Abstract
Background.
Global gains toward malaria elimination have been heterogeneous and have recently stalled. Interventions targeting afebrile malaria infections may be needed to address residual transmission. We studied the efficacy of repeated rounds of community-based mass testing and treatment (MTaT) on malaria infection prevalence in western Kenya.
Methods.
Twenty clusters were randomly assigned to 3 rounds of MTaT per year for 2 years or control (standard of care for testing and treatment at public health facilities along with government-sponsored mass long-lasting insecticidal net [LLIN] distributions). During rounds, community health volunteers visited all households in intervention clusters and tested all consenting individuals with a rapid diagnostic test. Those positive were treated with dihydroartemisinin-piperaquine. Cross-sectional community infection prevalence surveys were performed in both study arms at baseline and each year after 3 rounds of MTaT. The primary outcome was the effect size of MTaT on parasite prevalence by microscopy between arms by year, adjusted for age, reported LLIN use, enhanced vegetative index, and socioeconomic status.
Results.
Demographic and behavioral characteristics, including LLIN usage, were similar between arms at each survey. MTaT coverage across the 3 annual rounds ranged between 75.0% and 77.5% in year 1, and between 81.9% and 94.3% in year 2. The adjusted effect size of MTaT on the prevalence of parasitemia between arms was 0.93 (95% confidence interval [CI], .79–1.08) and 0.92 (95% CI, .76–1.10) after year 1 and year 2, respectively.
Conclusions.
MTaT performed 3 times per year over 2 years did not reduce malaria parasite prevalence in this high-transmission area.
Clinical Trials Registration.
Keywords: mass testing and treatment, mass drug administration, malaria transmission reduction, malaria in Kenya, asymptomatic malaria infections
From 2000 to 2015, Plasmodium falciparum infection prevalence halved and the incidence of clinical malaria decreased by 40% in sub-Saharan Africa [1]. These gains have been heterogeneous and in certain settings progress has stalled [2]. The population of individuals with afebrile infections, which represents ≥ 60% of all malaria infections in endemic settings, may contribute substantially to ongoing transmission [3]. These individuals are less likely to seek care at health facilities or to be treated through active fever screening strategies, and may remain infected for prolonged periods, sustaining a human parasite reservoir [3, 4].
Mass drug administration (MDA), where all members of a community are treated with an antimalarial without testing, and mass testing and treatment (MTaT), where all community members are first tested and those with positive test results are treated, are 2 strategies that specifically target afebrile infections. MDA has been implemented or tested on different scales and transmission settings for more than a century. In 1981, a nationwide MDA in Nicaragua reduced P. falciparum incidence rates for up to 7 months [5]. In the Garki Project, conducted in northern Nigeria between 1971 and 1975, indoor residual spraying combined with high-frequency MDA (every 2 weeks during the wet season, and every 10 weeks during the dry season) rapidly reduced P. falciparum prevalence from > 50% to < 1%, and it remained below 5% for the duration of the intervention [6]. However, after withdrawal of these interventions, and in the absence of sustained control measures, parasite prevalence returned to baseline levels within 1 year [6]. Most MDA trials have corroborated these findings of a large, rapid reduction in parasite prevalence with a return to baseline levels within 6 months in the absence of robust malaria preventive services [7].
The availability of sensitive point-of-care rapid diagnostic tests (RDTs) and artemisinin-based combination therapies with a prolonged posttreatment prophylaxis window initiated interest in the evaluation of MTaT for rapid malaria reduction in a moderate- to high-transmission area where sustained malaria control measures were in place. We conducted a cluster randomized controlled trial (RCT) to evaluate the efficacy of MTaT on malaria infection prevalence in an area of high malaria transmission.
METHODS
Study Site
The study was performed within the Kenya Medical Research Institute (KEMRI) and Centers for Disease Control and Prevention (CDC) Health and Demographic Surveillance System (HDSS) in Siaya County, Kenya [8, 9]. Malaria transmission is high and perennial with peak prevalence during May–July and November–December, following the long and short rainy seasons, respectively. In July 2012, the population prevalence of malaria was 30.6% by microscopy, and 80.2% by 18S-nucleic acid sequence–based amplification [10]. In 2013, 55% of individuals with microscopically confirmed malaria infections reported being afebrile in the preceding 2 weeks, and increased with age to > 90% [11].
Following the 2014 Ministry of Health’s long-lasting insecticidal net (LLIN) distribution, 54.4% of households had access (1 LLIN for every 2 household inhabitants) to LLINs [12]; indoor residual spraying has never been conducted programmatically in this area. Artemether-lumefantrine was scaled up as the first-line antimalarial in 2006 [13], and while community case management of malaria was initially implemented in 2013 [14], in 2015, only 3.6% of febrile children aged < 5 years who sought care did so from a community health volunteer (CHV) [12].
Mass Testing and Treatment Design, Procedures, and Evaluation
A detailed description of the study procedures and methodology has been published [9]. In brief, 10 health facilities in the HDSS were purposively selected and adjacent villages within 3 km of each facility were grouped into 3 clusters that were randomly assigned to intervention, control, or future intervention. We decided not to implement a future intervention and the third cluster was merged with the control cluster for a total of 10 clusters per arm (Figure 1). To reduce the impact of parasite migration on the analyses, only individuals residing in compounds within a core area of each cluster, defined as ≥ 300 m from the cluster perimeter, were considered for sampling [15].
Figure 1.
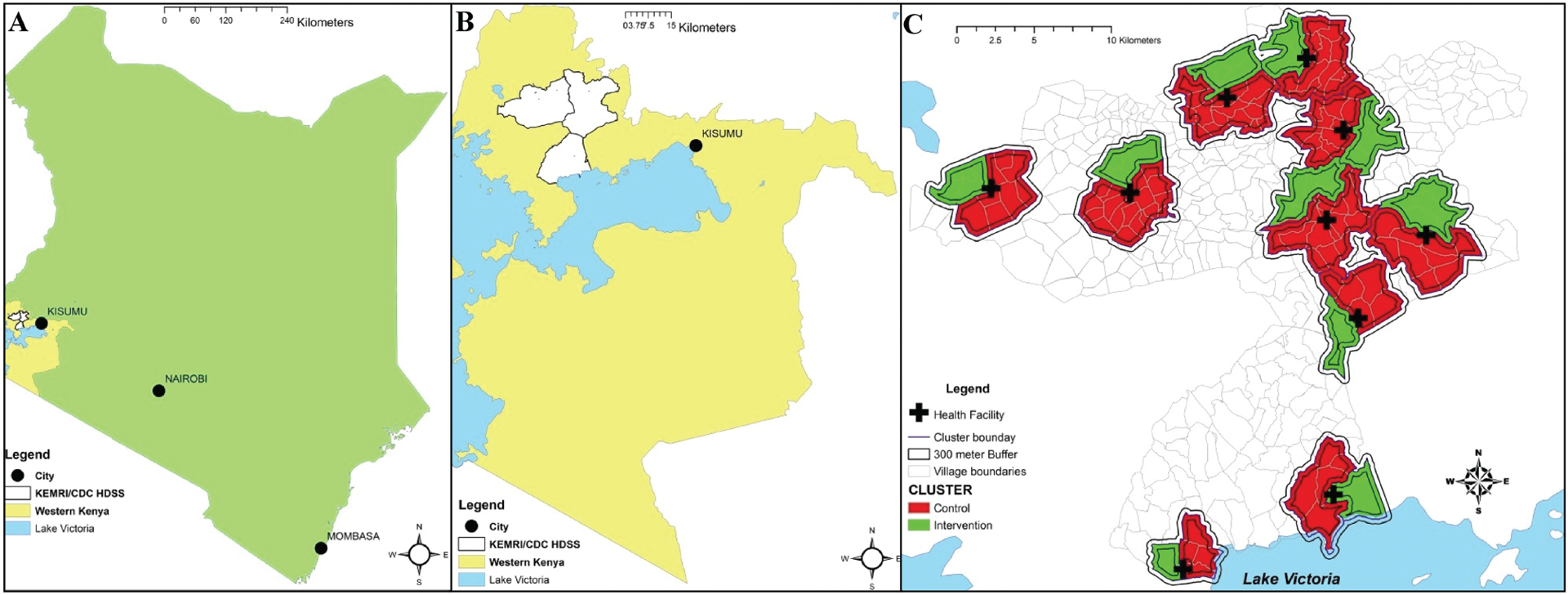
Study site and clusters, including core areas. A, Study site in relation to Kenya. B, Health and Demographic Surveillance System (HDSS) in relation to western Kenya. C, Clusters within HDSS, core areas within clusters, and study health facility location. Figure reprinted from Samuels et al [9] (open access; https://creativecommons.org/licenses/by/4.0/); no changes were made. Abbreviations: CDC, Centers for Disease Control and Prevention; HDSS, Health and Demographic Surveillance System; KEMRI, Kenya Medical Research Institute.
Six rounds of MTaT were performed in the intervention clusters between September 2013 and April 2015 (Supplementary Figure 1). The selection of the number and timing of rounds was informed by a mathematical model [9]. During MTaT rounds, CHVs visited every household in intervention clusters until they had tested each household member ≥ 1 month of age by RDT (Carestart Malaria HRP-2/pLDH [Pf/PAN] Combo Test RDT; Somerset, New Jersey), or made 3 attempts to do so. RDTs were used for MTaT rounds as point-of-care tests are needed for treatment decisions. This RDT was selected because it was the RDT procured and distributed by the Kenya Ministry of Health for use in public facilities and by CHVs at this time, received a positive recommendation by the World Health Organization (WHO), and had a sensitivity of 95%–99% and 99%–100% during WHO testing in samples with 200 parasites/μL and 2000 parasites/μL, respectively [16]. Those positive by RDT were treated with dihydroartemisinin-piperaquine (Eurartesim, Sigma-Tau, Pomezia, Italy; or Duo-Cotecxin, Holley-Cotec, China), selected due to its prolonged posttreatment prophylaxis window, or according to the study algorithm [9]. Dried blood spots were prepared on filter paper for future real time quantitative polymerase chain reaction (qPCR). This manuscript describes the impact of MTaT on malaria prevalence; the impact on malaria incidence is described elsewhere [17].
MTaT Rounds Data
Population coverage of MTaT, adherence to treatment, and in- and out-migration by round have been published previously [17] and are presented in Supplementary Figure 2. In brief, MTaT coverage was defined as follows:
Coverage ranged between 75.0% – 77.5% during year 1 rounds and increased to 81.9%–94.3% in year 2. Test positivity rate across the 6 rounds ranged from 35.6% to 48.6%, and self-reported adherence to treatment courses ranged from 91.5% to 95.4%. In- and out-migration were measured in each household at each round and ranged between 25.1% and 35.9%.
We estimated the number of infections missed by MTaT during rounds due to the limit of detection (LoD) of RDTs (compared to qPCR) and incomplete intervention coverage using previously published data [17]. The equations, assumptions, and results are presented in the Supplementary Methods and Supplementary Table.
Ethical Considerations
The protocol was approved by the KEMRI institutional review board (IRB), the CDC IRB relied on KEMRI for approval, and the Kenya Pharmacy and Poisons Board approved the protocol and importation of Eurartesim. The trial was retrospectively registered at ClinicalTrials.gov (NCT02987270). Written informed consent was obtained from adult participants and parents/guardians of participating children. Additionally, written informed assent was sought for children 13–17 years of age.
Sample Size
The sample size was calculated using Bennett and Hayes’s [18] formula for RCTs assuming a malaria infection prevalence of 40% in the control arm, a type I error rate of 5%, and 80% power to detect a relative difference in malaria prevalence of 50% between arms in the final cross-sectional study. A coefficient of variation of 0.3 for between-cluster and compound variance was used. We performed a simple random sample of compounds, sampling all constituents. Assuming an average compound constituency of 4.5 individuals, we selected 20 compounds per cluster to attain our calculated sample size.
Cross-sectional Community Infection Prevalence Evaluation
Cross-sectional studies were performed annually at peak malaria transmission seasons in July prior to the first round of MTaT in September 2013, and then 2 months after the completion of the last of 3 MTaT rounds in years 1 and 2. CHVs visited the randomly selected compounds from the core areas of each cluster, enrolled all residents ≥ 1 month of age, and administered a questionnaire to each participant or their caregiver. Excepting children ≤ 4 months of age, only data from individuals having lived in the study area for at least 4 months (usual residents) were included in the analyses. Global Positioning System geocoordinates were collected for each compound. CHVs collected a blood sample for RDT to prepare a thick and thin blood smear for microscopy, and RDT-positive individuals were treated according to an algorithm that incorporated age, pregnancy status, and history of drug reaction [9].
Laboratory Procedures
Preparation and examination of blood smears are described in detail elsewhere [9]. In brief, all blood smears were read by 2 microscopists who were blinded to study arm; discordant reads were evaluated by a third blinded microscopist. The procedures for qPCR × have been described elsewhere [19].
Statistical Analysis
Prevalence estimates and 95% confidence intervals (CIs) accounting for clustering at the level of the health facility were calculated using Taylor series linearization [20]. Socioeconomic status (SES) wealth quintiles were assigned using multiple correspondence analysis models from data of household assets collected during cross-sectional surveys [21]. Values for enhanced vegetative index (eVI) were accessed for the 3 months preceding each survey [22] using compound geocoordinates; values associated with the best model fit were assigned to each observation.
The primary outcome was the all-ages effect size of MTaT on P. falciparum infection prevalence by microscopy from the cross-sectional studies between arms for each year with the baseline serving as the reference. The effect sizes were calculated as adjusted ratio of prevalence ratios (aRPRs) with 95% CIs of the exponentiated parameter estimates of the interaction between study arm and year by a log-binomial model using generalized estimating equations to account for clustering at the health facility level [23]. The aRPRs represent a ratio in the change in prevalence from baseline for each of the 2 study arms. A 3-way interaction term between reported net use, study arm, and year was assessed for effect modification. In the absence of evidence of this (P > .05), reported net use was included as a variable in the model along with age (categorized as < 5 years, 5–14 years inclusive, and ≥ 15 years), SES, and eVI [24].
As a planned secondary analysis, we performed age-stratified analyses of the primary outcome, and post hoc secondary analyses of clinical malaria prevalence, defined as microscopically confirmed malaria in the presence of axillary fever ≥ 37.5°C or history of fever in the previous 2 weeks, and clinical malaria as a proportion of all with malaria infection.
RESULTS
Two hundred compounds were randomly selected in each study arm at each of the 3 study surveys. Compound enrollment ranged between 179 and 190 compounds (Figure 2); the coefficient of variation was 0.154. The most common reasons for not enrolling were that the compound was vacant, destroyed, could not be found, or no one was home after 3 visits. Compound head refusals were < 3% in each round. A total of 1927 of 1954 (98.6%), 1912 of 2044 (93.5%), and 1748 of 1849 (94.5%) eligible individuals from selected compounds were enrolled and provided data at each survey.
Figure 2.

Compound and individual study enrollment by survey and arm.
Population Characteristics by Survey and Study Arms
Demographic characteristics, reported history of fever and LLIN use, SES, and eVI were similar between arms at each round (Table 1). Sex and age structures of the sampled population were similar to those of the overall HDSS population in 2012 [25]. Approximately 55% of the population was female, 15% were aged < 5 years, 33% were aged 5–14 years, and 52% were aged ≥ 15 years. Reported LLIN use increased significantly in both arms in year 2 to 87.5% (95% CI, 82.4%–92.6%) vs 87.0% (95% CI, 82.1%–91.9%), after the Ministry of Health sponsored mass LLIN distribution. Reported LLIN use was lowest in those aged 5–14 years and highest in those aged ≥ 15 years.
Table 1.
Population Characteristics by Survey Year and Study Arm
| Characteristic | Baseline | Year 1 | Year 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |||||||
| (n = 1008 [52.3%]) | (n = 919 [47.7%]) | (n = 1016 [53.1%]) | (n = 896 [46.9%]) | (n = 907 [51.9%]) | (n = 841 [48.1%]) | |||||||
| No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | |
| Age (n = 1927) | ||||||||||||
| <5 y | 151 | 15.0 (11.9–18.1) | 152 | 16.5 (13.8–19.2) | 140 | 13.8 (11.3–16.3) | 141 | 15.7 (13.3–18.2) | 136 | 15.0 (12.2–17.8) | 117 | 13.9 (12.7–15.1) |
| 5–14 y | 321 | 31.8 (29.7–34.0) | 297 | 32.3 (27.5–37.2) | 358 | 35.2 (32.7–37.8) | 303 | 33.8 (30.1–37.5) | 289 | 31.9 (28.3–35.4) | 278 | 33.1 (29.3–36.9) |
| ≥15 y | 536 | 53.2 (49.6–56.8) | 470 | 51.1 (46.5–55.8) | 518 | 51.0 (49.3–52.7) | 452 | 50.4 (46.8–54.1) | 482 | 53.1 (49.5–56.8) | 446 | 53 (48.8–57.3) |
| Sex (female) (n = 1927) | 546 | 54.2 (50.7–57.7) | 527 | 57.3 (53.7–60.9) | 575 | 56.6 (53.2–60.0) | 493 | 55.0 (53.1–57.0) | 509 | 56.1 (53.6–58.6) | 449 | 53.4 (51.3–55.5) |
| Reported fever in previous 2 wk (n = 1882) | ||||||||||||
| All ages | 452 | 45.9 (40.5–51.3) | 426 | 47.5 (40.3–54.7) | 451 | 44.4 (39.3–49.5) | 412 | 45.9 (39.2–52.8) | 327 | 36.1 (28.7–43.4) | 314 | 37.3 (33.7–41.0) |
| <5 y | 75 | 49.7 (35.5–63.9) | 85 | 55.9 (44.3–67.5) | 74 | 52.9 (46.0–59.7) | 64 | 45.4 (34.9–55.9) | 69 | 50.7 (36.8–64.7) | 59 | 50.4 (40.9–60.0) |
| 5–14 y | 122 | 39 (33.9–44.0) | 128 | 45.1 (33.2–56.9) | 143 | 39.9 (32.9–47.0) | 140 | 46.2 (38.2–54.2) | 75 | 26.0 (18.9–33.1) | 92 | 33.1 (26.7–39.5) |
| ≥15 y | 255 | 48.9 (42.5–55.3) | 213 | 46.2 (40.5–51.9) | 234 | 45.2 (38.9–51.4) | 208 | 46.0 (39.5–52.5) | 183 | 38.0 (30.4–45.6) | 163 | 36.5 (31.0–42.1) |
| Reported LLIN use the previous night (n = 1865) | ||||||||||||
| All ages | 621 | 63.8 (56.6–71.0) | 563 | 63.1 (54.5–71.8) | 642 | 63.2 (57.5–68.8) | 604 | 67.4 (60.4–74.4) | 789 | 87.0 (82.1–91.9) | 736 | 87.5 (82.4–92.6) |
| <5 y | 97 | 66.4 (52.4–80.5) | 101 | 66.9 (52.7–81.1) | 108 | 77.1 (66.9–87.4) | 94 | 66.7 (56.9–76.4) | 124 | 91.2 (83.0–99.3) | 110 | 94.0 (89.4–98.6) |
| 5–14 y | 161 | 51.6 (40.9–62.3) | 136 | 48.2 (40.7–55.8) | 165 | 46.1 (38.2–54.0) | 181 | 59.7 (47.8–71.7) | 247 | 85.5 (78.1–92.8) | 225 | 80.9 (72.8–89.1) |
| ≥15 y | 363 | 70.5 (63.6–77.4) | 326 | 71.0 (62.1–80.0) | 369 | 71.2 (66.0–76.4) | 329 | 72.8 (65.3–80.3) | 418 | 86.7 (81.6–91.8) | 401 | 89.9 (85.4–94.4) |
| Household wealth quintile (n = 1875) | (n = 1900) | |||||||||||
| 1 (poorest) | 191 | 19.4 (15.0–23.8) | 184 | 20.7 (15.9–25.5) | 211 | 20.9 (13.9–27.9) | 169 | 19.0 (12.6–25.4) | 196 | 21.6 (15.1–28.1) | 152 | 18.1 (11.1–25.1) |
| 2 | 186 | 18.9 (14.6–23.1) | 188 | 21.1 (16.2–26.1) | 200 | 19.8 (14.7–24.9) | 179 | 20.1 (11.8–28.4) | 192 | 21.2 (13.9–28.4) | 158 | 18.8 (12.1–25.5) |
| 3 | 210 | 21.3 (17.2–25.4) | 166 | 18.7 (11.6–25.7) | 190 | 18.8 (12.2–25.5) | 187 | 21 (14.6–27.4) | 181 | 20.0 (11.5–28.5) | 168 | 20.0 (12.3–27.6) |
| 4 | 192 | 19.5 (15.3–23.6) | 183 | 20.6 (16.2–24.9) | 200 | 19.8 (14.5–25.2) | 182 | 20.4 (12.8–28.1) | 184 | 20.3 (14.3–26.2) | 162 | 19.3 (14.4–24.1) |
| 5 (least poor) | 207 | 21 (16.7–25.3) | 168 | 18.9 (12.4–25.4) | 208 | 20.6 (13.1–28.1) | 174 | 19.5 (12.9–26.2) | 154 | 17.0 (10.3–23.7) | 201 | 23.9 (18.6–29.2) |
| eVI (n = 1880) | (n = 1905) | |||||||||||
| 992 | 0.48 (.48–.49) | 888 | 0.48 (.47–.48) | 1014 | 0.39 (.38–.39) | 891 | 0.37 (.37–.38) | 906 | 0.36 (.36–.37) | 836 | 0.35 (.34–.35) | |
Unless otherwise noted, each analysis is conducted with the full sample size from intervention and control arms for that year.
Abbreviations: CI, confidence interval; eVI, enhanced vegetative index; LLIN, long-lasting insecticidal net.
Malaria Microscopy Results
Parasite Prevalence
Parasite prevalence by microscopy did not significantly change in the intervention or control arms across years (Table 2). Parasite prevalence in the intervention and control arms, respectively, was 33.9% (95% CI, 28.0%–39.9%) vs 36.8% (95% CI, 32.0%–41.6%) at baseline; 31.8% (95% CI, 25.8%–37.8%) vs 39.4% (95% CI, 34.2%–44.5%) after year 1; and 29.8% (95% CI, 24.0%–35.7%) vs 36.1% (95% CI, 30.2%–41.9%) after year 2. Prevalence in the 5–14 year age group was consistently highest in each arm and year, ranging from 42.8% to 55.6% and 57.4% to 61.2% in the intervention and control arms, respectively.
Table 2.
Microscopy Results by Survey, Study Arm, and Age Category
| Characteristic | Baseline | Year 1 | Year 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | |||||||
| Tested | % Positive | Tested | % Positive | Tested | % Positive | Tested | % Positive | Tested | % Positive | Tested | % Positive | |
| Blood smear results | ||||||||||||
| All ages | 1008 | 36.8 (32.0–41.6) | 919 | 33.9 (28.0–39.9) | 1016 | 39.4 (34.2–44.5) | 896 | 31.8 (25.8–37.8) | 907 | 36.1 (30.2–41.9) | 841 | 29.8 (24.0–35.7) |
| <5 y | 151 | 43.7 (29.4–58.0) | 152 | 31.6 (17.0–46.2) | 140 | 32.9 (23.3—42.4) | 142 | 31.2 (22.7–39.7) | 136 | 39.0 (30.7–47.2) | 117 | 29.1 (21.4–36.7) |
| 5–14 y | 321 | 57.9 (51.5–64.4) | 297 | 55.6 (43.5–67.6) | 358 | 61.2 (53.6–68.7) | 303 | 49.5 (43.5–55.6) | 289 | 57.4 (47.4–67.5) | 278 | 42.8 (30.6–55.0) |
| ≥15 y | 536 | 22.2 (18.2–26.2) | 470 | 21.1 (18.3–23.9) | 518 | 26.1 (19.9–32.2) | 456 | 20.1 (13.2–27.0) | 482 | 22.4 (16.7–28.1) | 446 | 22.0 (18.0–25.9) |
| Clinical malariaa | ||||||||||||
| All ages | 985 | 14.4 (11.9–17.0) | 897 | 15.2 (11.1–19.2) | 1016 | 15.6 (12.5–18.6) | 896 | 12.6 (10.4–14.8) | 907 | 11.4 (8.8–13.9) | 841 | 10.7 (8.4–13.0) |
| <5 y | 151 | 22.5 (13.5–31.5) | 152 | 21.1 (9.6–32.5) | 140 | 15.0 (8.6–21.4) | 141 | 14.2 (9.2–19.2) | 136 | 20.6 (14.4–26.8) | 117 | 18.8 (11.8–25.8) |
| 5–14 y | 313 | 20.4 (16.4–24.5) | 284 | 22.9 (13.0–32.7) | 358 | 22.1 (15.2–29.0) | 303 | 18.2 (14.7–21.6) | 289 | 12.8 (9.5–16.1) | 278 | 13.3 (9.5–17.1) |
| ≥15 y | 521 | 8.4 (5.8–11.1) | 461 | 8.5 (7.2–9.8) | 518 | 11.2 (5.1–17.3) | 452 | 8.4 (4.6–12.2) | 482 | 7.9 (5.0–10.7) | 446 | 7.0 (4.7–9.2) |
| Clinical malaria among those infected | ||||||||||||
| All ages | 364 | 39.0 (34.9–43.0) | 302 | 45.0 (34.9–55.2) | 400 | 39.5 (34.5–44.5) | 285 | 39.6 (31.6–47.7) | 327 | 31.5 (26.3–36.7) | 251 | 35.9 (31.1–40.6) |
| <5 y | 66 | 51.5 (33.0–70.0) | 48 | 66.7 (51.1–82.2) | 46 | 45.7 (30.9–60.4) | 44 | 45.5 (30.3–60.6) | 53 | 52.8 (37.8–67.9) | 34 | 64.7 (47.6–81.8) |
| 5–14 y | 183 | 35.0 (28.1–41.8) | 158 | 41.1 (26.3–56.0) | 219 | 36.1 (27.3–44.8) | 150 | 36.7 (27.3–46.0) | 166 | 22.3 (16.4–28.2) | 119 | 31.1 (23.9–38.3) |
| ≥15 y | 115 | 38.3 (28.6–47.9) | 96 | 40.6 (32.0–49.3) | 135 | 43.0 (27.1–58.8) | 91 | 41.8 (29.1–54.4) | 108 | 35.2 (25.5–44.6) | 98 | 31.6 (22.0–41.3) |
Parasite densities were log-transformed and are expressed as parasites per microliter. Values in parentheses represent 95% confidence intervals.
Clinical malaria is defined as individuals with a positive blood smear who reported a fever within the previous 2 weeks
Clinical Malaria
The proportion of individuals with clinical malaria did not significantly change in either arm (Table 2). In the intervention arm, clinical malaria was 15.2% (95% CI, 11.1%–19.2%), 12.6% (95% CI, 10.4%–14.8%), and 10.7% (95% CI, 8.4%–13.0%) at baseline, year 1, and year 2, respectively. In the control arm, the prevalence was 14.4% (95% CI, 11.9%–17.0%), 15.6% (95% CI, 12.5%–18.6%), and 11.4% (95% CI, 8.8%–13.9%) at baseline, and after years 1 and 2, respectively.
The crude proportion of individuals with clinical malaria among those infected did not significantly change in either arm across years. In the intervention arm, the proportion was 45.0% (95% CI, 34.9%–55.2%), 39.6% (95% CI, 31.6%–47.7%), and 35.9% (95% CI, 31.1%–40.6%) at baseline, and after years 1 and 2, respectively. In the control arm, the proportion was 39.0% (95% CI, 34.9%–43.0%), 39.5% (95% CI, 34.5%–44.5%), and 31.5% (95% CI, 26.3%–36.7%) at baseline, and after years 1 and 2, respectively.
Effect Size of MTaT
The effect size of MTaT on the primary outcome of all-age malaria microscopy prevalence was nonsignificant after year 1 (aRPR, 0.93 [95% CI, .79–1.1]) and year 2 (aRPR, 0.92 [95% CI, .76–1.1]) (Figure 3). Though the study was not powered for age-stratified evaluations, there was a consistent, though not statistically significant, protective effect of MTaT in the age group 5–14 years (0.85 [95% CI, .68–1.07] and 0.80 [95% CI, .63–1.02] after year 1 and year 2, respectively).
Figure 3.

Effect size of mass testing and treatment on blood smear prevalence, clinical malaria, and clinical malaria as a proportion of malaria. *Sample size insufficient for < 5-year age category. Abbreviations: CI, 95% confidence interval; MTaT, mass testing and treatment; Yr, year.
The effect size of MTaT on the prevalence of clinical malaria was not significant. There was a significant reduction in the proportion of individuals with clinical malaria among those infected with malaria between year 1 and baseline (0.81 [95% CI, .66–.99]); however, there was no effect after 2 years (Figure 3).
Missed Infections
The total number of individuals tested per round ranged between 23 226 and 26 342. We estimated that 12.6%–19.6% and 5.7%–25.0% of the infections were missed due to the LoD of RDTs as compared to qPCR among those tested and due to incomplete coverage by round, respectively (Supplementary Methods and Supplementary Table). Combining these, we estimate that 24.2%–36.9% of all of the infections were missed per round.
DISCUSSION
Despite high levels of community coverage and self-reported adherence to treatment, MTaT did not significantly reduce malaria infection or clinical malaria prevalence over 2 years. Our results are consistent with recent findings from another high-transmission area [26], and support the 2015 WHO Malaria Policy Advisory Committee position not to recommend MTaT with the current LoD of RDTs [27]. Insufficient number of MTaT rounds and suboptimal levels of malaria control interventions likely contributed to the lack of efficacy. Additionally, it is possible that the stability of the artemisinin derivative in dihydroartemisinin-piperaquine could have been compromised during MTaT rounds when carried for days by CHVs [28]. However, we believe that missed infections during rounds and parasite migration from nonintervention to intervention areas were the primary drivers.
Missed Infections
Missed infections are primarily due to the LoD of the diagnostic test used and incomplete coverage during rounds [29]. We estimate that we missed a total of 24.2%–36.9% of all circulating infections in each round; 12.6%–19.6% due to the LoD of RDTs, and 5.7%–25.0% of all infections due to incomplete intervention coverage (Table 1). A minimal cutoff of 80% coverage is suggested for effective rounds [7], which we did not achieve until year 2. However, despite coverage ranging from 81.9% to 94.3% in year 2, there was no evidence of increased efficacy; the aRPRs in year 1 and year 2 to baseline were 0.93 (95% CI, .79–1.08) and 0.92 (95% CI, .76–1.10), respectively. MTaT with ultrasensitive RDTs, which were not available at the time of this study, likely would have reduced the number of missed infections. However, it may be that in areas with high parasite reproductive rates, higher coverage levels with MDA, which in addition to treating all reached infections provides a chemoprophylactic effect on all treated, may be necessary to effectively reduce transmission [30].
Parasite Migration
We attempted to limit the impact of parasite migration on the analyses by selecting compounds from cluster core areas. Epidemiological [31] and entomological [32] data from our study area that indirectly demonstrated the mass effect of a community-based intervention (LLINs) extended to approximately 300-m informed our choice of distance; this distance may have been insufficient.
Additionally, modeling studies have concluded that parasite migration through human mobility is an important factor toward the success of MDA [33]. We found that an average of 31% of the population in our clusters migrated in or out between each round, a large proportion of which likely carried parasites into intervention arms. Additionally, individuals may have been exposed to infectious bites through daily commuting activities to a market, place of work, or school outside the cluster of residence as increased vector biting has been documented in this area in the early evening and late mornings when individuals are unlikely to be under bednets [34–36]. These exposures were unmeasured in our study. Our cluster size (3 villages in the intervention arms) and buffer area may not have been large enough to minimize the impact from these events, and may partially explain the differing results from our trial and one performed in a moderate- to high-transmission setting in Zambia, which found a significant impact of a single year of 3 rounds of MTaT on the prevalence of malaria in children < 5 years of age (adjusted odds ratio [aOR], 0.47 [95% CI, .24–.90]) [37]. There, cluster sizes were much larger (2–3 health facility catchment areas), and rather than a 300-m buffer zone, they had a 5-km buffer [37].
Clinical Malaria and Age-stratified Analyses
The effect size of MTaT on infection prevalence and clinical malaria did not change. These findings were corroborated with those from the incidence cohort (incidence rate ratio [IRR], 0.95 [95% CI, .87–1.04]) and from passive surveillance of clinical malaria at facilities (IRR, 0.79 [95% CI, .61–1.02]) [17]. However, clinical malaria was transiently reduced after the dry season (after rounds 2 and 5; IRR, 0.73 [95% CI, .54–.98] and 0.66 [95% CI, .49–.87], respectively) [17], supporting the importance of timing of rounds in relation to malaria seasonality. Additionally, after year 1 of MTaT, individuals with malaria infection were less likely to report febrile events within the previous 2 weeks (aRPR, 0.81 [95% CI, .66–.99]). While this may be interpreted as MTaT impacting the clinical presentation of malaria, it is difficult to make any conclusions as this finding did not persist after year 1.
We did not power our trial to assess age-stratified effects of MTaT; however, there was a consistent nonsignificant protective point estimate of MTaT (aRPR, 0.80 and 0.85) in the 5–14 age category after each year. This age category harbors the highest prevalence of infection and is the least likely to have clinical malaria and thus seek care when infected, and least likely to report LLIN use the previous night. While a trial of MTaT in Kenya among school-aged children showed a nonsignificant reduction on malaria parasitemia after 12 months of follow-up (aOR, 0.76 [95% CI, .46–1.11]), the authors suggest that this may have been the result of the intervention only being carried out in 2 classes within the school [38]. Our findings suggest that an active approach, rather than an intervention predicated on consistent and repeated behavioral patterns by the end user (such as LLIN use), may be effective in reducing malaria in this age category and could be trialed.
In summary, MTaT utilizing traditional RDTs performed 3 times per year for 2 years in an area of high transmission was not efficacious in reducing the prevalence of malaria infection. This is likely due to several factors including missed infections and the impact of human movement on parasite migration.
Supplementary Material
Acknowledgments.
The authors thank the Siaya County Department of Health and community health volunteers who made this study possible and all of the individuals living within the study area for participating. This manuscript was published with the permission of the Director of the Kenya Medical Research Institute (KEMRI).
Financial support.
This study was made possible by funding from the United States President’s Malaria Initiative (US Agency for International Development [grant number GHN-T-00-06-00001-00]) and the US-based CDC (grant number 5U01GH000048) through a cooperative agreement with the Kenya Medical Research Institute.
Footnotes
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Publisher's Disclaimer: Disclaimer. The findings and conclusions presented in this manuscript are those of the authors and do not necessarily reflect the official position of the US Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World malaria report 2017. Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 3.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 2013; 11:623–39. [DOI] [PubMed] [Google Scholar]
- 4.Ashley EA, White NJ. The duration of Plasmodium falciparum infections. Malar J 2014; 13:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garfield RM, Vermund SH. Changes in malaria incidence after mass drug administration in Nicaragua. Lancet 1983; 2:500–3. [DOI] [PubMed] [Google Scholar]
- 6.Molineaux L, Gramiccia G. The Garki project: research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. Geneva, Switzerland: World Health Organization, 1980. [Google Scholar]
- 7.Newby G, Hwang J, Koita K, et al. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg 2015; 93:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odhiambo FO, Laserson KF, Sewe M, et al. Profile: the KEMRI/CDC health and demographic surveillance system—western Kenya. Int J Epidemiol 2012; 41:977–87. [DOI] [PubMed] [Google Scholar]
- 9.Samuels AM, Awino N, Odongo W, et al. Community-based intermittent mass testing and treatment for malaria in an area of high transmission intensity, western Kenya: study design and methodology for a cluster randomized controlled trial. Malar J 2017; 16:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Mitchell RM, Kariuki S, et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J 2016; 15:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samuels AM, Odero NA, Onyango W, et al. Baseline epidemiological characteristics of participants enrolled in a trial of intermittent mass screening and treatment for malaria in western Kenya [abstract 1503]. In: American Society of Tropical Medicine and Hygiene Annual Conference, New Orleans, LA, 2014. [Google Scholar]
- 12.National Malaria Control Programme (NMCP), Kenya National Bureau of Statistics (KNBS), and ICF International. Kenya malaria indicator survey 2015. Nairobi, Kenya, and Rockville, MD: NMCP, KNBS, and ICF International, 2016. [Google Scholar]
- 13.Amin AA, Zurovac D, Kangwana BB, et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar J 2007; 6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juma PA, Owuor K, Bennett S. Integrated community case management for childhood illnesses: explaining policy resistance in Kenya. Health Policy Plan 2015; 30(Suppl 2):ii65–73. [DOI] [PubMed] [Google Scholar]
- 15.Delrieu I, Leboulleux D, Ivinson K, Gessner BD; Malaria Transmission Blocking Vaccine Technical Consultation Group. Design of a phase III cluster randomized trial to assess the efficacy and safety of a malaria transmission blocking vaccine. Vaccine 2015; 33:1518–26. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs: round 7 (2015–2016). Geneva, Switzerland: WHO, 2017. [Google Scholar]
- 17.Desai M, Samuels A, Odongo W, et al. Impact of intermittent mass testing and treatment on incidence of malaria infection in a high transmission area of western kenya. Am J Trop Med Hyg 2020. doi: 10.4269/ajtmh.19-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 1999; 28:319–26. [DOI] [PubMed] [Google Scholar]
- 19.Mayor A, Serra-Casas E, Bardají A, et al. Sub-microscopic infections and long-term recrudescence of Plasmodium falciparum in Mozambican pregnant women. Malar J 2009; 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binder DA. On the variances of asymptotically normal estimators from complex surveys. Int Stat Rev 1983; 51:279–92. [Google Scholar]
- 21.Traissac P, Martin-Prevel Y. Alternatives to principal components analysis to derive asset-based indices to measure socio-economic position in low- and middle-income countries: the case for multiple correspondence analysis. Int J Epidemiol 2012; 41:1207–8; author reply 1209–10. [DOI] [PubMed] [Google Scholar]
- 22.National Aeronautics and Space Administration. MODIS: moderate resolution imaging spectroradiometer. Available at: https://modis.gsfc.nasa.gov/. Accessed 18 April 2018.
- 23.Yelland LNS, Amy B, Philip R. Relative risk estimation in cluster randomized trials: a comparison of generalized estimating equation methods. Int J Biostat 2011; 7:27. [Google Scholar]
- 24.Weiss DJ, Mappin B, Dalrymple U, et al. Re-examining environmental correlates of Plasmodium falciparum malaria endemicity: a data-intensive variable selection approach. Malar J 2015; 14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kenya Medical Research Institute/Centers for Disease Control and Prevention. Health and Demographic Surveillance System report for 2012. XXX, XXX: KEMRI/CDC, 2013. [Google Scholar]
- 26.Tiono AB, Ouédraogo A, Ogutu B, et al. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J 2013; 12:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Evidence review group meeting report: mass drug administration, mass screening and treatment and focal screening and treatment for malaria. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 28.Jansen FH. The pharmaceutical death-ride of dihydroartemisinin. Malar J 2010; 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuckey EM, Miller JM, Littrell M, Chitnis N, Steketee R. Operational strategies of anti-malarial drug campaigns for malaria elimination in Zambia’s southern province: a simulation study. Malar J 2016; 15:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pemberton-Ross P, Chitnis N, Pothin E, Smith TA. A stochastic model for the probability of malaria extinction by mass drug administration. Malar J 2017; 16:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawley WA, Phillips-Howard PA, ter Kuile FO, et al. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg 2003; 68:121–7. [PubMed] [Google Scholar]
- 32.Gimnig JE, Kolczak MS, Hightower AW, et al. Effect of permethrin-treated bed nets on the spatial distribution of malaria vectors in western Kenya. Am J Trop Med Hyg 2003; 68:115–20. [PubMed] [Google Scholar]
- 33.Gerardin J, Bertozzi-Villa A, Eckhoff PA, Wenger EA. Impact of mass drug administration campaigns depends on interaction with seasonal human movement. Int Health 2018; 10:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayoh MN, Walker ED, Kosgei J, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors 2014; 7:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J 2015; 14:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooke MK, Kahindi SC, Oriango RM, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J 2015; 14:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen DA, Bennett A, Silumbe K, et al. Population-wide malaria testing and treatment with rapid diagnostic tests and artemether-lumefantrine in southern Zambia: a community randomized step-wedge control trial design. Am J Trop Med Hyg 2015; 92:913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliday KE, Okello G, Turner EL, et al. Impact of intermittent screening and treatment for malaria among school children in Kenya: a cluster randomised trial. PLoS Med 2014; 11:e1001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


