Abstract
Breast cancer survivors have a high risk of a second primary contralateral breast cancer (CBC), but there are few studies of CBC risk in minority populations. We examined whether the incidence and risk factors for CBC differed by race/ethnicity in the United States. Women with a first invasive stage I-IIB breast cancer diagnosis at ages 20–74 years between 2000 and 2015 in the Surveillance, Epidemiology, and End Results Program (SEER) 18 registries were followed through 2016 for a diagnosis of invasive CBC ≥1 year after the first breast cancer diagnosis. We used cause-specific Cox proportional hazards models to test the association between race/ethnicity and CBC, adjusting for age, hormone receptor status, radiation therapy, chemotherapy, and stage at first diagnosis, and evaluated the impact of contralateral prophylactic mastectomy, socioeconomic status, and insurance status on the association. After a median follow-up of 5.9 years, 9,247 women (2.0%) were diagnosed with CBC. Relative to non-Hispanic (NH) White women, CBC risk was increased in NH Black women (hazard ratio = 1.44, 95% CI 1.35–1.54) and Hispanic women (1.11, 95% CI 1.02–1.20), with the largest differences among women diagnosed at younger ages. Adjustment for contralateral prophylactic mastectomy, socioeconomic status, and health insurance did not explain the associations. Therefore, non-Hispanic Black and Hispanic women have an increased risk of CBC that is not explained by clinical or socioeconomic factors collected in SEER. Large studies of diverse breast cancer survivors with detailed data on treatment delivery and adherence are needed to inform interventions to reduce this disparity.
Keywords: contralateral breast cancer, second primary cancer, race, ethnicity, survivorship
INTRODUCTION
In 2019 there were over 3 million survivors of female breast cancer in the United States (U.S.)1 who are at risk of a second, primary, contralateral breast cancer (CBC). The 5-year cumulative incidence of CBC is 2.5% overall and 9.7% among those carrying pathogenic mutations in BRCA1 or BRCA2.2, 3 Risk factors for CBC have been investigated in largely non-Hispanic (NH) White populations,3–6 and it is not clear whether incidence of CBC or the risk factors for CBC differ by race/ethnicity.
While the incidence of first primary breast cancer is similar for NH White women and NH Black women, and lower among Hispanic and NH Asian women in the U.S.,1 several studies have reported elevated age-adjusted incidence of second primary breast cancer following a diagnosis of invasive or in situ breast cancer for Black women relative to white women.7–10 However, others have failed to detect differences in CBC risk by race/ethnicity after adjusting for tumor characteristics and treatment.11–13 Racial/ethnic differences in risk of second cancer after lobular or ductal carcinoma in situ have also been observed.14, 15 It remains unknown whether reported differences in CBC risk are explained or modified by socioeconomic status (SES), health insurance, differences in receipt of chemotherapy, or differences in uptake of contralateral prophylactic mastectomy (CPM) after a first breast cancer diagnosis.16
In this study we evaluated whether invasive CBC risk was associated with race/ethnicity among survivors of a first primary invasive breast cancer in the population-based Surveillance, Epidemiology and End Results (SEER) Program 18 registries. With wide coverage of diverse populations in the U.S., extensive follow-up of breast cancer cases, and linkage with U.S. Census data, SEER is uniquely positioned to investigate racial/ethnic disparities in CBC risk. While SEER databases do not currently include some important CBC risk factors such as cancer family history or germline mutation status, this is the only resource available with sufficient follow-up of racially/ethnically diverse breast cancer survivors to evaluate disparities in CBC risk in the U.S. Furthermore, SEER data permit the evaluation of whether differential uptake of CPM after a first primary breast cancer16 modifies or explains observed associations between race/ethnicity and CBC risk in the U.S.
We estimated the absolute and relative risks of CBC by race/ethnicity and evaluated whether differences were explained or modified by age at diagnosis, estrogen receptor (ER) status, radiation therapy or chemotherapy for the first breast cancer, neighborhood SES, insurance status, or recent changes in uptake of CPM.
METHODS
We identified women diagnosed with a first primary invasive breast cancer in the SEER 18 incidence database (excluding the Alaska Native Tumor Registry) using a “Multiple Primary-Standardized Incidence Ratios” session in SEER*Stat software.17 We used the most recent SEER 18 data release, with first invasive diagnoses between 2000 and 2015 and follow-up through 2016, as the data in the SEER 21 registries do not yet permit analysis of second primary cancers. We restricted the study sample to women with a first breast cancer diagnosis before age 75 years and ended follow-up at attained age of 80 years due to underreporting of second primary cancers among elderly women.18 We began follow-up for second primary CBC one year after diagnosis of the first breast cancer and limited the first diagnosis to local or regional disease (American Joint Committee on Cancer Stage I-IIB) to reduce the chance that an apparent CBC was a metastasis.8, 19 Women with a second primary invasive cancer within the first year of follow-up were excluded and any women with a second primary ipsilateral breast cancer at least one year after the first diagnosis were censored at the date of the second diagnosis (see Statistical Methods). All CBCs were coded as primary tumors by SEER, which maintains a centralized data management system to identify duplicate patient reports within any of the participating SEER registries. There may be patients that migrated into or out of SEER registry regions during the study time which could result in a small number of missed CBCs. Data on the receipt of first-course radiation and chemotherapy were obtained from SEER as “Yes” vs “No/Unknown”. Insurance status was available for diagnoses made after 2006. We collapsed the insurance data to “Insured” (including Medicaid) vs “Uninsured” or missing. The data were exported to R software (Version 3.6.1)20 for additional data preparation and analysis. Follow-up time was censored at a diagnosis of ipsilateral second primary invasive breast cancer, other second primary invasive cancer, death, attained age of 80 years, or end of follow-up (December 31st, 2016).
Statistical Methods
To compare the age-adjusted incidence rate of CBC among racial/ethnic minority women relative to NH White women, we designated NH White women with a first local or regional breast cancer between 2000 and 2015 as the standard population and calculated age-adjusted incidence rates of CBC by the indirect method for each group. Confidence intervals were estimated by the method developed by Anderson and Rosenberg.21 Standardized incidence ratios (SIRs) were calculated as a ratio of the age-adjusted incidence rate of the race/ethnicity group of interest over the incidence rate among NH White women. The absolute excess risk of CBC for each race/ethnicity group was calculated by . Next, we estimated the cumulative incidence of CBC accounting for competing risks (ipsilateral second invasive primary breast cancer, other second primary invasive cancer, or death), and tested for differences between cumulative incidence of CBC by race/ethnicity.22
Cause-specific multivariable Cox proportional hazard models were used to estimate the association between race/ethnicity and CBC risk. In all models we adjusted for age at diagnosis (continuous), ER and progesterone receptor (PR) status (positive, negative, missing; 0.14% of first primaries with ER or PR results were submitted to SEER as ‘borderline’ receptor status and were recoded as negative for this study), receipt of chemotherapy (Yes vs No/Unknown), receipt of radiotherapy (Yes vs No/Unknown), and stage (I, IIA, or IIB) at diagnosis. All adjustment variables refer to characteristics of the first primary breast cancer diagnosis. Observations missing data for any of the covariates were excluded from the multivariable models. We used Akaike’s Information Criterion to determine whether restricted cubic splines allowing for non-linearity of the age term improved the fit of the models. Proportional hazards for each model were checked by testing the independence of Schoenfeld residuals and follow-up time. Multivariable models were then estimated separately by age at first diagnosis (10-year groups) and ER status to determine if associations with race/ethnicity were modified by these factors.
There were several variables that were added to SEER during the follow-up period. First, among women diagnosed with a first breast cancer after 2006, we fit the final overall model adjusting for insurance status. Second, among women diagnosed with a first breast cancer in or after 2010, we adjusted for tumor subtype including human epidermal growth factor 2 (HER2) expression, defined as follows: luminal A, hormone receptor (HR)-positive and HER2-negative; luminal B, HR-positive and HER2-positive; HER2-enriched, HR-negative and HER2-positive; and triple negative, HR-negative and HER2-negative.
In further analysis we excluded women receiving CPM following the first breast cancer to determine whether differences in CPM uptake explained associations between race/ethnicity and CBC risk. CPM was defined as any surgery involving complete removal of all breast tissue, the nipple, and the areolar complex of the unaffected contralateral breast.
To determine whether SES explained differences in CBC risk, we used a “neighborhood” SES score developed by Yost et al., available for all diagnoses through 2016, which is a composite of U.S. census-block-level characteristics including an education index, proportion with blue-collar jobs, proportion unemployed, proportion below 200% of the poverty level, median rent, and median house value.23 This variable is comprised of census-block-level data allowing for neighborhood-level control of socioeconomic factors. We adjusted the overall model for neighborhood SES, and also re-fit the neighborhood SES-adjusted model limiting to women with a diagnosis of stage I first breast cancer to rule out any effect of lead-time bias.24 We also fit this model separately by race/ethnicity for those groups with sufficient CBCs for stratified multivariable analysis (NH White, NH Black, Hispanic, and NH Asian / Pacific Islander) to determine whether CBC risk factors differed across race/ethnicity categories. In all models, coefficients were estimated conditionally on year of the first breast cancer diagnosis in order to account for unmeasured changes in the reporting of diagnoses throughout the follow-up time. To minimize the probability of patient identifiability, all analyses including neighborhood SES were carried out with a dataset that excluded other geographic variables, such as SEER registry.25
In additional subgroup analyses, we (1) stratified the models by neighborhood SES tertile; (2) restricted to women who received radiotherapy for the first primary breast cancer; and (3) restricted to women who received chemotherapy for the first primary breast cancer.
RESULTS
There were 459,916 women with a first invasive local/regional breast cancer in SEER 18 between 2000 and 2015 and with complete information on both race and ethnicity. Median follow-up time was 6.1 years for NH White women, 5.1 years for NH Black, Hispanic, and NH American Indian/Alaska Native women, and 5.4 years for NH Asian women. In total, 9,247 women (2.0%) developed a CBC, of whom 56 (0.6%) underwent CPM after their first breast cancer but nonetheless developed a CBC, presumably in residual breast tissue. In addition, 4,786 women received a diagnosis of second primary invasive ipsilateral breast cancer at least one year after their first breast cancer diagnosis; their follow-up time was censored at the time of diagnosis of the second primary diagnosis. Of the women with a first primary breast cancer, 71% were NH White, 10% were NH Black, 10% were Hispanic of any race, 8% were NH Asian/Pacific Islander and 0.4% were American Indian/Alaska Native. The median age at first breast cancer diagnosis was lower among NH Black women (55 years), Hispanic women (53 years) and NH Asian/women (54 years) compared to NH White women (58 years) and NH American Indian / Alaska Native women (56 years). Seventeen percent of NH White women had an ER-negative first breast cancer, compared to 32% of NH Black women and 22% of Hispanic women. CPM following the first breast cancer was most common among NH White (10%) and NH American Indian / Alaska Native women (9%). Additional details of the study sample are given in Supplementary Table 1.
NH Black women had the highest age-adjusted incidence rate of CBC (IR = 441 per 100,000 person-years, 95% CI 416–467), followed by Hispanic women (IR = 329 per 100,000 person-years, 95% CI 307–351) (Table 1). In Figure 1, we present the cumulative incidence of CBC by race/ethnicity, showing that NH Black women have the highest cumulative incidence of CBC throughout the follow-up time, while NH White women had the lowest. In Figure 2, we present the age-specific incidence of CBC per 100,000 person-years by race/ethnicity, as well as the age-specific incidence of first primary breast cancer, for reference. NH Black women had the highest incidence rate of CBC compared to Hispanic and NH White women for all age-at-diagnosis categories, with the largest differences among women diagnosed with CBC at age <40 years: 1,335 per 100,000 person-years for NH Black women compared to 956 per 100,000 person-years for Hispanic women and 686 per 100,000 person-years for non-Hispanic White women.
Table 1.
Age-Adjusted Incidence Rate, Standardized Incidence Ratios (SIRs), and Absolute Excess Risks of Contralateral Breast Cancer (CBC) by Race/Ethnicity in the United States, SEER 18 (2000 – 2016)
| Observed CBC | Expecteda CBC | Age-Adjusted Incidence Rate (95% CI)b | SIR (95% CI) | Absolute Excess Riskc | |
|---|---|---|---|---|---|
|
| |||||
| Non-Hispanic White | 6474 | Reference | 302 (295 – 309) | Reference | Reference |
| Non-Hispanic Black | 1156 | 792.1 | 441 (416 – 467) | 1.46 (1.38 – 1.55) | 134 |
| Hispanic (Any Race) | 859 | 788.9 | 329 (307 – 351) | 1.09 (1.02 – 1.16) | 26 |
| Non-Hispanic Asian or Pacific Islander | 723 | 682.6 | 320 (297 – 344) | 1.06 (0.98 – 1.14) | 17 |
| Non-Hispanic American Indian/Alaska Native | 35 | 33.1 | 319 (229 – 444) | 1.06 (0.76 – 1.47) | 17 |
Abbreviations. SEER, Surveillance, Epidemiology, and End Results Program; CI, confidence interval
Estimated based on the rates of contralateral breast cancer among non-Hispanic White women
Age-adjusted by the indirect method with non-Hispanic White women as the standard population and expressed per 100,000 person-years
Calculated as (Observed - Expected)/Person-Years*100,000
Figure 1:
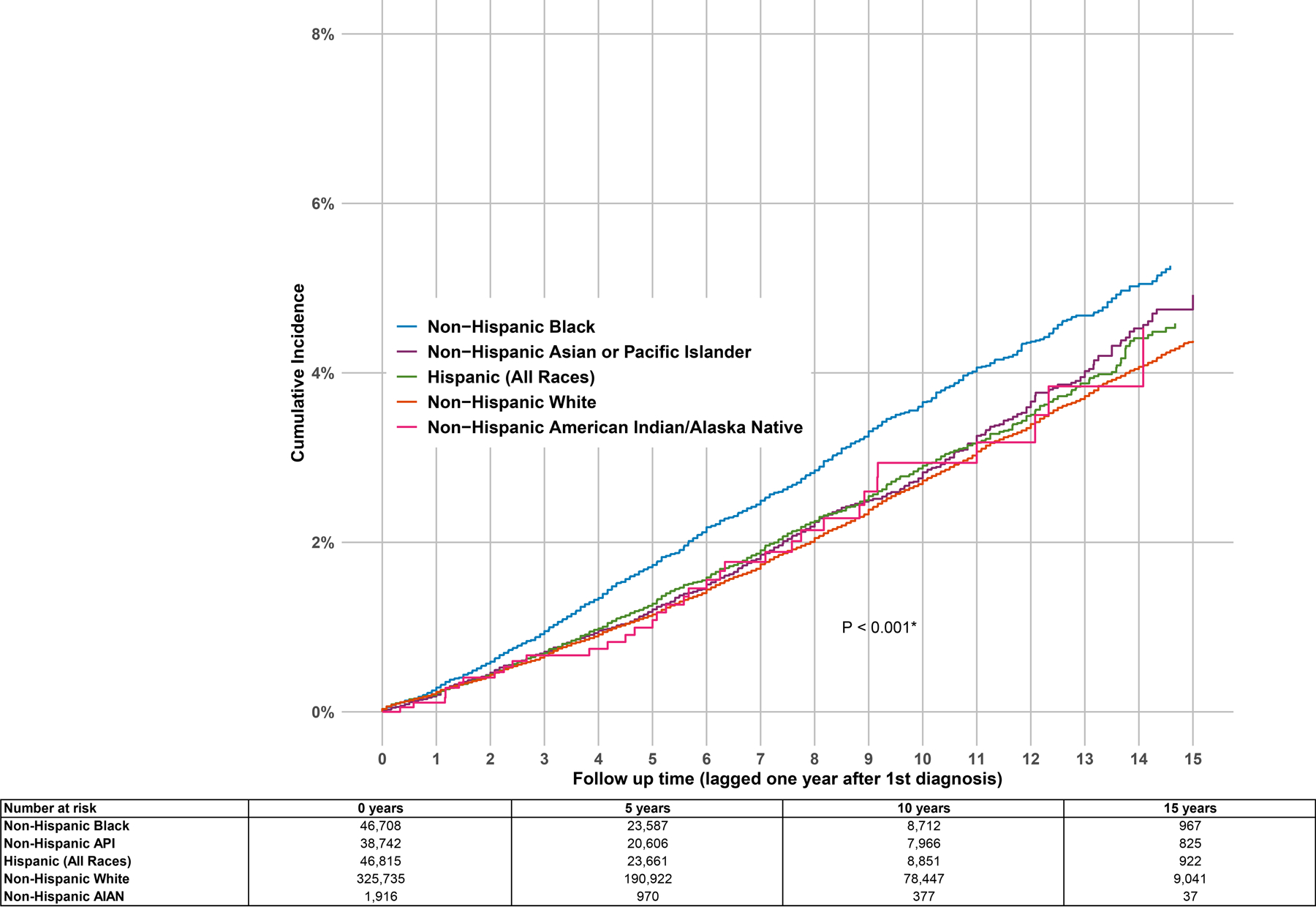
Abbreviations. CBC, contralateral breast cancer; API, Asian / Pacific Islander; AIAN, American Indian / Alaska Native
Cumulative incidence of CBC among one-year survivors of a first primary invasive breast cancer. Cumulative incidence estimates account for competing risks of death and other second primary invasive cancer diagnoses. The P-value is a test for difference of the cumulative incidence of CBC across race/ethnicity groups.
Figure 2:
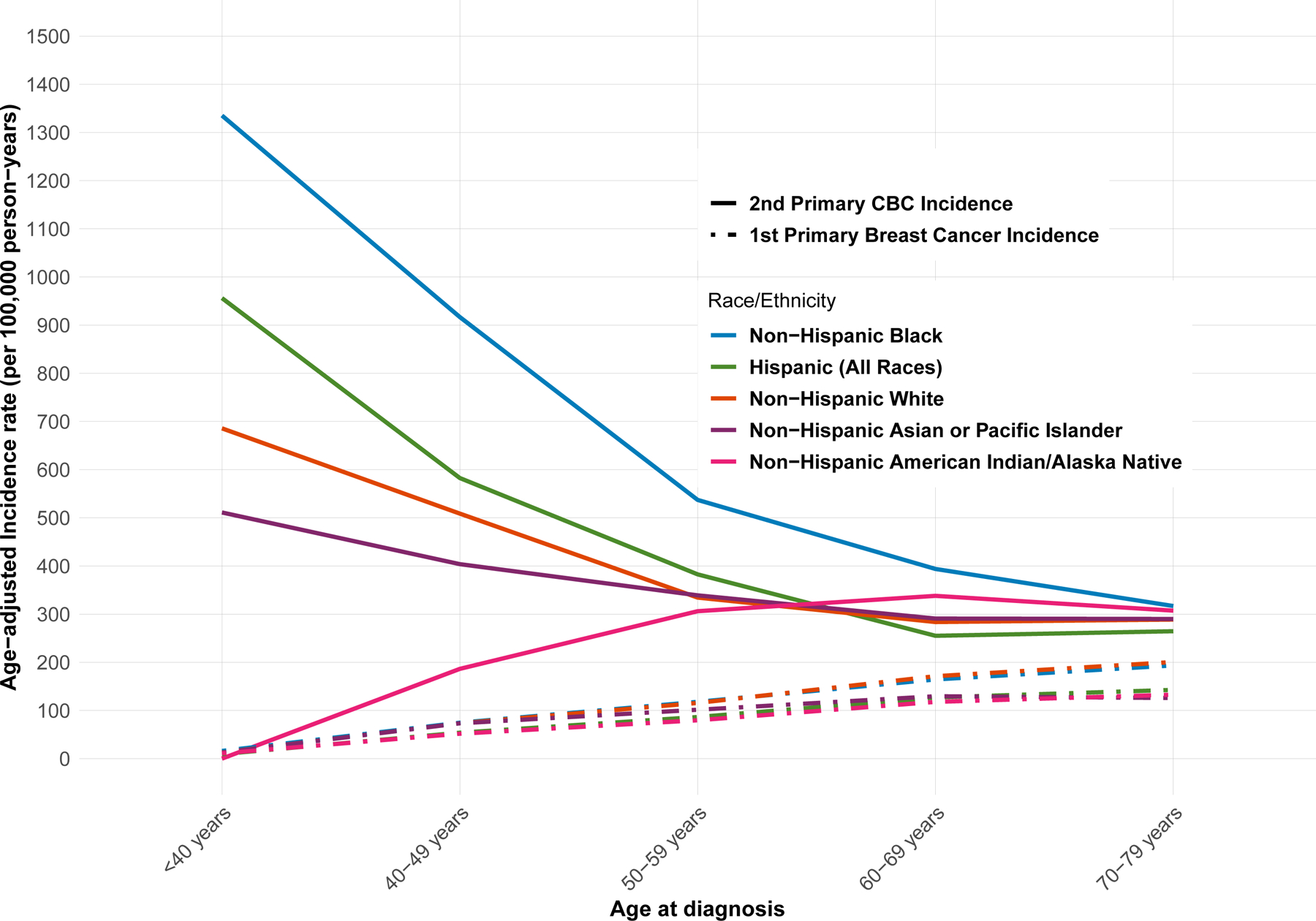
Abbreviations. CBC, contralateral breast cancer; NH, Non-Hispanic
Age-specific incidence per 100,000 person-years of 2nd primary breast cancer and first primary breast cancer in SEER 18 registries (2000 – 2016).
In multivariable models of CBC risk, 30,571 women (6.6%) missing ER status or PR status were excluded. Missingness of ER status was associated with year of first diagnosis; 13% of diagnoses between 2000 and 2004 were missing ER status, 4.1% of diagnoses between 2005 and 2009 and 1.6% of diagnoses between 2010 and 2015 (p < 0.001). NH Black women had a 44% increased hazard of CBC (HR 1.44, 95% CI 1.35–1.54), and Hispanic women had an 11% increased hazard of CBC (HR 1.11, 95% CI 1.02–1.20) relative to NH White women after adjustment for ER status, PR status, radiation therapy, chemotherapy, stage, age, and year of first breast cancer diagnosis (Table 2). There was a non-significant but suggestive association between NH Asian / Pacific Islander race/ethnicity and CBC (HR 1.07, 95% CI 0.99 – 1.17). ER-negative first cancers were associated with an increased hazard of CBC relative to ER-positive first cancers (HR 1.59, 95% CI 1.48 – 1.71). Radiation therapy for the first cancer was associated with increased risk of CBC (HR 1.16, 95% CI 1.11–1.22), whereas chemotherapy for the first cancer was associated with decreased risk (HR 0.85, 95% CI 0.81–0.90). The association between age and CBC risk was non-linear, and the best model fit was obtained with restricted cubic spline terms for age with 3 knots placed between each age quartile (see Supplementary Figure 1).
Table 2.
Multivariable-Adjusted Associations of Race/Ethnicity with Risk of Contralateral Breast Cancer Among Women Diagnosed with a First Primary Invasive Breast Cancer, United States, SEER 18 (2000 – 2015).
| N = 429,345 | HRa (95% CI) |
|---|---|
|
| |
| Race/Ethnicity | |
| Non-Hispanic White | Reference |
| Non-Hispanic Black | 1.44 (1.35 – 1.54) |
| Hispanic (Any race) | 1.11 (1.02 – 1.20) |
| Non-Hispanic Asian or Pacific Islander | 1.07 (0.99 – 1.17) |
| Non-Hispanic American Indian/Alaska Native | 1.09 (0.77 – 1.54) |
| ER status of 1st diagnosis | |
| ER+ | Reference |
| ER- | 1.59 (1.48 – 1.71) |
| PR status of 1st diagnosis | |
| PR+ | Reference |
| PR- | 0.96 (0.90 – 1.03) |
| Radiation therapy for 1st diagnosis b | |
| No/Unknown | Reference |
| Yes | 1.16 (1.11 – 1.22) |
| Chemotherapy for 1st diagnosisc | |
| No/Unknown | Reference |
| Yes | 0.85 (0.81 – 0.90) |
| Stage of 1st Diagnosis | |
| I | Reference |
| IIA | 1.01 (0.96 – 1.07) |
| IIB | 1.10 (1.02 – 1.18) |
Abbreviations. SEER, Surveillance, Epidemiology, and End Results Program; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor
Hazard ratios estimated in a Cox proportional hazards model with adjustment for variables in table, as well as age at first diagnosis (restricted cubic spline with 3 knots) with stratification by year of diagnosis.
Radiation and chemotherapy variables were provided by SEER with limited detail (“Yes” vs “No/Unknown”)
The increased risk of CBC for NH Black and Hispanic women, relative to NH White women, was modified by age at first breast cancer diagnosis (interaction p-value = 0.006; Figure 3A). Among women first diagnosed at age <40 years, NH Black women had a 2.5-fold increased risk of CBC relative to NH White women (HR = 2.51, 95% CI 2.05–3.07). For each increasing 10-year interval of age at first diagnosis, the increased risk of CBC associated with NH Black race/ethnicity diminished but was statistically significantly higher compared to NH White women, except for women first diagnosed at age 60 years or older. For Hispanic women, there was also a greater risk of CBC relative to NH White women among those first diagnosed at age <40 years (HR 1.94, 95% CI 1.56–2.41).
Figure 3:
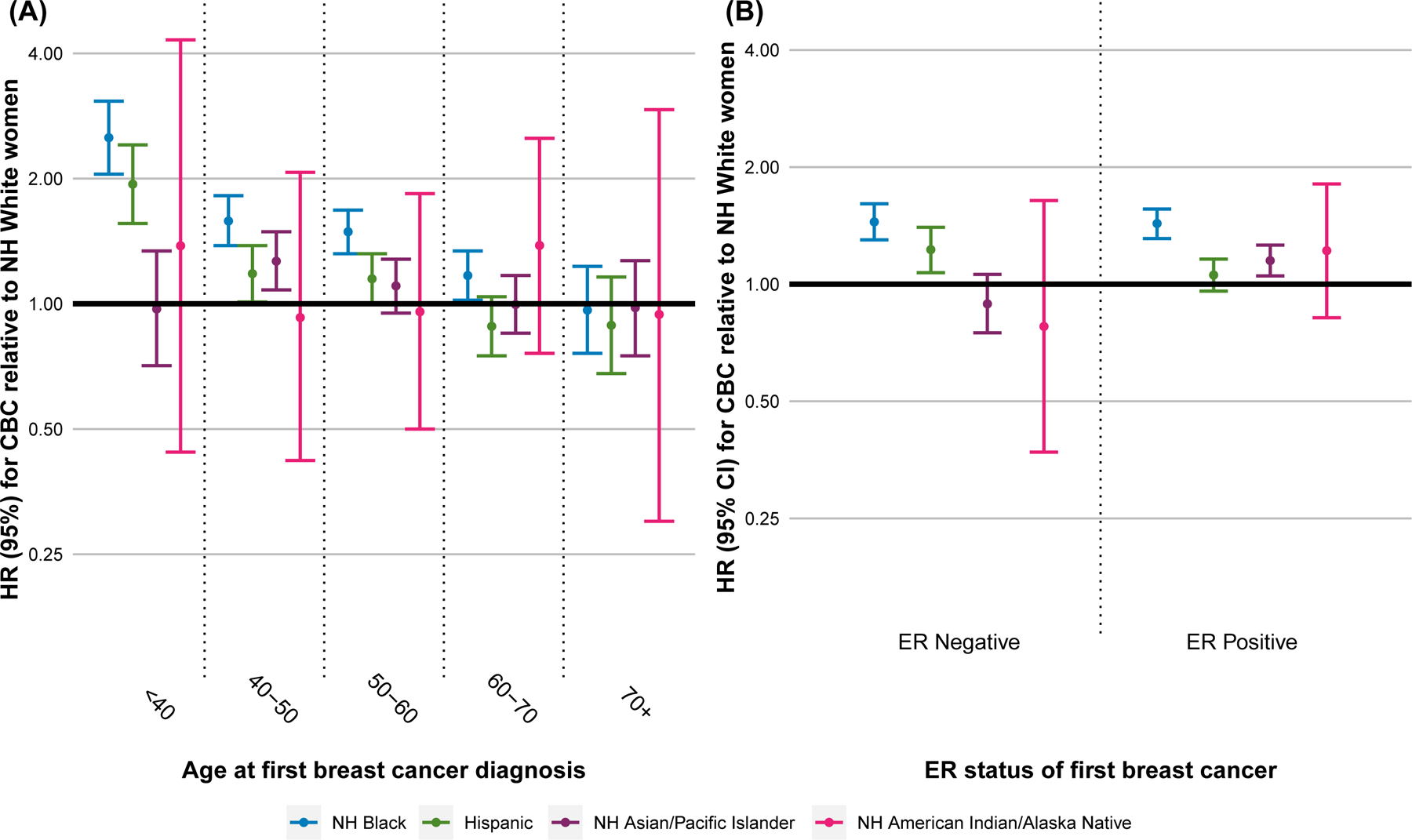
Abbreviations. Hazard Ratio, HR; NH, Non-Hispanic; ER, Estrogen Receptor.
HRs of CBC estimated for each race/ethnicity group in stratified multivariable Cox proportional hazards models relative to non-Hispanic White women and adjusting for ER status of the first breast cancer, PR status of the first breast cancer, radiation therapy for the first breast cancer, chemotherapy for the first breast cancer, stage of first breast cancer, with conditioning on year of diagnosis. (A) Multivariable models stratified by age at diagnosis of the first primary breast cancer. (B) Multivariable models stratified by ER status of the first primary breast cancer.
The increased risk of CBC associated with NH Black race/ethnicity was not modified by ER status of the first breast cancer (Figure 3B). In contrast, risk of CBC associated with Hispanic ethnicity was increased only among women with an ER-negative first breast cancer (interaction p-value = 0.02), whereas risk of CBC associated with NH Asian / Pacific Islander race/ethnicity was increased only among women with an ER-positive first breast cancer (interaction p-value = 0.04)
Insurance status at time of first diagnosis was not associated with CBC risk and adjustment did not materially change the results in the overall model (Supplementary Table 2). Excluding women receiving a CPM after the first breast cancer likewise did not alter the association between race/ethnicity and CBC risk. (Supplementary Table 3).
In the subset of women with HER2 expression data (N = 175,814; median follow-up time 2.6 years), CBC hazard was significantly increased for women with a first triple-negative breast cancer (HR 1.77, 95% CI 1.46–2.15) compared to women with a first luminal A breast cancer (Table 3). HER2-enriched first breast cancer had a borderline significant association with CBC (HR 1.37, 95% CI 0.99 – 1.89). NH Black race/ethnicity remained statistically significantly associated with CBC risk in this smaller subset (HR 1.46, 95% CI 1.21–1.76).
Table 3.
Multivariable-Adjusted Associations of Race/Ethnicity with Risk of Contralateral Breast Cancer Among Women Diagnosed with a First Primary Invasive Breast Cancer, among women with HER2 expression data, United States, SEER 18 (2010 – 2016).
| N = 175,814 | HRa (95% CI) |
|---|---|
|
| |
| Race/Ethnicity | |
| Non-Hispanic White | Reference |
| Non-Hispanic Black | 1.46 (1.21 – 1.76) |
| Hispanic (Any race) | 1.17 (0.95 – 1.44) |
| Non-Hispanic Asian or Pacific Islander | 1.21 (0.97 – 1.50) |
| Non-Hispanic American Indian/Alaska Native | 1.47 (0.66 – 3.29) |
| Tumor subtype of first diagnosis | |
| Luminal A | Reference |
| Luminal B | 1.00 (0.78 – 1.28) |
| HER2-enriched | 1.37 (0.99 – 1.89) |
| Triple Negative | 1.77 (1.46 – 2.15) |
| Radiation therapy for 1st diagnosis b | |
| No/Unknown | Reference |
| Yes | 1.26 (1.11 – 1.43) |
| Chemotherapy for 1st diagnosisc | |
| No/Unknown | Reference |
| Yes | 0.62 (0.53 – 0.73) |
| Stage of 1st Diagnosis | |
| I | Reference |
| IIA | 1.05 (0.91 – 1.23) |
| IIB | 0.93 (0.75 – 1.16) |
Abbreviations. SEER, Surveillance, Epidemiology, and End Results Program; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor
Hazard ratios estimated in a Cox proportional hazards model with adjustment for variables in table, as well as age at first diagnosis (restricted cubic spline with 3 knots) with stratification by year of diagnosis.
Radiation therapy and chemotherapy variables were provided by SEER with limited detail (“Yes” vs “No/Unknown”)
In the multivariable model adjusting for neighborhood SES at the time of first diagnosis, the association between hazard of CBC and NH Black race/ethnicity (HR 1.36, 95% CI 1.30–1.43) and Hispanic ethnicity (HR 1.08, 95% CI 0.99–1.18) remained statistically significant (Table 4). Relative to residing in low SES neighborhoods at first diagnosis, residing in middle SES neighborhoods (HR 0.95, 95% CI 0.91–0.99) or high SES neighborhoods (HR 0.85, 95% CI 0.81–0.88) was associated with a reduced risk of CBC. When restricting to stage I first breast cancers, the results did not differ markedly from the overall model, suggesting a negligible impact of lead time bias (data not shown). Stratifying by race/ethnicity, the positive association between ER-negative first tumors and CBC risk was consistent across race/ethnicity categories. Receipt of chemotherapy for the first breast cancer was statistically significantly associated with reduced CBC risk among all race/ethnicity groups except for NH Black women.
Table 4.
Multivariable-Adjusted Associations of Race/Ethnicity with Risk of Contralateral Breast Cancer Among Women Diagnosed with a First Primary Invasive Breast Cancer, adjusting for neighborhood SES, SEER 18 (2000 – 2016).
| Overall | Non-Hispanic White women | Non-Hispanic Black women | Hispanic women | Non-Hispanic Asian / Pacific Islander women | |
|---|---|---|---|---|---|
| (N = 452,615) | (N = 322,414) | (N = 46,095) | (N = 44,365) | (N = 38,113) | |
|
| |||||
| HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | HRa (95% CI) | ||
|
| |||||
| Race/Ethnicity | |||||
|
| |||||
| Non-Hispanic White | Reference | - | - | - | - |
| Non-Hispanic Black | 1.36 (1.30 – 1.43) | ||||
| Hispanic (Any Race) | 1.07 (1.02 – 1.14) | ||||
| Non-Hispanic Asian or Pacific Islander | 1.05 (0.99 – 1.12) | ||||
| Non-Hispanic American Indian/Alaska Native | 1.13 (0.88 – 1.47) | ||||
|
| |||||
| ER status of 1st diagnosis | |||||
| ER+ | Reference | Reference | Reference | Reference | Reference |
| ER- | 1.58 (1.50 – 1.66) | 1.60 (1.50 – 1.70) | 1.55 (1.36 – 1.77) | 1.74 (1.48 – 2.05) | 1.29 (1.07 – 1.56) |
| PR status of 1st diagnosis | |||||
| PR+ | Reference | Reference | Reference | Reference | Reference |
| PR- | 0.98 (0.93 – 1.02) | 0.98 (0.92 – 1.03) | 0.94 (0.82 – 1.07) | 1.04 (0.89 – 1.21) | 0.92 (0.78 – 1.09) |
| Radiation therapy for 1st diagnosis | |||||
| No/Unknown | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.16 (1.12 – 1.19) | 1.18 (1.13 – 1.22) | 1.13 (1.04 – 1.24) | 1.10 (0.99 – 1.22) | 1.02 (0.91 – 1.14) |
| Chemotherapy for 1st diagnosis | |||||
| No/Unknown | Reference | Reference | Reference | Reference | Reference |
| Yes | 0.85 (0.82 – 0.89) | 0.86 (0.82 – 0.89) | 0.98 (0.89 – 1.09) | 0.80 (0.71 – 0.90) | 0.76 (0.67 – 0.87) |
| Stage of 1st diagnosis | |||||
| I | Reference | Reference | Reference | Reference | Reference |
| IIA | 1.01 (0.97 – 1.05) | 1.00 (0.96 – 1.05) | 0.94 (0.85 – 1.04) | 1.12 (0.99 – 1.26) | 1.09 (0.95 – 1.24) |
| IIB | 1.11 (1.05– 1.16) | 1.06 (0.99 – 1.13) | 1.18 (1.05 – 1.34) | 1.19 (1.02 – 1.39) | 1.14 (0.95 – 1.36) |
| Neighborhood SES Tertile at 1st diagnosis | |||||
| Low | Reference | Reference | Reference | Reference | Reference |
| Middle | 0.95 (0.91 – 0.99) | 0.91 (0.86 – 0.95) | 0.95 (0.86 – 1.05) | 0.98 (0.87 – 1.11) | 1.08 (0.93 – 1.25) |
| High | 0.85 (0.81 – 0.88) | 0.81 (0.77 – 0.85) | 0.93 (0.83 – 1.05) | 1.06 (0.93 – 1.20) | 0.81 (0.70 – 0.94) |
Abbreviations. SEER, Surveillance, Epidemiology, and End Results Program; SES, socioeconomic status; HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor
Hazard ratios estimated in a Cox proportional hazards model with adjustment for variables in table, as well as age at first diagnosis (restricted cubic spline with 3 knots) with stratification by year of diagnosis.
Within neighborhood SES tertiles (Supplementary Table 4), there were statistically significant associations between NH Black race/ethnicity and CBC risk within each neighborhood SES group, with a statistically significantly greater increased risk for NH Black women residing in high SES neighborhoods (HR 1.55, 95% CI 1.39–1.72) compared to NH black women in low SES neighborhoods (HR 1.25, 95% CI 1.15–1.34). Hispanic ethnicity was associated with increased CBC risk only among women residing in high SES neighborhoods (HR 1.34, 95% CI 1.15–1.57).
After stratifying by first-course treatment received, among women treated with radiation therapy (Supplementary Table 3), NH Black race/ethnicity was associated with increased CBC risk (HR 1.40, 95% CI 1.27–1.54), but there was no association between CBC risk and Hispanic ethnicity (HR 0.98, 95% CI 0.87–1.10). Among women treated with chemotherapy, NH Black race/ethnicity remained associated with increased CBC risk (HR 1.57, 95% CI 1.42–1.74), as did Hispanic ethnicity (HR 1.20, 95% CI 1.06–1.35).
DISCUSSION
This study indicates that NH Black women in the U.S. have an increased risk of CBC after accounting for ER status, age at diagnosis, radiation therapy, chemotherapy, and neighborhood SES, and the association was not modified by ER status of the first breast cancer. Hispanic women likewise have an increased risk of CBC relative to NH White women, but only those with ER-negative first breast cancers. Additionally, NH Asian / Pacific Islander women have a CBC risk at least as high as NH White women, despite having a lower incidence of first primary breast cancer. There were relatively few NH American Indian / Alaska Native women in this study, resulting in wide confidence intervals for estimates of CBC incidence, but the point estimates for CBC incidence for this group are similar to those for NH Asian / Pacific Islander or Hispanic women. Importantly, our results suggest that the lower uptake of CPM among NH Black and Hispanic women does not explain their increased risk of CBC. The disparities in CBC risk were greatest for women diagnosed with their first breast cancer at age <40 years, suggesting that the drivers of this disparity are especially important for younger breast cancer survivors. This finding is concordant with the age-modified breast cancer mortality disparity observed for NH Black compared to NH White women.1
ER-negative first breast cancer was associated with an increased risk of CBC irrespective of race/ethnicity. A positive association between ER-negative first breast cancers and CBC risk, independent of BRCA1 and BRCA2 mutation status, has been reported for mainly NH White populations, but had not previously been confirmed for other racial/ethnic groups among whom ER-negative cancers are more frequent.4, 5 Our results also confirm prior studies demonstrating that women with triple negative first breast cancers have an increased risk of CBC.4 In contrast to previous work, however, the present study suggests NH Black race/ethnicity is consistently associated with CBC risk independently of tumor subtype defined by hormone receptor status and HER2 expression.
Among women with ER-negative first tumors, CBC risk was increased for both NH Black and Hispanic women relative to NH White women, but not for NH Asian / Pacific Islander women. However, among women with ER-positive first tumors, CBC risk was increased for NH Black and NH Asian / Pacific Islander women only, whereas Hispanic women had a similar risk of CBC as NH White women. These results, which are adjusted for known risk factors of CBC available in the SEER database, contrast with previous work reporting that all groups other than NH White women in the U.S. had increased risk of CBC regardless of ER status of the first primary breast cancer.13 Of relevance, previous studies have shown that endocrine therapy for a first primary breast cancer reduces the risk of CBC,26 but that NH Black women have lower uptake of and adherence to endocrine therapy compared to NH White women, which may contribute to the CBC disparity among women with ER-positive first breast cancers.27 Additionally, obesity, which is associated with increased risk of postmenopausal first primary breast cancer,28 recurrence,29, 30 CBC risk,31 and mortality,32 is more prevalent among NH Black and Hispanic women than NH White women33 and may contribute to CBC disparities. However, to test these hypotheses and develop evidence-based interventions to reduce the observed CBC disparities, large studies which comprise diverse populations of women, with detailed data including receipt of adjuvant cancer therapies, body composition, and reproductive factors, are needed.
We are the first to report that residence in higher SES neighborhoods at the time of first breast cancer diagnosis was associated with a decreased risk of CBC. Adjustment for neighborhood SES attenuated the HR estimate for NH Black women from 1.44 to 1.36, suggesting that the racial disparity in CBC risk is only partially explained by differences in neighborhood SES. When stratifying by neighborhood SES, we found that the association between NH Black and Hispanic race/ethnicity and CBC risk was slightly stronger among women living in high SES neighborhoods, indicating that residence in a higher-SES region does not ameliorate racial/ethnic disparities in CBC risk. We also demonstrated that insurance status is not an important predictor of CBC risk. Other social determinants of health likely contribute to the risk of CBC,34 but data to study these determinants in the CBC context are currently not available from any data source.
The positive association of CBC risk with radiation therapy and the negative association with chemotherapy are well established for NH White women,3, 35, 36 but have not previously been evaluated in racial/ethnic minority populations. We did not observe significant associations between CBC risk and either radiation or chemotherapy among NH Black women. While the sensitivity of SEER treatment data is limited,37 this observation is potentially important and could represent differences in completeness of data, treatment delivery or adherence, baseline risk, or other unmeasured factors. For instance, previous work has shown that NH Black women are less likely to receive a complete regimen of neoadjuvant therapy38 or adjuvant therapy.39, 40 Data were not available in SEER to determine whether these factors explain the increased risk of CBC among minority women in the U.S.
The current study is limited by the availability of data in the SEER Program. First, SEER treatment data are currently limited to first-course treatment and no longer include endocrine therapy. This limitation is an important consideration for future studies of CBC risk, as receipt of a complete course of endocrine therapy for treatment of a first breast cancer is known to be associated with both race/ethnicity and lower risk of CBC.27, 35 However, accounting for endocrine therapy in this study would not explain the increased risk of CBC among NH Black and Hispanic women with an ER-negative first breast cancer. In addition, our validated approach37 to complete subgroup analyses restricting to women receiving radiation or chemotherapy suggests that the disparity in CBC risk among NH Black women is not explained by differences in first-course therapy. Second, we based our analyses on SEER 18 data, which included first diagnoses between 2000 and 2015 only. This dataset has a shorter median follow-up time than previous SEER-based studies of CBC, but less heterogeneity associated with changes in screening and treatment practices prior to 2000 (i.e., introduction of tamoxifen, identification of the BRCA1 and BRCA2 susceptibility genes) and is arguably more generalizable to present-day disparities in CBC risk. Third, SEER’s aggregation of NH Asian / Pacific Islander women with diverse ancestries into a single group may have masked differences in CBC risk, similar to differences in risk of first primary breast cancer that are evident within specific Asian ancestral groups.41 Fourth, SEER does not include breast cancer screening data. NH Black and Hispanic women in the U.S. have lower rates of breast cancer screening relative to NH White women,42 which could contribute to the observed disparity in invasive CBC, particularly among women over 40 years of age for whom screening is indicated. Controlling for neighborhood SES, as a proxy for screening,43 may partially account for these differences. Finally, germline mutation data in breast cancer predisposition genes are not available in SEER. Further studies in large, diverse cohorts with germline mutation data, information on additional CBC risk factors, and breast cancer screening information are needed to determine whether CBC disparities can be explained by these factors.
In conclusion, NH Black women, regardless of ER status of their first breast cancer, have an increased risk of CBC relative to NH White women that is not explained by age at first diagnosis, radiation and chemotherapy for the first cancer, CPM, insurance status, or neighborhood SES. Hispanic women may also have a slightly increased risk of CBC relative to NH White women, but only those with ER-negative first breast cancers. Therefore, studies of CBC risk factors in U.S. minority groups are needed to collect evidence to inform interventions aimed at reducing these disparities in CBC risk.
Supplementary Material
Novelty and Impact.
Non-Hispanic Black survivors of breast cancer in the United States have an unexplained increased risk of second primary contralateral breast cancer. This study shows that the increased risk is not explained by age at diagnosis, receipt of chemotherapy or radiation therapy, socioeconomic or insurance status, or estrogen receptor status of the first breast cancer. Studies of diverse breast cancer survivors are needed to identify modifiable predictors of CBC to develop interventions to reduce this disparity.
Acknowledgments:
We would like to thank Dr. Colin C. Begg and Ms. Renee L. Gennarelli for their advice on this study, and Mr. Joseph Kanik for assistance with figures. In addition, we are grateful to the SEER staff for their help in creating custom databases for this study.
FUNDING
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers R01 CA129639, U01 CA083178, R01 CA097397, R01 CA114236, and P30 CA008748).
List of Abbreviations:
- CBC
Contralateral breast cancer
- CPM
Contralateral prophylactic mastectomy
- ER
Estrogen receptor
- HER2
Human epidermal growth factor 2
- HR
Hazard ratio
- NH
Non-Hispanic
- PR
Progesterone receptor
- SEER
Surveillance, Epidemiology, and End Results Program
- SES
Socioeconomic status
- SIR
Standardized incidence ratio
- U.S.
United States
Footnotes
Conflicts of Interest: The authors report no conflicts of interest related to this work.
NOTES
Ethical approval: The analysis of these data was not subject to institutional review board approval.
Prior Presentations: None
Data Availability Statement:
The data that support the findings of this study are openly available in the Surveillance, Epidemiology, and End Results Program at https://seer.cancer.gov/data/. Datasets used in this analysis are available from the corresponding author upon reasonable request.
REFERENCES
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin 2019;69: 438–51. [DOI] [PubMed] [Google Scholar]
- 2.Malone KE, Begg CB, Haile RW, Borg A, Concannon P, Tellhed L, Xue S, Teraoka S, Bernstein L, Capanu M, Reiner AS, Riedel ER, et al. Population-based study of the risk of second primary contralateral breast cancer associated with carrying a mutation in BRCA1 or BRCA2. J Clin Oncol 2010;28: 2404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein JL, Thompson WD, Risch N, Holford TR. Risk factors predicting the incidence of second primary breast cancer among women diagnosed with a first primary breast cancer. Am J Epidemiol 1992;136: 925–36. [DOI] [PubMed] [Google Scholar]
- 4.Bessonova L, Taylor TH, Mehta RS, Zell JA, Anton-Culver H. Risk of a second breast cancer associated with hormone-receptor and HER2/neu status of the first breast cancer. Cancer Epidemiol Biomarkers Prev 2011;20: 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiner AS, Lynch CF, Sisti JS, John EM, Brooks JD, Bernstein L, Knight JA, Hsu L, Concannon P, Mellemkjær L, Tischkowitz M, Haile RW, et al. Hormone receptor status of a first primary breast cancer predicts contralateral breast cancer risk in the WECARE study population. Breast Cancer Res 2017;19: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lizarraga IM, Sugg SL, Weigel RJ, Scott-Conner CE. Review of risk factors for the development of contralateral breast cancer. Am J Surg 2013;206: 704–8. [DOI] [PubMed] [Google Scholar]
- 7.Calip GS, Law EH, Ko NY. Racial and ethnic differences in risk of second primary cancers among breast cancer survivors. Breast Cancer Res Treat 2015;151: 687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nsouli-Maktabi HH, Henson DE, Younes N, Young HA, Cleary SD. Second primary breast, endometrial, and ovarian cancers in Black and White breast cancer survivors over a 35-year time span: effect of age. Breast Cancer Res Treat 2011;129: 963–9. [DOI] [PubMed] [Google Scholar]
- 9.Diab N, Clark G, Langer L, Wang Y, Hamlington B, Brzeskiewicz L, O’Shaughnessy J, Diab S, Jabbour SK. Impact of race and tumor subtype on second malignancy risk in women with breast cancer. Springerplus 2016;5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiong Z, Yang L, Deng G, Huang X, Li X, Xie X, Wang J, Shuang Z, Wang X. Patterns of Occurrence and Outcomes of Contralateral Breast Cancer: Analysis of SEER Data. J Clin Med 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mruthyunjayappa S, Zhang K, Zhang L, Eltoum I-EA, Siegal GP, Wei S. Synchronous and metachronous bilateral breast cancer: clinicopathologic characteristics and prognostic outcomes. Hum Pathol 2019;92: 1–9. [DOI] [PubMed] [Google Scholar]
- 12.Haque R, Xu X, Shi J, Kwan ML, Chlebowski RT. Breast Cancer Outcomes in a Racially and Ethnically Diverse Cohort of Insured Women. Ethn Dis 2018;28: 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurian AW, McClure LA, John EM, Horn-Ross PL, Ford JM, Clarke CA. Second primary breast cancer occurrence according to hormone receptor status. J Natl Cancer Inst 2009;101: 1058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Colditz GA, Gehlert S, Goodman M. Racial disparities in risk of second breast tumors after ductal carcinoma in situ. Breast Cancer Res Treat 2014;148: 163–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao K, Yang Y, Wu W, Liang S, Deng H, Liu J. Risk of second breast cancers after lobular carcinoma in situ according to hormone receptor status. PLoS One 2017;12: e0176417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017;265: 581–9. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute D, Surveillance Research Program. [Google Scholar]
- 18.Curtis R, Freedman D, Ron E, Ries L, Hacker D, Edwards B, Tucker M, FJ Jr, New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. National Cancer Institute, 2006. [Google Scholar]
- 19.Bernstein JL, Langholz B, Haile RW, Bernstein L, Thomas DC, Stovall M, Malone KE, Lynch CF, Olsen JH, Anton-Culver H, Shore RE, Boice JD, et al. Study design: Evaluating gene-environment interactions in the etiology of breast cancer - the WECARE study. Breast Cancer Res 2004;6: R199–R214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computinged. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 21.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep 1998;47: 1–16, 20. [PubMed] [Google Scholar]
- 22.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988;16: 1141–54. [Google Scholar]
- 23.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12: 703–11. [DOI] [PubMed] [Google Scholar]
- 24.Haas JS, Earle CC, Orav JE, Brawarsky P, Keohane M, Neville BA, Williams DR. Racial segregation and disparities in breast cancer care and mortality. Cancer 2008;113: 2166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M, Tatalovich Z, Gibson JT, Cronin KAJCC, Control. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes & Control 2014;25: 81–92. [DOI] [PubMed] [Google Scholar]
- 26.Kramer I, Schaapveld M, Oldenburg HSA, Sonke GS, McCool D, van Leeuwen FE, Van de Vijver KK, Russell NS, Linn SC, Siesling S, Menke-van der Houven van Oordt CW, Schmidt MK. The influence of adjuvant systemic regimens on contralateral breast cancer risk and receptor subtype. J Natl Cancer Inst 2019. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L, Olshan A, Tse CK, Bell ME, Weinberger M, Reeder-Hayes KE. Endocrine Therapy Nonadherence and Discontinuation in Black and White Women. J Natl Cancer Inst 2019;111: 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body Mass Index and Breast Cancer Risk According to Postmenopausal Estrogen-Progestin Use and Hormone Receptor Status. Epidemiol Rev 2014;36: 114–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Azambuja E, McCaskill-Stevens W, Francis P, Quinaux E, Crown JP, Vicente M, Giuliani R, Nordenskjold B, Gutierez J, Andersson M, Vila MM, Jakesz R, et al. The effect of body mass index on overall and disease-free survival in node-positive breast cancer patients treated with docetaxel and doxorubicin-containing adjuvant chemotherapy: the experience of the BIG 02–98 trial. Breast Cancer Res Treat 2010;119: 145–53. [DOI] [PubMed] [Google Scholar]
- 30.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134: 769–81. [DOI] [PubMed] [Google Scholar]
- 31.Brooks JD, John EM, Mellemkjaer L, Lynch CF, Knight JA, Malone KE, Reiner AS, Bernstein L, Liang X, Shore RE, Stovall M, Bernstein JL. Body mass index, weight change, and risk of second primary breast cancer in the WECARE study: influence of estrogen receptor status of the first breast cancer. Cancer Med 2016;5: 3282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat 2010;123: 627–35. [DOI] [PubMed] [Google Scholar]
- 33.Flegal KM, Carroll MD, Kit BK, Ogden CL. PRevalence of obesity and trends in the distribution of body mass index among us adults, 1999–2010. JAMA 2012;307: 491–7. [DOI] [PubMed] [Google Scholar]
- 34.Dean LT, Gehlert S, Neuhouser ML, Oh A, Zanetti K, Goodman M, Thompson B, Visvanathan K, Schmitz KH. Social factors matter in cancer risk and survivorship. Cancer Causes Control 2018;29: 611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertelsen L, Bernstein L, Olsen JH, Mellemkjaer L, Haile RW, Lynch CF, Malone KE, Anton-Culver H, Christensen J, Langholz B, Thomas DC, Begg CB, et al. Effect of systemic adjuvant treatment on risk for contralateral breast cancer in the Women’s Environment, Cancer and Radiation Epidemiology Study. J Natl Cancer Inst 2008;100: 32–40. [DOI] [PubMed] [Google Scholar]
- 36.Stovall M, Smith SA, Langholz BM, Boice JD Jr., Shore RE, Andersson M, Buchholz TA, Capanu M, Bernstein L, Lynch CF, Malone KE, Anton-Culver H, et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys 2008;72: 1021–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noone A-M, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL. Comparison of SEER Treatment Data With Medicare Claims. Med Care 2016;54: e55–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knisely AT, Michaels AD, Mehaffey JH, Hassinger TE, Krebs ED, Brenin DR, Schroen AT, Showalter SL. Race is associated with completion of neoadjuvant chemotherapy for breast cancer. Surgery 2018;164: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedewa SA, Ward EM, Stewart AK, Edge SB. Delays in adjuvant chemotherapy treatment among patients with breast cancer are more likely in African American and Hispanic populations: a national cohort study 2004–2006. J Clin Oncol 2010;28: 4135–41. [DOI] [PubMed] [Google Scholar]
- 40.Reyes SA, King TA, Fei K, Franco R, Bickell NA. Factors Affecting the Completion of Adjuvant Chemotherapy in Early-Stage Breast Cancer. Ann Surg Oncol 2016;23: 1537–42. [DOI] [PubMed] [Google Scholar]
- 41.Gomez SL, Von Behren J, McKinley M, Clarke CA, Shariff-Marco S, Cheng I, Reynolds P, Glaser SL. Breast cancer in Asian Americans in California, 1988–2013: increasing incidence trends and recent data on breast cancer subtypes. Breast Cancer Res Treat 2017;164: 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed AT, Welch BT, Brinjikji W, Farah WH, Henrichsen TL, Murad MH, Knudsen JM. Racial Disparities in Screening Mammography in the United States: A Systematic Review and Meta-analysis. J Am Coll Radiol 2017;14: 157–65.e9. [DOI] [PubMed] [Google Scholar]
- 43.Pruitt SL, Shim MJ, Mullen PD, Vernon SW, Amick BC 3rd. Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: a systematic review. Cancer Epidemiol Biomarkers Prev 2009;18: 2579–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in the Surveillance, Epidemiology, and End Results Program at https://seer.cancer.gov/data/. Datasets used in this analysis are available from the corresponding author upon reasonable request.


