NASH, and infiltration of different subsets of inflammatory cells is the hallmark of steatohepatitis.25 In this study, we found that the percentage of DNT cells in the liver was significantly higher than that in the spleen or peripheral blood, indicating the importance of DNT cells in liver immunity. However, the function of these specific T cells in liver physiology and pathology is still unclear. DNT cells play critical and diverse roles in the immune system, and they are reported to have both proinflammatory and anti-inflammatory functions. Many studies have reported that DNT cells display a unique immunoregulatory ability,9,10,17,26 and the immune regulation of DNT cells mainly relies on the high expression of the cytotoxic molecules GZMB and perforin.10,13,26 In this study, we found that among hepatic DNT cells, TCRαβ+ DNT cells are the major source of GZMB and contribute to the maintenance of liver homeostasis. However, during NAFLD development, TCRαβ+ DNT cells and their GZMB production are decreased, which results in the weakened immune regulation of TCRαβ+ DNT cells and promotes NAFLD/NASH progression. Adoptive transfer of natural hepatic TCRαβ+ DNT cells inhibited liver inflammation, enhanced PD-1+CD122+CD8-Treg cell liver infiltration, restored liver homeostasis, and significantly ameliorated NAFLD.
In this study, we also revealed that the level of IL17 secreted by hepatic TCRγδ+ DNT cells was much higher than that secreted by TCRαβ+ DNT cells in normal mice. During NAFLD development, IL17 production was markedly enhanced by TCRγδ+ DNT cells but not TCRαβ+ DNT cells, which might aggravate liver inflammation and facilitate NAFLD/NASH progression. When we eliminated TCRγδ+ DNT cells, IL17 secretion by intrahepatic DNT cells was sharply decreased in the mice with NAFLD. Many studies have reported that TCRγδ+ DNT cells exert significant protective effects on self-tolerance27,28 and tumor formation29 in mice. Human peripheral, tumor-infiltrating, small intestinal TCRγδ+ DNT cells also possess immunosuppressive potential in tumors, celiac disease, and systemic lupus erythematosis.30, 31, 32, 33, 34 IL17A induces Kupffer cell activation, macrophage activation, and subsequent cytokine secretion, and neutrophil accumulation plays important roles in ischemia and reperfusion injury and liver fibrosis.35 In our study, we found that TCRγδ+ DNT cell deletion decreased liver injury and M1 monocyte proportions, which indicated that these cells played important roles in the proinflammatory effects of NAFLD.
Hepatic FA metabolic disorder manifests as an imbalance between harmful FAs and beneficial FAs in NAFLD. FA synthesis, catabolism, and oxidation are pivotal for the proliferation, survival, differentiation, and function of T cells.36,37 FA catabolism and oxidation are pivotal for the development and function of CD8+ T-cell memory as well as for the differentiation of CD4+ regulatory T cells.36 Moreover, FA oxidation is an essential factor for CD8+ tissue-resident memory T-cell survival in gastric adenocarcinoma.37 In this study, we found that the harmful FA AA may be the key factor in regulating the balance of TCRαβ+ DNT cells and TCRγδ+ DNT cell survival and functions.
Leukotriene B4, prostaglandin E2, and eicosanoids induced by AA are typical inflammatory mediators, and they regulate T- and B-lymphocyte functions.38, 39, 40 Moreover, AA reduces T-cell proliferation in human peripheral blood.41 Our results showed that AA could induce intrahepatic DNT cell apoptosis and down-regulate GZMB expression in TCRαβ+ DNT cells. The AKT and NF-κB signaling pathways play a key role in regulating the immune response of immune cells, and we have also found that these signaling pathways play an important role in the regulation of TCRαβ+ DNT cells by AA. We also found that in contrast to the effect on TCRαβ+ DNT cells, AA promoted the secretion of IL17A by TCRγδ+ DNT cells, suggesting that it also promoted the inflammatory response and that the NF-κB signaling pathway may be involved. The discrepant effects of FAs on TCRαβ+ and TCRγδ+ DNT cells need in-depth exploration in the future.
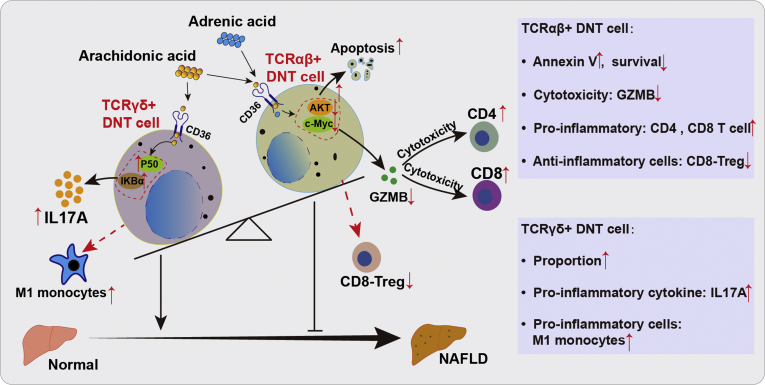
In conclusion, our data revealed that AA and ADA induced apoptosis of TCRαβ+ DNT cells and decreased their immunosuppressive function, which was mainly associated with the AKT signaling pathway during NAFLD development. AA also facilitated IL17A secretion by TCRγδ+ DNT cells, which was mainly associated with the NF-κB signaling pathway (Figure 11). Exploring the different functions of TCR subtypes would help elucidate the role of DNT cells in the progression of NAFLD, and potentially controlling the different DNT subtypes will contribute to NAFLD therapy.
Figure 11.
Intrinsic mechanisms of AA and ADA regulation on TCRαβ+ and TCRγδ+ DNT cells during NAFLD development. AA and ADA induced apoptosis of TCRαβ+ DNT cells and decreased their immunosuppressive function, which were mainly associated with the AKT signaling pathway during NAFLD development. AA also facilitated IL17A secretion by TCRγδ+ DNT cells, which was mainly associated with the NF-κB signaling pathway.
Methods
Animals and Treatment
Eight-week-old weight-matched male C57BL/6 mice and CD45.1 congenic C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). MCD has short modeling duration, low cost, and easy acquisition, which can effectively induce steatosis and steatohepatitis, and the lipid distribution in liver can simulate human NAFLD well.42, 43, 44 One of the most important disadvantages of the MCD model is that the extrahepatic metabolic performance differs from that of the vast majority of human NAFLD patients, with this model displaying significant loss weight gain and decreased blood glucose levels.44 In addition, CDHFD-fed mice also developed well to mimic human NAFLD and fibrosis disease.44 Because of its long duration, it has a high chance of progressing to liver cancer.45 Thus, we mainly used these 2 diets to induce NAFLD models. After being allowed to adapt for 5 days, the C57BL/6 mice were fed either a NCD or a MCD (10401; Beijing HFK Bioscience, Beijing, China) for 5 weeks or a CDHFD for 18 weeks. The mice were housed in a pathogen-free, comfortable temperature environment with a 12-hour light/dark cycle. All animal studies were performed in compliance with the ethical guidelines for animal studies and approved by the animal ethics committee of Beijing Friendship Hospital, Capital Medical University.
Enzyme-Linked Immunosorbent Assay and H&E, Oil Red O, and Immunohistochemistry Staining of α-SMA
Serum AST and ALT enzyme-linked immunosorbent assay kits were obtained from Jiancheng (Nanjing, China). All experiments followed the manufacturer’s instructions. The left lobes of livers were fixed with 4% paraformaldehyde and then stained with H&E, Oil Red O staining, and immunohistochemistry for α-SMA by Servicebio Technology (Wuhan, China).
Flow Cytometry
The isolation and detection of hepatic immune cells were conducted as previously described.46 Immune cells were harvested, and the expression of various cell surface, intracellular, and intranuclear markers was determined (Table 1). A FACS Aria II flow cytometer (BD Biosciences, San Jose, CA) was applied to measure all samples, and FlowJo software version V10 data were used to analyze the data.
Table 1.
Various Cell Surface, Intracellular, and Intranuclear Markers in This Study
| Manufacturer | Mouse antibody | Clone/catalog number |
|---|---|---|
| BioLegend (USA) | CD45 (PE/Cyanine7) | S18009F |
| CD45 (FITC) | QA17A26 | |
| CD45 (BV510) | 30-F11 | |
| CD45(APC/Cyanine7) | 30-F11 | |
| CD45 (BV605) | 30-F11 | |
| CD3 (Percp/Cyanine5.5) | 17A2 | |
| CD3 (APC) | 17A2 | |
| CD3 (PE/Cyanine7) | 17A2 | |
| CD3 (PE) | 17A2 | |
| CD3 (BV785) | 17A2 | |
| NK1.1 (PE) | S17016D | |
| NK1.1(FITC) | S17016D | |
| NK1.1 (BV421) | PK136 | |
| NK1.1 (BV605) | PK136 | |
| CD4 (PE) | GK1.5 | |
| CD4 (Percp) | GK1.5 | |
| CD4 (PE/Cyanine7) | GK1.5 | |
| CD4 (BV785) | GK1.5 | |
| CD4 (Pacific Blue) | GK1.5 | |
| CD4 (FITC) | GK1.5 | |
| CD4 (BV650) | GK1.5 | |
| CD8a (APC) | 53-6.7 | |
| CD8a (PE) | 53-6.7 | |
| CD8a (APC/Cyanine7) | 53-6.7 | |
| CD8a (FITC) | 53-6.7 | |
| Annexin V (APC) | 640941 | |
| Annexin V (PE) | 640934 | |
| Annexin V (FITC) | 640906 | |
| 7-AAD (Percp/ Cyanine5.5) | 420404 | |
| Ki67(FITC) | 16A8 | |
| TCRβ (FITC) | H57-597 | |
| TCRβ (PE/Cyanine7) | H57-597 | |
| TCRβ (Percp/Cyanine5.5) | H57-597 | |
| TCRγδ (PE) | UC7-13D5 | |
| TCRγδ (APC) | GL3 | |
| CD36 (APC) | HM36 | |
| Ly6G (APC/Cyanine7) | 1A8 | |
| CD11b (BV421) | M1/70 | |
| F4/80 (PE) | BM8 | |
| CD11c (FITC) | N418 | |
| CD206 (APC) | C068C2 | |
| IL17A (PE) | TC11-18H10.1 | |
| GZMB (FITC) | GB11 | |
| GZMB (PE) | QA16A02 | |
| GZMB (APC) | QA16A02 | |
| IFNγ (BV711) | XMG1.2 | |
| eBioscience | NKG2D (PE) | A10 |
| CD122 (PE) | TM-b1(TM-beta1) | |
| P-AKT(APC) | SDRNR | |
| Abcam | NFκB P105/P50 | ab19285 |
| P-IKB αlphα | ab32518 | |
| Goat pAb to Rb IgG (Alexa Fluor 488) | ab150077 | |
| Invitrogen | BODIPYTM 493/503 | D3922 |
Transcriptome Sequencing Analysis
Intrahepatic DNT cells from the NCD- and MCD-fed mice were sorted by a FACS Aria II flow cytometer, and total RNA was isolated. Transcriptome sequencing libraries were sequenced on an Illumina HiSeq 2000 platform (Illumina, San Diego, CA) following the manufacturer’s recommendations. After the measurement of fragments per kilobase per million mapped reads value, we performed a differential expression analysis of the transcripts with P value <.05 and fold change ≤0.5 or fold change ≥1.5 between the NCD- and MCD-fed mice, and these transcripts were submitted to GO and KEGG enrichment analysis, which uses unbiased methods to assess pathway enrichment.
Quantitative Real-Time Reverse Transcription–PCR
Total RNA of primary DNT cells from NCD- or MCD-fed mouse livers was isolated using TRIzol Reagent (T9424-200ML; Sigma-Aldrich, St Louis, MO), and first strand cDNA was synthesized using PrimeScript RT Master Mix (RR037A; TaKaRa, Japan) according to the manufacturer’s instructions. The cDNA was subjected to PCR cycles with SYBR Green quantitative PCR reagent (11202ES08; Yeasen, Shanghai, China), and assays were conducted using the ABI 7500 sequence detection system (Applied Biosystems, Waltham, MA). The primers are presented in Table 2.
Table 2.
Primers in This Study
| Sequence | ||
|---|---|---|
| GAPDH | Forward Primer | AAGGTCATCCCAGAGCTGAA |
| Reverse Primer | CTGCTTCACCACCTTCTTGA | |
| Bik | Forward Primer | CTGCGTCAACACTGCCAGATA |
| Reverse Primer | CTTGTCCTTAATGCGGCTCAG | |
| Arrb1 | Forward Primer | AAGGGACACGAGTGTTCAAGA |
| Reverse Primer | CCCGCTTTCCCAGGTAGAC | |
| Atf6 | Forward Primer | TGGAGCAGGATGTCCCGTT |
| Reverse Primer | CTGTGGAAAGATGTGAGGACTC | |
| Rarg | Forward Primer | ATGTACGACTGCATGGAATCG |
| Reverse Primer | CCAGTGGCTCTGCGTAGTAA | |
| Top2a | Forward Primer | CAACTGGAACATATACTGCTCCG |
| Reverse Primer | GGGTCCCTTTGTTTGTTATCAGC | |
| Ccna2 | Forward Primer | GCCTTCACCATTCATGTGGAT |
| Reverse Primer | TTGCTCCGGGTAAAGAGACAG | |
| Ppp2ca | Forward Primer | ATGGACGAGAAGTTGTTCACC |
| Reverse Primer | CAGTGACTGGACATCGAACCT | |
| Ect2 | Forward Primer | AACTTGTGCTTGGCGTCTACT |
| Reverse Primer | TTCCTCCGATTTTCCAGGACA | |
| Dusp6 | Forward Primer | ATAGATACGCTCAGACCCGTG |
| Reverse Primer | ATCAGCAGAAGCCGTTCGTT | |
| Mcl1 | Forward Primer | AAAGGCGGCTGCATAAGTC |
| Reverse Primer | TGGCGGTATAGGTCGTCCTC | |
| Ncr1 | Forward Primer | ATGCTGCCAACACTCACTG |
| Reverse Primer | GATGTTCACCGAGTTTCCATTTG | |
| Klrb1c | Forward Primer | ATGGACACAGCAAGTATCTACCT |
| Reverse Primer | AGCTCTCAGGAGTCACTTTATCT | |
| Klrg1 | Forward Primer | TCTCATCCCTTCCTCTGC |
| Reverse Primer | TTGCGTCTTTCTGTCTTGT | |
| Havcr2 | Forward Primer | ACTGGTGACCCTCCATAATAACA |
| Reverse Primer | GCAGTTCTGATCGTTTCTCCA | |
| Fyn | Forward Primer | TCAACACGGGGAGTAACCC |
| Reverse Primer | CGAGCTTTGTCCTGCAACT | |
| Klra1 | Forward Primer | GTTCCTTCAGACGTTTGCTGG |
| Reverse Primer | ATTGTCAACCCATGACCAATCTT | |
| IL1R1 | Forward Primer | GTGCTACTGGGGCTCATTTGT |
| Reverse Primer | GGAGTAAGAGGACACTTGCGAAT | |
| IL2Ra | Forward Primer | AACCATAGTACCCAGTTGTCGG |
| Reverse Primer | TCCTAAGCAACGCATATAGACCA | |
| Ahr | Forward Primer | ACATACGCCGGTAGGAAGAGA |
| Reverse Primer | GGTCCAGCTCTGTATTGAGGC | |
| Rorc | Forward Primer | AGGAGAGCGTTCCAAGACCA |
| Reverse Primer | AGTTTCCGCTTCATGCTACTG | |
| Il17a | Forward Primer | GCTCCAGAAGGCCCTCAGACT |
| Reverse Primer | CCAGCTTTCCCTCCGCATTGA | |
| Il17f | Forward Primer | TGCTACTGTTGATGTTGGGAC |
| Reverse Primer | AATGCCCTGGTTTTGGTTGAA | |
| Ifngr2 | Forward Primer | TCCTCGCCAGACTCGTTTTC |
| Reverse Primer | GTCTTGGGTCATTGCTGGAAG | |
| IL21R | Forward Primer | GGCTGCCTTACTCCTGCTG |
| Reverse Primer | TCATCTTGCCAGGTGAGACTG | |
| IL-23R | Forward Primer | TTCAGATGGGCATGAATGTTTCT |
| Reverse Primer | CCAAATCCGAGCTGTTGTTCTAT | |
| CD36 | Forward Primer | ATGGGCTGTGATCGGAACTG |
| Reverse Primer | GTCTTCCCAATAAGCATGTCTCC | |
| Pdcd1 | Forward Primer | TTCACCTGCAGCTTGTCCAA |
| Reverse Primer | TGGGCAGCTGTATGATCTGG | |
| Fasl | Forward Primer | TGAATTACCCATGTCCCCAG |
| Reverse Primer | AAACTGACCCTGGAGGAGCC | |
| Perforin | Forward Primer | CTGCCACTCGGTCAGAATG |
| Reverse Primer | CGGAGGGTAGTCACATCCAT | |
| Granzyme B | Forward Primer | ATCCTGCTCTGATTACCCATCGT |
| Reverse Primer | ATGGACATGAAGCCAGTCTTTGC | |
| IFN-γ | Forward Primer | GGCCATCAGCAACAACATAAGCGT |
| Reverse Primer | TGGGTTGTTGACCTCAAACTTGGC | |
| Klrk1 | Forward Primer | ACTCAGAGATGAGCAAATGCC |
| Reverse Primer | CAGGTTGACTGGTAGTTAGTGC | |
| Klra7 | Forward Primer | GCAGAAACTAGTGAGGACTGAG |
| Reverse Primer | TTACCAGGAGAAGGAAACAGAAG | |
| Klrd1 | Forward Primer | TCTAGGATCACTCGGTGGAGA |
| Reverse Primer | CACTTGTCCAGGCAAACACAG | |
| Klrc1 | Forward Primer | AGAAACTCATTGCTGGTA |
| Reverse Primer | CCTTTGCTTCGGTAT | |
| Ifngr1 | Forward Primer | TTGACGAGCACTGAGGA |
| Reverse Primer | AGGAACCCGAATACACC | |
| IL-4 | Forward Primer | GTCATCCTGCTCTTCTTT |
| Reverse Primer | ATGGCGTCCCTTCTC | |
| Jak2 | Forward Primer | CCACGGCCCAATATCAATG |
| Reverse Primer | CCCGCCTTCTTTAGTTTGCTA | |
| Cxcl16 | Forward Primer | CCTTGTCTCTTGCGTTCTTCC |
| Reverse Primer | TCCAAAGTACCCTGCGGTATC | |
| c-Myc | Forward Primer | TCTCCACTCACCAGCACAACTACG |
| Reverse Primer | ATCTGCTTCAGGACCCT | |
| Nfkb2 | Forward Primer | GGCCGGAAGACCTATCCTACT |
| Reverse Primer | CTACAGACACAGCGCACACT |
TCRγδ+ DNT Depletion Assay
Injections with 400 μg anti-mouse TCRγ/δ (BE0070-25MG; Bio X Cell, Lebanon, NH) were administered on day –2, and administration of 200 μg InVivoMab anti-mouse TCRγ/δ was repeated on days –1, 3, 6, 10, 13, 17, 20, 24, 27, 31, 34, 38, and 41. Phosphate-buffered saline served as the control group. On day 43, mice were killed for the study of progressive NAFLD as described above.
TCRαβ+ DNT Cells Adoptive Transfer Administration in Vivo
TCRαβ+ DNT cells were isolated from C57BL/6 mouse liver by a fluorescence-activated cell sorter (FACS Aria II; BD Biosciences). In the MCD-fed NAFLD model, the C57BL/6 mice received an adoptive transfer of 3 × 106 CD45.1-positive DNT (purity > 97%) and were fed the MCD for 5 weeks; then liver inflammation and lymphocytes proportion were detected as described above.
Metabonomics and FA Stimulation Assay
A liver sample (50 mg) from the NCD- and MCD-fed mice was subjected to identification and quantification of the 65 FFAs using a Q300 Metabolite Array Kit (Metabo-Profile, Shanghai, China). CD3+ T cells were enriched from C57BL/6 mouse spleens by a mouse CD3+ T-cell enrichment column (R&D Systems, Minneapolis, MN). Isolated CD3+ T cells were cultured in wells precoated with the anti-CD3 monoclonal antibody (5 μg/mL; BD Biosciences) and the anti-CD28 monoclonal antibody (2 μg/mL; BD Biosciences) and then with 25 or 50 μmol/L ADA, AA (MCE, Monmouth Junction, NJ), or dimethyl sulfoxide at 37°C with 5% CO2 for 48 hours, and 25 μmol/L ADA group was sent to transcriptome sequencing after extracting RNA by RNasey Plus Micro Kit according to instructions (Qiagen, Hilden. Germany). Then, flow cytometry was conducted to assess apoptosis and cell surface markers of DNT cells. One μmol/L, 5 μmol/L AKT activator SC-79 (MCE) or 1 nmol/L, 5 nmol/L NF-κB signaling pathway inhibitor BAY 11-7082 (MCE) were used to interrupt effect induced by AA in TCRαβ+ DNT cells or TCRγδ+ DNT cells for 48 hours.
Clinical Study
Seven patients diagnosed with NAFLD were confirmed by liver biopsy specimen at Capital Medical University, Beijing Friendship Hospital. NAFLD patients were consuming less than 10 g alcohol/day for women and 20 g alcohol/day for men, and other causes of steatosis or chronic liver disease were excluded. Healthy liver tissues were obtained from 6 donors whose livers were subsequently used for liver transplantation. Written informed consent was obtained from all patients before their enrollment, and the study protocol was approved by the Human Institutional Review Board of Beijing Friendship Hospital (No. 2017-P2-131-03).
Human liver tissues were fixed in 4% paraformaldehyde for 48 hours and then embedded in paraffin, sectioned at 5 μm for immunofluorescence staining. After deparaffinization and rehydration, we performed antigen unmasking using heat treatment with citrate solution, followed by incubating with 10% H2O2 to neutralize endogenous peroxidase activity. We blocked nonspecific binding sites with goat serum for 1 hour at 37°C. Then the sections were incubated overnight at 4°C with the primary antibodies against TCRα (Santa Cruz Biotechnology, Dallas, TX; sc-515719, 1:50), TCRδ (Santa Cruz Biotechnology; sc-100289, 1:50), CD4 (Beijing Zhongshan Golden Bridge Biotechnology Co; TA500481, 1:50), CD8 (Cell Signaling Technology, Danvers, MA; 70306s, 1:50), and CD56 (Cell Signaling Technology; 99746s, 1:50). The secondary antibodies (Beijing Zhongshan Golden Bridge Biotechnology Co) were added and then incubated at room temperature for 1 hour before TSA reaction. The sections were observed by microscopy (FV1000; Olympus, Tokyo, Japan).
Statistical Analysis
Statistical analysis was performed by using SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0; Armonk, NY) and Prism 8.0 software (GraphPad Software, San Diego, CA). Values are expressed as the mean ± standard error of the mean. The normal distribution of variables was tested with the Shapiro–Wilk test. Differences between 2 groups were compared by t tests for normal variables and Kruskal–Wallis tests for non-normal variables. One-way analysis of variance with post hoc test for normal variables and Kruskal–Wallis tests for non-normal variables were used in multiple comparisons. Two-sided P values <.05 were considered significant.
Acknowledgments
CRediT Authorship Contributions
Changying Li (Data curation: Lead; Formal analysis: Lead; Investigation: Lead; Methodology: Lead; Project administration: Lead; Resources: Equal; Software: Lead; Validation: Lead; Visualization: Lead; Writing – original draft: Lead; Writing – review & editing: Supporting)
Xiaonan Du (Data curation: Equal; Formal analysis: Equal; Investigation: Equal; Methodology: Equal)
Zongshan Shen (Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting)
Yunxiong Wei (Data curation: Supporting; Investigation: Supporting; Resources: Supporting; Supervision: Supporting)
Yaning Wang (Data curation: Supporting)
Xiaotong Han (Data curation: Supporting)
Hua Jin (Data curation: Supporting)
Chunpan Zhang (Data curation: Supporting)
Mengyi Li (Methodology: Supporting; Resources: Supporting; Supervision: Supporting)
Zhongtao Zhang (Project administration: Supporting; Supervision: Supporting)
Songlin Wang (Supervision: Supporting)
Dong Zhang (Funding acquisition: Equal; Methodology: Supporting; Project administration: Lead; Resources: Lead; Writing – review & editing: Equal)
Guangyong Sun (Funding acquisition: Lead; Resources: Equal; Supervision: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by grants from the National Natural Science Foundation of China (No. 81870399, 81970503, 82001694 and 82070580), the Beijing Natural Science Foundation (7192046), and Youth Beijing Scholar (No. 035).
Contributor Information
Dong Zhang, Email: zhangd@ccmu.edu.cn.
Guangyong Sun, Email: sungy@ccmu.edu.cn.
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Petroni M.L., Brodosi L., Bugianesi E., Marchesini G. Management of non-alcoholic fatty liver disease. BMJ. 2021;372 doi: 10.1136/bmj.m4747. [DOI] [PubMed] [Google Scholar]
- 3.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 4.Cai J., Zhang X.J., Li H. The role of innate immune cells in nonalcoholic steatohepatitis. Hepatology. 2019;70:1026–1037. doi: 10.1002/hep.30506. [DOI] [PubMed] [Google Scholar]
- 5.Sutti S., Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81–92. doi: 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt D., Hedrich C.M. TCRalphabeta(+)CD3(+)CD4(-)CD8(-) (double negative) T cells in autoimmunity. Autoimmun Rev. 2018;17:422–430. doi: 10.1016/j.autrev.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Juvet S.C., Zhang L. Double negative regulatory T cells in transplantation and autoimmunity: recent progress and future directions. J Mol Cell Biol. 2012;4:48–58. doi: 10.1093/jmcb/mjr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillhouse E.E., Lesage S. A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. J Autoimmun. 2013;40:58–65. doi: 10.1016/j.jaut.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Fischer K., Voelkl S., Heymann J., Przybylski G.K., Mondal K., Laumer M., Kunz-Schughart L., Schmidt C.A., Andreesen R., Mackensen A. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(-)CD8- double-negative regulatory T cells. Blood. 2005;105:2828–2835. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
- 10.Zhang D., Yang W., Degauque N., Tian Y., Mikita A., Zheng X.X. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood. 2007;109:4071–4079. doi: 10.1182/blood-2006-10-050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D., Zhang W., Ng T.W., Wang Y., Liu Q., Gorantla V., Lakkis F., Zheng X.X. Adoptive cell therapy using antigen-specific CD4(-)CD8(-)T regulatory cells to prevent autoimmune diabetes and promote islet allograft survival in NOD mice. Diabetologia. 2011;54:2082–2092. doi: 10.1007/s00125-011-2179-4. [DOI] [PubMed] [Google Scholar]
- 12.Maccari M.E., Fuchs S., Kury P., Andrieux G., Volkl S., Bengsch B., Lorenz M.R., Heeg M., Rohr J., Jagle S., Castro C.N., Gross M., Warthorst U., Konig C., Fuchs I., Speckmann C., Thalhammer J., Kapp F.G., Seidel M.G., Duckers G., Schonberger S., Schutz C., Fuhrer M., Kobbe R., Holzinger D., Klemann C., Smisek P., Owens S., Horneff G., Kolb R., Naumann-Bartsch N., Miano M., Staniek J., Rizzi M., Kalina T., Schneider P., Erxleben A., Backofen R., Ekici A., Niemeyer C.M., Warnatz K., Grimbacher B., Eibel H., Mackensen A., Frei A.P., Schwarz K., Boerries M., Ehl S., Rensing-Ehl A. A distinct CD38+CD45RA+ population of CD4+, CD8+, and double-negative T cells is controlled by FAS. J Exp Med. 2021:218. doi: 10.1084/jem.20192191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z.X., Ma Y., Wang H., Arp J., Jiang J., Huang X., He K.M., Garcia B., Madrenas J., Zhong R. Double-negative T cells, activated by xenoantigen, lyse autologous B and T cells using a perforin/granzyme-dependent, Fas-Fas ligand-independent pathway. J Immunol. 2006;177:6920–6929. doi: 10.4049/jimmunol.177.10.6920. [DOI] [PubMed] [Google Scholar]
- 14.Li W., Tian Y., Li Z., Gao J., Shi W., Zhu J., Zhang D. Ex vivo converted double negative T cells suppress activated B cells. Int Immunopharmacol. 2014;20:164–169. doi: 10.1016/j.intimp.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Gao J.F., McIntyre M.S., Juvet S.C., Diao J., Li X., Vanama R.B., Mak T.W., Cattral M.S., Zhang L. Regulation of antigen-expressing dendritic cells by double negative regulatory T cells. Eur J Immunol. 2011;41:2699–2708. doi: 10.1002/eji.201141428. [DOI] [PubMed] [Google Scholar]
- 16.Su Y., Huang X., Wang S., Min W.P., Yin Z., Jevnikar A.M., Zhang Z.X. Double negative Treg cells promote nonmyeloablative bone marrow chimerism by inducing T-cell clonal deletion and suppressing NK cell function. Eur J Immunol. 2012;42:1216–1225. doi: 10.1002/eji.201141808. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z.X., Yang L., Young K.J., DuTemple B., Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–789. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 18.Voelkl S., Gary R., Mackensen A. Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4- CD8- double-negative T cells. Eur J Immunol. 2011;41:739–748. doi: 10.1002/eji.201040982. [DOI] [PubMed] [Google Scholar]
- 19.Crispin J.C., Oukka M., Bayliss G., Cohen R.A., Van Beek C.A., Stillman I.E., Kyttaris V.C., Juang Y.T., Tsokos G.C. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alunno A., Bistoni O., Bartoloni Bocci E., Caterbi S., Bigerna B., Pucciarini A., Tabarrini A., Mannucci R., Beghelli D., Falini B., Gerli R. IL-17-producing double-negative T cells are expanded in the peripheral blood, infiltrate the salivary gland and are partially resistant to corticosteroid therapy in patients with Sjogren’s syndrome. Reumatismo. 2013;65:192–198. doi: 10.4081/reumatismo.2013.192. [DOI] [PubMed] [Google Scholar]
- 21.Li H., Adamopoulos I.E., Moulton V.R., Stillman I.E., Herbert Z., Moon J.J., Sharabi A., Krishfield S., Tsokos M.G., Tsokos G.C. Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat Commun. 2020;11:2859. doi: 10.1038/s41467-020-16636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun G., Zhao X., Li M., Zhang C., Jin H., Li C., Liu L., Wang Y., Shi W., Tian D., Xu H., Tian Y., Wu Y., Liu K., Zhang Z., Zhang D. CD4 derived double negative T cells prevent the development and progression of nonalcoholic steatohepatitis. Nat Commun. 2021;12:650. doi: 10.1038/s41467-021-20941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Wang H., Peters M., Ding N., Ribback S., Utpatel K., Cigliano A., Dombrowski F., Xu M., Chen X., Song X., Che L., Evert M., Cossu A., Gordan J., Zeng Y., Chen X., Calvisi D.F. Loss of Fbxw7 synergizes with activated Akt signaling to promote c-Myc dependent cholangiocarcinogenesis. J Hepatol. 2019;71:742–752. doi: 10.1016/j.jhep.2019.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F., Li K., Yao X., Wang H., Li W., Wu J., Li M., Zhou R., Xu L., Zhao L. A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine. 2019;44:311–321. doi: 10.1016/j.ebiom.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X., Sun G., Sun X., Tian D., Liu K., Liu T., Cong M., Xu H., Li X., Shi W., Tian Y., Yao J., Guo H., Zhang D. A novel differentiation pathway from CD4(+) T cells to CD4(-) T cells for maintaining immune system homeostasis. Cell Death Dis. 2016;7 doi: 10.1038/cddis.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locke N.R., Stankovic S., Funda D.P., Harrison L.C. TCR gamma delta intraepithelial lymphocytes are required for self-tolerance. J Immunol. 2006;176:6553–6559. doi: 10.4049/jimmunol.176.11.6553. [DOI] [PubMed] [Google Scholar]
- 28.Ashour H.M., Niederkorn J.Y. Gammadelta T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J Immunol. 2006;177:8331–8337. doi: 10.4049/jimmunol.177.12.8331. [DOI] [PubMed] [Google Scholar]
- 29.Seo N., Tokura Y., Takigawa M., Egawa K. Depletion of IL-10- and TGF-beta-producing regulatory gamma delta T cells by administering a daunomycin-conjugated specific monoclonal antibody in early tumor lesions augments the activity of CTLs and NK cells. J Immunol. 1999;163:242–249. [PubMed] [Google Scholar]
- 30.Kuhl A.A., Pawlowski N.N., Grollich K., Blessenohl M., Westermann J., Zeitz M., Loddenkemper C., Hoffmann J.C. Human peripheral gammadelta T cells possess regulatory potential. Immunology. 2009;128:580–588. doi: 10.1111/j.1365-2567.2009.03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng G., Wang H.Y., Peng W., Kiniwa Y., Seo K.H., Wang R.F. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27 doi: 10.1016/j.immuni.2007.05.020. 334–248. [DOI] [PubMed] [Google Scholar]
- 32.Bhagat G., Naiyer A.J., Shah J.G., Harper J., Jabri B., Wang T.C., Green P.H., Manavalan J.S. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–293. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Kang N., Zhang X., Dong X., Wei W., Cui L., Ba D., He W. Generation of human regulatory gammadelta T cells by TCRgammadelta stimulation in the presence of TGF-beta and their involvement in the pathogenesis of systemic lupus erythematosus. J Immunol. 2011;186:6693–6700. doi: 10.4049/jimmunol.1002776. [DOI] [PubMed] [Google Scholar]
- 34.Ye J., Ma C., Hsueh E.C., Eickhoff C.S., Zhang Y., Varvares M.A., Hoft D.F., Peng G. Tumor-derived gammadelta regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol. 2013;190:2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giles D.A., Moreno-Fernandez M.E., Divanovic S. IL-17 axis driven inflammation in non-alcoholic fatty liver disease progression. Curr Drug Targets. 2015;16:1315–1323. doi: 10.2174/1389450116666150531153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lochner M., Berod L., Sparwasser T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015;36:81–91. doi: 10.1016/j.it.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Lin R., Zhang H., Yuan Y., He Q., Zhou J., Li S., Sun Y., Li D.Y., Qiu H.B., Wang W., Zhuang Z., Chen B., Huang Y., Liu C., Wang Y., Cai S., Ke Z., He W. Fatty acid oxidation controls CD8(+) tissue-resident memory T-cell survival in gastric adenocarcinoma. Cancer Immunol Res. 2020;8:479–492. doi: 10.1158/2326-6066.CIR-19-0702. [DOI] [PubMed] [Google Scholar]
- 38.Calder P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77:327–335. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Valitutti S., Castellino F., Aiello F.B., Ricci R., Patrignani P., Musiani P. The role of arachidonic acid metabolite PGE2 on T cell proliferative response. J Clin Lab Immunol. 1989;29:167–173. [PubMed] [Google Scholar]
- 40.Kawahara K., Hohjoh H., Inazumi T., Tsuchiya S., Sugimoto Y. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta. 2015;1851:414–421. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Zurier R.B., Rossetti R.G., Seiler C.M., Laposata M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: effects of unsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1999;60:371–375. doi: 10.1016/s0952-3278(99)80015-5. [DOI] [PubMed] [Google Scholar]
- 42.Marcolin E., Forgiarini L.F., Tieppo J., Dias A.S., Freitas L.A., Marroni N.P. Methionine- and choline-deficient diet induces hepatic changes characteristic of non-alcoholic steatohepatitis. Arq Gastroenterol. 2011;48:72–79. doi: 10.1590/s0004-28032011000100015. [DOI] [PubMed] [Google Scholar]
- 43.Haberl E.M., Pohl R., Rein-Fischboeck L., Horing M., Krautbauer S., Liebisch G., Buechler C. Hepatic lipid profile in mice fed a choline-deficient, low-methionine diet resembles human non-alcoholic fatty liver disease. Lipids Health Dis. 2020;19:250. doi: 10.1186/s12944-020-01425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jahn D., Kircher S., Hermanns H.M., Geier A. Animal models of NAFLD from a hepatologist’s point of view. Biochim Biophys Acta Mol Basis Dis. 2019;1865:943–953. doi: 10.1016/j.bbadis.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 45.Marquez-Quiroga L.V., Arellanes-Robledo J., Vasquez-Garzon V.R., Villa-Trevino S., Muriel P. Models of nonalcoholic steatohepatitis potentiated by chemical inducers leading to hepatocellular carcinoma. Biochem Pharmacol. 2022;195:114845. doi: 10.1016/j.bcp.2021.114845. [DOI] [PubMed] [Google Scholar]
- 46.Sun G., Jin H., Zhang C., Meng H., Zhao X., Wei D., Ou X., Wang Q., Li S., Wang T., Sun X., Shi W., Tian D., Liu K., Xu H., Tian Y., Li X., Guo W., Jia J., Zhang Z., Zhang D. OX40 regulates both innate and adaptive immunity and promotes nonalcoholic steatohepatitis. Cell Rep. 2018;25:3786–3799 e4. doi: 10.1016/j.celrep.2018.12.006. [DOI] [PubMed] [Google Scholar]