Abstract
Background
Artificial intelligence (AI) is defined as the study of algorithms that allow machines to reason and perform cognitive functions such as problem-solving, objects, images, word recognition, and decision-making. This study aimed to review the published articles and the comprehensive clinical relevance of AI-based tools used before, during, and after knee arthroplasty.
Methods
The search was conducted through PubMed, EMBASE, and MEDLINE databases from 2000 to 2021 using the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol (PRISMA).
Results
A total of 731 potential articles were reviewed, and 132 were included based on the inclusion criteria and exclusion criteria. Some steps of the knee arthroplasty procedure were assisted and improved by using AI-based tools. Before surgery, machine learning was used to aid surgeons in optimizing decision-making. During surgery, the robotic-assisted systems improved the accuracy of knee alignment, implant positioning, and ligamentous balance. After surgery, remote patient monitoring platforms helped to capture patients’ functional data.
Conclusion
In knee arthroplasty, the AI-based tools improve the decision-making process, surgical planning, accuracy, and repeatability of surgical procedures.
Keywords: Knee arthroplasty, Artificial intelligence, Machine learning, Predictive models, Augmented reality, Robotic surgery
Introduction
Artificial intelligence (AI) refers to machine algorithms giving the ability to reason and perform cognitive functions [1]. Over the past 70 years, AI has evolved rapidly, with computer models and algorithms designed to replicate human intelligence and performs specific tasks within various industries [2, 3]. Surgeons are key stakeholders in adopting AI-based technologies for medical care. Health-care professionals can help data scientists and engineers develop clinically relevant software.
In orthopedic surgery, the AI technology enables surgeons to provide patient-specific knee arthroplasty in clinical decision making, preoperative health optimization, resource allocation, decision support, and early intervention. However, the safety and effectiveness of AI-based knee arthroplasty are still challenging. A rigorous validation process and a clinical relevance analysis are required with new technologies. This process aims to distinguish which AI-based tool is clinically relevant and which is just hype. Many studies reporting the interest of AI-based tools in the orthopedic field have been published during the past years with the growing interest in AI-based tools in knee arthroplasty. Nevertheless, the interest and the understanding for AI in knee arthroplasty remain little-known and underused.
This study aimed to review the published articles on the comprehensive clinical relevance of AI-based tools used before, during, and after knee arthroplasty.
Material and methods
Article identification and selection process
In May 2021, we performed a query to identify available articles describing AI tools for knee arthroplasty pre-, intra-, and postoperatively. We searched PubMed, EMBASE, and MEDLINE databases from 2000 to 2021 using the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol (PRISMA). We used the following terms: “knee arthroplasty” or “knee replacement”; “artificial intelligence” or “predictive model” or “predictive modeling” or “analytic model” or “machine learning” or “remote patient monitoring” or “augmented reality” or “mixed reality” or “virtual reality” or “robotic” or “robotically-assisted”.
The inclusion criteria were English language studies reporting on AI tools in knee arthroplasty. The exclusion criteria were: (1) editorial articles; (2) systematic reviews or meta-analyses; and (3) studies evaluating joints other than the knee. Two investigators independently reviewed the abstracts of the identified articles. Discrepancies were settled by discussion between the reviewers or consultation with a third reviewer. Articles were excluded if the title and abstract did not include AI tools used in knee arthroplasty. Full-text articles were assessed if necessary.
Definition and description of AI tools
The tools evaluated in this analysis include different sub-groups of AI tools defined and described below: Predictive modeling is a discipline of AI where algorithms generate estimates for a defined target output. Predictive models are “trained” to identify relationships between a set of features (e.g., age, body mass index (BMI), sex) and the target (e.g., the occurrence of myocardial infarction) [4]. Statistical models (e.g., regression models) and machine learning techniques (e.g., random forest models or neural networks) are used to learn the target-predictors relationship among the data [5]. In its most simplistic form, predictive modeling involved using real-world data sets to predict or estimate an outcome. Machine learning allowed a computer to utilize partial labeling of data (supervised learning) or the structure detected in the data itself (unsupervised learning) to explain or make predictions about the data without explicit programming. Deep learning models (e.g., neural networks with several hidden layers) have seen wide success in image recognition and classification where the input is represented by unstructured data (e.g., pixel values) [6]. Predictive models were usually deployed in contexts where the measurement of the output is complicated, time-demanding, expensive, or when an early estimate of the target can trigger a proactive intervention to modify the course of action and, for instance, avoid adverse events (e.g., a readmission to the hospital). The predictive models and machine learning can be used in several domains in surgical management (decision-making, aid to surgical planning).
Natural language processing aimed to understand human language and was crucial for large-scale content analyses such as electronic medical record data such as physicians’ narrative documentation [e.g., data collection of clinical scores after TKA]. Computer vision described machine understanding of images and videos, and advances have resulted in machines achieving human-level capabilities in object and scene recognition [e.g., screening of implants loosening on radiographs]. More recently, digital technologies used in augmented and mixed realities have been developed to interact with the human senses. These technologies enabled user projection into a reality described through a digital memory. Augmented reality (AR) technologies aimed to introduce virtual elements into the user’s environment [e.g., superimposition of the values of bone resection axis and the virtual bone cuts onto real-knee surfaces during total knee arthroplasty (TKA)] by measuring and understanding the user’s reality, processing, and then computing the information required, and finally rendering it to project this information to the user in correlation with reality. Mixed reality presents the surgeon with holographic elements that align with the real world, and the surgeon can manipulate the digital content generated by the mixed reality device.
Results
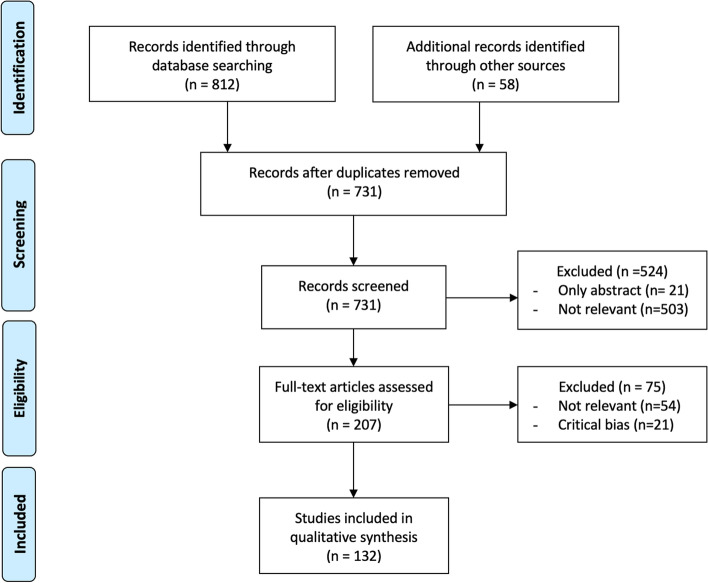
The PRISMA flow diagram shows the study selection (Fig. 1). Of 731 abstracts, 21 were excluded due to the lack of a full-text article, and 503 irrelevant abstracts were also excluded. Of 207 full-text articles, 54 irrelevant articles were excluded, and 21 articles with a serious risk of bias were also excluded [7]. Finally, 132 articles were included in this study.
Fig. 1.
Flow chart showing initial literature search for data extraction from the final list of included studies
Review and discussion
Based on the 132 articles, we reviewed AI used before, during, and after knee arthroplasty.
AI used before knee arthroplasty
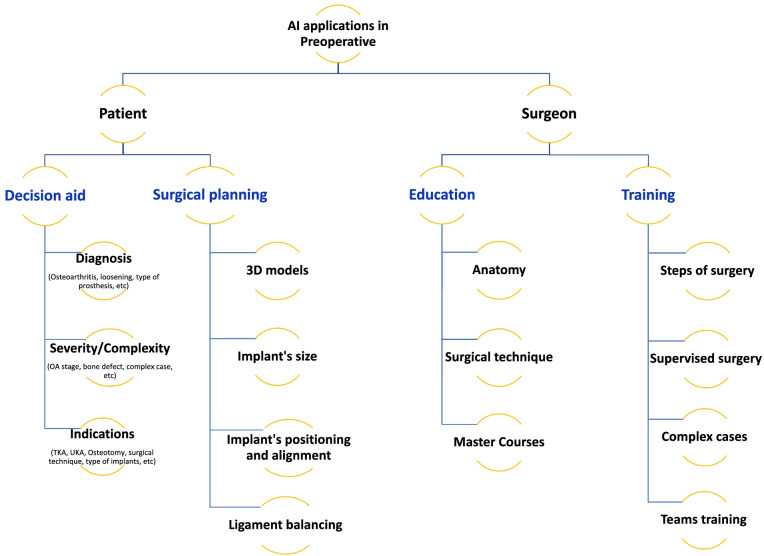
Patient selection is the first priority for successful knee arthroplasty. However, 33% of patients may be improperly selected, resulting in unsatisfactory outcomes [8]. The American Academy of Orthopedic Surgeons develops the best practice guidelines and machine learning-based algorithm to standardize the patient selection process based on the medical history, symptom severity, osteoarthritis severity, failures of previous treatment, etc [9, 10]. The predictive algorithm models require effective communication between the patients and surgeons to develop a relationship that promotes the integration of patient preferences, values, and needs, with the transfer of knowledge regarding treatments, risks, benefits, and alternatives before making informed decisions [11, 12]. The decision-making process is based on relevant and validated predictive factors, for example, demographics or preoperative patient-reported outcome measurements (PROMs) [13]. The AI-based algorithms can be used to specify the preoperative parameters, grade severity of knee osteoarthritis, and reduce inter-observer variability [14]. In revision knee arthroplasty, it can be used to diagnose prosthetic loosening on X-ray (with a precision of > 95%) and identify implant models [15]. (Table 1 and Fig. 2).
Table 1.
Diverse systems of AI in knee arthroplasty management
| Authors | Patients | Year | Type | Time | Assessment | Factors | Conclusion |
|---|---|---|---|---|---|---|---|
| Patient Decision Aid | |||||||
| Ramkumar et al. [16] | 175,042 | 2019 | Predictive of perioperative parameters (ANN) | Preop |
Predict LOS, inpatient discharge Propose a risk-based plan for complex cases |
Preop variables | Model can predict perioperative management |
| Bansback et al. [17] | 280 | 2019 | Patient Decision | Preop | Decision quality | PROMS, demographics | Predictive model with decision aid |
| Jayakumar et al. [18] | 150 | 2020 | Patient Decision | Preop | Decision quality, patient outcomes | PROMS, demographics | Presentation of RCT |
| Shah et al. [15] | 697 | 2020 | Patient decision | Preop | TKA loosening | Preop radiographs | Detection of implants loosening THA > TKA (Se 70%, Sp 96%) |
| Jayakumar et al. [19] | 129 | 2021 | Patient Decision | Preop | Decision quality, patient experience, functional outcomes | Education, preference assessment, PROMs | Better decision quality, satisfaction, improved PROMs |
| Yi et al. [14] | 237–274 | 2019 | TKA identification | Preop | Difference of TKA, UKA | Radiographs | Identification of TKA on X-ray and distinguish 2 models of TKA |
| Karnuta et al. [20] | 424 | 2020 | TKA identification | Preop | TKA models | Radiographs | Valid |
| Schwartz et al. [21] | 326 | 2020 | OA classification | Preop | OA stage | Preop radiographs | Convolutional neural network (CNN) and classify knee OA |
| Surgical training | |||||||
| Aim et al. [22] | 330 | 2016 | VR training in arthroscopy | Preop | Review | Few assessments of VR training but promising | |
| Goh et al. [23] | 2021 | VR and AR training in knee arthroplasty | Preop | Review | Few assessments of VR training but promising | ||
| Preoperative planning | |||||||
| Wallace et al. [24] | 382 | 2020 | PM Implant Size | Preop | Component size prediction | Sex, height, weight, age, and ethnicity | More accurate than radiographic templating |
| Kunze et al. [25] | 17,283 | 2021 | PM Implant size | Preop | Component size prediction | Demographic variables (age, height, weight, BMI, sex) |
Good to excellent performance for predicting TKA component Size. Main factor: sex Free app: https://orthopedics.shinyapps.io/TKASizing_Calculator/ |
| Li et al. [26] | 200 | 2021 | 3D reconstruction | Preop | AI-based 3D model construction | CT scan | As accurate as operator reconstruction. Faster than operator construction |
| Surgery | |||||||
| Tsukada et al. [27] | 10 | 2019 | Augmented reality in surgery | Intraop | Tibial bone resection with AR | AR-KNEE system | Insufficient accuracy of bone cuts |
| Pokhrel et al. [28] | 15 | 2019 | Augmented reality in surgery | Intraop | Accuracy of bone cuts | Augmented reality system | Reliable accuracy |
| Verstraete et al. [29] | 479 | 2020 | ML PM | Intraop | Intraop planning (load) | Intraop alignment – tibiofemoral load | Validated ML algorithm |
| Remote patient monitoring | |||||||
| Chiang et al. [30] | 18 | 2017 | Patient Monitoring | Postop | APDM sensors | Postop ROM |
Continuous monitoring of ROM progress after TKA |
| Kang et al. [31] | 60 | 2018 | Patient Monitoring | Postop | Rehabilitation training instrument NEO-GAIT | VAS, ROM, HSS | NEO-GAIT plays more active and effective role in promoting rehabilitation after TKA |
| Ramkumar et al. [32] | 25 | 2019 | Remote Patient Monitoring | Postop | Feasibility – ROM – PROMs – exercise compliance | RPM mobile application | Pilot study — acquisition of continuous data |
| Mehta et al. [33] | 242 | 2020 | Remote Patient Monitoring | Postop | rate of discharge to home and clinical outcomes after hip or knee arthroplasty. | RPM mobile application | No significant difference in the rate of discharge to home. Significant reduction in rehospitalization rate with RPM |
| Bovonratwet et al. [34] | 319 | 2020 | NLP | Postop | Satisfaction | Patient narratives | Not efficient |
| Sagheb et al. [35] | 20,000 | 2020 | NLP | Postop | Identify data in OR report | OR report | NLP algorithms efficient |
AR Augmented reality, ML Machine learning, NLP Natural language processing, OA Osteoarthritis, OR Operating room, PROMs Patient-reported outcome measurements, PM Predictive monitoring, RCT Randomized control trial, ROM Range of motion, RPM Remote patient monitoring, SDM Shared decision-making, THA Total hip arthroplasty, TKA Total knee arthroplasty, UKA Unicompartmental knee arthroplasty, VR Virtual reality
Fig. 2.
Structure chart resuming the preoperative major AI applications
Surgical training is traditionally performed on real patients in the operating room and supervised by senior surgeons. It is undergoing substantial change with AI development. Immersive virtual reality is an AI-based teaching tool that provides all access levels to many surgical techniques in a 360-degree viewing mode. The surgeons can simultaneously evaluate decisions on implant choice and placement, track procedural errors and efficiency, minimize completion time, and use adjunct operating equipment (such as fluoroscope) or instruments (such as retractor). By obtaining technical and cognitive practice, the surgeons can reduce implant mal-alignment and surgical complications in primary and revision knee arthroplasty [36]. Using the assistive mode, the surgeon can obtain feedback on key steps (planning bone resection, implant positioning, sizing, assessing the virtual range of motion, and gap balancing) to anticipate difficulties or achieve a surgical target [23]. Immersive virtual reality has advantages. The surgeons can obtain learning experience without direct supervision and collect data all along the training process. The replicate real-life procedures do not carry the risk of injury or costly resources (e.g., cadavers). The downsides included limited image quality, degree of presence, cyber-sickness, haptic realism, device-related issues (e.g., battery capacity and wireless technology), and access/cost considerations [22, 37, 38].
Preoperative planning and modelization include limb alignment, implant positioning, gap balancing, and implant size. Preoperatively, proper TKA component size can be predicted by using the formulas based on the demographic data such as sex, height, weight, age, ethnicity/race, and shoe size. Still, the limited predictive factors and limited size of certain products are the drawbacks [39–41]. Demographic-based multivariate linear regression models can be used to predict more accurate implant size than digitally-templated sizes for femoral (P = 0.04) and tibial (P < 0.01) components [24]. The regression models are created using the stochastic gradient boosting model, allowing users to input data and receive individualized sizing predictions and explanations [25]. This application is made freely accessible at the following link: https://orthopedics.shinyapps.io/TKASizing_Calculator/.
Segmentation tools are developed to reduce operative time and human involvement. The tools consequently reduce the process cost and are more efficient than the usual operator [26]. The tools can be used in knee arthroplasty, spine surgery, and trauma surgery based on the three-dimensional (3D) models obtained from bone-mapping (imageless system), CT scans, and specific X-rays (image-based system) [42, 43]. As a result, more accurate component alignment, ligament balance, and implant size prediction are achieved in robotic-assisted TKA than image-less robotic-TKA [42, 44, 45].
AI used during knee arthroplasty
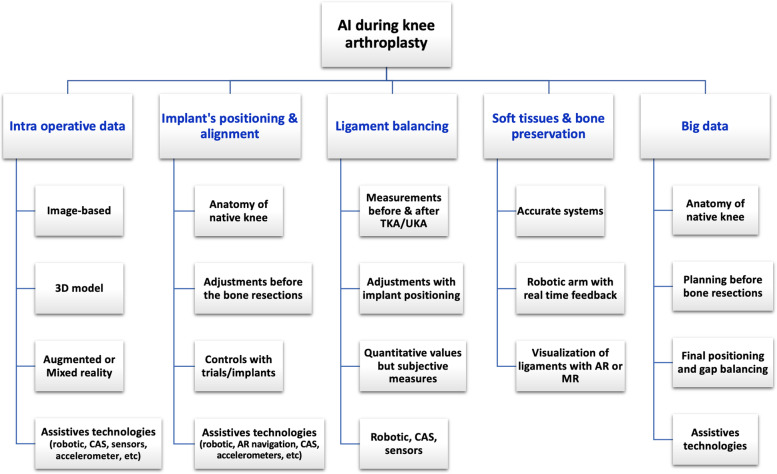
Robotic-assisted knee arthroplasty is defined as machine capable of automatically carrying out complex actions, especially programmable by a computer. This system integrates information from preoperative imaging or intraoperative surface mapping, specific bone landmarks (bone shape, tibial and femoral alignment) and the ligament balancing intraoperatively. There are three categories of robotic systems, i.e., passive, semi-autonomous, and autonomous robotic systems. A passive system provides a 3D virtual model allowing accurate preoperative planning but does not prepare the bone. The autonomous and semi-autonomous systems incorporate safeguards against bone removal beyond the 3D plane. The semi-autonomous robotic-assisted system combines the benefits of a navigation system and an autonomous robotic system, and is a typical example of an AI-based tool. The collected data include bone and implant alignment algorithms and soft-tissue balance to propose surgical planning, secondarily adjusted according to the surgeon’s requests and targets. A robotic arm allows performing bone resections or positioning a cutting guide with a real-time automatic feedback system following knee movements or cut progression. Progressively, the algorithms of robotic system integrate machine learning models to improve surgical planning according to the previous surgery. During surgery, a feedback loop is created when bone cutting is controlled or the cutting guide is positioned. This control improves the surgeon’s accuracy and decreases the risk of errors. The robotic systems do not aim to replace surgeons but to be an accurate and consistent delivery tool. The major benefit of robotic systems is accurate and reproducible bone preparation thanks to the robotic interface, regardless of the system used [42, 46]. Most currently available robotic platforms assess the ligament balancing according to intraoperative bone cutting and implant positioning. The advantages of robotic-assisted TKA are accurate knee alignment, implant positioning, ligamentous balance, and soft tissue protection [47–50]. Most controlled studies suggested better short-term functional outcomes than mid- or long-term outcomes, compared to the conventional TKA [51–56]. The downsides of robotic-assisted TKA include cost-related capital investment and the consumables in the operating room, an amount of surgeon and staff’s education to optimize safety and efficiency of robotics, unpractical specific hardware with bony trackers and a bulky robotic unit, longer operative time, learning curve required, and compromised cost-efficiency. Moreover, a robotic system is usually compatible only with one type of implant. The laxity assessment at the beginning of surgery is manual and thus lacks accuracy. (Table 1 and Fig. 3).
Fig. 3.
Structure chart presenting the intraoperative major interests of AI tools
Augmented reality-based navigation systems superimpose clinical information into the surgeon’s sight and have been developed to guide TKA implantation. Augmented reality platforms require three processes, i.e., tracking, computing, and visualization. Tracking of object position is achieved by semi-contact, or contactless methods. The semi-contact system involves attachment to the anatomy and marker and a contactless link between the features and cameras. In the contactless systems, tracking is done without the need for attachment to the patient and has been made possible with the apparition of depth cameras [57]. The computing requires to register the anatomical features tracked with the preoperative images, and to compute the clinical index from raw information, which compares the actual situation with the preoperative plan. The visualization produces an image for the user. The digital image must align with surgeons’ reality. Compared to the robotic-assisted systems, the advantages are a smaller physical footprint, a lower cost, the ability to have the intraoperative data in the same field of view, the absence of intraosseous trackers, and an easier workflow [58]. In a study examining an AR-assisted system, the preoperative CT scan was superimposed on the bone exposed during the surgery [28]. This system was found to be accurate in a cadaveric pilot study. The Pixee Medical system is a computer-assisted orthopedic surgery solution using AR to support TKA (Pixee Medical, Besancon, France). The connected glasses precisely calculate the 3D coordinates of the instruments thanks to the analysis of their specific markers (QR-Code), filmed by the integrated camera. The navigation information is displayed in the surgeon’s field of vision, which interacts with the application thanks to the glasses’ accelerometers. The NextAR™ system (Medacta, Castel San Pietro, Switzerland) requires sensors to be anchored to the femur and tibia using pins inserted within the surgical wound. A preoperative plan is generated based on CT imaging and a dedicated algorithm used to identify ligament origin and insertion to monitor balance during intraoperative navigation. To date, to our knowledge, no clinical studies have yet been published on the accuracy and the clinical efficiency of these novel devices. Despite a current important mediatization, whether these devices lead to improved patient outcomes and/or are cost-effective remains unclear.
AI used after knee arthroplasty
Remote monitoring via smartphones can be used to obtain continuous subjective and objective data postoperatively. The first platforms have been limited by the absence of interconnectivity between applications, poor user engagement, high cost of sensors, and inability to scale [30, 31, 59]. Recently, a machine learning-based remote patient monitoring system for smartphones has been developed. These devices allow for real-time tracking of patient participation in physical therapy and home exercise programs through the patient’s smartphone. The surgical team can thus follow their rehabilitation progress and intervene with an additional clinic visit or a phone call if patients are not meeting postoperative milestones [60, 61]. A pilot study of 25 patients who underwent TKA has been validated, demonstrating the ability of this technology to passively collect data from each patient’s smartphone without interruptions [32]. A recent randomized clinical trial on 242 patients operated on hip or knee arthroplasty found no significant difference in the rate of discharge to home between the usual care arm and the remote patient monitoring arm, but a statistically significant reduction in rehospitalization rate in the remote patient monitoring arm [33].
Predictive models and machine learning can be used to estimate postoperative improvement and patient satisfaction and to learn for future patient and surgeon decision-making [62]. The increasing availability of large digital healthcare datasets facilitates the development of predictive models for postoperative outcomes after TKA (Table 2). These predictive models examine how variables such as patient-specific attributes, functional scores and preoperative pain [95], comorbidities [96], psychological features [63, 97, 98], socioeconomic indicators [63] or perioperative recovery location influence clinical outcomes. The main preoperative predictive factors are pain scores (VAS and back pain), knee specific PROMs (such as KOOS and WOMAC), range of motion, quality of life PROMs (EQ-5D), and mental health (assessed by anxiety and depression scales and SF-12). Other factors that have also been evaluated are comorbidities (ASA score), demographic data (such as BMI, sex, age), previous knee surgery, the severity of osteoarthritis, and preoperative knee alignment [99]. Predictive models using data from very large populations, including several centers or countries, and objective preoperative 3D anatomy assessment are more reliable than those built on limited data sets [78]. So far, none of the available predictive models have replicated surgeon clinical acumen [100] or become a practical tool for clinical use yet. These predictive models are still in their research/pre-clinical phase [101].
Table 2.
Predictive models for knee arthroplasty management
| Authors | Patients | Year | Type | Assessment | Factors | Conclusion/Algorithms |
|---|---|---|---|---|---|---|
| Judge et al. [63] | 1991 | 2012 | PM | Satisfaction, OKS | Age, sex, BMI, Primary diagnosis, ASA score, Index of Multiple Deprivation, OKS, EQ. 5D | Strongest determinants of outcome: pain/function (less severe preop disease obtain best outcomes); diagnosis in relation to pain outcome (RA > OA); deprivation (poorer areas = worse outcomes); anxiety/depression (=worse pain) |
| Lungu et al. [64] | 141 | 2014 | PM | WOMAC | 5 preoperative WOMAC questions: difficulty of taking off socks, getting on/off toilet, performing light domestic duties and rising from bed as well as degree of morning stiffness after the first wakening | Predictive rule, based on 5 preop WOMAC questions |
| Dowsey et al. [65] | 615 | 2016 | PM |
WOMAC (using OMERACT-OARSI responder criteria) |
BMI, radiographic degree of OA (K.L. scale), WOMAC, SF-12, sex, age, ASA score, Charlson comorbidity, smoking status, etiology, SEIFA, rurality, contralateral TKA, constraint, patella, computer navigation, LOS, discharge destination, complication/adverse event | Better probability of clinical response with lower BMI, lower SF-12 MCS disability level, lower K.L., higher (worse) preoperative WOMAC |
| Pua et al. [66] | 1096 | 2016 | PM | Walking limitations (time before severe difficulty) | Age, BMI, hypertension, fall history, walking aids, contralateral knee pain, reconstruction specialist, walking ability, fast gait speed and knee pain, sex | Lower risk of walking< 15 min with younger age, lower BMI, no HTA, less fall history, less preop walking aids, no contralateral knee pain, adult reconstruction specialist surgeon, better preop walking ability, faster 1-month gait speed, lower 1-month knee |
| Van Onsem et al. [67] | 113 | 2016 | PM | KSS satisfaction score | Questions selections based on KOOS, OKS, PCS, EQ-5D, KSS, age and sex |
Algorithm: Satisfaction at M3 = 26.10 + 2.3*sex+ 0.13*age + 1.58*Q3–1.40*Q4–1.08*Q5–0.75*Q6–1*Q7–1.12*Q8–0.88*Q9–1.10*Q10 |
| To et al. [68] | 737 | 2017 | PM | Transfusion | Preop variables | Valid |
| Garriga et al. [69] | 221 | 2018 | PM | Non-satisfaction | Demographic preop pain, function | Country dependent |
| Shim et al. [70] | 721 | 2018 | PM | OKS (score less than 26 classified as poor). | OKS, chronic widespread pain, high expectations of knee pain after recovery, lack of active coping | Better (higher) postop OKS with better preop OKS, less chronic widespread pain, lower expectations of knee pain after recovery, better active coping strategies |
| Kunze et al. [71] | 484 | 2018 | PM | Satisfaction after TKA | 97.5% sensitivity, 95.7% VPN | |
| Navarro et al. [72] | 141,446 | 2018 | PM | LOS, Cost | Age, race, sex, comorbidity scores | Excellent validity |
| Sanchez et al. [73] | 1649 | 2018 | PM | OKS | Age, sex, marital status, Index of Multiple Deprivation, BMI, anxiety/depression, OKS, ASA score, etiology, previous knee arthroscopy, flexion contracture, ACL status | Better (higher) postoperative OKS with better (higher) preoperative OKS, no anxiety/depression (E.Q. 5D-3L Q5), fit and healthy ASA grade, no other conditions affective mobility, no previous arthroscopy, lower IMD 2004 score, lower BMI, presence of fixed flexion deformity, damaged/absent ACL, females aged < 80 or males aged > 60. |
| Van Onsem et al. [74] | 57 | 2018 | PM | KOOS, KSS, OKS | Preop ROM, quadriceps and hamstring force, sit-to-stand test, 6-min walk test |
High postop PROMs showed higher postop functional outcomes. A model to predict the cluster allocation contained sex, ROM improvement and 6MWT improvement (sensitivity 91.1%, specificity 75%) |
| Calkins et al. [75] | 145 | 2019 | PM | Satisfaction (KSS satisfaction subscale, score less than 20 classified as unsatisfied). | KOOS, OKS, PCS, EQ-5D, new KSS, age, sex, diagnosis, previous surgery on knee, BMI, radiographic degree of OA, coronal alignment | Higher KSS score with male sex, older age, higher pain (EQ-5D-5L Q4), less knee joint stiffness (KOOS Sy1), less grinding/clicking noise (KOOS Sy4), knee felt ‘normal’ (KSS: Symptoms Q3), less awareness of knee problem (KOOS Q1), less anxiety/depression (EQ-5D-5L Q5), pain not on mind (PCS Q9), less worried about serious problem occurring (PCS Q13) |
| Zabawa et al. [76] | 203 | 2019 | PM | Patient dissatisfaction following TKA | KOOS, OKS, PCS, EQ-5D, new KSS, age, sex, diagnosis, previous surgery on knee, BMI, radiographic degree of OA, coronal alignment, payment method, education, income, diabetes mellitus, HTA, hyperlipidemia, insurance provider, comorbidities | External validation of a new prediction model; Less pain prior to surgery (Q3), lesser anxiety/depression prior to surgery (Q9) and better ability to control pain symptoms (Q9); Also found lower BMI and past medical history of hypertension through additional analysis |
| Twiggs et al. [77] | 330 | 2019 | PM | Knee pain | Age, sex, KOOS items, back pain, occurrence of hip pain, occurrence of falls in past year |
Predictive model with a web application KOOS: activities of daily living, pain and symptom subscores, pain when pivoting on knee, pain when standing, difficulty bending the knee fully, frequency of back pain, severity of back pain, occurrence of hip pain, occurrence of falls in preceding year, age, sex |
| Tolk et al. [78] | 7071 | 2019 | PM | Residual symptoms (pain at rest and activity, sit-to-stand movement, stair negotiation, walking, performance of activities of daily living, kneeling and squatting) | Age, sex, ASA score, BMI, smoking, previous knee surgery, Charnley score, KOOS-PS, OKS, EuroQoL 5D-3L, NRS | Predictive model for residual symptoms |
| Kunze et al. [71] | 484 | 2019 | PM |
Patient-reported health state, KSS, ROM, satisfaction = > Knee survey score |
BMI, drug allergies, osteophytes, soft tissue thickness, flexion contracture, diabetes, opioid use, comorbidities, previous knee surgery, surgical indication, smoking |
Knee survey score on 110 pts; 4 risks of experiencing postoperative dissatisfaction: Score 96.5–110 = low risk Score 75–96.4 = mild risk Score 60–74.9 = medium risk Score < 60 = high risk |
| Huber et al. [79] | 34,110 | 2019 | PM | EQ-VAS (MID), OKS (MID). | All 81 variables in NHS dataset (April 2015 – March 2016); including sociodemographic information such as living status, age groups, sex, disease affliction, EQ-5D-3L, EQ-VAS, OKS scores | Preop OKS score, often limping (OKS Q6), preop EQ-VAS, revision surgery, no disability, not interfering with work (OKS Q9), no previous knee surgery, no diabetes, extreme difficulty doing shopping (OKS Q11), age 50–59 |
| Gronbeck et al. [80] | 61,284 | 2019 | PM | Inpatient admission after TKA | Demographic, comorbidity, perioperative variables | Reliable identification of candidates for inpatient admission |
| Bini et al. [5] | 22 | 2019 | PM | PROMs | 35 variables (PROMS, demographic …) | Valid |
| Jo et al. [81] | 1686 | 2019 | PM | Transfusion after TKA | 43 preop variables | Validated – good performance |
| Pua et al. [82] | 4026 | 2019 | PM | Walking limitation | Socio-demographic data outcomes | Better (higher) postop score with lower preop knee pain levels, lower preop depression levels, lower preop knee flexion range and Chinese race |
| Itou et al. [83] | 50 | 2020 | PM | satisfaction | KSS FJS12 | Low utility |
| Li et al. [84] | 1826 | 2020 | PM | LOS | ASA, diabetes, comorbidities, anesthesia, operation time | LOS prediction model for TKA |
| Kunze et al. [85] | 430 | 2020 | PM | Dissatisfaction after TKA | Demographics, medical history, flexion contracture, knee flexion, outcome scores | Good discriminative capacity |
| Turcotte et al. [86] | 2266 | 2020 | PM | Ambulatory surgery for TKA | Demographics, comorbidities | Good validity |
| Harris et al. [87] | 587 | 2020 | PM | PROMs Improvement | PROMs health data | Improve decision support and decision making |
| Goltz et al. [88] | 10,155 | 2020 | PM | Risk prediction of TKA for discharge location | 45 variables (sociodemographic data, postop labs, comorbidity) | Excellent accuracy to predict discharge location |
| Farooq et al. [89] | 897 | 2020 | PM | Satisfaction | 15 variables (sociodemographic – surgery) | Valid - multifactorial |
| El Galaly et al. [90] | 25,104 | 2020 | PM | Revision TKA | Patient’s characteristics and surgical information | Inable to predict revision |
| Anis et al. [91] | 5958–2391 | 2020 | PM | LOS, 90 days readmission, PROMs | Age, sex, BMI, race, educational level, smoking, comorbidities, KOOS items, 12PCS, 12MCS |
Scalable predictive tools Can accurately estimate the likelihood of improved pain, function, and quality of life 1 year after TKA as well as LOS and 90 day readmission. |
| Ko et al. [92] | 5757 | 2020 | PM | Acute kidney injury | 18 variables | 6 major variables – valid |
| Andersen et al. [93] | 538 | 2021 | PM | Revision TKA | Age, EQ-5D, comorbidities | Partially validated |
| Han et al. [94] | 1298 | 2021 | PM | LOS | 36 variables | Valid |
BMI Body mass index, EQ-5D Euro QOL score, KOOS Knee injury and osteoarthritis outcome score, KSS Knee society score, LOS Length of stay, OA Osteoarthritis, OKS Oxford knee score, PCS Pain catastrophizing scale, PM Predictive model, PROMs Patient-reported outcome measurements, RA Rheumatoid arthritis, ROM Range of motion, TKA Total knee arthroplasty, WOMAC Western Ontario and McmMaster Universities osteoarthritis index
Limitations and future expectations
The review is not comprehensive enough to include all the available technologies but has described the basic and current AI applications in knee arthroplasty. The outputs of machine learning and AI analyses are limited by the types and accuracy of available data sets. Systematic biases in clinical data collection affect the recognition or prediction of AI patterns, such as women and racial minorities due to long-standing under-representation in a clinical trial and patient registry populations. Ethical considerations regarding the ownership and the use of AI data remain unanswered. The robotic platform storing surgeon and patient information sometimes lacks the patient’s express consent, and is then used for product development. Although the aggregate data are deidentified, who access the data and what purposes are still debatable. The European Commission has proposed a regulatory framework (released on April 2021) to monitor AI with this aim.
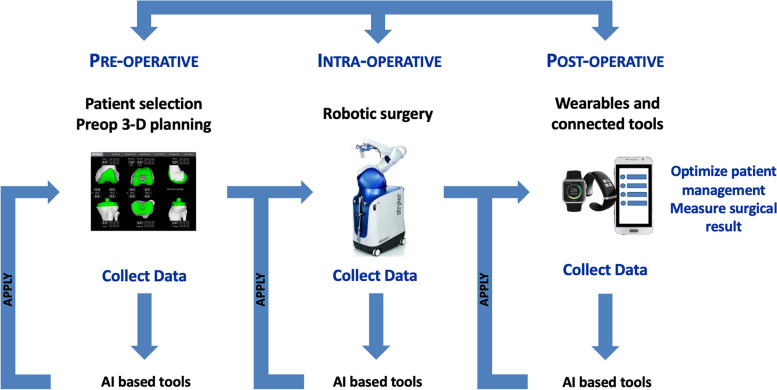
The next challenge will be to “close the loop” using accurate interconnected data sets and predictive monitoring during the different phases of the patient path (before, during and after knee arthroplasty) to help surgeons and health-care providers in their decision-making [102] (Fig. 4). The goal is not to replace the health-care providers but to assist the medical decision collaboratively, combining the doctor’s experience and the AI-based tools. The answer is probably collaborative intelligence to adjust the patient management using predictive models and clinical experience and make the subsequent surgery better for every patient.
Fig. 4.
Diagram explaining the principle of the feedback loop, which interconnects data collection (before, during, and after surgery) via the connected tools to create mega data information for adjusting the surgical plan
Conclusion
In knee arthroplasty, the AI-based tools improve the decision-making process, surgical planning, accuracy and repeatability of surgical procedures. More clinical evidence is needed to confirm the benefits.
Acknowledgments
None.
Conflict of interest
CB: Institutional research support to Lepine. Grant from SoFCOT (Société Francaise de Chirurgie Orthopédique et Traumatologique).
JS, ESM: declare that they have no conflict of interest.
ES: Consultant for Corin.
SP: Royalties for Zimmer Biomet and Newclip; Consultant for Zimmer Biomet; Treasurer for European Knee Society.
SL: Consultant for Stryker, Smith Nephew, Heraeus, Depuy Synthes; Institutional research support from Groupe Lepine, Amplitude; Editorial Board for Journal of Bone and Joint Surgery (Am)
Abbreviations
- AI
Artificial Intelligence
- AR
Augmented Reality
- BMI
Body Mass Index
- EMR
Electronic Medical Record
- ML
Machine Learning
- MR
Mixed Reality
- NLP
Natural Language Processing
- PROMs
Patient-Reported Outcome Measurements
- PM
Predictive Monitoring
- ROM
Range Of Motion
- RPM
Remote Patient Monitoring
- SDM
Shared Decision-Making
- TKA
Total Knee Arthroplasty
- UKA
Unicompartmental Knee Arthroplasty
Authors’ contributions
CB: Study design, literature review and manuscript writing. JS: Literature review and manuscript editing. ESM: Literature review and manuscript editing. ES: Manuscript editing. SP: Study design, literature review and manuscript editing. SL: Study design, manuscript editing, and supervisor. The author(s) read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Sébastien Lustig is a member of the Editorial Board of Arthroplasty and other authors declare that they have no competing interests. All authors were not involved in the journal’s review of or decisions related to, this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cécile Batailler, Email: cecile-batailler@hotmail.fr.
Jobe Shatrov, Email: jobeshatrov1@gmail.com.
Elliot Sappey-Marinier, Email: elliot.sappey-marinier@chu-lyon.fr.
Elvire Servien, Email: elvire.servien@chu-lyon.fr.
Sébastien Parratte, Email: sebastien.parratte@gmail.com.
Sébastien Lustig, Email: sebastien.lustig@gmail.com.
References
- 1.Bellman R. An introduction to artificial intelligence: Can computers think? 1978. [Google Scholar]
- 2.Jones LD, Golan D, Hanna SA, Ramachandran M. Artificial intelligence, machine learning and the evolution of healthcare: a bright future or cause for concern? Bone Joint Res. 2018;7(3):223–225. doi: 10.1302/2046-3758.73.BJR-2017-0147.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panchmatia JR, Visenio MR, Panch T. The role of artificial intelligence in orthopaedic surgery. Br J Hosp Med (Lond) 2018;79(12):676–681. doi: 10.12968/hmed.2018.79.12.676. [DOI] [PubMed] [Google Scholar]
- 4.Bini SA. Artificial intelligence, machine learning, deep learning, and cognitive computing: what do these terms mean and how will they impact health care? J Arthroplast. 2018;33(8):2358–2361. doi: 10.1016/j.arth.2018.02.067. [DOI] [PubMed] [Google Scholar]
- 5.Bini SA, Shah RF, Bendich I, Patterson JT, Hwang KM, Zaid MB. Machine learning algorithms can use wearable sensor data to accurately predict six-week patient-reported outcome scores following joint replacement in a prospective trial. J Arthroplast. 2019;34(10):2242–2247. doi: 10.1016/j.arth.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Shahid N, Rappon T, Berta W. Applications of artificial neural networks in health care organizational decision-making: a scoping review. Plos One. 2019;14(2):e0212356. doi: 10.1371/journal.pone.0212356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355i4919. 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed]
- 8.Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134–2143. doi: 10.1002/art.38685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle DL, Perera RA. Appropriateness and total knee arthroplasty: an examination of the American Academy of Orthopaedic surgeons appropriateness rating system. Osteoarthr Cartil. 2017;25(12):1994–1998. doi: 10.1016/j.joca.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Katz JN, Winter AR, Hawker G. Measures of the appropriateness of elective Orthopaedic joint and spine procedures. J Bone Joint Surg Am. 2017;99(4):e15. doi: 10.2106/JBJS.16.00473. [DOI] [PubMed] [Google Scholar]
- 11.Barry MJ, Edgman-Levitan S. Shared decision making-pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 12.Rabi DM, Kunneman M, Montori VM. When guidelines recommend shared decision-making. JAMA. 2020;323(14):1345–1346. doi: 10.1001/jama.2020.1525. [DOI] [PubMed] [Google Scholar]
- 13.Noonan VK, Lyddiatt A, Ware P, et al. Montreal accord on patient-reported outcomes (PROs) use series - paper 3: patient-reported outcomes can facilitate shared decision-making and guide self-management. J Clin Epidemiol. 2017:89125–35. 10.1016/j.jclinepi.2017.04.017. [DOI] [PubMed]
- 14.Yi PH, Wei J, Kim TK, et al. Automated detection & classification of knee arthroplasty using deep learning. Knee. 2020;27(2):535–542. doi: 10.1016/j.knee.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Shah RF, Bini SA, Martinez AM, Pedoia V, Vail TP. Incremental inputs improve the automated detection of implant loosening using machine-learning algorithms. Bone Joint J. 2020;102-B(6_Supple_A):101–106. doi: 10.1302/0301-620X.102B6.BJJ-2019-1577.R1. [DOI] [PubMed] [Google Scholar]
- 16.Ramkumar PN, Karnuta JM, Navarro SM, et al. Deep learning preoperatively predicts value metrics for primary Total knee arthroplasty: development and validation of an artificial neural network model. J Arthroplast. 2019;34(10):2220–2227 e2221. doi: 10.1016/j.arth.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Bansback N, Trenaman L, MacDonald KV, et al. An individualized patient-reported outcome measure (PROM) based patient decision aid and surgeon report for patients considering total knee arthroplasty: protocol for a pragmatic randomized controlled trial. BMC Musculoskelet Disord. 2019;20(1):89. doi: 10.1186/s12891-019-2434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayakumar P, Bozic KJ. Advanced decision-making using patient-reported outcome measures in total joint replacement. J Orthop Res. 2020;38(7):1414–1422. doi: 10.1002/jor.24614. [DOI] [PubMed] [Google Scholar]
- 19.Jayakumar P, Moore MG, Furlough KA, et al. Comparison of an artificial intelligence-enabled patient decision aid vs educational material on decision quality, shared decision-making, patient experience, and functional outcomes in adults with knee osteoarthritis: a randomized clinical trial. JAMA Netw Open. 2021;4(2):e2037107. doi: 10.1001/jamanetworkopen.2020.37107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karnuta JM, Luu BC, Roth AL, et al. Artificial intelligence to identify arthroplasty implants from radiographs of the knee. J Arthroplast. 2021;36(3):935–940. doi: 10.1016/j.arth.2020.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz AJ, Clarke HD, Spangehl MJ, Bingham JS, Etzioni DA, Neville MR. Can a convolutional neural network classify knee osteoarthritis on plain radiographs as accurately as fellowship-trained knee arthroplasty surgeons? J Arthroplast. 2020;35(9):2423–2428. doi: 10.1016/j.arth.2020.04.059. [DOI] [PubMed] [Google Scholar]
- 22.Aim F, Lonjon G, Hannouche D, Nizard R. Effectiveness of virtual reality training in Orthopaedic surgery. Arthroscopy. 2016;32(1):224–232. doi: 10.1016/j.arthro.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 23.Goh GS, Lohre R, Parvizi J, Goel DP. Virtual and augmented reality for surgical training and simulation in knee arthroplasty. Arch Orthop Trauma Surg. 2021;141(12):2303–12. 10.1007/s00402-021-04037-1. [DOI] [PubMed]
- 24.Wallace SJ, Murphy MP, Schiffman CJ, Hopkinson WJ, Brown NM. Demographic data is more predictive of component size than digital radiographic templating in total knee arthroplasty. Knee Surg Relat Res. 2020;32(1):63. doi: 10.1186/s43019-020-00075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunze KN, Polce EM, Patel A, Courtney PM, Levine BR. Validation and performance of a machine-learning derived prediction guide for total knee arthroplasty component sizing. Arch Orthop Trauma Surg. 2021;141(12):2235–44. 10.1007/s00402-021-04041-5. [DOI] [PubMed]
- 26.Li Z, Zhang X, Ding L, et al. Deep learning approach for guiding three-dimensional computed tomography reconstruction of lower limbs for robotically-assisted total knee arthroplasty. Int J Med Robot. 2021:e2300. 10.1002/rcs.2300. [DOI] [PubMed]
- 27.Tsukada S, Ogawa H, Nishino M, Kurosaka K, Hirasawa N. Augmented reality-based navigation system applied to tibial bone resection in total knee arthroplasty. J Exp Orthop. 2019;6(1):44. doi: 10.1186/s40634-019-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pokhrel S, Alsadoon A, Prasad PWC, Paul M. A novel augmented reality (AR) scheme for knee replacement surgery by considering cutting error accuracy. Int J Med Robot. 2019;15(1):e1958. doi: 10.1002/rcs.1958. [DOI] [PubMed] [Google Scholar]
- 29.Verstraete MA, Moore RE, Roche M, Conditt MA. The application of machine learning to balance a total knee arthroplasty. Bone Jt Open. 2020;1(6):236–244. doi: 10.1302/2633-1462.16.BJO-2020-0056.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiang CY, Chen KH, Liu KC, Hsu SJ, Chan CT. Data collection and analysis using wearable sensors for monitoring knee range of motion after Total knee arthroplasty. Sensors (Basel). 2017;17(2). 10.3390/s17020418. [DOI] [PMC free article] [PubMed]
- 31.Kang K, Geng Q, Xu HT, et al. Clinical study of a new wearable device for rehabilitation after total knee arthroplasty. Zhonghua Yi Xue Za Zhi. 2018;98(15):1162–1165. doi: 10.3760/cma.j.issn.0376-2491.2018.15.008. [DOI] [PubMed] [Google Scholar]
- 32.Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote patient monitoring using Mobile health for Total knee arthroplasty: validation of a wearable and machine learning-based surveillance platform. J Arthroplast. 2019;34(10):2253–2259. doi: 10.1016/j.arth.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SJ, Hume E, Troxel AB, et al. Effect of remote monitoring on discharge to home, return to activity, and Rehospitalization after hip and knee arthroplasty: a randomized clinical trial. JAMA Netw Open. 2020;3(12):e2028328. doi: 10.1001/jamanetworkopen.2020.28328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bovonratwet P, Shen TS, Islam W, Ast MP, Haas SB, Su EP. Natural language processing of patient-experience comments after primary Total knee arthroplasty. J Arthroplast. 2021;36(3):927–934. doi: 10.1016/j.arth.2020.09.055. [DOI] [PubMed] [Google Scholar]
- 35.Sagheb E, Ramazanian T, Tafti AP, et al. Use of natural language processing algorithms to identify common data elements in operative notes for knee arthroplasty. J Arthroplast. 2021;36(3):922–926. doi: 10.1016/j.arth.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kazarian GS, Lawrie CM, Barrack TN, et al. The impact of surgeon volume and training status on implant alignment in Total knee arthroplasty. J Bone Joint Surg Am. 2019;101(19):1713–1723. doi: 10.2106/JBJS.18.01205. [DOI] [PubMed] [Google Scholar]
- 37.Bartlett JD, Lawrence JE, Stewart ME, Nakano N, Khanduja V. Does virtual reality simulation have a role in training trauma and orthopaedic surgeons? Bone Joint J. 2018;100-B(5):559–565. doi: 10.1302/0301-620X.100B5.BJJ-2017-1439. [DOI] [PubMed] [Google Scholar]
- 38.Clarke E. Virtual reality simulation-the future of orthopaedic training? A systematic review and narrative analysis. Adv Simul (Lond) 2021;6(1):2. doi: 10.1186/s41077-020-00153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren AN, Neher RE, Bell T, Grimm J. Using patient demographics and statistical modeling to predict knee tibia component sizing in Total knee arthroplasty. J Arthroplast. 2018;33(6):1732–1736. doi: 10.1016/j.arth.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 40.Sershon RA, Courtney PM, Rosenthal BD, Sporer SM, Levine BR. Can demographic variables accurately predict component sizing in primary Total knee arthroplasty? J Arthroplast. 2017;32(10):3004–3008. doi: 10.1016/j.arth.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Bhowmik-Stoker M, Scholl L, Khlopas A, et al. Accurately predicting Total knee component size without preoperative radiographs. Surg Technol Int. 2018;33:337–42. [PubMed]
- 42.Batailler C, Fernandez A, Swan J, et al. MAKO CT-based robotic arm-assisted system is a reliable procedure for total knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3585–98. 10.1007/s00167-020-06283-z. [DOI] [PubMed]
- 43.Batailler C, Hannouche D, Benazzo F, Parratte S. Concepts and techniques of a new robotically assisted technique for total knee arthroplasty: the ROSA knee system. Arch Orthop Trauma Surg. 2021;141(12):2049–58. 10.1007/s00402-021-04048-y. [DOI] [PubMed]
- 44.Batailler C, Bordes M, Lording T, et al. Improved sizing with image-based robotic-assisted system compared to image-free and conventional techniques in medial unicompartmental knee arthroplasty. Bone Joint J. 2021;103-B(4):610–618. doi: 10.1302/0301-620X.103B4.BJJ-2020-1453.R1. [DOI] [PubMed] [Google Scholar]
- 45.Jacofsky DJ, Allen M. Robotics in arthroplasty: a comprehensive review. J Arthroplast. 2016;31(10):2353–2363. doi: 10.1016/j.arth.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 46.van der List JP, Chawla H, Joskowicz L, Pearle AD. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24(11):3482–3495. doi: 10.1007/s00167-016-4305-9. [DOI] [PubMed] [Google Scholar]
- 47.Kayani B, Konan S, Pietrzak JRT, Haddad FS. Iatrogenic bone and soft tissue trauma in robotic-arm assisted Total knee arthroplasty compared with conventional jig-based Total knee arthroplasty: a prospective cohort study and validation of a new classification system. J Arthroplast. 2018;33(8):2496–2501. doi: 10.1016/j.arth.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 48.Sires JD, Wilson CJ. CT validation of intraoperative implant position and knee alignment as determined by the MAKO Total knee arthroplasty system. J Knee Surg. 2021;34(10):1133–7. 10.1055/s-0040-1701447. [DOI] [PubMed]
- 49.Kayani B, Konan S, Huq SS, Tahmassebi J, Haddad FS. Robotic-arm assisted total knee arthroplasty has a learning curve of seven cases for integration into the surgical workflow but no learning curve effect for accuracy of implant positioning. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1132–1141. doi: 10.1007/s00167-018-5138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sultan AA, Samuel LT, Khlopas A, et al. Robotic-arm assisted Total knee arthroplasty more accurately restored the posterior condylar offset ratio and the Insall-Salvati index compared to the manual technique; a cohort-matched study. Surg Technol Int. 2019;34:409–13. [PubMed]
- 51.Gilmour A, MacLean AD, Rowe PJ, et al. Robotic-arm-assisted vs conventional Unicompartmental knee arthroplasty. The 2-year clinical outcomes of a randomized controlled trial. J Arthroplast. 2018;33(7S):S109–S115. doi: 10.1016/j.arth.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 52.Bhimani S, Bhimani R, Smith A, Eccles C, Smith L, Malkani A. Robotic-assisted total knee arthroplasty demonstrates decreased postoperative pain and opioid usage compared to conventional total knee arthroplasty. Bone Joint Open. 2020;2020(1–2):8–12. doi: 10.1302/2633-1462.12.BJO-2019-0004.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naziri Q, Cusson BC, Chaudhri M, Shah NV, Sastry A. Making the transition from traditional to robotic-arm assisted TKA: what to expect? A single-surgeon comparative-analysis of the first-40 consecutive cases. J Orthop. 2019;16(4):364–368. doi: 10.1016/j.jor.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batailler C, White N, Ranaldi FM, Neyret P, Servien E, Lustig S. Improved implant position and lower revision rate with robotic-assisted unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2018;27(4):1232–40. 10.1007/s00167-018-5081-5. [DOI] [PubMed]
- 55.Blyth MJG, Anthony I, Rowe P, Banger MS, MacLean A, Jones B. Robotic arm-assisted versus conventional unicompartmental knee arthroplasty: exploratory secondary analysis of a randomised controlled trial. Bone Joint Res. 2017;6(11):631–639. doi: 10.1302/2046-3758.611.BJR-2017-0060.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen DC, Kusuma SK, Palmer RM, Harris KB. Robotic guidance does not improve component position or short-term outcome in medial unicompartmental knee arthroplasty. J Arthroplast. 2014;29(9):1784–1789. doi: 10.1016/j.arth.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Auvinet E, Giles J, Rodriguez YBF. Augmented reality based navigation for computer assisted hip resurfacing: a proof of concept study. Ann Biomed Eng. 2018;46(10):1595–1605. doi: 10.1007/s10439-018-2055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Auvinet E, Maillot C, Uzoho C. Augmented reality Technology for Joint Replacement. In: Riviere C, Vendittoli PA, editors. Personalized hip and knee joint replacement. Cham: Springer; 2020. p. 321–8. [PubMed]
- 59.Kline PW, Melanson EL, Sullivan WJ, et al. Improving physical activity through adjunct Telerehabilitation following Total knee arthroplasty: randomized controlled trial protocol. Phys Ther. 2019;99(1):37–45. doi: 10.1093/ptj/pzy119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang S, Summers RM. Machine learning and radiology. Med Image Anal. 2012;16(5):933–951. doi: 10.1016/j.media.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;23. 10.1186/2047-2501-2-3. [DOI] [PMC free article] [PubMed]
- 63.Judge A, Arden NK, Cooper C, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology (Oxford) 2012;51(10):1804–1813. doi: 10.1093/rheumatology/kes075. [DOI] [PubMed] [Google Scholar]
- 64.Lungu E, Desmeules F, Dionne CE, Belzile EL, Vendittoli PA. Prediction of poor outcomes six months following total knee arthroplasty in patients awaiting surgery. BMC Musculoskelet Disord. 2014;15299. 10.1186/1471-2474-15-299. [DOI] [PMC free article] [PubMed]
- 65.Dowsey MM, Spelman T, Choong PF. Development of a prognostic nomogram for predicting the probability of nonresponse to Total knee arthroplasty 1 year after surgery. J Arthroplast. 2016;31(8):1654–1660. doi: 10.1016/j.arth.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Pua YH, Seah FJ, Clark RA, Poon CL, Tan JW, Chong HC. Development of a prediction model to estimate the risk of walking limitations in patients with Total knee arthroplasty. J Rheumatol. 2016;43(2):419–426. doi: 10.3899/jrheum.150724. [DOI] [PubMed] [Google Scholar]
- 67.Van Onsem S, Van Der Straeten C, Arnout N, Deprez P, Van Damme G, Victor J. A new prediction model for patient satisfaction after Total knee arthroplasty. J Arthroplast. 2016;31(12):2660–2667 e2661. doi: 10.1016/j.arth.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 68.To J, Sinha R, Kim SW, et al. Predicting perioperative transfusion in elective hip and knee arthroplasty: a validated predictive model. Anesthesiology. 2017;127(2):317–325. doi: 10.1097/ALN.0000000000001709. [DOI] [PubMed] [Google Scholar]
- 69.Garriga C, Sanchez-Santos MT, Judge A, et al. Development of a model predicting non-satisfaction 1 year after primary total knee replacement in the UK and transportation to Switzerland. Sci Rep. 2018;8(1):3380. doi: 10.1038/s41598-018-21713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shim J, McLernon DJ, Hamilton D, Simpson HA, Beasley M, Macfarlane GJ. Development of a clinical risk score for pain and function following total knee arthroplasty: results from the TRIO study. Rheumatol Adv Pract. 2018;2(2):rky021. doi: 10.1093/rap/rky021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kunze KN, Akram F, Fuller BC, Zabawa L, Sporer SM, Levine BR. Internal validation of a predictive model for satisfaction after primary Total knee arthroplasty. J Arthroplast. 2019;34(4):663–670. doi: 10.1016/j.arth.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 72.Navarro SM, Wang EY, Haeberle HS, et al. Machine learning and primary Total knee arthroplasty: patient forecasting for a patient-specific payment model. J Arthroplast. 2018;33(12):3617–3623. doi: 10.1016/j.arth.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez-Santos MT, Garriga C, Judge A, et al. Development and validation of a clinical prediction model for patient-reported pain and function after primary total knee replacement surgery. Sci Rep. 2018;8(1):3381. doi: 10.1038/s41598-018-21714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Onsem S, Verstraete M, Dhont S, Zwaenepoel B, Van Der Straeten C, Victor J. Improved walking distance and range of motion predict patient satisfaction after TKA. Knee Surg Sports Traumatol Arthrosc. 2018;26(11):3272–3279. doi: 10.1007/s00167-018-4856-z. [DOI] [PubMed] [Google Scholar]
- 75.Calkins TE, Culvern C, Nahhas CR, et al. External validity of a new prediction model for patient satisfaction after Total knee arthroplasty. J Arthroplast. 2019;34(8):1677–1681. doi: 10.1016/j.arth.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 76.Zabawa L, Li K, Chmell S. Patient dissatisfaction following total knee arthroplasty: external validation of a new prediction model. Eur J Orthop Surg Traumatol. 2019;29(4):861–867. doi: 10.1007/s00590-019-02375-w. [DOI] [PubMed] [Google Scholar]
- 77.Twiggs JG, Wakelin EA, Fritsch BA, et al. Clinical and statistical validation of a probabilistic prediction tool of Total knee arthroplasty outcome. J Arthroplast. 2019;34(11):2624–2631. doi: 10.1016/j.arth.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 78.Tolk JJ, Waarsing JEH, Janssen RPA, van Steenbergen LN, Bierma-Zeinstra SMA, Reijman M. Development of preoperative prediction models for pain and functional outcome after Total knee arthroplasty using the Dutch arthroplasty register data. J Arthroplast. 2020;35(3):690–698 e692. doi: 10.1016/j.arth.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 79.Huber M, Kurz C, Leidl R. Predicting patient-reported outcomes following hip and knee replacement surgery using supervised machine learning. BMC Med Inform Decis Mak. 2019;19(1):3. doi: 10.1186/s12911-018-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gronbeck C, Cote MP, Halawi MJ. Predicting inpatient status after primary Total knee arthroplasty in Medicare-aged patients. J Arthroplast. 2019;34(7):1322–1327. doi: 10.1016/j.arth.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Jo C, Ko S, Shin WC, et al. Transfusion after total knee arthroplasty can be predicted using the machine learning algorithm. Knee Surg Sports Traumatol Arthrosc. 2020;28(6):1757–1764. doi: 10.1007/s00167-019-05602-3. [DOI] [PubMed] [Google Scholar]
- 82.Pua YH, Kang H, Thumboo J, et al. Machine learning methods are comparable to logistic regression techniques in predicting severe walking limitation following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3207–3216. doi: 10.1007/s00167-019-05822-7. [DOI] [PubMed] [Google Scholar]
- 83.Itou J, Itoh M, Kuwashima U, Okazaki K. Assessing the validity of a new prediction model for patient satisfaction after Total knee arthroplasty: a retrospective cross-sectional study. Orthop Res Rev. 2020:12133–7. 10.2147/ORR.S271253. [DOI] [PMC free article] [PubMed]
- 84.Li H, Jiao J, Zhang S, Tang H, Qu X, Yue B. Construction and comparison of predictive models for length of stay after Total knee arthroplasty: regression model and machine learning analysis based on 1,826 cases in a single Singapore center. J Knee Surg. 2020. 10.1055/s-0040-1710573. [DOI] [PubMed]
- 85.Kunze KN, Polce EM, Sadauskas AJ, Levine BR. Development of machine learning algorithms to predict patient dissatisfaction after primary Total knee arthroplasty. J Arthroplast. 2020;35(11):3117–3122. doi: 10.1016/j.arth.2020.05.061. [DOI] [PubMed] [Google Scholar]
- 86.Turcotte JJ, Menon N, Kelly ME, Grover JJ, King PJ, MacDonald JH. Preoperative predictors of same-day discharge after Total knee arthroplasty. Arthroplast Today. 2021:7182–7. 10.1016/j.artd.2020.12.006. [DOI] [PMC free article] [PubMed]
- 87.Harris AHS, Kuo AC, Bowe TR, Manfredi L, Lalani NF, Giori NJ. Can machine learning methods produce accurate and easy-to-use preoperative prediction models of one-year improvements in pain and functioning after knee arthroplasty? J Arthroplast. 2021;36(1):112–117 e116. doi: 10.1016/j.arth.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 88.Goltz DE, Ryan SP, Attarian DE, Jiranek WA, Bolognesi MP, Seyler TM. A preoperative risk prediction tool for discharge to a skilled nursing or rehabilitation facility after Total joint arthroplasty. J Arthroplast. 2021;36(4):1212–1219. doi: 10.1016/j.arth.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 89.Farooq H, Deckard ER, Ziemba-Davis M, Madsen A, Meneghini RM. Predictors of patient satisfaction following primary Total knee arthroplasty: results from a traditional statistical model and a machine learning algorithm. J Arthroplast. 2020;35(11):3123–3130. doi: 10.1016/j.arth.2020.05.077. [DOI] [PubMed] [Google Scholar]
- 90.El-Galaly A, Grazal C, Kappel A, Nielsen PT, Jensen SL, Forsberg JA. Can machine-learning algorithms predict early revision TKA in the Danish knee arthroplasty registry? Clin Orthop Relat Res. 2020;478(9):2088–2101. doi: 10.1097/CORR.0000000000001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anis HK, Strnad GJ, Klika AK, et al. Developing a personalized outcome prediction tool for knee arthroplasty. Bone Joint J. 2020;102-B(9):1183–1193. doi: 10.1302/0301-620X.102B9.BJJ-2019-1642.R1. [DOI] [PubMed] [Google Scholar]
- 92.Ko S, Jo C, Chang CB, et al. A web-based machine-learning algorithm predicting postoperative acute kidney injury after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2020. 10.1007/s00167-020-06258-0. [DOI] [PubMed]
- 93.Andersen JD, Hangaard S, Buus AAO, Laursen M, Hejlesen OK, El-Galaly A. Development of a multivariable prediction model for early revision of total knee arthroplasty - the effect of including patient-reported outcome measures. J Orthop. 2021:24216–21. 10.1016/j.jor.2021.03.001. [DOI] [PMC free article] [PubMed]
- 94.Han C, Liu J, Wu Y, Chong Y, Chai X, Weng X. To predict the length of hospital stay after Total knee arthroplasty in an orthopedic Center in China: the use of machine learning algorithms. Front Surg. 2021;8606038. 10.3389/fsurg.2021.606038. [DOI] [PMC free article] [PubMed]
- 95.Baker PN, van der Meulen JH, Lewsey J, Gregg PJ, National Joint Registry for E, Wales The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br. 2007;89(7):893–900. doi: 10.1302/0301-620X.89B7.19091. [DOI] [PubMed] [Google Scholar]
- 96.Baker PN, Deehan DJ, Lees D, et al. The effect of surgical factors on early patient-reported outcome measures (PROMS) following total knee replacement. J Bone Joint Surg Br. 2012;94(8):1058–1066. doi: 10.1302/0301-620X.94B8.28786. [DOI] [PubMed] [Google Scholar]
- 97.Brander VA, Stulberg SD, Adams AD, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;416:27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 98.Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res. 2011;97(2):139–144. doi: 10.1016/j.otsr.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Batailler C, Lording T, De Massari D, Witvoet-Braam S, Bini S, Lustig S. Predictive models for clinical outcomes in Total knee arthroplasty: a systematic analysis. Arthroplast Today. 2021:91–15. 10.1016/j.artd.2021.03.013. [DOI] [PMC free article] [PubMed]
- 100.Escobar A, Quintana JM, Bilbao A, et al. Development of explicit criteria for prioritization of hip and knee replacement. J Eval Clin Pract. 2007;13(3):429–434. doi: 10.1111/j.1365-2753.2006.00733.x. [DOI] [PubMed] [Google Scholar]
- 101.Riddle DL, Perera RA, Jiranek WA, Dumenci L. Using surgical appropriateness criteria to examine outcomes of total knee arthroplasty in a United States sample. Arthritis Care Res (Hoboken) 2015;67(3):349–357. doi: 10.1002/acr.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.