Abstract
BACKGROUND:
Cisplatin-induced hearing loss (CIHL) is a common and debilitating toxicity in cancer survivors, particularly impacting children, adolescents, and young adults (AYA). The aim of this study was to address persistent knowledge gaps in CIHL by establishing benchmarks for the prevalence and risk factors for CIHL.
METHODS:
Children and AYA patients diagnosed with a cisplatin-treated tumor from children’s cancer centers and consortia located in North America were included. Audiology was centrally reviewed and CIHL graded using the consensus International Society of Pediatric Oncology (SIOP) Ototoxicity Scale. Primary endpoints determined the prevalence of moderate/severe CIHL (SIOP Grade ≥2) among demographic, diagnosis, and treatment groups and their relative contributions to risk for CIHL; secondary endpoints explored associations of cisplatin dose reductions and CIHL with survival.
FINDINGS:
From 1,481 cisplatin-treated patients identified at participating sites, 1,414 had audiometry at latest follow-up. Forty-four percent developed moderate/severe CIHL. Youngest patients (<5 years) and those with a brain tumor, hepatic tumor, or neuroblastoma had the highest prevalence of CIHL (all ≥50%). After accounting for cumulative cisplatin dose, higher fractionated doses were associated with risk for CIHL (for each +10mg/m2/day, adjusted odds ratio [aOR] 1·15, 95%CI [1·07–1·25]; for each +50mg/m2/cycle aOR 2·16 [1·37–3·51]). Vincristine exposure was newly identified as a risk factor for CIHL. Dose reductions and moderate/severe CIHL were not significantly associated with survival differences.
INTERPRETATION:
Using this large multicenter cohort, benchmarks were established for the prevalence of CIHL among cisplatin-treated patient groups. Variations in cisplatin dosing confer additive risk for developing CIHL and warrant investigation as a potential approach to decrease the burden of therapy.
FUNDING:
NIH/NIDCD, NIH/NCI, St. Baldrick’s Foundation, Genome Canada, Genome British Columbia, Canadian Institutes of Health Research, the Canada Foundation for Innovation, University of British Columbia, BC Children’s Hospital Research Institute, BC Provincial Health Services Authority, Health Canada, C17 Research Network.
INTRODUCTION
Therapeutic advances for childhood cancers have improved survival.1 However, greater treatment intensity is often at the expense of increased toxicity.2 Cisplatin is a highly effective chemotherapeutic agent used against a wide range of childhood and adult cancers.3 Unfortunately, cisplatin crosses into the cochlea, often resulting in permanent sensorineural cisplatin-induced hearing loss (CIHL).4 Hearing loss results in neurocognitive deficits along with profound academic, social, and behavioral challenges affecting quality of life.5–8 Younger children may be at particularly high risk for deficits from CIHL as hearing is critical to language development and decreased auditory stimuli broadly affects development of the central nervous system.6
Despite cisplatin’s longstanding incorporation in treatment regimens and the burden of CIHL on cancer survivors, major knowledge gaps remain. Foremost, the prevalence of CIHL is unknown. Reported rates of CIHL following cisplatin exposure vary widely from 1.7–90.1%.9 Risk factors for developing CIHL are not well-characterized; apart from cumulative cisplatin dose, very few risk factors for CIHL have been consistently identified across case series.3,9–14 Identification of populations and risk factors for CIHL from past reports is complicated by studies with relatively small numbers of cisplatin-treated patients, single-institution cohorts, and variability among studies in timing of audiology assessments, methods of audiologic evaluation, and even the definition of hearing loss.9–12,14 Similarly, genomic studies of pediatric CIHL have used relatively small and varied cohorts, regimens, and hearing endpoints. The resultant inconsistent phenotyping has resulted in few genes implicated in CIHL being replicated among studies.13,15 The lack of generalizable data has thus limited our understanding of which patients are at greatest risk for CIHL and hampered investigations into underlying genetic variation.
These knowledge gaps for CIHL have precluded our abilities to develop risk-adjusted approaches to cisplatin delivery and to better target new otoprotective agents, such as sodium thiosulfate (STS).16,17 To overcome limitations from past reporting on CIHL and refine our understanding of who is at risk for ototoxicity, we assembled a large, diverse cisplatin-treated cohort drawn from across the United States and Canada through the use of a common hearing endpoint, the consensus International Society of Pediatric Oncology (SIOP) Boston Ototoxicity Scale.3 Our overarching aim was to establish benchmarks for the prevalence of CIHL in children, adolescents, and young adults and to identify risk factors for CIHL to guide otoprotective interventions. We hypothesized that, as the cochlea is mature at birth before cisplatin exposure,18 the prevalence and severity of CIHL is primarily associated with dosing strategies for cisplatin and concomitant antineoplastic therapies, and not associated with age or other demographic factors.
METHODS
Patient population
All research was approved by the Institutional Review Board (or Research Ethics Board) at each institution and conducted in accordance with Good Clinical Practice. Patient-level data were collected for cisplatin-treated patients from 16 sites through two multicenter consortia in Canada (Canadian Pharmacogenomics Network for Drug Safety19 [patients treated 1984–2015] and the Applying Biomarkers to Minimize Long-Term Effects of Childhood/Adolescent Cancer Treatment [2013–2017])20 and from three independent children’s cancer centers in the United States (U.S.): Children’s Hospital Los Angeles (2003 to 2016), Oregon Health & Science University Pediatric Hospital (1995 to 2017), and the Vanderbilt Reach for Cancer Survivorship Program (1991 to 2009) (see Appendix, p.2). Eligibility for inclusion in the cohort was a cancer diagnosis during childhood or during adolescence and young adulthood (AYA, defined as 15–39 years of age),21 treatment with the drug cisplatin, availability of cisplatin dosing information, and primary audiology data for central review. A uniform data collection instrument was designed for this study and used to collate data input across all sites. Demographics, cancer diagnosis, treatment regimen, ventriculoperitoneal shunting (VPS), other antineoplastic therapy (vincristine exposure, carboplatin exposure, cranial radiation [cRT] and dose [Gy], stem cell transplantation or rescue [SCT]), and outcome (disease progression, vital status) were collected. Documented cisplatin dosing was extracted from individual patient records and included total cumulative cisplatin dose delivered (mg/m2), prescribed cisplatin dose per cycle (mg/m2), prescribed dose per day (mg/m2), infusion time (hours), dose reduction, dose-reduction reason (hearing loss, renal toxicity, other), and use of otoprotective agents. Patients receiving an otoprotectant were excluded from analyses of audiology outcomes.
Audiology assessments
All patients received serial audiometry as routinely performed during and after chemotherapy. Baseline audiometry results were not collected. Audiometry consisted of ear-specific audiograms at minimum in the frequencies 2000–8000 Hz. Tympanometry was routinely used to assess middle ear dysfunction. Criteria for bone-conduction measurements varied by site but were generally performed up to 4000Hz for hearing loss >20 dB and/or when middle ear dysfunction was present. Soundfield audiometry or frequency-specific tone-burst auditory brainstem responses were substituted where ear-specific behavioral testing was not feasible. All audiology results were centrally reviewed by study investigators from each site who assigned a SIOP grade (Appendix p.3).4,22 Where discrepancies between ears were present, grading conservatively reflected hearing in the better ear. Moderate or severe hearing loss typically associated with communication impairment sufficient to require audiology intervention (i.e., hearing aid, cochlear implant) was defined as a SIOP grade of 2 or greater as previously described.4 Hearing was assessed at end of therapy (EOT) and at most recent post-treatment follow-up (latest follow-up, LFU). The EOT audiology assessment was defined as the first audiometry following all cisplatin doses and completion of the first treatment attempt, or for those receiving SCT, before SCT. Prescription for hearing aids was assessed at LFU as a distinct measure of CIHL as this determination is based upon a variety of factors interpreted by the audiologist including cognition, vision, other comorbidities, and family input.
Statistical approach:
The primary endpoint for the study was prevalence of moderate/severe CIHL at LFU; key secondary endpoints were prevalence of CIHL at EOT, and associations of cisplatin dosing and CIHL with progression-free survival (PFS) or overall survival (OS). Prevalence of moderate/severe CIHL was calculated within demographic and treatment variables. To understand the impact of age during cisplatin exposure, age at diagnosis was used for all analyses. Univariable logistic regression was used to test each independent variable on audiology outcomes. Multivariable logistic regression models were constructed to evaluate treatment exposures as direct candidate risk factors for the endpoint of moderate/severe CIHL present at EOT or at LFU and for recommendation for hearing aids. Serial models were constructed evaluating progressively granular aspects of cisplatin delivery, in the order of cumulative dose, dose per cycle, dose per day, and dose rate per hour. Significance of cisplatin administration parameters adjusted for covariables in these nested models was evaluated using likelihood ratio tests. All analyses at time of LFU controlled for duration from diagnosis. Carboplatin exposure and SCT were excluded from the EOT analyses because SCT, often inclusive of myeloablative carboplatin, by definition occurred only following EOT. PFS was defined as time from diagnosis to LFU or disease progression (as determined by the treating physician), relapse, or death. OS was calculated as time from diagnosis to LFU or death. Kaplan-Meir curves were constructed for OS and PFS, stratified by underlying malignancy, and compared using Wald tests of the restricted mean survival time (RMST) ratio with Greenwood plug-in variance.23 Univariable Cox regression was used to test each independent variable on survival outcomes. Multivariable Cox models stratified on malignancy were constructed to examine the association of treatment exposures and cisplatin delivery with PFS or OS. Individual cisplatin dosing parameters were imputed only for those patients with partially missing cisplatin data using documented cisplatin information for that patient and only if cisplatin dosing parameters could reasonably be estimated from conventional treatment regimens. If all cisplatin dosing information was not available, the patient was excluded from the cohort as per above eligibility. Sensitivity analysis excluding patients with imputed data showed no significant differences in the models for primary and secondary hearing endpoints. Secondary analyses explored risk factors of moderate/severe CIHL in subsets of those with or without cRT and in those without an underlying central nervous system (CNS) malignancy. All statistical tests were two sided with a significance level of 0·05 and performed with R software version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
Funding by sponsors was limited to providing for time and effort to collect and analyze data. All authors had access to the data for the study and accept responsibility for publication.
RESULTS:
Description of cohort
Of 1,549 cisplatin-treated patients identified by sites, 1,481 met eligibility criteria for inclusion in at least one analysis (Appendix p.2). The resulting cohort was representative of the overall treatment population, including common cisplatin-treated childhood and AYA tumors, proportional inclusion of AYA patients,21 and those of Hispanic ethnicity (Table 1). Patients who were black were underrepresented in the cohort. Cumulative delivered cisplatin doses ranged between 50–1,600 mg/m2 (mean cumulative dose 410 mg/m2 [standard deviation [SD] 150]), were divided among 1–12 cycles (mean cycle dose 115 mg/m2 [SD 40]), delivered between 1–5 days/cycle (mean daily dose 69 mg/m2 [SD 29]), and over 0·5–24 hours (mean dose rate 22·3 mg/m2/hour [SD 13·8]). Documentation of presence or absence of dose reductions were recorded for 773/1,481 (52%) of patients; of these, 190/773 (25%) had cisplatin dose reduced or eliminated during therapy. Hearing loss was the most common reported reason for dose reduction (n=120) followed by marrow suppression/other (n= 52), and nephrotoxicity (n=18). Use of an otoprotectant was rare (20/1,481, 1%).
Table 1:
Summary of demographics and risk factors
| Total N=1481 |
|
|---|---|
| Sex | |
| Female | 646/1481 (44%) |
| Male | 835/1481 (56%) |
| Age at diagnosis, years | |
| <5 | 644/1480 (44%) |
| ≥5 to <15 | 630/1480 (43%) |
| ≥15 | 206/1480 (14%) |
| Race | |
| White | 867/1481 (59%) |
| Black | 35/1481 (2%) |
| Asian | 126/1481 (9%) |
| Other/unknown | 453/1481 (31%) |
| Ethnicity | |
| Non-Hispanic | 928/1481 (63%) |
| Hispanic | 381/1481 (26%) |
| Unknown | 172/1481 (12%) |
| Underlying malignancy | |
| Central nervous system | 457/1481 (31%) |
| Germ cell tumor | 207/1481 (14%) |
| Hepatoblastoma | 177/1481 (12%) |
| Neuroblastoma | 258/1481 (17%) |
| Osteosarcoma | 297/1481 (20%) |
| Other | 85/1481 (6%) |
| Vincristine exposure | |
| No | 673/1481 (45%) |
| Yes | 785/1481 (53%) |
| Unknown | 23/1481 (2%) |
| Cranial radiation therapy dose, Gy | |
| 0 | 1078/1454 (74%) |
| >0 to ≤50 | 56/1454 (4%) |
| >50 | 320/1454 (22%) |
| VP shunt | |
| No | 658/1481 (44%) |
| Yes | 120/1481 (8%) |
| Unknown | 703/1481 (47%) |
| Stem cell transplant | |
| No | 1046/1481 (71%) |
| Yes | 265/1481 (18%) |
| Unknown | 170/1481 (11%) |
| Carboplatin exposure | |
| No | 637/1481 (43%) |
| Yes | 219/1481 (15%) |
| Unknown | 625/1481 (42%) |
Prevalence of CIHL
In the cohort, 1,280/1,481 (86%) and 1,414/1,481 (96%) of patients underwent audiology assessment at EOT and at LFU, respectively. A mean of 3·9 years (SD 4·2) elapsed from diagnosis to the LFU audiology assessment, and 1·5 years (SD 2·3) between EOT and LFU assessments; 132/1,414 (9%) of audiology assessments at LFU occurred ≥10 years from diagnosis. Moderate/severe CIHL was present in 505/1,280 (39%) of patients by EOT and in 624/1,414 (44%) at LFU. Audiologists recommended hearing aids for 364/1,414 (26%) of patients at LFU. SIOP grade at LFU was associated with hearing aid recommendation (Grade ≥2 versus Grade <2, 346/570 [61%] vs. 18/733 [2%], p<0.0001). At LFU, CIHL was most common in the youngest children (Figure 1A) with decreasing prevalence at older ages. Over half of patients treated with cisplatin for CNS tumors, hepatic tumors, and neuroblastoma had moderate/severe CIHL at LFU, as compared to less than a third of patients treated with cisplatin for germ cell tumors, osteosarcoma, or other cancers (Figure 1B).
Figure 1: Prevalence of moderate or severe cisplatin-induced hearing loss by age and underlying malignancy.
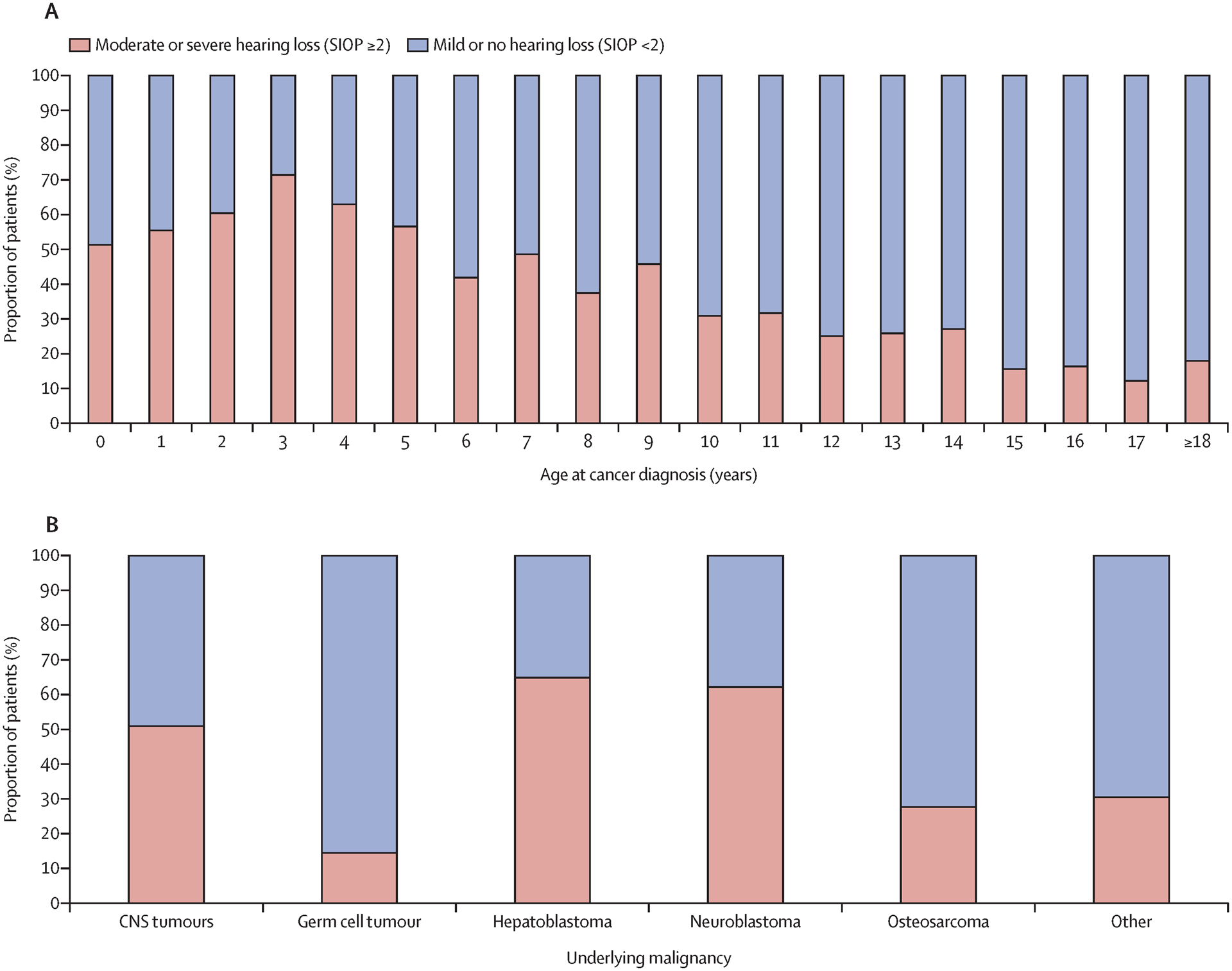
Prevalence of hearing loss at latest audiometry by age at cancer diagnosis in years (A) and underlying malignancy (B). χ2 tested for differences by age in part A and by category in part B; p<0.001 in both. SIOP=International Society of Pediatric Oncology Ototoxicity Grade (0–4).
Risk factors for developing CIHL during or after therapy
In multivariable analysis (Table 2), risk for moderate/severe CIHL at EOT and at LFU increased by 10–20% for every 100 mg/m2of cumulative cisplatin dose delivered (adjusted odds ratio [aOR] 1·11, 95% Confidence Interval [95%CI] 1·01–1·23; and aOR 1·19, 95%CI 1·08–1·31, respectively). Differences in cisplatin dosing also conferred additive risk for moderate/severe CIHL at LFU, from cisplatin dose per cycle (for each +50 mg/m2/cycle, aOR 2·16, 95%CI 1·37–3·51) and cisplatin dose per day (for each +10mg/m2/day, aOR 1·15, 95%CI 1·07–1·25). Increasing the cisplatin infusion rate (i.e., shorter infusion) decreased the risk for moderate/severe CIHL (+1mg/m2/hour, aOR = 0·98, 95%CI 0·95–1·00). For the corresponding endpoint of hearing aid recommendation at LFU, dose/cycle and dose/day remained significant (Appendix, p. 4). Analysis of cisplatin administration parameters and risk for moderate/severe CIHL at EOT showed dose/cycle was similarly significant (Table 2). From the non-cisplatin related treatment variables, use of SCT and carboplatin exposure were associated with risk of moderate/severe CIHL at LFU. Underlying malignancy, VPS, and high doses of pre-cisplatin cRT (>50 Gy) were associated with moderate/severe CIHL at EOT, but not at LFU. Vincristine exposure was associated with a greater than two-fold increased risk of moderate/severe CIHL at both time points; this finding was confirmed in subset analyses of patients treated with cRT (aOR 4·58, 95%CI 1·43–16·63), without cRT (aOR 3·36, 95%CI 1·86–6·17), and in patients with non-CNS tumors (aOR 2·83, 95%CI 1·78–4·53) (Appendix, pp. 5–6).
Table 2:
Association of therapy approach with moderate/severe cisplatin-induced hearing loss at end of therapy and at latest follow-up
| End of therapy2 | Latest follow-up2 | |||||
|---|---|---|---|---|---|---|
| N | aOR1 [95%CI] | p-value | N | aOR1 [95%CI] | p-value | |
| Vincristine exposure | ||||||
| No | 513 | Ref | 566 | Ref | ||
| Yes | 602 | 2·58 [1·60, 4·22] | 0·00012 | 679 | 3·55 [2·19, 5·84] | <0·0001 |
| Unknown | 14 | 1·36 [0·36, 4·69] | 0·63 | 16 | 2·08 [0·86, 9·34] | 0·087 |
| CRT dose, Gy | ||||||
| 0 | 842 | Ref | 923 | Ref | ||
| >0 to ≤ 50 | 51 | 1·50 [0·70, 3·23] | 0·29 | 53 | 1·09 [0·50, 2·39] | 0·83 |
| >50 | 236 | 2·08 [1·20, 3·64] | 0·0096 | 285 | 1·28 [0·72, 2·24] | 0·40 |
| VP shunt | ||||||
| No | 437 | Ref | 517 | Ref | ||
| Yes | 85 | 1·92 [1·11, 3·36] | 0·021 | 104 | 1·59 [0·94, 2·73] | 0·087 |
| Unknown | 607 | 1·62 [1·16, 2·26] | 0·0046 | 640 | 1·13 [0·74, 1·70] | 0·58 |
| Stem cell transplant | ||||||
| No | 882 | Ref | ||||
| Yes | 246 | 0·94 [0·59, 1·49] | 0·78 | |||
| Unknown | 133 | 2·15 [1·13, 4·11] | 0·020 | |||
| Carboplatin exposure | ||||||
| No | 566 | Ref | ||||
| Yes | 199 | 2·13 [1·34, 3·42] | 0·0016 | |||
| Unknown | 496 | 0·79 [0·50, 1·23] | 0·30 | |||
| Time from diagnosis to audiogram, years | 1261 | 1·11 [1·07, 1·15] | <0·0001 | |||
| Cisplatin dosing parameters | ||||||
| Cisplatin total cumulative dose, +100 mg/m2 |
1129 | 1·11 [1·01, 1·23] | 0·037 | 1261 | 1·19 [1·08, 1·31] | 0·00064 |
| Cisplatin prescribed cycle dose, +50 mg/m2/cycle |
1129 | 1·73 [1·12, 2·76] | 0·017 | 1261 | 2·16 [1·37, 3·51] | 0·0012 |
| Cisplatin prescribed daily dose, +10 mg/m2/day |
1129 | 1·08 [1·00, 1·17] | 0·068 | 1261 | 1·15 [1·07, 1·25] | 0·00046 |
| Cisplatin dose rate, +1 mg/m2/hour |
1129 | 0·98 [0·95, 1·00] | 0·082 | 1261 | 0·98 [0·95, 1·00] | 0·048 |
aOR = adjusted Odds Ratio: multivariable model includes independent variables as depicted and is additionally adjusted for sex, age at diagnosis, race, ethnicity, and underlying malignancy (central nervous system, germ cell tumor, hepatoblastoma or hepatocellular carcinoma, neuroblastoma, osteosarcoma, other).
Cisplatin dosing parameters were evaluated in a series of a series of nested regression models including progressively more granular dosing (Model 2: +cumulative dose; Model 3: +cumulative dose, cycle dose; Model 4: +cumulative dose, cycle dose, daily dose; Model 5: +cumulative dose, cycle dose, daily dose, dose rate); Likelihood Ratio Tests compared each model at EOT (Base Model vs. Model 2, p= 0·051; Models 2 vs. 3, p= 0·096; Models 3 vs. 4, p= 0·28; Models 4 vs. 5, p= 0·067) and at latest follow-up (Base Model vs. Model 2, p= 0·0015; Models 2 vs. 3, p= 0·0053; Models 3 vs. 4, p=0·0031; Models 4 vs. 5, p= 0·037). VP shunt= ventriculoperitoneal (or other ventricular) shunt. CRT= cranial radiotherapy.
Progression-free and overall survival
After stratifying by underlying malignancy, no differences in PFS or OS were present between patients with and without documented cisplatin dose reductions (Figure 2A–B, Appendix pp.7–8) nor when compared within each underlying malignancy (Appendix, pp. 9–10). However, in disease-stratified multivariable Cox models for PFS and OS including all patients (i.e., even those with unknown dose-reductions), increasing cumulative cisplatin dose delivered was protective against death (+100mg/m2, hazard ratio [HR] 0·85, 95%CI 0·75–0·97) though not against disease progression (+100mg/m2, HR 0·92, 95%CI 0·83–1·02) (Appendix, pp.11–12). In examining CIHL as a surrogate marker of chemotherapy tumor efficacy, there was no difference in PFS or OS between patients who developed moderate/severe CIHL at EOT versus those who did not (Figures 2C–D, Appendix, pp. 13–14).
Figure 2: Association of cisplatin dose reduction or cisplatin-induced hearing loss with survival.
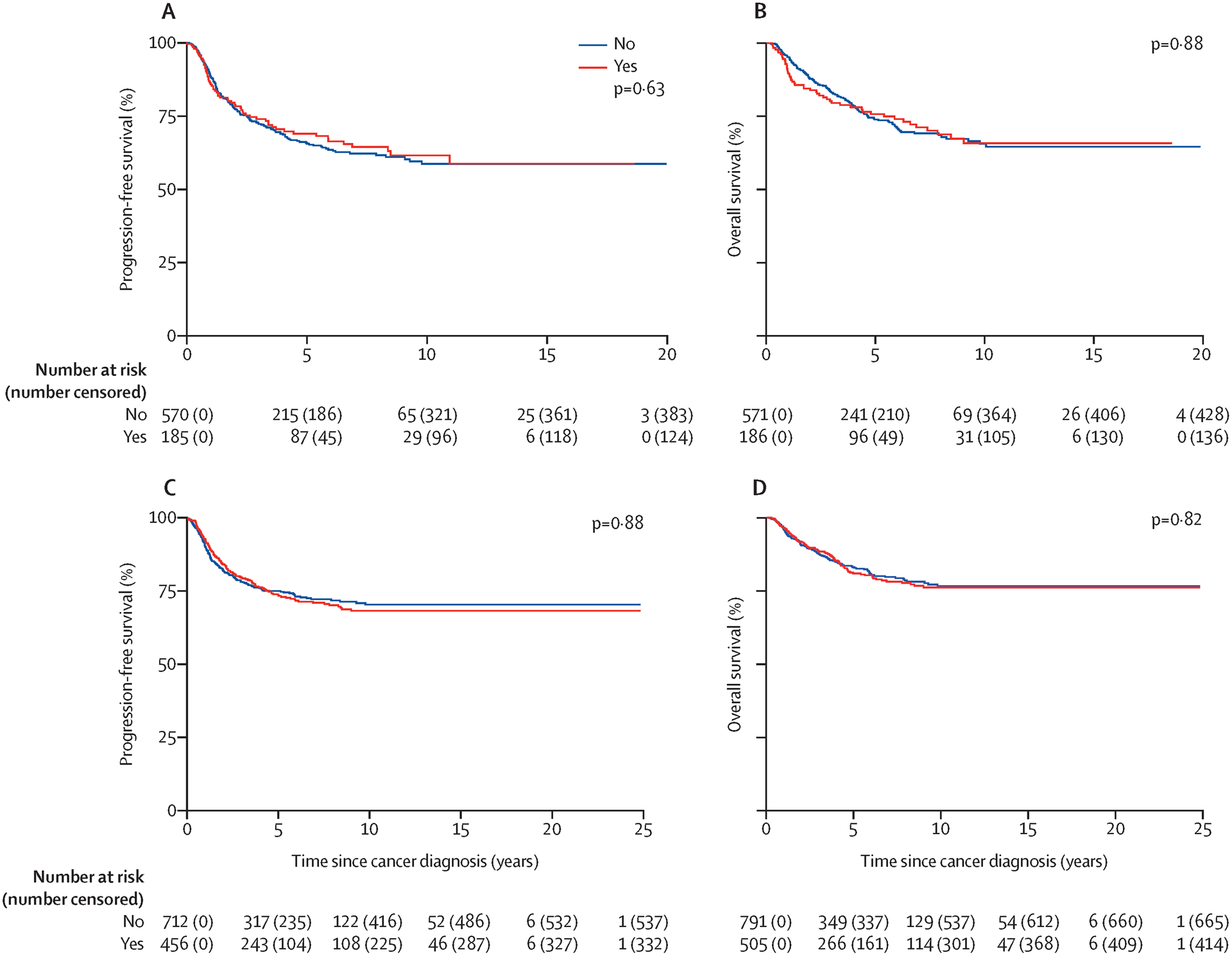
Effect of dose reduction on progression-free survival (A) and overall survival (B). No significant difference in progression-free survival or overall survival in those with dose-reductions of cisplatin versus those receiving planned dosing. Effect of moderate or severe hearing loss at end of treatment on progression-free survival (C) and overall survival (D). No significant difference in progression-free survival or overall survival in those with moderate or severe hearing loss at end of therapy versus those with mild or no hearing loss. Significance assessed using restricted mean survival time.
DISCUSSION
Prior reports describing CIHL in children and adolescents have been limited to small cohorts, and the risk for and burden of CIHL have thus remained uncertain. In this study, we establish benchmarks for the prevalence and risk factors for moderate/severe CIHL using a large and robust cohort of children and AYAs treated with cisplatin-containing regimens. Historically, cumulative cisplatin dose has been thought to be the predominant dose-dependent risk factor for CIHL. However, this study clearly shows for the first time that, while cumulative dose is important, how cisplatin is administered confers a significant and additive impact on development of CIHL. Higher daily and cycle doses of cisplatin, such as those used in regimens to treat liver tumors, neuroblastoma, and CNS tumors, increased the risk for CIHL above that conferred by cumulative dose alone. Incidence of CIHL in the cohort was associated with diagnosis and inversely related to age. After adjusting for host and disease, risk for CIHL remained associated with differences in cisplatin dosing and exposure to other therapies. Together, these findings identify patient populations at greatest risk for the debilitating effects from CIHL, and thus those who may benefit most from otoprotective strategies and agents.16,24,25
Along with cumulative cisplatin dose, our study found that CIHL is positively associated with dosing intensity of cisplatin regimens. Our results suggest this may be secondary to higher circulating levels of free (unbound) cisplatin. Population pharmacokinetic models of cisplatin elimination have demonstrated clearance of unbound cisplatin inversely correlates with dose.26 We hypothesize that higher daily and cycle doses may increase cochlear drug delivery through saturation of active and passive transport mechanisms into the cochlea. Once in the cochlea, cisplatin accumulates and is retained, particularly following repeated high doses.27 Tumor cytotoxicity is thought to occur primarily from initial cisplatin exposure;28 retained cisplatin therefore likely only contributes to long-term toxicity. Circulating cisplatin has been detected in the plasma of survivors even decades after therapy, and these retained cisplatin levels correlate with toxicities.29 Further investigation is warranted into potential interventions to reduce CIHL by altering cisplatin dosing intensity with or without the introduction of otoprotective agents (e.g., STS) while maintaining the cytotoxicity and curative potential of cumulative dosing.
Additional risk factors for developing early moderate/severe CIHL during therapy (i.e., prior to the EOT timepoint) were exposure to preceding cRT and/or placement of a VPS at diagnosis. However, these factors did not independently convey additional risk for CIHL at the later LFU audiology assessments. This suggests that cRT and VPS (likely as a surrogate for changes in intracranial pressure11) may accelerate cochlear damage from cisplatin, but their additive effects are attenuated over time as cisplatin accumulates in the cochlea and eventually causes damage even in non-irradiated and non-shunted patients. In addition, vincristine was newly identified as a potential ototoxic chemotherapy agent that considerably increased the risk of CIHL. In patients with CNS tumors receiving cRT, vincristine is often used as a radiation-sensitizer to improve cytotoxicity of cRT on the tumor; here, we identified the off-target effect of this approach on the cochlea. Patients receiving vincristine and cRT were at more than four-fold risk for CIHL versus those receiving cRT without vincristine exposure. Moreover, the effect of vincristine was found not merely to be exacerbating cRT. Even in patients without CNS tumors or cRT exposure, vincristine was found to increase the risk of hearing loss over two-fold. While vincristine is known to be neurotoxic, and isolated cases reports have ascribed ototoxicity to the chemotherapy class of vinca alkaloids,15,30 these findings reveal the ototoxic potential of vincristine, one previously obscured from its use primarily in multiagent and multimodal regimens. As the data for vincristine in the study was limited to exposure only (i.e., yes/no), further research is necessary to better delineate the influence of vincristine dosing and frequency to characterize vincristine’s pathophysiology and ototoxic potential.
In the exploratory survival analyses, after accounting for underlying malignancy, we did not find that PFS or OS differed between those with documented cisplatin dose reductions and those without. Although retrospective data must be interpreted with caution, for practitioners seeking to balance toxicity and cure, this is reassuring that it might be possible to incorporate necessary dose-reductions for toxicity without adversely impacting survival. However, in multivariable analysis, higher cumulative cisplatin dose delivered remained protective for survival, a confounding finding at odds with documented dose-reductions. While one might hypothesize this discrepancy was due to increased cisplatin exposure and/or cytotoxic susceptibility in those patients developing toxicity, we found that the development of CIHL during therapy was also not positively associated with higher survival in our dataset. This further suggests that CIHL represents an off-target effect independent from cisplatin’s tumor cytotoxicity and that host susceptibility to toxicity from cisplatin does not reflect tumor sensitivity. With management recommendations widely varying for cisplatin dosing when confronted by treatment toxicity, these findings highlight the absence of clear data to guide clinical decision making and the need for prospective investigation into the optimal management of cisplatin-associated toxicities during therapy.
This study has multiple strengths. Foremost, as cisplatin-treated pediatric tumors are rare, this cohort represents the first study with a sufficiently large population to study the detailed impact of cisplatin in children and AYAs. Studying patients treated at multiple cancer centers spread across Canada and the United States promotes the generalizability of these findings to aid both oncologists and audiologists in complex treatment discussions with patients and families. The use of centrally reviewed audiology, along with a uniform, consensus audiology endpoint, was crucial to ensure the validity of the audiology outcomes and to enable cross-center comparisons. This study fills a knowledge gap resulting from the use of different grading scales (Munster, Brock, Chang, U.S. National Cancer Institute Common Technology Criteria for Adverse Events)12,14,22 and definitions of CIHL among previous studies. Finally, our ability to study survival outcomes provides insights for providers seeking to balance the risk for CIHL with goals of cure as well as the basis for hypotheses to investigate how to optimize this balance within cisplatin-dosing regimens.
These strengths were, of necessity, balanced by several limitations. The rarity of these tumors necessitated including patients treated over a wide range of years, though of note, cisplatin administration and associated supportive care has not changed meaningfully during the included treatment era. As commonly used for pediatric cancer trials reporting on CIHL,4,12,14,17 our study relies on cross-sectional assessment of post-therapy audiometry. Pre-treatment bilateral hearing loss is rare in children and is therefore unlikely to impact rates of CIHL in this large cohort, but without baseline audiometry, it is possible small numbers of patients with preexisting hearing loss were included. Similarly, as data was collected from routine audiometry performed during therapy, details for the specific equipment, calibration, and techniques used were not available, nor was a single testing battery used across all sites. While testing was performed and reviewed by pediatric audiologists, there is likely some variation in methodology and equipment. Second, some patients required partial imputation of prescribed cisplatin dosing by the recorded treatment regimen; however, all patients had some dosing data available, data was imputed only for known regimens, and no differences were found in sensitivity analyses excluding these data. With data gathered across so many sites, detailed dosing data for concomitant potential ototoxic agents (e.g., furosemide, aminoglycosides, etc.) were also not available. Specific details for VPS and increased intracranial pressure at diagnosis of CNS tumors were not reported, thus precluding our ability to evaluate their impact on CIHL directly. As per above, some patients with VPS may also have had preexisting hearing loss not attributable to cisplatin. Comparison of audiology outcomes in those with and without a CNS tumor (or VPS) suggest patients with VPS are nonetheless at increased risk for CIHL. Prospective study to understand the etiology and course of VPS-associated hearing loss is essential to determine how best to address this additive risk factor. Finally, while dosing parameters are a logical surrogate to approximate relative differences in drug levels in the blood, direct measurement of cisplatin pharmacokinetics were not available. Despite these limitations, this study provides the largest and most comprehensive assessment of host and treatment-related risk factors to date for CIHL in children and AYA patients. These findings not only identify those continuing to be at greatest risk for CIHL, but also suggest potential avenues to investigate different dosing regimens to limit CIHL while preserving efficacy for cure. Establishing these detailed phenotypic data for CIHL now provides the foundation for combining clinical factors with the emerging genetic framework of CIHL as the next step to predict, prevent, and alleviate this comorbidity in cisplatin-treated cancer survivors.
Supplementary Material
RESEARCH IN CONTEXT:
Evidence before this study:
Cisplatin-induced hearing loss (CIHL) is a debilitating toxicity commonly experienced by cancer survivors. The majority of reports on the burden of and risk factors for CIHL are limited by small numbers, single institution cohorts, variability in grading and defining hearing loss, and conflicting results. A Pubmed search without language restrictions from 1970 through October 2020 using the criteria “hearing loss” AND “cisplatin” AND “pediatric OR childhood” yielded 176 publications. Included in these results was a recent Cochrane systematic review which evaluated the evidence from 1945 through September 2015 for the associations between platinum chemotherapy (cisplatin, carboplatin) in children and CIHL. Results from this database review included 13 cohort studies. The quality of studies was poor, prevalence of CIHL ranged widely, and formal multivariable assessments of risk factors for CIHL were performed in only two of these studies. Since September 2015, we identified an additional eight studies that examined demographic or treatment associated risk factors for CIHL, but only two studies assessed these candidate risk factors using multivariable models. Findings from the two multivariable analyses supported that young age, higher cumulative cisplatin dose, hospitalization for infection, and cotreatment with myeloablative carboplatin or furosemide are independent risk factors for CIHL. However, these studies continued to have limited generalizability secondary to selection criteria, variability in timing of audiology assessments, methods of audiologic evaluation, hearing loss assessment scales, and definitions of hearing loss. Similarly, genomic studies of hearing loss have failed to yield consistent genetic variation associated with CIHL between cohorts due to phenotyping limitations from inconsistent covariables and hearing endpoints. It therefore remains unclear who are most at risk and who might benefit from preventive strategies.
Added value of this study:
This study analyzed data for CIHL from the largest cohort of cisplatin-treated children, adolescents, and young adults to be evaluated by primary audiology data, central review, and a uniform grading scale to determine CIHL. By doing so, benchmarks for prevalence of CIHL were established to guide treatment discussions and future research into CIHL prevention; moderate/severe CIHL was most common (prevalence ≥50%) in young patients exposed to cisplatin (<5 years old), and by diagnosis, in those who were treated for hepatoblastoma, neuroblastoma, or brain tumors. The study newly identified that risk for CIHL transcends cumulative dose alone and demonstrates that how cisplatin is administered confers a significant and additive independent risk for CIHL. Vincristine and male sex were also newly identified as risk factors for CIHL. Exploratory analyses of survival data for the cohort demonstrated that CIHL and cisplatin dose reductions are not clearly associated with differences in relapse or survival, though prospective validation is necessary.
Implications of all the available evidence:
Despite decades of cisplatin use, a major knowledge gap has persisted for which patients are at risk for CIHL. The current study addresses this gap and delineates clear at-risk populations along with the relative contributions from therapy modalities to CIHL risk. This information is essential to guide practitioner discussions, devise treatment plans balancing cure and toxicity, and to provide additional avenues for research into preventing CIHL. Careful consideration should be given to use of available otoprotectants, such as sodium thiosulfate, in patients at greatest risk (i.e., younger than five years receiving regimens at high-risk for CIHL). The large impact of dose delivery on risk for CIHL highlights the necessity of further research into understanding the mechanism and pharmacokinetics of cisplatin ototoxicity to prevent CIHL without sacrificing tumor cytotoxicity.
Acknowledgements:
This work was funded by the NIH/NIDCD (EO: 1K23DC014291); St. Baldrick’s Foundation (DJM); NIH/NCI CL, JM: P30CA014089); Canadian Institutes of Health Research (CIHR) (SRR). CPNDS support: Genome Canada, Genome British Columbia, CIHR, the Canada Foundation for Innovation; University of British Columbia, BC Children’s Hospital Research Institute, BC Provincial Health Services Authority, Health Canada, and C17 Research Network.
Footnotes
Declaration of interests: EO served on an advisory board for Servier Pharmaceutical outside the scope of this work (leukemia). No other authors have disclosures to report.
Data sharing statement:
Deidentified data will be made available for a period of three years from publication date upon reasonable request to the corresponding author (eorgel@chla.usc.edu) with a formal proposal within the scope of the dataset objectives to understand CIHL, review and approval of the submitted proposal by coauthors, and following establishment of a data governance agreement.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD: National Cancer Institute, 2019. [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355(15): 1572–82. [DOI] [PubMed] [Google Scholar]
- 3.Clemens E, van den Heuvel-Eibrink MM, Mulder RL, et al. Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol 2019; 20(1): e29–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 2012; 30(19): 2408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol 2005; 23(34): 8588–96. [DOI] [PubMed] [Google Scholar]
- 6.Gurney JG, Tersak JM, Ness KK, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics 2007; 120(5): e1229–36. [DOI] [PubMed] [Google Scholar]
- 7.Brinkman TM, Bass JK, Li Z, et al. Treatment-induced hearing loss and adult social outcomes in survivors of childhood CNS and non-CNS solid tumors: Results from the St. Jude Lifetime Cohort Study. Cancer 2015; 121(22): 4053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orgel E, O’Neil SH, Kayser K, et al. Effect of Sensorineural Hearing Loss on Neurocognitive Functioning in Pediatric Brain Tumor Survivors. Pediatr Blood Cancer 2016; 63(3): 527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van As JW, van den Berg H, van Dalen EC. Platinum-induced hearing loss after treatment for childhood cancer. Cochrane Database Syst Rev 2016; (8): CD010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushner BH, Budnick A, Kramer K, Modak S, Cheung NK. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer 2006; 107(2): 417–22. [DOI] [PubMed] [Google Scholar]
- 11.Guillaume DJ, Knight K, Marquez C, Kraemer DF, Bardo DM, Neuwelt EA. Cerebrospinal fluid shunting and hearing loss in patients treated for medulloblastoma. J Neurosurg Pediatr 2012; 9(4): 421–7. [DOI] [PubMed] [Google Scholar]
- 12.Landier W, Knight K, Wong FL, et al. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children’s Oncology Group. J Clin Oncol 2014; 32(6): 527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olgun Y, Aktaş S, Altun Z, et al. Analysis of genetic and non genetic risk factors for cisplatin ototoxicity in pediatric patients. Int J Pediatr Otorhinolaryngol 2016; 90: 64–9. [DOI] [PubMed] [Google Scholar]
- 14.Clemens E, de Vries AC, Pluijm SF, et al. Determinants of ototoxicity in 451 platinum-treated Dutch survivors of childhood cancer: A DCOG late-effects study. Eur J Cancer 2016; 69: 77–85. [DOI] [PubMed] [Google Scholar]
- 15.Carleton BC, Ross CJ, Pussegoda K, et al. Genetic markers of cisplatin-induced hearing loss in children. Clin Pharmacol Ther 2014; 96(3): 296–8. [DOI] [PubMed] [Google Scholar]
- 16.Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017; 18(1): 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brock PR, Maibach R, Childs M, et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N Engl J Med 2018; 378(25): 2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall JW. Development of the ear and hearing. J Perinatol 2000; 20(8 Pt 2): S12–20. [DOI] [PubMed] [Google Scholar]
- 19.Ross CJ, Visscher H, Sistonen J, et al. The Canadian Pharmacogenomics Network for Drug Safety: a model for safety pharmacology. Thyroid : official journal of the American Thyroid Association 2010; 20(7): 681–7. [DOI] [PubMed] [Google Scholar]
- 20.McMahon KR, Rod Rassekh S, Schultz KR, et al. Design and Methods of the Pan-Canadian Applying Biomarkers to Minimize Long-Term Effects of Childhood/Adolescent Cancer Treatment (ABLE) Nephrotoxicity Study: A Prospective Observational Cohort Study. Canadian journal of kidney health and disease 2017; 4: 2054358117690338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freyer DR, Felgenhauer J, Perentesis J, Committee CAaYAOD. Children’s Oncology Group’s 2013 blueprint for research: adolescent and young adult oncology. Pediatr Blood Cancer 2013; 60(6): 1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight KR, Chen L, Freyer D, et al. Group-Wide, Prospective Study of Ototoxicity Assessment in Children Receiving Cisplatin Chemotherapy (ACCL05C1): A Report From the Children’s Oncology Group. J Clin Oncol 2017; 35(4): 440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uno H, Tian L, Horiguchi M, Cronin A, Battioui C, Bell J. survRM2: Comparing Restricted Mean Survival Time. 2020. https://CRAN.R-project.org/package=survRM2.
- 24.Freyer DR, Brock P, Knight K, et al. Interventions for cisplatin-induced hearing loss in children and adolescents with cancer. Lancet Child Adolesc Health 2019; 3(8): 578–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freyer DR, Brock PR, Chang KW, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. Lancet Child Adolesc Health 2020; 4(2): 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urien S, Lokiec F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br J Clin Pharmacol 2004; 57(6): 756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breglio AM, Rusheen AE, Shide ED, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nature Communications 2017; 8(1): 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Kareh AW, Secomb TW. A mathematical model for cisplatin cellular pharmacodynamics. Neoplasia (New York, NY) 2003; 5(2): 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in Cisplatin-treated survivors of testicular cancer. J Clin Oncol 2012; 30(3): 300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lugassy G, Shapira A. Sensorineural hearing loss associated with vincristine treatment. Blut 1990; 61(5): 320–1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data will be made available for a period of three years from publication date upon reasonable request to the corresponding author (eorgel@chla.usc.edu) with a formal proposal within the scope of the dataset objectives to understand CIHL, review and approval of the submitted proposal by coauthors, and following establishment of a data governance agreement.


