Abstract
Hydration modulates every aspect of protein structure and function. However, studying water structures in hydration shells remains challenging mostly due to overwhelming background from bulk water. We used vibrational sum frequency generation (SFG) spectroscopy to characterize hydrated films of an anti-parallel β-sheet peptide (LK7β) adsorbed on glass slides. The hydrated films give chiral SFG response from water only when the peptide self-assembles into anti-parallel β-sheets. Experiments of isotopic labeling, isotopic dilution of water, and H2O-D2O exchange kinetics corroborate the assignments of the chiral SFG response to water stretching modes. Since individual water molecules are achiral, the chiral SFG response indicates formation of chiral superstructures of water around the anti-parallel β-sheet, implying that a protein secondary structure can imprint its chirality onto the surrounding water. This result demonstrates chiral SFG spectroscopy as a promising tool for probing water structures in protein hydration and addressing fundamental questions of protein structure-function.
Graphical Abstract
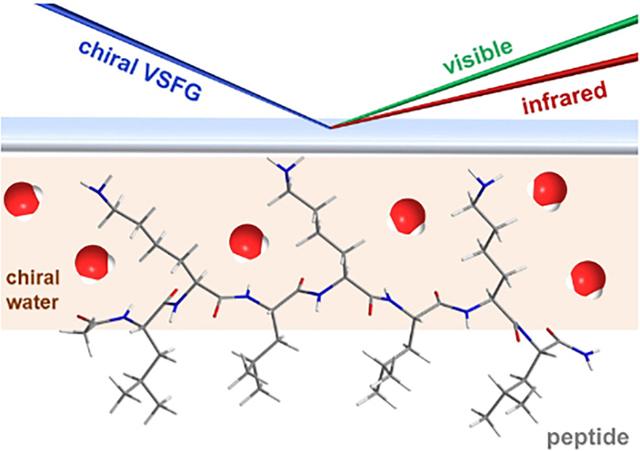
Water molecules are integral parts of proteins, influencing protein structures, dynamics, and functions. (1–3) Probing waters in protein hydration remains challenging due to background from a large population of water molecules in bulk solution. X-ray crystallography has shown that ordered water structures occur in the first hydration shell of proteins, (4) and that ordered water H-bonding networks can be found in protein reaction centers that are critical for protein function. (5,6) However, these ordered water structures were not observed under ambient conditions. NMR, (7) neutron scattering, (8) and terahertz spectroscopy (9,10) can report on the collective motions of waters near proteins but may not be sensitive to the architectures of waters surrounding proteins. Molecular dynamics (11) and quantum mechanics (12) simulations have yielded insights into water structures and dynamics at protein surfaces. Still, additional experimental validations of computational results are needed.
Here, we provide experimental evidence that vibrational sum frequency generation (SFG) can probe water superstructures around protein. SFG is a non-linear optical spectroscopy that selectively probes the second-order susceptibility χ(2) of non-centrosymmetric media, such as interfaces. (13) SFG has become one of the major tools for investigating water structures and dynamics at biological interfaces. (14–18) Chiral SFG is a variant of SFG spectroscopy that is selective for the vibrational modes of chiral macromolecular structures. Theoretical treatments of chiral SFG suggest achiral molecular entities that form extended chiral macromolecular or supermolecular structures can give chiral SFG response. (19) Chiral SFG has been increasingly used to study biomacromolecules, e.g., distinguishing the handedness of dsDNA molecules (20) and characterizing protein secondary structures at interfaces. (21–24)
In two recent studies, Petersen and co-workers used chiral SFG and observed a chiral “spine of hydration” of water molecules in the minor groove of the DNA double helix, (25) and chiral “water wires” in artificial channels spanning supported lipid bilayers. (26) The chiral SFG technique allowed for detecting this water superstructure without bulk water background. The existence of a chiral water superstructure around DNA engenders foundational research questions. (27) Most pressing is whether chiral water superstructures also exist around proteins and other biomacromolecules.
In this study, we present evidence for chiral water superstructures surrounding the LK7β peptide. This peptide has the sequence Acetyl-LKLKLKL-NH2. (28) It forms amphiphilic antiparallel β-sheet with hydrophobic leucine and hydrophilic lysine side chains residing on opposite sides of the β-sheet. This peptide has been characterized by vibrational circular dichroism (VCD), attenuated total reflectance-infrared (ATR-IR), (28) achiral SFG, (29,30) and chiral SFG. (30,31) Here, we present studies of this peptide using chiral SFG in conjunction with isotopic labeling, isotopic dilution, and kinetic measurements of H2O-D2O exchange. The results support the assignments of the O-D and O-H stretches of water in the chiral SFG spectra. Since individual water molecules are achiral, the chiroptical responses of the water stretching modes indicate water molecules form chiral supermolecular structures around the anti-parallel β-sheet. Therefore, the results introduce a new concept that protein secondary structures can orient the surrounding water molecules into chiral superstructures. Since water stretching modes are highly sensitive to H-bonding environments, chiral SFG can be a promising tool for probing in situ and real time local H-bonding interactions of water with proteins under ambient conditions. Thus, it can potentially be used to study the interplay of water structures in hydration with protein structures and dynamics, addressing fundamental questions of how local water structures modulate protein folding, aggregation, and denaturation, as well as protein structural-functional correlations.
For all SFG measurements, LK7β was dissolved in water solvent and adsorbed onto glass slides by evaporation, giving a hydrated thin film (see Experimental Methods). We first obtained the chiral amide I spectrum of LK7β using H2O on glass to examine the folding of LK7β. The spectrum (red, Figure 1) shows an intense vibrational band at ~1618 cm−1. This band is characteristic of the B2 modes of antiparallel β-sheets. (31) This band is significantly reduced when the LK7β sample was prepared using HCl aqueous solution at ~pH 2 as solvent (blue, Figure 1), indicating acid denaturation. Moreover, instead of using LK7β made of native L-form amino acids, we also used the LK7β peptide made of mixed L-leucine and D-lysine amino acids. Since proteins made of alternate L- and D-forms of amino acids cannot form antiparallel β-sheet structures, (32) the chiral amide I spectrum is silent (green, Figure 1). These results support that the LK7β peptide made of native L-form amino acids is in antiparallel β-sheet and the observed chiral SFG signals are correlated with formation of β-sheets.
Figure 1.
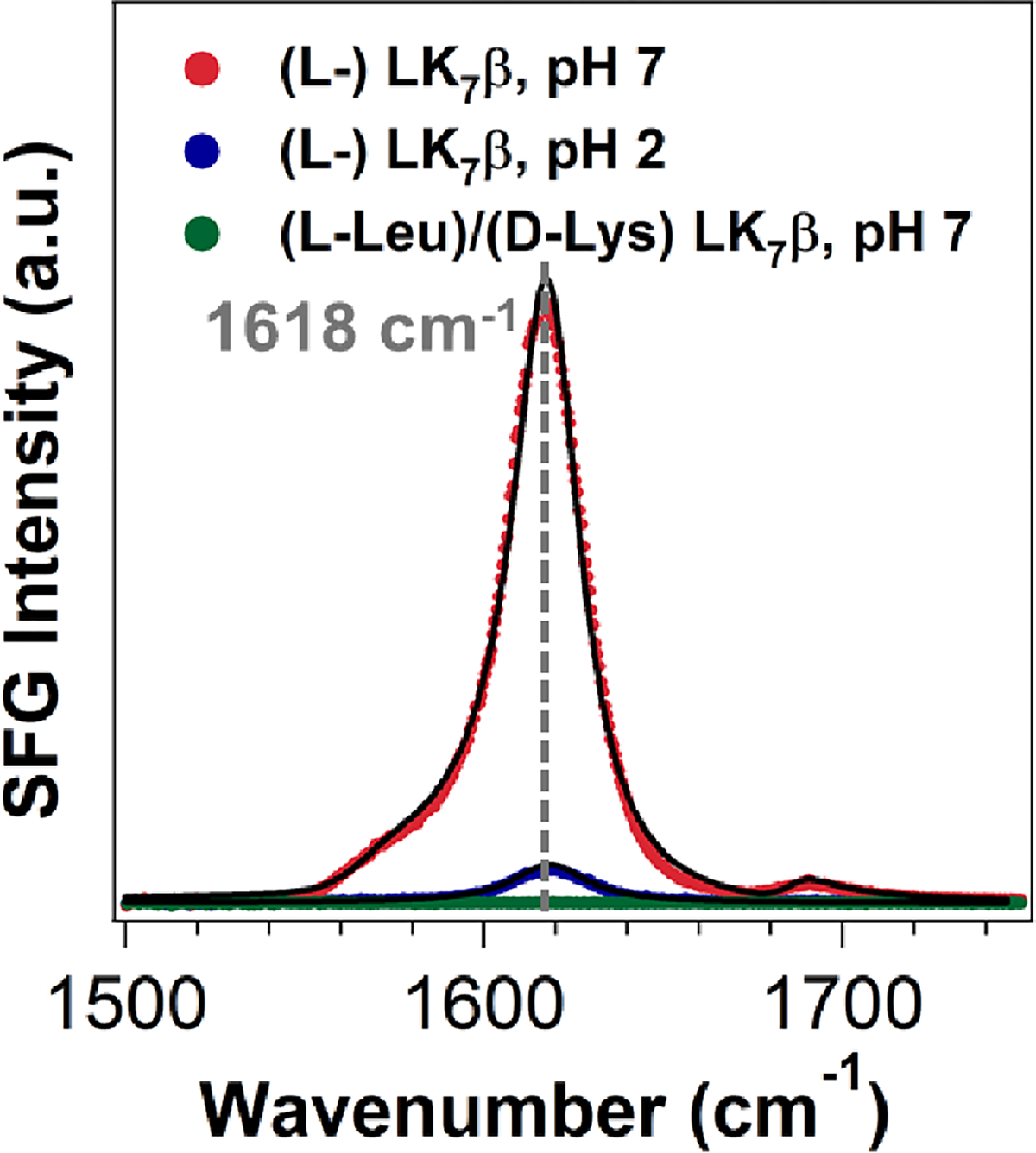
Chiral SFG amide I spectra of the LK7β thin films on glass slides: L-form LK7β prepared using H2O as solvent (red), L-form LK7β prepared using HCl(aq) at pH 2 as solvent (blue), and mixed (L-Leu)/(D-Lys) LK7β prepared using H2O as solvent (green). The fitting parameters and assignments are given in Table S1.
We obtained the chiral SFG spectra in the O-H stretch and O-D stretch regions for the hydrated thin films of LK7β made with the native L-form amino acids in the H2O and D2O solvents, respectively (Figures 2a and 2b). Each spectrum exhibits a major and a minor vibrational band. These vibrational bands are muted for the hydrated thin film of LK7β made with the mixed (L-Leu)/(D-Lys) residues (Figure S2). To fit the spectra (Figure 2), we used Eq. 1 and obtained the fitting results presented in the left-hand column of Table 1. We further performed three experiments: (i) O18-isotopic shifts, (ii) H2O/D2O isotopic dilution, and (iii) kinetic studies of H2O-D2O exchange. Based on the results of these three experiments, we assign the vibrational bands (left-hand column, Table 1). In the LK7β-H2O spectrum (Figure 2a), the peak at 3269 cm−1 is due to N-H stretch of the LK7β peptide and the peak at 3191 cm−1 to the O-H stretch of H2O. In the LK7β-D2O spectrum (Figure 2b), the peaks at 2401 cm−1 and 2454 cm−1 are both due to O-D stretches of D2O. Below, we provide details of the three experiments and discuss the results in the context of these spectral assignments.
Figure 2.
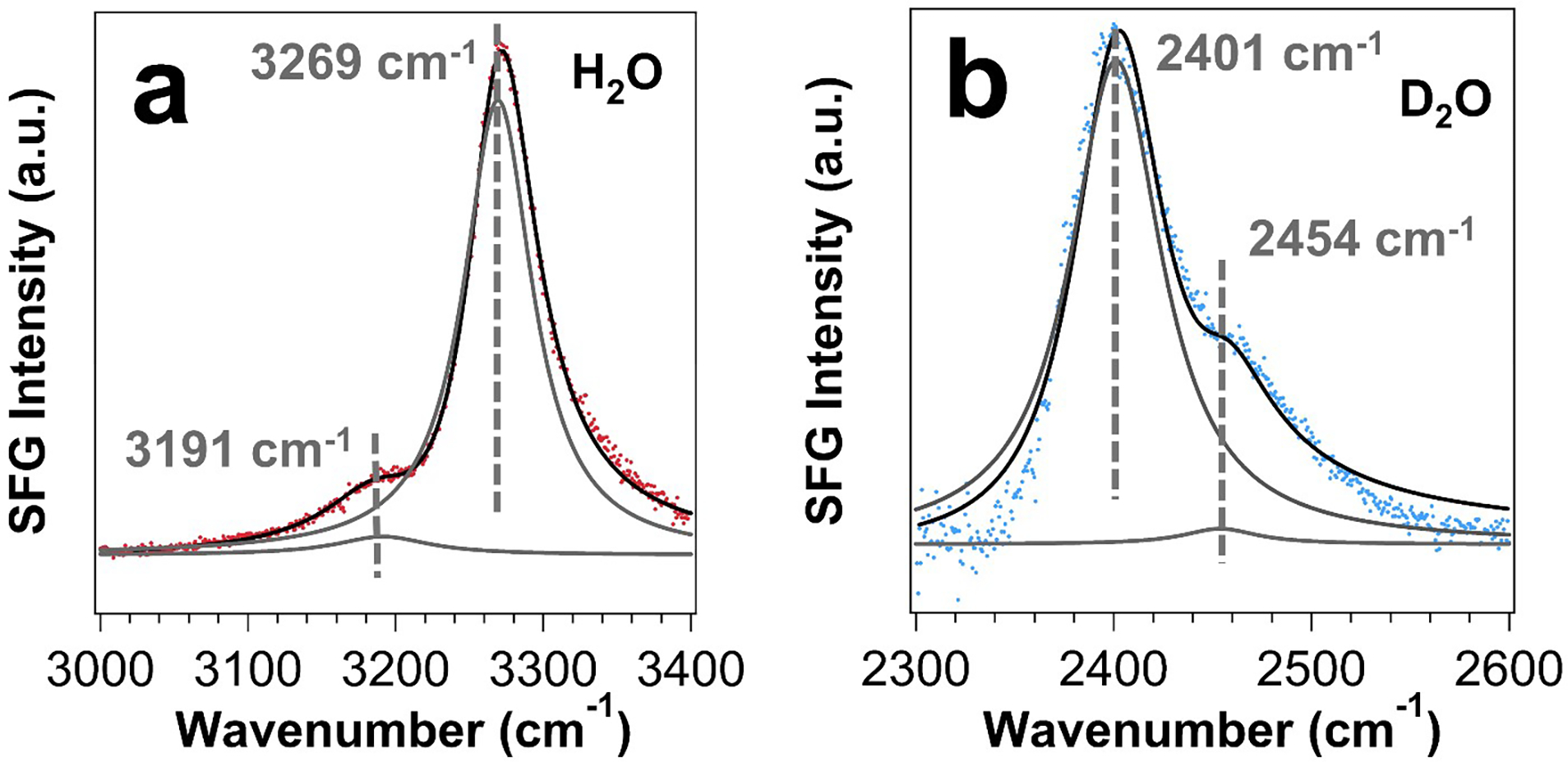
Chiral SFG spectra of (a) LK7β-H2O and (b) LK7β-D2O on glass slides. The fitting parameters and assignments are given in Table 1. Fits to equation 1 are shown in black lines with component peaks in solid grey lines.
Table 1.
| Samples | Parameters | Fittings | Assignments | Samples | Parameters | Fittings | Assignments |
|---|---|---|---|---|---|---|---|
|
| |||||||
| LK7β-H2O (2a) | 0.01 ± 0.01 | - | LK7β-H218O (3a) | 0.02 ± 0.01 | - | ||
|
|
|
||||||
| ω1 (cm−1) | 3191 ± 1 | O-H (H2O) | ω1 (cm−1) | 3179 ± 3 | O-H (H218O) | ||
| A1 (a.u.) | 1.59 ± 0.11 | A1 (a.u.) | 1.06 ± 0.23 | ||||
| Γ1 (cm−1) | 38.62 ± 2.14 | Γ1 (cm−1) | 33.80 ± 6.95 | ||||
|
|
|
||||||
| ω2 (cm−1) | 3269 ± 1 | N-H (LK7β) | ω2 (cm−1) | 3267 ± 1 | N-H (LK7β) | ||
| A2 (a.u.) | 5.82 ± 0.06 | A2 (a.u.) | 9.86 ± 0.12 | ||||
| Γ2 (cm−1) | 28.31 ± 0.21 | Γ2 (cm−1) | 32.03 ± 0.33 | ||||
|
| |||||||
| LK7β-D2O (2b) | 0.02 ± 0.01 | - | LK7β-D218O (3b) | 0.02 ± 0.01 | - | ||
|
|
|
||||||
| ω1 (cm−1) | 2401 ± 1 | O-D (D2O) | ω1 (cm−1) | 2390 ± 1 | O-D (D218O) | ||
| A1 (a.u.) | 4.27 ± 0.04 | A1 (a.u.) | 5.75 ± 0.05 | ||||
| Γ1 (cm−1) | 28.18 ± 2.19 | Γ1 (cm−1) | 28.82 ± 1.33 | ||||
|
|
|
||||||
| ω2 (cm−1) | 2454 ± 4 | O-D (D2O) | ω2 (cm−1) | 2442 ± 3 | O-D (D218O) | ||
| A2 (a.u.) | 0.64 ± 0.03 | A2 (a.u.) | 0.88 ± 0.06 | ||||
| Γ2 (cm−1) | 22.82 ± 9.42 | Γ2 (cm−1) | 26.54 ± 9.84 | ||||
In the first experiment, we used H218O and D218O as solvent to isolate the water vibrational bands. The frequency of the water O-H (or O-D) stretch overlaps with the peptide N-H (or N-D) stretch. Thus, both water and the peptide can contribute to the chiral SFG spectra (Figure 2). However, the greater atomic mass of 18O is expected to red shift the O-H or O-D stretch by ~12 cm−1, (33)33 but not the peptide N-H and ND stretches. (34) Thus, the 18O-labeled water will allow for unambiguous assignments of water vibrational modes.
We used H218O as solvent to prepare the thin film of LK7β on a glass slide and obtained the chiral SFG spectrum. This LK7β-H218O spectrum (Figure 3a) exhibits a major peak at 3267 cm−1 and a minor peak at 3179 cm−1 (right-hand column, Table 1). Relative to the LK7β-H2O spectrum (Figure 2a; left-hand column, Table 1), the 18O substitution does not shift the major peak (within error ± 1 cm−1), but red-shifts the minor peak by 12 cm−1 (Table 1). Thus, the minor band is assigned to water O-H stretch and the major band is assigned to peptide N-H stretch.
Figure 3.
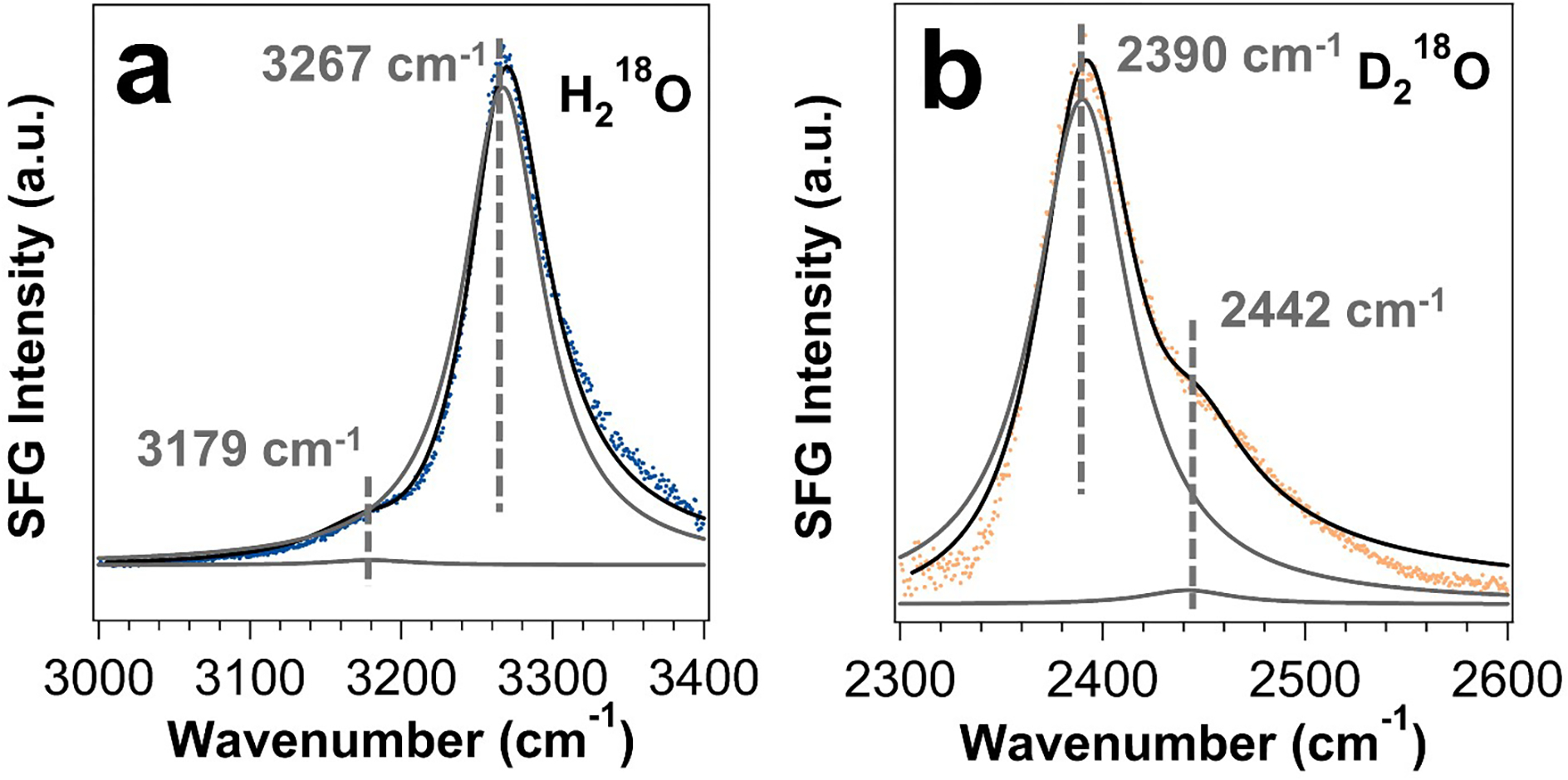
Chiral SFG spectra of (a) LK7β-H218O and (b) LK7β-D218O on glass slides. The fitting parameters and assignments are given in Table 1. Fits to equation 1 are shown in solid black lines with component peaks in solid grey lines.
In addition, we used D218O as solvent in the sample preparation and obtained the chiral SFG spectrum. The resulting LK7β-D218O spectrum (Figure 3b) exhibits a major peak at 2390 cm−1 and a minor peak at 2442 cm−1 (right-hand column, Table 1). Relative to the LK7β-D2O spectrum (Figure 2b), both peaks are red-shifted by 11–12 cm−1 (left-hand column, Table 1). Thus, both peaks are assigned to O-D water stretching modes (Table 1). Our assignments agree with previous achiral SFG studies of peptide at the air-D2O interface. (35) Thus, the results of 18O isotopic shifts allow assigning both the major and minor bands of the LK7β-D2O spectrum (Figure 2b) to water stretching modes (Table 1).
The spectral assignments of water vibrational bands in the LK7β-H2O and LK7β-D2O spectra (Figure 2) are further supported by the second experiment of isotopic dilution. Isotopic dilution is carried out using mixtures of H2O and D2O at various ratios (see Experimental Methods). It is often used in vibrational studies to modulate intramolecular vibrational coupling of water molecules. (36) Mixing H2O and D2O at a ratio of x:y introduces HOD at an anticipated ratio of [H2O]:[HOD]:[D2O] = x2:2xy:y2. (37) In HOD, the difference in the stretching frequencies of O-H and O-D removes the intramolecular vibrational coupling that otherwise occurs between two O-H bonds in pure H2O, or two O-D bonds in pure D2O. Although intermolecular couplings cannot be discounted under isotopic dilution, if water stretching contributes to the chiral SFG spectra of the hydrated LK7β thin films (Figure 2), the spectra should be changed by isotopic dilution.
In the isotopic dilution experiments, we mixed H2O and D2O at volumetric ratios (v/v) of 1:1, 4:1, and 1:4, which result in [H2O]:[HOD]:[D2O] = 1:2:1, 16:8:1, and 1:8:16, respectively. We used these mixtures as solvent to prepare the LK7β thin film and obtained the chiral SFG spectra in the O-H stretching region (Figure 4a) and O-D stretching region (Figure 4b). We present these spectra in the order of the HOD content relative to H2O (D2O) that contributes to the O-H (O-D) stretching region as indicated in Figure 4a (Figure 4b). Both Figures 4a and 4b show that higher HOD content results in larger deviation from the pure H2O or D2O spectrum. For example, in the spectrum (Figure 4a, blue) with the highest relative HOD content, the N-H stretch peak at 3269 cm−1 shows lower SFG intensity. This decrease in intensity is expected, as the protons along the peptide backbone can be exchanged to deuterons. Moreover, fitting this spectrum (Figure S3 and Table S2) reveals a third peak emerging at 3304 ± 5 cm−1, which was previously assigned by Shen and co-workers to the O-H stretch of HOD. (38) Altogether, the results support that water stretching modes contribute to the chiral SFG spectra of the hydrated LK7β thin films (Figure 2), in agreement with the spectral assignment of water stretching bands in Figure 2 and Table 1.
Figure 4.
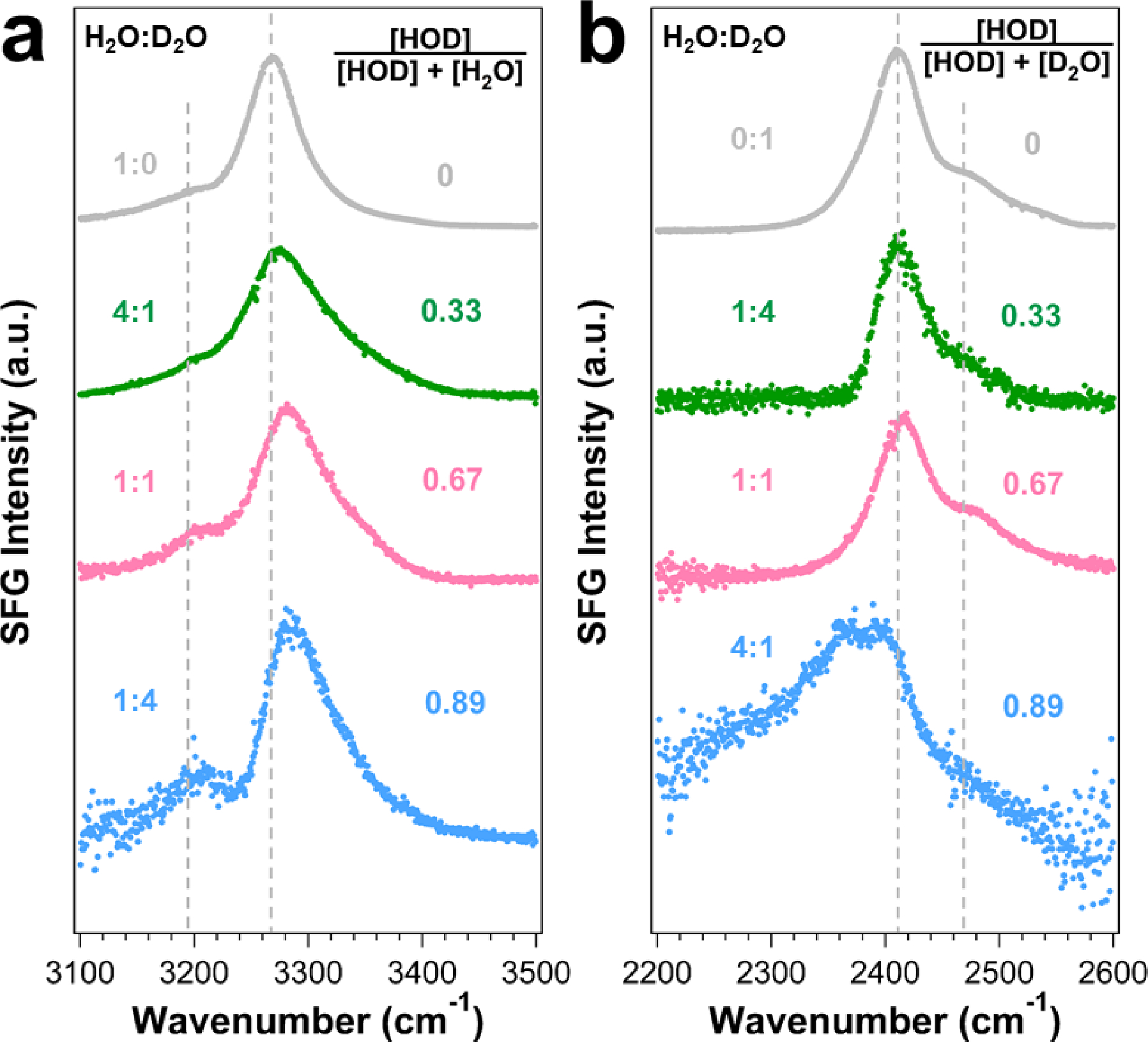
Isotopic dilution of the LK7β water superstructures probed by chiral SFG. Chiral SFG spectra of samples with increasing HOD content in the (a) O-H and (b) O-D stretching regions. The dashed lines correspond to peak positions (Table 1) of the pure H2O and pure D2O samples.
Finally, the third experiment involved kinetic studies of H2O-D2O exchange in the hydrated LK7β films, providing results in agreement with the spectral assignments of the chiral water stretching modes (Figure 2 and Table 1). We monitored the SFG spectra in the O-H and O-D stretching regions upon exposing the LK7β-H2O thin film to D2O vapor (see Experimental Methods and Figure S4). Figures 5a show the chiral SFG spectra in the O-H stretching region before and after exposure to D2O vapor. The major peak at 3269 cm−1 remains prominent while the minor peak at 3191 cm−1 disappears over time (Figure 5a). To analyze the kinetics, we extract the amplitudes (Aq) (see Experimental Methods) from the time-dependent chiral SFG spectra. Figure 5b plots the amplitudes for the minor and major vibrational bands against time. The amplitude of the minor band at 3191 cm−1 undergoes a single exponential decay with a time constant (τminor) of 0.6 ± 0.1 min (Figure 5b, top), while the amplitude of the major band at 3269 cm−1 fluctuates around its initial value (Figure 5b, bottom). Since the two vibrational bands do not show the same kinetics, they must originate from two different molecular species. The result therefore agrees with the assignments of the minor band to water stretching modes and the major band to the N-H stretching mode of the LK7β peptide (Figure 2a and Table 1).
Figure 5.
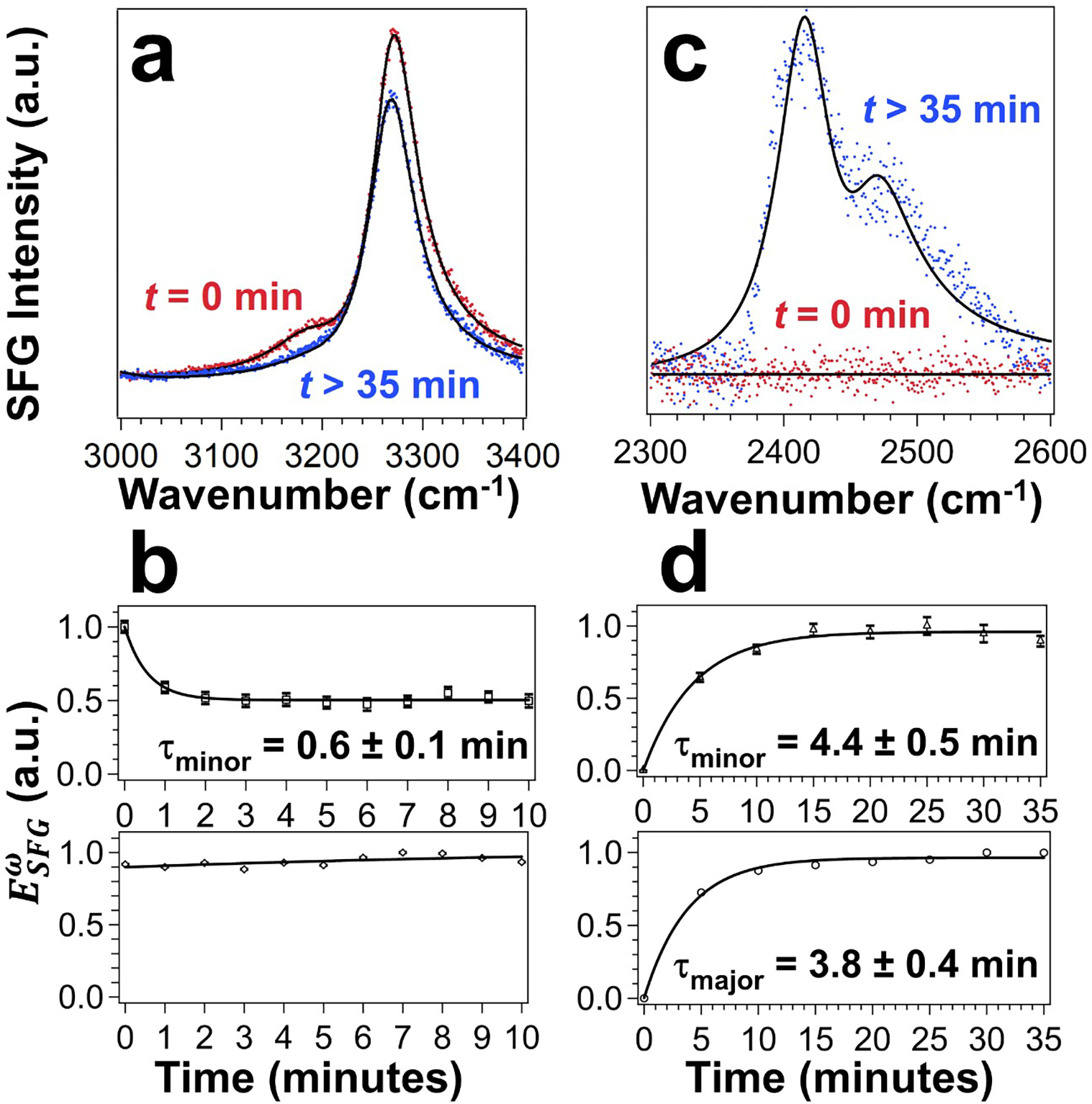
H2O-to-D2O exchange via the vapor phase monitored by chiral SFG. (a) Response in the O-H/N-H region (red) before and (blue) after exposure to D2O vapor. See Figure S5 and Table S3 for fittings of time-dependent spectra. (b) Amplitudes over time for the minor peak at 3191 cm−1 (top) and major peak at 3269 cm−1 (bottom). (c) Response in the O-D region before and after exposure to D2O vapor. (d) Amplitudes over time for the minor peak at 2401 cm−1 (top) and major peak at 2454 cm−1 (bottom). Solid lines in (b) and (d) are single exponential fits.
Figure 5c shows the spectra of LK7β-H2O in the region of O-D stretch before and after exposure to D2O vapor. Similarly, we extracted the amplitudes of the major and minor vibrational bands. Figure 5d plots the amplitudes as a function of time. Both the major and minor peaks undergo a single exponential increase with time constants for the minor peak (τminor) of 4.4 ± 0.5 minutes and major peak (τmajor) of 3.8 ± 0.4 minutes. These time constants are indistinguishable within one standard deviation, suggesting that both vibrational bands likely originate from a single species, consistent with the assignments of both vibrational peaks in the LK7β-D2O spectrum to water stretching modes (Figure 2b and Table 1).
The results of the three experiments – 18O isotopic shift (Figure 3), H2O/D2O isotope dilution (Figure 4), and kinetic studies of H2O-D2O exchange (Figure 5) – corroborate the assignments of the minor band (3191 cm−1) in the LK7β-H2O spectrum (Figure 3a) to the water O-H stretch and both the major (2401 cm−1) and minor (2454 cm−1) bands in the LK7β-D2O spectrum (Figure 3b) to water O-D stretch. The presence of one water band when the peptide is solvated in H2O (Figure 1a) versus two water bands when the peptide is solvated in D2O might be because the intense N-H stretch response obscures another H2O band in the spectrum, or because H2O and D2O could be differently structured around the peptide due to different H-bonding strengths. Computational studies in conjunction with experimental work will be needed for further exploration.
We attribute the chiral SFG signals of water stretches to chiral supermolecular structures of water surrounding the β-sheet LK7β peptide. These chiral water signals cannot be observed using the LK7β peptide made with mixed L/D-forms of amino acids (Figure S2). Since the mixed (L-Leu)/(D-Lys)-LK7β peptide contains the same side chains but cannot fold into β-sheet structures, the observed chiral SFG responses of water stretching is not due to local interactions of the side-chains but associated with the antiparallel β-sheet structure. These chiral SFG responses arise from orthogonal second-order susceptibility elements (i.e., , where 𝑖 ≠ 𝑗 ≠ 𝑘) and these orthogonal elements can be non-zero only for chiral molecular systems. Since individual water molecules are achiral, the chiral SFG signals of water stretching modes must be generated from water molecules arranged in chiral superstructures.
The chiral SFG theory and results of control experiments suggest that the bulk of the LK7β thin films can contribute to the observed chiral SFG signals. According to the theoretical framework established by Simpson and coworkers, orthogonal χ(2) elements can originate from achiral molecular entities arranged in chiral macromolecular structures at anisotropic interfaces, giving rise to strong surface-specific SFG signals. Such surface-specific chiral signals have been increasingly used to study chiral biomacromolecular structures at interfaces. (20,21,23,39,40) Further elaboration concerning the orthogonal element based on a symmetry argument is detailed in a recent review. (23) The analysis suggests that under dipole approximation and without electronic resonance an anisotropic chiral bulk system can also give rise to strong chiral SFG signals that are detected in reflection geometry (Figure S6). Such systems can include (i) reflection from surfaces of chiral crystals and (ii) reflection from solid or liquid interfaces adsorbed with layers of anisotropic chiral macromolecules. In these cases, the depth of bulk contribution can be roughly equal to the coherence length. Unlike chiral SFG generated from isotropic bulk media (e.g., chiral liquid) that is generally small and requires electronic resonance for detection, (41,42) these chiral signals generated from anisotropic media can be of a magnitude comparable to or even higher intensity than the surface-specific SFG signal. (43) In addition, the hydrated thin film of LK7β is indeed anisotropic on glass surface with a preferred orientation of the β-strand axis, as shown by our independent measurements using polarized attenuated total reflectance-Fourier transform infrared (polarized ATR-FTIR) spectroscopy (Figure S7). Hence, the anisotropic bulk of the LK7β thin film likely contributes to the observed chiral SFG signals.
The observation of water stretching modes by chiral SFG spectroscopy suggests that water molecules can follow the contour of protein secondary structures and form chiral supermolecular structures. Although duplex DNA orienting surrounding water molecules into chiral structures has for decades been a subject of intense interest, (11,44–47) the ability of proteins to imprint their macromolecular chirality onto surrounding waters has not been investigated. In general, there are 1.2 water molecules per amino acid in proteins and around 80% of ordered water structures are found in the first hydration shell of proteins. (4) The chiral supermolecular structures observed by chiral SFG are likely composed of these ordered water molecules. Indeed, X-ray structures of amyloid-like fibrils have revealed ordered arrangements of water oxygen atoms along surfaces of β-sheets.(48–50) Further experimental and theoretical efforts are pertinent to elucidate the molecular arrangements and H-bonding configurations in the chiral water superstructures. For example, temperature-dependent kinetic studies of H2O-D2O exchange can potentially reveal the energetics of H-bonding interactions essential for formation of water superstructures.
Our prior study reported chiral SFG spectra of LK7β at the air-H2O and air-D2O interfaces in the O-H and O-D spectral regions, respectively. (22,31) In this prior study, ab initio calculations guided the assignments of all vibrational bands of LK7β at the air-water interface to the N-H or N-D stretches of the LK7β peptide. With the current results, these assignments need to be re-examined in future experiments. Nonetheless, the current results confirm the assignment of the major band at 3269 cm−1 to the N-H stretches of the LK7β peptide, supporting the applications of this vibrational band for characterizing protein structures.(21–23,31,51–54)
The work reported here suggests that SFG can be a promising tool for probing protein hydration under ambient conditions. The water stretching modes are highly sensitive to H-bonding environments. Thus, chiral SFG can be used to solve a wide range of problems related to how H-bonding interactions with water molecules modulate protein structures and dynamics, potentially providing molecular insights into the role of hydration in protein aggregation, folding, and denaturation as well as structural-functional correlation of proteins. As a vibrational method, chiral SFG is label-free, non-invasive, in situ, and real time. It does not require large amount of sample or growth of crystal. Thus, it can be a powerful method complementing the current techniques (e.g., NMR and X-ray crystallography) to probe energetics and dynamics of protein hydration. Being a second-order optical method, chiral SFG offers unique selectivity and high sensitivity for detecting chiral macromolecular structures and can potentially offer new biophysical descriptions of hydration of proteins and other biomacromolecules (e.g., DNA, RNA, and polysaccharides).
EXPERIMENTAL METHODS
Sample preparation.
The LK7β peptide was obtained as a lyophilized powder (GL Biochem Ltd.). The powder was dissolved in H2O, H218O (Sigma Aldrich; 97 atom % 18O), D2O (Cambridge Isotope Laboratories, Inc., Andover, MA; 99.9 atom % D), D218O (Sigma Aldrich; 99 atom % D, 95 atom % 18O), or a mixture of H2O and D2O at 1 mM concentration in 100 μL aliquots. The aliquots were frozen in liquid nitrogen and then stored at −80 °C until time of use. Plain glass microscope slides (Thermo Scientific, Portsmouth, NH) were plasma-cleaned (Harrick Plasma, Ithaca, NY; Plasma Cleaner PDC-32G), and 100 μL of defrosted LK7β solution was pipetted onto the slide. The slides were then dried in a sealed container with desiccant and purged with dry nitrogen to avoid replacement of water isotopes by atmospheric water. The LK7β peptide formed a hydrated thin film on the glass slides for the SFG experiments.(55–57)
SFG experiments.
All SFG spectra were collected in reflection geometry using a homebuilt broad-bandwidth SFG spectrometer with ~120-fs pulsed IR and ~2-ps pulsed visible beams at a repetition rate of 5 kHz (complete details about the spectrometer can be found in Ma et al.).(58) The psp and ssp polarization settings were used for obtaining chiral and achiral SFG spectra, respectively (see section III in Supporting Information). The spectra in the O-H stretch and O-D stretch regions were collected with the LK7β thin film on glass slides facing down in the reflection geometry. The SFG spectra in the amide I region were collected with the LK7β thin film facing up to avoid attenuation of the IR beam by the glass slides. The spectra were collected with an integration time of 10 minutes.
Kinetics of H2O-D2O Exchange.
The glass slide with adsorbed LK7β thin film was supported by a Teflon beaker on a stage with adjustable height. A plastic cuvette was sitting inside the Teflon beaker. At t = 0 min, 1.6 mL of D2O was carefully pipetted into the cuvette through a gap between the glass slide and the wall of the Teflon beaker without disturbing the slide (Figure S1). The adsorbed LK7β thin film was situated approximately 1 cm from the surface of the D2O. The use of the plastic cuvette was to avoid the artifact of water crawling on the wall of the Teflon beaker that supported the glass slide. Evaporation of D2O from the plastic cuvette induced H2O-to-D2O exchange in the LK7β thin film. Chiral SFG spectra were collected in the O-H stretch and O-D stretch regions until the SFG intensity reached stable plateaus.
Analysis of spectra.
The raw spectral data were processed as detailed in Ma et al. (58) The resulting SFG spectra were fitted to:
| (1) |
with the SFG intensity ISFG, the non-resonant second-order susceptibility , the frequency of the incident IR beam ωIR, and the amplitude, frequency, and damping factor of the qth resonant vibrational mode, Aq, ωq, and Γq, respectively. To extract the amplitudes (Aq) for H2O-D2O exchange kinetics, the highest intensity spectrum was first fit to Eq. 1. After a best fit of the spectrum was achieved, the background , frequency ωq, and damping Γq parameters were fixed for fitting the remaining spectra to obtain the amplitude values for kinetic analyses.
Supplementary Material
ACKNOWLEDGMENTS
EAP is supported by the NIH (5T32GM008283-30). The authors thank Profs. Mark A. Johnson, Andrew D. Miranker, Ziad Ganim, and Dr. Zahra Sohrabpour at Yale University for insightful discussions.
Footnotes
Notes
The authors declare no competing financial interests.
ASSOCIATED CONTENT
Supporting Information. Methods of H2O-D2O kinetic exchange experiments; chiral SFG spectra of (L-Leu)/(D-Lys) LK7β; 1:4 H2O:D2O isotopic dilution spectrum with fits; H2O-D2O exchange spectra with fits; chiral SFG theoretical background; polarized ATR-FTIR spectra. (PDF)
REFERENCES
- 1.Bellissent-Funel M-C; Hassanali A; Havenith M; Henchman R; Pohl P; Sterpone F; van der Spoel D; Xu Y; Garcia AE Water determines the structure and dynamics of proteins. Chem. Rev 2016, 116, 7673–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cremer PS; Flood AH; Gibb BC; Mobley DL Collaborative routes to clarifying the murky waters of aqueous supramolecular chemistry. Nat. Chem 2018, 10, 8. [Google Scholar]
- 3.Kuriyan J; Konforti B; Wemmer D The Molecules of Life: Physical and Chemical Principles. Garland Science: New York; 2012. [Google Scholar]
- 4.Lee J; Kim S-H Water polygons in high-resolution protein crystal structures. Protein Sci. 2009, 18, 1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ermler U; Fritzsch G; Buchanan SK; Michel H Structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.65 Å resolution: cofactors and protein-cofactor interactions. Structure 1994, 2, 925–936. [DOI] [PubMed] [Google Scholar]
- 6.Baciou L; Michel H Interruption of the water chain in the reaction center from Rhodobacter sphaeroides reduces the rates of the proton uptake and of the second electron transfer to QB. Biochemistry 1995, 34, 7967–7972. [DOI] [PubMed] [Google Scholar]
- 7.Nucci NV; Pometun MS; Wand AJ Site-resolved measurement of water-protein interactions by solution NMR. Nat. Struct. Mol. Biol 2011, 18, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svergun D; Richard S; Koch M; Sayers Z; Kuprin S; Zaccai G Protein hydration in solution: experimental observation by x-ray and neutron scattering. Proc. Natl. Acad. Sci. U. S. A 1998, 95, 2267–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebbinghaus S; Kim SJ; Heyden M; Yu X; Heugen U; Gruebele M; Leitner DM; Havenith M An extended dynamical hydration shell around proteins. Proc. Natl. Acad. Sci. U. S. A 2007, 104, 20749–20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti Nibali V; Havenith M New insights into the role of water in biological function: Studying solvated biomolecules using terahertz absorption spectroscopy in conjunction with molecular dynamics simulations. J. Am. Chem. Soc 2014, 136, 12800–12807. [DOI] [PubMed] [Google Scholar]
- 11.Laage D; Elsaesser T; Hynes JT Water dynamics in the hydration shells of biomolecules. Chem. Rev 2017, 117, 10694–10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf S; Freier E; Cui Q; Gerwert K Infrared spectral marker bands characterizing a transient water wire inside a hydrophobic membrane protein. J. Chem. Phys 2014, 141, 12B625_1. [Google Scholar]
- 13.Shen Y Surface properties probed by second-harmonic and sum-frequency generation. Nature 1989, 337, 519. [Google Scholar]
- 14.Jena KC; Hore DK Water structure at solid surfaces and its implications for biomolecule adsorption. Phys. Chem. Chem. Phys 2010, 12, 14383–14404. [DOI] [PubMed] [Google Scholar]
- 15.Vácha R; Rick SW; Jungwirth P; de Beer AG; de Aguiar HB; Samson J-S; Roke S The orientation and charge of water at the hydrophobic oil droplet–water interface. J. Am. Chem. Soc 2011, 133, 10204–10210. [DOI] [PubMed] [Google Scholar]
- 16.Pandey R; Usui K; Livingstone RA; Fischer SA; Pfaendtner J; Backus EH; Nagata Y; Fröhlich-Nowoisky J; Schmüser L; Mauri S Ice-nucleating bacteria control the order and dynamics of interfacial water. Sci. Adv 2016, 2, e1501630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medders GR; Paesani F Dissecting the molecular structure of the air/water interface from quantum simulations of the sum-frequency generation spectrum. J. Am. Chem. Soc 2016, 138, 3912–3919. [DOI] [PubMed] [Google Scholar]
- 18.Okur HI; Hladilkova J; Rembert KB; Cho Y; Heyda J; Dzubiella J; Cremer PS; Jungwirth P Beyond the Hofmeister series: Ion-specific effects on proteins and their biological functions. J. Phys. Chem. B 2017, 121, 1997–2014. [DOI] [PubMed] [Google Scholar]
- 19.Simpson GJ Nonlinear Optical Polarization Analysis in Chemistry and Biology. Cambridge University Press: Cambridge; 2017. [Google Scholar]
- 20.Stokes GY; Gibbs-Davis JM; Boman FC; Stepp BR; Condie AG; Nguyen ST; Geiger FM Making “sense” of DNA. J. Am. Chem. Soc 2007, 129, 7492–7493. [DOI] [PubMed] [Google Scholar]
- 21.Wang J; Chen X; Clarke ML; Chen Z Detection of chiral sum frequency generation vibrational spectra of proteins and peptides at interfaces in situ. Proc. Natl. Acad. Sci. U. S. A 2005, 102, 4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu L; Liu J; Yan EC Chiral sum frequency generation spectroscopy for characterizing protein secondary structures at interfaces. J. Am. Chem. Soc 2011, 133, 8094–8097. [DOI] [PubMed] [Google Scholar]
- 23.Yan EC; Fu L; Wang Z; Liu W Biological macromolecules at interfaces probed by chiral vibrational sum frequency generation spectroscopy. Chem. Rev 2014, 114, 8471–8498. [DOI] [PubMed] [Google Scholar]
- 24.Yan EC; Wang Z; Fu L Proteins at interfaces probed by chiral vibrational sum frequency generation spectroscopy. J. Phys. Chem. B 2015, 119, 2769–2785. [DOI] [PubMed] [Google Scholar]
- 25.McDermott ML; Vanselous H; Corcelli SA; Petersen PB DNA’s chiral spine of hydration. ACS Cent. Sci 2017, 3, 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocsis I; Sorci M; Vanselous H; Murail S; Sanders SE; Licsandru E; Legrand Y-M; van der Lee A; Baaden M; Petersen PB Oriented chiral water wires in artificial transmembrane channels. Sci. Adv 2018, 4, eaao5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perets EA; Yan EC The H2O Helix: The Chiral Water Superstructure Surrounding DNA. ACS Cent. Sci 2017, 3, 683–685.: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeGrado W; Lear J Induction of peptide conformation at apolar water interfaces. 1. A study with model peptides of defined hydrophobic periodicity. J. Am. Chem. Soc 1985, 107, 7684–7689. [Google Scholar]
- 29.Phillips DC; York RL; Mermut O; McCrea KR; Ward RS; Somorjai GA Side chain, chain length, and sequence effects on amphiphilic peptide adsorption at hydrophobic and hydrophilic surfaces studied by sum-frequency generation vibrational spectroscopy and quartz crystal microbalance. J. Phys. Chem. C 2007, 111, 255–261. [Google Scholar]
- 30.Wang Z; Fu L; Yan EC C–H stretch for probing kinetics of self-assembly into macromolecular chiral structures at interfaces by chiral sum frequency generation spectroscopy. Langmuir 2013, 29, 4077–4083. [DOI] [PubMed] [Google Scholar]
- 31.Fu L; Xiao D; Wang Z; Batista VS; Yan EC Chiral sum frequency generation for in situ probing proton exchange in antiparallel β-sheets at interfaces. J. Am. Chem. Soc 2013, 135, 3592–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahalakshmi R; Balaram P D-Amino Acids: A New Frontier in Amino Acids and Protein Research–Practical Methods and Protocols. Nova Science Publisher: New York; 2007. [Google Scholar]
- 33.Jung S-Y; Lim S-M; Albertorio F; Kim G; Gurau MC; Yang RD; Holden MA; Cremer PS The Vroman effect: a molecular level description of fibrinogen displacement. J. Am. Chem. Soc 2003, 125, 12782–12786. [DOI] [PubMed] [Google Scholar]
- 34.Englander SW; Sosnick TR; Englander JJ; Mayne L Mechanisms and uses of hydrogen exchange. Curr. Opin. Struct. Biol 1996, 6, 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonn M; Bakker HJ; Tong Y; Backus EH No ice-like water at aqueous biological interfaces. Biointerphases 2012, 7, 20. [DOI] [PubMed] [Google Scholar]
- 36.Stiopkin IV; Weeraman C; Pieniazek PA; Shalhout FY; Skinner JL; Benderskii AV Hydrogen bonding at the water surface revealed by isotopic dilution spectroscopy. Nature 2011, 474, 192. [DOI] [PubMed] [Google Scholar]
- 37.Nihonyanagi S; Ishiyama T; Lee T.-k.; Yamaguchi S; Bonn M; Morita A; Tahara T Unified molecular view of the air/water interface based on experimental and theoretical χ (2) spectra of an isotopically diluted water surface. J. Am. Chem. Soc 2011, 133, 16875–16880. [DOI] [PubMed] [Google Scholar]
- 38.Tian C-S; Shen YR Isotopic dilution study of the water/vapor interface by phase-sensitive sum-frequency vibrational spectroscopy. J. Am. Chem. Soc 2009, 131, 2790–2791. [DOI] [PubMed] [Google Scholar]
- 39.Liu W; Fu L; Wang Z; Sohrabpour Z; Li X; Liu Y; Wang H.-f.; Yan EC Two dimensional crowding effects on protein folding at interfaces observed by chiral vibrational sum frequency generation spectroscopy. Phys. Chem. Chem. Phys 2018, 20, 22421–22426. [DOI] [PubMed] [Google Scholar]
- 40.Tan J; Zhang J; Luo Y; Ye S Misfolding of Human Islet Amyloid Polypeptide at Lipid Membrane Populates through β-Sheet Conformers without Involving α-Helical Intermediates. J. Am. Chem. Soc 2019, 141, 1941–1948. [DOI] [PubMed] [Google Scholar]
- 41.Belkin MA; Kulakov TA; Ernst KH; Yan L; Shen YR Sum-Frequency Vibrational Spectroscopy on Chiral Liquids: A Novel Technique to Probe Molecular Chirality. Phys. Rev. Lett 2000, 85, 4474–4477. [DOI] [PubMed] [Google Scholar]
- 42.Lee T; Rhee H; Cho M Femtosecond Vibrational Sum-Frequency Generation Spectroscopy of Chiral Molecules in Isotropic Liquid. J. Phys. Chem. Lett 2018, 9, 6723–6730. [DOI] [PubMed] [Google Scholar]
- 43.Nagahara T; Kisoda K; Harima H; Aida M; Ishibashi T.-a. Chiral sum frequency spectroscopy of thin films of porphyrin J-aggregates. J. Phys. Chem. B 2009, 113, 5098–5103. [DOI] [PubMed] [Google Scholar]
- 44.Kopka ML; Fratini AV; Drew HR; Dickerson RE Ordered water structure around a B-DNA dodecamer: A quantitative study. J. Mol. Biol 1983, 163, 129–146. [DOI] [PubMed] [Google Scholar]
- 45.Liepinsh E; Otting G; Wüthrich K NMR observation of individual molecules of hydration water bound to DNA duplexes: direct evidence for a spine of hydration water present in aqueous solution. Nucleic Acids Res 1992, 20, 6549–6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furse K; Corcelli S Effects of an unnatural base pair replacement on the structure and dynamics of DNA and neighboring water and ions. J. Phys. Chem B 2010, 114, 9934–9945. [DOI] [PubMed] [Google Scholar]
- 47.Duboué-Dijon E; Fogarty AC; Hynes JT; Laage D Dynamical disorder in the DNA hydration shell. J. Am. Chem. Soc 2016, 138, 7610–7620. [DOI] [PubMed] [Google Scholar]
- 48.Nelson R; Sawaya MR; Balbirnie M; Madsen AØ; Riekel C; Grothe R; Eisenberg D Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawaya MR; Sambashivan S; Nelson R; Ivanova MI; Sievers SA; Apostol MI; Thompson MJ; Balbirnie M; Wiltzius JJ; McFarlane HT et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature 2007, 447, 453. [DOI] [PubMed] [Google Scholar]
- 50.Liu C; Zhao M; Jiang L; Cheng P-N; Park J; Sawaya MR; Pensalfini A; Gou D; Berk AJ; Glabe CG; Nowick J; Eisenberg D Out-of-register β-sheets suggest a pathway to toxic amyloid aggregates. Proc. Natl. Acad. Sci U. S. A 2012, 109, 20913–20918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mermut O; Phillips DC; York RL; McCrea KR; Ward RS; Somorjai GA In situ adsorption studies of a 14-amino acid leucine-lysine peptide onto hydrophobic polystyrene and hydrophilic silica surfaces using quartz crystal microbalance, atomic force microscopy, and sum frequency generation vibrational spectroscopy. J. Am. Chem. Soc 2006, 128, 3598–3607. [DOI] [PubMed] [Google Scholar]
- 52.Breen NF; Weidner T; Li K; Castner DG; Drobny GP A solid-state deuterium NMR and sum-frequency generation study of the side-chain dynamics of peptides adsorbed onto surfaces. J. Am. Chem. Soc 2009, 131, 14148–14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weidner T; Breen NF; Drobny GP; Castner DG Amide or amine: determining the origin of the 3300 cm− 1 NH mode in protein SFG spectra using 15N isotope labels. J. Phys. Chem B 2009, 113, 15423–15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meister K; Paananen A; Bakker H Identification of the response of protein N–H vibrations in vibrational sum-frequency generation spectroscopy of aqueous protein films. Phys. Chem. Chem. Phys 2017, 19, 10804–10807. [DOI] [PubMed] [Google Scholar]
- 55.Frank HS Low-Pressure Adsorption on a Washed Glass Surface. J. Phys. Chem 1929, 33, 970–976. [Google Scholar]
- 56.Razouk RI; Salem AS The adsorption of water vapor on glass surfaces. J. Phys. Chem 1948, 52, 1208–1227. [Google Scholar]
- 57.Pauling L The adsorption of water by proteins. J. Am. Chem. Soc 1945, 67, 555–557. [Google Scholar]
- 58.Ma G; Liu J; Fu L; Yan EC Probing water and biomolecules at the air–water interface with a broad bandwidth vibrational sum frequency generation spectrometer from 3800 to 900 cm−1. Appl. Spectrosc 2009, 63, 528–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


