Abstract
Background and Aims
The burden of respiratory syncytial virus (RSV) infection in adults is of growing concern. This study was designed to quantify disease burden, treatment approaches, and outcomes associated with RSV infections in adult subpopulations, from prehospitalization to hospital discharge.
Methods
A retrospective chart analysis was conducted to collect patient‐case data from hospitalized US adults (aged >18 years) with RSV infection during two RSV seasons. Patients were categorized into risk groups: comorbid lung disease, immunocompromised, older adults (aged ≥65 years), and other adults (aged <65 years). Physicians reported diagnosis, treatment choices including respiratory supportive therapy (oxygen and fluid supplementation), and outcome variables using a standardized online case form.
Results
The majority (277/379; 73%) of patients presented to the emergency room, with a mean age of 60 years. Once hospitalized, the median length of stay was 6.0 days (3.0–9.0), with disease severity having the greatest impact on duration of stay. No significant between‐group differences in rates of patients requiring management in intensive care units were found (comorbid lung disease, 28%; immunocompromised, 36%; older adults, 26%; and other adults, 23%). Overall, respiratory supportive therapy was the most commonly used form of treatment. Antibiotics were administered in over half of all risk groups (comorbid lung disease, 61%; immunocompromised, 59%; older adults, 59%; and other adults, 51%). Patients usually required follow‐up visits following discharge, with 10%–16% requiring skilled nursing care and approximately 25% requiring assistance from a social worker.
Conclusion
RSV in adult subpopulations, irrespective of age, is a significant burden to healthcare systems.
Keywords: adult, burden of disease, respiratory syncytial virus, retrospective
1. INTRODUCTION
The recognition of the prevalence and burden of respiratory syncytial virus (RSV) in adults is increasing, especially in older adults, those with chronic respiratory or cardiopulmonary disease, and those who are immunocompromised. 1 , 2 , 3 The significant morbidity and mortality associated with RSV infection places a substantial burden on healthcare systems, 3 while the clinical impact of RSV infection in adults at increased risk for serious diseases can approach that of influenza in some seasons. 4 , 5 , 6 Treatment for RSV and measures to prevent infection, particularly in more vulnerable patients, could therefore have a major impact on RSV disease burden in adults.
Progress in the development of RSV therapeutics and vaccines, however, has been slow compared with that for influenza and other viral infections. 7 Barriers to the development of RSV therapies include difficulties in trial design, primarily a lack of established clinically meaningful endpoints, and concerns that antiviral therapies may be ineffective against a disease driven mainly by virus‐induced inflammatory cascades. 3 , 8
Management of RSV infection in adults includes supportive care (e.g., supplemental oxygen, fluid replacement, and mechanical ventilation), bronchodilators, corticosteroids, and non‐RSV‐specific antiviral therapy. 8 In addition, antibiotics are often administered. 8 Aerosolized ribavirin is the only drug approved for RSV treatment in the United Kingdom 9 and United States 10 ; however, it is only indicated for hospitalized infants with severe lower respiratory tract infection. Ribavirin has unclear efficacy and questionable safety. 7 Palivizumab, which specifically targets RSV infection, is also licensed for prophylactic use in selected pediatric populations. 7 , 8 Several RSV vaccines are in development, although none have yet been approved. 7
This review of patient charts was conducted to further quantify disease burden, the use of diagnostics, and current treatment practices for RSV infection in adults in the United States. Clinical characteristics, presenting symptoms, and virological diagnosis from this study have been previously reported. 11 Herein, we report the burden of RSV infection in adults during and posthospitalization, as demonstrated by the rates of intensive care unit (ICU) admissions, hospital length of stay (LOS), treatment of RSV infection, and the follow‐up care required.
2. METHODS
Complete details of the methods, including physician and patient inclusion criteria, used for this physician chart survey have been previously reported. 11 The following provides an overview.
2.1. Survey design
This was a retrospective chart review of individual patient data with RSV infections in hospitalized adults presenting to a US hospital‐based physician between October 1, 2014 and October 21, 2016 (i.e., including two winter seasons). Patient data were recorded by the treating physician onto a standardized online case form capturing standard medical records.
2.2. Inclusion criteria of responding physicians
Physicians were contacted and invited to participate through market research panels. Responding physicians were required to fulfill nine key eligibility criteria to participate in the survey, including being able to provide one to three confirmed cases of RSV in adults for whom they were the primary treating hospital physician. Physicians indicated if they were from an integrated delivery network (IDN).
2.3. Inclusion criteria of RSV cases
Case selection was based on searches of patient files according to an allocated random letter of the alphabet matching the patient's last name. Eligible cases included hospitalized patients ≥18 years of age diagnosed with RSV, confirmed via laboratory or point‐of‐care diagnostics within the past two RSV seasons, and not enrolled in a clinical trial at the time.
Cases were categorized into one of four mutually exclusive risk groups, including: (1) Patients who were immunocompromised (regardless of other comorbidities) due to hematological malignancy in remission; hematological malignancy not in remission, not on chemotherapy; hematological malignancy on chemotherapy at the time of RSV diagnosis; solid tumor on chemotherapy at the time of RSV diagnosis; pulmonary fibrosis on immunosuppressive therapy at the time of RSV diagnosis; autologous or allogeneic hematopoietic stem cell transplant; solid organ transplant; connective tissue disorder; inflammatory bowel disease on immunosuppressive therapy at the time of RSV diagnosis; or vasculitis. (2) Patients who were not immunocompromised but had an underlying chronic lung disease: chronic obstructive pulmonary disease (COPD, including chronic bronchitis or emphysema); treated tuberculosis; interstitial lung disease; cystic fibrosis; asthma; bronchiectasis; or other lung comorbid condition. (3) Older adults (≥65 years of age) who were not categorized into either of the first two groups. (4) Remaining adult patients not categorized into any of the first three groups.
Illness severity for each patient was characterized by the reporting physician based on clinical judgment as mild, moderate, or severe. These terms were not defined in the survey. Improvements in clinical outcomes were categorized qualitatively (either: no improvement, slight improvement, moderate improvement, extreme improvement, don't know, or none of the above) at the treating physician's discretion. For each hospitalization, the reason for hospitalization was also categorized as not likely, somewhat likely, or very likely to be RSV infection by the treating physician, according to their clinical judgment.
2.4. Data analyses
A sample size of 135 respondents was required to detect reasonable, statistically significant differences in the burden of RSV infection between the adult risk groups (comorbid lung disease, immunocompromised patients, older adults, and other adults) with acceptable power. This sample size enabled an α of 5% (p < 0.05) and 80% power when comparing differences between‐risk groups.
Statistical analyses were performed using IBM SPSS Statistics Version 23 software. Descriptive results of therapy during hospitalization, occurrence of clinically or microbiologically suspected bacterial coinfections and antibiotic use, ICU admission and duration, length of hospitalization, and recovery of follow‐up care posthospitalization are reported for all cases in the four risk groups. Due to the retrospective nature of the analysis, patients were not actively followed‐up over time; patient outcomes were collected according to data availability. Baseline case demographics, comorbidities, presenting symptoms, diagnostic test procedures, time intervals of diagnostic testing and reporting, hospital LOS, and other aspects of antibiotic use are reported. 11 Mean time to clinical stability was calculated based on time for normalization of clinical conditions: blood oxygen, oral feeding, respiratory rate, and heart rate, and is displayed in days ± SD. Percentages and mean ± SD are reported where appropriate. Statistically significant means for key inputs into adult burden of disease metrics were measured via two‐tailed t‐tests for comparisons between two variables, and one‐way analysis of variance (ANOVA) across multiple burden of disease metrics. A p value of <0.05 was considered statistically significant.
2.5. Ethics approval statement
The analysis was conducted according to the guidelines of the US Health Insurance Portability and Accountability Act (1996), and was exempt from protocol review by the New England Independent Review Board.
2.6. Patient consent statement
Since data were collected retrospectively with no identifying patient characteristics, informed consent by patients was not required.
3. RESULTS
3.1. Responding physician and patient case demographics
Of the 13,000 physicians invited to participate in the study (Figure S1), 132 physicians completed screening, met inclusion criteria, and provided data on hospitalized adult patients with a confirmed diagnosis of RSV infection. Half of the responding physicians were pulmonologists (n = 34; 25.8%) or infectious disease specialists (n = 32; 23.9%). Of the 379 patient cases submitted, 126 (33.2%) had comorbid lung disease, 90 (23.7%) were immunocompromised, 110 (29.0%) were older adults, and 53 (14.0%) were other adults. Physicians from IDN submitted 213 (56.2%) patient cases, of which 76 (35.7%) had comorbid lung disease, 42 (19.7%) were immunocompromised, 72 (33.8%) were older adults, and 23 (10.8%) were other adults. An overview of patient demographic data is presented in Table 1. Detailed physician and patient demographic data have been presented previously. 11
Table 1.
Patient case demographics, clinical characteristics, and medical resource utilization (n = 379)
| Comorbid lung diseasea (n = 126) | Immunocompromised (n = 90) | Older adults (n = 110) | Other adults (n = 53) | Overall (n = 379) | |
|---|---|---|---|---|---|
| Mean ± SD (range), age (years) | 63 ± 15 (18–95) | 57 ± 15 (19–82) | 70 ± 5 (65–88) | 41 ± 12 (20–64) | 60 ± 16 (18–95) |
| Male, n (%) | 68 (54) | 51 (57) | 60 (55) | 32 (60) | 211 (55.6) |
| Smoking status, n (%) | |||||
| Current smoker | 47 (37.3) | 19 (21.1) | 19 (17.3) | 27 (50.9) | 112 (29.6) |
| Previous smoker | 60 (47.6) | 38 (42.2) | 59 (53.6) | 14 (26.4) | 171 (45.1) |
| Never smoked | 16 (12.7) | 30 (33.3) | 28 (25.5) | 10 (18.9) | 84 (22.2) |
| Don't know | 3 (2.4) | 3 (3.3) | 4 (3.6) | 2 (3.8) | 12 (3.2) |
| Caregiver requirements before hospitalization, n (%) | |||||
| Did not need caregiver | 66 (52.3) | 53 (58.8) | 59 (53.6) | 38 (71.6) | 216 (57.0) |
| Relied on spouse/partner | 42 (33.3) | 24 (26.7) | 33 (30.0) | 11 (20.8) | 110 (29.0) |
| Relied on child | 9 (7.1) | 6 (6.7) | 8 (7.2) | 4 (7.5) | 27 (7.1) |
| Relied on professional caregiver/in‐home nurse | 9 (7.1) | 7 (7.8) | 10 (9.1) | 0 (0) | 26 (6.7) |
| Median LOS in hospital, days (IQR) | 6.0 (4.0–9.0) | 6.0 (4.0–9.0) | 6.0 (3.0–8.5) | 5.0 (3.0–7.75) | 6.0 (3.0–9.0) |
| ICU admissionsb (%) | 29 | 36 | 26 | 20 | ‐ |
| Median ICU LOS, days (IQR) | 4.0 (3.0–7.0) | 3.0 (2.0–5.75) | 2.5 (1.25–4.0) | 6.0 (1.5–9.5) | 3.0 (2.0–6.0) |
Note: Table adapted from Lee et al. 11
Abbreviations: ICU, intensive care unit; IQR, interquartile range; LOS, length of stay.
Types of lung disease included chronic obstructive pulmonary disease (chronic bronchitis or emphysema), treated tuberculosis, interstitial lung disease, cystic fibrosis, asthma, bronchiectasis, or other.
Samples sizes for this analysis were comorbid lung disease (n = 119), immunocompromised (n = 90), older adults (n = 109), and other adults (n = 59).
3.2. Treatment before hospitalization
Patients generally did not receive any treatment for RSV infection before hospital admission. The patient cases reported by the IDN physicians (n = 213) were comprised of 13/76 (17%) patients with comorbid lung disease, 14/42 (33%) immunocompromised patients, 15/72 (21%) older adults, and 8/23 (35%) other adults who received medical care before hospital admission. Of the patients who sought outpatient care before hospitalization (n = 35), most were admitted to hospital immediately, with almost all (25/35; 71%) admitted within 2 days of the initial physician visit.
3.3. Hospitalization and hospital LOS
The majority (340/379; 92%) of patients were hospitalized within 5 days of the initial onset of RSV symptoms (Figure S2); of these patients, 74/379 (20%) were hospitalized on the same day as symptom onset. RSV was considered by the treating physician to be at least somewhat likely to be the cause of hospitalization in almost all patients (n = 279/291; 96%); the cause was considered to be very likely in 162/291 (55%) patients across the risk groups. Overall median LOS in hospital was 6.0 (interquartile range [IQR] 3.0–9.0) days (n = 376). Median hospital LOS was 6.0 (4.0–9.0) days for patients with comorbid lung disease, 6.0 (4.0–9.0) days for immunocompromised patients, 6.0 (3.0–8.5) days for older adults, and 5.0 (3.0–7.75) days for other adults. Physician assessment of illness severity was reported as severe in 65/379 (17%) patients overall, and severe in 19/126 (15%), 21/90 (23%), 19/109 (17%), and 5/52 (9%) in the comorbid lung disease, immunocompromised, older adult, and other adult groups, respectively.
Of the patients in any risk group, most (277/379; 73%) presented to the emergency room (ER) before hospital admission, while the remainder (102/379; 27%) were admitted directly to a hospital. ER stays were typically 3–8 h. Numbers of patients remaining in the ER < 8 h were 40/45 (89%), 21/26 (81%), 45/51 (88%), and 15/17 (88%) in the comorbid lung disease, immunocompromised, older adult, and other adult groups, respectively.
A total of 108/379 (28%) patients were admitted to the ICU, the most common reasons being respiratory difficulty, abnormal respiratory rate, or low blood oxygen level (Figure 1A). There was no significant difference in rates of ICU admission between risk groups; 35/126 (28%), 32/90 (36%), 29/109 (26%), and 12/52 (23%) in the comorbid lung disease, immunocompromised, older adult, and other adult groups, respectively. Rates of ICU admission were 6% (6/104), 25% (52/210), and 81% (50/62) in patients with mild, moderate, and severe infection, respectively. The overall median ICU LOS was 3.0 days (IQR 2.0–6.0). A significantly greater proportion of patients with comorbid lung disease had an ICU stay over 3 days than older adults (p = 0.03) (Figure 1B).
Figure 1.
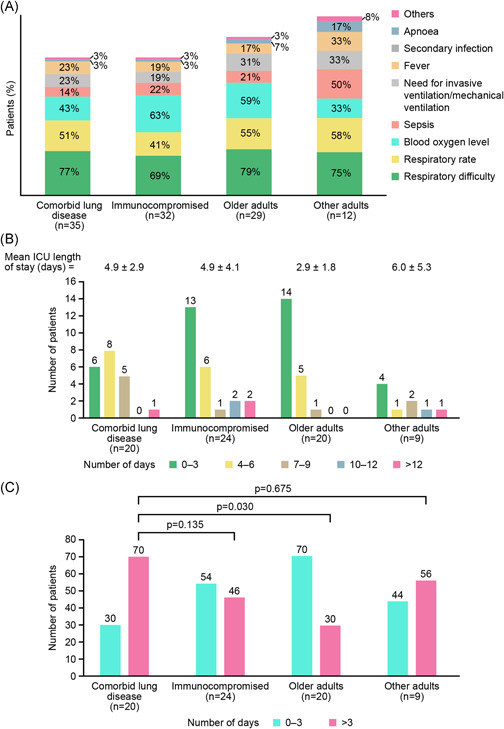
(A) Reasons for ICU admission, (B) ICU LOS (days) for RSV‐infected patients, and (C) proportion of patients with an ICU stay of 0–3 days or >3 days. Note: Patients could have multiple reasons for ICU admission. A cut‐off of 3 days was selected based on an overall median ICU LOS of 3.0 days. ICU, intensive care unit; LOS, length of stay; RSV, respiratory syncytial virus
4. TREATMENT
Irrespective of the risk group, supportive respiratory therapies (including, supplemental oxygen and fluids, bronchodilators, mechanical ventilation) were the primary treatment for RSV infection. Overall, the most common therapies during hospitalization (both in hospital and in the ER) were supplemental oxygen and bronchodilators followed by corticosteroids, antibiotics, and ribavirin (Figure 2A,B). Patients with comorbid lung disease were frequently treated with supplemental oxygen and bronchodilators; immunocompromised patients more frequently received ribavirin and immunoglobulin than other patients; and older adults received respiratory support and antibiotics during hospital stay more often than younger other adults. The proportion of immunocompromised patients receiving ribavirin (40%) was higher than in the comorbid lung disease group (30%; p = 0.15) and significantly higher than in the older adults group (26%; p = 0.05; Figure 2A). A greater proportion of severely ill patients required treatment with bronchodilators and mechanical ventilation compared with moderately or mildly ill patients (Figure 3).
Figure 2.
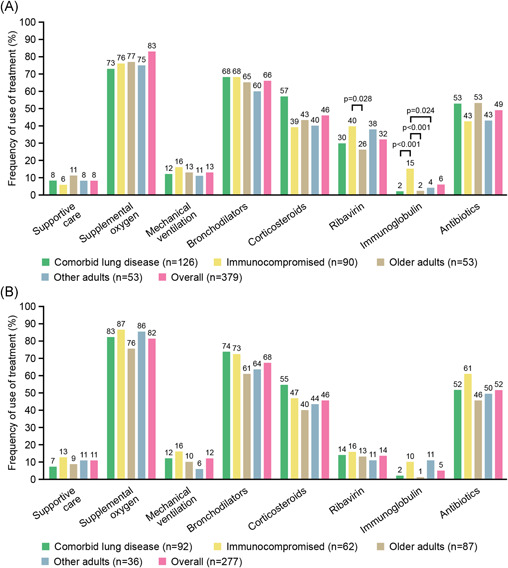
Comparison of treatments used in (A) hospital and (B) in the ER by risk group and overall among patients with RSV infection. Note: “Supportive care” (as defined by the treating physician) was a specific option in the case form, and the option was nonexclusive. p values shown between population groups were significant; all other comparisons were not significant. ER, emergency room; RSV, respiratory syncytial virus
Figure 3.
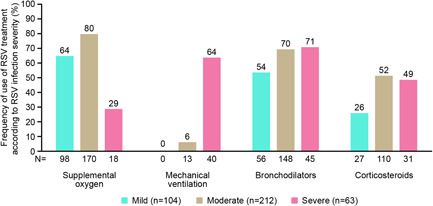
Treatment of RSV infection in hospital according to RSV infection severity. RSV, respiratory syncytial virus
4.1. Potential or suspected bacterial coinfection and antibiotic usage
Bacterial coinfections, confirmed by routine diagnostic testing, were reported in 87/379 (23%) patients, with a further 132/379 (32%) patients with suspected but unconfirmed bacterial infection. The proportion of patients with either suspected or confirmed bacterial coinfections was highest in the comorbid lung disease group and the older adult group (34% and 25%, respectively, for both groups) (Figure 4A). Significantly more patients in the ICU had suspected bacterial coinfections compared with non‐ICU patients (47/108; 44% vs. 75/268; 28%; p = 0.004).
Figure 4.
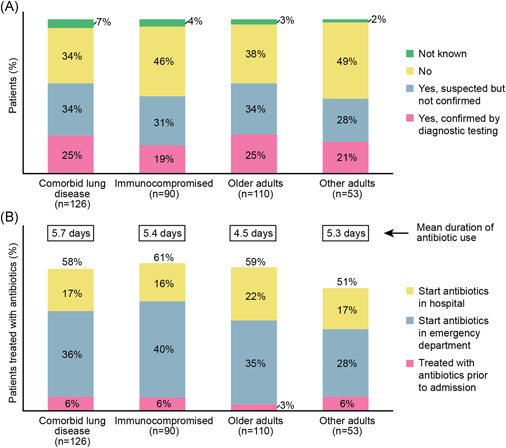
(A) RSV‐infected patients with confirmed or suspected bacterial coinfection and (B) proportion of patients with reported antibiotic usage. Note: (B) adapted from Lee et al. 11 RSV, respiratory syncytial virus
Antibiotics were used in over half of all patients in each of the risk groups 73/126 (58%), 55/90 (61%), 65/110 (59%), and 27/53 (51%) in the comorbid lung disease, immunocompromised, older adult, and other adult groups, respectively, often starting in the emergency department (Figure 4B). Antibiotic usage was continued by 79/114 (69%) patients for at least 1 day after RSV diagnosis.
Significant differences were observed between the percentage of mild (45/104; 43%), moderate (123/212; 58%; p = 0.031 vs. mild), and severe (52/63; 83% p < 0.001 vs. mild, p = 0.001 vs. moderate) patients who received antibiotics. Patients admitted to the ICU were more likely to receive antibiotics than those who were not admitted (83/108; 76% vs. 135/268; 50%, respectively). Furthermore, urban practice settings were significantly more likely to use antibiotics than suburban practices (142/220; 65% vs. 77/154; 50%, respectively; p = 0.006). Of the patients treated with antibiotics in the hospital, n = 46/187 (25%) had a laboratory‐confirmed bacterial infection and n = 88/187 (47%) had a suspected bacterial infection.
4.2. Clinical outcomes and posthospital follow‐up care
Improvements in clinical outcomes were categorized qualitatively at the treating physician's discretion. Approximately three‐quarters of all patients showed at least moderate improvement following inpatient care, with moderate‐to‐high improvement reported in 47/49 (96%) of patients with comorbid lung disease, 21/23 (91%) of immunocompromised patients, 37/45 (82%) of older adults, and 11/14 (79%) of other adults. Mean ± SD time to clinical stability was 4.0 ± 9.6, 5.0 ± 4.6, 4.0 ± 2.1, and 5.0 ± 8.1 days in the comorbid lung disease, immunocompromised, older adult, and other adult groups, respectively. In‐hospital mortality rates were low: 1/74 (1.4%) of patients with comorbid lung disease, 2/41 (4.9%) of immunocompromised patients, 1/62 (1.6%) of older adults, and 1/23 (4.3%) of other adults. Of patients hospitalized with RSV, the majority were alive after 60 days; 70/72 (97%) of patients with comorbid lung disease, 39/41 (95%) of immunocompromised, 60/62 (97%) of older adults, and 22/23 (96%) of other adults.
The majority of patients required follow‐up care, with more than 90% (68/75) of the comorbid lung disease group requiring care related to RSV infection postdischarge (Figure 5). Most patients required follow‐up visits with healthcare providers only, while 10%–16% required skilled nursing (either at home or discharged to assisted care/nursing facility), compared with 6.7% before admission. A social worker was required to manage 24%–27% of patients in each group.
Figure 5.
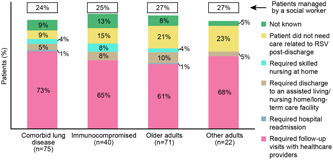
Comparison of types of follow‐up care posthospitalization required by RSV‐infected patients by risk group. RSV, respiratory syncytial virus
5. DISCUSSION
This chart review analysis quantified burden of disease, treatment approaches, and outcomes associated with RSV infections requiring hospitalization in US adults. Our approach created a rich database of case details, focusing on four risk groups. Three groups were considered to be vulnerable to RSV infection—comorbid lung disease, immunocompromised, and older adults—while one was at lower risk—other adults. These findings contribute to the growing body of evidence highlighting that RSV can be a serious cause of illness in adults. 12 , 13
RSV causes an estimated 177,000 annual hospitalizations and 14,000 annual deaths in US adults ≥65 years of age. 14 RSV infection is more likely to occur in older adults, those with chronic respiratory disease or cardiopulmonary disease, or those who are immunocompromised. 1 , 2 , 3 The burden may also be skewed towards older adults, particularly those aged ≥65 years due to an age‐related reduction in cell‐mediated immunity. 15 , 16 , 17
The majority of hospitalized adults with RSV infection were older adults, had underlying comorbidities, or a combination of both. This is similar to prior studies of adults hospitalized with RSV infection, with the exception of the larger number of immunocompromised patients. 4 , 18 , 19 Many patients in each group initially presented to the ER before being admitted to hospital, with ER stays shorter than 8 h, and there was no difference in proportions of patients admitted to the ICU. However, other patient characteristics may have had an impact on the burden of RSV infection. For example, over half of the other adult group were current smokers, suggesting that at least some may have had early‐stage airway disease. 11 Additionally, 18% of the other adult group reported having cardiovascular disease, which has been associated with higher rates of healthcare utilization for RSV‐related illnesses and poorer disease outcomes, including LOS and admission to the ICU. 20
Patients with RSV were generally hospitalized within a week of symptom onset. Severity of illness and underlying comorbidities may be important drivers of hospital LOS, although the length of time from admission to confirmed diagnosis may have also played a role. 11 Our findings are consistent with other RSV‐associated hospitalization studies, which demonstrate that median hospital LOS ranges from 3 to 9 days. 4 , 18 , 19 , 20 , 21 , 22
Between 23% and 36% of patients were admitted to the ICU, although there was no significant difference in rates between patient risk groups. The need for intensive care was reported more frequently for patients in this more vulnerable study population than in other studies of RSV‐hospitalized patients. 22 , 23 The higher incidence of ICU admission in this study compared with previous data may potentially represent a selection bias by physicians for those patients with more severe illnesses.
Treatment was not usually initiated before hospital admission, which occurred soon after visiting a physician. RSV treatment typically involves respiratory supportive care for common symptoms, such as dyspnea, wheezing, bronchitis, and upper respiratory infection, including bronchodilators and antibiotics. 23 In our study, the primary treatments were supplemental oxygen and bronchodilators, which were received by >70% and ≥60% of patients, respectively. Antibiotics were used for the treatment of RSV infection and for suspected or proven bacterial coinfection in many patients in all risk groups, starting in the ER and continuing throughout hospitalization. High antibiotic use in RSV infection is well documented. 15 , 21 , 23 , 24 Inappropriate antibiotic prescribing for patients with RSV has been suggested, 15 , 21 , 23 although use is reasonable in RSV‐infected individuals with confirmed or highly suspected bacterial pathogens. 8 Ribavirin use was common in the immunocompromised group, consistent with other published studies of immunocompromised RSV patients, including hematopoietic stem cell transplant recipients. 21 , 25 Surprisingly, ribavirin was also used frequently in the other risk groups, although information regarding the route of ribavirin administration was not collected. In the United States and Europe, ribavirin is not indicated for use in adults with RSV infection. 10 , 26 Routine use of ribavirin is not recommended due to questionable evidence regarding its safety and efficacy. 7 , 27
Most patients with RSV required follow‐up visits after hospital discharge and demonstrated at least moderate improvement with this care. Sixty‐day mortality was lower in this study (3.5%) than reported in other RSV studies (5%–8%). 2 , 17 , 19 , 28 It is possible that detection of posthospitalization deaths was incomplete due to limitations of physician visibility into patient outcomes postdischarge, even at IDNs.
Our analysis provides useful insights into the burden of RSV infections in adult patients across hospitals in the United States, including IDNs. IDNs are organizations that own and operate a network of healthcare facilities, and thereby provide a continuum of care for patients as they transition through different disease states. The patient journey may differ depending on whether they receive care from an IDN. Importantly, IDNs and non‐IDN settings were both represented in this survey. This study has several limitations. This was a retrospective analysis, which relied on the records of responding physicians. Unavoidable case selection bias may have occurred despite attempts to minimize physicians specifically selecting cases for inclusion; reported data assumes that physicians faithfully entered data from the patient record. In contrast to a prospective design, a retrospective design may underestimate specific symptoms and/or treatment modalities. The study design also does not allow an estimate of the actual RSV incidence in the population studied, and thus the total burden of RSV in adults. Severity was not assessed using a clear objective rating scale. A further limitation was the limited visibility of physicians for patient outcomes beyond inpatient care.
Although not designed to provide incidence data, the study demonstrates that the healthcare burden of RSV infection in adults is substantial. RSV infection in older adults resulted in longer hospital stays, antibiotic usage, ICU admissions, and respiratory support treatments compared with other adults. While most patients with RSV infection recovered well posthospitalization, the significant burden associated with RSV infection during hospitalization is apparent. Hospital resource utilization is notable, with long hospital stays even in patients without underlying high‐risk comorbidities. Antibiotics are used in most hospitalized patients with RSV infection, often without a confirmed bacterial infection. ICU admissions, usually for respiratory support treatments, averaged 4.5 ± 3.6 days. The data identified the need for ongoing care following hospital discharge, with an increase in the number of patients requiring long‐term care. These findings suggest that there is a large unmet clinical need for new effective and selective antiviral treatments for RSV, along with vaccines to significantly reduce the burden of RSV in adults.
CONFLICTS OF INTEREST
Nelson Lee has previously received honoraria for consultancy work, speaking in educational programs, and/or travel support from Shionogi Inc., Janssen, Sanofi Pasteur Ltd., F. Hoffmann‐La Roche Ltd., Genentech Inc., CIDARA Therapeutics Inc. Edward Walsh has research contracts from Gilead, Janssen, Merck Sharp & Dohme and unpaid consultation to Novavax, and Pfizer. Robert Stolper and Jessica Zakar are employees of IQVIA, a healthcare consulting firm engaged by Janssen Pharmaceuticals. Ian Sander is a former employee of IQVIA and a current employee of Ironwood Pharmaceuticals. Veronique Wyffels and Roman Fleischhackl are employees of Janssen Pharmaceuticals. David Myers is a former employee of Janssen Pharmaceuticals. Veronique Wyffels and Roman Fleischhackl may be Johnson & Johnson stockholders.
AUTHOR CONTRIBUTIONS
All authors have read and approved the final version of the manuscript. Roman Fleischhackl had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We thank Arnaud Chéret, David Rivas, and Katia Boven for their review comments on the manuscript. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Patrick Hoggard of Ashfield MedComms, and funded by Janssen. This study was supported by Janssen Pharmaceuticals. The sponsor was involved in the study design, collection and interpretation of the data, revision of the manuscript, and the decision to submit the report for publication.
Walsh E, Lee N, Sander I, et al. RSV‐associated hospitalization in adults in the USA: a retrospective chart review investigating burden, management strategies, and outcomes. Health Sci. Rep. 2022;5:e556. 10.1002/hsr2.556
DATA AVAILABILITY STATEMENT
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu
REFERENCES
- 1. Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infect Disord Drug Targets. 2012;12(2):98‐102. [DOI] [PubMed] [Google Scholar]
- 2. Pilie P, Werbel WA, Riddell J, Shu X, Schaubel D, Gregg KS 4th. Adult patients with respiratory syncytial virus infection: impact of solid organ and hematopoietic stem cell transplantation on outcomes. Transpl Infect Dis. 2015;17(4):551‐557. [DOI] [PubMed] [Google Scholar]
- 3. Simões EA, DeVincenzo JP, Boeckh M, et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J Infect Dis. 2015;211(suppl 1):S1‐S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high‐risk adults. N Engl J Med. 2005;352(17):1749‐1759. [DOI] [PubMed] [Google Scholar]
- 5. Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993‐2008. Clin Infect Dis. 2012;54(10):1427‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Asten L, van den Wijngaard C, van Pelt W, et al. Mortality attributable to 9 common infections: significant effect of influenza A, respiratory syncytial virus, influenza B, norovirus, and parainfluenza in elderly persons. J Infect Dis. 2012;206(5):628‐639. [DOI] [PubMed] [Google Scholar]
- 7. Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. BNF . Respiratory syncytial virus: Management in children. NICE. 2020. Accessed January 2021. https://bnf.nice.org.uk/treatment-summary/respiratory-syncytial-virus.html
- 10. FDA . Respiratory syncytial virus infection: developing antiviral drugs for prophylaxis and treatment (guidance for industry). 2017. Accessed January 2021. https://www.fda.gov/media/108437/download
- 11. Lee N, Walsh EE, Sander I, et al. Delayed diagnosis of respiratory syncytial virus infections in hospitalized adults: individual patient data, Chart Review Analysis and Physician Survey in the USA. J Infect Dis. 2019;220(6):969‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coultas JA, Smyth R, Openshaw PJ. Respiratory syncytial virus (RSV): a scourge from infancy to old age. Thorax. 2019;74(10):986‐993. [DOI] [PubMed] [Google Scholar]
- 13. Falsey AR, Walsh EE, Esser MT, Shoemaker K, Yu L, Griffin MP. Respiratory syncytial virus‐associated illness in adults with advanced chronic obstructive pulmonary disease and/or congestive heart failure. J Med Virol. 2019;91(1):65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . Respiratory syncytial virus infection (RSV): trends and surveillance. Accessed October 2018. www.cdc.gov/rsv/research/us-surveillance.html#f2
- 15. Fleming DM, Taylor RJ, Lustig RL, et al. Modelling estimates of the burden of respiratory syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect Dis. 2015;15:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalisation burden associated with influenza and respiratory syncytial virus in New York City, 2003‐2011. Influenza Other Respir Viruses. 2015;9(5):225‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matias G, Taylor R, Haguinet F, Schuck‐Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997‐2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses. 2014;8(5):507‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee N, Lui GCY, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069‐1077. [DOI] [PubMed] [Google Scholar]
- 19. Belongia EA, King JP, Kieke BA, et al. Clinical features, severity, and incidence of RSV illness during 12 consecutive seasons in a community cohort of adults ≥60 years old. Open Forum Infect Dis. 2018;5(12):ofy316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ivey KS, Edwards KM, Talbot HK. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol. 2018;71(14):1574‐1583. [DOI] [PubMed] [Google Scholar]
- 21. Loubet P, Lenzi N, Valette M, et al. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza‐like illness in France. Clin Microbiol Infect. 2017;23(4):253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Volling C, Hassan K, Mazzulli T, et al. Respiratory syncytial virus infection‐associated hospitalisation in adults: a retrospective cohort study. BMC Infect Dis. 2014;14:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalisations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012;206(1):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee N, Chan MCW, Lui GCY, et al. High viral load and respiratory failure in adults hospitalized for respiratory syncytial virus infections. J Infect Dis. 2015;212(8):1237‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim YJ, Guthrie KA, Waghmare A, et al. Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. J Infect Dis. 2014;209(8):1195‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Guideline on the clinical evaluation of medicinal products indicated for the prophylaxis or treatment of respiratory syncytial virus disease. 2018. Accessed January 2021. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-evaluation-medicinal-products-indicated-prophylaxis-treatment-respiratory_en.pdf
- 27. Empey KM, Peebles RS Jr., Kolls JK. Pharmacologic advances in the treatment and prevention of respiratory syncytial virus. Clin Infect Dis. 2010;50(9):1258‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pastula ST, Hackett J, Coalson J, et al. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997‐2012. Open Forum Infect Dis. 2017;4(1):ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu


