Abstract
Study Objectives:
It is unknown whether sleep quality improvements after repetitive transcranial magnetic stimulation (rTMS) are inherent to the intervention or related to improvements in depressive symptoms. This retrospective study examined sleep quality in patients with major depressive disorder before and after treatment with rTMS, adjusting for age, sex, sedative-hypnotic use, number of rTMS treatments, depression severity, and changes in depressive symptoms.
Methods:
Adults with major depressive disorder underwent a 6-week course of 10 Hz rTMS over the left dorsolateral prefrontal cortex. Patients completed the Patient Health Questionnaire-9 depression rating scale and the Pittsburgh Sleep Quality Index before and after treatment. To limit confounding, analysis of depressive symptoms occurred without item 3 (the sleep item) of the Patient Health Questionnaire-9.
Results:
Twenty-one patients completed the study, with a mean (± standard deviation) baseline Pittsburgh Sleep Quality Index score of 12.0 (± 3.8), compared to 10.5 (± 4.3) posttreatment (P = .01). The mean baseline Patient Health Questionnaire-9 score without item 3 was 17.3 (± 3.0), compared to 12.2 (± 4.9) posttreatment (P = .0001). Pittsburgh Sleep Quality Index and modified Patient Health Questionnaire-9 changes were uncorrelated in nonadjusted and adjusted linear regression models and in the Spearman rank-order correlation.
Conclusions:
Mood and sleep quality improved independently after rTMS treatment, even after adjusting for age, sex, sedative-hypnotic use, number of rTMS treatments, and depression severity. These findings suggest that rTMS exerts direct effects on both mood and sleep in patients with major depressive disorder.
Citation:
Collins AR, Cheung J, Croarkin PA, Kolla BP, Kung S. Effects of transcranial magnetic stimulation on sleep quality and mood in patients with major depressive disorder. J Clin Sleep Med. 2022;18(5):1297–1305.
Keywords: repetitive transcranial magnetic stimulation, major depressive disorder, sleep quality, insomnia, neuroplasticity
BRIEF SUMMARY
Current Knowledge/Study Rationale: The effects of repetitive transcranial magnetic stimulation (rTMS) on sleep are not well understood, and results from studies evaluating the effects of rTMS on sleep quality in patients with major depression have been mixed. In particular, it is uncertain whether sleep benefits from rTMS in patients with major depression are mediated by the intervention itself or by treatment of the underlying mood disorder.
Study Impact: We found that self-reported sleep quality improved after rTMS in patients with major depressive disorder and that this improvement was independent of changes in mood. This study offers evidence that rTMS may directly alleviate sleep impairment in patients with major depressive disorder.
INTRODUCTION
The effects of repetitive transcranial magnetic stimulation (rTMS) on sleep are not well understood. rTMS is thought to affect sleep by regulating altered cortical states and increasing slow-wave activity, a marker of synaptic strength. 1– 3 Compared to healthy control patients or sham stimulation, rTMS has shown benefit for patients with narcolepsy, restless legs syndrome, obstructive sleep apnea, and primary insomnia. 4 However, a recent systematic review of rTMS in primary insomnia found that 73.5% of the effect of active rTMS was produced by sham rTMS. 5 Although multiple studies have indicated self-reported sleep improvements after rTMS, objective sleep improvements, such as those measured by polysomnography and actigraphy, have been inconsistent. 6
Studies exploring the effects of rTMS on sleep quality in patients with depression have been mixed. Some studies report that rTMS improves sleep quality in patients with major depressive disorder (MDD), 7, 9– 12 even in those who experience no improvements in the underlying mood disorder. 13 Others report that rTMS relieves depressive symptoms yet has no direct effects on sleep in patients with depression. 14, 15 The latter finding has led researchers to conclude that rTMS is an “arousal-neutral” antidepressant. Biological differences between primary sleep disorders and sleep impairments in depression may underpin these divergent findings. 16 A complicating factor is the reality that mood, anxiety, and sleep disturbances are often bidirectionally related, such that the initial disturbance or cause-effect relationship may remain imperceptible. 17
Existing literature on rTMS, sleep, and depression points to several possible pathways. rTMS may have no effect on sleep in patients with MDD, may improve sleep through a direct mechanism, or may improve sleep through relief of depression. Teasing apart this relationship is important both for exploring rTMS as a future treatment modality for patients with insomnia and for treating patients with overlapping sleep and mood impairments. If sleep alteration is an early marker of depression recurrence, 7 then earlier treatment with a potentially sleep-modifying modality may lead to better outcomes. This study sought to evaluate sleep quality in patients with MDD before and after treatment with rTMS, adjusting for age, sex, sedative-hypnotic use, number of rTMS treatments, depression severity, and changes in depressive symptoms, with the hypothesis that rTMS affects mood and sleep independently.
METHODS
Participants
We conducted a retrospective study of adult patients aged 18 or older who received rTMS for MDD between January 1, 2020, and February 28, 2021, at Mayo Clinic in Rochester, Minnesota. Patients met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition, criteria for moderate to severe MDD and had tried at least 4 antidepressants and psychotherapy. Only participants who provided consent for research were included. 8 Those who received rTMS with nonstandard parameters were excluded, as were those who did not complete both pre- and posttreatment Patient Health Questionnaire-9 (PHQ-9) and Pittsburgh Sleep Quality Index (PSQI) rating scales. The study was carried out in accordance with the latest version of the Declaration of Helsinki and was approved by our Institutional Review Board.
Rating scales
Depressive symptoms were assessed through the PHQ-9, which consists of 9 self-rated questions scored from 0–3 points each, for a total score ranging from 0–27 points. 18 The questions assess anhedonia, mood, sleep, energy, appetite, guilt, concentration, psychomotor agitation or slowing, and suicidal ideation. Higher scores suggest more severe depression. Scores < 5 are considered normal. To remove the confounding factor of sleep in the mood ratings, PHQ-9 item 3, an assessment of sleep impairment, was removed from the PHQ-9 total score to analyze mood independently of sleep.
Sleep was assessed through the PSQI, which consists of 19 self-rated questions and 5 questions rated by a bed partner or roommate, if available. 19 Only self-rated questions are scored. The self-rated questions are combined to form 7 components, each scored from 0–3 points, for a total score ranging from 0–21 points. The components assess self-reported sleep quality, sleep latency and duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Higher scores indicate worse sleep quality. Scores < 5 are considered normal.
rTMS treatment protocol
The NeuroStar (Neuronetics, Inc., Malvern, PA) device was used with standard MDD rTMS parameters of 4-second 10 Hz stimulation trains with 26-second intertrain intervals. Stimulation was applied to the left dorsolateral prefrontal cortex guided by the 5.5-cm rule, with a target intensity of 120% of the motor threshold. A total of 3,000 pulses were given daily (Monday– Friday) within a 37.5-minute treatment window. Up to 30 treatments and 6 taper sessions were offered.
Procedures
The PHQ-9 and PSQI were completed before the initiation of rTMS, usually on the first day of treatment. Both were completed at the conclusion of the acute course of rTMS treatments, immediately after the last treatment. Although the PHQ-9 might be given again at the end of a taper that followed the acute course of treatment, the PSQI was not given at the end of a taper. The PHQ-9 at the end of the acute course was used as the posttreatment score. Because usual clinical care occurred during the course of rTMS, patients could utilize prescribed and over-the-counter sleep aids as needed.
Outcomes
The primary outcome was a change in sleep quality after rTMS treatment, as measured by the total PSQI score. Secondary outcomes were a change in depression severity after rTMS treatment, as measured by the modified PHQ-9, and a change in PSQI component scores.
Statistical analysis
Data values were reported as mean ± standard deviation. Group means were compared using paired t tests or 1- or 2-way analyses of variance (ANOVAs) with Bonferroni post hoc correction. Shapiro-Wilk tests were performed to determine normality. Cohen’s d values were calculated as the difference in 2 means divided by the pooled standard deviation.
To identify potential predictors of positive or negative sleep quality response, post hoc assessments of pre- and posttreatment PSQI scores were performed using paired t tests across binary subsets of participants. These subsets were defined by sex, age, depression severity, self-reported sedative-hypnotic use, and depression response to rTMS. Modified PHQ-9 score changes were assessed similarly. A cutoff of age 50 years was chosen because it provided the most even split of participants (n = 9 participants older than age 50 years, n = 12 participants younger than age 50 years). Severe depression was defined by scores of 20 or greater on the original (unmodified) PHQ-9. Depression response was defined as a reduction in the unmodified PHQ-9 score by 50% or more. Scores on component 6 of the PSQI, a measure of frequency of sedative-hypnotic use, were used to divide the sample into those with less frequent sedative-hypnotic use (answers of 0 or 1) and those with more frequent sedative-hypnotic use (answers of 2 or 3).
To evaluate the effects of antidepressant and sedative-hypnotic medications on the PSQI and modified PHQ-9 score changes, participants were divided into subgroups based on the most common regimens used. Outcomes were compared using a 1-way ANOVA with Bonferroni posthoc correction. Pre- and posttreatment PSQI component scores were compared using a 2-way ANOVA with Bonferroni posthoc correction.
Spearman rank-order correlations were calculated to assess for correlation between the PSQI score change and modified PHQ-9 score change across the total sample and the subgroups of participants. Simple linear regressions were performed to model the relationship between the PSQI score change and the modified PHQ-9 score change across the total sample and the subgroups of participants.
To determine the maximum number of predictor variables to use in each multiple linear regression analysis, the total sample size was divided by 10 (21 participants ÷ 10 ≈ 2 predictor variables). Multiple linear regression was performed to relate the modified PHQ-9 score change coupled with 1 covariate to the PSQI score change. Covariates included age, sex, frequency of sedative-hypnotic use, number of rTMS treatments, and depression severity. The 2-tailed significance level was set to P < .05. All statistical analyses were performed using BlueSky Statistics (version 7.20, Chicago, IL) and GraphPad Prism (version 9.2, San Diego, CA).
RESULTS
Participants
Twenty-one participants were included in the study. The mean age was 43.9 (± 14.8) years, with a range of ages 19–71 years. Fourteen participants (66.7%) were female and 7 (33.3%) were male. Nine participants (42.9%) were employed, 4 (19.0%) were unemployed, and 3 (14.3%) were retired. Sixteen participants (76.2%) were referred by local providers and 5 (23.8%) were from out of state. Nine (42.9%) were insured through commercial insurance, 7 (33.3%) were insured through Medicare, and 5 (23.8%) were insured through Medicaid.
rTMS associated with improved sleep quality per PSQI score
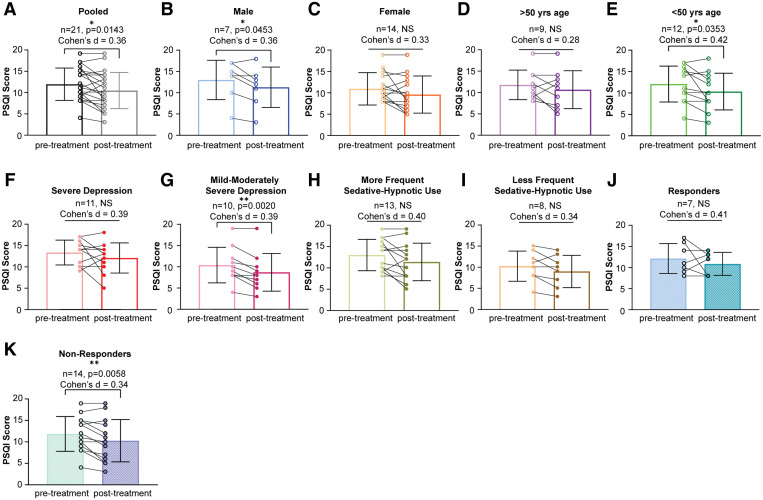
In our total participant sample (n = 21), rTMS treatment was associated with significantly improved sleep quality as measured by paired t test comparison of pre- and posttreatment PSQI scores (P = .0143; Figure 1A). In the subgroup analysis, treatment with rTMS was associated with significantly improved sleep quality in male patients (P = .0453; Figure 1B), those younger than age 50 years (P = .0353; Figure 1E), those with mild to moderately severe depression (P = .0020; Figure 1G), and those whose change in depressive symptoms did not meet the criteria for response to rTMS (P = .0058; Figure 1K). Female participants, those older than age 50 years, those with severe depression, those with more and less frequent sedative-hypnotic use, and those whose depression responded to rTMS did not see significant improvement in sleep quality (Figure 1C, Figure 1D, Figure 1F, Figure 1H, Figure 1I, and Figure 1J). Cohen’s d analyses revealed small to medium effect sizes in all treatment groups (d = 0.2–0.5).
Figure 1. Effects of rTMS on sleep quality, as measured by paired t test comparisons of pre- and post-treatment PSQI scores.
(A) Pooled sample data. (B) Male patients. (C) Female patients. (D) Age > 50 years. (E) Age < 50 years. (F) Severe depression. (G) Mild to moderately severe depression. (H) More frequent sedative-hypnotic use. (I) Less frequent sedative-hypnotic use. (J) Criteria for depression response met. (K) Criteria for depression response not met. NS = not significant, PSQI = Pittsburgh Sleep Quality Index, rTMS = repetitive transcranial magnetic stimulation. *P < .05. **P < .01.
rTMS associated with improved mood per PHQ-9 score minus sleep item
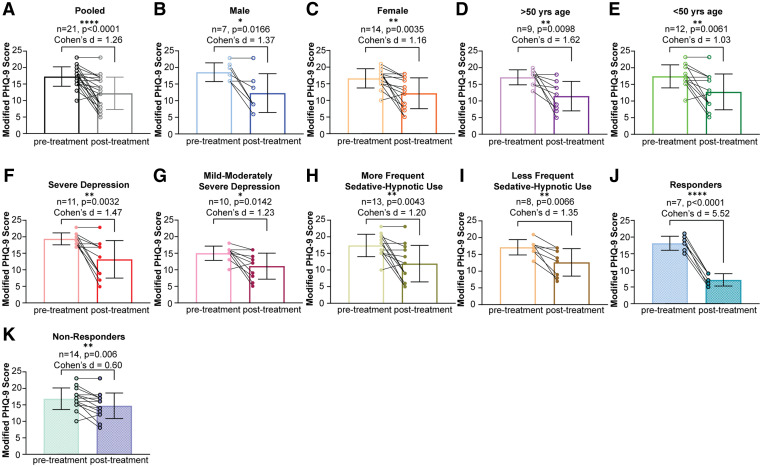
rTMS treatment was associated with significantly improved depressive symptoms in the total participant sample (P < .0001; Figure 2A) and in all subsets of participants: men (P = .0166; Figure 2B), women (P = .0035; Figure 2C), those older than age 50 years (P = .0098; Figure 2D), those younger than age 50 years (P = .0061; Figure 2E), those with severe depression (P = .0032; Figure 2F), those with mild to moderately severe depression (P = .0142; Figure 2G), those with less frequent sedative-hypnotic use (P = .0083; Figure 2H), those with more frequent sedative-hypnotic use (P = .0173; Figure 2I), those whose depression responded to rTMS (P < .0001; Figure 2J), and those whose depression did not respond to rTMS (P = .006; Figure 2K). Cohen’s d analyses revealed a large effect size in all treatment groups (d > 1.0).
Figure 2. Effects of rTMS on depressive symptoms, as measured by paired t test comparisons of pre- and posttreatment modified PHQ-9 scores.
(A) Pooled sample data. (B) Male patients. (C) Female patients. (D) Age > 50 years. (E) Age < 50 years. (F) Severe depression. (G) Mild to moderately severe depression. (H) More frequent sedative-hypnotic use. (I) Less frequent sedative-hypnotic use. (J) Criteria for depression response met. (K) Criteria for depression response not met. PHQ-9 = Patient Health Questionnaire-9, rTMS = repetitive transcranial magnetic stimulation. *P < .05. **P < .01. ***P < .0001.
Post-rTMS improvements in depression and sleep: separable outcomes
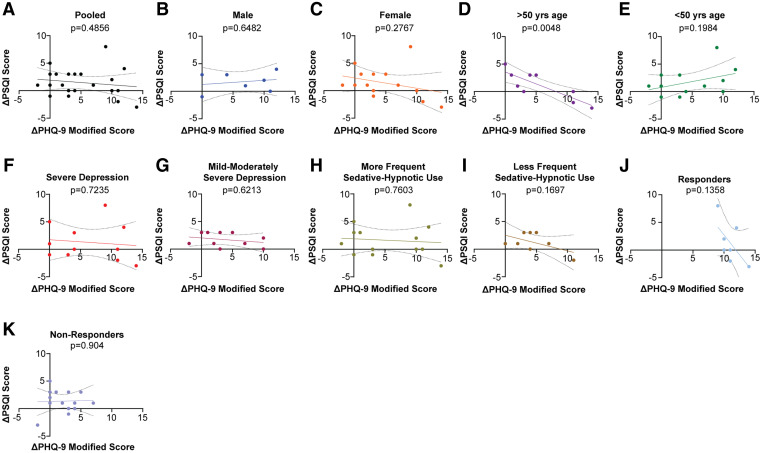
To determine whether post-rTMS improvements in depression and sleep were correlated, we performed simple linear regression to evaluate the modified PHQ-9 score change as a predictor of the PSQI score change in the total sample and in each subset of participants (Figure 3). We found 1 statistically significant correlation: In patients older than age 50 years, the modified PHQ-9 score change negatively predicted the PSQI score change (P = .0048; see Figure 3D). In all other subgroups, there was no significant relationship. We also assessed for correlation between the modified PHQ-9 score change and the PSQI score change by calculating the Spearman rank correlation ρ. In the total sample, the Spearman ρ was –0.1590 (P = .6019), suggesting no significant correlation. Across the subgroups, the Spearman ρ remained nonsignificant, except in participants older than age 50 years (ρ = –0.7319; P = .0488). Multiple linear regression was performed to relate the modified PHQ-9 score change coupled with 1 additional covariate to the PSQI score change. A maximum of 2 predictor variables was considered at a time because of the small total sample size (n = 21). Even after adjusting for age, sex, frequency of sedative-hypnotic use, number of rTMS treatments, and depression severity, no relationships were found to be statistically significant. The results of the multiple linear regression analyses are reported in Table 1.
Figure 3. Simple linear regression models of modified PHQ-9 score change as a predictor of PSQI score change.
(A) Pooled sample data. (B) Male patients. (C) Female patients. (D) Age > 50 years. (E) Age < 50 years. (F) Severe depression. (G) Mild to moderately severe depression. (H) More frequent sedative-hypnotic use. (I) Less frequent sedative-hypnotic use. (J) Criteria for depression response met. (K) Criteria for depression response not met. PHQ-9 = Patient Health Questionnaire-9, PSQI = Pittsburgh Sleep Quality Index.
Table 1.
Results of multiple linear regressions modeling modified PHQ-9 score change + 1 covariate as predictors of PSQI score change.
| Covariate | R 2 | F-Statistic | Intercept | 95% CI | P |
|---|---|---|---|---|---|
| Age | 0.02682 | 0.2480 | 2.129 | –1.912 to 6.169 | .7830 |
| Sex | 0.03573 | 0.3335 | 1.781 | –0.09450 to 3.657 | .7207 |
| Frequency of sedative-hypnotic use | 0.03100 | 0.2879 | 1.700 | –0.5818 to 3.979 | .7532 |
| Number of rTMS treatments | 0.1129 | 1.15 | –5.382 | –17.04 to 6.274 | .3403 |
| Depression severity | 0.04627 | 0.4366 | 3.987 | –3.281 to 11.26 | .6529 |
The relationship between the modified PHQ-9 score change (predictor variable) coupled with 1 additional covariate (predictor variable) and the PSQI score change (outcome variable) was evaluated across the total sample. No relationships were found to be statistically significant. CI = confidence interval around the intercept, F-statistic = variance of group means ÷ mean of within group variances, PHQ-9 = Patient Health Questionnaire-9, PSQI = Pittsburgh Sleep Quality Index, R2 = coefficient of determination, rTMS = repetitive transcranial magnetic stimulation.
Mood and sleep outcomes after rTMS
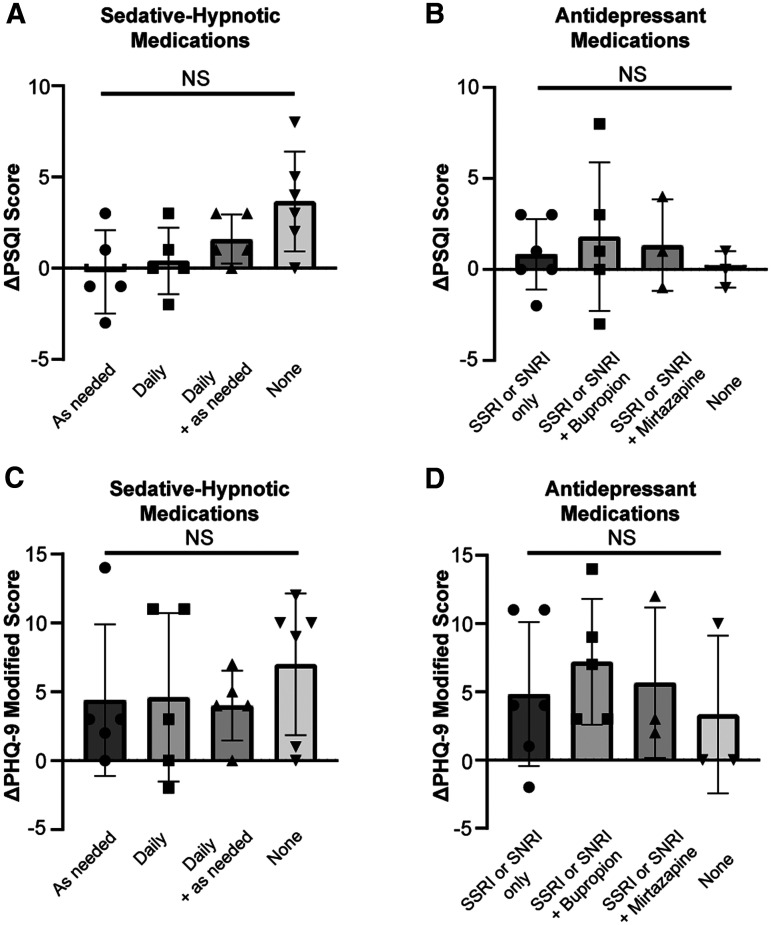
To evaluate whether PSQI and modified PHQ-9 change scores differed significantly between participants based on their medications, we divided the total sample into 4 groups according to the antidepressant regimens and sedative-hypnotic regimens used. We found that most patients (n = 17; 81.0%) fell into 1 of the following 4 antidepressant groups: (1) selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor alone (n = 6), (2) selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor + bupropion (n = 5), (3) selective serotonin reuptake inhibitor or serotonin-norepinephrine reuptake inhibitor + mirtazapine (n = 3), or (4) no antidepressant (n = 3). One participant was on a tricyclic antidepressant, 2 were on serotonin modulators, and 1 was on mirtazapine alone. These patients were excluded from the analysis because we did not have a minimum number of 3 patients per antidepressant regimen to perform ANOVA. All patients fell into 1 of the following 4 sedative-hypnotic groups: (1) as-needed prescription sleep aid (n = 5), (2) daily medication with sedative-hypnotic adverse effects (n = 5), (3) both as-needed prescription sleep aid and daily medication with sedative-hypnotic adverse effects (n = 6), or (4) no sedative-hypnotic medication (n = 5). A 1-way ANOVA with Bonferroni posthoc correction revealed no statistically significant differences in PSQI or modified PHQ-9 outcomes among these subgroups (Figure 4).
Figure 4. One-way ANOVA with Bonferroni posthoc correction depicting changes in sleep quality and mood by medication regimen after rTMS.
(A) PSQI scores across sedative-hypnotic groups. (B) Modified PHQ-9 scores across sedative-hypnotic groups. (C) PSQI scores across antidepressant groups. (D) Modified PHQ-9 scores across antidepressant groups. ANOVA = analysis of variance, NS = not significant, PHQ-9 = Patient Health Questionnaire-9, PSQI = Pittsburgh Sleep Quality Index, rTMS = repetitive transcranial magnetic stimulation, SNRI = serotonin-norepinephrine reuptake inhibitor, SSRI = selective serotonin reuptake inhibitor.
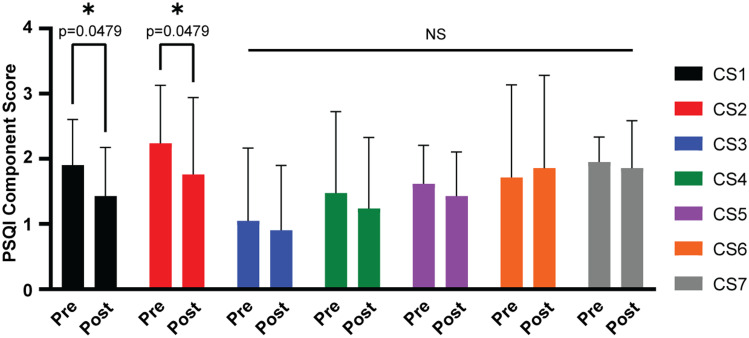
rTMS and improvement in 2 PSQI component scores
To evaluate whether rTMS treatment was associated with improvements in specific elements of sleep quality, we compared pre- and posttreatment scores on each component of the PSQI. A 2-way ANOVA with Bonferroni posthoc correction revealed that component score 1 (a measure of overall sleep quality) and component score 2 (a measure of sleep latency) of the PSQI significantly improved after rTMS (Figure 5).
Figure 5. Two-way ANOVA with Bonferroni post hoc correction depicting PSQI component score changes after rTMS.
ANOVA = analysis of variance, CS1–7 = component scores 1–7, NS = not significant, PSQI = Pittsburgh Sleep Quality Index, rTMS = repetitive transcranial magnetic stimulation. *P < .05.
DISCUSSION
Sleep quality as measured by the total PSQI score significantly improved in this sample of 21 patients with MDD after rTMS treatment. The PSQI subscale scores for component 1 and component 2 showed significant improvement, whereas the changes in the other subscale scores were not significant. This improvement in sleep was independent of improvements in depression scores as measured by the modified PHQ-9.
A recent meta-analysis of 28 rTMS studies involving 2,357 patients with primary insomnia found that compared with sham rTMS, active rTMS led to significantly improved total PSQI scores and scores on each of the 7 component scales. 20 Compared to other treatments, rTMS alone or as an adjunct to other therapy led to significantly improved total PSQI scores, with limited data suggesting improved scores in the 7 components. These results suggest that rTMS may have more extensive effects on self-reported sleep quality in patients with primary insomnia than in patients with depression. Across rTMS studies in depression and primary sleep disorders, the total PSQI score seems to be the most consistent aspect of the PSQI to improve after rTMS treatment. 10, 15, 20
In subgroup analyses, we found that the total PSQI scores improved significantly in male participants, those younger than age 50 years, those with nonsevere-range depression, and those whose depression did not improve by ≥ 50% after rTMS. The total PSQI scores did not improve significantly in female participants, those older than age 50 years, those with severe-range depression, or those whose depression improved by ≥ 50% after rTMS. Modified PHQ-9 scores improved across every subgroup. Because most study participants were prescribed a combination of antidepressants and medications with sedative-hypnotic properties, we did not have adequate sample sizes of participants who were not on antidepressants or medications with sedative-hypnotic properties to generate paired comparisons. However, we were able to divide the total sample into 4 groups according to the combinations of antidepressant medications used and into an additional 4 groups according to the combinations of sedative-hypnotic medications used. We found no statistically significant difference in PSQI outcomes across these medication-based groupings.
The finding of a sex difference in sleep quality response to rTMS among patients with depression has not been reported in the literature. One sham-controlled study showed sleep quality and mood improvement after a 6-week course of rTMS in male inpatients with substance dependence during abstinence. 21 There is general consensus that sex is not a significant factor in rTMS treatment response in depression. 22 However, 1 study showed that postmenopausal women responded less well to rTMS than men or premenopausal women with depression. 23
Our finding that sleep quality in older patients (aged > 50 years) did not significantly improve after rTMS contrasts with several studies indicating the sleep benefits of rTMS in older patients (aged 60 to 80 years). 24, 25 However, studies of rTMS and sleep in older patients are relatively sparse compared to similar studies in younger patients. 26 We speculate that because slow-wave activity declines with age, patients of older age may see less sleep benefit from rTMS, which acts on slow-wave sleep, than younger patients. Further, our sample of adults older than age 50 years was smaller than that of prior studies. 24, 25 Our finding that rTMS was associated with significantly improved mood in patients older than age 50 years is consistent with the results of a recent systematic review and meta-analysis showing that rTMS is effective for the treatment of MDD in the same age group. 27 Nevertheless, younger age is generally regarded as a positive predictor of response to rTMS. 28
Sleep quality did not improve significantly in participants with baseline severe depression, defined by a PHQ-9 score ≥ 20. However, mood symptoms improved significantly in this subgroup. Sleep quality also did not improve significantly in participants who showed a clinical response to rTMS treatment, defined by a reduction in the PHQ-9 score of ≥ 50%. These outcomes challenge the principle that rTMS-induced mood improvement drives sleep improvement in patients with MDD. Furthermore, sleep quality improved significantly in participants whose depression did not indicate a clinical response to rTMS treatment. This finding is in line with prior evidence that rTMS improves sleep quality in patients with major depression, 8– 12 even in those who experience no improvement in the underlying mood disorder. 13
Linear regression models showed that the PSQI improvement was not significantly predicted by the modified PHQ-9 change, even with adjustment for patient age, sex, sedative-hypnotic use, number of rTMS treatments, or initial depression severity. In patients older than age 50 years, linear regression analysis of the modified PHQ-9 score change as a predictor of PSQI score change revealed a significant negative correlation. That is, larger changes in depressive symptoms predicted smaller changes in sleep-related symptoms. We are not sure how to explain this finding. All other subgroup regressions showed no significant relationship.
Taken together, these results suggest that mood and sleep improved independently after rTMS in our study population. Because of the small sample size of 21 participants in this study, larger studies are required to confirm these findings. Nevertheless, the finding that sleep quality improved alongside improvements in mood challenges the common perception of rTMS as an arousal-neutral antidepressant and is consistent with several previous studies. 7, 9– 12, 14, 15 Sleep abnormalities in depression are thought to result from the same biological imbalances that underlie cognitive and affective symptoms of depression. 29 Pathways involving corticotropin-releasing hormone, brain-derived neurotrophic factor, adenosine, and cyclic adenosine monophosphate response element binding protein seem to be dysregulated in both affective and sleep disorders. 30 Yet if mood and sleep impairments in depression share a common biology, then one might expect the mood and sleep changes elicited by rTMS to be statistically correlated. The lack of correlation between mood and sleep changes in our study may suggest divergent mechanisms of action of rTMS for mood and sleep disturbances in patients with depression. In addition, the question of why sleep in depression differs from that in primary sleep disorders, and how these differences may interact with rTMS, remains of interest. 16
An uncertainty complicating the present analysis of PSQI results is whether overall sleep quality, or a more specific aspect of self-reported sleep experience, promotes neuroplasticity. If rTMS promotes neuroplasticity by increasing slow-wave sleep, then how is this increase experienced or described by patients? Actigraphy studies have not shown a significant change in sleep continuity after rTMS, leading investigators to speculate that total sleep time and sleep continuity are necessary but insufficient to increase plasticity through slow-wave sleep. 31 In addition, therapeutic sleep deprivation is known to provide rapid antidepressant effects in patients with depression, possibly by increasing plasticity in the hippocampus. 32, 33 Thus, sleep duration is not a linear predictor of overall sleep quality or neuroplasticity. This detail is lost in the PSQI, which assigns better scores to patients with longer sleep duration.
The limitations of this study include a small sample size, a limited follow-up period (acute treatment only), no control group, no consideration of potential comorbidities, and no objective measures of sleep or related neurophysiology. The lack of objective sleep variables is notable, both because the effects of rTMS on objective sleep variables are inconsistent and because patients with MDD are observed to have poor insight into their sleep quality, frequently misestimating sleep latency, sleep times, and sleep duration. 6, 34 Because this was an open-label study, a placebo effect may have contributed to both mood and sleep quality improvements.
In summary, we found that sleep quality improved in our small sample of patients with MDD receiving rTMS and that the improvement was independent of change in mood. There are several potential implications of our findings. In patients with a history of major depression, rTMS may be helpful in the prodromal phase of a recurrent episode, when sleep disturbances are often the earliest symptoms to manifest. 35 rTMS may lessen the need for over-the-counter or prescription sleep aids in patients with depression and may present a more attractive option than therapeutic sleep deprivation, the benefits of which are transient. 32, 33 Finally, rTMS may be helpful in depressive episodes marked by significant sleep impairment. Many uncertainties remain to be clarified, including the mechanisms of rTMS in primary sleep disorders vs sleep impairment related to depression, how rTMS interacts with antidepressant and sleep medications, and whether the optimal rTMS parameters might differ in patients with depression based on the degree of sleep impairment. Because subgroup analyses uncovered significant relationships, a next step might include analyzing potential predictors of sleep improvement after rTMS in patients with mood disorders. Evaluation of both self-reported and objective measures of sleep changes would also be of interest. Further studies exploring the effects of rTMS on sleep are needed.
ACKNOWLEDGMENTS
The authors thank Jeremy Syrjanen and Dr. Andrew Chapp for expert statistical assistance.
DISCLOSURE STATEMENT
All authors have read and approved this manuscript. Work for this study was performed at Mayo Clinic Rochester, Rochester, Minnesota, and Mayo Clinic Jacksonville, Jacksonville, Florida. This study was funded by grant number UL1 TR002377 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Dr. Croarkin has received research support from Pfizer, Inc.; Neuronetics, Inc.; and NeoSync, Inc. He has received equipment support from Neuronetics, Inc., and MagVenture, Inc., for investigator-initiated studies, including off-label use of repetitive transcranial magnetic stimulation for children and adolescents. He received genotyping supplies and services from Assurex for an investigator-initiated study. Dr. Croarkin has served as a paid consultant for Engrail Therapeutics, Myriad Neuroscience, Procter & Gamble Company, and Sunovion. Dr. Kung has received equipment support from Neuronetics, Inc., for a study of repetitive transcranial magnetic stimulation and bipolar depression. Ms. Collins, Dr. Cheung, and Dr. Kolla report no conflicts of interest.
ABBREVIATIONS
- ANOVA
analysis of variance
- MDD
major depressive disorder
- PHQ-9
Patient Health Questionnaire-9
- PSQI
Pittsburgh Sleep Quality Index
- rTMS
repetitive transcranial magnetic stimulation
REFERENCES
- 1. Huber R , Esser SK , Ferrarelli F , Massimini M , Peterson MJ , Tononi G . TMS-induced cortical potentiation during wakefulness locally increases slow wave activity during sleep . PLoS One. 2007. ; 2 ( 3 ): e276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui J , Tremblay S , Daskalakis ZJ . The current and future potential of transcranial magnetic stimulation with electroencephalography in psychiatry . Clin Pharmacol Ther. 2019. ; 106 ( 4 ): 734 – 746 . [DOI] [PubMed] [Google Scholar]
- 3. Luber B , Steffener J , Tucker A , et al . Extended remediation of sleep deprived-induced working memory deficits using fMRI-guided transcranial magnetic stimulation . Sleep. 2013. ; 36 ( 6 ): 857 – 871 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lanza G . Repetitive TMS for sleep disorders: are we ready? Sleep Med. 2020. ; 71 : 111 – 112 . [DOI] [PubMed] [Google Scholar]
- 5. Jiang B , He D , Guo Z , Mu Q , Zhang L . Efficacy and placebo response of repetitive transcranial magnetic stimulation for primary insomnia . Sleep Med. 2019. ; 63 : 9 – 13 . [DOI] [PubMed] [Google Scholar]
- 6. Oroz R , Kung S , Croarkin PE , Cheung J . Transcranial magnetic stimulation therapeutic applications on sleep and insomnia: a review . Sleep Sci Pract. 2021. ; 5 ( 3 ): 3 . [Google Scholar]
- 7. Pellicciari MC , Cordone S , Marzano C , et al . Dorsolateral prefrontal transcranial magnetic stimulation in patients with major depression locally affects alpha power of REM sleep . Front Hum Neurosci. 2013. ; 7 : 433 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- 9. Brakemeier EL , Luborzewski A , Danker-Hopfe H , Kathmann N , Bajbouj M . Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS) . J Psychiatr Res. 2007. ; 41 ( 5 ): 395 – 403 . [DOI] [PubMed] [Google Scholar]
- 10. Nishida M , Kikuchi S , Nisijima K , Suda S . Actigraphy in patients with major depressive disorder undergoing repetitive transcranial magnetic stimulation: an open label pilot study . J ECT. 2017. ; 33 ( 1 ): 36 – 42 . [DOI] [PubMed] [Google Scholar]
- 11. Lowe A , Rajaratnam SM , Hoy K , Taffe J , Fitzgerald PB . Can sleep disturbance in depression predict repetitive transcranial magnetic stimulation (rTMS) treatment response? Psychiatry Res. 2013. ; 210 ( 1 ): 121 – 126 . [DOI] [PubMed] [Google Scholar]
- 12. Grunhaus L , Schreiber S , Dolberg OT , Polak D , Dannon PN . A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression . Biol Psychiatry. 2003. ; 53 ( 4 ): 324 – 331 . [DOI] [PubMed] [Google Scholar]
- 13. Sonmez AI , Kucuker MU , Lewis CP , et al . Improvement in hypersomnia with high frequency repetitive transcranial magnetic stimulation in depressed adolescents: preliminary evidence from an open-label study . Prog Neuropsychopharmacol Biol Psychiatry. 2020. ; 97 : 109763 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenquist PB , Krystal A , Heart KL , Demitrack MA , McCall WV . Left dorsolateral prefrontal transcranial magnetic stimulation (TMS): sleep factor changes during treatment in patients with pharmacoresistant major depressive disorder . Psychiatry Res. 2013. ; 205 ( 1-2 ): 67 – 73 . [DOI] [PubMed] [Google Scholar]
- 15. Antczak JM , Poleszczyk A , Wichniak A , Rakowicz M , Parnowski TJ . The influence of the repetitive transcranial magnetic stimulation on sleep quality in depression. Article in English and Polish . Psychiatr Pol. 2017. ; 51 ( 5 ): 845 – 857 . [DOI] [PubMed] [Google Scholar]
- 16. Sun Q , Tan L . Comparing primary insomnia to the insomnia occurring in major depression and general anxiety disorder . Psychiatry Res. 2019. ; 282 : 112514 . [DOI] [PubMed] [Google Scholar]
- 17. Alvaro PK , Roberts RM , Harris JK . A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression . Sleep. 2013. ; 36 ( 7 ): 1059 – 1068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K , Spitzer RL , Williams JB . The PHQ-9: validity of a brief depression severity measure . J Gen Intern Med. 2001. ; 16 ( 9 ): 606 – 613 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buysse DJ , Reynolds CF III , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 20. Sun N , He Y , Wang Z , Zou W , Liu X . The effect of repetitive transcranial magnetic stimulation for insomnia: a systematic review and meta-analysis . Sleep Med. 2021. ; 77 : 226 – 237 . [DOI] [PubMed] [Google Scholar]
- 21. Lin J , Liu X , Li H , et al . Chronic repetitive transcranial magnetic stimulation (rTMS) on sleeping quality and mood status in drug dependent male inpatients during abstinence . Sleep Med. 2019. ; 58 : 7 – 12 . [DOI] [PubMed] [Google Scholar]
- 22. Rostami R , Kazemi R , Nitsche MA , Gholipour F , Salehinejad MA . Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders . Clin Neurophysiol. 2017. ; 128 ( 10 ): 1961 – 1970 . [DOI] [PubMed] [Google Scholar]
- 23. Huang CC , Wei IH , Chou YH , Su TP . Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression . Psychoneuroendocrinology. 2008. ; 33 ( 6 ): 821 – 831 . [DOI] [PubMed] [Google Scholar]
- 24. Dai L , Wang P , Zhang P , et al . The therapeutic effect of repetitive transcranial magnetic stimulation in elderly depression patients . Medicine (Baltimore). 2020. ; 99 ( 32 ): e21493 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qin BY , Dai LL , Zheng Y . Efficacy of repetitive transcranial magnetic stimulation for alleviating clinical symptoms and suicidal ideation in elderly depressive patients: a randomized controlled trial [in Chinese] . Nan Fang Yi Ke Da Xue Xue Bao. 2017. ; 37 ( 1 ): 97 – 101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimaldi D , Papalambros NA , Zee PC , Malkani RG . Neurostimulation techniques to enhance sleep and improve cognition in aging . Neurobiol Dis. 2020. ; 141 : 104865 . [DOI] [PubMed] [Google Scholar]
- 27. Valiengo L , Maia A , Cotovio G , et al . Repetitive transcranial magnetic stimulation for major depressive disorder in older adults: systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. Published online August 25, 2021. . doi: 10.1093/gerona/glab235. [DOI] [PubMed]
- 28. Kar SK . Predictors of response to repetitive transcranial magnetic stimulation in depression: a review of recent updates . Clin Psychopharmacol Neurosci. 2019. ; 17 ( 1 ): 25 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sculthorpe LD , Douglass AB . Sleep pathologies in depression and the clinical utility of polysomnography . Can J Psychiatry. 2010. ; 55 ( 7 ): 413 – 421 . [DOI] [PubMed] [Google Scholar]
- 30. Kuhn M , Wolf E , Maier JG , et al . Sleep recalibrates homeostatic and associative synaptic plasticity in the human cortex . Nat Commun. 2016. ; 7 : 12455 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centorino MB , Bajor LA , Gootam PK , Nakase-Richardson R , Kozel FA . The relationship of transcranial magnetic stimulation with sleep and plasticity . J Psychiatr Pract. 2020. ; 26 ( 6 ): 434 – 443 . [DOI] [PubMed] [Google Scholar]
- 32. Zhang MQ , Li R , Wang YQ , Huang ZL . Neural plasticity is involved in physiological sleep, depressive sleep disturbances, and antidepressant treatments . Neural Plast. 2017. ; 2017 : 5870735 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wolf E , Kuhn M , Normann C , et al . Synaptic plasticity model of therapeutic sleep deprivation in major depression . Sleep Med Rev. 2016. ; 30 : 53 – 62 . [DOI] [PubMed] [Google Scholar]
- 34. Murphy MJ , Peterson MJ . Sleep disturbances in depression . Sleep Med Clin. 2015. ; 10 ( 1 ): 17 – 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perlis ML , Giles DE , Buysse DJ , Tu X , Kupfer DJ . Self-reported sleep disturbance as a prodromal symptom in recurrent depression . J Affect Disord. 1997. ; 42 ( 2–3 ): 209 – 212 . [DOI] [PubMed] [Google Scholar]