Abstract
Background
Coronavirus disease 2019 (COVID‐19) is associated with hematological abnormalities of variable severity. The full blood count (FBC) and leukocyte differential count (DIFF) could facilitate the prediction of disease severity and outcome in COVID‐19. This study aimed to assess the hematological parameters in early severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and their correlation with disease outcome.
Methods
A retrospective cross‐sectional descriptive study was performed. Adults with a FBC and positive SARS‐CoV‐2 polymerase chain reaction results between March 1, and June 31, 2020 were reviewed. Basic hematological parameters (FBC, DIFF) and human immunodeficiency virus (HIV) status were recorded. Outcome measures were admission to a general ward or intensive care unit (ICU), recovery or death.
Results
Six hundred and eighty‐five cases median age 51 years, were analyzed. Forty‐four percent were males and fourteen percent were HIV‐positive with no association between death and/or ICU admission (p = 0.522 and p = 0.830, respectively). Leucocytosis was predictive of ICU admission (odds ratio [OR]: 2.4, confidence interval [CI]: 1.77–3.8186) and neutrophilia, of both mortality (OR: 1.5, CI: 1.0440–2.0899) and ICU admission (OR: 4, CI: 2.5933–6.475). Median lymphocyte count was decreased and d‐dimer raised, showing no significant association with outcome. Raised neutrophil‐to‐lymphocyte‐ratio (NLR) was associated with increased odds of mortality (OR: 2.5, CI: 1.3556–3.2503) and ICU admission (OR: 4.8, CI: 2.4307–9.5430) as was monocyte‐to‐lymphocyte‐ratio (MLR) (OR: 2, CI: 1.3132–2.9064) and (OR: 2.3, CI: 1.0608–1.9935), respectively. Hospital admission and older age were significantly associated with mortality (p = 0.0008 and p < 0.0001), respectively.
Conclusion
Evidence‐based interpretation of routine laboratory parameters, readily available in resource‐constrained settings, may identify patients at increased risk of mortality. The FBC, DIFF, NLR, and MLR should form part of the early COVID‐19 investigation.
Keywords: COVID‐19, d‐dimer, HIV, leucocytosis, neutrophil‐lymphocyte‐ratio, SARS‐CoV‐2
1. INTRODUCTION
Since the first cluster of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infections and the emergence of the ensuing global pandemic, there has been an exponential increase in the understanding of the virus, its clinical presentation, laboratory features and management. 1 , 2 , 3 , 4 , 5 Coronavirus disease 2019 (COVID‐19) is associated with multisystem complications and hematological abnormalities of variable severity. 4 , 5 , 6 The correlation between early changes in hematological parameters and disease outcome, is under active investigation. 4 , 5 , 7 , 8 , 9
Hematological changes reported include changes in full blood count (FBC) parameters, abnormal morphological characteristics of blood cells on peripheral blood smear examination and derangement of hemostatic parameters, referred to as COVID‐19‐associated coagulopathy (CAC). 4 , 7 , 10 FBC findings associated with SARS‐CoV‐2 infection include anemia, neutrophilia, lymphopenia, monocytosis, eosinopenia, mild thrombocytopenia, and less frequently thrombocytosis. 2 , 4 , 5 , 7 , 9 Lymphopenia, increased platelet‐to‐lymphocyte ratio, increased neutrophil‐to‐lymphocyte ratio (NLR), low B lymphocytes, low CD8‐positive T lymphocytes, and elevated CD4/CD8 ratio have been associated with adverse disease outcomes. 11 , 12 , 13 Changes reported on peripheral blood smears are predominantly nonspecific features seen in infective/inflammatory states. 7 Reported coagulation parameter changes include elevated d‐dimer and fibrinogen levels with relatively normal partial thromboplastin time (PTT) and thrombin time. 13 , 14 , 15 , 16 However, coagulation parameters are dynamic and differ depending on the severity of disease and coagulopathy. 16 Coagulopathy is associated with increased mortality. 10 , 16 , 17
South Africa's diverse ethnicity and socioeconomic status of its inhabitants pose a particular challenge in terms of predicting the epidemiological and clinicopathological features of COVID‐19. The burden of the human immunodeficiency virus (HIV) and tuberculosis (TB) infections, compounded by the high prevalence of noncommunicable diseases, increase the difficulty in extrapolating data from other COVID‐19 ravaged countries, to the South African situation. Widely available routine basic laboratory parameters, accessible in low resource settings could facilitate prediction of disease severity and outcome in COVID‐19. 5 , 9 , 18 This study aimed to assess the hematological parameters in early COVID‐19 and their correlation with outcome in a resource‐constrained setting.
2. METHODS
The study is a retrospective cross‐sectional descriptive study performed at the National Health Laboratory Service (NHLS), Tygerberg Hospital (TBH). TBH is a 1384 bed hospital providing tertiary healthcare and is the main teaching hospital of the University of Stellenbosch Faculty of Medicine and Health Sciences.
All SARS‐CoV‐2 polymerase chain reaction (PCR) positive adults, diagnosed at TBH NHLS between March 1, 2020 and June 31, 2020 with a recorded FBC, were included in this study. The first FBC result, obtained closest to or within 7 days of the positive SARS‐CoV‐2 PCR result was recorded. Outcomes were recorded as reflected on TBH clinical database as discharge or death. If no admission was reflected around the time of data collection, the patient was recorded as an outpatient.
The following exclusion criteria was applied
2.1.
1. Age less than 18 years old.
2. Lack of FBC results during the illness period.
Data were collected from the NHLS Laboratory Information System and captured on Research Electronic Data Capture (REDCap). 19 Outcomes included admission to the general ward or intensive care unit (ICU) and death or recovery. The evaluated parameters included patient age, sex, admission status, HIV status, CD4 count, white blood cell count, hemoglobin (Hb), mean corpuscular volume, mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet, neutrophil, lymphocyte, monocyte, eosinophil and basophil counts, d‐dimer, prothrombin time (PT) and activated partial thromboplastin time. The TBH laboratory is accredited with the South African National Accreditation System and adheres to International Standards Organization ISO15189 requirements for medical laboratory quality. The Siemens ADVIA 2120i® (Siemens AG Healthcare, 91052 Elarngen, Germany) was used for FBC and differential count analysis and Sysmex CS‐2000i™ (Sysmex Medical Electronics, Germany), for d‐dimer, PT, and PTT following NHLS approved standard operating procedures.
The study was approved by the Ethics Committee of the University of Stellenbosch (project identification number 15331) and was performed according to the Helsinki Declaration (2000) guidelines. A waiver of patient informed consent was granted in light of the study's retrospective nature (see Appendix S1).
2.2. Statistical analysis
Data were exported from the REDCap database and imported into Microsoft Excel (2003) and STATA 17 (StataCorp, 2017, Stata Statistical Software: Release 15; StataCorp LLC) for analysis. Descriptive statistics were performed on early laboratory parameters for all patients within the inclusion criteria. Where appropriate, standard deviations or median and interquartile ranges (IQRs) were reported. Empirical diagnostic cut‐off points were determined for hematological markers using receiver operating characteristic (ROC) curves to maximize the product of sensitivity and specificity, positive predictive value, and negative predictive value (NPV) where the ratio of cases in the positive and negative groups reflected the prevalence of the disease. For inferential statistics, the chi‐square test was used, or Fisher's exact test (depending on assumptions) where appropriate. For multivariate analysis, significant predictors at the bivariate level were included, statistical significance was considered at p < 0.05.
3. RESULTS
Seven hundred and thirty‐eight SARS‐CoV‐2 cases were initially included, and fifty‐three cases were excluded. Excluded cases did not have an FBC after the collection of positive SARS‐CoV2 test or had an FBC result after recovery (see Figure 1). The median and IQR of laboratory parameters of the total group is summarized in Table 1 and the odds ratio (OR) in Table 2.
Figure 1.
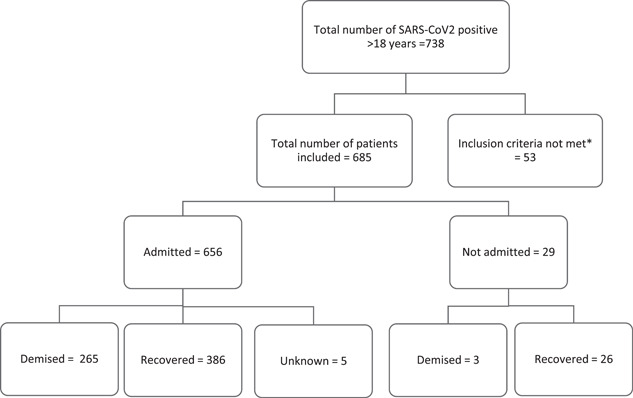
Total number of cases included in the study. *Excluded cases did not have a full blood count result available in the required period under review. SARS‐CoV2, severe acute respiratory syndrome coronavirus 2
Table 1.
Descriptive analysis of both hematological indices and demographics
| Indices | Overall median (IQR) | Died (n = 269 [39%]) median (IQR) | Recovered (n = 412 [60%]) median (IQR) | Admitted to general ward (n = 444 [65%]) median IQR | Admitted to ICU (n = 150 [22%]) median (IQR) |
|---|---|---|---|---|---|
| Age | 51 (39–60) | 53 (43–61) | 49 (37–59) | 50 (39–61) | 53 (42–60) |
| Male | 304 (44%) | 123 (46%) | 177 (43% | 187 (42%) | 81 (54%) |
| HIV‐positive | 95 (14%) | 40 (15%) | 55 (14%) | 62 (15% | 20 (13%) |
| Length of stay | 7 (3–11) | 7 (3–12) | 6 (3–11) | 6 (3–11) | 8 (4–11) |
| White cell count (3.92–10.40 × 109/L) | 8.28 (5.92–11.73) | 8.94 (6.39–12.36) | 7.6 (5.66–11.34) | 7.51 (5.63–10.84) | 10.83 (8.07–13.66) |
| Red cell count (3.80–4.80 × 1012/L) | 4.51 (4.01–4.94) | 4.48 (4.09–4.92) | 4.43 (3.9–4.91) | 4.63 (4.26–5) | |
| Hemoglobin (male: 13.0–17.0 g/dl) (female: 12.0–15.0 g/dl) | 13 (11.4–14) | 13.1 (11.5–14.1) | 13 (11.4–14) | 12.8 (11.1–13.9) | 13.4 (12.6–14.2) |
| Hematocrit (0.360–0.460 L/L) | 0.4 (0.36–0.44) | 0.4 (0.36–0.44) | 0.4 (0.35–0.43) | 0.395 (0.35–0.43) | 0.41 (0.38–0.44) |
| MCV (male: 83.1–101.6 fl) (female: 78.9–98.5 fl) | 89.4 (84.7–92.6) | 89.2 (85–92.1) | 89.5 (84.53–93) | 89.5 (84.8–92.9) | 89.2 (84.6–91.65) |
| MCH (male: 27.8–38.8) (female: 26.1–33.5 pg) | 28.7 (27.1–30.2) | 28.9 (27.3–30.4) | 28.5 (27.12–30.1) | 28.5 (27.1–30.2) | 29 (27.78–30.13) |
| MCHC (male: 33.0–35.0 pg) (female: 32.7–34.9 pg) | 32.2 (31.2–33.2) | 32.4 (31.38–33.4) | 32.15 (31.1–33.1) | 32 (31.2–33) | 32.7 (31.4–33.8) |
| RDW (males: 12.1–16.3%) (female: 12.4–17.3%) | 13.8 (13.2–14.8) | 13.8 (13.2–14.8) | 13.3 (13.8–4.9) | 13.8 (13.3–15) | 13.8 (13.2–14.6) |
| Platelets (186–454 × 109/L) | 234 (160–345) | 251 (189–324) | 252 (190–316) | 240 (176–299) | 238 (179–349) |
| Neutrophils (1.60–6.98 × 109/L) | 6.41 (4.26–9.45) | 7 (4.84–10.06) | 6.33 (4.0–9.39) | 5.56 (3.8–8.6) | 8.28 (6.55–11.3) |
| Lymphocytes (1.40–4.20 × 109/L) | 1.05 (0.74–1.48) | 1.03 (0.78–1.53) | 1.06 (0.76–1.45) | 1.04 (0.72–1.44) | 1.09 (0.54–1.52) |
| NLR (0.78–3.53) | 5.92 (3.72–9.9) | 6.52 (4.28–11.05) | 5.82 (3.41–9.56) | 5.46 (3.23–9.3) | 7.01 (5.07–11.41) |
| Monocytes (0.30–0.80 × 109/L) | 0.41 (0.28– 0.58) | 0.45 (0.3–0.6) | 0.4 (0.28–0.58) | 0.41 (0.26–0.55) | 0.46 (0.23–0.72) |
| MLR | 0.39 (0.25–0.6) | 0.41 (0.27–0.68) | 0.37 (0.24–0.55) | 0.38 (0.25–0.57) | 0.41 (0.28–0.63) |
| Eosinophils (0.00–0.95 × 109/L) | 0.02 (0.01– 0.05) | 0.02 (0.01– 0.05) | 0.02 (0.01–0.06) | 0.02 (0.01–0.04) | 0.02 (0.01‐0.7) |
| Basophils (0.00–0.10 × 109/L) | 0.04 (0.02–0.08) | 0.04 (0.02–0.08) | 0.04 (0.02–0.07) | 0.03 (0.02–0.07) | 0.05 (0.02–0.08) |
| d‐Dimer (0.00–0.25 mg/L) | 0.94 (0.45–2.91) | 1.04 (0.52–3.67) | 0.79 (0.4–2.1) | 0.66 (0.41–2.08) | 0.97 (0.5–4.03) |
| PT (9.9–12.3 s) | 13.3 (12.4–14.6) | 13 (12.1–14.58) | 13.4 (12.5–14.45) | 14 (12.75–15.35) | 13 (12.5–14) |
| aPTT | 29.55 (27.4–38.4) | 27.5 (26.65–36.9) | 30.05 (27.9–38.4) | 29.9 (27.6–37.9) | 27.5 (26.9–29.05) |
| Fibrinogen (2–4 g/L) | 4.7 (3.85–5.3) | 4.9 (4.55–5.2) | 4.45 (3.45–5.73) | 4.8 (4.075–5.43) | 3.75 (2.88–4.62) |
Note: Patients admitted to a general ward in cases where a high care or ICU level of care was not required.
Reference ranges used by the South African National Health Laboratory Services (NHLS) for the South African population.
Abbreviations: aPTT, activated partial thromboplastin time; CI, confidence interval; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; MCV, mean cell volume; MCHC, mean corpuscular hemoglobin concentration; MCH, mean cell hemoglobin; MLR, monocyte–lymphocyte ratio; NLR, neutrophil–lymphocyte ratio; PT, prothrombin time; RDW, red cell distribution width.
Table 2.
Odds ratio (OR) analysis of outcome association (mortality and ICU admission) with hematological indices
| OR died or recovered | (CI) p value | OR general ward or ICU | (CI) p value | |
|---|---|---|---|---|
| White cell count (>10 × 109/L) | 1.7 | (0.95–1.78) p = 0.089 | 2.4 | (1.77–3.82) p < 0.001 |
| Red cell count (<3.80 × 1012/L) | 0.6 | (0.41–0.99) p = 0.046 | 0.3 | (0.14–0.54) p = 0.002 |
| Hemoglobin (<12 g/dl) | 1.0 | (0.57–1.12) p = 0.192 | 0.3 | (0.19–0.49) p < 0.001 |
| Hematocrit (<0.360 L/L) | 0.8 | (0.60–1.27) p = 0.490 | 0.3 | (0.19–0.55) p < 0.001 |
| MCV (<80 fl) | 0.9 | (0.52–1.62) p = 0.768 | 0.6 | (0.29–1.34) p = 0.232 |
| MCH (<26 pg) | 1.0 | (0.65–1.59) p = 0.943 | 0.7 | (0.39–1.25) p = 0.223 |
| MCHC (<33 pg) | 0.7 | (0.49–0.98) p = 0.041 | 0.5 | (0.31–0.70) p = 0.002 |
| RDW (>17%) | 0.8 | (0.38–1.73) p = 0.595 | 0.6 | (0.24–1.73) p = 0.382 |
| Platelets | ||||
| Thrombocytopenia (<180 × 109/L) | 1.1 | (0.76–1.59) p = 0.608 | 0.4 | (0.27–0.74) p = 0.002 |
| Thrombocytosis (>450 × 109/L) | 1.5 | (0.74–3.02) p = 0.269) | 1.1 | (0.50–2.56) p = 0.716 |
| Neutrophils (neutrophilia > 7 × 109/L) | 1.5 | (1.04–2.09) p = 0.028 | 4.0 | (2.59–6.15) p < 0.0001 |
| Lymphocytes (<1.40 × 109/L) | 0.7 | (0.51–1.05) p = 0.088 | 0.6 | (0.41–0.95) p = 0.028 |
| Raised NLR > 3.8 | 2.5 | (1.36–3.25) p < 0.0001 | 4.8 | (2.43–9.54) p < 0.0001 |
| Monocytes (<0.30 × 109/L) | 0.7 | (0.52–1.02) p = 0.063 | 0.5 | (0.31–0.74) p < 0.0001 |
| Raised MLR > 0.26 | 2.3 | (1.06–1.99) p = 0.020 | 2 | (1.31–2.91) p = 0.001 |
| Eosinophils (<0.1 × 109/L) | 1 | (0.7–1.14) p = 0.987 | 0.2 | (0.14–0.31) p < 0.0001 |
| Basophils (0 × 109/L) | 0.8 | (0.59–1.23) p = 0.384 | 0.1 | (0.05–0.13) p < 0.0001 |
| d‐Dimer | ||||
| (>0.25 mg/L) | 1.7 | (0.53–5.20) p = 0.378 | 1.0 | (0.38–2.41) p = 0.925 |
| (>0.5 mg/L) | 2.1 | (0.66–6.71) p = 0.208 | 1.5 | (0.41–5.32) p = 0.548 |
| PT (9.9–12.3 s) | 0.4 | (0.18–0.94) p = 0.934 | 1.0 | (0.19–1.13) p = 0.089 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; MCH, mean cell hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume; MLR, monocyte–lymphocyte ratio; NLR, neutrophil–lymphocyte ratio; PT, prothrombin time; RDW, red cell distribution width.
A total of 685 cases were analyzed; 656 (96%) were admitted of which 150 (22%) were ICU admissions and 29 (4%) were outpatients. About 14% of the cases were HIV‐positive, 65% were HIV‐negative and the HIV status of 21%, was unknown. HIV‐positive status was not significantly associated with death (p = 0.521). Male gender was significantly associated with ICU admission (p = 0.007) but not with death overall (p = 0.522) or death in ICU (p = 0.157; see Table 3). The majority of recoveries were in the 18–39 years age group while most deaths were in the 50–59 years age group (see Figure 2). The median age of deceased patients was 53 years compared to 49 years in the recovered group. ICU admission (mean: 51.347, SD: 11.494; p < 0.001) and death (mean: 52.078, SD: 13.113; p < 0.001) were significantly associated with older age.
Table 3.
χ 2 Test to show the association of sex and HIV status with ICU admission and death
| Outcome | p Value | |
|---|---|---|
| ICU admission | ||
| Male, n = 81 (54% of ICU admissions) | 0.007 | |
| HIV‐positive, n = 20 (13% of ICU admissions) | 0.830 | |
| Death | ||
| Male, n = 123 (46% of deaths) | 0.522 | |
| HIV‐positive, n = 40 (15% of deaths) | 0.522 | |
| Admitted, n = 265 (39% of total admitted) | <0.001 | |
Abbreviations: HIV, human immunodeficiency virus; ICU, intensive care unit.
Figure 2.
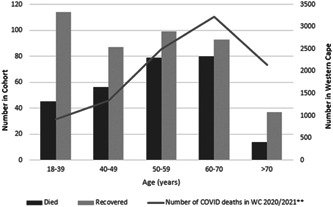
Coronavirus disease 2019 (COVID‐19) deaths per age category compared to local (South Africa, Western Cape) statistics. **Western Cape (WC) Government COVID‐19 statistics. https://coronavirus.westerncape.gov.za/covid-19-dashboard. Published 2021. Accessed June 4, 2021
Deceased patients had higher neutrophil counts and NLR; (OR: 1.5, confidence interval [CI]: 1.04–2.09) and (OR: 2.5, CI: 1.36–3.25), respectively. Furthermore, the differential counts impacted admission to ICU compared to general wards in which the ICU group had a higher white cell count (WCC), 10.83 × 109/L compared to 7.51 × 109/L in the general wards group (OR: 2.4, CI: 1.77–3.82) and neutrophil count 8.28 × 109/L for the ICU group compared to 5.56 × 109/L for general ward group (OR: 4.0, CI: 2.59–6.15). The likelihood of ICU admission increased with WCC, >10 × 109/L (OR: 2.6, CI: 1.77–3.82) and >11.5 × 109/L (OR: 2.4, CI: 1.58–3.69) seen in Figure 3. Most patients with leucocytosis had neutrophilia, except six cases where leucocytosis was secondary to increased lymphocyte count. The difference in length of stay (LOS) between those that recovered (mean: 8.9148, SD: 9.2257) and those that died (mean: 9.0538, SD: 8.36687) was not statistically significant (p = 0.847). Total leukocyte count did not influence LOS (mean: 8.8027, SD: 8.7605; p = 0.8143). HIV‐positive status did not influence LOS: general ward (mean: 9.9831, SD: 9.0918) compared to ICU ward (mean: 9.1500, SD: 9.0918), p = 0.7340, recovered (mean: 11.208, SD: 10.8147) compared to died (mean: 7.8974, SD: 6.2987) p = 0.1142.
Figure 3.
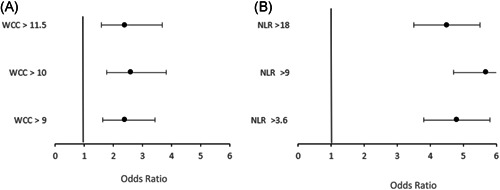
(A) Odds of admission to intensive care unit (ICU) compared to general ward according to total white cell count (WCC). (B) Odds of admission to general ward compared to intensive care unit (ICU) according to neutrophil‐to‐lymphocyte ratio (NLR). Both leucocytosis and raised NLR were associated with increased odds of ICU admission
Early lymphopenia and platelet count did not predict outcome although median lymphocyte count was low with a median of 1.03 × 109/L (IQR: 0.78–1.53) in those that died and those admitted to ICU with a median of 1.09 × 109/L (IQR: 0.54–1.52). Likelihood of death and ICU admission increased with NLR above 3.8 (OR: 2.5, CI: 1.36–3.25) and (OR: 4.8, CI: 2.43–9.54), neutrophil count (OR: 2.5, CI: 1.04–2.90) and (OR: 4.0, CI: 2.59–6.14), respectively. Raised MLR was associated with an increased likelihood of both death (OR: 2.3, CI: 1.06–1.99) and ICU admission (OR: 2.0, CI: 1.31–2.91), see Figure 4 for ROC curves.
Figure 4.
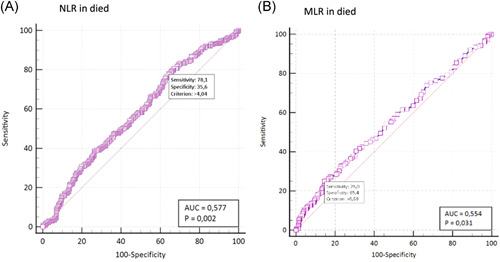
Receiver operating characteristic curves for neutrophil‐to‐lymphocyte ratio (NLR) and monocyte‐to‐lymphocyte ratio (MLR) and association with death. A raised NLR (optimal cut‐off of >4.04) showed a higher sensitivity for death while MLR (cut‐off > 0.68) showed higher specificity for death. AUC, area under the ROC curve
Platelet count, red cell indices, anemia, thrombocytopenia, or thrombocytosis, had no influence on outcome. Median d‐dimer levels (0.94 mg/L) were raised across all groups. In those that died, median d‐dimer levels were higher, 1.04 mg/L (IQR: 0.52–3.67) than those that recovered, median 0.79 mg/L (IQR: 0.4–2.1). However, the odds of raised d‐dimer and death were not statistically significant, (OR: 1.7, CI: 0.53–5.20). Those admitted, had a raised d‐dimer, general ward median of 0.66 mg/L, (IQR: 0.41–2.08) and ICU, median of 0.97 mg/L (IQR: 0.5–4.03) however the odds of admission to ICU with raised d‐dimer were not significant (OR: 1.0, CI: 0.38–2.41). Prolongation in the PT was not associated with increased odds of death or ICU admission. The prolonged PT was not corrected for use of anticoagulants (see Tables 1 and 2).
4. DISCUSSION
This study evaluated the available hematology laboratory results and demographic data in a tertiary public healthcare facility in South Africa and their relation to ICU admission, death, length of hospital and recovery in patients diagnosed with COVID‐19 over a 3‐month period.
Similar to previously published studies, advanced patient age at presentation was directly associated with worse outcomes in this cohort (see Figure 2). Adults older than 70 years represent only 13.6% of the Western Cape Province population. 22 , 23 Disproportionately, the highest death frequency was noted in patients above the age of 70 years (36.6%), which is similar to the findings of other studies during the first wave of the COVID‐19 pandemic. 1 , 24 The overall COVID‐19 mortality rate in South Africa in July 2020 was 1.7% which is considerably lower than the figure Italy reported during the first wave of the pandemic, 15.2%. 23 , 25 In comparison to Italian demographics, the South African population is younger which may be a significant contributory factor to the comparatively better South African outcomes despite the more advanced healthcare system in Italy. 23
The mortality was increased in the HIV‐positive group in our study; however, this was not statistically significant, probably due to the small number in this cohort. Viral suppression and anti‐retroviral (ARV) medication status were not analyzed, this, coupled with the large proportion of unknown viral status may be contributory. The impact of HIV infection on the severity of COVID‐19 is controversial. 29 , 30 Theoretically, patients living with HIV may have reduced risk of severe COVID‐19 due to dysfunction of cellular immunity with a lower risk of cytokine storm, therefore less COVID‐19 side effects. 26 However, HIV‐positive patients may have HIV‐related immunosuppression which may cause more severe illness. An increased risk of COVID‐19 associated death irrespective of viral suppression has been reported in South Africa. 20 These patients are at higher risk of concomitant TB and other comorbidities which might cause an over‐estimation of COVID‐19 death in HIV‐positive patients. 20
Hematological parameters that were associated with worse outcomes in our study included leucocytosis and neutrophilia in particular. Higher leukocyte counts have been associated with increased mortality in COVID‐19. 3 , 6 , 24 , 32 Neutrophils play a critical function in the clearance of pathogens and an important role in the inflammatory response in COVID‐19. 6 , 33 Recognition of the virus by the neutrophils occurs through pattern recognition receptors and Toll‐like receptors. Stimulation of these receptors activates downstream signaling cascades, causing the production of inflammatory cytokines. This inflammatory response may be severe enough to cause a cytokine storm which is one of the aetiologies of many COVID‐19 related complications. 12 , 33 Studies have demonstrated that higher values of proinflammatory markers are found in ICU admitted COVID‐19 patients. 29 Increased NLR has been associated with severe disease, enhancing the evidence of the increased inflammatory response in COVID‐19. 6 , 12 Raised NLR significantly increased likelihood of death or ICU admission. Sensitivity and NPV for ICU admission were good, however less so for death. This suggests that a raised NLR may be of use in risk stratification of patients with early SARS‐CoV‐2 infection.
Lymphopenia was not significantly associated with the outcome, however, median and IQRs were below normal reference ranges in all groups. Lymphopenia and dysregulation of lymphocyte subsets have been reported in COVID‐19. 35 , 36 , 37 This could be due to direct infection of lymphocytes, deregulation of cytokines or exudation of circulatory lymphocytes into inflamed lung tissues. 35 , 36 , 37 Although lower lymphocyte counts have been associated with severe disease, similar to previous studies, there was no significant difference in lymphocyte count between deceased and recovered patients. This could be attributed to the fact that early stages of infection were assessed. 27 , 33 Monocytes play an important role in the innate immune response to infection and elevated counts have been reported in COVID‐19. 3 , 33 An increased MLR was associated with both death and ICU admission.
Platelet production by megakaryocytes is regulated mainly through thrombopoietin, produced by the liver, and other inflammatory cytokines such as interleukin‐3 (IL‐3), IL‐6, and stem cell factor. 34 These cytokines can promote the production of platelets during inflammation, which may explain the reported elevation of platelet counts in the early phase of COVID‐19. 3 , 6 , 35 Changes in platelet count in COVID‐19 can be due to several reasons including the effect of cytokines, inhibition of platelet production by SARS‐CoV2 infection, platelet consumption in COVID‐19‐associated lung damage and associated‐thrombosis, and the effect of therapeutic agents. 7 Using platelet count as a predictor of COVID‐19 severity has shown contradicting findings. 24 , 32 In early SARS‐CoV2 infection, platelet counts have not correlated with the outcome which probably explains the normal platelet count in this cohort. 5 , 9 , 36
Anemia could develop during the course of COVID‐19 either due to direct SARS‐CoV‐2 infection, iatrogenic blood loss during admission, or dysregulation of iron metabolism. 5 , 7 Unlike other studies, Hb level, RDW and MCHC were not associated with worse outcome in our cohort. 36 , 37
COVID‐19 is associated with clinically significant coagulopathy termed CAC. CAC usually presents with thrombotic complications and is of multifactorial etiology. 1 , 2 It has been associated with ICU admission, poorer prognosis and mortality. 1 , 3 Although d‐dimer levels were raised in both the ICU admission and the death groups, there was no significant association with outcome.
Leucocytosis, neutrophilia, and raised NLR could be used as early markers to predict a worse outcome in SARS‐CoV‐2 infection. The availability and affordability of the FBC and differential WCC at public healthcare facilities provide an attractive modality of predicting disease outcome in the resources constrained setting such as South Africa.
This study provides local data in a resource‐constrained setting and reviews the hematological changes associated with early SARS‐CoV‐2 infection and their association with outcome. The cohort included a heterogeneous group of patients ranging from outpatients to critically ill ICU patients. The hematological parameters were assessed at an early stage of disease and dynamic changes throughout the course of the disease were beyond the scope of this study. Therefore, this data analysis could facilitate in predicting patients with increased odds of developing severe disease at time of presentation.
The limitations of this study include its retrospective nature and contributes to an uncontrolled risk of selection bias as some patients may have presented for the first time at a late stage of the disease. Only data from the NHLS LIS was used, with limited access to clinical data. The observed association between laboratory parameters and outcome may be confounded by clinical factors including various therapeutic modalities, such as lymphopenia due to the use of corticosteroid or the effect of ARV management on the degree of immune suppression or CD4 count in HIV‐positive group.
5. CONCLUSION
This study highlights the use of affordable and widely available laboratory tests in assessment of COVID‐19 in the South African setting. It was found that advanced patient age, leucocytosis, neutrophilia, raised NLR, and MLR are significantly associated with worse COVID‐19 outcome. These parameters form part of routine testing in public healthcare facilities in South Africa and other resource‐constrained settings and therefore could be used as early indicators of disease severity.
It is recommended that the FBC and differential count, NLR and MLR should form part of early clinical assessment in COVID‐19 infection.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Zivanai C. Chapanduka, Ibtisam Abdullah, and Helena M. Cornelissen conceptualized the study, wrote the study protocol, and drafted the manuscript. Ibtisam Abdullah and Helena M. Cornelissen coordinated all practical aspects of the conduct of the study. All the authors participated in the conduct of the study, data collection, and editing of the manuscript.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors would like to acknowledge the Tygerberg Hospital NHLS Hematology medical laboratory technologists particularly; Ms Fazlin Kolia, Mr. Wessel Kleinhans, Mrs. Lynda Killian, and Mrs. Amanda Truter as well as Mrs. Zaakiyyah Ryan and Ina Du Plessis. This study was self‐funded.
Abdullah I, Cornelissen HM, Musekwa E, et al. Hematological findings in adult patients with SARS CoV‐2 infection at Tygerberg Hospital Cape Town South Africa. Health Sci Rep. 2022;5:e550. 10.1002/hsr2.550
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1‐13. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fan BE, Chong VCL, Chan SSW, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95:131. 10.1002/ajh.25774 [DOI] [PubMed] [Google Scholar]
- 3. Qian GQ, Yang NB, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID‐19 in Zhejiang, China: a retrospective, multi‐centre case series. QJM. 2020;113:474‐481. 10.1093/qjmed/hcaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agbuduwe C, Basu S. Haematological manifestations of COVID‐19: from cytopenia to coagulopathy. Eur J Haematol. 2020;105(5):540‐546. 10.1111/ejh.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wiggill TM, Mayne ES, Vaughan JL, Louw S. Overview of the haematological effects of COVID‐19 infection. Adv Exp Med Biol. 2021;1321:163‐172. 10.1007/978-3-030-59261-5_14 [DOI] [PubMed] [Google Scholar]
- 6. Bai B, Xu Z, Hu Y, et al. Patient hematology during hospitalization for viral pneumonia caused by SARS‐CoV‐2 and non‐SARS‐CoV‐2 agents: a retrospective study. Eur J Med Res. 2021;26(1):45. 10.1186/s40001-021-00515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdullah I, Chapanduka Z. The pathophysiology of the haematological manifestations of COVID‐19: a review. J Med Lab Sci Technol South Africa. 2020;2(2):1‐6. 10.36303/JMLSTSA.2020.2.2.48 [DOI] [Google Scholar]
- 8. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frater JL, Zini G, d'Onofrio G, Rogers HJ. COVID‐19 and the clinical hematology laboratory. Int J Lab Hematol. 2020;42(S1):11‐18. 10.1111/ijlh.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID‐19. J Thromb Haemost. 2020;18:0‐1. 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qu R, Ling Y, Zhang YH, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020;92:0‐3. 10.1002/jmv.25767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng ZY, Feng SD, Chen GP, Wu JN. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID‐19: a prospective cohort study. BMC Infect Dis. 2021;21(1):1‐6. 10.1186/s12879-021-05796-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taj S, Kashif A, Arzinda Fatima S, Imran S, Lone A, Ahmed Q. Role of hematological parameters in the stratification of COVID‐19 disease severity. Ann Med Surg. 2021;62:68‐72. 10.1016/j.amsu.2020.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiong M, Liang X, Wei Y. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. Br J Haematol. 2020;189:0‐2. 10.1111/bjh.16725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95:834‐847. 10.1002/ajh.25829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connors J, States U, Levy J. COVID‐19 and its implications for thrombosis and anticoagulation COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrari D, Seveso A, Sabetta E, et al. Role of time‐normalized laboratory findings in predicting COVID‐19 outcome. Diagnosis. 2020;7(4):387‐394. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boulle AA, Davies M, Hussey H, et al. Risk factors for COVID‐19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020;73(7):1‐31. 10.1093/cid/ciaa1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. COVID‐19 Response . Western Cape Government COVID‐19 statistics. 2021. Accessed June 4, 2021. https://coronavirus.westerncape.gov.za/covid-19-dashboard
- 22. Zhang J‐J, Dong X, Cao Y‐Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 23. Statistics South Africa . Midyear Population Estimate. July 2019. p. 24.
- 24. Vizcarra P, Pérez‐Elías MJ, Quereda C, et al. Description of COVID‐19 in HIV‐infected individuals: a single‐centre, prospective cohort. Lancet HIV. 2020;7(8):e554‐e564. 10.1016/S2352-3018(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laurence J. Why aren't people living with HIV at higher risk for developing severe coronavirus disease 2019 (COVID‐19)? AIDS Patient Care STDS. 2020;34(6):247‐248. 10.1001/jama.2020.6548.8 [DOI] [PubMed] [Google Scholar]
- 27. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73(11):e4208‐e4213. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borges L, Pithon‐Curi TC, Curi R, Hatanaka E. COVID‐19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediators Inflamm. 2020;2020:8829674. 10.1155/2020/8829674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Wo J, Shao J, et al. SARS‐coronavirus replicates in mononuclear cells of peripheral blood (PBMCs) from SARS patients. J Clin Virol. 2003;28(3):239‐244. 10.1016/S1386-6532(03)00195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan PKS, Chen GG. Mechanisms of lymphocyte loss in SARS coronavirus infection. Hong Kong Med J. 2008;14:21‐26. [PubMed] [Google Scholar]
- 33. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;1:1‐8. 10.1093/infdis/jiaa150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hesse R, van der Westhuizen D, George J. COVID‐19 related laboratory analyte changes and the relationship between SARS‐CoV‐2 and HIV, TB and HbA1c in South Africa. Adv Exp Med Biol. 2021; 1321:183‐197. 10.13140/RG.2.2.20854.01600/2 [DOI] [PubMed] [Google Scholar]
- 36. Wang C, Zhang H, Cao X, et al. Red cell distribution width (RDW): a prognostic indicator of severe COVID‐19. Ann Transl Med. 2020;8(19):1230. 10.21037/atm-20-6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tao Z, Xu J, Chen W, et al. Anemia is associated with severe illness in COVID‐19: a retrospective cohort study. J Med Virol. 2021;93(3):1478‐1488. 10.1002/jmv.26444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


