Abstract
Antifungal susceptibilities (NCCLS, approved standard M27-A, 1997) were determined for the reference strain ATCC 90028 and 21 clinical isolates of Candida albicans with varying levels of fluconazole susceptibility using RPMI 1640 (RPMI) and 80% fresh human serum–20% RPMI (serum). Sixty-four percent (14 of 22) of the isolates tested demonstrated significant decreases (≥4-fold) in fluconazole MICs in the presence of serum, and the remaining eight isolates exhibited no change. Itraconazole and ketoconazole, two highly protein-bound antifungal agents, had MICs in serum that were increased or unchanged for 46% (10 of 22) and 41% (9 of 22) of the isolates, respectively. All 10 isolates tested against an investigational antifungal agent, LY303366, demonstrated significant increases in the MIC required in serum, while differences in amphotericin B MICs in the two media were not observed. Four of 10 isolates tested demonstrated fourfold higher flucytosine MICs in serum than in RPMI. Postantifungal effects (PAFEs) and 24-h kill curves were determined by standard methods for selected isolates. At the MIC, fluconazole, itraconazole, ketoconazole, flucytosine, and LY303366 kill curves and PAFEs in RPMI were similar to those in serum. Isolates of fluconazole-resistant C. albicans required lower MICs in serum than in RPMI, without relative increases in fungal killing or PAFEs. Isolates tested against amphotericin B demonstrated significantly reduced killing and shorter PAFEs in serum than in RPMI without observable changes in MIC. In conclusion, antifungal pharmacodynamics in RPMI did not consistently predict antifungal activity in serum for azoles and amphotericin B. Generally speaking, antifungal agents with high protein binding exhibited some form of reduced activity (MIC, killing, or PAFE) in the presence of serum compared to those with low protein binding.
Rates of fungal infection are rising worldwide and may be attributable to increasing numbers of immunosuppressed patients, such as those who have AIDS, are receiving cancer chemotherapy, or are undergoing organ transplantation (6). Standardized antifungal susceptibility testing is now available (10), and evidence has been published correlating the clinical outcome for patients suffering from oropharyngeal candidiasis with azole susceptibilities of infecting isolates (12). However, the information gleaned from in vitro susceptibility testing has limitations, as the MIC provides only a point-in-time, static measurement of antimicrobial effect in a defined medium (1). Other pharmacodynamic parameters such as time-kill curve and postantifungal effect (PAFE) determinations may also be useful to clinicians as they assist in defining in greater detail the relationship between drug concentrations, their fluctuation with time, and resultant pharmacologic effects (1). Previous work has demonstrated that the application of antibacterial pharmacodynamic parameters to dosing regimens improves clinical outcome and minimizes the development of drug resistance (4, 15) and that biological fluids such as human serum and urine can have profound effects on antibacterial pharmacodynamics (2, 19). Pharmacodynamic parameters of antifungal agents, however, remain poorly described, particularly concerning the influence of biological fluids (9, 13, 14). The goal of this study was to characterize pharmacodynamic parameters of commonly prescribed systemic antifungal agents in the defined medium RPMI 1640 (RPMI) (10) and in 80% human serum–20% RPMI (serum) against Candida albicans, the most commonly isolated human fungal pathogen (6).
MATERIALS AND METHODS
Isolates of C. albicans.
One reference strain, ATCC 90028, and 21 clinical isolates of C. albicans were tested. Eleven isolates (CA12, CA38, JK1, JK9, JK13, JK23, JK27, JK28, JK31, JK32, and JK37) were from the Department of Clinical Microbiology, Health Sciences Centre, Winnipeg, Canada, and eight isolates (TO1, TO3, TO5, TO6, TO8, TO10, TO16, and TO17) were from the Hospital for Sick Children, Toronto, Ontario, Canada. Two previously described isolates of C. albicans, 2-76 and 12-99, were also included (17). All isolates were stocked in skim milk at −80°C and subcultured twice on Sabouraud dextrose agar plates before use.
Media.
All experiments were performed in RPMI (10) as a control. Test media also included RPMI supplemented with bovine serum albumin (BSA) (Sigma, St. Louis, Mo.) at serum-equivalent concentrations (50 mg/ml), fresh serum (80% human serum–20% RPMI), heat-inactivated serum, and proteinase K (Sigma)-treated serum. Fresh serum was prepared daily from plasma (Canadian Blood Services, Winnipeg, Canada) by recalcification with 2 ml of 1 M CaCl2 per 100 ml of plasma, pH adjusted to 7.4, and filter sterilized (19). Biochemical analysis revealed similar osmolalities for all media. Heat-inactivated serum was prepared by heating at 56°C for 30 min and proteinase K was utilized to degrade serum protein according to the manufacturer's instructions. Sabouraud dextrose agar plates were used for isolate subculture and colony counts.
Antifungal agents and susceptibility testing.
Antifungal agents were selected on the basis of clinical relevance and extent of protein binding, including fluconazole (protein binding, 11%), itraconazole (99.8%), ketoconazole (99%), amphotericin B (91 to 95%), flucytosine (<10%), and LY303366, an investigational antifungal agent (84%) (8, 14, 18). Antifungal stock solutions were prepared from standard powders according to NCCLS M27-A guidelines (10) and as previously described for LY303366 (18). MICs were determined at least in duplicate and interpreted according to NCCLS guidelines (10). A fourfold (two-doubling dilution) increase or decrease in the MIC in serum relative to in RPMI alone was deemed a significant difference.
Time-kill curve determinations.
Three fluconazole-resistant (MIC, ≥64 μg/ml) clinical isolates (TO3, TO17, and CA38) were selected from the isolate collection, and 24-h kill curves were performed for all six antifungal agents at concentrations equal to the MICs in RPMI and in serum. Experiments were performed at least in duplicate for each isolate with each antifungal agent on different days. Additional 24-h kill curves were performed with amphotericin B at concentrations equal to the MIC in RPMI, RPMI supplemented with BSA, serum, heat-inactivated serum, and proteinase K-treated serum. Each culture was incubated at 37°C in a shaking water bath and sampled at 0, 1, 2, 3, 4, 6, 12, and 24 h by plating on Sabouraud dextrose agar. CFU were counted after 18 h of incubation at 35°C (5).
PAFE determinations.
One fluconazole-resistant isolate, TO3, was selected for PAFE study in RPMI and in serum with six antifungal agents, each at its MIC (see Table 3). PAFEs were determined by a standard method and repeated in triplicate for each antifungal agent (2, 19). The PAFE was calculated as the time difference required for a culture to grow 1 log10 following exposure to and removal of an antifungal agent at its MIC compared to the time required for 1 log10 of growth in an unexposed control culture. Cultures were exposed to the antifungal agent for 1 h and then diluted 1:100 to remove the drug. Residual antifungal concentration controls were performed simultaneously to ensure that diluted antifungal concentrations were insignificant. Cultures were incubated at 37°C in a shaking water bath and sampled immediately before the addition of the antifungal agent, 1 h after the addition of the antifungal agent, immediately following dilution, 2 h following dilution, and then hourly thereafter until cultures were ascertained to have increased by at least 1 log10 (2, 19).
TABLE 3.
PAFE determinations for fluconazole, itraconazole, ketoconazole, amphotericin B, flucytosine, and LY303366 with C. albicans TO3 in RPMI and in human serum
| Antifungal agent | PAFE (h) (mean ± SE)
|
|
|---|---|---|
| RPMI | Human serum | |
| Fluconazole | 0 | 0 |
| Itraconazole | 0 | 0 |
| Ketoconazole | 0 | 0 |
| Amphotericin B | 8.6 ± 0.3 | 3.0 ± 0.3a |
| Flucytosine | 0.7 ± 0.2 | 1.2 ± 0.6 |
| LY303366 | 0.4 ± 0.1 | 0.9 ± 0.3 |
PAFE in human serum was significantly shorter than in RPMI alone (P < 0.05).
Statistical analysis.
Time-kill curves were analyzed at 0, 6 and 24 h by analysis of variance and PAFEs were analyzed by the two-sample t test.
RESULTS
Of the 22 isolates, 64% (14 of 22) demonstrated significant (≥4-fold) decreases in fluconazole MICs in the presence of human serum, and the remaining 36% (8 of 22) showed no significant change (Table 1). Itraconazole and ketoconazole, two highly protein-bound antifungal agents, had MICs in serum that were increased or unchanged for 46% (10 of 22) and 41% (9 of 22) of the isolates, respectively. The other three isolates (JK28, TO1, and TO10), all fluconazole resistant, demonstrated decreases in all three azole MICs. Isolates JK28, TO1, and TO10 showed 64- to 1,024-fold reductions in fluconazole MICs, 16- to 32-fold reductions in itraconazole MICs, and 16- to 64-fold reductions in ketoconazole MICs in serum relative to in RPMI (Table 1). Amphotericin B MICs were not significantly altered by the presence of serum for any of the 10 isolates tested (Table 2). Four of the 10 isolates demonstrated fourfold higher flucytosine MICs in serum than in RPMI. LY303366 MICs for all 10 isolates tested were 8- to 32-fold higher in serum than in RPMI.
TABLE 1.
Azole susceptibilities of ATCC 90028 and 21 clinical isolates of C. albicans in RPMI and in serum
| Isolate | MIC (μg/ml)
|
||
|---|---|---|---|
| Fluconazole | Itraconazole | Ketoconazole | |
| Fluconazole susceptible | |||
| ATCC 90028 | |||
| RPMI | 0.25 | 0.03 | 0.125 |
| Serum | 0.25 | 0.25 | 0.25 |
| JK23 | |||
| RPMI | 8 | 0.25 | 0.5 |
| Serum | 2 | 2 | 2 |
| JK27 | |||
| RPMI | 4 | 0.125 | 0.25 |
| Serum | 2 | 2 | 1 |
| 2-76 | |||
| RPMI | 0.25 | 0.03 | 0.125 |
| Serum | 0.25 | 1 | 0.5 |
| Fluconazole susceptible, dose dependent | |||
| CA12 | |||
| RPMI | 32 | 1 | 1 |
| Serum | 2 | 1 | 1 |
| JK1 | |||
| RPMI | 32 | 2 | 1 |
| Serum | 8 | 4 | 2 |
| JK9 | |||
| RPMI | 32 | 1 | 1 |
| Serum | 8 | 2 | 2 |
| TO16 | |||
| RPMI | 32 | 2 | 1 |
| Serum | 16 | 4 | 4 |
| Fluconazole resistant | |||
| CA38 | |||
| RPMI | 128 | 1 | 2 |
| Serum | 32 | 4 | 4 |
| JK13 | |||
| RPMI | 128 | 2 | 0.5 |
| Serum | 64 | 2 | 4 |
| JK28 | |||
| RPMI | >128 | 16 | >16 |
| Serum | 4 | 2 | 1 |
| JK31 | |||
| RPMI | 128 | 0.5 | 0.5 |
| Serum | 0.25 | 0.5 | 0.5 |
| JK32 | |||
| RPMI | 64 | 0.5 | 0.5 |
| Serum | 16 | 1 | 2 |
| JK37 | |||
| RPMI | 128 | 0.5 | 0.5 |
| Serum | 0.25 | 0.5 | 0.5 |
| TO1 | |||
| RPMI | 128 | 4 | 4 |
| Serum | 0.25 | 0.5 | 0.5 |
| TO3 | |||
| RPMI | 128 | 4 | 8 |
| Serum | 32 | 16 | 16 |
| TO5 | |||
| RPMI | 64 | 1 | 1 |
| Serum | 64 | 8 | 16 |
| TO6 | |||
| RPMI | 64 | 1 | 1 |
| Serum | 32 | 8 | 8 |
| TO8 | |||
| RPMI | >128 | 2 | 8 |
| Serum | 64 | 2 | 8 |
| TO10 | |||
| RPMI | >128 | >16 | 16 |
| Serum | 1 | 1 | 0.5 |
| TO17 | |||
| RPMI | 64 | 1 | 4 |
| Serum | 16 | 4 | 8 |
| 12-99 | |||
| RPMI | 64 | 0.25 | 1 |
| Serum | 32 | 8 | 4 |
TABLE 2.
Amphotericin B, flucytosine, and LY303366 susceptibilities of ATCC 90028 and 21 clinical isolates of C. albicans in RPMI and in human serum
| Isolate | MIC (μg/ml)
|
||
|---|---|---|---|
| Amphotericin B | Flucytosine | LY303366 | |
| ATCC 90028 | |||
| RPMI | 1 | 0.5 | 0.125 |
| Serum | 1 | 2 | 1 |
| CA38 | |||
| RPMI | 2 | 0.125 | 0.25 |
| Serum | 2 | 0.25 | 2 |
| JK23 | |||
| RPMI | 1 | 0.25 | 0.25 |
| Serum | 1 | 0.5 | 2 |
| JK27 | |||
| RPMI | 1 | 0.25 | 0.25 |
| Serum | 1 | 0.5 | 2 |
| JK28 | |||
| RPMI | 1 | 0.5 | 0.125 |
| Serum | 1 | 1 | 1 |
| JK32 | |||
| RPMI | 1 | 1 | 0.125 |
| Serum | 1 | 1 | 1 |
| TO3 | |||
| RPMI | 1 | 0.125 | 0.125 |
| Serum | 1 | 0.5 | 1 |
| TO17 | |||
| RPMI | 1 | 0.125 | 0.125 |
| Serum | 1 | 0.5 | 1 |
| 2-76 | |||
| RPMI | 1 | 0.25 | 0.125 |
| Serum | 1 | 1 | 1 |
| 12-99 | |||
| RPMI | 1 | 1 | 0.125 |
| Serum | 1 | 2 | 4 |
The influence of RPMI supplemented with BSA (50 mg/ml) on MICs was tested for 10 isolates (ATCC 90028, CA38, JK23, JK27, JK28, JK32, TO3, TO17, 2-76, and 12-99). BSA supplementation did not significantly alter the MICs for fluconazole, itraconazole, ketoconazole, amphotericin B, or flucytosine from those determined in RPMI alone (data not shown). Consistent four- to eightfold increases in MICs similar to those present with serum were observed for all 10 isolates tested with LY303366.
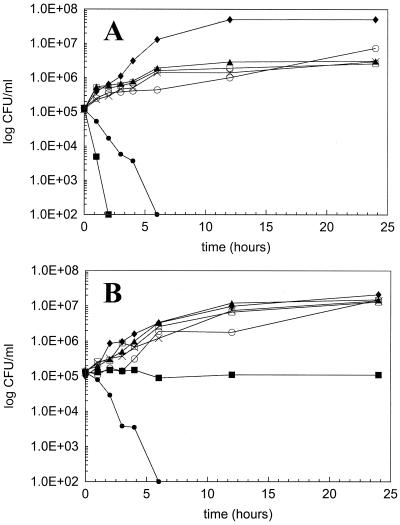
Similar growth control curves for CA38, TO3, and TO17 were observed in RPMI and in serum. Fluconazole, itraconazole, ketoconazole, and flucytosine kill curves for all three isolates demonstrated stasis, with growth mirroring the growth control over the 24-h duration. Serum tended to reduce the killing activity of LY303366, but this did not reach statistical significance. Amphotericin B killing was observed to be fungicidal (>3 log10 killed) against all three isolates in RPMI but demonstrated significantly reduced killing activity in serum against isolates TO3 and TO17. Representative kill curves for isolate TO3 are depicted in Fig. 1.
FIG. 1.
Twenty-four-hour kill curves for fluconazole, itraconazole, ketoconazole, amphotericin B, flucytosine, and LY303366 at concentrations equivalent to their MICs against C. albicans TO3 in RPMI (A) and serum (B). Symbols: ⧫, growth control; ▴, fluconazole; □, itraconazole; ×, ketoconazole; ■, amphotericin B; ○, flucytosine; ●, LY303366.
One fluconazole-resistant isolate, TO3, was selected for PAFE study in RPMI and in serum, with experiments repeated in triplicate for each of the six antifungal agents (Table 3). A PAFE was not observed with any of the azoles tested in either medium. Significant differences in PAFE were also not observed with flucytosine or LY303366. However, amphotericin B demonstrated significantly (P < 0.05) reduced PAFEs in the presence of serum.
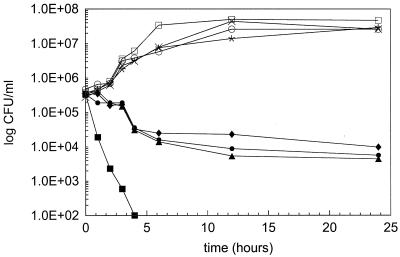
To investigate the reductions in amphotericin B killing further, kill curve experiments with amphotericin B against isolate TO3 in RPMI, serum, RPMI supplemented with BSA, heat-inactivated serum, and proteinase K-treated serum were performed (Fig. 2). Each medium tested revealed significantly reduced killing relative to RPMI. MIC determinations were also performed with amphotericin B and isolates TO3 and TO17 in RPMI, serum, heat-inactivated serum, and proteinase K-treated serum. Amphotericin B MICs remained unchanged from those in RPMI in all other media tested (data not shown).
FIG. 2.
Twenty-four-hour kill curves for amphotericin B at a concentration equivalent to its MIC against C. albicans TO3 in various media. Symbols for amphotericin B: ■, in RPMI; ▴, in serum; ●, in heat-inactivated serum; ⧫, in proteinase K-treated serum. Symbols for growth control: □, in RPMI; ×, in serum; ○, in heat-inactivated serum; ✕⃒, in proteinase K-treated serum.
DISCUSSION
In assessing the effect of human serum on azole MICs, it was noted that there was considerable variability between isolates; that is, effects were not universally observed. For example, three of the four fluconazole-susceptible isolates had similar fluconazole MICs in RPMI and in serum, while isolate JK23 demonstrated a reproducible fourfold reduction in the fluconazole MIC in the presence of serum. Of the four fluconazole-intermediate isolates, only three demonstrated a fourfold or greater reduction in the fluconazole MIC in the presence of serum. Ten (71.4%) of the 14 fluconazole-resistant isolates tested exhibited a fourfold or greater reduction in the fluconazole MIC in the presence of serum. These 10 isolates could be further divided into two distinct groups, one with a fourfold reduction (n = 5) and one with a ≥32-fold reduction (n = 5) in the fluconazole MIC. Variations and anomalies are also present among the itraconazole and ketoconazole MICs (Table 1). However, it can be concluded that the presence of serum exhibited reduced activity (MIC) with highly protein-bound antifungal agents relative to those with low protein binding. Albumin binding did not influence fluconazole, ketoconazole, or itraconazole MICs as has been previously reported (14). It is unclear why the MIC was not increased in the presence of albumin with highly protein-bound antifungal agents like itraconazole and ketoconazole. This is clearly not consistent with the “free drug hypothesis” (14). It should be mentioned that albumin is a nonspecific binding sink regardless of whether it is human or bovine; however, human albumin alone was not tested. Perhaps the binding of azoles to albumin is influenced by the presence of other serum proteins such as α-globulins which are absent in purified albumin (14). Thus, it is clear that for highly protein-bound antifungal agents such as itraconazole and ketoconazole, being protein-bound does not mean that they are inactivated.
The significant decreases in fluconazole MICs in the presence of serum did not result in changes in fungal killing or PAFE for three fluconazole-resistant isolates, TO3, TO17, and CA38. Fungal killing activity analyzed by analysis of variance at 0, 6, and 24 h (Fig. 1) did not have significant differences between RPMI and serum, but there did appear to be a trend toward less azole inhibition in the presence of human serum (Fig. 1). PAFEs were absent for all azoles tested in both RPMI and serum, which reflects the static, inhibitory effect of azole antifungal agents (5). The fungal killing and PAFE data collected in the present study are consistent with previous reports in the literature and imply that the ratio of the area under the concentration-time curve to the MIC and the time above the MIC are important pharmacodynamic parameters for azoles (3, 5, 7, 13, 14). The reductions in MIC without increased fungal killing may suggest improved uptake of azoles into cells and/or a more rapid inhibition of cellular replication in the presence of serum.
LY303366 MICs for all 10 isolates tested were 8- to 32-fold higher in serum than in RPMI. Consistent four- to eightfold increases in LY303366 MICs were also observed with all 10 isolates tested in RPMI supplemented with BSA (50 mg/ml), suggesting that albumin may bind LY303366 in human serum. Serum tended to reduce the killing activity of LY303366, but this did not reach statistical significance. In addition, significant differences in PAFE were not observed with LY303366 in the presence of serum.
Amphotericin B, a highly protein-bound antifungal agent, demonstrated unchanged MICs in the presence of human serum relative to in RPMI yet exhibited significantly reduced killing activity and shorter PAFE in serum. Results described in previous studies contrast with those presented here in that the antifungal activity of amphotericin B was reported to be reduced 1 order of magnitude in serum (11, 16). Data from the present study suggest that in vitro pharmacodynamic parameters studied for amphotericin B in RPMI may not be predictive of in vivo activity. Further investigations with isolate TO3 which attempted to discern the cause of reduced amphotericin B activity in the presence of human serum determined that albumin, the most abundant protein in serum, or other specific, intact, functional serum proteins were not associated with the observed decreases in killing activity and PAFE. Biochemical analysis and pH testing revealed that RPMI and serum were equivalent. The possibility of a nonprotein serum factor interacting with amphotericin B and resulting in decreased activity against C. albicans needs to be studied further.
In conclusion, antifungal pharmacodynamic parameters in RPMI may not always predict antifungal activity in serum for azoles and amphotericin B. The differences that arise may depend upon both the isolate and the antifungal agent tested. However, in general antifungal agents with high protein binding exhibited some form of reduced activity (MIC, killing, or PAFE) in the presence of serum compared to those with low protein binding.
Identifying the mechanisms responsible for isolate differences in antifungal susceptibilities in the presence and absence of serum and assessing their importance in vivo will enhance our understanding of the pharmacodynamics of systemic antifungal agents.
ACKNOWLEDGMENTS
We thank the MRC-Burroughs Wellcome Fund for the financial support of this project and the Manitoba Medical Services Foundation for the Dr. Jack Wilt Memorial Award. G.G.Z. is supported by a Merck Frosst Chair in Pharmaceutical Microbiology.
We thank Lorne Sargeant of Clinical Chemistry, Health Sciences Centre, Winnipeg, Canada, for his biochemical expertise, and the Canadian Blood Services for supplying the plasma.
REFERENCES
- 1.Andes D, Craig W A. Pharmacokinetics and pharmacodynamics of outpatient intravenous antimicrobial therapy. Infect Dis Clin N Am. 1998;12:849–860. doi: 10.1016/s0891-5520(05)70024-6. [DOI] [PubMed] [Google Scholar]
- 2.Drobot G R, Karlowsky J A, Hoban D J, Zhanel G G. Antibiotic activity in microbiological media versus that in human urine: comparison of ampicillin, ciprofloxacin, and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 1996;40:237–240. doi: 10.1128/aac.40.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst E J, Klepser M E, Pfaller M A. Postantifungal effects of echinocandin, azole, and polyene antifungal agents against Candida albicans and Cryptococcus neoformans. Antimicrob Agents Chemother. 2000;44:1108–1111. doi: 10.1128/aac.44.4.1108-1111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlowsky J A, Harding G A J, Zelenitsky S A, Hoban D J, Kabani A, Balko T V, Turik M, Zhanel G G. In vitro kill curves of a new semisynthetic echinocandin, LY-303366, against fluconazole-sensitive and -resistant Candida species. Antimicrob Agents Chemother. 1997;41:2576–2578. doi: 10.1128/aac.41.11.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlowsky J A, Zhanel G G, Klym K A, Hoban D J, Kabani A M. Candidemia in a Canadian tertiary care hospital from 1976 to 1996. Diagn Microbiol Infect Dis. 1997;28:5–9. doi: 10.1016/s0732-8893(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 7.Louie A, Drusano G L, Banerjee P, Liu O-F, Liu W, Kaw P, Shayegani M, Taber H, Miller M H. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother. 1998;42:1105–1109. doi: 10.1128/aac.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyman C A, Walsh T J. Systematically administered antifungal agents: a review of their clinical pharmacology and therapeutic application. Drugs. 1992;44:9–35. doi: 10.2165/00003495-199244010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Minguez F, Lima J E, Garcia M T, Prieto J. Influence of human serum on the postantifungal effect of four antifungal agents on Candida albicans. Chemotherapy. 1996;42:273–279. doi: 10.1159/000239455. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Nugent K M, Couchot K R. Effects of sublethal concentrations of amphotericin B on Candida albicans. J Infect Dis. 1986;154:665–669. doi: 10.1093/infdis/154.4.665. [DOI] [PubMed] [Google Scholar]
- 12.Pfaller M A, Rex J H, Rinaldi M G. Antifungal susceptibility testing: technical advances and potential clinical applications. Clin Infect Dis. 1997;24:776–784. doi: 10.1093/clinids/24.5.776. [DOI] [PubMed] [Google Scholar]
- 13.Schafer-Korting M, Korting H C, Amman F, Peuser R, Lukacs A. Influence of albumin on itraconazole and ketoconazole antifungal activity: results of a dynamic in vitro study. Antimicrob Agents Chemother. 1991;35:2053–2056. doi: 10.1128/aac.35.10.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer-Korting M, Korting H C, Rittler W, Obermuller W. Influence of serum protein binding on the in vitro activity of antifungal agents. Infection. 1995;23:292–297. doi: 10.1007/BF01716289. [DOI] [PubMed] [Google Scholar]
- 15.Thomas J K, Forrest A, Bhavnani S M, Hyatt J M, Cheng A, Ballow C H, Schentag J J. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients' drug therapy. Antimicrob Agents Chemother. 1998;42:521–527. doi: 10.1128/aac.42.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Etten E W M, van de Rhee N E, van Kampen K M, Bakker-Woudenberg I A J M. Effects of amphotericin B and fluconazole on the extracellular and intracellular growth of Candida albicans. Antimicrob Agents Chemother. 1991;35:2275–2281. doi: 10.1128/aac.35.11.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhanel G G, Karlowsky J A, Harding G A J, Balko T V, Zelenitsky S A, Friesen M, Kabani A, Turik M, Hoban D J. In vitro activity of a new semisynthetic echinocandin, LY-303366, against systemic isolates of Candida species, Cryptococcus neoformans, Blastomyces dermatitidis, and Aspergillus species. Antimicrob Agents Chemother. 1997;41:863–865. doi: 10.1128/aac.41.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhanel G G, Kirkpatrick I D C, Hoban D J, Kabani A M, Karlowsky J A. Influence of human serum on pharmacodynamic properties of an investigational glycopeptide, LY333328, and comparator agents against Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:2427–2430. doi: 10.1128/aac.42.9.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]