Abstract
Introduction:
Single-dose rotavirus vaccines, which are used by a majority of countries, are some of the largest-sized vaccines in immunization programs, and have been shown to constrain supply chains and cause bottlenecks. Efforts have been made to reduce the size of the single-dose vaccines; however, with two-dose, five-dose and ten-dose options available, the question then is whether using multi-dose instead of single-dose rotavirus vaccines will improve vaccine availability.
Methods:
We used HERMES-generated simulation models of the vaccine supply chains of the Republic of Benin, Mozambique, and Bihar, a state in India, to evaluate the operational and economic impact of implementing each of the nine different rotavirus vaccine presentations.
Results:
Among single-dose rotavirus vaccines, using Rotarix RV1 MMP (multi-monodose presentation) led to the highest rotavirus vaccine availability (49–80%) and total vaccine availability (56–79%), and decreased total costs per dose administered ($0.02-$0.10) compared to using any other single-dose rotavirus vaccine. Using two-dose ROTASIIL decreased rotavirus vaccine availability by 3–6% across each supply chain compared to Rotarix RV1 MMP, the smallest single-dose vaccine. Using a five-dose rotavirus vaccine improved rotavirus vaccine availability (52–92%) and total vaccine availability (60–85%) compared to single-dose and two-dose vaccines. Further, using the ten-dose vaccine led to the highest rotavirus vaccine availability compared to all other rotavirus vaccines in both Benin and Bihar.
Conclusion:
Our results show that countries that implement five-dose or ten-dose rotavirus vaccines consistently reduce cold chain constraints and achieve higher rotavirus and total vaccine availability compared to using either single-dose or two-dose rotavirus vaccines.
Keywords: Rotavirus, Vaccine, Supply chains, Multi-dose vials, Modeling
1. Introduction
Single-dose rotavirus vaccines, which are used by a majority of countries, [1] are some of the largest-sized vaccines in many immunization programs, and have been shown to constrain supply chains and cause bottlenecks [2-6]. Since the rotavirus vaccine first became available in 2006, manufacturers have developed smaller single-dose rotavirus vaccines, yet with two-dose, five-dose and ten-dose presentations now available, the question is whether using multi-dose rotavirus vaccines instead of single-dose vaccines will improve vaccine availability (i.e., the number of successful immunizations administered as a percentage of total immunizations needed). According to data collected by the Johns Hopkins International Vaccine Access Center (IVAC), [1] as of 2020, only six of the 114 countries reporting the rotavirus presentation introduced or planned for introduction report using multi-dose rotavirus vaccines. While all six of these countries are Gavi-supported, the vast majority of low- and middle-income countries (LMICs) use single-dose rotavirus vaccines [1]. Our past work has shown that vaccines with more doses per vial can reduce cold chain constraints, improve vaccine availability and reduce costs [3,4,7-11]. However, these previous analyses focused on one supply chain system at a time, and did not consider the impact of using multi-dose rotavirus vaccines. As such, there is limited evidence for immunization programs who have been using single-dose vaccines for many years to decide whether to begin using a multi-dose rotavirus presentation.
Determining which rotavirus presentation to implement using traditional methodologies (e.g., economic analyses) may not capture the complete systems-wide effects on the vaccine supply chain and, thus, the comprehensive operational and economic value of selecting a certain rotavirus vaccine presentation [12,13]. In order to assess whether countries should switch to using multi-dose rotavirus vaccines, we used our Highly Extensible Resource for Modeling Supply Chains (HERMES) simulation modeling platform to evaluate the operational and economic impact of nine different rotavirus vaccine vial presentations across the vaccine supply chains of Benin, Bihar (India), and Mozambique.
2. Methods
For this study, we utilized the HERMES software platform. As described in our previous publications, [7,9,11,14-16] HERMES allows users to generate detailed discrete-event simulation models of any vaccine supply chain over a one-year period. Each supply chain model contains a virtual representation of all storage facilities and devices (including buildings, refrigerators, and freezers), vehicles and routes (including vehicle types, travel frequency, and travel distance), human resources (including logisticians, drivers, and vaccinators), vaccines, supply chain policies, and associated costs for each component. HERMES tracks each simulated vial as it moves through a supply chain and provides a range of outputs, including how many vaccine doses are administered, the location and magnitude of storage and transport constraints, and vaccine wastage due to expiry of unopened vials or unused doses in an opened vial.
3. Description of HERMES models
We used models of the vaccine supply chains of the Republic of Benin, Mozambique, and Bihar, India. Descriptive details for each of these models are found in previous publications [14-17]. Briefly, the supply chain model for the Republic of Benin includes 637 vaccination locations and a birth cohort population of 461,536 representing the estimated population for 2020. The supply chain model for Mozambique includes 1,797 vaccination locations and a birth cohort population of 1,147,765. For Bihar, India, we represented a subsection of the supply chain that includes 161 fixed vaccination locations and a birth cohort population of 1,240,553. In order to show the current, potential demand for rotavirus vaccine in each supply chain, we include the most recent population data. While each vaccine supply chain modeled may have made changes or updates to their vaccine supply chains in certain areas since our data was collected, our models use the most recently available, comprehensive data on each vaccine supply chain.
Each storage facility in a model is assigned cold chain equipment (e.g., refrigerators and freezers) based on available data, with each refrigerator and freezer having a specific cold storage volume, depreciation cost, energy usage rate and cost, and lifetime usage duration. Additionally, each shipping route is assigned a vehicle based on available data, with each vehicle having a specific cold storage volume, depreciation cost, gasoline usage and cost (if applicable), and lifetime mileage. The vaccines included in each supply chain model are based on each respective immunization program, and each vaccine is assigned a specific cold chain volume per dose, number of doses per vial, cost per dose, and storage specification, based on available data. Table 1 provides specific details on each of these components.
Table 1.
Key Vaccine Supply Chain Characteristics.
| Characteristics | Vaccine Supply Chain | ||
|---|---|---|---|
| Benin | Bihar | Mozambique | |
| Number of supply chain levels modeled | 4 | 4 | 4 |
| Number of vaccination locations modeled | 637 | 161 | 1,797 |
| Number of vaccines in routine immunization program | 9 | 11 | 8 |
| Birth cohort population (2020) | 461,536 | 1,240,553 | 1,147,765 |
| Range of total refrigerator storage volume across all storage locations (m3) | 18 to 15,000 | 14 to 17,280 | 10 to 49,000 |
| Range of total freezer storage volume across all storage locations (m3) | 0 to 5,907 | 0 to 11,520 | 84 to 14,850 |
As described in our past publications, each model includes the unit costs of each supply chain component and calculates the cumulative total logistics, procurement, and disposal costs over the course of a simulation [7,11,14,15,17]. Logistics costs include the costs of vaccine storage (i.e., annualized cost of each refrigerator and freezer, energy costs for each storage unit, amount of energy used), vaccine transport (i.e., fuel costs for each vaccine shipment, annualized cost of each vehicle, fuel costs, per diems for each driver), and supply chain personnel (i.e., percentage of salary specific to immunization activities). Vaccine procurement costs include the total costs of all doses of vaccines procured over the course of a simulation. The costs per dose of each routine vaccine were extracted from UNICEF and Gavi databases [18,19]. Vaccine waste disposal costs include a standard cost of waste disposal per kilogram of vaccine waste (i.e., vials and syringes) [8].
We updated the populations and costs in each model to 2020 estimates using the average country-associated population growth rate between 2012 and 2018 and standard inflation rates provided by the World Bank [20,21]. For the Bihar model, we inflated costs to 2020 Indian rupees (INR) before converting to USD by multiplying INR by the 2020 exchange rate of 0.014 [22].
4. Characteristics of currently available rotavirus vaccines
Table 2 lists the rotavirus vaccines evaluated in this analysis and their associated characteristics [23]. We included rotavirus vaccine presentations currently supported by Gavi, including formulations currently in the process of being prequalified by the World Health Organization (WHO). The rotavirus vaccines modeled include five single-dose vaccines [Rotarix RV1, Rotarix RV1 MMP (multi-monodose presentation), single-dose Rotavac 5D, single-dose, lyophilized ROTASIIL RV5, and single-dose, liquid ROTASIIL RV5], and four multi-dose vaccines (two-dose, lyophilized ROTASIIL RV5, five-dose Rotavac, ten-dose Rotavac, and five-dose Rotavac 5D). Although ROTASIIL Thermo is supported by Gavi, we do not include it in the model since this analysis is focused on rotavirus vaccines that require storage within existing cold chains. Seven of the nine rotavirus presentations modeled require refrigerator storage, while two vaccines (five-dose and ten-dose Rotavac) are designed for storage in both freezers and refrigerators. Rotarix RV1 and Rotarix RV1 MMP require two doses per child, while the remaining vaccines require three. Each of the multi-dose rotavirus vaccines are currently recommended by the WHO to be discarded six hours after opening, though a proposed policy change would allow five-dose and ten-dose Rotavac vaccines to remain open for 28 days, which is in accordance with the multi-dose vial policy for many other multi-dose liquid vaccines [24].
Table 2.
Characteristics of modeled rotavirus vaccines.
| Vaccine | Volume per FIC* (cm3) |
Volume per dose (cm3) |
Doses per vial |
Doses per child |
Price per dose (2020 $USD) |
Storage temperature |
Open vial policy |
|---|---|---|---|---|---|---|---|
| ROTASIIL RV5 (single-dose, liquid) | 59.5 | 19.8 | 1 | 3 | 1.55 | 2-8C | NA |
| ROTASIIL RV5, (single-dose)^ | 52.8 | 17.6 | 1 | 3 | 1.55 | 2-8C | NA |
| Rotavac 5D (single-dose) | 48 | 16 | 1 | 3 | 1.58 | 2-8C | NA |
| Rotarix (RV1) | 34.2 | 17.1 | 1 | 2 | 2.2 | 2-8C | NA |
| ROTASIIL RV5 (two-dose)^ | 31.5 | 10.5 | 2 | 3 | 0.95 | 2-8C | Discard after six hours |
| Rotarix (RV1, MMP) | 23.6 | 11.8 | 1 | 2 | 2.2 | 2-8C | NA |
| Rotavac 5D (five-dose) | 12.6 | 4.2 | 5 | 3 | 1.14 | 2-8C | Discard after six hours / 28 days** |
| Rotavac (five-dose) | 12.6 | 4.2 | 5 | 3 | 0.85 | −20C, 2-8C | Discard after six hours / 28 days** |
| Rotavac (ten-dose) | 9.6 | 3.2 | 10 | 3 | 0.85 | −20C, 2-8C | Discard after six hours / 28 days** |
FIC: Fully-immunized child.
Requires reconstitution.
Five-dose and ten-dose rotavirus vaccine simulations included a sensitivity analysis allowing these vaccines to remain open for 28 days.
We assume that a multi-dose vial will be opened regardless of the vaccination session size, e.g., even if only one child is present, according to WHO recommendations [25]. Rather than input a predetermined wastage rate, our model calculates open vial wastage over the course of the simulation based on the number of doses per vial of each vaccine, the number of children who arrive to be vaccinated each day, and how long a vial can remain open. In the model, procurement of multidose vaccines is based on expected population demand across each vaccination location, and a 25% buffer stock is added to account for potential open vial wastage.
5. Experimental scenarios
Each experiment simulated the operations of an immunization supply chain over the course of one year. For each experiment, the routine immunization schedule used in each of the three immunization programs (Benin, Bihar, and Mozambique) included one of the nine rotavirus vaccine presentations. We ran 12 experimental scenarios for each of the three supply chain models. The first nine scenarios simulated the impact of introducing each one of the nine rotavirus vaccines, one at a time, into the routine immunization program under existing open vial policy guidelines. Three additional scenarios explored the impact of allowing the five-dose and ten-dose Rotavac and five-dose Rotavac 5D to remain open for 28 days. While we compared results across all scenarios, the results report comparisons for rotavirus vaccine presentations of equal or lesser number of doses. For each single-dose scenario, the results report comparisons to other single-dose scenarios; the two-dose scenario, results report comparisons to each of the single-dose scenarios, for each five-dose scenario, results report comparisons to each of the single-dose, two-dose, and five-dose scenarios, and for each ten-dose scenario, results reports comparisons to the all other scenarios.
Outcomes for each scenario included rotavirus vaccine availability (i.e., the number of successful rotavirus immunizations administered as a percentage of total rotavirus immunizations needed), total vaccine availability (i.e., the number of successful immunizations administered as a percentage of all of the immunizations needed), open vial wastage (i.e., partially used doses expired as a percentage of all doses opened), average peak refrigerator capacity utilization (i.e., the maximum percentage of available refrigerator capacity occupied by products at any time), average peak freezer capacity utilization (i.e., the maximum percentage of available freezer capacity occupied by products at any time), average peak transport capacity utilization (i.e., the maximum percentage of available transport capacity needed to complete any shipment, averaged across all shipping routes), and each of the cost components described above. Average peak transport capacity utilization can go beyond 100% (size of vaccine shipment equates to the entire capacity of vehicle) to indicate that the size of the shipment is larger than the available transport capacity, and additional trips may be needed. For each of the cost components, we calculate the costs per dose administered across all vaccines (e.g., the logistics cost per dose administered is the total logistics costs for all vaccines divided by the total number of doses of all vaccines administered).
6. Results
6.1. Impact of implementing a single-dose rotavirus vaccine
Across all three supply chains, using Rotarix RV1 MMP, the single-dose vaccine with the smallest volume-per-fully-immunized child (FIC), led to the highest rotavirus vaccine availability (49%-80%) and total vaccine availability (56%-79%) across the three supply chains, compared to implementing any other single-dose rotavirus vaccine (Fig. 1). Conversely, using the liquid ROTASIIL RV5, with the largest volume-per-FIC, resulted in the lowest rotavirus vaccine availability and total vaccine availability (31%-66% and 42%-68%, respectively). In Benin, for example, using the Rotarix RV1 MMP compared to the liquid ROTASIIL RV5 increased rotavirus vaccine availability by 14% and total vaccine availability by 11%.
Fig. 1.
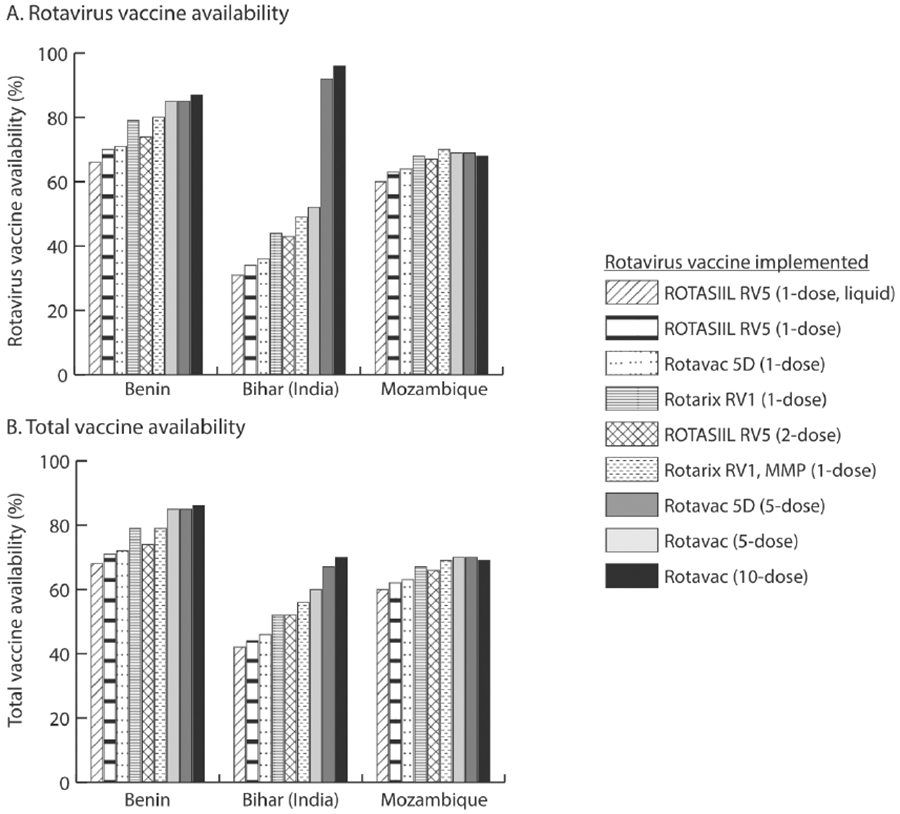
Rotavirus and total vaccine availability across different rotavirus vaccine scenarios.
Reducing the volume-per-FIC of single-dose vaccines reduced the amount of cold chain space being utilized per rotavirus vaccine, which increased the space available for procurement and storage of rotavirus and other vaccines (Fig. 2). For example, in Bihar, the single-dose Rotarix RV1 MMP vaccine reduced peak transport capacity utilization (i.e., the maximum percentage of available transport capacity needed to complete any shipment) by 50% across all shipping routes, and peak refrigerator capacity utilization (i.e., the maximum percentage of available refrigerator capacity occupied by products at any time) by 8% across all storage locations, compared to the liquid ROTASIIL (Fig. 2). Additionally, in Benin, using the Rotarix RV1 MMP reduced peak transport capacity utilization by 67% and peak refrigerator capacity utilization by 3%.
Fig. 2.
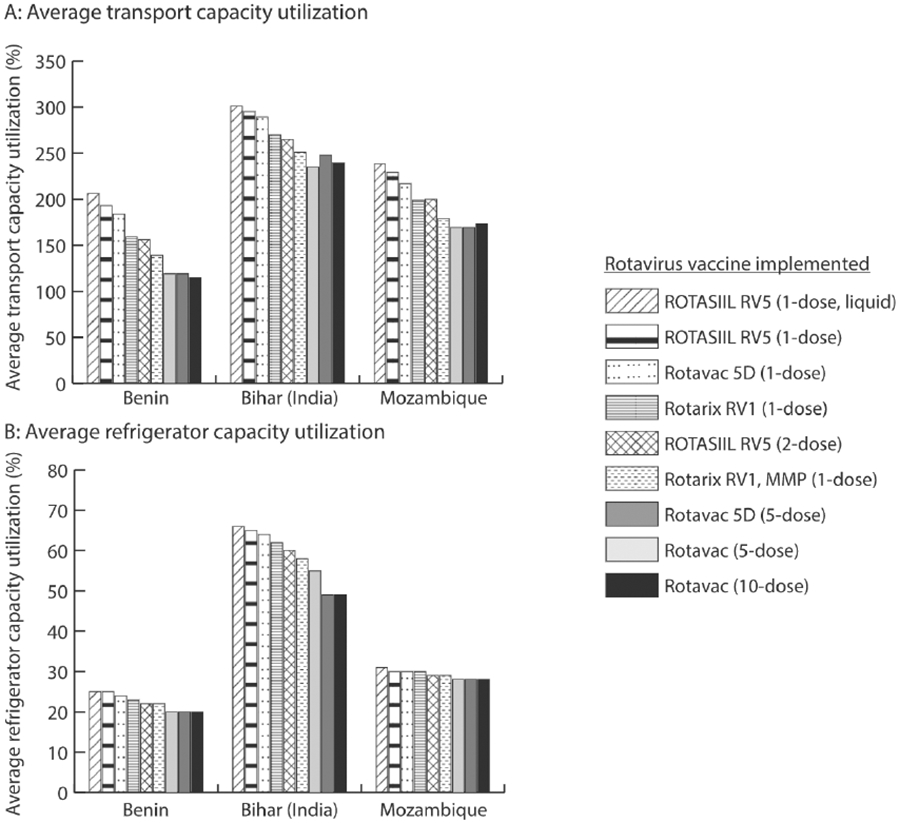
Average transport and refrigerator capacity utilization across different rotavirus vaccine scenarios.
Using the single-dose Rotarix vaccines (RV1 and RV1 MMP) resulted in slightly higher total costs per dose administered (i.e., the combined costs of logistics, vaccine procurement, and vaccine disposal) between $0.02 and $0.10 across the supply chains compared to the other single-dose vaccines (Fig. 3), due to its slightly higher price-per-dose (Table 2). Further, as the only vaccine requiring reconstitution and therefore requiring a reconstitution syringe, using the single-dose ROTASIIL vaccine increased the disposal cost per dose administered by $0.01 compared to other single-dose vaccines.
Fig. 3.
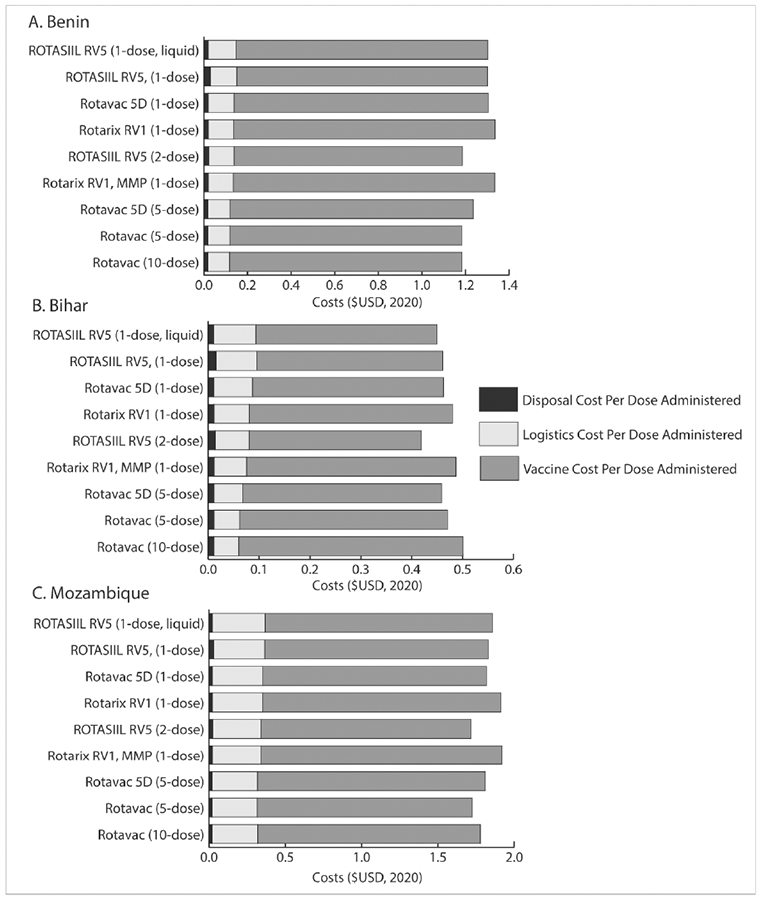
Total and component costs per dose administered ($USD) across different rotavirus vaccine scenarios.
NB: Cost per dose administered is across all vaccine doses administered in the immunization program
7. Impact of implementing a two-dose rotavirus vaccine
In all three supply chains, using two-dose ROTASIIL led to higher rotavirus vaccine availability and total vaccine availability than using any single-dose ROTASIIL (liquid or lyophilized) or single-dose Rotavac 5D vaccine. For example, in Bihar, using two-dose ROTASIIL compared to single-dose, liquid ROTASIIL resulted in 10% higher total vaccine availability and 12% higher rotavirus vaccine availability. However, in comparison to both single-dose Rotarix vaccines, two-dose ROTASIIL resulted in up to 3% lower vaccine availability in Mozambique and up to 6% lower vaccine availability in Benin and Bihar (Fig. 1). While the single-dose Rotarix RV1 had slightly higher volume-per-FIC, the increase in rotavirus open vial wastage from 0% to between 2% and 5% when using the two-dose ROTASIIL vaccine resulted in decreased overall vaccine availability.
Using two-dose ROTASIIL led to decreased transport capacity utilization and refrigerator capacity utilization (due to its smaller volume-per-FIC) compared to four of the single-dose rotavirus vaccines (Fig. 2). However, compared to the Rotarix RV1 MMP vaccine, two-dose ROTASIIL transport utilization was 14%, 17%, and 21% higher and refrigerator capacity utilization was 1.5%, 0.5%, 0.4% higher in Bihar, Benin, and Mozambique, respectively, as the Rotarix RV1 MMP has a smaller total volume-per-FIC than two-dose ROTASIIL. Overall, the two-dose ROTASIIL vaccine resulted in a decreased total cost per dose administered between $0.03 and $0.20 compared to any single-dose vaccine, which reduced logistics costs, and its lower price-per-dose (Table 2).
8. Impact of implementing a five-dose rotavirus vaccine
Implementing either the five-dose Rotavac or Rotavac 5D increased rotavirus and total vaccine availability compared to single- and two-dose rotavirus vaccines (Fig. 1), except in Mozambique, where using the single-dose Rotarix RV1 MMP resulted in a 1% higher rotavirus vaccine availability compared to five-dose vaccines. Even though rotavirus open vial wastage increased 6% to 13% compared to using two-dose ROTASIIL, using five-dose vaccines led to more efficient utilization of already available physical storage across each supply chain (Fig. 2) and improved overall vaccine availability between 1% and 11%. In Benin, implementing five-dose Rotavac, which is stored primarily in freezers, reduced refrigerator utilization at the central store from 100% (i.e., fully utilized) to 93%, which freed up space for other vaccines and increased overall vaccine availability by 6%. This switch increased the peak freezer capacity utilization from 2.5% to 11.6% on average across all storage locations in Benin. In Bihar, storing five-dose Rotavac in freezers compared to refrigerators, increased rotavirus vaccine availability by 40%. This switch increased average peak freezer capacity utilization from 4% to 15% in Bihar.
Using either five-dose rotavirus vaccines lowered logistics cost per dose between $0.01-$0.02 compared to Rotarix RV1 MMP and two-dose ROTASIIL. Both of the five-dose vaccines resulted in lower total costs per dose administered ($0.02 - $0.20) compared to Rotarix RV1 MMP. However, only the five-dose Rotavac vaccine resulted in similar total cost per dose administered compared to two-dose ROTASIIL.
When the five-dose rotavirus vaccines remained open for 28 days (instead of six hours) the impact of using five-dose Rotavac and Rotavac 5D compared to single-dose and two-dose rotavirus vaccines further increased. In all the three supply chains, open vial wastage due to rotavirus decreased to 0% (a 16%-18% reduction). While vaccine availability increased by less than 1%, fewer vials are needed, resulting in a lower total cost per dose administered compared to all single-dose vaccines. In Mozambique, implementing a five-dose Rotavac vaccine that could remain open for 28 days resulted in the highest total vaccine availability (71%) compared to all other rotavirus vaccines.
9. Impact of implementing a ten-dose rotavirus vaccine
Implementing ten-dose Rotavac resulted in the highest rotavirus and total vaccine availability in Benin and Bihar compared to all other rotavirus vaccines, but led to a decreased rotavirus and total vaccine availability in Mozambique compared to either of the five-dose vaccines (Fig. 1). In Benin, using ten-dose Rotavac resulted in the most efficient utilization of available physical storage within the cold chain system, reducing transport utilization by 4% and refrigerator utilization by about 1% compared to using five-dose Rotavac. This vaccine also resulted in the lowest total cost per dose administered. In Bihar, using the ten-dose Rotavac freed up 6% of constrained refrigerator space compared to the five-dose Rotavac 5D (resulting in an increase of freezer capacity utilization of approximately the same amount), increasing total vaccine availability by 10%, yet the increase in open vial wastage resulted in higher vaccine costs and the highest total cost per dose administered across all rotavirus vaccines. In Mozambique, where there was more refrigerator capacity, the increase in rotavirus open vial wastage of 18% compared to using the five-dose rotavirus vaccines outweighed the benefits of the decreased volume-per-FIC. This resulted in a 1% decrease in total vaccine availability compared to implementing the five-dose rotavirus vaccines, and a $0.06 increase in the total cost per dose administered.
Allowing the ten-dose rotavirus vaccine to remain open for 28 days (instead of six hours) resulted in the highest vaccine availability and lowest total cost per dose administered compared to all other rotavirus vaccines in Benin and Bihar (except the two-dose rotavirus vaccine in Bihar, which was slightly cheaper). For example, in Benin, the total vaccine availability was at least 1% higher than when any other vaccine was implemented, with more than 100,000 additional vaccine doses administered. Even in Mozambique, where the five-dose Rotavac resulted in slightly higher vaccine availability, implementing the ten-dose Rotavac resulted in a further $0.02 decrease in the total cost per dose administered compared to the five-dose Rotavac.
10. Discussion
Our results show that countries that implement five-dose or ten-dose rotavirus vaccines consistently achieve both higher rotavirus and total vaccine availability compared to using either single-dose or two-dose rotavirus vaccines. However, in locations like Mozambique, with relatively fewer storage constraints and smaller vaccination session sizes, using the smallest single-dose rotavirus vaccine presentation resulted in similar levels of vaccine availability compared to the five- and ten-dose rotavirus vaccines. Across all three supply chain systems, using five- and ten-dose rotavirus vaccines consistently reduced refrigerator and transport utilization, freeing up space for all vaccines. Further, implementing five- and ten-dose rotavirus vaccines often resulted in lower total costs per dose administered, including in the costs of logistics, vaccine procurement, and vaccine waste. Allowing the five- and ten-dose vaccines to remain open for 28 days (compared to six hours), resulted in the highest total vaccine availability of all scenarios explored, with five-dose Rotavac achieving the highest vaccine availability in Mozambique and ten-dose Rotavac achieving the highest vaccine availability in Benin and Bihar. However, this policy has not yet been approved by the WHO.
While the benefits of using multi-dose rotavirus vials are clear in each vaccine supply chain, the location with the highest number of people per vaccination site (i.e., Bihar, India) showed the clearest benefits from switching to multi-dose vials (11%-25% increase in vaccine availability in Bihar using 5-dose Rotavac compared to single-dose vaccines, compared to a 1%-11% increase in Mozambique). The high demand per location increases stress on the storage capacity, particularly at the service delivery level, which is relieved by switching to multi-dose vials. Further, the greater number of children seeking vaccination each day means that fewer doses of multidose vaccines will be wasted.
As many vaccine supply chains face storage and transport constraints, including outdated or nonfunctional refrigerators and freezers and a lack of vehicles for vaccine shipments, optimizing the use of existing cold chain infrastructure will be critical [16,26,27]. Further, with the ongoing and planned introduction of new vaccines (e.g., human papilloma virus vaccine, COVID-19 vaccines) to many immunization programs, vaccine supply chains could be further strained [28,29]. This is especially important as countries transitioning from Gavi support may have fewer resources to purchase new cold chain equipment or properly maintain existing equipment [30,31]. Even for countries who may not have information on the extent of constraints within their system, our results indicate that using multi-dose rotavirus vaccines will provide benefits across a variety of supply chain systems.
Across all three supply chains, switching to multi-dose vials consistently decreased the total logistics costs per dose administered. The switch to multi-dose vials decreased refrigerator and transport utilization and, therefore, reduced the number of shipments that needed to be made to meet demand, driving down logistics costs. In Benin and Mozambique, the switch to five- and ten-dose Rotavac also reduced the vaccine procurement costs per dose administered. However, in Bihar, where total vaccine procurement costs increased substantially as a result of large increases in total vaccine availability, vaccine procurement costs per dose administered actually increased slightly. Further, although the vaccine procurement costs per dose administered were consistently higher for the Rotarix vaccines, these two vaccines require only two doses to fully immunize a child, while other rotavirus vaccine presentations require three.
When deciding which vaccines to use in their immunization program, countries may tend to focus on clinic-level factors, such as open vial wastage, [32-34] and fail to see the broader systems-wide effects (e.g., how a vaccine’s size may constrain storage space for other vaccines). By taking a systems approach and looking at the supply chain as a whole, we show that while wastage does increase when switching to multi-dose vials, the reductions in cold chain utilization have a net positive effect on the availability of all vaccines and the costs per dose administered. Thus, making decisions based on clinic-level or limited evidence, rather than the potential systems-wide effects, may have unintended negative consequences, such as a reduction in cold chain space and decreased vaccine availability.
11. Limitations
The models developed for this study aim to represent complex vaccine supply chain systems, including infrastructural components, human resources, and policies; however, based on the inherent nature of computational modeling, not all factors influencing vaccine delivery can be captured. Further, vaccine supply chains are constantly changing and adapting, and therefore, models may not reflect the specifics of the current supply chain in each location. Our analysis did not consider the potential effect on patient safety of switching to multi-dose vials (e.g., re-use of needles, improper handling/storage) and subsequent costs of these. Further, our analysis did not consider the potential costs of training associated with switching from a single-dose to multidose vial or the difference in time it may take to administer one rotavirus presentation compared to another. While our model does capture the disposal costs of vaccine supplies, our model does not include the transport costs of vaccine supplies that are transported outside of the cold chain (e.g., syringes). Additionally, as it is not required and practices may vary between countries, we modeled storing the diluent for lyophilized rotavirus vaccines outside the cold chain.
12. Conclusion
When deciding which vaccines to use in their immunization program, countries may tend to focus on clinic-level factors, such as open vial wastage, and fail to see the broader systems-wide effects (e.g., how a vaccine’s size may constrain storage space for other vaccines). Our results show that countries that implement five-dose or ten-dose rotavirus vaccines could consistently achieve both higher rotavirus and total vaccine availability compared to using either single-dose or two-dose rotavirus vaccines.
Acknowledgements
This work was supported in part by Bharat Biotech International Limited and by the National Institute of General Medical Sciences (NIGMS) as part of the Models of Infectious Disease Agent Study (MIDAS) network under grant R01 GM127512. The funders did not have any role in the study design, collection, analysis and interpretation of data, writing the report, and the decision to submit the report for publication. The authors of this manuscript are responsible for its content, including data analysis.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].International Vaccine Access Center (IVAC) Johns Hopkins Bloomberg School of Public Health. VIEW-hub. 2020. [Google Scholar]
- [2].Flannery B, Samad S, de Moraes JC, Tate JE, Danovaro-Holliday MC, de Oliveira LH, et al. Uptake of oral rotavirus vaccine and timeliness of routine immunization in Brazil’s National Immunization Program. Vaccine 2013;31:1523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee BY, Assi TM, Rajgopal J, Norman BA, Chen SI, Brown ST, et al. Impact of introducing the pneumococcal and rotavirus vaccines into the routine immunization program in Niger. Am J Public Health 2012;102:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee BY, Assi TM, Rookkapan K, Wateska AR, Rajgopal J, Sornsrivichai V, et al. Maintaining vaccine delivery following the introduction of the rotavirus and pneumococcal vaccines in Thailand. PLoS ONE 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010;28:2806–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].UNICEF. Rotavirus Vaccine: Supply and Demand Update; 2020. [Google Scholar]
- [7].Assi TM, Brown ST, Djibo A, Norman BA, Rajgopal J, Welling JS, et al. Impact of changing the measles vaccine vial size on Niger’s vaccine supply chain: A computational model. BMC Public Health 2011;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee BY, Norman BA, Assi TM, Chen SI, Bailey RR, Rajgopal J, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine 2010;28:5292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wedlock PT, Mitgang EA, Haidari LA, Prosser W, Brown ST, Krudwig K, et al. The value of tailoring vial sizes to populations and locations. Vaccine 2019;37:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haidari LA, Wahl B, Brown ST, Privor-Dumm L, Wallman-Stokes C, Gorham K, et al. One size does not fit all: the impact of primary vaccine container size on vaccine distribution and delivery. Vaccine 2015;33:3242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee BY, Assi TM, Rookkapan K, Connor DL, Rajgopal J, Sornsrivichai V, et al. Replacing the measles ten-dose vaccine presentation with the single-dose presentation in Thailand. Vaccine 2011;29:3811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pecenka C, Debellut F, Bar-Zeev N, Anwari P, Nonvignon J, Shamsuzzaman M, et al. Re-evaluating the cost and cost-effectiveness of rotavirus vaccination in Bangladesh, Ghana, and Malawi: a comparison of three rotavirus vaccines. Vaccine 2018;36:7472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee BY, Mueller LE, Tilchin CG. A systems approach to vaccine decision making. Vaccine 2017;35:A36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brown ST, Schreiber B, Cakouros BE, Wateska AR, Dicko HM, Connor DL, et al. The benefits of redesigning Benin’s vaccine supply chain. Vaccine 2014;32:4097–103. [DOI] [PubMed] [Google Scholar]
- [15].Lee BY, Haidari LA, Prosser W, Connor DL, Bechtel R, Dipuve A, et al. Re-designing the Mozambique vaccine supply chain to improve access to vaccines. Vaccine 2016;34:4998–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee BY, Wedlock PT, Mitgang EA, Cox SN, Haidari LA Das MK, et al. How coping can hide larger systems problems: the routine immunisation supply chain in Bihar, India. BMJ Glob Health 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Brown ST, Lee BY. Unless changes are made in Benin, multiple storage and transport bottlenecks may prevent vaccines from reaching the population. Vaccine. 2014;32:2518–9. [DOI] [PubMed] [Google Scholar]
- [18].UNICEF. Vaccine Pricing Data. 2020. [Google Scholar]
- [19].Gavi. Detailed Product Profiles for WHO Prequalified Vaccines. 2020. [Google Scholar]
- [20].Bank W. World Bank National Accounts: Population. Growth. 2018. [Google Scholar]
- [21].Bank W. Inflation, consumer prices (annual %). 2018. [Google Scholar]
- [22].Indian Rupee (INR) to US Dollar (USD) Historical Exchange Rates; 2020. [Google Scholar]
- [23].Gavi. Gavi-supported rotavirus vaccines profiles to support country decision making. In: Gavi Secretariat and partners PaRWG, editor.2019. [Google Scholar]
- [24].Organization WH. WHO policy statement: multi-dose vial policy (MDVP): handling of multi-dose vaccine vials after opening. World Health Organization; 2014. [Google Scholar]
- [25].World Health Organization. Monitoring vaccine wastage at country level: guidelines for programme managers; 2005. [Google Scholar]
- [26].Zaffran M, Vandelaer J, Kristensen D, Melgaard B, Yadav P, Antwi-Agyei K, et al. The imperative for stronger vaccine supply and logistics systems. Vaccine 2013;31:B73–80. [DOI] [PubMed] [Google Scholar]
- [27].Lee BY, Haidari LA. The importance of vaccine supply chains to everyone in the vaccine world. Vaccine. 2017;35:4475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. The Lancet 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Organization WH. Controlled temperature chain: strategic roadmap for priority vaccines 2017–2020. World Health Organization; 2017. [Google Scholar]
- [30].Cernuschi T, Gaglione S, Bozzani F. Challenges to sustainable immunization systems in Gavi transitioning countries. Vaccine 2018;36:6858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saxenian H, Hecht R, Kaddar M, Schmitt S, Ryckman T, Cornejo S. Overcoming challenges to sustainable immunization financing: early experiences from GAVI graduating countries. Health Pol Plan 2015;30:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wallace AS, Willis F, Nwaze E, Dieng B, Sipilanyambe N, Daniels D, et al. Vaccine wastage in Nigeria: an assessment of wastage rates and related vaccinator knowledge, attitudes and practices. Vaccine 2017;35:6751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Byberg S, Fisker AB, Thysen SM, Rodrigues A, Enemark U, Aaby P, et al. Cost-effectiveness of providing measles vaccination to all children in Guinea-Bissau. Global Health Action 2017;10:1329968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kanagat N, Krudwig K, Wilkins KA, Kaweme S, Phiri G, Mwansa FD, et al. Health care worker preferences and perspectives on doses per container for 2 lyophilized vaccines in Senegal, Vietnam, and Zambia. Glob Health Sci Pract 2020;8:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


