Abstract
Background:
There is a strong bidirectional relationship between the use of alcohol and cigarettes which results in various challenges for treating those who co-use both substances. While varenicline and naltrexone each have FDA-approval for nicotine and alcohol use disorder, respectively, there is evidence that their clinical benefit may extend across the two disorders. Critically, the effect of combined varenicline and naltrexone on neural reactivity to alcohol cues among heavy drinking smokers has not yet been studied. Probing the effect of the combination therapy on alcohol cue-reactivity may give insight to the mechanisms underlying its efficacy.
Methods:
Forty-seven heavy drinking smokers enrolled in two medication studies were randomized to receive varenicline alone (n = 11), varenicline plus naltrexone (n = 11), or placebo (n = 25). Participants completed an fMRI alcohol cue-reactivity task and rated their in-scanner alcohol craving. Whole-brain analyses examined the effect of medication on alcohol cue-elicited neural response.
Results:
Varenicline plus naltrexone attenuated alcohol cue-elicited activation in mesolimbic regions relative to varenicline alone and to placebo (Z > 2.3, p < 0.05). The combination varenicline and naltrexone group also endorsed lower in-scanner alcohol craving relative to varenicline alone group (p = 0.04).
Conclusions:
These findings provide evidence for the benefit of combined therapy of varenicline and naltrexone over varenicline alone for the attenuation of alcohol cue-elicited neural activation. This study provides a preliminary proof-of-mechanism for this combination pharmacotherapy and suggests that naltrexone may be driving the reductions in cue-elicited alcohol craving in the brain. Further clinical studies using the combined therapy to treat heavy drinking smokers are warranted.
Keywords: Varenicline, Naltrexone, fMRI, Alcohol cue reactivity, Heavy drinking smoker
1. Introduction
Alcohol and cigarettes are two of the most frequently used recreational substances in the US. Results from the 2019 National Survey on Drug Use and Health (NSDUH) revealed that 25.8% of individuals reported engaging in a binge drinking episode (≥4 drinks per occasion for females, ≥5 drinks per occasion for males), and 6.3% reported engaging in heavy drinking defined as binge drinking on 5 or more days in the past month (U.S. Department of Health and Human Servies, 2019). The same survey also found that over the past month 18.1% reported cigarette use, with 10.7% of adults reported daily cigarette use (U.S. Department of Health and Human Servies, 2019). Critically, smokers are more likely to have an alcohol use disorder (AUD) than non-smokers, and the majority of individuals with an AUD smoke (Cross et al., 2017). Notably, over the last 5 years the use of e-cigarettes (“vaping”) has increased among adolescents and young adults (Chadi et al., 2019; Grant et al., 2019). The “gateway” hypothesis suggests that youth who otherwise would not have used tobacco products are using e-cigarettes (Barrington-Trimis et al., 2016; Dutra and Glantz, 2017), and the use of e-cigarettes is increasing intentions to use traditional cigarettes compared to those who never smoked any type of cigarette (Bunnell et al., 2015). E-cigarette use has been associated with progression to traditional cigarette use among adolescents and young adults (Leventhal et al., 2015; Primack et al., 2015). The high rate of co-use, and potential for an increase in cigarette users stemming from youth who are using e-cigarettes, underscores the importance of directing attention to this unique group of smokers who are also heavy drinkers.
The concurrent use of alcohol and cigarettes impacts motivation for using the respective substance. A recent overview of effects of alcohol and nicotine in the human laboratory environment summarized how alcohol use increases urge to smoke and decreases time to initiate smoking behavior, while nicotine increases alcohol craving and decreases the subjective effects of alcohol (Verplaetse and McKee, 2017). These effects of nicotine on alcohol use have been observed even with use of a transdermal nicotine patch increasing the positive effects of alcohol (Kouri et al., 2004). Alcohol has a dose-dependent effect on smoking behavior as greater quantities of alcohol have also been associated with earlier initiation of smoking behavior that was partially mediated by smoking urge (Kahler et al., 2014). In the natural environment, momentary assessment has also demonstrated that alcohol increases craving for cigarettes and greater satisfaction with the most recent cigarette (Piasecki et al., 2011, 2008). A recent examination of momentary influences on traditional cigarette use found greater odds of alcohol use was associated with traditional cigarette use, whereas greater tobacco cravings among women was predictive of use of an electronic nicotine delivery system product such as e-cigarettes (Berg et al., 2019). While less is known about the associations between e-cigarettes and alcohol used concurrently, another recent study found that alcohol consumption increased use of traditional cigarettes more than e-cigarettes, with participants reporting significantly greater pleasure from traditional cigarettes (Thrul et al., 2019). The strong associations between alcohol and cigarettes on predictors of use demonstrate a strong bidirectional relationship between these two substances.
This bidirectional relationship results in various challenges for treating those who co-use alcohol and cigarettes. For individuals attempting to quit smoking, smoking lapses that involve alcohol have been shown to be qualitatively different than those not involving alcohol, and consuming alcohol at moderate doses was associated with a 4 times greater risk of a smoking lapse (Kahler et al., 2010). Further, heavy drinking has been associated with significantly lower rates of smoking cessation (Kahler et al., 2009). A prospective analysis of drinking and smoking lapses found that higher urges to smoke, decreased positive affect, and lower confidence to resist smoking preceded smoking lapses (Holt et al., 2012). Analysis of the time-varying effects of alcohol on smoking lapses demonstrated that association between drinking and smoking lapse peaked early in smoking abstinence, then declined, highlighting how interventions to prevent alcohol-related relapses early during a smoking quit attempt could be beneficial (Dermody and Shiffman, 2020). Extending into existing treatments, heavy drinking smokers have been shown to be less responsive to pharmacological treatments for smoking cessation (Roche et al., 2016).
Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, is the most effective pharmacotherapy for smoking cessation (Anthenelli et al., 2016), with an estimated 1 in 8 patients reaching smoking abstinence at 1-year follow-up (Cahill et al., 2007). Studies have shown that in addition to its smoking cessation benefits, varenicline may also reduce alcohol consumption (Litten et al., 2013; O’Malley et al., 2018). Naltrexone, an opioid receptor antagonist, is an FDA-approved pharmacotherapy for alcohol use disorder (Anton et al., 2006; Donoghue et al., 2015), which has been shown to increases smoking cessation rates, particularly among heavy drinking smokers (Fridberg et al., 2014; King et al., 2009). While varenicline and naltrexone each have FDA-approval for nicotine and alcohol use disorder, respectively, there is evidence that their clinical benefit may extend across the two disorders.
Varenicline and naltrexone have individually been examined in functional neuroimaging studies of daily smokers and heavy drinkers. In smokers, varenicline increased activation in the dorsal anterior cingulate, medial frontal cortex, and dorsolateral prefrontal cortex during emotional processing (Loughead et al., 2013) and working memory (Loughead et al., 2010) tasks. Varenicline has also shown to decrease activation to smoking cues in the ventral striatum and medial orbitofrontal cortex (Franklin et al., 2011), and to reduce neural response to alcohol cues in the medial orbitofrontal cortex (Schacht et al., 2014). Recently, varenicline was found to decrease activation in striato-cortico-limbic regions associated with motivation and incentive salience of alcohol (Vatsalya et al., 2015).
Whereas the majority of varenicline neuroimaging studies have focused on smoking outcomes, the literature on naltrexone and neural activation to alcohol cues is more substantial (Grodin and Ray, 2019). Naltrexone has been shown to reduce ventral striatal activation to alcohol cues in alcohol-dependent individuals (Myrick et al., 2008; Schacht et al., 2017), and this effect predicted treatment outcomes, such that individuals with greater reduction in VS cue-reactivity while receiving naltrexone experienced less heavy drinking in the following 14 weeks (Schacht et al., 2017). Extended-release naltrexone attenuated the salience of alcohol-related cues, decreasing cue-reactivity in the orbital, cingulate, inferior and medial frontal gyri (Lukas et al., 2013a, b). Moreover, naltrexone reduced alcohol cue-reactivity in the left putamen, and high neural reactivity to alcohol cues in the left putamen predicted both NTX response and relapse risk (Bach et al., 2020).
While naltrexone and varenicline have each been studied using functional neuroimaging methods, the combined use of varenicline and naltrexone remains under-studied. Preliminary research from our group found that combined varenicline and low-dose naltrexone (i.e., 25 mg) was associated with reduced neural response to cigarette cues in the bilateral anterior cingulate cortex as compared to placebo and to naltrexone alone in a sample of heavy-drinking daily smokers (Ray et al., 2015). All three medication conditions (varenicline, naltrexone, and their combination) also suppressed smoking cue-elicited activation in the left nucleus accumbens, compared to placebo (Ray et al., 2015). Critically, the effect of combined varenicline and naltrexone on neural reactivity to alcohol cues among heavy drinking smokers has not yet been studied.
The current neuroimaging study was part of a randomized, double-blind, parallel group, superiority trial of treatment with varenicline plus naltrexone compared to varenicline alone, in outpatient heavy drinking smokers seeking treatment for smoking cessation and drinking reduction. This study found a reduction in drinks per drinking day in the varenicline plus naltrexone group compared to the varenicline alone group across the 12-week treatment phase (Ray et al., 2021). A subset of participants in the parent clinical trial completed an alcohol cue-reactivity neuroimaging paradigm to test the effect of combined varenicline and naltrexone compared to varenicline alone and placebo on neural alcohol cue reactivity. As the primary trial did not include a placebo group, we also included a placebo group from a separate trial which used the same alcohol cue-reactivity paradigm. Given our previous neuroimaging work and the results of the main study trial, we hypothesized that individuals treated with combined varenicline and naltrexone would show attenuated mesocorticolimbic activation to alcohol cues, relative to individuals treated with varenicline alone, and relative to those treated with placebo.
2. Materials and methods
2.1. Participants
A total of 47 individuals (22–65, 16 females) participated in this study. They were recruited for 2 separate projects which were approved by the University of California, Los Angeles Institutional Review Board. In both studies, participants completed the Alcohol Cues Task (Schacht et al., 2011) during fMRI using identical neuroimaging procedures while under treatment of an active medication or placebo. Participants from study 1 (n = 22) were part of a 26-week randomized clinical trial of varenicline alone versus varenicline plus naltrexone for smoking cessation and drinking reduction in a community sample of heavy drinking smokers (Ray et al., 2021; ClinicalTrials.gov identifier: NCT02698215). Neuroimaging was added to study 1 in March 2018; participants meeting MRI eligibility criteria were invited to participate in the neuroimaging portion of the study. Participants from study 2 (n = 25) were part of a 2-week randomized clinical trial of ibudilast for drinking reduction in a community sample of heavy drinkers (Grodin et al., 2021; ClinicalTrials.gov identifier: NCT03489850); only individuals treated with placebo were included in the current study. Neuroimaging data was collected from the majority of placebo participants in study 2 (25/28).
Participants for both studies were recruited through print, radio, social media, and mass transit advertisements. Participants were screened for initial eligibility over the telephone; eligible participants were invited to the laboratory for an in-person intake screening. In both studies participants were required to meet the definition of heavy drinking set by the National Institute of Alcohol Abuse and Alcoholism: >14 drinks/week for men and >7 drinks/week for women, in the 30 days prior to screening. Both studies excluded participants with past year DSM-5 diagnoses of substance use disorder (excluding alcohol), lifetime diagnoses of schizophrenia, bipolar disorder, or any psychotic disorder, and clinically significant alcohol withdrawal symptoms, as indicated by a score ≥ 10 on the Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) (Sullivan et al., 1989). Neuroimaging exclusion criteria for both studies were: claustrophobia, non-removable ferromagnetic objects in body; and serious head injury or prolonged period of unconsciousness (>30 min). Participants were also excluded if they had a medical condition thought to interfere with safe participation and if they reported recent use of medications contraindicated with varenicline or varenicline plus naltrexone (study 1) or ibudilast (study 2). Women of a childbearing age had to be practicing effective contraception and could not be pregnant or nursing. For study 1 participants were also required to smoke ≥5 cigarettes per day and to have a carbon monoxide reading ≥4 ppm to verify smoker status. Both studies required a physical exam with the study physician which included vital signs, E.K.G., and clinical laboratory exams to ensure medical eligibility. At each in-person visit, participants were required to have a breath alcohol concentration of 0.00 mg/dL, test negative on a urine toxicology screen for all drugs of abuse (except cannabis), and test negative on a pregnancy test (women only). Blood pressure and heart rate were assessed at screening and at each visit.
2.2. Assessments
Participants completed a series of assessments for eligibility and individual differences. These measures included the Structured Clinical Interview for DSM-5 (SCID-5)(First et al., 2015) to assess for alcohol use disorder (AUD) and other substance use disorders, the CIWA-Ar (Sullivan et al., 1989) to evaluate alcohol withdrawal symptoms, the Fagerstrom Test for Nicotine Dependence (FNTD)(Heatherton et al., 1991) which measures nicotine dependence severity, the Alcohol Use Disorder Identification Test (AUDIT)(Saunders et al., 1993), which assess severity of alcohol use problems, and the 30-day Timeline Followback Interview (TLFB)(Sobell and Sobell, 1992) for alcohol, cigarettes, and cannabis. From the TLFB interview the following items were calculated: total number of alcoholic drinks consumed over 30 days, drinks per drinking day, total number of cigarettes smoked over 30 days, and cigarettes per smoking day.
2.3. Neuroimaging procedures
Neuroimaging for both studies took place at the UCLA Center for Cognitive Neuroscience on a 3.0 T Siemens Prisma Scanner (Siemens Medical Solutions USA, Inc., Malvern, PA). The neuroimaging visit took place on study day 10–14 for Study 1 and on study day 8 for Study 2. A T2-weighted, high-resolution matched-bandwidth (MBW) anatomical scan (time to repetition (TR) = 5000 ms, time to echo (TE) =34 ms, flip angle = 90°, voxel size: 1.5 mm × 1.5 mm × 4 mm, field of view (FOV) = 192 mm2, 34 slices, ~1.5 min) and a T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence (TR = 2530 ms, TE =1.74 ms, time to inversion = 1260 ms, flip angle = 7°, voxel size: 1 mm3, FOV = 256 mm2, ~6.2 min) were acquired for co-registration to the functional data. A T2*-weighted echo planar imaging (EPI) scan (TR = 2200 ms, TE =35 ms, flip angle = 90°, FOV = 192 mm, slices = 36, 3.0 mm, ~12 min) was acquired to examine the blood oxygen-level dependent (BOLD) signal during the alcohol cue reactivity task.
2.4. Alcohol cue reactivity task
Participants completed a 720-s-long alcohol cue-reactivity task (Schacht et al., 2011), in which they were presented with 24 pseudo-randomly interspersed blocks of alcoholic beverage images (ALC), non-alcoholic beverage images (BEV), blurred images to serve as visual controls, and a fixation cross. Each block was composed of 5 individual pictures of the same type, each presented for 4.8 s, for a total of 24-seconds. Each block was followed by a 6-second washout period during which participants reported on the urge to drink using an MRI compatible 4-button response pad (ratings ranged from 1 to 4, with 1 representing no craving and 4 representing high craving, with each response reflected by a colored button on the response pad). Alcoholic beverage blocks were distributed between images of beer, wine, and liquor (2 of each).
2.5. Neuroimaging processing
Preprocessing followed conventional procedures as implemented in FMRIB Software (FSL v6.0.1 http://www.fmrib.ox.ac.uk/fsl). This included motion correction, high-pass temporal filtering (100-second cut-off), and smoothing with a 5-mm full-width, half-maximum Gaussian kernel (Jenkinson et al., 2002). Functional and structural data were skull-stripped to remove non-brain tissue. Each subject’s functional images were registered to their MBW, followed by their MPRAGE using affine linear transformations, and then were normalized to the Montreal Neurological Institute (MNI) 152-brain-average template through non-linear registration (Andersson et al., 2007).
2.6. Data analysis
A series of analyses of variance (ANOVAs) were conducted as omnibus tests comparing the medication groups on continuous demographic and clinical measures. Similarly, chi-square tests were used to compare medication groups on categorical measures. Tukey-Kramer t-tests were used to follow-up significant omnibus ANOVAs to identify the specific group differences. Group comparison analyses were conducted in SPSS 26.
For the neuroimaging data, whole-brain statistical analysis was performed using FSL’s FEAT software (Woolrich et al., 2001). The primary contrast of interest, ALC vs. BEV, was defined in the first-level models for each subject. FSL’s FLAME 1 (Woolrich et al., 2004) was used to conduct group-level analyses. Specifically, a 1-way ANOVA was conducted to evaluate the main effect of group (VAR, VAR + NTX, PLAC) on alcohol cue-elicited brain activation. Follow-up analyses evaluated group differences. Z-statistic images were thresholded using a cluster threshold of Z > 2.3 and a (corrected) cluster significance threshold of p = .05 (Worsley et al., 2001). Age, AUDIT total scores, and smoking status were examined as potential covariates in separate whole brain analyses. Only age was a significant covariate for the whole-brain analysis and is reported below.
For the exploratory alcohol craving analysis, the peak alcohol craving during the scan was collected for each participant. Given the significant differences between VAR alone and VAR + NTX on alcohol cue-elicited neural activation, the effect of the combination of medications compared to varenicline alone on alcohol craving was examined using a t-test.
3. Results
3.1. Participants
There were significant differences between groups on several clinical and demographic characteristics. Specifically, the placebo group was younger, had fewer smokers, and had less severe nicotine dependence compared to active medication groups (VAR and VAR + NTX). Regarding alcohol use, the groups did not differ on quantity or frequency of drinks or on AUDIT scores. There were no significant differences between the active medication groups on any demographic or clinical variable (see Table 1).
Table 1.
Demographic and Clinical Characteristics.
| Characteristic | VAR (n = 11) | VAR + NTX (n = 11) | PLAC (n = 25) | Statistic | p-value |
|---|---|---|---|---|---|
| Age a,b | 41.73 ± 11.41 | 39.73 ± 11.74 | 31.16 ± 7.80 | F = 5.81 | 0.006 |
| Sex (m/f) | 7/4 | 9/2 | 15/10 | X2 = 1.65 | 0.44 |
| Race (%) | X2 = 15.21 | 0.23 | |||
| White | 5 (45.45%) | 3 (27.27%) | 12 (48%) | ||
| Black | 3 (27.27%) | 5 (45.45%) | 4 (16%) | ||
| American Indian | 0 (0%) | 1 (9.09%) | 0 (0%) | ||
| Asian | 0 (0%) | 0 (0%) | 4 (16%) | ||
| Pacific Islander | 1 (9.09%) | 1 (9.09%) | 0 (0%) | ||
| Mixed | 0 (0%) | 1 (9.09%) | 2 (8%) | ||
| Other/Unknown | 2 (18.18%) | 0 (0%) | 3 (12%) | ||
| Hispanic/Latino | 3 (27.27%) | 2 (18.18%) | 10 (40%) | X2 = 1.82 | 0.40 |
| THC+ (%) | 7 (63.64%) | 4 (36.36%) | 6 (24%) | X2 = 5.20 | 0.07 |
| Smoker (y/n) | 11/0 | 11/0 | 13/12 | X2 = 14.18 | 0.001 |
| FTND a,b | 4.73 ± 2.24 | 5.00 ± 2.53 | 1.15 ± 1.57 | F = 12.55 | <0.001 |
| Total Cigarettes (30 Days) a,b | 477.27 ± 351.90 | 393.55 ± 292.67 | 140.62 ± 212.16 | F = 4.55 | 0.02 |
| Cigarettes Per Smoking Day | 16.03 ± 11.65 | 13.64 ± 9.39 | 7.30 ± 6.78 | F = 2.84 | 0.07 |
| AUDIT Score | 21.09 ± 6.19 | 14.91 ± 6.26 | 16.40 ± 6.26 | F = 2.95 | 0.06 |
| Total Drinks (30 Days) | 122.67 ± 57.62 | 108.57 ± 88.03 | 115.40 ± 95.35 | F = 0.06 | 0.94 |
| Drinks Per Drinking Day | 6.24 ± 3.10 | 5.70 ± 2.31 | 5.36 ± 3.72 | F = 0.27 | 0.76 |
| Heavy Drinking Days | 12.64 ± 8.06 | 10.82 ± 11.15 | 8.88 ± 8.31 | F = 0.70 | 0.50 |
Data are presented as mean ± standard deviation or as number of participants (percent of sample. VAR = varenicline only; VAR + NTX = varenicline + naltrexone; PLAC = placebo; FTND = Fagerstrom Test for Nicotine Dependence; AUDIT = Alcohol Use Disorder Identification Test.
VAR > PLAC.
VAR + NTX > PLAC.
3.2. Alcohol cue reactivity – whole brain analysis
There was a significant main effect of group on alcohol cue reactivity in several reward-associated brain regions, including the medial prefrontal cortex (mPFC), cingulate, and caudate (see Table 2 and Fig. 1A). To probe this main effect, a series of post-hoc tests were conducted to localize group differences. Specifically, the VAR group showed significantly greater alcohol cue-elicited activation in the caudate and thalamus as compared to the combined VAR + NTX group (see Table 2 and Fig. 1B). The PLAC group also showed significantly greater alcohol cue reactivity relative to the VAR + NTX group in a cluster extending from the parahippocampus to the thalamus (see Table 2 and Fig. 1C). There were no significant differences between the VAR group and the placebo group. The average alcohol vs. beverage activation for each group can be found in the Supplementary Materials (Table S1 and Figure S1).
Table 2.
Whole-Brain Group Results for Alcohol vs. Beverage Contrast.
| Comparison | Voxels | Region | X | Y | Z | Peak Z | p-value |
|---|---|---|---|---|---|---|---|
| Main Effect of Group | |||||||
| 1628 | Bilateral niPFC | −4 | 60 | 20 | 4.52 | <0.001 | |
| 1079 | Bilateral Cingulate | −2 | −22 | 34 | 4.31 | <0.001 | |
| 908 | L Caudate | −10 | 18 | 12 | 4.09 | <0.001 | |
| 407 | R Midbrain/VTA | 6 | −24 | −10 | 3.64 | 0.02 | |
| 356 | R Superior Frontal Gyrus | 12 | 12 | 62 | 3.46 | 0.04 | |
| 347 | L Superior Frontal Gyrus | −14 | 18 | 60 | 3.83 | 0.04 | |
| VAR > VAR + NTX | |||||||
| 711 | R Thalamus | 20 | −30 | −2 | 3.72 | <0.001 | |
| 345 | L Caudate | −14 | 8 | 14 | 3.53 | 0.04 | |
| VAR + NTX > VAR | |||||||
| N/A | |||||||
| VAR > PLAC | |||||||
| N/A | |||||||
| PLAC > VAR | |||||||
| N/A | |||||||
| VAR + NTX > PLAC | |||||||
| N/A | |||||||
| PLAC > VAR + NTX | |||||||
| 525 | R Parahippocampus, Hippocampus, Thalamus | 18 | −30 | −6 | 4.2 | 0.004 | |
| Age + | |||||||
| 890 | L Superior Parietal Lobule | −30 | −54 | 70 | 4.24 | <0.001 | |
| Age − | |||||||
| N/A |
VAR = varenicline; VAR + NTX = varenicline and naltrexone; PLAC = placebo; L = left; R = right; Coordinates are in Montreal Neurological Institute (MNI) space.
Fig. 1. Whole-Brain Alcohol vs. Beverage Results.
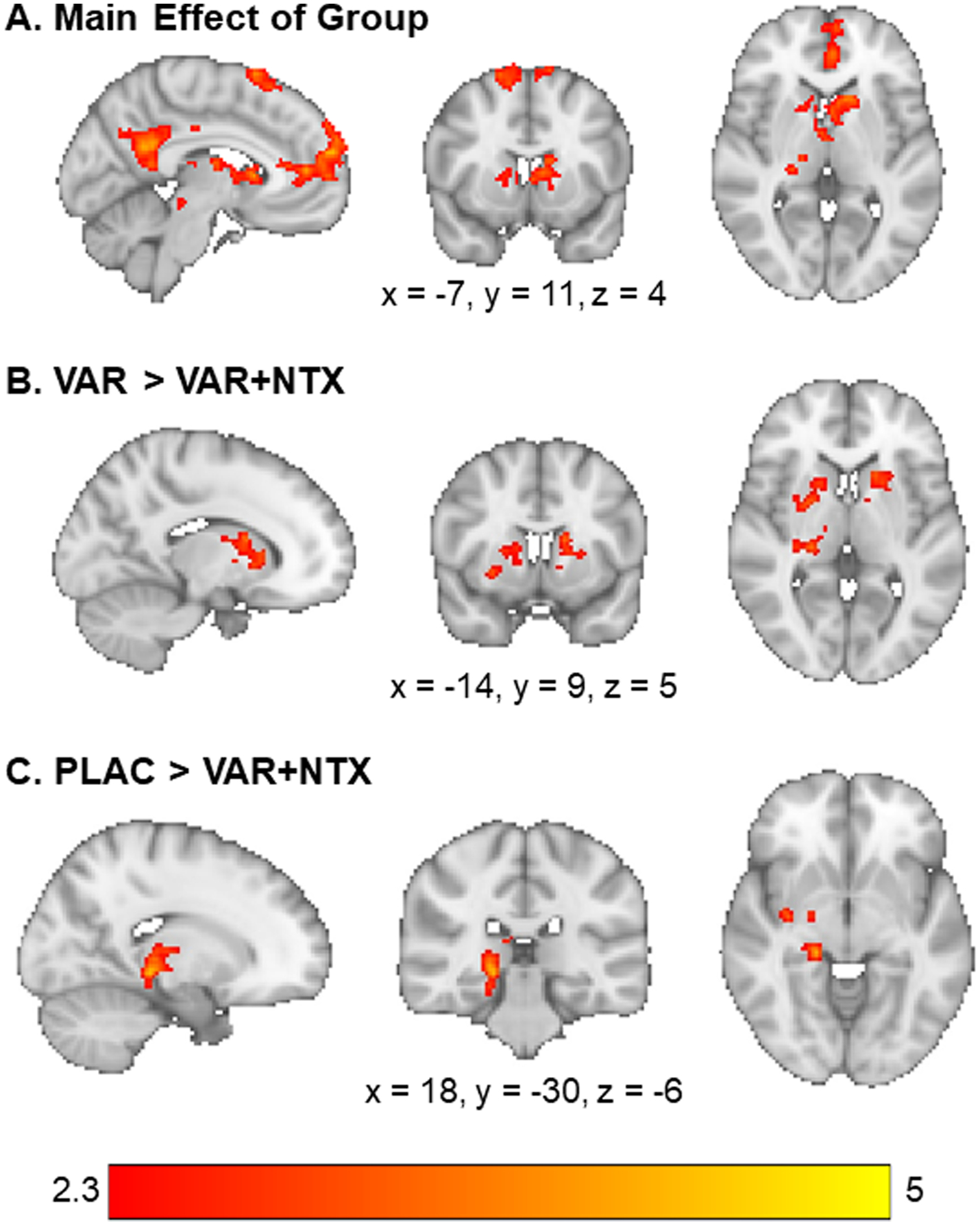
(A) There was a main effect of group on activation to alcohol vs, beverage cues in the medial prefrontal cortex, cingulate gyrus, caudate, and midbrain. (B) The varenicline only group had higher activation to alcohol vs. beverage cues in the caudate and thalamus relative to the varenicline + naltrexone group. (C) The placebo group had higher activation to alcohol vs. beverage cues in the thalamus, parahippocampus, and hippocampus, relative to the varenicline + naltrexone group. Z-statistic maps are whole-brain cluster corrected, Z > 2.3, p = .05. Coordinates are in Montreal Neurological Institute space. Brain is displayed in radiological convention (L=R).
Age was a significant covariate, such that age was positively correlated with increased activation in the left superior parietal lobule when viewing alcohol vs. beverage cues. There were no regions which showed a significant negative correlation with age and activation.
In order to ensure that medication effects were specific to alcohol cues and not a blunting of activation to all cue types, analyses were performed to examine whole-brain activation to alcohol and non-alcoholic beverage cues separately. For alcohol cues alone, there was a significant activation in mesocorticolimbic reward circuitry and occipital/temporal circuitry across all participants (Table S2). Each group had significant activation to alcohol cues in broad visual, temporal, and prefrontal circuitry (Table S2). The VAR group had significantly greater alcohol cue activation in the insula and putamen relative to the VAR + NTX group (Table S2 and Figure S2). For beverage cues alone, there was a significant activation in mesocorticolimbic reward circuitry occipital/temporal circuitry across all participants (Table S3). Similar to the alcohol cue results, each group had significant activation to non-alcoholic beverage cues in broad visual, temporal, and prefrontal circuitry (Table S3). The VAR group had significantly greater alcohol cue activation in the insula and postcentral gyrus relative to the VAR + NTX group, while the VAR + NTX group had greater activity in the lateral occipital cortex (Table S3 and Figure S3). The VAR group had significantly greater activation in the pre- and postcentral gyrus and insula relative to the placebo group (Table S3 and Figure S3). The VAR + NTX group had significantly greater activation in the superior and middle frontal gyrus, occipital cortex, and precentral gyrus relative to placebo (Table S3 and Figure S3).
3.3. Alcohol craving
The medication groups (VAR + NTX and VAR) significantly differed on peak in-scanner alcohol craving (t(1,18) = 2.24, p = 0.04), such that the combined VAR + NTX group had lower ratings than the VAR alone group (VAR alone craving = 3.00 ± 1.05; VAR + NTX craving = 2.00 ± 0.94; range 1–4). Only 1 participant endorsed the highest craving rating in the combined VAR + NTX group (9.09%); while 4 participants in the monotherapy group reported the highest level of craving (36.36%). The peak craving rating for the medication group was 2.56 ± 1.16; with 7 participants endorsing the highest craving rating (28%). There were no significant differences between either medication group and placebo on peak craving ratings.
4. Discussion
This neuroimaging study investigated the effect of combined varenicline and naltrexone compared to varenicline alone and placebo on the neural response to alcohol cues in heavy drinking smokers. Results revealed that that the combination of varenicline and naltrexone attenuated alcohol cue-elicited neural responses in mesolimbic reward circuitry relative to varenicline alone and placebo. The combined medication also reduced in-scanner alcohol craving relative to varenicline alone. This finding is consistent with results from the larger randomized controlled trial, which found a reduction in drinks per drinking day in the combined medication group, compared to varenicline only (Ray et al., 2021). Together these preliminary findings are in line with the notion that the combination of varenicline plus naltrexone may reduce craving for alcohol and neural activation to alcohol, versus control, cues in the mesolimbic pathway.
As hypothesized, the brain localization of the cue-reactivity attenuation effects largely aligned with previous neuroimaging literature on these medications. Both varenicline and naltrexone have individually been shown to affect neural activation in corticolimbic (Schacht et al., 2014; Loughead et al., 2013; Lukas et al., 2013a,b) and striatal (Vatsalya et al., 2015; Bach et al., 2020) reward-associated regions. Furthermore, the separate alcohol and non-alcoholic beverage cue analyses indicate that these results are not due to a blunting of overall reward response or medication-induced somnolence, as both medication groups showed a significant brain activation to non-alcoholic beverage cues alone. Additionally, reduced alcohol cue-reactivity in these mesocorticolimbic regions aligns with previous research in which combined varenicline and naltrexone attenuated neural reactivity to smoking cues (Ray et al., 2015). Consistent with the role of functional neuroimaging studies in medications development (Grodin and Ray, 2019), this study provides a proof-of-mechanism for this combination pharmacotherapy and suggests that naltrexone may be driving the reductions in cue-elicited alcohol craving in the brain. The potential mechanism driving these effects may be dopamine related. In rodent models, the co-administration of nicotine and alcohol in the ventral tegmental area results in an additive effect on the release of dopamine in the nucleus accumbens (Tizabi et al., 2002), indicating that alcohol’s reinforcing effects may be partially mediated through nicotinic receptors in the ventral tegmental area. Naltrexone has been shown to decrease striatal dopamine (Benjamin et al., 1993; Gonzales and Weiss, 1998). Therefore, the combination of varenicline and naltrexone may reduce dopamine in the striatum, resulting in a decrease in alcohol cue-elicited neural activation. Furthermore, cue-induced craving was significantly lower in the combined varenicline and naltrexone group relative to the varenicline alone group. These preliminary findings are in line with previous work investigating the effect of varenicline, naltrexone, and their combination on alcohol craving. Naltrexone has been shown to consistently blunt alcohol craving across cue-reactivity paradigms (Davidson et al., 1999a, b; Lukas et al., 2013a,b; McCaul et al., 2000a,b; Monti et al., 1999a,b; Ooteman et al., 2007a,b). Findings with varenicline have been mixed. A human laboratory study found that varenicline blunted alcohol cue-elicited craving (Roberts et al., 2017a,b); while a recent multisite trial of varenicline found no benefit of varenicline in reducing cue-induced alcohol craving (Miranda et al., 2020a,b). A human laboratory study of the combination of varenicline and naltrexone in heavy drinking smokers found no medication effects on alcohol craving after 12 h of nicotine abstinence (Ray et al., 2014a,b); however, this effect was not primed with an alcohol cue. Of note, the ratings of craving in the study were limited to a range of 1–4 and it is possible that the lower craving found in the combined medication group may not reflect a biologically-relevant difference between the medication groups, particularly as only a small portion of the study sample endorsed the highest level of craving.
Outside of the neuroimaging literature, these findings also support recent work indicating that naltrexone may be a more efficacious treatment for individuals with an AUD who also smoke cigarettes. In individuals who with AUD who smoke cigarettes, NTX decreased percent heavy drinking days compared to placebo (Anton et al., 2018; Schacht et al., 2017), and in a separate study increased percent days abstinent and reduced drinking-related consequences (Fucito et al., 2012). Importantly, these effects were not present in non-smoking individuals with AUD treated with NTX. Similar effects have been seen in smoking cessation studies, where NTX has been shown to reduce heavy drinking rates in treatment-seeking smokers who also drink heavily (Fridberg et al., 2014; King et al., 2009; O’Malley et al., 2009).
While this study is preliminary, it is important to note that this study advances the field through its investigation of potential concurrent treatment of smoking and heavy drinking. The majority of studies in the field focus on the treatment of single disorders and often have strict exclusion criteria (Blanco et al., 2008). While smoking is often not exclusionary in AUD treatment trials, there have been few studies which try to concurrently treat both smoking and alcohol using a single medication or a combination of pharmacotherapies. Given that a major goal of clinical studies is to translate to clinical practice, these studies, including fMRI pharmacotherapy studies, should enroll representative samples with co-occurring health conditions, which may help accelerate the acceptance and use of evidence-based treatments in clinical settings. Due to the limited number (<10%) of participants in our sample that used e-cigarettes, we cannot say with certainty that our results could extend to the co-use of e-cigarettes and alcohol. Initial evidence suggests there may be some overlap between the co-use of alcohol and traditional versus e-cigarettes (Hershberger and Cyders, 2017). However, various differences exist, such as e-cigarette accessibility to adolescents, social acceptability of e-cigarettes, and differences in the “high” from e-cigarettes versus traditional cigarettes (Hershberger and Cyders, 2017). These are all key factors that could impact the effects of medication on cue-elicited activation in mesolimbic regions. Given that many e-cigarette users transition to traditional cigarettes (Leventhal et al., 2015; Primack et al., 2015), these results are still highly relevant to smokers broadly and future studies could examine the effects of medication on alcohol cue-elicited neural response among alcohol and e-cigarette users.
This study should be interpreted in the context of its strengths and weaknesses. Strengths include the use of neuroimaging in a combination pharmacotherapy trial and the enrollment of a heavy drinking smoking sample. The main limitation of this study is its small sample size, particularly for the active treatment groups, which lead these findings to be classified as preliminary. Another study limitation is the lack of an active smoking cue condition, given that the study population consisted of heavy drinking smokers. Future studies with co-using samples should use alcohol cue reactivity neuroimaging paradigms that include a smoking cue condition and a combined alcohol and smoking cue condition to fully investigate the combination of varenicline and naltrexone in larger samples. Additionally, the placebo group was included from a separate pharmacotherapy study, as the main combination pharmacotherapy trial was a superiority trial and did not include a placebo arm. This resulted in the individuals in the placebo group not matching in age or smoking severity measures relative to the varenicline alone and varenicline plus naltrexone groups. Finally, in-scanner craving was measured using a 4-point visual analogue scale measured using a 4-button response box, which may have limited our ability to assess a full range of craving (i.e., a ceiling effect). Future studies should include a craving measurement with a wider range to better capture craving elicited during this task.
In conclusion, these preliminary results advance medication development for heavy drinking smokers, suggesting that the combination of varenicline and naltrexone is superior to monotherapy alone in attenuating alcohol-cue elicited mesolimbic activation and alcohol craving. Further study of the combination of these pharmacotherapies in heavy drinking smokers, a difficult to treat population, are warranted.
Supplementary Material
Acknowledgements
This research was supported in part by the National Institute of Drug Abuse (R01DA041226 to LAR]), the National Institute of Alcohol Abuse and Alcoholism (K24AA025704 to LAR; F32AA027699 to ENG; F31AA028976 to EMB), and the Tobacco-Related Disease Research Program (T30DT0950 to RG and T29DT0371 to AL) Pfizer and MediciNova provided the study medication and placebo, respectively. The funders had no role in the design, analysis, interpretation, or writing of the report.
Footnotes
Disclosures
All authors report no financial relationships with commercial interests.
Declaration of Competing Interest
No conflict declared.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2021.108825.
References
- Andersson JL, Jenkinson M, Smith S, 2007. Non-Linear Registration Aka Spatial Normalisation FMRIB Technial Report TR07JA2 FMRIB Analysis Group of the University of Oxford, pp. 1–22. [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE, 2016. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet (London, England) 387 (10037), 2507–2520. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, 2006. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295 (17), 2003–2017. [DOI] [PubMed] [Google Scholar]
- Anton RF, Latham PK, Voronin KE, Randall PK, Book SW, Hoffman M, Schacht JP, 2018. Nicotine-Use/Smoking is associated with the efficacy of naltrexone in the treatment of alcohol dependence. Alcohol. Clin. Exp. Res 42 (4), 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach P, Weil G, Pompili E, Hoffmann S, Hermann D, Vollstädt-Klein S, Mann K, Perez-Ramirez U, Moratal D, Canals S, Dursun SM, Greenshaw AJ, Kirsch P, Kiefer F, Sommer WH, 2020. Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict. Biol 25 (1), e12717. [DOI] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Urman R, Leventhal AM, Gauderman WJ, Cruz TB, Gilreath TD, Howland S, Unger JB, Berhane K, Samet JM, McConnell R, 2016. E-cigarettes, cigarettes, and the prevalence of adolescent tobacco use. Pediatrics 138 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D, Grant ER, Pohorecky LA, 1993. Naltrexone reverses ethanol-induced dopamine release in the nucleus accumbens in awake, freely moving rats. Brain Res 621 (1), 137–140. [DOI] [PubMed] [Google Scholar]
- Berg CJ, Haardörfer R, Payne JB, Getachew B, Vu M, Guttentag A, Kirchner TR, 2019. Ecological momentary assessment of various tobacco product use among young adults. Addict. Behav 92, 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Olfson M, Okuda M, Nunes EV, Liu SM, Hasin DS, 2008. Generalizability of clinical trials for alcohol dependence to community samples. Drug Alcohol Depend 98 (1–2), 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, Coleman BN, Dube SR, King BA, 2015. Intentions to smoke cigarettes among never-smoking US middle and high school electronic cigarette users: national Youth Tobacco Survey, 2011–2013. Nicotine Tob. Res 17 (2), 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T, 2007. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev 1. CD006103. [DOI] [PubMed] [Google Scholar]
- Chadi N, Hadland SE, Harris SK, 2019. Understanding the implications of the “vaping epidemic” among adolescents and young adults: a call for action. Subst. Abus 40 (1), 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Lotfipour S, Leslie FM, 2017. Mechanisms and genetic factors underlying co-use of nicotine and alcohol or other drugs of abuse. Am. J. Drug Alcohol Abuse 43 (2), 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R, 1999a. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol. Clin. Exp. Res 23 (2), 195–203. [PubMed] [Google Scholar]
- Davidson D, Palfai T, Bird C, Swift R, 1999b. Effects of naltrexone on alcohol self-administration in heavy drinkers. Alcohol. Clin. Exp. Res 23 (2), 195–203. [PubMed] [Google Scholar]
- Dermody SS, Shiffman S, 2020. The time-varying effect of alcohol use on cigarette smoking relapse risk. Addict. Behav 102, 106192. [DOI] [PubMed] [Google Scholar]
- Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C, 2015. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction 110 (6), 920–930. [DOI] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA, 2017. E-cigarettes and national adolescent cigarette use: 2004–2014. Pediatrics 139 (2), e20162450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, Spitzer R, 2015. Structured Clinical Interview for DSM5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) American Psychiatric Association, Arlington, VA, pp. 1–94. [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, Cruz J, Li Y, Goldman M, Detre JA, O’Brien CP, Childress AR, 2011. Effects of varenicline on smoking cue–triggered neural and craving responses. Arch. Gen. Psychiatry 68 (5), 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg DJ, Cao D, Grant JE, King AC, 2014. Naltrexone improves quit rates, attenuates smoking urge, and reduces alcohol use in heavy drinking smokers attempting to quit smoking. Alcohol. Clin. Exp. Res 38 (10), 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucito LM, Park A, Gulliver SB, Mattson ME, Gueorguieva RV, O’Malley SS, 2012. Cigarette smoking predicts differential benefit from naltrexone for alcohol dependence. Biol. Psychiatry 72 (10), 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F, 1998. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J. Neurosci 18 (24), 10663–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Lust K, Fridberg DJ, King AC, Chamberlain SR, 2019. E-cigarette use (vaping) is associated with illicit drug use, mental health problems, and impulsivity in university students. Ann. Clin. Psychiatry 31 (1), 27–35. [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Ray LA, 2019. The use of functional magnetic resonance imaging to test pharmacotherapies for alcohol use disorder: a systematic review. Alcohol. Clin. Exp. Res 43 (10), 2038–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Bujarski S, Towns B, et al. , 2021. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial. Transl. Psychiatry 11 (355). 10.1038/s41398-021-01478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, FAGERSTROM KO, 1991. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict 86 (9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hershberger A, Cyders MA, 2017. Essentially, all models are wrong, but some are useful”: a preliminary conceptual model of Co-occurring E-Cig and alcohol use. Curr. Addict. Rep 4 (2), 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LJ, Litt MD, Cooney NL, 2012. Prospective analysis of early lapse to drinking and smoking among individuals in concurrent alcohol and tobacco treatment. Psychol. Addict. Behav 26 (3), 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2), 825–841. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Borland R, Hyland A, McKee SA, Thompson ME, Cummings KM, 2009. Alcohol consumption and quitting smoking in the international tobacco control (ITC) four country survey. Drug Alcohol Depend 100 (3), 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Metrik J, 2010. Alcohol use and initial smoking lapses among heavy drinkers in smoking cessation treatment. Nicotine Tob. Res 12 (7), 781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Day A, Leventhal AM, McKee SA, Tidey JW, McGeary JE, Knopik VS, Rohsenow DJ, 2014. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacology 231 (24), 4649–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Cao D, Vanier C, Wilcox T, 2009. Naltrexone decreases heavy drinking rates in smoking cessation treatment: an exploratory study. Alcohol. Clin. Exp. Res 33 (6), 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE, 2004. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend 75 (1), 55–65. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J, 2015. Association of electronic cigarette use with initiation of combustible tobacco product smoking in early adolescence. Jama 314 (7), 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid-Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R, 2013. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J. Addict. Med 7 (4), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C, 2010. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol. Psychiatry 67 (8), 715–721. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, O’Donnell GP, Senecal N, Siegel S, Gur RC, Lerman C, 2013. Brain activity and emotional processing in smokers treated with varenicline. Addict. Biol 18 (4), 732–738. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM, 2013a. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. NeuroImage 78, 176–185. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM, 2013b. Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. NeuroImage 78, 176–185. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ, 2000a. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 22 (5), 480–492. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ, 2000b. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 22 (5), 480–492. [DOI] [PubMed] [Google Scholar]
- Miranda R Jr, O’Malley SS, Treloar Padovano H, Wu R, Falk DE, Ryan ML, Fertig JB, Chun TH, Muvvala SB, Litten RZ, 2020a. Effects of alcohol cue reactivity on subsequent treatment outcomes among treatment-seeking individuals with alcohol use disorder: a multisite randomized, double-blind, placebo-controlled clinical trial of varenicline. Alcohol. Clin. Exp. Res 44 (7), 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr, O’Malley SS, Treloar Padovano H, Wu R, Falk DE, Ryan ML, Fertig JB, Chun TH, Muvvala SB, Litten RZ, 2020b. Effects of Alcohol Cue Reactivity on Subsequent Treatment Outcomes Among Treatment-Seeking Individuals with Alcohol Use Disorder: A Multisite Randomized, Double-Blind, Placebo-Controlled Clinical Trial of Varenicline. Alcohol. Clin. Exp. Res 44 (7), 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB, 1999a. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol. Clin. Exp. Res 23 (8), 1386–1394. [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, Colby SM, Brown RA, Gulliver SB, Gordon A, Abrams DB, 1999b. Naltrexone’s effect on cue-elicited craving among alcoholics in treatment. Alcohol. Clin. Exp. Res 23 (8), 1386–1394. [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K, 2008. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch. Gen. Psychiatry 65 (4), 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, McKee SA, Leeman RF, Cooney NL, Meandzija B, Wu R, Makuch RW, 2009. Dose-dependent reduction of hazardous alcohol use in a placebo-controlled trial of naltrexone for smoking cessation. Int. J. Neuropsychopharmacol 12 (5), 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, Bold KW, Petrakis I, Muvvala S, Jatlow P, Gueorguieva R, 2018. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry 75 (2), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM, van den Brink W, 2007a. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur. Neuropsychopharmacol 17 (8), 558–566. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM, van den Brink W, 2007b. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur. Neuropsychopharmacol 17 (8), 558–566. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB, 2008. Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: an ecological study. Psychol. Addict. Behav 22 (2), 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Rohrbaugh JW, Heath AC, Shiffman S, Sher KJ, 2011. The subjective effects of alcohol–tobacco co-use: An ecological momentary assessment investigation. J. Abnorm. Psychol 120 (3), 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD, 2015. Progression to traditional cigarette smoking after electronic cigarette use among US adolescents and young adults. JAMA Pediatr 169 (11), 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED, 2014a. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology 231 (19), 3843–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED, 2014b. Varenicline, low dose naltrexone, and their combination for heavy-drinking smokers: human laboratory findings. Psychopharmacology 231 (19), 3843–3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED, 2015. Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am. J. Drug Alcohol Abuse 41 (1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Leventhal AM, Enders C, Grodin EN, Li G, Lim A, Hartwell E, Venegas A, Meredith L, Nieto SJ, Shoptaw S, Miotto K, 2021. Efficacy of combining varenicline and naltrexone for smoking cessation and drinking reduction: a randomized clinical trial. Am. J. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Harrison ELR, McKee SA, 2017a. Effects of varenicline on alcohol cue reactivity in heavy drinkers. Psychopharmacology (Berl.) 234 (18), 2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Harrison ELR, McKee SA, 2017b. Effects of varenicline on alcohol cue reactivity in heavy drinkers. Psychopharmacology (Berl.) 234 (18), 2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Ray LA, Yardley MM, King AC, 2016. Current insights into the mechanisms and development of treatments for heavy-drinking cigarette smokers. Curr. Addict. Rep 3 (1), 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M, 1993. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 88 (6), 791–804. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H, 2011. Stability of fMRI striatal response to alcohol cues: a hierarchical linear modeling approach. Neuroimage 56 (1), 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H, 2014. Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 231 (18), 3799–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Randall PK, Latham PK, Voronin KE, Book SW, Myrick H, Anton RF, 2017. Predictors of naltrexone response in a randomized trial: reward-related brain activation, OPRM1 genotype, and smoking status. Neuropsychopharmacology 42 (13), 2640–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-back, Measuring Alcohol Consumption Springer, pp. 41–72. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, 1989. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br. J. Addict 84 (11), 1353–1357. [DOI] [PubMed] [Google Scholar]
- Thrul J, Gubner NR, Tice CL, Lisha NE, Ling PM, 2019. Young adults report increased pleasure from using e-cigarettes and smoking tobacco cigarettes when drinking alcohol. Addict. Behav 93, 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL Jr, Louis VA, Taylor RE, 2002. Effects of combined systemic alcohol and central nicotine administration into Ventral Tegmental Area on dopamine release in the nucleus accumbens. Alcohol. Clin. Exp. Res 26 (3), 394–399. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Servies, S.A.a.M.H.S.A., 2019. 2019 National Survey on Drug Use and Health (NSDUH) Table 2.1B—Tobacco Product and Alcohol Use in Lifetime, Past Year, and Past Month Among Persons Aged 12 or Older, by Age Group: Percentages, 2018 and 2019.
- Vatsalya V, Gowin JL, Schwandt ML, Momenan R, Coe MA, Cooke ME, Hommer DW, Bartlett S, Heilig M, Ramchandani VA, 2015. Effects of varenicline on neural correlates of alcohol salience in heavy drinkers. Int. J. Neuropsychopharmacol 18 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, McKee SA, 2017. An overview of alcohol and tobacco/nicotine interactions in the human laboratory. Am. J. Drug Alcohol Abuse 43 (2), 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM, 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14 (6), 1370–1386. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM, 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21 (4), 1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley K, Jezzard P, Matthews P, Smith S, 2001. Functional MRI: an Introduction to Methods
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


