Introduction
The COVID-19 vaccination rollout is well underway. While common cutaneous side effects primarily pertain to transient injection site reactions,1 rare cutaneous side effects continue to be reported. Here we describe a case of pityriasis rubra pilaris (PRP) following administration of the AstraZeneca COVID-19 vaccine.
Case report
A 65-year-old Caucasian man presented to the emergency department with erythroderma associated with palmoplantar keratoderma 8 weeks following the first dose of the AstraZeneca ChAdOx1 nCoV-19 Vaxzevria (AZ) vaccine.
The condition began with scattered erythematous plaques over his face 2 days following administration of the AZ vaccine. This condition spread to his chest over the next 3 weeks. Biopsies revealed mild spongiosis, parakeratosis, and a perivascular lymphocytic infiltrate with sparse necrotic keratinocytes. Direct immunofluorescence test result was negative. This presentation was managed as a nonspecific vaccine-related reaction, and the patient was commenced on prednisolone and topical corticosteroids. He had not commenced any new medications, and his long-standing medications, including perindopril, amlodipine, and atorvastatin, were ceased. He had no coryzal symptoms suggestive of an infective prodrome. There was no personal or family history of skin disorders.
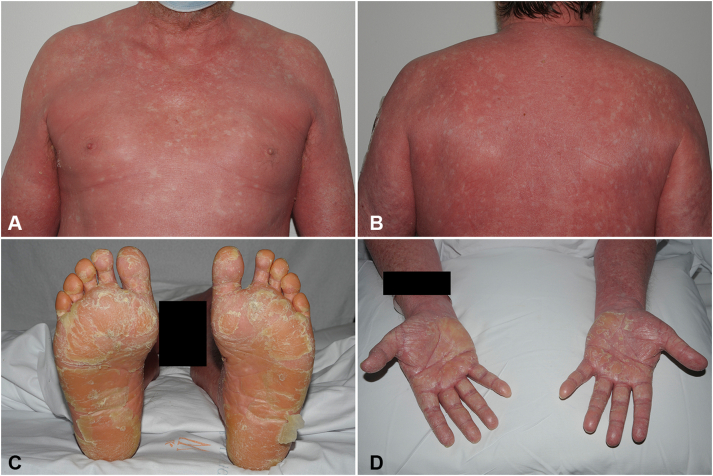
Despite treatment, the eruption spread in a cephalocaudal manner over the subsequent weeks, leading to his presentation at the emergency department 8 weeks following initial vaccine exposure. On examination, he had erythroderma characterized by erythematous-to-orange scaly plaques with islands of sparing, follicular keratotic red-brown papules over both thighs, and waxy red-orange palmoplantar keratoderma with fissuring of the soles (Fig 1). He had splinter hemorrhages and mild onycholysis of his fingernails. The eruption spared his scalp. There was no oral, ocular, or genital mucosal involvement and no lymphadenopathy.
Fig 1.
Pityriasis rubra pilaris clinical presentation. Erythematous-to-orange scaly plaques with islands of sparing over the anterior (A) and posterior (B) aspects of the trunk. C and D, Waxy, red-orange palmoplantar keratoderma. A black box was placed over the right forearm to maintain patient confidentiality.
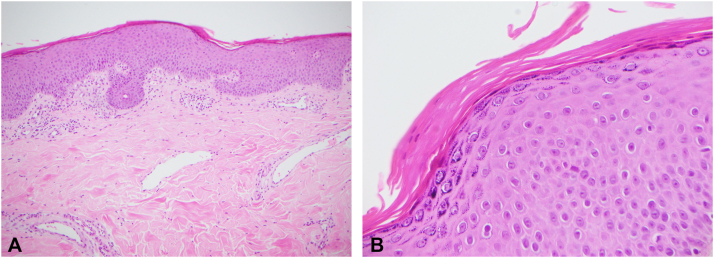
Initial biopsies performed on admission revealed nonspecific features, including focal mild spongiosis and a mild-to-moderate papillary dermal perivascular lymphoplasmacytic inflammatory infiltrate. Due to subsequent high clinical suspicion for PRP, repeat biopsies were performed. Histopathology revealed epidermal thickening with mild psoriasiform hyperplasia, broad rete ridges, and thick suprapapillary plates. There was a prominent granular layer and alternating orthokeratosis and parakeratosis in a horizontal and vertical direction (Fig 2). There were no spongiotic or lichenoid changes and few scattered eosinophils. No atypical lymphocytes or epidermotropism were present. A complete blood cell count, kidney and liver function tests, and lipid profile studies were unremarkable, as was serology for HIV and hepatitis B and C.
Fig 2.
Pityriasis rubra pilaris (PRP) histopathology. Skin biopsy revealed mild psoriasiform hyperplasia and a “checkerboard” pattern typical of PRP, with alternating orthokeratosis and parakeratosis in both the horizontal and vertical directions (A and B, hematoxylin-eosin stain; original magnifications: A, ×10; B, ×40).
The clinical features and histopathology were consistent with a diagnosis of PRP. Given the temporal relationship and lack of known preceding infection or new medication, the proposed trigger for this eruption was the AZ vaccine. Acitretin 25 mg daily was commenced with topical corticosteroids and wet dressings with partial relief of symptoms.
Discussion
To date, 5 known cases of post-COVID-19 vaccination PRP have been reported, following administration of AstraZeneca Vaxzevria,2 AstraZeneca Covishield,3 Pfizer-BioNTech BNT163b24 and BNT162,5 and Moderna 2019-nCoV Vaccine messenger RNA-12735 vaccines (Table I). We present a sixth case of COVID-19 vaccination-induced PRP. Prior to these reports associated with COVID-19 vaccination, only 4 known cases of vaccine-related PRP had been reported, following the measles-mumps-rubella vaccine,6 Vaxigrip Tetra,7 diphtheria-tetanus-pertussis vaccine,8,9 and oral poliovirus vaccine.8
Table I.
Reported cases of pityriasis rubra pilaris following COVID-19 vaccination.
| Case report | Patient demographics | Vaccine type | Vaccine dose | Onset of PRP following vaccine |
|---|---|---|---|---|
| Wada et al, 2022 | 65 y, man | ChAdOx1 nCoV-19 Vaxzevria | 1st | 2 d |
| Llado et al, 20212 | 63 y, woman | ChAdOx1 nCoV-19 Vaxzevria | 1st | 11 d |
| Sahni et al, 20213 | 72 y, man | ChAdOx1 nCoV-19 Covishield | 1st, nil recurrence with 2nd dose | 3 wk |
| Hunjan et al, 20224 | 51 y, man | BNT163b2 (Pfizer-BioNTech) | 1st, worsened following 2nd | 3 d |
| Sechi et al, 20225 | 82 y, woman | Pfizer-BioNTech BNT162 | 1st | 7 d |
| Sechi et al, 20225 | 62 y, woman | Moderna 2019-nCoV Vaccine mRNA-1273 | 1st | 5 d |
d, days; mRNA, messenger RNA; PRP, pityriasis rubra pilaris; wk, weeks.
The 6 patients reported to have PRP following COVID-19 vaccination were aged 51 to 82 years, reflecting the population that has so far received the majority of the vaccines. The time to onset of PRP ranges from 2 days to 3 weeks. Similar to the presentations described by Sahni et al,3 Hunjan et al,4 and Sechi et al,5 our patient developed the typical, erythrodermic findings of classic adult-onset PRP. Llado et al2 described a case of circumscribed PRP, confined to the patient’s palmoplantar and extensor surfaces. Our patient experienced improvement in PRP symptoms over a 3-month period while receiving acitretin treatment. The cutaneous findings of the other cases also improved with acitretin,2,4 subcutaneous methotrexate,5 and oral and topical corticosteroids.3,4 The prognosis of COVID-19 vaccine-related PRP is unknown.
In our case, the clinical progression of the eruption coincided with the histopathologic evolution. Initial biopsies demonstrated features suggestive of a drug eruption, despite the absence of an oral medication trigger. The onset of the rash 2 days after the COVID-19 vaccination and further progression following cessation of all oral medications supports a causal relationship. Ross et al10 stated that the variable cutaneous findings of early PRP and a slower rate of eruption progression can contribute to delayed diagnosis, often necessitating multiple biopsies for histopathologic diagnosis, as was required for our patient. The histopathology of other reported COVID-vaccine-induced PRP cases also showed the typical “checkerboard” pattern of alternating orthokeratosis and parakeratosis.2, 3, 4, 5 From these reports, there seems to be no clear histopathologic difference between vaccine-induced PRP as compared to classical, idiopathic PRP.
It remains unknown, whether subsequent doses of COVID-19 vaccination will cause worsening or recurrence of PRP. Sahni et al3 reported no recurrence following the second dose of the ChAdOx1 nCoV-19 Covishield vaccine; however, Hunjan et al4 described worsening of PRP following the second dose of the Pfizer-BioNTech BNT163b2 vaccine. We recommend avoiding the further doses of the same vaccine, with consideration to receive an alternative.
The COVID-19 vaccines currently in use worldwide have been demonstrated to be extremely safe; however, rare side effects continue to be reported. Our case adds to the existing reports of PRP as a rare complication of COVID-19 vaccination. Early presentations of this eruption may not be associated with typical histologic findings and therefore repeat skin biopsies may be required for histopathologic confirmation of the diagnosis.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Wei T.S., Tam Y.C., Pang S.M. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178–186. doi: 10.1016/j.jdin.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lladó I., Butrón B., Sampedro-Ruiz R., Fraga J., de Argila D. Pityriasis rubra pilaris after Vaxzevria COVID-19 vaccine. J Eur Acad Dermatol Venereol. 2021;35(12):e833–e835. doi: 10.1111/jdv.17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahni M.K., Roy K., Asati D.P., Khurana U. An old entity, a new trigger: post COVID-19 vaccine pityriasis rubra pilaris. Int J Risk Saf Med. 2021;32(4):261–264. doi: 10.3233/JRS-210048. [DOI] [PubMed] [Google Scholar]
- 4.Hunjan M.K., Roberts C., Karim S., Hague J. Pityriasis rubra pilaris-like eruption following administration of the BNT163b2 (Pfizer-BioNTech) mRNA COVID-19 vaccine. Clin Exp Dermatol. 2022;47(1):188–190. doi: 10.1111/ced.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sechi A., Pierobon E., Pezzolo E., et al. Abrupt onset of Sweet syndrome, pityriasis rubra pilaris, pityriasis lichenoides et varioliformis acuta and erythema multiforme: unravelling a possible common trigger, the COVID-19 vaccine. Clin Exp Dermatol. 2022;47(2):437–440. doi: 10.1111/ced.14970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennani B.N., Rouhou H.C., Waton J., et al. Pityriasis rubra pilaris after vaccination. Article in French. Ann Dermatol Venereol. 2011;138(11):753–756. doi: 10.1016/j.annder.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 7.Bitbol-Duneton V., Mikita M., Lebas D., Bularca S., Wiart T., Modiano P. Pityriasis rubra pilaris triggered by Tetragrip vaccine. Ann Dermatol Venerol. 2006;133:S280–S281. [Google Scholar]
- 8.Mohamed M., Belhadjali H., Hammedi F., Ben Meriem C., Zili J. Pityriasis rubra pilaris occurring after vaccination with diphtheria-pertussis-tetanus and oral poliovirus vaccines. Indian J Dermatol Venereol Leprol. 2015;81(6):618–620. doi: 10.4103/0378-6323.168326. [DOI] [PubMed] [Google Scholar]
- 9.Musette P., Senet P., Verola O., Dubertret L. A case of pityriasis rubra pilaris induced by DTPolio vaccination. Ann Dermatol Venerol. 1997;124:S226. [Google Scholar]
- 10.Ross N.A., Chung H.J., Li Q., Andrews J.P., Keller M.S., Uitto J. Epidemiologic, clinicopathologic, diagnostic, and management challenges of pityriasis rubra pilaris: a case series of 100 patients. JAMA Dermatol. 2016;152(6):670–675. doi: 10.1001/jamadermatol.2016.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]