Abstract
Study Objectives:
Lifestyle-induced weight loss is a complementary therapeutic approach for obstructive sleep apnea (OSA). We aimed at identifying the dose-response relationship between weight loss and OSA severity improvement.
Methods:
This is a secondary analysis of a 6-month clinical trial in 180 adult, overweight/obese moderate-to-severe OSA patients. Participants were randomized to a standard care, a Mediterranean diet, or a Mediterranean lifestyle arm. All patients were prescribed with continuous positive airway pressure (CPAP), while intervention arms additionally participated in a weight-loss dietary/lifestyle intervention. Based on percent change in weight at 6 months, participants were categorized into a weight-stable/gain (WS/GG) group or 3 weight-loss groups (WLG): < 5%WLG, 5%–10%WLG, and ≥ 10%WLG. Polysomnographic data and OSA symptoms were evaluated preintervention and postintervention.
Results:
Respiratory events and oximetry indices improved only in patients who lost weight and improvements were proportional to the degree of weight loss. Median percent change in apnea-hypopnea index (AHI) was −11.7%, − 37.9%, and − 49.3% in the < 5%WLG, 5%–10%WLG, and ≥ 10%WLG, respectively (P < .001). Compared to the WS/GG, the age-, sex-, baseline-, and CPAP use–adjusted relative risk (95% confidence interval) of severe OSA (AHI ≥ 30 events/h) was 0.45 (0.23–0.87) in the 5%–10%WLG and 0.32 (0.17–0.64) in the ≥ 10%WLG; the risk was also lower in the ≥ 10%WLG vs the < 5%WLG (0.42 [0.22–0.82]). Insomnia and daytime sleepiness also improved more in participants exhibiting ≥ 5% weight loss.
Conclusions:
Even a < 5% weight loss can reduce respiratory events, but a ≥ 5% and ideally ≥ 10% weight loss is necessary for reducing the prevalence of severe OSA.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: Mediterranean Diet/Lifestyle Intervention in Obstructive Sleep Apnea; URL: https://clinicaltrials.gov/ct2/show/NCT02515357; Identifier: NCT02515357.
Citation:
Georgoulis M, Yiannakouris N, Kechribari I, et al. Dose-response relationship between weight loss and improvements in obstructive sleep apnea severity after a diet/lifestyle intervention: secondary analyses of the “MIMOSA” randomized clinical trial. J Clin Sleep Med. 2022;18(5):1251–1261.
Keywords: sleep apnea, apnea-hypopnea index, oximetry, weight loss, dose-response analysis
BRIEF SUMMARY
Current Knowledge/Study Rationale: Weight loss is essential for obstructive sleep apnea (OSA) management. Although a positive association between the amount of weight loss and improvements in OSA severity has been reported, this dose-response relationship is not supported by all available data and optimal weight loss goals for OSA require further research.
Study Impact: Our results confirm a dose-response relationship between the degree of weight loss achieved through a dietary/lifestyle intervention and improvements in OSA severity. Even a < 5% weight loss was sufficient for improvements in respiratory events and oximetry indices, but the prevalence of severe OSA reduced only after a ≥ 5% weight loss, and patients achieving a ≥ 10% weight loss exhibited the greatest benefits compared to weight-stable/gain patients.
INTRODUCTION
Obstructive sleep apnea (OSA) represents one of the most common and serious sleep-related breathing disorders, with a high worldwide prevalence of almost 1 billion people 1 and well-established cardiometabolic consequences. 2 Excess body weight has emerged as the strongest modifiable predictor of the onset and severity of OSA. 3 The prevalence of OSA in adults with obesity is almost twice compared to adults of normal weight, and approximately 40% of adult OSA cases are attributable to obesity. 4 The involvement of obesity in the pathophysiology of OSA can be explained through several mechanisms, including (1) central fat accumulation, mainly in abdominal viscera and around the neck, leading to increased mechanical load on upper airways; (2) increased leptin resistance, which can impair central respiratory control and lead to abnormal hypercapnic ventilatory response; (3) expression of proinflammatory cytokines, which negatively impact upper airway neuromuscular control; and (4) a state of increased oxidative stress, which reduces the force-generating capacity of upper airway muscles. 5
On the basis of the well-established causal association between obesity and OSA, lifestyle-induced weight loss is currently advocated as an ancillary treatment for the disease. Available clinical trials exploring the effects of weight loss through lifestyle modifications (usually involving very-low-calorie or low-calorie diets, prescribed either solely or in combination with counseling for increasing physical activity and behavioral change toward reduced caloric intake and healthier dietary habits) on overweight/obese patients with OSA have consistently exhibited significant improvements in weight status, respiratory events, and OSA symptoms (eg, daytime sleepiness). 6– 10 In light of this evidence, guidelines for OSA management from various health organizations recommend weight loss through lifestyle modification for all overweight/obese patients. 11– 14 However, contrariwise to other chronic diseases of cardiometabolic nature, the optimal degree of weight loss for improving OSA severity or eliminating sleep-disordered breathing remains less studied. Previous longitudinal epidemiological studies 15, 16 and a few randomized clinical trials 17– 19 reported a positive association between the magnitude of weight loss and improvements in OSA severity; however, this dose-response relationship is not supported by all available data. 7, 20
Current guidelines for obesity management suggest a 5%–10% weight loss over 6 months as a realistic goal sufficient to produce health benefits in most overweight/obese individuals; an even greater degree of weight loss of ≥10% may be suitable for patients with higher degrees of obesity; however, even a mild weight loss of 3%–5% is considered beneficial for health, especially for obese patients at high risk of or with established obesity-related chronic diseases. 21– 23 Whether these weight loss goals are suitable for the management of sleep-disordered breathing requires further investigation. The aim of the present study was to explore the dose-response relationship between the degree of weight loss and improvements in OSA severity.
METHODS
This is a secondary analysis of the Mediterranean diet/lifestyle Intervention for the Management of Obstructive Sleep Apnea (MIMOSA) study, which was designed as a single-center, single-blind, parallel, randomized, controlled clinical trial to evaluate the effectiveness of a weight-loss Mediterranean dietary/lifestyle intervention on managing OSA. The study protocol has been presented in detail in previous reports 24– 26 and was approved by the Scientific Board of Evangelismos Hospital, Athens, Greece and the Bioethics Committee of Harokopio University, Athens, Greece. The MIMOSA study is registered in the National Institutes of Health database (ClinicalTrials.gov identifier: NCT02515357) and was conducted in agreement with the ethical principles of the Declaration of Helsinki. 27
Study sample and interventions
Eligible candidates were adult (18–65 years old) individuals with a body mass index (BMI) of ≥ 25 kg/m2 and a diagnosis of moderate-to-severe OSA (apnea-hypopnea index [AHI] ≥ 15 events/h) based on an attended overnight in-hospital polysomnography (PSG). Participants were free of major comorbidities, such as diabetes mellitus, cancer, or cardiovascular disease, and had stable lifestyle habits, ie, were not on a weight-loss diet and did not report any recent (within 6 months prior to enrollment) significant change in habitual dietary intake or physical activity level. After providing written consent, participants were randomly allocated to a standard care, a Mediterranean diet, or a Mediterranean lifestyle group. Randomization was restricted 28 based on age, sex, BMI, and AHI levels.
All 3 study groups were prescribed with continuous positive airway pressure (CPAP) therapy as the standard care for OSA management. After the initial PSG for OSA diagnosis, participants underwent an overnight in-laboratory CPAP titration sleep study and were accordingly prescribed with the same auto-CPAP device on the basis of current recommendations for OSA management. 11, 12 Patients were asked to obtain the prescribed CPAP device; were given detailed instructions on CPAP technical standards, cleaning, and maintenance; and were instructed to use it during night sleep on a daily basis throughout the 6-month study period. Patients were also requested to self-monitor and record their daily CPAP use in print forms during the study to evaluate adherence with CPAP therapy. In addition to CPAP prescription, patients of the standard care group were only provided with written general healthy lifestyle advice, while those in the Mediterranean diet/lifestyle groups participated in a dietitian-led behavioral intervention (in groups of 3–5 patients), aiming at a healthier body weight and improving dietary/lifestyle habits according to the Mediterranean pattern. 29 The intervention was structured in 7 1-hour counseling sessions performed over 6 months, each covering specific topics and goals of behavioral change toward weight loss and healthy dietary/lifestyle modification. 24– 26 A detailed description of the dietary/lifestyle intervention is presented in the supplemental material.
Assessments
Body weight was measured preintervention and postintervention to the nearest 100 g using a digital scale (Tanita HD-351, Tokyo, Japan) placed on a hard, flat surface, with participants wearing light clothing and going barefoot. BMI was calculated as (weight [kg] ÷ height2 [m]), and patients were categorized as overweight or obese according to the established BMI cutoff values for Caucasian adults. 30 Percent weight change at the 6-month re-evaluation was calculated as ([{follow-up weight – baseline weight} ÷ baseline weight] × 100), based on which participants were categorized into 4 groups: weight-stable/gain (WS/GG); < 5% weight loss (< 5%WLG); ≥ 5 to < 10% weight loss (5%–10%WLG), and ≥ 10% weight loss (≥ 10%WLG). Waist circumference (WC) was measured to the nearest 0.1 cm between the lowest rib and the superior border of the iliac crest at the end of normal expiration, using a nonelastic measuring tape positioned parallel to the floor and with the participant standing; WC change at the 6-month reevaluation was calculated as (follow-up WC – baseline WC). Participants’ lifestyle habits were also evaluated preintervention and postintervention. Habitual food group consumption was estimated through a validated semiquantitative food frequency questionnaire, 31 and adherence to the Mediterranean diet was assessed through the Mediterranean Diet Score (MedDietScore). 32 The International Physical Activity Questionnaire short form 33 was used to evaluate participants’ physical activity habits, and total daily time (min/day) of physical activity was calculated for each patient. Daily duration of night sleep (hours/day) was self-reported.
OSA diagnosis and severity assessment were based on an attended overnight PSG, performed both at baseline and at the 6-month follow-up at the Center of Sleep Disorders of Evangelismos Hospital following standard procedures. 34 For the follow-up PSG, participants were asked to refrain from CPAP use for 2 nights before testing to partly eliminate the CPAP washout effect that could lead to an underestimation of OSA severity 35, 36 and allow for valid between-group comparisons in OSA severity at the end of the study. In the context of the PSG, brain, eye, and muscle activity; heart rate; respiratory airflow; respiratory effort; and blood oxygen levels were assessed, allowing for a detailed evaluation of sleep architecture and sleep-disordered breathing. All data were simultaneously recorded on a multichannel digital system, and a sole credentialed sleep technologist monitored the recordings and blindly scored all PSG data. Respiratory events were classified as apneas and hypopneas according to the guidelines of the American Academy of Sleep Medicine; apneas were defined as a drop in airflow by ≥ 90% of baseline for ≥ 10 seconds, while hypopneas were defined as a drop in airflow by ≥ 30% of baseline for ≥ 10 seconds in association with a ≥ 3% arterial oxygen desaturation or an arousal. 34 The AHI was calculated as (apneas and hypopneas [events] ÷ total sleep time [hours]). OSA severity was classified as mild, moderate, or severe if the AHI received values of 5–14, 15–29, or ≥ 30 events/h of sleep, respectively. 37 Other respiratory indices, ie, the oxygen desaturation index (ODI), the apnea index (AI), the hypopnea index (HI), and the AHI during nonrapid eye movement sleep (NREM-AHI) and rapid eye movement sleep (REM-AHI) were also recorded and measurements of the minimum oxygen saturation (SaO2) and time with SaO2 < 90% (% of total sleep duration) were obtained through pulse oximetry. For all PSG variables, percent change at the 6-month follow-up was estimated as ([{follow-up value – baseline value} ÷ baseline value] × 100).
Symptoms typically associated with OSA were also evaluated at baseline and the 6-month follow-up. The intensity of insomnia symptoms was assessed through the Athens Insomnia Scale (AIS); the scale ranges from 0 to 24, with higher values indicating greater difficulty in sleep induction and maintenance, and values > 6 are diagnostic of insomnia. 38 Participants’ daytime sleepiness was also assessed through the Epworth Sleepiness Scale (ESS); the scale ranges from 0 to 24, with higher values indicating a greater chance of dozing off or involuntarily falling asleep during various routine daytime activities/situations, and values > 10 are diagnostic of excessive daytime sleepiness. 39 For both scales, percent change at the 6-month follow-up was estimated as ([{follow-up value – baseline value} ÷ baseline value] × 100).
Statistical analysis
The present work is a secondary analysis of the MIMOSA study, which was designed to explore postintervention differences in the AHI between original study groups. A priori power analysis revealed that a sample of 180 patients (45 patients per study group plus a 30% expected dropout rate) would be sufficient to detect a significant difference in postintervention values of the AHI between intervention arms (Mediterranean diet/lifestyle groups) and the standard care group, with at least 80% statistical power and a 5% significance level of 2-sided hypotheses. All analyses were performed in SPSS version 23.0 (IBM Corp. 2015, Armonk, NY) according to the intention-to-treat principle. Missing values were predicted using estimating-equation methods, which involved fitting a statistical model to the observed data with the use of the missing-at-random method. 40 In specific, for every outcome, a statistical equation linking baseline and postintervention data was created for each of the original study groups (standard care, Mediterranean diet, and Mediterranean lifestyle group) based on the data of study completers. The equations were subsequently used to predict missing data for dropouts at the 6-month follow-up.
The Shapiro-Wilk test was applied to evaluate the normality of numerical variables, based on which normally distributed variables are presented as mean ± standard deviation and skewed variables as median (1st, 3rd quartile). Categorical variables are presented as absolute number (relative frequency). Correlations between skewed numerical variables were tested through the Spearman’s correlation coefficient (rho), and a scatterplot was created to illustrate the relationship between percent body weight and percent AHI change. Changes within weight change groups were tested through the paired-samples t-test for normally distributed numerical variables, the Wilcoxon signed-rank test for skewed numerical variables, and the McNemar test for categorical variables. The analysis of variance, the Kruskal-Wallis test, and the chi-square test were used to test differences between groups in normally distributed numerical variables, skewed numerical variables, and categorical variables, respectively, both preintervention and postintervention. Pairwise differences between weight change groups in percent AHI, AIS, and ESS change were tested through the Kruskal-Wallis 1-way analysis of variance. Differences between groups in the follow-up levels of the AHI and the risk of severe OSA were also tested in multivariable models adjusted for participants’ age, sex, baseline levels of the dependent variables, and CPAP use (hours/day) through the analysis of covariance and generalized linear models; results are presented as adjusted mean difference and relative risk with their 95% confidence intervals, respectively. For all pairwise comparisons, the Bonferroni correction was used to adjust for multiplicity. The statistical significance level was set at .05.
RESULTS
From September 2015 to January 2019, 260 patients with OSA were screened for eligibility, of whom 12 declined participation and 61 were noneligible. After enrollment, 7 participants were additionally excluded from analyses on the basis of chronic disease diagnosis (diabetes mellitus [n = 2] and malignancy [n = 1]) or prohibited concomitant care (OSA surgery [n = 2], bariatric surgery [n = 1], and supervised weight loss [n = 1]), leaving a final sample of 180 middle-aged (mean age: 49 ± 10 years), predominantly male (75%), overweight/obese (mean BMI: 35.4 ± 5.9 kg/m2) patients with moderate-to-severe OSA (median AHI: 58 [30, 82] events/h) (Table 1). Of those 180 patients, 53 (29%) were lost to follow-up and their 6-month data were predicted using estimating-equation methods and included in intention-to-treat analysis.
Table 1.
Baseline characteristics of the total study sample and weight-change groups.
| Total (n = 180) | WS/GG (n = 43) | < 5%WLG (n = 38) | 5%–10%WLG (n = 52) | ≥ 10%WLG (n = 47) | P a | |
|---|---|---|---|---|---|---|
| Study group, n (%) | ||||||
| Standard care | 62 (34) | 38 (88) | 20 (52) | 3 (6) | 1 (2) | <.001 |
| Mediterranean diet | 59 (33) | 3 (7) | 9 (24) | 35 (67) | 12 (26) | |
| Mediterranean lifestyle | 59 (33) | 2 (5) | 9 (24) | 14 (27) | 34 (72) | |
| Age, y | 49 ± 10 | 48 ± 11 | 47 ± 10# | 53 ± 9* | 47 ± 9# | .008 |
| Male sex, n (%) | 135 (75) | 31 (72) | 30 (79) | 39 (75) | 35 (75) | .915 |
| Education, y | 14 ± 3 | 13 ± 4 | 15 ± 3 | 14 ± 3 | 15 ± 3 | .107 |
| Annual income, n (%)b | ||||||
| Low | 68 (38) | 16 (37) | 13 (34) | 20 (39) | 19 (40) | .572 |
| Medium | 82 (46) | 21 (49) | 17 (45) | 23 (44) | 21 (45) | |
| High | 30 (16) | 6 (14) | 8 (21) | 9 (17) | 7 (15) | |
| Current smokers, n (%) | 58 (32) | 11 (26) | 10 (26) | 23 (44) | 14 (30) | .169 |
| BMI, kg/m2 | 35.4 ± 5.9 | 33.9 ± 5.6# | 36.1 ± 6.2 | 34.1 ± 5.8# | 37.6 ± 5.6* | .006 |
| Obesity, n (%)c | 143 (79) | 30 (70) | 33 (87) | 36 (70) | 44 (94) | .005 |
| WC, cm | 118 ± 15 | 114 ± 14# | 118 ± 13 | 115 ± 14# | 124 ± 16* | .010 |
| Severe OSA, n (%)d | 136 (76) | 31 (72) | 30 (79) | 39 (75) | 36 (77) | .907 |
| AIS (0–24) | 8.9 ± 4.9 | 8.7 ± 4.7 | 9.6 ± 5.5 | 9.7 ± 4.8 | 7.7 ± 4.6 | .177 |
| Insomnia, n (%)e | 135 (75) | 34 (79) | 28 (74) | 41 (79) | 32 (68) | .567 |
| ESS (0–24) | 11.1 ± 5.3 | 10.3 ± 4.9 | 11.3 ± 5.7 | 11.2 ± 5.2 | 11.4 ± 5.3 | .793 |
| Sleepiness, n (%)f | 93 (52) | 23 (53) | 19 (50) | 27 (53) | 24 (53) | .989 |
| CPAP therapy, n (%) | 135 (75) | 34 (79) | 32 (84) | 36 (69) | 33 (70) | .248 |
Normally distributed and skewed numerical variables are presented as mean ± standard deviation and median (1st, 3rd quartile), respectively, and categorical variables as absolute and relative frequency. Analyses were performed in the intention-to-treat population (n = 180). aP value for differences between weight change groups, as derived from the analysis of variance or the Kruskal-Wallis signed-rank test for normally distributed and skewed numerical variables, respectively, or the chi-square test for categorical variables. P values < .050 indicate statistically significant results. bLow: ≤ 10,000 euros/year; medium: 10,000–20,000 euros/year; high: > 20,000 euros/year. cBMI ≥ 30 kg/m2. dApnea-hypopnea index ≥ 30 events/h of sleep. eAIS > 6. fESS > 10. *,#Different symbols indicate statistically significant differences between groups (P < .050) according to posthoc pairwise comparisons. AIS = Athens Insomnia Scale, BMI = body mass index, CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale, OSA = obstructive sleep apnea, WC = waist circumference, WLG = weight-loss group, WS/GG = weight-stable/gain group.
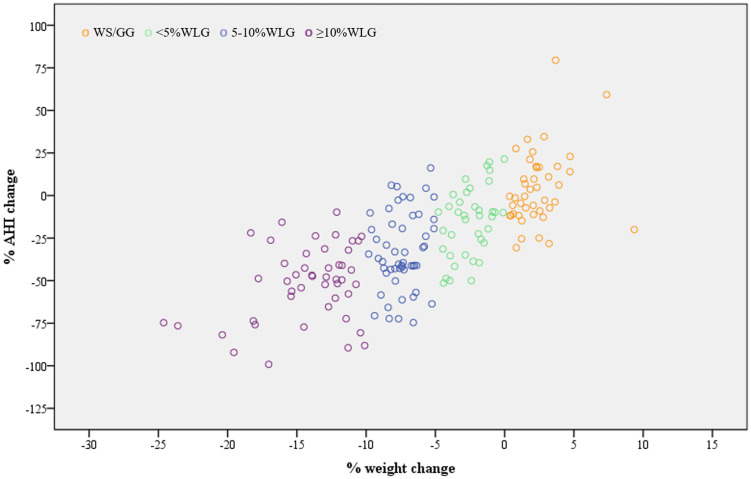
In the total analyzed study sample (n = 180), percent change in body weight and percent change in AHI showed a strong positive correlation (rho: 0.723, P < .001). This was evident both in men (rho: 0.712) and women (rho: 0.751), in patients who were overweight (rho: 0.706) and obese (rho: 0.721), as well as in patients with moderate (rho: 0.699) and severe OSA (rho: 0.742) (all P < .001). A scatterplot graphical illustration (Loess curve) of the association between percent change in body weight and percent change in AHI is presented in Figure 1. Percent change in body weight also correlated positively with percent change in NREM-AHI (rho: 0.649, P < .001), REM-AHI (rho: 0.253, P = .012), AI (rho: 0.519, P < .001), ODI (rho: 0.592, P < .001), % time with SaO2 < 90% (rho = 0.344, P < .001), AIS (rho = 0.474, P < .001), and ESS (rho = 0.510, P < .001) and negatively with percent change in lowest SaO2 (rho: −0.194, P = .010). A significant correlation was also observed between WC change and percent change in AHI (rho = 0.578, P < .001), NREM-AHI (rho = 0.470, P < .001), REM-AHI (rho = 0.239, P = .018), AI (rho = 0.373, P < .001), ODI (rho = 0.385, P < .001), AIS (rho = 0.322, P < .001), ESS (rho = 0.338, P < .001), lowest SaO2 (rho: −0.175, P = .020), and % time with SaO2 < 90% (rho = 0.355, P < .001).
Figure 1. Scatterplot for the association between percent change in body weight and percent change in AHI.
Analyses were performed in the intention-to-treat population (n = 180). AHI = apnea-hypopnea index, WLG = weight-loss group, WS/GG = weight-stable/gain group.
During the 6-month study period, 43 patients (24%) were either weight stable or gained weight, while 38 (21%), 52 (29%), and 47 (26%) exhibited < 5%, 5%–10%, and ≥ 10% weight loss, respectively (Table 1). The majority of patients in the WS/GG were originally assigned to the standard care group (88%), whereas most patients who exhibited a ≥ 5% weight loss were originally allocated to intervention arms (5%–10%WLG: 94%, ≥ 10%WLG: 98%). A significant difference between weight change groups was observed in age, BMI, WC, and obesity presence at baseline (all P < .050). Posthoc comparisons revealed that patients in the 5%–10%WLG were older compared to those in the < 5%WLG (P = .029) and the ≥ 10%WLG (P = .017), while patients in the ≥ 10%WLG had higher baseline BMI and WC values compared to those in the WS/GG (P = .016 and P = .023) and the 5%–10%WLG (both P = .020). On the contrary, there were no significant between-group differences in baseline AHI (P = .587) and the percentage of patients with severe OSA (P = .907). Although all patients were prescribed with CPAP, only 135 started the treatment at baseline; however, the percentage of users was not statistically different among weight change groups (P = .248). During the 6-month study period, mean CPAP use was 3.7 ± 2.3 hours/day in the WS/GG, 4.6 ± 2.5 hours/day in the < 5%WLG, 3.2 ± 2.2 hours/day in the 5%–10%WLG, and 3.7 ± 2.5 hours/day in the ≥ 10%WLG (P = .068).
Median (1st, 3rd quartile) body weight change was 1.08% (0.22, 2.17) in the WS/GG, −2.50% (−3.74, −1.25) in the < 5%WLG, −7.41% (−8.35, −6.53) in the 5%–10%WLG, and −13.0% (−5.9, −11.7) in the ≥ 10%WLG. Obesity prevalence (BMI ≥ 30 kg/m2) was not altered in the WS/GG (from 70% to 72%, P > .999) and in the < 5%WLG (from 87% to 79%, P = .250) but decreased significantly in the 5%–10%WLG (from 69% to 52%, P = .003) and in the ≥ 10%WLG (from 94% to 64%, P < .001). WC values remained unchanged in the WS/GG (from 114 ± 14 cm to 115 ± 16 cm, P = .058) and in the < 5%WLG (from 118 ± 13 cm to 117 ± 14 cm, P = .439) but decreased significantly in the 5%–10%WLG (from 115 ± 14 cm to 111 ± 13 cm, P < .001) and in the ≥ 10%WLG (from 124 ± 16 cm to 113 ± 12 cm, P < .001). With regard to lifestyle habits, the WS/GG did not present any significant changes throughout the study (data not shown). On the contrary, all 3 weight loss groups exhibited a significant increase in mean MedDietScore values (< 5%WLG: from 32.8 ± 4.8 to 35.5 ± 6.4, P = .010; 5%–10%WLG: from 32.5 ± 4.4 to 40.0 ± 4.3, P < .001; ≥ 10%WLG: from 31.3 ± 4.7 to 41.6 ± 3.8, P < .001). The 5%–10%WLG and the ≥ 10%WLG additionally showed a significant increase in median (1st, 3rd quartile) daily time spent in physical activity (from 9.64 [0.00, 32.1] minutes/day to 16.8 [7.50, 43.9] minutes/day, P = .014 and from 15.0 [8.57, 38.6] minutes/day to 49.8 [30.0, 60.0] minutes/day, P = .006, respectively), and mean nighttime sleep duration (from 6.1 ± 1.2 hours/day to 6.6 ± 0.9 hours/day, P = .001 and from 6.2 ± 1.6 hours/day to 7.0 ± 0.8 hours/day, P < .001, respectively).
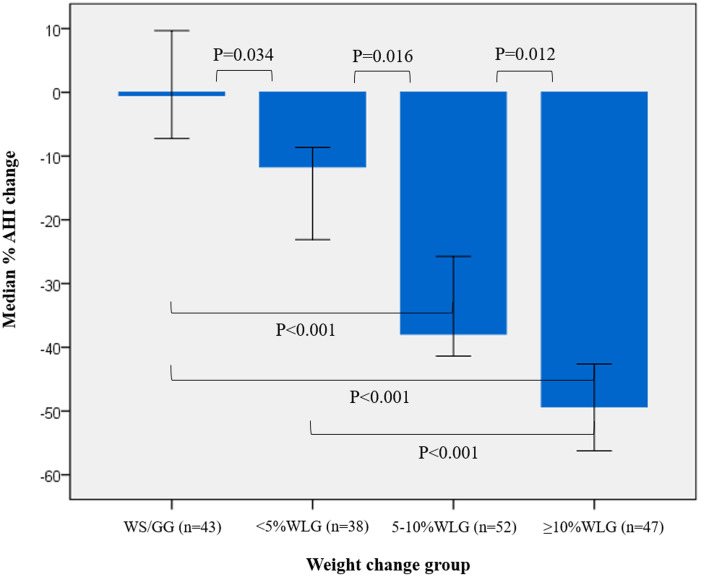
Changes for each weight change group and differences between groups in respiratory and oximetry parameters are presented in Table 2. Median AHI remained unchanged in the WS/GG (P = .488) but decreased significantly in all 3 weight loss groups (all P < .001). Median percent AHI change ranged from −0.50% in the WS/GG to −49.3% in the ≥ 10%WLG, with a significant between-group difference (P < .001). According to pairwise comparisons (Figure 2), a significant dose-response relationship between the degree of weight loss and percent AHI change was evident across all groups, with patients in the ≥ 10%WLG exhibiting the greatest improvement (all P for pairwise comparisons < .050). Besides the AHI, other PSG indices, namely NREM-AHI, REM-AHI, AI, ODI, as well as lowest SaO2 and % time with SaO2 < 90%, also improved only in patients who lost weight, and improvements were proportional to the degree of weight loss achieved (all P for between-group differences < .050) (Table 2). With regard to OSA symptoms, median percent change in AIS was −23.6% (−43.3, 15.0), −35.4% (−65.2, 0.00), −61.3% (−77.7, −37.5), and −64.6% (−80.5, −56.9), while median percent change in ESS was −19.1% (−37.8, 5.54), −32.9% (−52.6, −4.69), −46.9% (−69.7, -31.0), and −70.7% (−81.0, −44.0) in the WS/GG, < 5%WLG, 5%–10%WLG, and ≥ 10%WLG, respectively (both P between groups < .001). Posthoc comparisons revealed that median percent decline in AIS was greater in the 5%–10%WLG and the ≥ 10%WLG compared to both the WS/GG (P = .001 and P = .015, respectively) and the < 5%WLG (P = .024 and P = .002, respectively), while median percent decline in ESS was greater in the 5%–10%WLG compared to the WS/GG (P < .001) and in the ≥ 10%WLG compared to both the WS/GG (P < .001) and the < 5%WLG (P < .001). At the 6-month follow-up, the prevalence of insomnia (AIS > 6) was 56%, 47%, 14%, and 6%, while the prevalence of excessive daytime sleepiness (ESS > 10) was 28%, 24%, 4%, and 2% in the WS/GG, < 5%WLG, 5%–10%WLG, and ≥ 10%WLG, respectively (both P between groups ≤ .001).
Table 2.
Within-group changes and between-group differences in polysomnographic data.
| WS/GG (n = 43) | P a | < 5%WLG (n = 38) | P a | 5%–10% WLG (n = 52) | P a | ≥ 10%WLG (n = 47) | P a | P b | |
|---|---|---|---|---|---|---|---|---|---|
| AHI, events/h | |||||||||
| Baseline | 56 (25, 80) | .488 | 57 (31, 80) | < .001 | 61 (29, 82) | < .001 | 59 (28, 84) | < .001 | .587 |
| 6 months | 54 (29, 75) | 45 (27, 62) | 33 (20, 49) | 23 (15, 40) | < .001 | ||||
| %Δ | −0.50 (−11.1, 16.7) | −11.7 (−32.3, −2.78) | −37.9 (−43.5, −14.7) | −49.3 (−72.3, −34.1) | < .001 | ||||
| NREM-AHI, events/h | |||||||||
| Baseline | 55 (31, 81) | .402 | 54 (31, 74) | .023 | 56 (31, 84) | < .001 | 58 (33, 82) | < .001 | .494 |
| 6 months | 56 (37, 74) | 40 (26, 63) | 27 (19, 43) | 20 (12, 46) | .004 | ||||
| %Δ | 1.40 (−10.5, 17.3) | −8.11 (−34.1, 16.6) | −34.7 (−58.4, −16.81) | −48.0 (−75.5, −29.1) | < .001 | ||||
| REM-AHI, events/h | |||||||||
| Baseline | 63 (31, 94) | .253 | 62 (33, 90) | .004 | 65 (25, 78) | .002 | 64 (30, 95) | < .001 | .905 |
| 6 months | 53 (32, 60) | 45 (17, 64) | 36 (23, 53) | 27 (8, 43) | .045 | ||||
| %Δ | −8.11 (−36.0, 31.7) | −25.4 (−52.7, 26.8) | −38.3 (−74.6, 1.84) | −52.5 (−58.8, −23.63) | .007 | ||||
| AI, events/h | |||||||||
| Baseline | 38 (11, 62) | .481 | 39 (14, 65) | .026 | 44 (15, 70) | < .001 | 42 (14, 65) | < .001 | .209 |
| 6 months | 35 (13, 58) | 28 (14, 37) | 20 (10, 35) | 13 (5, 30) | .122 | ||||
| %Δ | −10.8 (−31.1, 86.6) | −18.5 (−48.7, 32.0) | −42.1 (−66.1, −13.2) | −51.6 (−80.3, −26.1) | < .001 | ||||
| HI, events/h | |||||||||
| Baseline | 16 (8, 25) | .469 | 18 (9, 27) | .898 | 13 (5, 22) | .672 | 14 (8, 22) | .087 | .206 |
| 6 months | 20 (13, 23) | 19 (12, 26) | 12 (10, 20) | 8 (4, 11) | < .001 | ||||
| %Δ | 15.9 (−48.3, 82.5) | 17.1 (−47.3, 98.4) | −3.02 (−44.5, 27.3) | −23.9 (−65.1, 37.8) | .570 | ||||
| ODI, events/h | |||||||||
| Baseline | 49 (27, 64) | .450 | 48 (17, 74) | .029 | 53 (23, 72) | < .001 | 54 (20, 73) | < .001 | .671 |
| 6 months | 47 (24, 51) | 35 (21, 49) | 26 (14, 41) | 16 (7, 40) | .002 | ||||
| %Δ | −6.28 (−24.9, 21.4) | −21.0 (−44.4, 43.6) | −42.9 (−60.3, −22.0) | −63.6 (−75.1, −39.7) | < .001 | ||||
| Minimum SaO2, % | |||||||||
| Baseline | 81 (76, 84) | .155 | 82 (76, 86) | .005 | 81 (72, 86) | < .001 | 78 (71, 85) | < .001 | .414 |
| 6 months | 83 (78, 84) | 84 (79, 86) | 84 (80, 87) | 83 (79, 88) | .403 | ||||
| %Δ | 1.19 (−2.41, 6.04) | 2.47 (−0.24, 7.32) | 4.53 (0.22, 11.4) | 6.02 (1.16, 12.7) | .031 | ||||
| SaO2 < 90%, % TSP | |||||||||
| Baseline | 3.66 (1.11, 10.0) | .372 | 4.00 (0.77, 7.32) | .042 | 3.98 (0.81, 10.8) | .001 | 4.29 (0.80, 8.99) | < .001 | .942 |
| 6 months | 3.40 (0,74, 11.5) | 3.18 (0.73, 6.53) | 2.60 (0.31, 4.59) | 1.85 (0.03, 3.00) | .007 | ||||
| %Δ | 3.63 (−27.3, 30.1) | −11.1 (−26.6, 17.6) | −45.6 (−75.0, 6.99) | −69.7 (−97.5, −20.1) | < .001 | ||||
Variables are presented as median (1st, 3rd quartile). Analyses were performed in the intention-to-treat population (n = 180). aP value for changes within groups, as derived from the Wilcoxon signed-rank test. bP value for differences between weight-change groups, as derived from the Kruskal-Wallis signed-rank-test. a,bP values < .050 indicate statistically significant results. %Δ = percent change, AHI = apnea-hypopnea index, AI = apnea index, HI = hypopnea index; NREM = nonrapid eye movement, ODI = oxygen desaturation index, REM = rapid eye movement, SaO2 = oxygen saturation, TSP = total sleep period, WLG = weight-loss group, WS/GG = weight-stable/gain group.
Figure 2. Median percent change in AHI for different categories of weight change.
I-bars represent 95% confidence intervals for medians. Analyses were performed in the intention-to-treat population (n = 180). Between-group differences were tested through the Kruskal-Wallis 1-way analysis of variance. The Bonferroni correction was applied to adjust for multiple pairwise comparisons. P values < .050 indicate statistically significant results. AHI = apnea-hypopnea index, WLG = weight-loss group, WS/GG = weight-stable/gain group.
With regard to OSA severity, the prevalence of severe disease (AHI ≥ 30 events/h) remained unchanged in the WS/GG (from 72% to 74%, P = .980) and the < 5%WLG (from 79% to 71%, P = .250), but decreased significantly in the 5%–10%WLG (from 75% to 54%, P = .001) and the ≥ 10%WLG (from 77% to 40%, P < .001). At the end of the 6-month intervention, a total of 20 patients moved from moderate/severe (AHI ≥ 15 events/h) to mild OSA (AHI: 5–15 events/h), ie, 1 in the WS/GG (2%), 3 in the < 5%WLG (8%), 5 in the 55–10%WLG (10%), and 11 in the ≥ 10%WLG (23%), with a significant between-group difference (P = .006). Among those 20 patients, only 3 had insomnia (AIS > 6) or excessive daytime sleepiness (ESS > 10) at the 6-month follow-up, suggesting that the remaining 17 no longer met the criteria for OSA and/or need of relevant treatment. Moreover, 3 patients, all in the ≥ 10%WLG (6%), achieved a full remission of OSA (AHI < 5 events/h).
At the 6-month follow-up, significant differences between weight change groups were observed in the AHI and the risk of severe OSA in multivariable models adjusted for participants’ age, sex, CPAP use, and baseline status (Table 3). Compared to the WS/GG, patients in the 5%–10%WLG and the ≥ 10%WLG exhibited lower AHI (P = .006 and P < .001, respectively) and a lower relative risk for severe OSA (P = .018 and P = .001, respectively). The 5%–10%WLG also exhibited lower AHI compared to the < 5%WLG (P = .023), while the ≥ 10%WLG additionally exhibited lower AHI compared to both the < 5%WLG (P < .001) and the 5%–10%WLG (P = .010), and a lower risk for severe OSA compared to the < 5%WLG (P = .012).
Table 3.
Adjusted pairwise differences in the AHI and the risk of severe OSA between weight-change groups at the end of the study.
| Reference Group | Weight Change Group | AHI (events/h) | Severe OSA | ||||
|---|---|---|---|---|---|---|---|
| MDa | 95% CIa | P a | RRa | 95% CIa | P a | ||
| WS/GG | < 5%WLG | −5.83 | −14.8 to 3.12 | .504 | 0.77 | 0.37 to 1.59 | .478 |
| 5%–10%WLG | −15.6 | −23.9 to −7.38 | .006 | 0.45 | 0.23 to 0.87 | .018 | |
| ≥ 10%WLG | −29.8 | −38.1 to −21.4 | < .001 | 0.32 | 0.17 to 0.64 | .001 | |
| < 5%WLG | 5%–10%WLG | −9.79 | −18.7 to −0.86 | .023 | 0.59 | 0.30 to 1.14 | .115 |
| ≥ 10%WLG | −23.9 | −32.9 to −15.0 | < .001 | 0.42 | 0.22 to 0.82 | .012 | |
| 5%–10%WLG | ≥ 10%WLG | −14.1 | −22.3 to −5.96 | .010 | 0.72 | 0.40 to 1.29 | .268 |
Results are presented as adjusted mean differences for the AHI and adjusted relative risk for severe OSA at the 6-month follow-up, with their corresponding 95% confidence intervals, as derived from the analysis of covariance and generalized linear models, respectively. Analyses were performed in the intention-to-treat population (n = 180). aAdjusted for age, sex, baseline levels of the dependent variable, and continuous positive airway pressure use (hours/day). The Bonferroni correction was applied to adjust for multiple pairwise comparisons. P values < .050 indicate statistically significant results. AHI = apnea-hypopnea index, CI = confidence interval, MD = mean difference, OSA = obstructive sleep apnea, RR = relative risk, WLG = weight-loss group, WS/GG = weight-stable/gain group.
DISCUSSION
The present study aimed at exploring the relationship between the magnitude of weight loss and improvements in OSA severity after a dietary/lifestyle intervention in adult, overweight patients with moderate-to-severe disease. Respiratory events and oximetry indices improved only in patients who lost weight and improvements were proportional to the degree of weight loss. When percent change in weight was categorized according to commonly used weight loss targets for obesity management, even a < 5% weight loss was sufficient for small but statistically significant improvements in respiratory events and oximetry indices. However, the prevalence of severe OSA reduced only after a ≥ 5% weight loss and patients achieving a ≥ 10% weight loss exhibited the greatest benefits, ie, a ∼50% decline in median AHI, a > 60% decline in OSA-related symptoms (AIS and ESS), and a ∼70% lower risk of severe OSA compared to weight-stable/gain patients. Improvements after weight loss were significant even though a healthy body weight was not achieved, and the dose-response relationship between weight loss and improvement in OSA severity was evident regardless of self-reported CPAP use.
Weight gain and obesity are well-established risk factors for OSA. The Wisconsin Sleep Cohort study was the first large-scale prospective study to test longitudinal associations between weight change and OSA indices. 15 In a sample of 690 participants from Wisconsin who were followed for 4 years, compared to weight-stable conditions, a 5%, 10%, and 20% weight gain predicted a 15%, 32%, and 70% increase in the AHI, respectively, while weight loss was associated with analogous decreases in the AHI. 15 Similar observations were subsequently reported by the Sleep Heart Health Study among 2,968 middle-aged men and women from several U.S. communities; during a 5-year follow-up period, compared to weight-stable individuals, those who gained weight experienced increases in the respiratory disturbance index in a dose-response manner and vice versa. 16 Several case-control studies have also revealed a significant positive association between the presence of overweight/obesity and odds of OSA in both children and adults. 41 Given the aforementioned data, weight loss is considered an integral part of OSA management, along with CPAP. CPAP was introduced as a therapeutic approach for OSA in 1981 42 and has ever since been recommended as the first-line treatment for the disease. 11, 12 Although CPAP is efficient in minimizing respiratory events and improving OSA symptoms and quality of life, available clinical trials have revealed that, compared with control therapy, CPAP promotes a modest but significant increase in body weight. 43, 44 In our study, patients exhibiting a < 5% weight loss had the highest self-reported CPAP use compared to patients who lost more weight (≥ 5%). Although our study was not designed to examine the effect of CPAP on weight status, this observation might be indirect evidence of the positive association of CPAP with body weight or its potential weight-loss hindering effect, highlighting the need for efficient weight loss interventions in overweight OSA patients initiated on CPAP therapy.
In the context of lifestyle intervention, a mean weight loss of 10%–15% has been shown to reduce the AHI by 20%–50%, but available studies are characterized by a high degree of heterogeneity with regard to the design and nature of interventions applied. 18, 45– 48 A few meta-analyses of clinical trials have also suggested a positive correlation between the degree of weight loss and AHI decline, although this was weak/moderate due to the small number and high heterogeneity of the studies included. 7, 10, 49 Among the aforementioned interventional studies, a few have revealed a dose-response relationship between weight loss and improvements in OSA severity. Papandreou et al 17 randomized 40 obese, moderate-to-severe patients with OSA to a weight-loss Mediterranean diet or a weight-loss prudent diet for a 6-month period. In a secondary analysis of this trial, patients who achieved a ≥ 10% weight loss exhibited a greater decline in the AHI during REM sleep (but not in total AHI) and a greater increase in the lowest SaO2 compared with patients who achieved a < 5% weight loss; however, comparisons between the 5%–10% and < 5% weight loss groups did not reveal significant differences in OSA severity indices. In another controlled clinical trial, 18 81 patients with mild OSA were randomized to either a very-low-calorie diet with supervised lifestyle modification or a control group receiving routine lifestyle counseling for 1 year; at the 12-month follow-up, mean reduction in the AHI and percentage of OSA resolution increased as weight loss increased from 0–5 kg to 5–15 kg and > 15 kg, but statistical significance for within-group changes and between-group differences was not reported. In a subsequent 5-year follow-up analysis of the same patient dataset, 19 only patients who achieved a > 10% weight loss, but not those with < 5% and 5%–10% weight loss, experienced a statistically significant decline in the AHI, ODI, and obstruction and desaturation duration and severity, while OSA severity indices increased in patients who gained weight.
Our results are in line with the available literature showing that weight loss is accompanied by a significant reduction in respiratory events compared to weight stability or weight gain and that the greater the degree of weight loss the greater the improvements in OSA severity. A similar dose-response pattern has been reported for weight loss and improvements in cardiometabolic disorders, with which OSA frequently co-occurs or shares common pathogenetic mechanisms, such as type 2 diabetes mellitus, dyslipidemia, hypertension, and nonalcoholic fatty liver disease. 50 In our sample, improvements in the AHI were mostly attributed to a significant reduction of apneas rather than hypopneas, a fact that can partly be explained by the higher baseline AI values of our study sample compared to HI values or to the regression of some apneas to hypopneas (eg, decline in the percentage of airflow reduction) in the follow-up PSG after weight loss. Similar to previous interventional studies, we observed that a ≥ 10% weight loss is optimal for OSA management, leading to a ∼50% decline in median AHI and even to a complete OSA resolution (AHI < 5 events/h) or no further need of treatment (AHI < 15 events/h combined with resolution of OSA-related symptoms and the absence of comorbidities) in some patients. However, our analyses also revealed that even a lower degree of weight loss, ie, 5%–10% or even < 5%, can lead to significant improvements in respiratory and oximetry indices. This can be attributed to our study sample, mainly consisting of patients with severe OSA (median AHI: 58 events/h) allowing for improvements even with mild weight loss and to the beneficial effects of a healthy Mediterranean-style dietary/lifestyle intervention on OSA severity, which may be evident regardless of improvements in body weight status. 5, 25 As expected, participants who lost more weight had the greatest adherence with intervention goals, ie, exhibited the greatest improvements in lifestyle habits (significant increase in the level of adherence to the Mediterranean diet, physical activity level, and sleep duration), highlighting the need for efficient interventions to achieve long-term behavior change and lifestyle modification with concomitant health benefits in patients with OSA.
In contrast to the aforementioned studies and our results, Dixon et al 20 reported that laparoscopic adjustable gastric banding did not result in a statistically greater reduction in 2-year AHI levels compared to conventional weight loss therapy through dietary modification, despite an almost 6-fold greater weight loss, among 60 participants with obesity and a baseline AHI of ≥ 20 events/h. Moreover, they observed a strong positive correlation between weight loss and AHI decline in the conventional weight loss group, similar to that observed in our study, but not in the bariatric surgery group. The authors concluded that there was a nonlinear link between weight loss and OSA severity with great individual effect variability, as well as a complex rather than a pure mechanical-load pathogenesis of OSA in individuals with obesity. It must be noted that in the study of Dixon et al 20 mean (95% confidence interval) percent weight loss was 20.6% (15.4, 25.7) in the bariatric surgery group, whereas in the present study median (1st, 3rd quartile) percent weight loss was 13.0% (11.7, 15.9) in the ≥ 10%WLG. It is therefore possible that although mild to moderate weight loss is associated with proportional improvements in the AHI, as also supported by our results, the dose-response relationship eventually reaches a plateau with limited additional benefit in OSA severity from further weight reduction.
This is a secondary analysis of a randomized controlled clinical trial with a primary aim to explore the superiority of the combination of a weight-loss dietary/lifestyle intervention with CPAP prescription for OSA management compared to CPAP alone. 24– 26 Our findings confirm previous evidence of a dose-response relationship between the degree of weight loss and improvement in OSA severity assessed through an attended overnight PSG and indicate optimal weight loss goals for overweight/obese patients with OSA. The comparison of weight loss groups with a group of patients who either remained weight-stable or gained weight serving as control is also a strong point of the present work. However, the study sample consisted of predominantly male, overweight, otherwise healthy patients with moderate-to-severe OSA and the present findings cannot be generalized to the whole OSA population. Moreover, 76% of study participants had severe OSA, and although weight loss significantly improved OSA severity, a complete OSA resolution (AHI < 5 events/h) with no need for treatment was only achieved by a few patients; elimination of respiratory events might be more feasible in patients with mild OSA (AHI < 15 events/h) who were not included in the MIMOSA trial. The relatively short intervention period (6 months) and the self-reported CPAP use by study participants represent additional limitations of the present work. Last but not least, although all participants were strictly advised to abstain from CPAP therapy for 2 days prior to the follow-up PSG partly to eliminate the CPAP washout effect on respiratory events, this was not objectively evaluated or confirmed (eg, from CPAP memory data).
In conclusion, a dose-response relationship between the degree of weight loss and improvement in OSA severity and symptoms seems to exist. Although guidelines on OSA management recommend that weight loss should be encouraged in patients who are overweight, more data on the optimal degree of weight loss and appropriate weight-loss interventions for managing the wide spectrum of OSA severity are needed to guide clinical practice. The present analysis supports that even a small degree of weight loss can have a beneficial effect on respiratory events and oxygen desaturation in moderate-to-severe OSA, but clinicians should preferably aim at a ≥ 5% weight loss, and ideally a ≥ 10% weight loss, to achieve clinically meaningful reductions in OSA severity.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Material and financial support for the study was provided by the Postgraduate Program (Master of Science) “Applied Nutrition and Dietetics,” Department of Nutrition and Dietetics, School of Health Sciences and Education, Harokopio University, Athens, Greece. The funder was not involved in study design and methodology, data analyses and interpretation, manuscript preparation, or the authors’ decision to submit the present work for publication. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank A. Kallimanis, sleep technologist, Center of Sleep Disorders, Evangelismos Hospital, 1st Department of Critical Care and Pulmonary Services, Medical School of Athens University, for the processing and scoring of polysomnographic data. Author contributions: MDK, NY, and EV conceived the study’s research hypothesis. MDK and NY conceived the study’s aims, design, and methodology. MDK supervised the dietary/lifestyle intervention. MG, IK, KL, and EP recruited the study sample and performed assessments. MG led the dietary/lifestyle intervention, analyzed data, and drafted the initial manuscript. All authors critically reviewed the manuscript, contributed to further drafts, and approved the final version.
ABBREVIATIONS
- AIS
Athens Insomnia Scale
- AHI
apnea-hypopnea index
- AI
apnea index
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ESS
Epworth sleepiness scale
- HI
hypopnea index
- MD
mean difference
- MIMOSA
Mediterranean diet/lifestyle Intervention for the Management of Obstructive Sleep Apnea
- NREM
nonrapid eye movement
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- REM
rapid eye movement
- RR
relative risk
- SaO2
oxygen saturation
- TSP
total sleep period
- WC
waist circumference
- WLG
weight loss group
- WS/GG
weight-stable/gain group
REFERENCES
- 1. Benjafield AV , Ayas NT , Eastwood PR , et al . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis . Lancet Respir Med. 2019. ; 7 ( 8 ): 687 – 698 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sánchez-de-la-Torre M , Campos-Rodriguez F , Barbé F . Obstructive sleep apnoea and cardiovascular disease . Lancet Respir Med. 2013. ; 1 ( 1 ): 61 – 72 . [DOI] [PubMed] [Google Scholar]
- 3. Romero-Corral A , Caples SM , Lopez-Jimenez F , Somers VK . Interactions between obesity and obstructive sleep apnea: implications for treatment . Chest. 2010. ; 137 ( 3 ): 711 – 719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young T , Peppard PE , Taheri S . Excess weight and sleep-disordered breathing . J Appl Physiol (1985). 2005. ; 99 ( 4 ): 1592 – 1599 . [DOI] [PubMed] [Google Scholar]
- 5. Dobrosielski DA , Papandreou C , Patil SP , Salas-Salvadó J . Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk . Eur Respir Rev. 2017. ; 26 ( 144 ): 160110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomasouli MA , Brady EM , Davies MJ , et al . The impact of diet and lifestyle management strategies for obstructive sleep apnoea in adults: a systematic review and meta-analysis of randomised controlled trials . Sleep Breath. 2013. ; 17 ( 3 ): 925 – 935 . [DOI] [PubMed] [Google Scholar]
- 7. Araghi MH , Chen YF , Jagielski A , et al . Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis . Sleep. 2013. ; 36 ( 10 ): 1553 – 1562 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell LJ , Davidson ZE , Bonham M , O’Driscoll DM , Hamilton GS , Truby H . Weight loss from lifestyle interventions and severity of sleep apnoea: a systematic review and meta-analysis . Sleep Med. 2014. ; 15 ( 10 ): 1173 – 1183 . [DOI] [PubMed] [Google Scholar]
- 9. Carneiro-Barrera A , Díaz-Román A , Guillén-Riquelme A , Buela-Casal G . Weight loss and lifestyle interventions for obstructive sleep apnoea in adults: systematic review and meta-analysis . Obes Rev. 2019. ; 20 ( 5 ): 750 – 762 . [DOI] [PubMed] [Google Scholar]
- 10. Anandam A , Akinnusi M , Kufel T , Porhomayon J , El-Solh AA . Effects of dietary weight loss on obstructive sleep apnea: a meta-analysis . Sleep Breath. 2013. ; 17 ( 1 ): 227 – 234 . [DOI] [PubMed] [Google Scholar]
- 11. Epstein LJ , Kristo D , Strollo PJ Jr , et al . Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults . J Clin Sleep Med. 2009. ; 5 ( 3 ): 263 – 276 . [PMC free article] [PubMed] [Google Scholar]
- 12. Qaseem A , Holty JE , Owens DK , Dallas P , Starkey M , Shekelle P ; Clinical Guidelines Committee of the American College of Physicians . Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians . Ann Intern Med. 2013. ; 159 ( 7 ): 471 – 483 . [DOI] [PubMed] [Google Scholar]
- 13. Parati G , Lombardi C , Hedner J , et al . EU COST Action B26 members . Recommendations for the management of patients with obstructive sleep apnoea and hypertension . Eur Respir J. 2013. ; 41 ( 3 ): 523 – 538 . [DOI] [PubMed] [Google Scholar]
- 14. Hudgel DW , Patel SR , Ahasic AM , et al . American Thoracic Society Assembly on Sleep and Respiratory Neurobiology . The role of weight management in the treatment of adult obstructive sleep apnea. An official American Thoracic Society clinical practice guideline . Am J Respir Crit Care Med. 2018. ; 198 ( 6 ): e70 – e87 . [DOI] [PubMed] [Google Scholar]
- 15. Peppard PE , Young T , Palta M , Dempsey J , Skatrud J . Longitudinal study of moderate weight change and sleep-disordered breathing . JAMA. 2000. ; 284 ( 23 ): 3015 – 3021 . [DOI] [PubMed] [Google Scholar]
- 16. Newman AB , Foster G , Givelber R , Nieto FJ , Redline S , Young T . Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study . Arch Intern Med. 2005. ; 165 ( 20 ): 2408 – 2413 . [DOI] [PubMed] [Google Scholar]
- 17. Papandreou C , Hatzis CM , Fragkiadakis GA . Effects of different weight loss percentages on moderate to severe obstructive sleep apnoea syndrome . Chron Respir Dis. 2015. ; 12 ( 3 ): 276 – 278 . [DOI] [PubMed] [Google Scholar]
- 18. Tuomilehto HP , Seppä JM , Partinen MM , et al . Kuopio Sleep Apnea Group . Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea . Am J Respir Crit Care Med. 2009. ; 179 ( 4 ): 320 – 327 . [DOI] [PubMed] [Google Scholar]
- 19. Kulkas A , Leppänen T , Sahlman J , et al . Amount of weight loss or gain influences the severity of respiratory events in sleep apnea . Med Biol Eng Comput. 2015. ; 53 ( 10 ): 975 – 988 . [DOI] [PubMed] [Google Scholar]
- 20. Dixon JB , Schachter LM , O’Brien PE , et al . Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial . JAMA. 2012. ; 308 ( 11 ): 1142 – 1149 . [DOI] [PubMed] [Google Scholar]
- 21. Jensen MD , Ryan DH , Apovian CM , et al . Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society . Circulation. 2014. ; 129 ( 25 Suppl 2) : S102 – S138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yumuk V , Tsigos C , Fried M , et al .; Obesity Management Task Force of the European Association for the Study of Obesity . European Guidelines for obesity management in adults . Obes Facts. 2015. ; 8 ( 6 ): 402 – 424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stegenga H , Haines A , Jones K , Wilding J ; Guideline Development Group . Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance . BMJ. 2014. ; 349 : g6608 . [DOI] [PubMed] [Google Scholar]
- 24. Georgoulis M , Yiannakouris N , Kechribari I , et al . Cardiometabolic benefits of a weight-loss Mediterranean diet/lifestyle intervention in patients with obstructive sleep apnea: the “MIMOSA” randomized clinical trial . Nutrients. 2020. ; 12 ( 6 ): 1570 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Georgoulis M , Yiannakouris N , Kechribari I , et al . The effectiveness of a weight-loss Mediterranean diet/lifestyle Intervention in the Management of Obstructive Sleep Apnea: Results of the “MIMOSA” randomized clinical trial . Clin Nutr. 2021. ; 40 ( 3 ): 850 – 859 . [DOI] [PubMed] [Google Scholar]
- 26. Georgoulis M , Yiannakouris N , Tenta R , et al . A weight-loss Mediterranean diet/lifestyle intervention ameliorates inflammation and oxidative stress in patients with obstructive sleep apnea: results of the “MIMOSA” randomized clinical trial . Eur J Nutr. 2021. ; 60 ( 7 ): 3799 – 3810 . [DOI] [PubMed] [Google Scholar]
- 27. World Medical Association Declaration of Helsinki . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects . JAMA. 2000. ; 284 ( 23 ): 3043 – 3045 . [PubMed] [Google Scholar]
- 28. Han B , Enas NH , McEntegart D . Randomization by minimization for unbalanced treatment allocation . Stat Med. 2009. ; 28 ( 27 ): 3329 – 3346 . [DOI] [PubMed] [Google Scholar]
- 29. Bach-Faig A , Berry EM , Lairon D , et al . Mediterranean Diet Foundation Expert Group . Mediterranean diet pyramid today. Science and cultural updates . Public Health Nutr. 2011. ; 14 ( 12A ): 2274 – 2284 . [DOI] [PubMed] [Google Scholar]
- 30. Kushner RF . Clinical assessment and management of adult obesity . Circulation. 2012. ; 126 ( 24 ): 2870 – 2877 . [DOI] [PubMed] [Google Scholar]
- 31. Bountziouka V , Bathrellou E , Giotopoulou A , et al . Development, repeatability and validity regarding energy and macronutrient intake of a semi-quantitative food frequency questionnaire: methodological considerations . Nutr Metab Cardiovasc Dis. 2012. ; 22 ( 8 ): 659 – 667 . [DOI] [PubMed] [Google Scholar]
- 32. Panagiotakos DB , Pitsavos C , Arvaniti F , Stefanadis C . Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore . Prev Med. 2007. ; 44 ( 4 ): 335 – 340 . [DOI] [PubMed] [Google Scholar]
- 33. Papathanasiou G , Georgoudis G , Georgakopoulos D , Katsouras C , Kalfakakou V , Evangelou A . Criterion-related validity of the short International Physical Activity Questionnaire against exercise capacity in young adults . Eur J Cardiovasc Prev Rehabil. 2010. ; 17 ( 4 ): 380 – 386 . [DOI] [PubMed] [Google Scholar]
- 34. Berry RB , Budhiraja R , Gottlieb DJ , et al . Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kohler M , Stoewhas AC , Ayers L , et al . Effects of continuous positive airway pressure therapy withdrawal in patients with obstructive sleep apnea: a randomized controlled trial . Am J Respir Crit Care Med. 2011. ; 184 ( 10 ): 1192 – 1199 . [DOI] [PubMed] [Google Scholar]
- 36. Vroegop AV , Smithuis JW , Benoist LB , Vanderveken OM , de Vries N . CPAP washout prior to reevaluation polysomnography: a sleep surgeon’s perspective . Sleep Breath. 2015. ; 19 ( 2 ): 433 – 439 . [DOI] [PubMed] [Google Scholar]
- 37. Sateia MJ . International Classification of Sleep Disorders-third edition: highlights and modifications . Chest. 2014. ; 146 ( 5 ): 1387 – 1394 . [DOI] [PubMed] [Google Scholar]
- 38. Soldatos CR , Dikeos DG , Paparrigopoulos TJ . The diagnostic validity of the Athens Insomnia Scale . J Psychosom Res. 2003. ; 55 ( 3 ): 263 – 267 . [DOI] [PubMed] [Google Scholar]
- 39. Tsara V , Serasli E , Amfilochiou A , Constantinidis T , Christaki P . Greek version of the Epworth Sleepiness Scale . Sleep Breath. 2004. ; 8 ( 2 ): 91 – 95 . [DOI] [PubMed] [Google Scholar]
- 40. Little RJ , D’Agostino R , Cohen ML , et al . The prevention and treatment of missing data in clinical trials . N Engl J Med. 2012. ; 367 ( 14 ): 1355 – 1360 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dong Z , Xu X , Wang C , Cartledge S , Maddison R , Shariful Islam SM . Association of overweight and obesity with obstructive sleep apnoea: a systematic review and meta-analysis . Obes Med. 2020. ; 17 : 100185 . [Google Scholar]
- 42. Sullivan CE , Issa FG , Berthon-Jones M , Eves L . Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares . Lancet. 1981. ; 1 ( 8225 ): 862 – 865 . [DOI] [PubMed] [Google Scholar]
- 43. Drager LF , Brunoni AR , Jenner R , Lorenzi-Filho G , Benseñor IM , Lotufo PA . Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials . Thorax. 2015. ; 70 ( 3 ): 258 – 264 . [DOI] [PubMed] [Google Scholar]
- 44. Hoyos CM , Murugan SM , Melehan KL , et al . Dose-dependent effects of continuous positive airway pressure for sleep apnea on weight or metabolic function: individual patient-level clinical trial meta-analysis . J Sleep Res. 2019. ; 28 ( 5 ): e12788 . [DOI] [PubMed] [Google Scholar]
- 45. Johansson K , Neovius M , Lagerros YT , et al . Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial . BMJ. 2009. ; 339 : b4609 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Foster GD , Borradaile KE , Sanders MH , et al . Sleep AHEAD Research Group of Look AHEAD Research Group . A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study . Arch Intern Med. 2009. ; 169 ( 17 ): 1619 – 1626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nerfeldt P , Nilsson BY , Mayor L , Uddén J , Friberg D . A two-year weight reduction program in obese sleep apnea patients . J Clin Sleep Med. 2010. ; 6 ( 5 ): 479 – 486 . [PMC free article] [PubMed] [Google Scholar]
- 48. Shechter A , Foster GD , Lang W , et al . Sleep Ahead Research Group of the Look Ahead Research Group . Effects of a lifestyle intervention on REM sleep-related OSA severity in obese individuals with type 2 diabetes . J Sleep Res. 2017. ; 26 ( 6 ): 747 – 755 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sutherland K , Chapman JL , Cayanan EA , et al . Does craniofacial morphology relate to sleep apnea severity reduction following weight loss intervention? A patient-level meta-analysis . Sleep. 2021. ; 44 ( 3 ): zsaa207 . [DOI] [PubMed] [Google Scholar]
- 50. Ryan DH , Yockey SR . Weight loss and improvement in comorbidity: differences at 5%, 10%, 15%, and over . Curr Obes Rep. 2017. ; 6 ( 2 ): 187 – 194 . [DOI] [PMC free article] [PubMed] [Google Scholar]