Abstract
Aim
Arterial involvement has been implicated in the coronavirus disease of 2019 (COVID-19). Fluorine 18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging is a valuable tool for the assessment of aortic inflammation and is a predictor of outcome. We sought to prospectively assess the presence of aortic inflammation and its time-dependent trend in patients with COVID-19.
Methods
Between November 2020 and May 2021, in this pilot, case-control study, we recruited 20 patients with severe or critical COVID-19 (mean age of 59 ± 12 years), while 10 age and sex-matched individuals served as the control group. Aortic inflammation was assessed by measuring 18F-FDG uptake in PET/CT performed 20-120 days post-admission. Global aortic target to background ratio (GLA-TBR) was calculated as the sum of TBRs of ascending and descending aorta, aortic arch, and abdominal aorta divided by 4. Index aortic segment TBR (IAS-TBR) was designated as the aortic segment with the highest TBR.
Results
There was no significant difference in aortic 18F-FDG PET/CT uptake between patients and controls (GLA-TBR: 1.46 [1.40-1.57] vs. 1.43 [1.32-1.70], respectively, P = 0.422 and IAS-TBR: 1.60 [1.50-1.67] vs. 1.50 [1.42-1.61], respectively, P = 0.155). There was a moderate correlation between aortic TBR values (both GLA and IAS) and time distance from admission to 18F-FDG PET-CT scan (Spearman’s rho = − 0.528, P = 0.017 and Spearman’s rho = − 0.480, p = 0.032, respectively). Patients who were scanned less than or equal to 60 days from admission (n = 11) had significantly higher GLA-TBR values compared to patients that were examined more than 60 days post-admission (GLA-TBR: 1.53 [1.42-1.60] vs. 1.40 [1.33-1.45], respectively, P = 0.016 and IAS-TBR: 1.64 [1.51-1.74] vs. 1.52 [1.46-1.60], respectively, P = 0.038). There was a significant difference in IAS- TBR between patients scanned ≤ 60 days and controls (1.64 [1.51-1.74] vs. 1.50 [1.41-1.61], P = 0.036).
Conclusion
This is the first study suggesting that aortic inflammation, as assessed by 18F-FDG PET/CT imaging, is increased in the early post COVID phase in patients with severe or critical COVID-19 and largely resolves over time. Our findings may have important implications for the understanding of the course of the disease and for improving our preventive and therapeutic strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12350-022-02962-1.
Keywords: 18F-FDG PET/CT, Aortic inflammation, COVID 19, Cardiovascular risk, Endothelial dysfunction, Long COVID, CRP
Introduction
The coronavirus disease of 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the result of disruption of the immune, renin-angiotensin-aldosterone (RAA), and thrombotic balance.1 All these mechanisms converge on vascular dysfunction as a common pathway. The main organs involved in COVID-19, i.e., the lungs, the heart, and the kidneys, exhibit similar findings of endothelial dysfunction and vasculitis with monoclonal cells, lymphocyte infiltration and intravascular thrombosis.2 Transient increase of arterial stiffness that is associated with hospital stay length3 attests to the vascular involvement in COVID-19. Nevertheless, the patients that end up with irreversible myocardial damage at long-term follow constitute a small percentage.1,4,5
18-Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging is a valuable tool for the diagnosis and assessment of disease severity in different types of vascular and cardiac inflammation and infection6–9 and is a potential predictor of outcomes in different settings.7,10,11 We have shown that aortic FDG uptake is associated with disease severity and response to treatment in patients with lymphoma and hypercholesterolemia.12,13
There are very few observational studies regarding 18F-FDG PET/CT in COVID-19 affected patients focusing on the lung FDG uptake.14,15 We investigated the effects of COVID-19 on a vascular level, by assessing 18F-FDG PET/CT uptake in the aorta in patients with severe or critical illness.
Methods
Between November 2020 and May 2021, we recruited 20 patients from two dedicated COVID 19 hospitalization centers (General Hospital ‘Evaggelismos’ and Thoracic Diseases General Hospital ‘Sotiria’ in Athens, Greece), who were diagnosed and admitted with severe or critical COVID 19. The criteria for severe COVID 19 were (1) SpO2 < 94% on room air at sea level, (2) and a respiratory rate > 30 breaths/min, (3) PaO2/FiO2 < 300 mmHg or lung infiltrates > 50%. The criteria for critical COVID 19 were the presence of respiratory failure, septic shock, and/or multiple organ dysfunction.16 Patient inclusion criteria were 1. Positive PCR test for COVID 19, 2. Fulfillment of criteria for severe or critical COVID 19 as described above, 3. age > 18 years old, 4. a given written signed consent form, 5. glucose levels prior to 18F-FDG PET/CT < 200 mg/dL, 6. to be holders of national insurance number. Exclusion criteria were 1. Age < 18 or > 80 years old, 2. Pregnancy or breastfeeding, 3. Inability to comply with follow up instructions 4. Enrollment in interventional studies. Patients underwent whole body 18F-FDG PET/CT imaging between 20 to 120 days after hospital admission. Ten age and sex-matched individuals scheduled for 18F-FDG PET/CT imaging served as the control group. They had a prior history of malignancy but were free of active disease at the time of the 18F-FDG-PET/CT investigation. Image acquisition was obtained following recommended protocols and as previously described.9,17 Patients were classified according to their cardiovascular risk as low, intermediate, high and very high risk for cardiovascular disease as suggested by the European Society Guidelines on prevention of cardiovascular disease.18 Patients enrolled in the study had capacity to give informed consent ether at the time of admission, or they were enrolled in the study at the time that they were able to give informed consent. No surrogates were recruited to provide consent for the participants. The study was approved by the Institutional Research Ethics Committee and conducted according to institutional guidelines and the Declaration of Helsinki.
The study was approved by the Institutional Research Ethics Committee and conducted according to institutional guidelines and the Declaration of Helsinki.
Study outcomes
The primary endpoints of the study were: (1) the difference of aortic inflammation as assessed by 18F-FDG PET/CT between patients with recent COVID 19 and control subjects and (2) the association of aortic FDG uptake with time after admission in order to investigate the behavior of aortic inflammation over time post COVID 19. The secondary outcome was to investigate the association between aortic FDG uptake and disease severity biomarkers. In a post hoc analysis, we compared aortic FDG uptake in patients in early (≤ 60 days from admission) or late (> 60 days from admission) recovery phase vs. aortic FDG uptake in control subjects.
18F-FDG PET/CT imaging protocol
Image acquisition was obtained following recommended protocols and as previously described. None of the patients had blood glucose levels > 200 mg dL−1 before injection. FDG was injected intravenously (3-4 MBq/kg) and scanning was performed at 60-120 minutes post-injection for aortic tracer uptake assessment. A low dose CT scan in a supine position was obtained for attenuation correction and image fusion. No CT intravenous (IV) contrast was administered. PET data were reconstructed using an ordered subset expectation maximization iterative reconstruction algorithm. Regions of interest (ROI) around the aortic wall were manually drawn along the entire aorta in consecutive axial slices at intervals of 5 mm. Metabolic activity within each arterial ROI was measured by maximum standardized uptake value (SUVmax). Six consecutive circular ROIs of 3 mm diameter, were drawn within the superior vena cava and an average venous SUVmean value was calculated. The arterial target to background ratio (TBR) was then derived by dividing the mean aortic SUVmax to the average value of venous SUVmean. Finally, global aortic TBR (GLA-TBR) was calculated as the sum of TBRs of ascending and descending aorta, aortic arch, and abdominal aorta divided by 4. Index aortic segment TBR (IAS-TBR) was designated as the aortic segment with the highest TBR.
Blood measurements
Blood samples from the 20 COVID-19 patients were obtained < 48 hours after admission. On each blood sample, high sensitivity troponin I (hs-Troponin I) and CRP (hs-CRP) were measured in the serum. hsCRP and hs-Troponin I were promptly available from the laboratory results of the 2 hospitals.
Statistical analysis
Quantitative data are presented as mean values ± standard deviation (SD) or medians (interquartile range), while qualitative variables as absolute and relative frequencies. Parameters which exhibited a non-normally distribution were presented as the median (25th-75th percentile).
For between-groups comparisons, the Student’s t-test or χ2 test for continuous and categorical variables, respectively, were employed. For comparison of TBR values, Mann Whitney U test was performed. To assess the relation between aortic TBR and inflammatory markers, time distance since admission D-dimers and oxygen saturation (SO2), Spearman’s rho was employed. Linear regression analysis was performed using aortic TBR as the dependent variable and time distance from admission, age, sex and systematic inflammation as described by hs-CRP as covariates.19 Further linear regression analysis was performed using aortic TBR as the dependent variable and time distance from admission, age, sex and CVD risk according to European Society of Cardiology (ESC) guidelines as covariates (Supplementary material).
A two-tailed P-value < 0.05 was considered significant. All statistical analyses were performed with the SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Mean age of patients was 59 ± 12 years and 10 were males (60%). Ten patients had hypertension (50%), six had dyslipidemia (30%) and 6 were diabetics (30%). There were 6 smokers (60%) in the patient group. Six patients were on statins, six on angiotensin receptor blockers (ARBs) and one on aspirin (Table 1).
Table 1.
Sociodemographic and clinical characteristics of patients (n = 20) and age and sex-matched control subjects (n = 10)
| Patients (n = 20) |
Controls (n = 10) |
P-value | |
|---|---|---|---|
| Age | 59.3 (± 12.2) | 55.3 (± 15.2) | 0.500 |
| Male | 60% (n = 12) | 60% (n = 6) | 1.0 |
| Obesity* | 10% (n = 2) | n/a | – |
| Medication during hospitalization | |||
| Steroids | 100% (n = 20) | – | – |
| Remdesivir | 80% (n = 16) | – | – |
| COVID 19 severity indicators | |||
| SO2 on admission (%) | 90 (± 5) | – | – |
| Respiratory rate on admission (bpm) | 27.12 (± 6.26) | – | – |
| Admission to ICU | 65% (n = 13) | – | – |
| Hospitalization days | 19.7 (± 11.6) | – | – |
| Biomarkers on admission | |||
| hs-CRP (mg/L) | 9.77 (3.78–14.25) | – | – |
| hs-Troponin I (ng/L) | 6.50 (4.17–9.01) | – | – |
| D-dimers (mg/L) | 1.085 (0.59–2.02) | – | – |
| Target to blood ratio (TBR)** | |||
| Global Aortic TBR | 1.46 (1.40–1.57) | 1.43 (1.28–1.64) | 0.422 |
| Index aortic segment TBR*** | 1.60 (1.50–1.67) | 1.50 (1.42–1.61) | 0.155 |
| Ascending aorta TBR | 1.50 (1.45–1.65) | 1.41 (1.33–1.61) | 0.150 |
| Aortic arch TBR | 1.48 (1.42–1.53) | 1.41 (1.34–1.54) | 0.218 |
| Descending thoracic aorta TBR | 1.41 (1.30–1.49) | 1.38 (1.23–1.48) | 0.566 |
| Abdominal aorta TBR | 1.37 (1.29–1.62) | 1.40 (1.32–1.51) | 0.897 |
Categorical variables are presented as absolute and relative frequencies, while continuous variables as mean value ± SD for normally distributed and median value (25th–75th percentile) for skewed variables. Χ2 test was employed for between group comparisons of categorical variables, t-test for normally distributed continuous variables and *BMI > 30 kg/m2
**Mann Whitney U test was performed for comparison of aortic TBR values.
***Index aortic segment was determined as the aortic segment with the higher TBR value
Bpm, breaths per minute; CVD, cardiovascular disease; FHx, family History; CAD, coronary artery disease; ICU, intensive care unit; hs-CRP, high sensitivity C reactive protein; SD, Standard deviation; SO2, Oxygen saturation
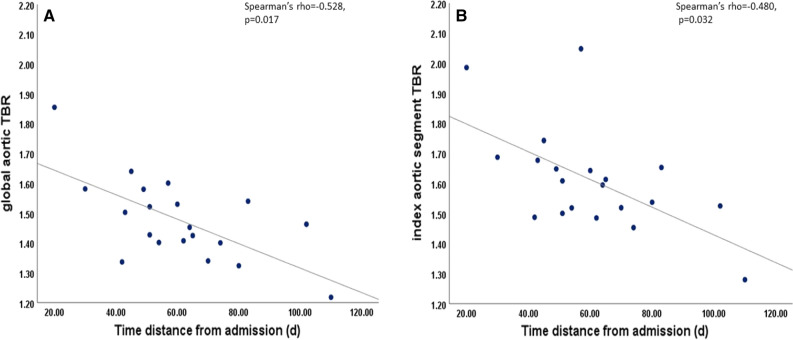
There was a moderate association between aortic TBR values (both GLA and IAS) and time distance from admission to 18F-FDG PET-CT scan (Spearman’s rho = − 0.528, P = 0.017 and Spearman’s rho = − 0.480, P = 0.032, respectively) (Figure 1). Multivariate analysis showed that both GLA and IAS-TBR remained moderately associated with time distance from admission to 18F-FDG PET/CT and with systemic inflammation assessed by hs-CRP, even after adjustment for sex and age (Tables 2 and 3). A multivariate analysis associating GLA and IAS–TBR with time distance from COVID 19 admission, using cardiovascular risk, age and sex as confounders is presented in the Supplement Tables 1 and 2, respectively.
Figure 1.
Association between aortic TBR values and time distance from admission to 18F-FDG PET/CT scan. A Moderate negative association of GLA-TBR values with time from admission for COVID 19 (Spearman’s rho − 0.528, P = 0.017). B Moderate negative association of IAS-TBR with time from admission for COVID 19 (Spearman’s rho = − 0.480, P = 0.032). d, days; TBR, target to background ratio; 18F-FDG PET/CT, Fluorine 18-fluorodoxyglucose positron emission/computed tomography
Table 2.
Multivariate analysis associating GLA-TBR with time distance of 18F-FDG PET/CT scan from admission, adjusted for sex, age, and systematic inflammation as indicated by hs-CRP
| Variables | b (95% CI) | SE b | β | P-value |
|---|---|---|---|---|
| (Dependent variable = GLA-TBR), Adjusted R2 of model = 0.741 | ||||
| Age |
0.002 (− 0.002 to 0.007) |
0.002 | 0.142 | 0.301 |
| Sex (male vs. female) |
0.070 (− 0.011 to 0.151) |
0.037 | 0.251 | 0.085 |
| hs-CRP |
0.009 (0.004 to 0.015) |
0.002 | 0.493 | 0.002 |
| Days from admission to 18F-FDG PET/CT |
− 0.004 (− 0.005 to − 0.002) |
0.001 | − 0.576 | 0.001 |
Aortic TBR remained moderately associated with time distance from admission of COVID 19 to 18F-FDG PET/CT and with systemic inflammation assessed by hs-CRP, even after adjustment for sex and age
hs-CRP, high sensitivity C reactive protein; TBR target to background ratio; 18F-FDG PET/CT Fluorine 18-fluorodoxyglucose positron emission/computed tomography
Table 3.
Multivariate analysis investigating association of IAS-TBR with time distance of 18F-FDG PET/CT scan from admission, adjusted for sex, age, and systematic inflammation as indicated by hs-CRP
| Variables | b (95% CI) | SE b | β | P-value |
|---|---|---|---|---|
| (Dependent variable = IAS-TBR), Adjusted R2 of model = 0.554 | ||||
| Age |
− 0.004 (− 0.007 to 0.001) |
0.003 | − 0.235 | 0.197 |
| Sex (male vs. female) |
0.068 (− 0.070 to 0.205) |
0.063 | 0.189 | 0.303 |
| hs-CRP |
0.011 (0.002 to 0.020) |
0.004 | 0.452 | 0.021 |
| Days from admission to 18F-FDG PET/CT |
− 0.004 (− 0.007 to − 0.001) |
0.001 | − 0.494 | 0.014 |
IAS-TBR remained moderately associated with time distance from admission of COVID 19 to 18F-FDG PET/CT and with systemic inflammation assessed by hs-CRP, even after adjustment for sex and age
hs-CRP, high sensitivity C reactive protein; TBR, target to background ratio; 18F-FDG PET/CT, Fluorine 18-fluorodoxyglucose positron emission/computed tomography
There was no significant difference in aortic 18F-FDG PET/CT uptake between patients and controls (GLA-TBRpatients = 1.46 [1.40-1.57] vs. GLA-TBRcontrol = 1.43 [1.32-1.70], respectively, P = 0.422). Similarly, there was no difference in IAS- TBR (IAS-TBRpatients = 1.60 [1.50-1.67] vs. IAS-TBRcontrols = 1.50 [1.42-1.61], P = 0.155).
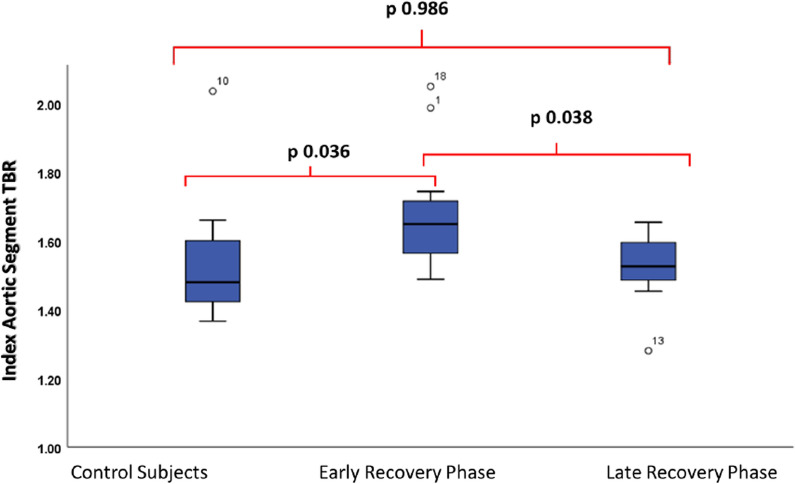
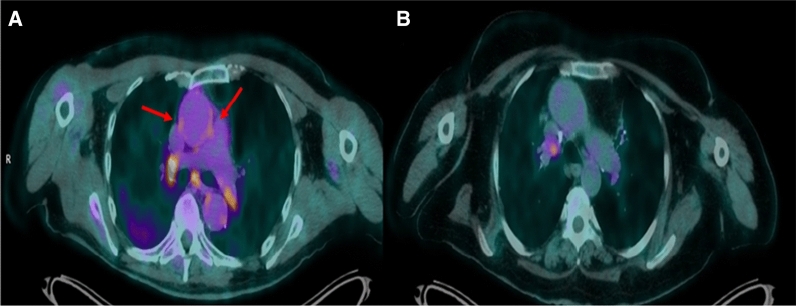
The median (interquartile range) time from admission to the 18F-FDG PET/CT was 58.5 (45-74) days. Patients who were scanned less than or equal to 60 days from admission (n = 11) had significantly higher GLA-TBR values compared to patients that were examined more than 60 days post-admission (GLA-TBR≤60 days = 1.53 [1.42-1.60] vs. GLA-TBR>60 days = 1.40 [1.33-1.45], P = 0.016. Similarly, using IAS for comparison of patients scanned less than or equal to 60 days with patients scanned more than 60 days revealed significantly higher values in the first group (IAS -TBR≤60 days = 1.64 [1.51-1.74], IAS-TBR>60 days = 1.52 [1.46-1.60], P = 0.038). These 11 patients scanned ≤ 60 days from admission, were also compared to controls and while there was not a statistically significant difference, there was a trend towards higher values of the patients scanned ≤ 60 days from admission compared with controls (GLA-TBR≤60 days = 1.55 [1.47-1.61] vs. GLA-TBRcontrol = 1.43 [1.32-1.70], P = 0.085). However, when the same analysis was performed using IAS -TBR there was a significant difference between the two groups (IAS-TBR≤60 days = 1.64 [1.51-1.74], IAS-TBRcontrol = 1.50 [1.41-1.61], P = 0.036) (Figure 2). Figure 3 demonstrates a patient assessed 20 days post-admission compared to a patient assessed 64 days after admission for COVID 19.
Figure 2.
Boxplot demonstrating IAS-TBR in control subjects, patients scanned ≤ 60 days and > 60 days from admission for COVID 19. Significant difference in IAS-TBR between control subjects and patients scanned ≤ 60 days (Early Recovery Phase) from admission for COVID 19. On the contrary patients scanned > 60 days (Late Recovery Phase) did not demonstrate significantly different values compared to controls
Figure 3.
Transaxial views of fused 18F-FDG PET/CT images of 2 patients post severe COVID 19. A Patient scanned twenty days post-admission of severe COVID 19. B Patient scanned sixty-four days after admission. In the former, there is increased 18F-FDG uptake in the wall of the ascending aorta (arrows) and there are also hypermetabolic hilar and mediastinal lymph nodes. 18F-FDG PET/CT: Fluorine 18-fluorodoxyglucose positron emission/computed tomography
Out of all patients, 65% (n = 13) had critical COVID 19 and required ICU admission. There was no significant difference in aortic TBR between patients admitted to ICU and patients with severe COVID disease not requiring ICU admission (global TBRICUpatients = 1.45 [1.40-1.54] vs global TBRnonICUpatients = 1.52 [1.34-1.58], P = 0.757). Similarly, there was no significant difference in index vessel TBR in patients admitted to ICU compared to patients that were not admitted to ICU (index Vessel TBRICUpatients = 1.59 [1.49-1.67] vs index vessel TBRnonICUpatients = 1.61 [1.52-1.69], P = 0.485).
Regarding localization of the index aortic segment in patients, higher FDG uptake values were observed more frequently in thoracic aorta (n = 14, 70%) compared to abdominal aorta (n = 6, 30%).
Regarding circulating biomarkers, there was a modest association between both global aortic TBR and index vessel TBR and CRP (Spearman’s rho = 0.662, P = 0.004 and Spearman’s rho = 0.559, P = 0.020, respectively). There was no association between neither global nor index vessel aortic FDG uptake and troponin or d-dimer levels (Table 4).
Table 4.
Associations of GLA-TBR/ISA-TBR and biomarkers of COVID-19 severity
| GLA-TBR | ISA-TBR | |||
|---|---|---|---|---|
| Spearman’s rho | P-value | Spearman’s rho | P-value | |
| hs-CRP | 0.662 | 0.004 | 0.559 | 0.020 |
| hs-Troponin | 0.044 | 0.871 | − 0.92 | 0.724 |
| D-dimers | − 0.182 | 0.499 | 0.029 | 0.914 |
| Admission SO2 | 0.095 | 0.708 | 0.289 | 0.244 |
hs-CRP, high sensitivity C reactive protein; TBR, target to blood ratio; GLA, global aortic; ISA, index segment aortic
Discussion
To the best of our knowledge, this is the first study that investigates prospectively the presence of aortic inflammation during early recovery in COVID-19 in severely or critically ill patients, as assessed by aortic 18F-FDG PET/CT uptake. More specifically, our findings suggest that patients scanned earlier than 60 days post COVID-19 admission, demonstrate higher aortic inflammation compared to either patients scanned later or to control subjects. The time pattern of this aortic involvement shows a largely transient involvement. Furthermore, aortic TBR values are associated with higher CRP levels during admission.
Previous knowledge
There is limited information on aortic FDG uptake in patients post COVID 19.11,12 Solini et al12 showed in 10 patients that although patients and control subjects had similar vascular scores, as assessed by a semi-quantitative method, there were higher aortic TBR scores in specific aortic regions in patients (thoracic aorta, right iliac artery, femoral arteries). A retrospective analysis of 18F-FDG PET/CT scans of 47 patients with long COVID-19 symptoms (persistent symptoms 4 weeks post infection), revealed that 10 patients had increased standard uptake values (SUVmax) mainly in the thoracic aorta.20 This finding is in agreement with our hypothesis that systemic inflammation is associated with aortic inflammation and that the presence of the later could be used to identify patients with long COVID-19 syndrome and late complications after infection. Regarding the time course, our results are in keeping with indirect evidence from FDG PET/CT imaging study of Minamimoto et al21 who showed an inflammatory response in mediastinal lymph nodes of patients post COVID-19, which decreased during 4 weeks of observation.
Clinical implications
Our results may have important clinical implications. 18F-FDG PET/CT is a valuable tool for assessment of inflammation in the aorta in conditions where inflammation is present, such as malignancies and hyperlipidaemia7,10,13 and can be used as an additional parameter for the evaluation of arterial atherosclerotic activity,22 as well as large vessel vasculitis.23 Furthermore, it can be used in the monitoring of disease since relapse was more common in patients with vasculitis when there was high aortic 18F-FDG uptake during clinical remission.24 Vascular involvement in patients with COVID-19 is common and even vaccination against SARS-CoV-2 has been associated with transient endothelial dysfunction.25 Accordingly, the findings of this study could expand to identify patients at risk both the early and late post COVID-19 period. The cytokine storm induced by SARS-CoV-2 results in lung injury and multi-organ failure.26 COVID-19-induced vasculitis can cause thrombosis (arterial or venous), as well as coronary or aortic dissection.1 Histopathological post-mortem findings in COVID-19 patients have shown increased inflammatory burden in patients with a severe clinical presentation and signs of endotheliitis in various affected organs.2,27 Importantly, a considerable percentage of patients develop long-standing COVID-19 symptoms often in the absence of elevated traditional inflammatory markers.1,5,6 Interestingly, this condition is mainly associated to endothelial dysfunction28 and some of these patients exhibit residual aortic inflammation,20 Accordingly, assessment of aortic inflammation with 18F-FDG PET/CT could predict, or serve as a marker of, longstanding COVID-19 mandating closer follow up and offering access to potentially available treatments.
Limitations
Our study is limited by its observational nature. No individual patient serial measurements with subsequent 18F-FDG PET/CT scans were taken and this hypothesis-generating study cannot prove causality. A further limitation is the relatively small number of patients. Practical shortcomings in the COVID-19 setting due to strict staff, and patient safety protocols, necessary to prevent spreading of disease, as well as enrolment of COVID-19 patients to numerous other interventional research protocols, led to a very cumbersome and challenging recruitment process that could explain the relatively small number of participants. Nevertheless, our study attests to the potential of this diagnostic modality to become clinically useful, when practical issues are addressed.
Our findings are provided by cross-sectional and not individual prospective data. While they overall suggest a time-related recovery of inflammation, factors of resolution and the exact time pattern still remain unknown. Further research is needed to disentangle the process of recovery of inflammation (aortic and systemic) in patients with COVID-19.
Conclusions
In conclusion, this is the first study suggesting by means of 18F-FDG PET/CT imaging that patients may demonstrate high aortic inflammation early post COVID-19; however, this aortic involvement is largely transient. 18F-FDG PET/CT imaging could recognise early patients that have residual aortic inflammation and may serve as a predictor of outcome both in the early and late post infection period. Larger prospective studies are needed to support this. In addition, our results could instigate investigation of even earlier phases of the disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Disclosures
There is no conflict of interest. There was no funding for this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com
All editorial decisions for this article, including selection of reviewers and the final decision, were made by guest editor Jeroen J Bax, MD.
Funding
There was no funding provided for this study.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Charalambos Vlachopoulos, Email: cvlachop@otenet.gr.
Constantinos Anagnostopoulos, Email: cdanagnostopoulos@bioacademy.gr.
References
- 1.Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeed S, Mancia G. Arterial stiffness and COVID-19: A bidirectional cause-effect relationship. J Clin Hypertens. 2021;23:1099. doi: 10.1111/jch.14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter MA, Melzer RA, Schindler C, et al. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32:674–681. doi: 10.1007/s00259-004-1757-9. [DOI] [PubMed] [Google Scholar]
- 7.Kung BT, Seraj SM, Zadeh MZ, et al. An update on the role of 18F-FDG-PET/CT in major infectious and inflammatory diseases. Am J Nucl Med Mol Imaging. 2019;9:255–273. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Song HC. Role of PET/CT in the evaluation of aortic disease. Chonnam Med J. 2018;54:143–152. doi: 10.4068/cmj.2018.54.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawal I, Sathekge M. F-18 FDG PET/CT imaging of cardiac and vascular inflammation and infection. Br Med Bull. 2016;120:55–74. doi: 10.1093/bmb/ldw035. [DOI] [PubMed] [Google Scholar]
- 10.Figureueroa AL, Abdelbaky A, Truong QA, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Higashigawa T, Ichikawa Y, Chino S, et al. 18F-FDG uptake as a predictive factor for progressive aortic enlargement in aortic dissection. Ann Nucl Med. 2020;34:636–642. doi: 10.1007/s12149-020-01487-2. [DOI] [PubMed] [Google Scholar]
- 12.Vlachopoulos C, Koutagiar I, et al. Lymphoma severity and type are associated with aortic FDG uptake by 18F-FDG PET/CT imaging original research. J Am Coll Cardiol. 2020;2:758–770. doi: 10.1016/j.jaccao.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlachopoulos C, Koutagiar I, Skoumas I, et al. Long-term administration of proprotein convertase subtilisin/kexin type 9 inhibitors reduces arterial FDG uptake. J Am Coll Cardiol Img. 2019;12:2573–3257. doi: 10.1016/j.jcmg.2019.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Charters PFP, Little D, Rodrigues JCL, et al. 18FDG-PET/CT findings in COVID-19: a single centre retrospective radiological review. BJR Case Rep. 2020;6(3):20200091. doi: 10.1259/bjrcr.20200091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sollini M, Ciccarelli M, Cecconi M, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [18F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging. 2021;48:1460–1466. doi: 10.1007/s00259-020-05084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed 10 June 21 [PubMed]
- 17.Bucerius J, Hyafil F, Verberne H, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43(4):780–792. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piepoli MF, Hoes AW, Agewall S, et al. ESC Scientific Document Group, 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akoglu H. User's guide to correlation coefficients. Turk J Emerg Med. 2018;18:91–93. doi: 10.1016/j.tjem.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudouet P, Cammilleri S, Guedj E, et al. Aortic 18F-FDG PET/CT hypermetabolism in patients with long COVID: A retrospective study. Clin Microbiol Infect. 2021;27:1873–1875. doi: 10.1016/j.cmi.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minamimoto R, Hotta M, Ishikane M, Inagaki T. FDG-PET/CT images of COVID-19: A comprehensive review. Glob Health Med. 2020;2:221–226. doi: 10.35772/ghm.2020.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elfattah Hassan Gadalla AA, Elsayed ND. The role of 18FDG PET/CT imaging of aortic atherosclerosis: Prospective study and technique optimization. Egypt J Radiol Nucl Med. 2020;51:13. doi: 10.1186/s43055-020-0137-1. [DOI] [Google Scholar]
- 23.Soussan M, Nicolas P, Schramm C, Katsahian S, et al. Management of large-vessel vasculitis with FDG-PET: A systematic literature review and meta-analysis. Medicine (Baltimore) 2015;94:e622. doi: 10.1097/MD.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grayson PC, Alehashemi S, Bagheri AA, et al. 18 F-fluorodeoxyglucose-positron emission tomography as an imaging biomarker in a prospective, longitudinal cohort of patients with large vessel vasculitis. Arthritis Rheumatol. 2018;70(3):439–449. doi: 10.1002/art.40379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terentes-Printzios D, Gardikioti V, Solomou E, et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hyper Res. 2022 doi: 10.1038/s41440-022-00876-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song P, Li W, Xie J, et al. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charfeddine S, Amor IH, et al. Long COVID 19 syndrome: Is it related to microcirculation and endothelial dysfunction, insights from TUN-EndCOV study. Front Cardiovasc Med. 2021 doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.