Abstract
Study Objectives:
To assess the frequency, determinants, and clinical impact of clinical rapid eye movement (REM) and non-REM (NREM) parasomnias in adult patients with narcolepsy type 1 (NT1), narcolepsy type 2 (NT2), and idiopathic hypersomnia compared with healthy controls.
Methods:
Familial and past and current personal parasomnias were assessed by questionnaire and medical interviews in 710 patients (220 NT1, 199 NT2, and 221 idiopathic hypersomnia) and 595 healthy controls.
Results:
Except for sleep-related eating disorder, current NREM parasomnias were rare in all patient groups and controls. Sleep-related eating disorder was more frequent in NT1 patients (7.9% vs 1.8% in NT2 patients, 2.1% in patients with idiopathic hypersomnia, and 1% in controls) and associated with disrupted nighttime sleep (odds ratio = 3.9) and nocturnal eating in full awareness (odds ratio = 6.9) but not with sex. Clinical REM sleep behavior disorder was more frequent in NT1 patients (41.4%, half being violent) than in NT2 patients (13.2%) and affected men more often than women (odds ratio = 2.4). It was associated with disrupted nighttime sleep, depressive symptoms, and antidepressant use. Frequent (> 1/week) nightmares were reported by 39% of patients with NT1, 29% with NT2, and 27.8% with idiopathic hypersomnia (vs 8.3% in controls) and were associated with depressive symptoms in narcolepsy. No parasomnia (except sleep-related hallucinations) worsened daytime sleepiness.
Conclusions:
In patients with central disorders of hypersomnolence, comorbid NREM parasomnias (except for sleep-related eating disorder) are rare and do not worsen sleepiness. In contrast, REM parasomnias are prevalent (especially in NT1) and are associated with male sex, disrupted nighttime sleep, depressive symptoms, and antidepressant use.
Citation:
Leu-Semenescu S, Maranci J-B, Lopez R, et al. Comorbid parasomnias in narcolepsy and idiopathic hypersomnia: more REM than NREM parasomnias. J Clin Sleep Med. 2022;18(5):1355–1364.
Keywords: idiopathic hypersomnia, narcolepsy, sleep-related eating disorder, sleepwalking, sleep terrors, sleep talking, REM sleep behavior disorder, nightmare disorder, antidepressants
BRIEF SUMMARY
Current Knowledge/Study Rationale: The frequency, determinants, and clinical impact of comorbid parasomnias (with the exception of REM parasomnias in narcolepsy type 1 [NT1]) have not been studied in central disorders of hypersomnolence. A large group of patients and healthy controls was interviewed about current sleepwalking, sleep terrors, sleep talking/shouting, sleep-related eating disorder, clinical REM sleep behavior disorder, sleep-related hallucinations, sleep paralysis, and nightmares.
Study Impact: NREM parasomnias were rare in patients and controls, except for sleep-related eating disorder (exclusively associated with NT1), whose determinants support a model combining fragmented sleep and a drive to eat during the night. REM parasomnias were more frequent in NT1 patients with depressive symptoms and in male patients (for REM sleep behavior disorder). Except for sleep-related hallucinations, no parasomnia worsened daytime sleepiness, but REM sleep behavior disorder caused injuries in 24% of NT1 patients.
INTRODUCTION
Narcolepsy and idiopathic hypersomnia (IH) are rare and disabling central disorders of hypersomnolence affecting mostly young individuals. 1 Patients with narcolepsy also experience abnormal rapid eye movement (REM) sleep manifestations, including cataplexy, which is present in narcolepsy type 1 (NT1) and absent in narcolepsy type 2 (NT2), sleep paralysis, and sleep-related hallucinations. Disrupted nighttime sleep and obesity affect one-third of patients with narcolepsy. In addition to excessive daytime sleepiness, patients with IH often report long sleep time, severe morning sleep inertia, impaired daytime alertness, and automatic behaviors. NT1 is a homogeneous disorder caused by a hypocretin deficiency of probable autoimmune origin, 2 whereas the causes of NT2 and IH are yet unknown.
Parasomnias belong to another category of sleep disorders characterized by undesirable behaviors or experiences that occur during entry into sleep, within sleep, or during arousal from sleep. 1 They may cause self-harm and bed-partner injuries, sleep disruption, and psychological consequences. Parasomnias may present as an isolated syndrome (eg, recurrent isolated sleep paralysis) or may be comorbid with other neurological disorders (eg, sleep paralysis is a classic symptom in NT1). They may occur in relation to non-REM (NREM) sleep, REM sleep, or both. NREM parasomnias include confusional arousal, sleep terrors, sleepwalking (or disorders of arousal), sleep-related eating disorder (SRED), and sexsomnia. SRED is characterized by frequent episodes of nocturnal eating with a partial or complete loss of conscious awareness and is usually not associated with waking eating disorder. 1, 3, 4 During REM sleep, REM sleep behavior disorder (RBD) is characterized by unpleasant dreams associated with violent behaviors during which the patients enact their dreams. 1 Other REM sleep–associated parasomnias include nightmare disorder, sleep paralysis, and sleep-related hallucinations. Sleep talking (from simple utterances to coherent conversation) may occur during both NREM and REM sleep. It may occur in normal individuals but is frequent in those with NREM and REM parasomnias, as well as in those with psychiatric disorders. 5
REM sleep parasomnias are frequent in patients with NT1 (and to a lesser degree in those with NT2) to the point that they constitute some of the core symptoms of NT1 and are considered markers of REM sleep boundary dyscontrol. Sleep paralysis has been reported by 53%–75% of patients with NT1 and 28%–47% of patients with NT2 6– 10 and in 3%–8% of the general population. 11 Sleep-related hallucinations are often associated with sleep paralysis and are found in 59%–87% of patients with NT1 as well as in 28%–53% of patients with NT2. 6– 10, 12 Up to 83% of patients with NT1 (and 41% with NT2) complain of recurrent nightmares. 10, 13 In studies based on questionnaires or clinical interviews, RBD prevalence is 7%–63% in patients with NT1 14, 15 and 15%–40% of patients with NT2. 6, 8 In contrast, NREM parasomnias have rarely been studied in narcolepsy, with the exception of isolated reports of SRED 7, 16 and occasional, simple gesturing in NREM sleep in children with NT1. 17 In IH, information on comorbid parasomnias is scarce, with the exception of sleep paralysis and sleep-related hallucinations, which were found in 4%–30% of patients in a large series. 18– 20
Most information regarding comorbid parasomnias in central disorders of hypersomnolence is focused on REM sleep parasomnias in NT1. There is insufficient information on REM sleep parasomnias in NT2 and IH and almost no information on NREM parasomnias in narcolepsy and IH. On the other hand, recent studies in primary NREM parasomnias have indicated that sleepwalking and sleep terrors in adults may be associated with significant daytime hypersomnolence, morning fatigue, and psychological consequences, in addition to injuries. 21– 23 Consequently, determining how comorbid NREM parasomnias impact the severity of daytime sleepiness in central disorders of hypersomnolence may be important. We aimed to assess the frequency of NREM and REM parasomnias, as well as demographic and clinical associations (determinants and impacts), in a large series of adults with NT1, NT2, and IH compared with healthy controls.
METHODS
Participants
Adult patients with central disorders of hypersomnolence were prospectively recruited among the cohort of patients followed in the national reference centers for narcolepsy and rare hypersomnias in 3 university hospitals (Pitié-Salpêtrière and Mondor Hospitals in Paris, Gui-de-Chauliac Hospital in Montpellier) between 2008 and 2011. To be included in the study, patients had to meet the international criteria of narcolepsy with or without cataplexy (later named NT1 and NT2) 1 or IH. 24 All patients had a systematic interview with a sleep neurologist, followed by a clinical examination and the completion of questionnaires. Their sleep recordings (nocturnal polysomnography followed by the Multiple Sleep Latency Test, which was followed by bed-rest extended sleep monitoring that lasted 24 to 36 hours) and biological tests (human leukocyte antigen [HLA] DQB1*06:02 genotyping) were performed at the time of diagnosis. Healthy controls were recruited by word of mouth in the same period and face-to-face interviews by clinicians, who ensured that they had no chronic excessive daytime sleepiness; no sleep monitoring was performed in the controls. Healthy controls (but not patients) were paid for their participation in the study. The participants signed an informed-consent form to take part in the study, which was included in a wider research program on rare hypersomnia diseases (NARCOBANK, PHRC AOM 2007, funded by the Health Ministry; principal investigator, Isabelle Arnulf) and approved by the ethics committee (Comité de Protection des Personnes—Ile de France 06).
Medical interview and questionnaires
At the time of the study, the patients underwent a standardized self-administered questionnaire followed by a face-to-face medical interview. 25, 26 The main collected information included sex, age, and body mass index (BMI) at the time of the study; age at sleepiness onset; disease course; the presence of severe morning sleep inertia and restless legs syndrome; and treatment with antidepressants, stimulants, and sodium oxybate. The patients completed the Epworth Sleepiness Scale 27 at the time of diagnosis (prior to any treatment) and then at the time of the study. They also completed the Beck Depression Inventory-II 28 (BDI-II) with results dichotomized as “none/mild symptoms” if the score was lower than or equal to 19 and “moderate/severe symptoms” if greater than 19) and the State-Trait Anxiety Inventory (STAI-Y) 29 at the time of the study.
The personal and family histories of NREM parasomnias were collected based on patients’ self-report on a questionnaire (including type and age at onset) and verified during the interview with the neurologist. Questionnaires included information about sleepwalking, sleep terror, SRED, sleep talking, and sleep shouting as well as REM parasomnias, including dream-enacted behavior, nightmare disorder, sleep paralysis, and sleep-related hallucinations. Current parasomnias were defined as episodes occurring during the year preceding the study. The final parasomnia diagnoses were based on clinical questionnaires and neurological interviews, but none were polysomnography-based. NREM parasomnias were defined based on international criteria 1 as disorders of arousal. These were further classified as sleepwalking if ambulation had occurred during some of the episodes of disorders of arousal and as sleep terror if the participants, in addition to a positive medical interview, reported screaming when asleep in the self-report questionnaire. SRED was defined using the following question: “Have you ever realized in the morning that you had unconsciously consumed food or drinks at night? had you a “food-oriented sleepwalking?” In addition, a specific interview question focused on episodes with simple nocturnal eating, characterized by eating food, without being compulsory and without compensatory behavior such as self-induced vomiting or purging activities (which would define a night eating syndrome). Information on nocturnal eating in full awareness was available in patients but not in controls. Sexsomnia and confusional arousals were not investigated at this time. Clinical RBD was defined as (1) repeated episodes of vocalization or complex, sometimes violent, motor behavior during sleep; (2) events that mostly occur during the last third of the major sleep episode; and (3) episodes that were associated with dream recall suggestive of RBD. Information on clinical RBD was not available in controls. Due to the lack of documentation by video-polysomnography or measures of REM sleep without atonia and to increase the diagnostic accuracy, supplemental clinical information was carefully searched by the clinician based on our previous studies about mental content during REM and NREM parasomnia. 30, 31 For example, complex dream mentation, patients being attacked and counter-attacking in their dream, dream settings out of the bedroom, and lying or having eyes closed throughout episodes were strongly suggestive of RBD (in this case, NREM parasomnias were excluded), whereas short, unpleasant dreamlike mentation, such as facing a disaster (eg, being buried alive or facing a tsunami, although still being in the bedroom), as well interacting with their immediate physical environment during an episode, having open eyes, standing up, and walking were suggestive of NREM parasomnias. 32
Statistical analysis
The sample was described using percentages for categorical measures and mean ± standard deviation (SD) for continuous measures. Personal and family histories of NREM/REM parasomnias were compared between controls and patients with hypersomnias as a whole group and then between groups of patients with each disorder. Logistic regression analysis using odds ratios (ORs) and 95% confidence intervals (CIs) was conducted to compare categorical measures between groups, and analyses of variance (ANOVAs) were performed to compare continuous measures. Because age and sex were different between groups and because anxiety and depression may influence parasomnias, a crude association model, further adjusted for sex, age, and BDI-II and STAI-Y scores, was built to compare patient and control groups. Comparisons between patient groups were performed using a crude association model further adjusted for sex and age. For the other analyses, comparisons of qualitative measures were performed with Fisher’s or chi-square tests; and for quantitative measures, Wilcoxon tests or t tests were performed based on the conditions of validity of the different tests. To describe any overlap between parasomnias, Spearman correlations were conducted between the 9 parasomnia types. Since the categories of parasomnias are qualitative and not quantitative, the value of the correlations is only indicative: The statistical association between the parasomnias was demonstrated by chi-square tests. Analyses were performed using the statistical software R Core Team (R Foundation for Statistical Computing, Vienna, Austria). 33
RESULTS
Demographic and clinical characteristics of the participants
Over 3 years, 710 consecutive patients with a diagnosis of central disorders of hypersomnolence took part in the study, of whom 290 had NT1, 199 had NT2, and 221 had IH. Among the 737 recruited healthy controls, 595 had completed questions related to parasomnias and were included in the analysis. There were between-group differences in age, sex, and BMI. The patients with NT1 were older and had a higher BMI, a longer disease course, a higher sleepiness score, and more frequent HLA DQB1*0602 positivity, and more among them were treated with sodium oxybate than the individuals in the other patient groups. As expected, antidepressants were taken more often in the NT1 group than in the NT2 and IH groups, mostly as a treatment for cataplexy (only 6/129 [5%] patients with NT1 used these drugs to treat both cataplexy and depression). In the IH group, there were more women than in the other 3 groups. The patients with IH had lower BMI and more frequent morning inertia, and were less frequently treated with stimulants at the time of inclusion (ie, time of diagnosis) than the NT1 patients. Restless legs syndrome was more frequent in the NT1 group than in the 3 other groups and more frequent in the NT2 and IH groups than in the control group. Higher anxiety scores (both trait and state), higher depression scores, and higher use of antidepressants were found in all 3 groups of patients than in the control group (Table 1).
Table 1.
Demographic and clinical characteristics of patients with central disorders of hypersomnolence, including NT1, NT2, and IH and healthy controls.
| NT1 | NT2 | IH | Controls | P* | |
|---|---|---|---|---|---|
| n | 290 | 199 | 221 | 595 | |
| Age, y | 41.2 ± 18.3a,b,c | 35.8 ± 13.9 | 35.9 ± 13.8 | 37.9 ± 15.5 | .0002 |
| BMI, kg/m2 | 26.4 ± 5.4a,b,c | 25 ± 5.1 | 24 ± 5.2 | 23.5 ± 5.4 | < .0001 |
| Women, % | 51.4a,c | 52.8a,c | 76a | 62.5 | < .0001 |
| Medication use, % (n) | |||||
| Stimulants | 69.3 (201)a,c | 63.3 (126)a | 56.1 (124)a | 0 | < .0001 |
| Antidepressants† | 44.5 (129)a,b,c | 9.5 (19)a | 9.9 (22)a | 1.2 (7) | < .0001 |
| Sodium oxybate | 22.4 (65)a,b,c | 10.1 (20)a,c | 1.8 (4) | 0 | < .0001 |
| Age at sleepiness onset, y | 23.6 ± 13.4 | 25.6 ± 12.7 | 22.5 ± 13.2 | NA | .68 |
| Disease course, y | 18.3 ± 16.6b, c | 11.9 ± 10.6 | 13.3 ± 12.2 | NA | < .0001 |
| Epworth Sleepiness Scale score (0–24) | |||||
| At diagnosis time | 18 ± 3.8b,c | 16.7 ± 3.7 | 15.9 ± 3.9 | NA | < .0001 |
| At study time | 16.6 ± 4.3a,b,c | 14.9 ± 4.6a | 15.2 ± 4.6a | 5 ± 3 | < .0001 |
| Morning sleep inertia, % | 26.9a,b,c | 42.1a,c | 65a | 0.8 | < .0001 |
| Restless legs syndrome, % | 16.8a,b,c | 10.1a | 6.8a | 2.4 | < .0001 |
| Psychological symptoms | |||||
| BDI-II, 0–63 | 13.1 ± 9.7a | 13.7 ± 10a | 12.8 ± 9.3a | 6.2 ± 6.6 | < .0001 |
| BDI-II cutoff > 19, % | 23.8a | 23.4a | 20.7a | 4.6 | < .0001 |
| STAI-Y, A and B score (total) (40–160) | 81.9 ± 21.8a | 85.9 ± 24.4a | 85.6 ± 23.7a | 69.8 ± 19.2 | < .0001 |
| STAI-Y, A | 38.5 ± 11.6a | 40.3 ± 13.5a | 40.4 ± 12.8a | 32.6 ± 9.9 | < .0001 |
| STAI-Y, B | 43.7 ± 11.9a | 45.6 ± 12.3a | 45.3 ± 11.9a | 37.2 ± 10.2 | < .0001 |
| HLA DQB1*06:02 positivity, % | 86.1b,c | 26.19c | 19.9 | Not done | < .0001 |
Values are presented as mean ± SD and % (n). *P for ANOVA with quantitative measures, or P for a logistic regression with qualitative measures, between the 4 groups. †Antidepressants were used for treating cataplexy in NT1 patients (in 6/129 NT1 patients, antidepressants were used to treat both cataplexy and depression) and for treating depression in NT2 and IH patients. aP < .05 for difference from controls. bP < .05 for difference from NT2. cP < .05 for difference from IH. ANOVA = analysis of variance, BDI-II = Beck Depression Inventory-II, BMI = body mass index, HLA = human leukocyte antigen, IH = idiopathic hypersomnia, NA = not applicable, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, SD = standard deviation, STAI-Y = State (A) Trait (B) Anxiety Inventory.
Frequency of comorbid NREM and REM parasomnias
All patient groups had a higher frequency of familial and personal history of NREM parasomnias than the control group (with ORs varying from 1.55 to 4.15 and no further differences between pairs of groups). However, the 3 patient groups had a similar frequency of current NREM parasomnia, with the exception of SRED, which was more frequent in the NT1 group than in the other groups (Table 2 and Table 3). The differences persisted after adjustments for age, sex, BMI, and STAI-Y scores. Night eating in full awareness was reported by 63 of 266 (23.7%) NT1 patients, 41 of 185 (22.2%) NT2 patients, and 33 of 203 (16.3%) IH patients (P adjusted for age and sex = .04). The patients with NT1 had more frequent episodes of night eating in full awareness than patients with IH (adjusted P = .02) but no more than NT2 patients.
Table 2.
Clinically assessed parasomnias in patients with NT1, NT2, and IH and healthy controls.
| Diagnosis | Model 1: Comparison | Model 2: Comparison | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT1 | NT2 | IH | Controls | Global P Value | NT1 vs Controls | NT2 vs Controls | IH vs Controls | Global P Value | NT1 vs Controls | NT2 vs Controls | IH vs Controls | |
| NREM parasomnias | ||||||||||||
| Current NREM parasomnias | 3.9 (9/234) | 3.6 (6/169) | 1.04 (2/193) | 1.4 (8/578) | .07 | — | — | — | .11 | — | — | — |
| Childhood NREM parasomnias | 17.7 (39/220) | 19.5 (31/159) | 22.3 (41/184) | 12.2 (71/582) | .004 | 1.55 [1.01–2.36]* | 1.74 [1.08–2.75]* | 2.06 [1.34–3.15]* | .02 | 1.62 [1.01–2.58]* | 1.69 [1.04–2.78]* | 1.93 [1.22–3.03]* |
| Family history of sleepwalking | 9.9 (26/263) | 9.7 (18/186) | 14.2 (29/204) | 5.1 (29/564) | .0005 | 2.02 [1.16–3.51]* | 1.98 [1.05–3.62]* | 3.06 [1.77–5.27]* | .0006 | 2.73 [1.5–4.9]* | 2.2 [1.1–4.27]* | 2.96 [1.63–5.34]* |
| Family history of NREM parasomnias | 16.2 (44/271) | 18.5 (35/189) | 24.3 (50/206) | 7.2 (41/572) | < .0001 | 2.51 [1.6–3.96]* | 2.94 [1.8–4.78]* | 4.15 [2.65–6.54]* | < .0001 | 3 [1.82–4.92]* | 3.11 [1.83–5.25]* | 3.89 [2.39–6.35]* |
| Sleep-related eating disorder | 7.9 (18/228) | 1.8 (3/168) | 2.1 (4/192) | 1 (6/573) | < .0001 | 8.1 [3.35–22.59]* | 1.72 [0.36–6.59 | 2.01 [0.51–7.11] | < .0001 | 5.81 [2.24–16.94]* | 1.14 [0.23–4.64] | 1.51 [0.37–5.63] |
| REM parasomnias | ||||||||||||
| Clinical REM sleep behavior disorder | 41.4 (115/278) | 13.2 (25/190) | 1.8 (4/217) | ND | — | — | — | — | — | — | — | — |
| Nightmares | 85.8 (200/233) | 80.4 (131/163 | 81.2 (151/186) | 77.9 (448/575) | .07 | — | — | — | .03 | 1.88 [1.18–3.08]* | 1.07 [0.66–1.8] | 0.9 [0.57–1.44] |
| Frequent nightmares† | 39 (78/200) | 29 (38/131) | 27.8 (42/151) | 8.3 (37/448) | < .0001 | 7.1 [4.6–11.13]* | 4.54 [2.74–7.55]* | 4.28 [2.63–7.01]* | < .0001 | 5.28 [3.29–8.58]* | 3.2 [1.86–5.51]* | 3.11 [1.84–5.51]* |
| Sleep paralysis | 45 (129/287) | 24.6 (49/199) | 10.9 (24/221) | 1.9 (11/594) | < .0001 | 43.2 [23.8–86.66]* | 17.28 [9.1–35.76]* | 6.45 [3.17–13.92]* | < .0001 | 46.4 [24.18–98.75]* | 16.66 [8.29–36.57]* | 6.99 [3.28–15.92]* |
| Sleep-related hallucinations | 53.8 (155/288) | 29.8 (59/198) | 10.9 (24/221) | 1 (6/594) | < .0001 | 114.21 [53.83–295.55]* | 41.6 [19.04–109.54]* | 11.94 [5.12–32.61]* | < .0001 | 96.33 [44.3–253.32]* | 32.26 [14.31–86.65]* | 10.17 [4.25–28.28]* |
| NREM/REM parasomnias (normal variants) | ||||||||||||
| Sleep talking | 79.7 (173/217) | 59.9 (85/142) | 51.1 (89/174) | 42.5 (247/581) | < .0001 | 5.32 [3.7–7.77]* | 2.02 [1.39–2.94]* | 1.42 [1.01–1.99]* | < .0001 | 5.33 [3.57–8.12]* | 1.76 [1.18–2.65]* | 1.07 [0.74–1.55] |
| Sleep shouting | 49.8 (107/215) | 25.2 (38/151) | 23.3 (43/183) | 10.4 (68/584) | < .0001 | 7.5 [5.21–10.89]* | 2.55 [1.62–3.96]* | 2.33 [1.51–3.55]* | < .0001 | 6.09 [4.1–9.11]* | 2.06 [1.27–3.32]* | 1.87 [1.18–2.93]* |
Values are presented as % (n) and odds ratio [95% confidence interval]. Model 1: crude associations; Model 2: adjusted for sex, age, BDI-II score, and STAI-Y total score. *P < .05. †Frequent nightmares: >1 episode per week. BDI-II = Beck Depression Inventory-II, IH = idiopathic hypersomnia, ND = not done, NREM = nonrapid eye movement, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, REM = rapid eye movement, STAI-Y = State-Trait Anxiety Inventory.
Table 3.
Results of the logistic regressions comparing parasomnia categories in NT1, NT2, and IH.
| Model 1: Comparison | Model 2: Comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| Global P Value | NT1 vs NT2 | NT1 vs IH | NT2 vs IH | Global P Value | NT1 vs NT2 | NT1 vs IH | NT2 vs IH | |
| NREM parasomnias | ||||||||
| Current | .13 | — | — | — | .07 | — | — | — |
| In childhood | .52 | — | — | — | .85 | — | — | — |
| Family history | .09 | — | — | — | .23 | — | — | — |
| Family history of sleepwalking | .26 | — | — | — | .53 | — | — | — |
| Sleep-related eating disorder | .003 | 4.71 [1.56–20.37]* | 4.03 [1.47–14.13]* | 0.85 [0.17–3.93] | .003 | 4.79 [1.57–20.78]* | 3.98 [1.41–14.24]* | 0.83 [0.16–3.87] |
| REM parasomnias | ||||||||
| Clinical REM sleep behavior disorder | < .0001 | 4.7 [2.9–7.7]* | 37.6 [15.4–124.3]* | 8.1 [3.1–27.8]* | < .0001 | 4.4 [2.7–7.4]* | 31.2 [12.7–103.5]* | 7 [2.6–24.4]* |
| Nightmares | .28 | — | — | — | .07 | — | — | — |
| Frequent nightmares† | .049 | 1.56 [0.98–2.53] | 1.66 [1.06–2.63] | 1.06 [0.63–1.78] | .07 | — | — | — |
| Sleep paralysis | < .0001 | 2.5 [1.69–3.74]* | 6.7 [4.2–11.08]* | 2.68 [1.59–4.63]* | < .0001 | 2.9 [1.94–4.4]* | 7.71 [4.75–12.97]* | 2.66 [1.56–4.63]* |
| Sleep-related hallucinations | < .0001 | 2.75 [1.88–4.04]* | 9.57 [6–15.82]* | 3.48 [2.09–5.96]* | < .0001 | 2.92 [1.98–4.33]* | 10.76 [6.65–18.05]* | 3.69 [2.2–6.36]* |
| Sleep talking | < .0001 | 2.64 [1.65–4.24]* | 3.76 [2.42–5.9]* | 1.42 [0.91–2.24] | < .0001 | 2.94 [1.81–4.83]* | 5.11 [3.17–8.38]* | 1.74 [1.09–2.8]* |
| Sleep shouting | < .0001 | 2.95 [1.88–4.68]* | 3.23 [2.1–5.01]* | 1.09 [0.66–1.81] | < .0001 | 2.88 [1.83–4.59]* | 3.29 [2.11–5.18]* | 1.14 [0.68–1.9] |
Values are presented as odds ratio [95% confidence interval]. Model 1: crude associations; Model 2: adjusted for sex and age. *P < .05. †Frequent nightmares: >1 episode per week. IH = idiopathic hypersomnia, NREM = nonrapid eye movement, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, REM = rapid eye movement.
With regard to REM sleep parasomnia, clinical RBD was more frequent in patients with narcolepsy than in patients with IH, and the highest frequency was observed in the NT1 patients (41.4%, which was 3 times higher than 13.2% in the NT2 patients; see Table 2 and Table 3). Although the presence of nightmares was similar across the 4 groups, the frequency of more than 1 nightmare per week (ie, frequent nightmares) was higher in all patient groups than in the control group, with no further difference between the 3 patient groups. As expected, the frequency of sleep-related hallucinations and sleep paralysis was higher in the patient groups than in the control group, with a decreasing order of frequency from NT1 to NT2 and then IH. Sleep talking was frequent in the controls, but its frequency was higher in the NT1 and NT2 (but not in the IH) groups than in the control group. Sleep shouting was higher in the patient groups than in the control group and higher in the NT1 group than in the NT2 and IH groups.
Finally, when comparing IH with vs without long sleep time groups, there was no difference in the frequency of these NREM and REM parasomnias (data not shown).
Overlap between parasomnias
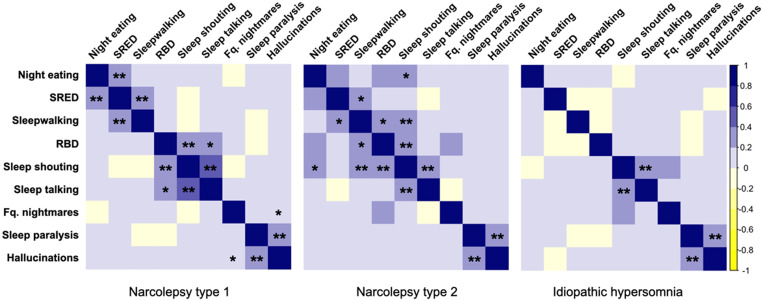
The correlation matrices between the parasomnias in NT1, NT2, and IH are shown in Figure 1. Three “clusters of association” were found in the NT1 patients. First, there were correlations between night eating in full awareness and SRED and between SRED and NREM parasomnias. A second “cluster” associated clinical RBD, sleep shouting, and sleep talking. A third “cluster” associated sleep paralysis and sleep-related hallucinations. In the NT2 group, there were less frequent overlaps between parasomnias than in the NT1 group. In the IH group, sleep talking was associated with sleep shouting and sleep paralysis with sleep-related hallucinations. There were no significant negative correlations.
Figure 1. Spearman correlation matrix between parasomnias in NT1, NT2, and IH.
The color of each box indicates the strength of the correlation (darkest = highest, as indicated in the right column), and asterisks indicate the significance of the correlation (*P < .01, **P < .001). IH = idiopathic hypersomnia, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, RBD = REM sleep behavior disorder, REM = rapid eye movement, SRED = sleep-related eating disorder.
Factors associated with comorbid parasomnias in NT1 patients
Current episodes of sleepwalking and night terrors (any of them) were rare (3.9%) in the NT1 group and as rare as in the control group. Therefore, no analysis of associated factors was performed. SRED (found in 18 patients with NT1) started at 20.2 ± 4.7 years old, on average, and occurred at a frequency of 2.9 ± 1.3 episodes/month. It was associated with disrupted nighttime sleep (OR = 3.9; 95% CI, 1.2–17.3; P = .02) and with night eating in full awareness (OR = 6.9; 95% CI, 2.5–21.2; P = .0002) but not with sex, depressive symptoms, or use of antidepressants. The patients with NT1 plus SRED had lower scores on the Epworth Sleepiness Scale (14.3 ± 5.6) than the NT1 patients without SRED (17.9 ± 3.5; P = .03) and more current NREM parasomnias (23.5% [4/17] vs 2.5% [5/200], respectively; P = .003) but no association with REM parasomnias (clinical RBD, sleep paralysis, and sleep-related hallucinations) or with sleep talking and sleep shouting. Although a higher percentage of NT1 patients with SRED (38.9% [7/18]) took sodium oxybate than NT1 patients without SRED (22% [47/210]), the between-group difference was not significant (P = .15).
The age at clinical RBD onset in the NT1 patients was 30.4 ± 16.5 (range: 23–76) years. Episodes of RBD occurred, on average, during 3.6 ± 1.2 nights per month. During the RBD episodes, 49 of 115 (42.6%) patients were aggressive toward themselves, resulting in self-injuries in 28 (24.3%) of them. The patients with clinical RBD were more frequently males (OR = 2.4; 95% CI, 1.5–4), had more frequently disrupted nighttime sleep, and had higher BDI-II scores and anxiety scores. No other clinical differences were noted for age, disease course, or frequency of restless legs syndrome (Table S1 (25.2KB, pdf) in the supplemental material). The NT1 patients with RBD took more antidepressant drugs (OR = 1.8; 95% CI, 1.1–2.9; P = .02) than those without RBD but no more stimulants or sodium oxybate.
Sleep-related hallucinations (but not sleep paralysis) were associated with frequent nightmares (OR = 2.3; P = .006). Sleep paralysis (OR = 0.6; 95% CI, 0.3–0.9; P = .02) and sleep shouting (OR = 0.5; 95% CI, 0.3–0.9; P = .03) were less frequent in NT1 patients treated with stimulants than in those without stimulant treatment. More NT1 patients treated with sodium oxybate than without sodium oxybate experienced frequent nightmares (OR = 2.3; 95% CI, 1.1–4.6; P = .02) and NREM parasomnias (OR = 4.6; 95% CI, 1.2–19.3; P = .03).
Factors associated with comorbid parasomnias in patients with NT2 and IH
The reduced number of patients who experienced comorbid NREM parasomnias (including SRED) in the NT2 and IH groups prevented any valid between-group comparisons. Night eating in full awareness in NT2 patients was strongly associated with the use of antidepressants (OR = 9.3; 95% CI, 3–33; P < .0001).
In the NT2 group, patients with clinical RBD were older, had a longer disease course, and had higher Epworth Sleepiness Scale scores at the time of diagnosis than those without clinical RBD. They had similar depression and anxiety scores (Table S1 (25.2KB, pdf) ), but those with clinical RBD took more antidepressant drugs (OR = 4; 95% CI, 1.1–13.2; P = .02). The presence of frequent nightmares in the NT2 group was associated with depressive symptoms (OR = 2.5; 95% CI, 1.1–5.9; P = .03) and antidepressant use (OR = 3.3; 95% CI, 1.01–10.7; P = .04) but not with sleep-related hallucinations (OR = 1.1; 95% CI, 0.5–2.5; P = .77) or sleep paralysis (OR = 1.1; 95% CI, 0.5–2.5; P = .82).
In the IH group, female sex was associated with sleep talking (OR = 2.8; 95% CI, 1.4–5; P = .003) and sleep shouting (OR = 2.8; 95% CI, 1.1–10; P = .029). Medications (stimulants, antidepressants, or sodium oxybate) were not associated with more frequent REM parasomnias in the IH group.
Parasomnias and sleepiness
We examined whether parasomnias exposed all groups of patients with central disorders of hypersomnolence to higher levels of sleepiness. Except for sleep-related hallucinations, which were associated with higher levels of sleepiness, no other parasomnia (once adjusted for NT1, NT2, and IH diagnosis) was associated with higher sleepiness (Table S2 (25.2KB, pdf) in the supplemental material).
DISCUSSION
In this large, controlled group of adult patients with well-defined NT1, NT2, and IH, current comorbid NREM parasomnias were as rare as in healthy controls, except for a higher frequency of SRED in the NT1 group. In contrast, REM parasomnias (including by order of frequency, sleep-related hallucinations, sleep paralysis, and frequent nightmares) were more frequent in all groups of patients than in the control group and more frequent in the NT1 group than in other patient groups. Clinical RBD was also more frequent in the NT1 group compared with the NT2 and IH groups, although information on this parasomnia was not available in controls. Treatments influenced parasomnias, particularly in NT1 patients. Antidepressants were more frequently associated with RBD, and sodium oxybate promoted nightmares and NREM parasomnias. Stimulants reduced the risk of sleep paralysis and sleep shouting. In NT2 patients, antidepressants were more frequently associated with RBD, nightmares, and night eating. In the IH group, the prevalence of parasomnias was not affected by medications.
Surprisingly, we found no increased frequency of current NREM parasomnias (except for SRED in the NT1 group) in all groups with central disorders of hypersomnolence. Even if this is a negative and unexpected finding (indeed, it is expected that there is an increased frequency of current NREM parasomnias via higher N3 amounts and sleep inertia in patients with IH, via higher sleep fragmentation in narcolepsy, 34 or via increased anxiety in the 3 hypersomnias), this is new information in the field, suggesting that the association of narcolepsy or IH with NREM parasomnias is by chance. One should note, however, that the 3 groups of patients reported more frequent familial history of NREM parasomnias and personal history of childhood NREM parasomnias than did the control group. Because this historical association of NREM parasomnias and central disorders of hypersomnolence disappeared at the time of the study, one may suggest that (1) patients have a (higher) recall bias of any familial and personal history of sleep disorders because they now experience a severe sleep disorder and (2) the treatments used in central disorders of hypersomnolence may reduce NREM parasomnias. Although nearly half of adults with primary NREM parasomnias have reported excessive daytime sleepiness (at least on questionnaires, 22, 23 but not on the Multiple Sleep Latency Test 21), the patients with central disorders of hypersomnolence had no higher sleepiness when they had or did not have comorbid parasomnias, suggesting no common pathophysiology and no additional parasomnia-related sleepiness in patients with NT1, NT2, and IH.
In this study, SRED was exclusively associated with NT1 but not with NT2 or IH. However, only 8% of NT1 patients reported an SRED. A single previous study found that one-third of 65 adults with NT1 had SRED, but it was unclear whether it was current or lifetime SRED. 7 Additionally, amnesia of eating episodes was not mandatory for the diagnosis at that time. 7 This prevalence discrepancy may be due to a more restrictive definition of SRED in our study, as we required a partial or complete loss of conscious awareness during the episode with subsequent impaired recall to meet the International Classification of Sleep Disorders, third edition (ICSD-3) diagnosis criteria of SRED, 1 which was not mandatory in the second edition of the ICSD. 24 In contrast with this previous study and with SRED in the general population, 35– 37 SRED was not associated with female sex or depression (nor with antidepressant treatment) in our sample, which suggests that SRED is linked to NT1 itself. New associations between SRED and NT1 included dyssomnia, night eating in full awareness, and the presence of other NREM parasomnias (but not with restless legs syndrome, as described in patients with primary restless legs syndrome), 4 which makes sense when one models SRED as a confusional arousal combined with eating disorders. 35, 36 Alternatively, sodium oxybate intake could be associated with food craving before sleeping and with higher drowsiness when searching for food. 38 Here, more NT1 patients with SRED than without SRED took sodium oxybate, but the association did not reach significance, possibly due to the reduced group sizes. Note that SRED was not associated with sleepiness severity and was rare in patients with NT2 and IH, suggesting that SRED was not a contributor to sleepiness. Eating disorders were not identified in our study, but several studies have previously reported their presence (mostly as “eating disorders not otherwise specified”) in NT1. 39, 40 Overall, we confirm in a large sample that SRED is more frequent in NT1 than in other central disorders of hypersomnolence and in healthy controls but that it remains an infrequent condition.
Clinical RBD in our study was reported by 41.4% of the NT1 patients, in concordance with previous studies in small groups, which reported, on average, an RBD prevalence of 30.2% (but with a wide range of 16%–71%). 41, 42 This result suggests that hypocretin deficiency is important 6, 43 but not sufficient for promoting RBD in NT1 patients. Patients with NT1 and RBD were more frequently men. This male predominance has previously been observed (albeit at a higher 82% frequency) in idiopathic RBD 44 and sometimes in narcolepsy. 14 The patients with NT1 and RBD had more depressive symptoms and higher use of antidepressants. Antidepressant use was also more frequent in NT2 patients with RBD. This suggests that antidepressant use mediated (at least in part) the association between narcolepsy and RBD because antidepressants increase REM sleep without atonia. 45 Although RBD is a manifestation of dissociated REM sleep, it was not associated with other symptoms of REM dissociation, including sleep-related hallucinations and sleep paralysis, except for cataplexy (RBD being more frequent in NT1 than NT2 groups), suggesting different mechanisms of dissociation. Eventually, we focused on the importance of clinical RBD in NT1 (which could be less severe on polysomnography than that in idiopathic RBD). 15 Indeed, even if the frequency of clinical RBD was less than once per week, almost half of patients had violent behaviors, and as many as 24% had injured themselves during RBD episodes. This result suggests that clinicians should pay more attention to diagnosing and treating RBD in patients with NT1.
As expected, the prevalence of frequent nightmares was high, both in the NT1 (39%) and NT2 (29%) groups. Previous studies found that nightmares were equally 13 or more 10 frequent in NT1 than in NT2 and that their weekly frequency was 10 times higher in narcolepsy than in controls. 46, 47 Patients with NT1 have vivid, bizarre, or frightening dreams, 48 a negative content that may favor their conversion into nightmares. Additionally, frequent nightmares were associated with depressive symptoms in the NT1 and NT2 groups. Indeed, nightmares are more prevalent in individuals with than without depressive mood in the general population. 47 What was less expected here was the high prevalence of frequent nightmares in patients with IH compared with controls (28% vs 8%, respectively), although patients with IH have a lower dream recall frequency than patients with narcolepsy. 49 The sleep mentation in IH has rarely been studied, except for the finding that 20% of patients with IH complained of “dreaming too much” (a term that could be equivalent to epic dreaming) and that another 50% complained of “black out” (no dream, no sense of time) during sleep. 49
Here, sleep-related hallucinations and sleep paralysis were more prevalent in the patients than in the controls, with a decreasing order of frequency from NT1 (54% and 45%, respectively) to NT2 (30% and 25%) and then IH (11% and 11%). These frequencies are concordant with those found in studies in patients with NT1, 8, 50, 51 NT2, 1, 8, 50, 52 and IH, 19, 20, 53 although the group sizes are larger here. Sleep-related hallucinations and sleep paralysis are often associated in patients with narcolepsy, 8 which was also true here. A new association identified here was the higher (OR = 2.3) prevalence of hallucinations in NT1 adults with depressive symptoms than in those without depressive symptoms, which parallels findings in children with NT1. 54
This study has some limitations. Most parasomnias are unconscious events, so our interviews and questionnaires captured only what the patients have heard from their sleep partners or sometimes felt, including the limits of long-term recall. Such reports may also underestimate episode frequency, especially if nonviolent, gentler behavioral episodes are overlooked. This interview was not confirmed with video-polysomnography. Arousals from N3 and their associated behaviors are important for supporting the diagnosis of NREM parasomnias, 55, 56 although this measure is not required in the current International Classification of Sleep Disorders. 1 Measures of REM sleep without atonia and behaviors on video are mandatory for meeting a diagnosis of proven RBD. 1 However, these biases were the same in all groups of patients and in controls. Additionally, the interviews were performed face to face in interaction with sleep neurologists, which reduced the risk of confounding NREM and REM parasomnias (eg, avoiding confusion between night eating and SRED or between sleep terrors, sleep shouting, and clinical RBD).
This study in patients with well-defined central disorders of hypersomnolence was sufficiently large to drive several robust, mostly confirmatory but also novel conclusions. First, SRED and REM parasomnias are more prevalent in NT1 than in NT2 and IH, which suggests an important role for hypocretin deficiency in their pathogenesis, whether via a general instability of motor regulation during sleep or via a higher sleep fragmentation. In contrast, sleepwalking and sleep terrors are as prevalent in central disorders of hypersomnolence as in the general population, which suggests that they overlap by chance. REM parasomnias, with the exception of frequent nightmares, rarely occur in patients with IH. Depressive symptoms are associated with nightmares in patients with narcolepsy and with hallucinations in NT1 patients. In terms of clinical impact, none of the parasomnias (except for sleep-related hallucinations) contribute to increased daytime sleepiness, but RBD caused injuries in one-quarter of NT1 patients, suggesting that clinicians should pay attention to this parasomnia in NT1.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at the Sleep Disorders Unit, Pitié-Salpêtrière University Hospital, Paris, France; Sleep Disorders Center, Department of Neurology, Gui-de-Chauliac Hospital, CHU Montpellier; and the Sleep Disorder Unit, Mondor Hospital, Créteil. This study was funded by PHRC AOM07-138 from the French Health Ministry (promoter: Assistance Publique-Hôpitaux de Paris; principal investigator: I.A.). Isabelle Arnulf received an honorarium from UCB Pharma for speaking engagements and was a consultant for Idorsia Pharma and Ono Pharma. These financial disclosures are unrelated to the present study and topic. Yves Dauvilliers received funds for seminars, board engagements, and travel to conferences from UCB Pharma, Jazz Pharma, Theranexus, Flamel, and Bioprojet Pharma. Régis Lopez received funds for speaking from UCB Pharma and Shire Pharma. Lucie Barateau and Smaranda Leu-Semenescu received funds for travel to conferences from UCB Pharma. Patricia Franco had speaking engagements with UCB Pharma and was a consultant for Biocodex Pharma. Michel Lecendreux has received consulting fees and honoraria and has been on advisory boards for Bioprojet Pharma, Jazz Pharma, UCB Pharma, and Alvadel Pharma. Ana Gales has received honoraria for speaking engagements from EISAI Pharma. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: The patients were diagnosed (clinical diagnosis and scoring) and followed up by I.A., S.L.-S., P.D., J.-B.M., X.D., R.L., L.B., A.G., M.L., P.F., and Y.D. The measures were collected by S.L.-S., who prepared the tables and figures. J.-B.M. performed the statistical analyses. S.L.-S. and I.A. drafted the manuscript, which was reviewed and approved by all authors.
ABBREVIATIONS
- CI
confidence interval
- IH
idiopathic hypersomnia
- NREM
nonrapid eye movement
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- OR
odds ratio
- RBD
REM sleep behavior disorder
- REM
rapid eye movement
- SRED
sleep-related eating disorder
- STAI-Y
State-Trait Anxiety Inventory
REFERENCES
- 1. Sateia MJ . International Classification of Sleep Disorders-Third Edition: highlights and modifications . Chest. 2014. ; 146 ( 5 ): 1387 – 1394 . [DOI] [PubMed] [Google Scholar]
- 2. Barateau L , Liblau R , Peyron C , Dauvilliers Y . Narcolepsy type 1 as an autoimmune disorder: evidence, and implications for pharmacological treatment . CNS Drugs. 2017. ; 31 ( 10 ): 821 – 834 . [DOI] [PubMed] [Google Scholar]
- 3. Winkelman JW . Sleep-related eating disorder and night eating syndrome: sleep disorders, eating disorders, or both? Sleep. 2006. ; 29 ( 7 ): 876 – 877 . [DOI] [PubMed] [Google Scholar]
- 4. Howell MJ , Schenck CH . Restless nocturnal eating: a common feature of Willis-Ekbom syndrome (RLS) . J Clin Sleep Med. 2012. ; 8 ( 4 ): 413 – 419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnulf I , Uguccioni G , Gay F , et al . What does the sleeping brain say? syntax and semantics of sleep talking in healthy subjects and in parasomnia patients . Sleep. 2017. ; 40 ( 11 ): zsx159 . [DOI] [PubMed] [Google Scholar]
- 6. Knudsen S , Gammeltoft S , Jennum PJ . Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency . Brain. 2010. ; 133 ( Pt 2 ): 568 – 579 . [DOI] [PubMed] [Google Scholar]
- 7. Palaia V , Poli F , Pizza F , et al . Narcolepsy with cataplexy associated with nocturnal compulsive behaviors: a case-control study . Sleep. 2011. ; 34 ( 10 ): 1365 – 1371 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leu-Semenescu S , De Cock VC , Le Masson VD , et al . Hallucinations in narcolepsy with and without cataplexy: contrasts with Parkinson’s disease . Sleep Med. 2011. ; 12 ( 5 ): 497 – 504 . [DOI] [PubMed] [Google Scholar]
- 9. Frauscher B , Ehrmann L , Mitterling T , et al . Delayed diagnosis, range of severity, and multiple sleep comorbidities: a clinical and polysomnographic analysis of 100 patients of the Innsbruck Narcolepsy Cohort . J Clin Sleep Med. 2013. ; 9 ( 8 ): 805 – 812 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dodet P , Chavez M , Leu-Semenescu S , Golmard J-L , Arnulf I . Lucid dreaming in narcolepsy . Sleep. 2015. ; 38 ( 3 ): 487 – 497 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharpless BA , Barber JP . Lifetime prevalence rates of sleep paralysis: a systematic review . Sleep Med Rev. 2011. ; 15 ( 5 ): 311 – 315 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goswami M . The influence of clinical symptoms on quality of life in patients with narcolepsy . Neurology. 1998. ; 50 ( 2 Suppl 1 ): S31 – S36 . [DOI] [PubMed] [Google Scholar]
- 13. Pisko J , Pastorek L , Buskova J , Sonka K , Nevsimalova S . Nightmares in narcolepsy: underinvestigated symptom? Sleep Med. 2014. ; 15 ( 8 ): 967 – 972 . [DOI] [PubMed] [Google Scholar]
- 14. Schenck CH , Mahowald MW . Motor dyscontrol in narcolepsy: rapid-eye-movement (REM) sleep without atonia and REM sleep behavior disorder . Ann Neurol. 1992. ; 32 ( 1 ): 3 – 10 . [DOI] [PubMed] [Google Scholar]
- 15. Dauvilliers Y , Rompré S , Gagnon JF , Vendette M , Petit D , Montplaisir J . REM sleep characteristics in narcolepsy and REM sleep behavior disorder . Sleep. 2007. ; 30 ( 7 ): 844 – 849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schenck CH , Hurwitz TD , Bundlie SR , Mahowald MW . Sleep-related eating disorders: polysomnographic correlates of a heterogeneous syndrome distinct from daytime eating disorders . Sleep. 1991. ; 14 ( 5 ): 419 – 431 . [DOI] [PubMed] [Google Scholar]
- 17. Antelmi E , Pizza F , Vandi S , et al . The spectrum of REM sleep-related episodes in children with type 1 narcolepsy . Brain. 2017. ; 140 ( 6 ): 1669 – 1679 . [DOI] [PubMed] [Google Scholar]
- 18. Anderson KN , Pilsworth S , Sharples LD , Smith IE , Shneerson JM . Idiopathic hypersomnia: a study of 77 cases . Sleep. 2007. ; 30 ( 10 ): 1274 – 1281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vernet C , Arnulf I . Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients . Sleep. 2009. ; 32 ( 6 ): 753 – 759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evangelista E , Lopez R , Barateau L , et al . Alternative diagnostic criteria for idiopathic hypersomnia: a 32-hour protocol . Ann Neurol. 2018. ; 83 ( 2 ): 235 – 247 . [DOI] [PubMed] [Google Scholar]
- 21. Lopez R , Jaussent I , Dauvilliers Y . Objective daytime sleepiness in patients with somnambulism or sleep terrors . Neurology. 2014. ; 83 ( 22 ): 2070 – 2076 . [DOI] [PubMed] [Google Scholar]
- 22. Lopez R , Jaussent I , Scholz S , Bayard S , Montplaisir J , Dauvilliers Y . Functional impairment in adult sleepwalkers: a case-control study . Sleep. 2013. ; 36 ( 3 ): 345 – 351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carrillo-Solano M , Leu-Semenescu S , Golmard JL , Groos E , Arnulf I . Sleepiness in sleepwalking and sleep terrors: a higher sleep pressure? Sleep Med. 2016. ; 26 : 54 – 59 . [DOI] [PubMed] [Google Scholar]
- 24. American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. .
- 25. Barateau L , Jaussent I , Lopez R , et al . Smoking, alcohol, drug use, abuse and dependence in narcolepsy and idiopathic hypersomnia: a case-control study . Sleep. 2016. ; 39 ( 3 ): 573 – 580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barateau L , Lopez R , Arnulf I , et al . Comorbidity between central disorders of hypersomnolence and immune-based disorders . Neurology. 2017. ; 88 ( 1 ): 93 – 100 . [DOI] [PubMed] [Google Scholar]
- 27. Johns MW . A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 28. Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory. 2nd ed. San Francisco: The Psychological Corporation; 1996.
- 29. Spielberger C. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
- 30. Uguccioni G , Golmard JL , de Fontréaux AN , Leu-Semenescu S , Brion A , Arnulf I . Fight or flight? Dream content during sleepwalking/sleep terrors vs. rapid eye movement sleep behavior disorder . Sleep Med. 2013. ; 14 ( 5 ): 391 – 398 . [DOI] [PubMed] [Google Scholar]
- 31. Oudiette D , Leu S , Pottier M , Buzare MA , Brion A , Arnulf I . Dreamlike mentations during sleepwalking and sleep terrors in adults . Sleep. 2009. ; 32 ( 12 ): 1621 – 1627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siclari F , Valli K , Arnulf I . Dreams and nightmares in healthy adults and in patients with sleep and neurological disorders . Lancet Neurol. 2020. ; 19 ( 10 ): 849 – 859 . [DOI] [PubMed] [Google Scholar]
- 33. R: a language and environment for statistical computing . Vienna, Austria: R Foundation for Statistical Computing Core Team; 2020. . Available from: https://www.r-project.org/ . Accessed January 18, 2022.
- 34. Barateau L , Lopez R , Chenini S , et al . Association of CSF orexin-A levels and nocturnal sleep stability in patients with hypersomnolence . Neurology. 2020. ; 95 ( 21 ): e2900 – e2911 . [DOI] [PubMed] [Google Scholar]
- 35. Howell MJ , Schenck CH , Crow SJ . A review of nighttime eating disorders . Sleep Med Rev. 2009. ; 13 ( 1 ): 23 – 34 . [DOI] [PubMed] [Google Scholar]
- 36. Brion A , Flamand M , Oudiette D , Voillery D , Golmard JL , Arnulf I . Sleep-related eating disorder versus sleepwalking: a controlled study . Sleep Med. 2012. ; 13 ( 8 ): 1094 – 1101 . [DOI] [PubMed] [Google Scholar]
- 37. Santin J , Mery V , Elso MJ , et al . Sleep-related eating disorder: a descriptive study in Chilean patients . Sleep Med. 2014. ; 15 ( 2 ): 163 – 167 . [DOI] [PubMed] [Google Scholar]
- 38. Wallace DM , Maze T , Shafazand S . Sodium oxybate-induced sleep driving and sleep-related eating disorder . J Clin Sleep Med. 2011. ; 7 ( 3 ): 310 – 311 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Holst RJ , van der Cruijsen L , van Mierlo P , et al . Aberrant food choices after satiation in human orexin-deficient narcolepsy type 1 . Sleep. 2016. ; 39 ( 11 ): 1951 – 1959 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fortuyn HA , Swinkels S , Buitelaar J , et al . High prevalence of eating disorders in narcolepsy with cataplexy: a case-control study . Sleep. 2008. ; 31 ( 3 ): 335 – 341 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antelmi E , Pizza F , Franceschini C , Ferri R , Plazzi G . REM sleep behavior disorder in narcolepsy: a secondary form or an intrinsic feature? Sleep Med Rev. 2020. ; 50 : 101254 . [DOI] [PubMed] [Google Scholar]
- 42. Nightingale S , Orgill JC , Ebrahim IO , de Lacy SF , Agrawal S , Williams AJ . The association between narcolepsy and REM behavior disorder (RBD) . Sleep Med. 2005. ; 6 ( 3 ): 253 – 258 . [DOI] [PubMed] [Google Scholar]
- 43. Dauvilliers Y , Jennum P , Plazzi G . Rapid eye movement sleep behavior disorder and rapid eye movement sleep without atonia in narcolepsy . Sleep Med. 2013. ; 14 ( 8 ): 775 – 781 . [DOI] [PubMed] [Google Scholar]
- 44. Postuma RB , Iranzo A , Hu M , et al . Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study . Brain. 2019. ; 142 ( 3 ): 744 – 759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang B , Hao Y , Jia F , et al . Sertraline and rapid eye movement sleep without atonia: an 8-week, open-label study of depressed patients . Prog Neuropsychopharmacol Biol Psychiatry. 2013. ; 47 : 85 – 92 . [DOI] [PubMed] [Google Scholar]
- 46. Rak M , Beitinger P , Steiger A , Schredl M , Dresler M . Increased lucid dreaming frequency in narcolepsy . Sleep. 2015. ; 38 ( 5 ): 787 – 792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bjorvatn B , Grønli J , Pallesen S . Prevalence of different parasomnias in the general population . Sleep Med. 2010. ; 11 ( 10 ): 1031 – 1034 . [DOI] [PubMed] [Google Scholar]
- 48. Fosse R . REM mentation in narcoleptics and normals: an empirical test of two neurocognitive theories . Conscious Cogn. 2000. ; 9 ( 4 ): 488 – 509 . [DOI] [PubMed] [Google Scholar]
- 49. Chabani E , Vionnet MC , Beauté R , Leu-Semenescu S , Dodet P , Arnulf I . Blackout of my nights: contentless, timeless and selfless report from the night in patients with central hypersomnias . Conscious Cogn. 2020. ; 81 : 102931 . [DOI] [PubMed] [Google Scholar]
- 50. Fortuyn HAD , Lappenschaar GA , Nienhuis FJ , et al . Psychotic symptoms in narcolepsy: phenomenology and a comparison with schizophrenia . Gen Hosp Psychiatry. 2009. ; 31 ( 2 ): 146 – 154 . [DOI] [PubMed] [Google Scholar]
- 51. Okun ML , Lin L , Pelin Z , Hong S , Mignot E . Clinical aspects of narcolepsy-cataplexy across ethnic groups . Sleep. 2002. ; 25 ( 1 ): 27 – 35 . [DOI] [PubMed] [Google Scholar]
- 52. Andlauer O , Moore H IV , Hong SC , et al . Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy . Sleep. 2012. ; 35 ( 9 ): 1247 – 1255 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ali M , Auger RR , Slocumb NL , Morgenthaler TI . Idiopathic hypersomnia: clinical features and response to treatment . J Clin Sleep Med. 2009. ; 5 ( 6 ): 562 – 568 . [PMC free article] [PubMed] [Google Scholar]
- 54. Inocente CO , Gustin MP , Lavault S , et al . Depressive feelings in children with narcolepsy . Sleep Med. 2014. ; 15 ( 3 ): 309 – 314 . [DOI] [PubMed] [Google Scholar]
- 55. Lopez R , Shen Y , Chenini S , et al . Diagnostic criteria for disorders of arousal: a video-polysomnographic assessment . Ann Neurol. 2018. ; 83 ( 2 ): 341 – 351 . [DOI] [PubMed] [Google Scholar]
- 56. Barros A , Uguccioni G , Salkin-Goux V , Leu-Semenescu S , Dodet P , Arnulf I . Simple behavioral criteria for the diagnosis of disorders of arousal . J Clin Sleep Med. 2020. ; 16 ( 1 ): 121 – 128 . [DOI] [PMC free article] [PubMed] [Google Scholar]