Abstract
Glucocorticoid resistance often dims glucocorticoid’s therapeutic efficacy in sepsis. However, the mechanism is incompletely understood. In this issue, Vandewalle et al. (2021) demonstrate that glucocorticoid resistance leads to hyperlactatemia and that this combination facilitates lethal sepsis. This insight gives important clarity to the pathophysiology of sepsis, while further suggesting therapeutic avenues for its treatment.
Sepsis is a multifactorial, complex pathological condition attributing to the maladaptive function of the metabolic, immune, vascular, coagulation, and endocrine systems. Current treatment options of sepsis are mainly palliative care, while focusing on controlling infections, maintaining blood pressure by fluid resuscitation and vasopressors, and providing mechanical support to failing organs (Lelubre and Vincent, 2018). Glucocorticoids (GCs) are steroid hormones widely used to treat inflammation and autoimmune diseases (Timmermans et al., 2019). However, the benefits of GC treatment in sepsis patients remain limited (Fang et al., 2019). GCs bind to the GC receptor (GR), which belongs to the nuclear receptor superfamily of transcription factors, to exert their physiological and therapeutic effects. GC binding to GR transduces potential anti-inflammatory signals by squelching pro-inflammatory transcription factors that promote the expression of inflammatory mediators and regulating the expression of proteins of gluconeogenesis (Cain and Cidlowski, 2017; Timmermans et al., 2019). During sepsis, hyperinflammation, hypoglycemia, and hyperlactatemia are the key indicators of poor prognosis, representing the abnormal homeostasis of metabolic and immune pathways (Vandewalle et al., 2021). During the first 12 h of the cecal ligation and puncture (CLP)-induced sepsis model in mice, hyperglycemia is observed, followed by severe hypoglycemia 24 h after surgery (Vandewalle et al., 2021). Several clinical trials with GCs in severe sepsis and septic shock reveal controversial findings, as some studies have described a survival benefit (Annane et al., 2018), while others have not (Venkatesh et al., 2018). Clinical trials with GCs focusing on the relationship between the timing of GC administration and survival benefit reveal a significant association between the time lag to GC therapy and mortality (Katsenos et al., 2014), pinpointing that the GC resistance appears to be the cause of unfavorable outcomes in septic shock. Moreover, reduced GR expression and function have been correlated with disease severity and mortality in sepsis. While the GR unresponsiveness has been identified as the critical factor for GC resistance and subsequent unsuccessful clinical trials with GC therapy, a detailed downstream mechanism of GC resistance involving the crosstalk of various physiological systems remains largely unknown. In their study using a genome-wide transcriptome assay in hepatic tissues, Vandewalle et al. have identified the decreased expression of gluconeogenesis regulatory genes following treatment of septic mice with steroids (Vandewalle et al., 2021). Thus, they identified a pivotal link between GC resistance, GR unresponsiveness, and maladaptive gluconeogenesis in sepsis.
Lactate is produced in excess in sepsis, which needs to be cleared from the liver by the Cori cycle to avoid hyperlactatemia. However, given the genome-wide GC resistance that quickly develops in septic livers, the expression levels of the rate-limiting enzymes of the gluconeogenesis pathway, phosphoenolpyruvate carboxykinase (PEPCK), and glucose 6-phosphatase (G6Pase) are downregulated, leading to lactate accumulation in the circulation. While hyperlactatemia serves as a biomarker of sepsis, interestingly enough, the increased level of lactate per se does not cause measurable detrimental effects in the CLP model unless the GC-GR pathway is also paralyzed, which is commonly encountered in sepsis. GR-deficient mice show mortality after direct lactate injection; however, normal mice do not.
A functional GC-GR axis maintains a normal degree of gluconeogenic cycling by utilizing lactate. The authors have shown that hyperlactatemia is harmful by causing the excess production of vascular endothelial growth factor (VEGF) through the upregulation of extracellular signal-regulated kinases (ERKs) and downregulation of mitogen-activated protein (MAP) kinase phosphatase 1 (MKP1), a negative regulator of MAP kinases. In light of other studies showing VEGF inhibition improves sepsis (Yano et al., 2006), the current findings of excess VEGF expression by lactate due to GC resistance to exaggerate sepsis is thus firmly established. Although this group has previously reported that sepsis induces GC resistance and GC therapy sometimes provides good or refractory outcomes in sepsis, the downstream factors that they identified in the current study (i.e., lactate and VEGF to link with the GC resistance phenomenon) are unique (Figure 1). By elucidating the detailed mechanism of GC resistance in sepsis, the interplay between the brain, adrenal gland, liver, vascular, and peripheral organ systems comprising immune, endocrine, and metabolic mediators in sepsis is firmly confirmed, opening a vast research area.
Figure 1. Effect of combined glucocorticoid resistance and hyperlactatemia in sepsis.
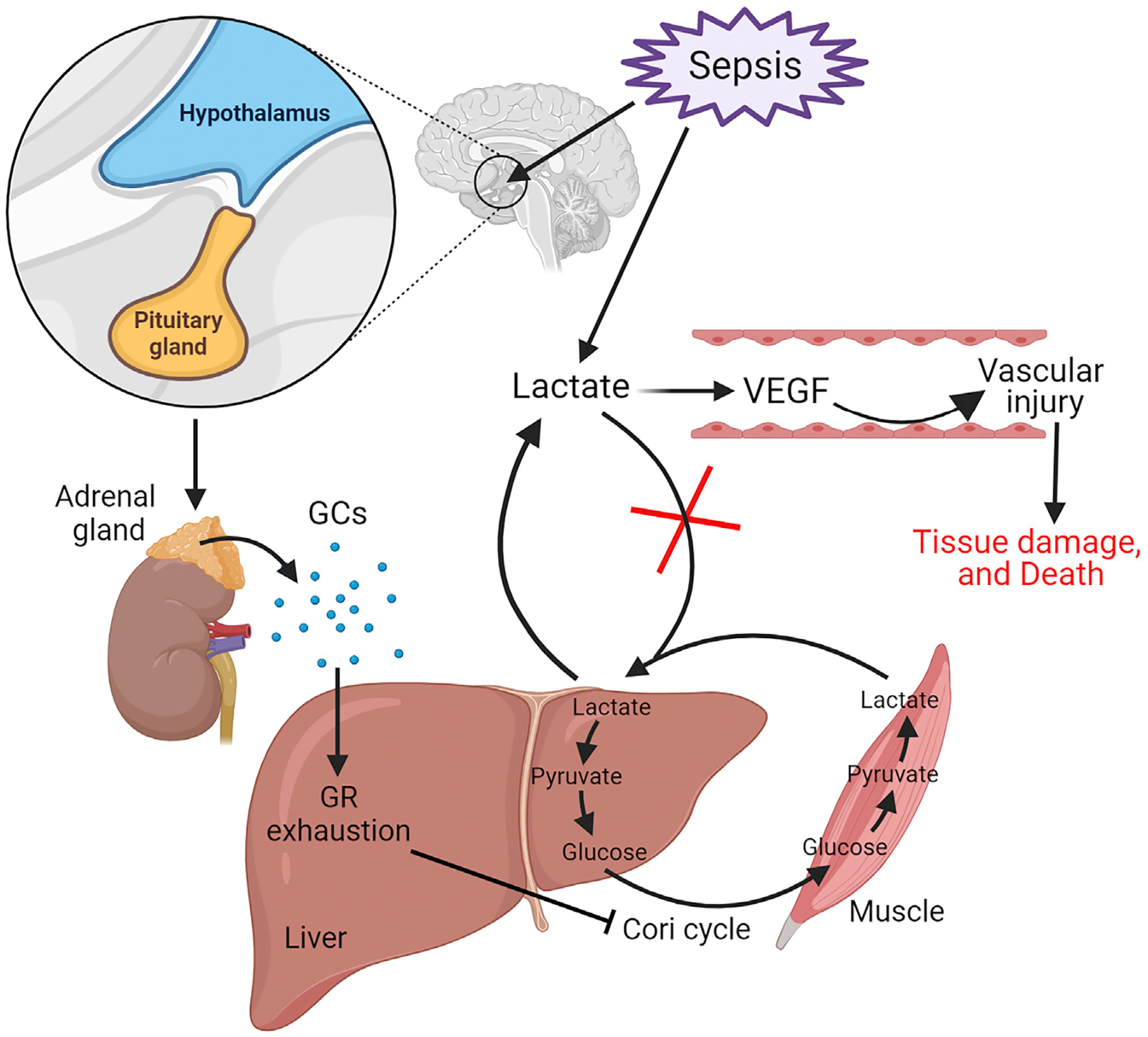
Sepsis stimulates the hypothalamic-pituitary-adrenal axis to release glucocorticoids (GCs). Sepsis increases lactate production in the blood and the muscle by glycolysis. Sepsis-induced genome-wide glucocorticoid resistance due to hyporesponsiveness (exhaustion) of the glucocorticoid receptor (GR) in the liver leads to decreased expression of the gluconeogenesis pathway’s genes and maladaptive function of the Cori cycle. As a result, lactate levels become elevated due to the failure of their clearance or utilization by the Cori cycle. Hyperlactatemia causes increased expression of vascular endothelial growth factor (VEGF), resulting in increased vascular permeability, tissue injury, hypotension, organ dysfunction, and death.
This study focuses on the murine CLP model of sepsis, which is debatable because it may not solely mimic clinical scenarios. Studies using other inflammatory models—such as bacteremia by inoculating Staphylococcus aureus and Streptococcus pneumonia, ischemia/reperfusion or even hemorrhagic shock—to determine the impact of the GC resistance-hyperlactatemia axis on GC therapy or overall disease progression will further add value to this pathway. Furthermore, only male mice were used in the study, and gender is an independent predictor influencing sepsis prognosis because males and females have distinct hormonal patterns (Sakr et al., 2013). As the GC resistance-hyperlactatemia phenomenon acts downstream of hyperactivation of the hypothalamic-pituitary-adrenal axis and excess release of GCs, differential hormonal patterns in males and females may influence the outcome of these pathways, suggesting future studies should be conducted in male and female mice.
Akin to macrophage and T cell immune tolerance, which can lead to secondary infection and death, GC resistance poses a mortality burden on patients with sepsis. Therefore, in spite of the limitations of this study, the discovery that an axis of GC resistance-hyperlactatemia and excess VEGF production occurs during sepsis may give insight into the most optimal timing of GC administration or ways to avoid its resistance to improve GC-based therapy for sepsis.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (NIH) grants R35GM118337 (P.W.) and R01GM129633 (M.A.).
REFERENCES
- Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, et al. ; CRICS-TRIGGERSEP Network (2018). Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N. Engl. J. Med 378, 809–818. 10.1056/NEJMoa1705716. [DOI] [PubMed] [Google Scholar]
- Cain DW, and Cidlowski JA (2017). Immune regulation by glucocorticoids. Nat. Rev. Immunol 17, 233–247. 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R, He J, Xu P, Faramand A, Xu J, and You C (2019). Association of Corticosteroid Treatment With Outcomes in Adult Patients With Sepsis: A Systematic Review and Meta-analysis. JAMA Intern. Med 179, 213–223. 10.1001/jamainternmed.2018.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsenos CS, Antonopoulou AN, Apostolidou EN, Ioakeimidou A, Kalpakou GT, Papanikolaou MN, Pistiki AC, Mpalla MC, Paraschos MD, Patrani MA, et al. ; Hellenic Sepsis Study Group (2014). Early administration of hydrocortisone replacement after the advent of septic shock: impact on survival and immune response. Crit. Care Med 42, 1651–1657. 10.1097/CCM.0000000000000318. [DOI] [PubMed] [Google Scholar]
- Lelubre C, and Vincent JL (2018). Mechanisms and treatment of organ failure in sepsis. Nat. Rev. Nephrol 14, 417–427. 10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, Livigni S, Fiore G, Filippini C, and Ranieri VM (2013). The influence of gender on the epidemiology of and outcome from severe sepsis. Crit. Care 17, R50. 10.1186/cc12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans S, Souffriau J, and Libert C (2019). A General Introduction to Glucocorticoid Biology. Front. Immunol 10, 1545. 10.3389/fimmu.2019.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle J, Timmermans S, Paakinaho V, Vancraeynest L, Dewyse L, Vanderhaeghen T, Wallaeys C, Van Wyngene L, Van Looveren K, Nuyttens L, et al. (2021). Combined glucocorticoid resistance and hyperlactatemia contributes to lethal shock in sepsis. Cell Metab. S1550–4131(21)00318–1. 10.1016/j.cmet.2021.07.002. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, Billot L, Correa M, Glass P, Harward M, et al. ; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group (2018). Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med 378, 797–808. 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, et al. (2006). Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J. Exp. Med 203, 1447–1458. 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]


