Abstract
Background
Although there are many hypotheses, the pathogenesis of Crohn's disease (CD) is not completely clear so far. Exclusive enteral nutrition (EEN) is a routine measure in the treatment of active CD. We aimed at investigating the impact of EEN on patients with active CD from microbial metabolomics.
Methods
16S-rDNA sequencing technology and gas chromatography–mass spectrometer analysis were employed to investigate the modification of the intestinal flora and fecal short-chain fatty acid (SCFA) during the EEN.
Results
Seven patients with CD, who conducted EEN, were followed up successfully in the present study. The 8-week EEN resulted in a remission of the condition of subjects with active CD, as revealed by a significant decrease in erythrocyte sedimentation rate (ESR) (P = 0.018), C-reactive protein (CRP) (P = 0.028), and Crohn’s disease activity index (CDAI) (P = 0.018). The nutrition of the subjects was improved after an 8-week treatment course with EEN, which was associated with an increase in body mess index (BMI) (P = 0.018) and serum albumin (ALB) (P = 0.018) levels. Furthermore, our investigations revealed a significantly increased abundance of Firmicutes paralleled by decreased levels of Proteobacteria. With respect to the genus, five species of bacteria including Ruminococcus (P = 0.01), Lachnospiraceae (P = 0.02), Anaerotruncus (P = 0.04), Flavonifractor (P = 0.04), and Novosphingobium (P = 0.05) showed significantly increased abundance. This was accompanied by relative changes in fecal short-chain fatty acids levels. Moreover, we successfully constructed a stable model by combining these five significantly different genera to predict the therapeutic effect of EEN on patients with CD (AUC = 0.9598).
Conclusions
The findings indicated that EEN can alleviate the condition and the nutrition of patients with active CD by regulating the intestinal flora and influencing the expression level of fecal short-chain fatty acids.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-022-02293-y.
Keywords: Crohn's disease, Exclusive enteral nutrition, 16S-rDNA, Intestinal flora, Short-chain fatty acid
Introduction
Crohn's disease (CD) is an inflammatory bowel disease (IBD), characterized by recurrent illness. The incidence and prevalence of CD have risen during the last decades, but the specific etiology and pathogenesis are still unclear [1]. The clinical manifestations of CD often occur as the alternation of disease remission and relapse [2]. The current clinical treatment of CD is mainly based on drug-based induction therapy such as glucocorticoids, immunosuppressant drugs, and biological agents.
Existing studies have confirmed that the application of exclusive enteral nutrition (EEN) had a significant effect on the healing of intestinal mucous membrane and recovery of intestinal function in patients with CD [3]. Early implementation of EEN has a higher therapeutic value for the long-term prognosis of CD [4]. According to domestic and foreign guidelines and consensus [5, 6], EEN is one of the preferences for patients with CD, severe cases included, and is the first-line induction therapy for children and adolescents. Based on available single-center cohort studies, some patients with CD can achieve a remission rate of up to 80% by applying EEN [6–9]. The therapeutic effect of EEN on patients with CD is not only inducing the remission of inflammation but also offering nutritional support [10, 11]. And EEN is also a relatively economic treatment. Plenty of patients cannot tolerate corticosteroid and immunosuppressive therapy [12]. Moreover, some cannot afford expensive biologics. Under such circumstances, EEN may even be the only effective treatment option.
Intestinal flora plays an important role in mucosal immune regulation. Intestinal symbiotic bacteria can inhibit the growth of pathogenic bacteria and maintain intestinal homeostasis [13]. Short-chain fatty acid (SCFA) represents metabolites produced by the intestinal flora and might occupy an important position in the occurrence and development of CD [14–16]. According to Ceylan Tanes et al. [17], EEN or other special dietary might change the structure of specific metabolites by affecting the microbiome of healthy volunteers, thus affecting the inflammatory process. Whether this phenomenon is the same in patients with CD needs further testing. The present study aimed to investigate the changes of the intestinal flora and fecal SCFAs level after the EEN in patients with CD.
Methods
Study subjects
The patients who were diagnosed with Crohn's disease in the Department of Gastroenterology, Zhongda Hospital Southeast University from October 2018 to December 2019 were included in the study. The selection of subjects was based on the Crohn's disease diagnostic criteria and Crohn's disease activity index (CDAI) in the ‘2018 Consensus Opinions on the Diagnosis and Treatment of Inflammatory Bowel Disease' released by the Inflammatory Bowel Disease Group of the Chinese Medical Association Gastroenterology Branch [5]. The Montreal classification was applied to type the subjects [18].
The inclusion criteria of our subjects were as follows: (1) patients with active Crohn's disease who had already been treated with infliximab (Janssen-Cilag AG, USA) for more than 12 weeks but responded poorly; (2) age ≤ 75 years old; (3) no administration of antibiotics or probiotics, for at least 1 month before entering the study; No additional treatment regimens (such as glucocorticoids, biological agents or surgery) were newly employed during the period of EEN; CDAI score ≥ 150 points, i.e. in the active phase; All participants approved the informed consent before conducting experiments. Infection before or during the study as well as incapability of taking oral or intestinal food and suffering from dairy allergy represented the exclusion criteria.
The enteral nutrition used was Enteral Nutritional Powder (TP) (ENSURE, Abbott Laboratories B.V., USA). This represents a compound preparation that contains protein, fat, carbohydrates, vitamins and minerals. 55.8 g of powder can be mixed with 200 ml of water to prepare a 250 ml dose per serving. It provides 1.06 kcal energy per milliliter, with a calorie distribution of 54% carbohydrate, 31.8% fat, 14.2% protein, and a variety of vitamins and minerals. This compound can be delivered either orally or tube-fed. All participants lived in Nanjing, Jiangsu Province, or in Nanjing's surrounding counties and cities for a long time with comparable lifestyles, climate environments and dietary habits. Our study was approved by the Clinical Research Ethics Committee of Zhongda Hospital Southeast University (Approval Number: 2020ZDSYLL129-P01).
Sample collection
Blood samples were collected before and after 8-week EEN to determine the inflammation index, namely erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). The fresh stool samples were taken and loaded into a sterile dry stool kit at baseline time and after 8 weeks of EEN. The approximate weight of the stool specimen was about 3.0 g. Upon collection and labeling, samples were quickly frozen in liquid nitrogen and stored in the refrigerator at minus 80 degrees centigrade.
Sample preparation and 16S-rDNA sequencing
The E.Z.N.A.®Stool DNA Kit for extraction of fecal bacterial DNA was used according to the manufacturer's instructions. During the whole process of DNA extraction, ultra-pure water was used to avoid false positive PCR results. The PCR products were purified by the AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA) and quantified by Qubit (Invitrogen, USA). Before sequencing, the size and quantity of the amplicon pool were evaluated on the Agilent 2100 Bioanalyzer (Agilent, USA) and Illumina (Kapa Biosciences, Woburn, MA, USA) library quantification kits. Finally, we sequenced the libraries on the NovaSeq PE250 platform.
Analysis of fecal short-chain fatty acids
Twenty milligrams of each fecal sample were drawn out and mixed with isobutanol solution in a volume ratio of 1:9 before grinding and centrifugation. Sodium hydroxide and chloroform were added to the supernatant before another centrifugation round. This supernatant was retrieved and subjected to derivatized with chloroformate. Finally, the ultimate supernatant was extracted with normal hexane to detect the level of SCFAs by GC–MS (Agilent, USA).
Statistical analysis
The sample sequencing in this study was performed on the Illumina NovaSeq platform provided by Lc-bio. All data calculations were performed using QIIME2. Data analysis and preparation of charts were performed using the R software package (v3.5.2) and online cloud platform (Lc-bio) get on. To accurately compare the similarities and differences in the composition of intestinal flora before and after treatment, we involved Multidimensional scaling analysis (NMDS analysis), Principal coordinates analysis (PCoA analysis) and Analysis of similarities (Anosim analysis) based on the OTUs of the sample. NMDS analysis under Weighted-UniFrac distance on the OTU levels of all fecal samples was conducted. The data collection and analysis of SCFAs involved in this study were performed using Mass Hunter software (Version B.08.00, Agilent, USA).
Results
Study subjects
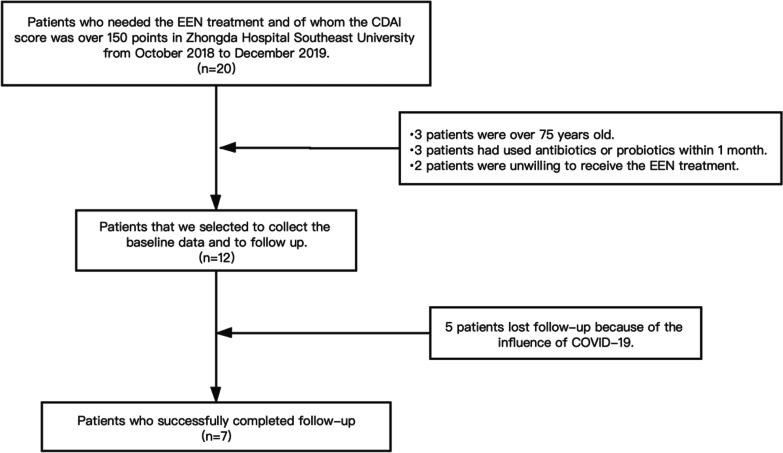
Twenty patients with active CD (CDAI > 150) who were going to be conducted EEN were initially entered into the study. Eight patients were excluded because of the eligibility criteria: three of them were > 75 years old; three had used antibiotics or probiotics within 1 month and two patients were unwilling to receive the EEN for a long time. The remaining 12 patients were subjected to a collection of baseline data and follow-up. Due to the COVID-19 pandemic, five patients were missed in the follow-up analysis. As a result, seven subjects successfully completed the follow-up analysis (Fig. 1).
Fig. 1.
Flow chart depicting the procedure of subjects' registration is presented
Seven patients with active CD (CDAI > 150) were selected for our study (Fig. 1). We applied EEN for 8 weeks to the 7 subjects, using ENSURE as the enteral nutrition. The cohort was included 5 males (72%) and 2 females (28%) with a median age of 25.0 years. According to the location of CD, 43% of the subjects featured ileal disease (L1), 14% displayed colonic disease (L2), while 43% showed ileocolonic Crohn's disease (L3) (Table 1). The median ESR before EEN (Group Prior) was 23 mm/h, while the median hypersensitive CRP was 7.67 mg/L. After 8 weeks of EEN (Group Post) revealed a decreased median ESR (4 mm/h) and CRP (0.86 mg/L). Wilcoxon rank-sum test was used to compare the ESR and CRP in the two groups. The result suggested that the level of ESR and CRP significantly decreased after the 8-week EEN (P = 0.018 and P = 0.028 respectively). At the same time, a significant decrease in CDAI after EEN (P = 0.018) was observed, suggesting that EEN was effective in treating patients with active CD in this study. In addition, various baseline parameters of the subjects related to our study, including weight, height, body mess index (BMI), and serum albumin (ALB) were documented (Table 1). Our investigations revealed increased BMI and ALB after the EEN, suggesting that the nutritional status of subjects was obviously improved (P = 0.018).
Table 1.
Clinical Characteristics of 7 patients with CD (Prior EEN/Post EEN)
| ID | Sex | Age | Montreal | Previous relevant medications | BMI (Pr*/Po*) | CDAI (Pr/Po) | ESR (mm/h) (Pr/Po) | CRP (mg/L) (Pr/Po) | ALB (g/L) (Pr/Po) |
|---|---|---|---|---|---|---|---|---|---|
| 01 | M | 16 | A1L3B1p | 5-ASA/IFX | 12.80/14.87 | 329.9/128.1 | 74/11 | 79.6/0.86 | 29.8/42.1 |
| 02 | F | 25 | A2L2B1 | Pred/5-ASA/IFX | 17.19/17.97 | 250.87/107.89 | 35/2 | 7.67/0.86 | 33.1/44.6 |
| 03 | F | 31 | A2L3B3p | 5-ASA/IFX | 16.41/18.75 | 257.44/118.24 | 23/18 | 7.38/0.86 | 35.3/38.3 |
| 04 | M | 23 | A2L1B2 | AZA/IFX | 16.98/17.63 | 249.37/129.2 | 3/2 | 1.2/1.58 | 48.8/50.5 |
| 05 | M | 15 | A1L1B1p | IFX | 14.80/17.36 | 293.71/144.47 | 8/4 | 32.47/2.01 | 32.1/39.6 |
| 06 | M | 51 | A3L3B3 | 5-ASA/IFX | 20.43/20.70 | 385.8/108.47 | 5/4 | 16.4/0.78 | 24.7/32.8 |
| 07 | M | 34 | A2L1B1 | AZA/IFX | 21.51/21.97 | 212.26/86.42 | 32.95/4 | 5.85/0.78 | 38.5/40.7 |
CD Crohn’s disease, M male, F female, Pr Prior, Po Post, BMI body mess index, 5-ASA 5-aminosalicylic acid, IFX infliximab, Pred Prednisone, AZA Azathioprine, CDAI Crohn's disease activity index, ESR erythrocyte sedimentation rate, CRP C-reactive protein, ALB serum albumin
*Pr represents the baseline collection time point and Po represents 8 weeks of EEN
Effective sequencing sequence statistics
Double-ended splicing, quality controlling, and mosaic filtering of the original off-machine data, were followed by high-quality data statistics. The effective sequencing sequence statistics are presented in Table S1 (Additional file 1).
The statistical analysis of the abundance indicates that the two groups have 230 identical bacteria in their respective stool sample flora as presented in the Venn diagram (Fig. 2). However, the two groups have their own relatively unique bacteria as well. Group Post has a higher number of unique bacteria than Group Prior (390 vs. 292). This indicates that the fecal flora species of patients with CD increased after the 8-week of EEN.
Fig. 2.
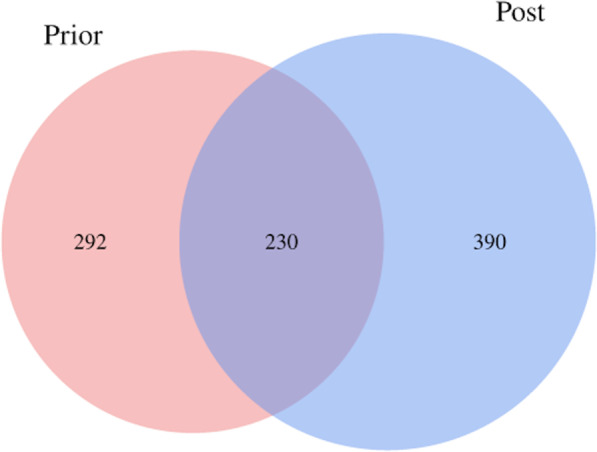
Venn diagram of the operational taxonomic units (OTUs) in group prior (red), in group post (green), and the overlap OTUs between the two groups. The number of OTUs of the samples before the EEN is 292, and that of the samples after 8-week EEN is 390. The number of the overlap OTUs is 230
Diversity analysis of the intestinal flora after the EEN
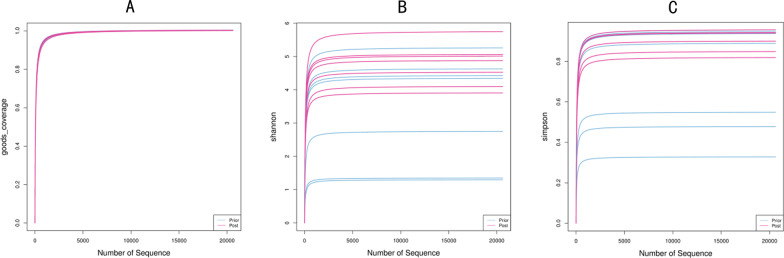
The Goods-coverage curve reflects that sample feature coverage is infinitely close to 100%. This suggests that the sequencing results may represent the true condition of these 14 samples (Fig. 3a). Upon applying Shannon and Simpson indexes to evaluate the alpha diversity of the intestinal flora of the two groups, we observed that the diversity of the Group Post is higher than that of Group Prior at the same sequencing depth. This indicates an improvement in the abundance and diversity of the intestinal flora of patients with CD after EEN (Fig. 3b, c). The alpha diversity dilution curve of this study gradually stabilized as the amount of sequencing data increased, indicating that the amount of sample sequencing has been saturated. The analysis of alpha diversity revealed that although both groups have high flora abundance and diversity, Group Post shows even higher levels. Our investigations indicate a certain degree of clustering of OTUs between the samples (Fig. 4) with obvious separation of the intestinal flora of Group Prior and Group Post. This means that the two groups of flora have low similarity which suggests that the composition of intestinal flora, (i.e. the intestinal microecology of patients with CD after EEN), has changed to a certain extent. Furthermore, the value of Stress (i.e. Stress = 0.1), namely the pressure coefficient, indicates the high reliability of the NMDS analysis results (Fig. 4a).
Fig. 3.
a Rarefaction curve of the alpha diversity in each sample is presented. b, c Alpha diversity analyses, including Shannon index and Simpson index, are shown. Shannon index represents the species diversity of the sample. Simpson index reflects the species richness and evenness of the sample
Fig. 4.
a NMDS analysis under weighted-Unifrac distance on the OTUs of Group Prior (blue) and Group Post (red) are presented. b PCoA under weighted-Unifrac distance on the OTUs of Group Prior (blue) and Group Post (red) are shown
These findings were substantiated by PCoA analysis of the samples under Weighted-UniFrac distance. We found that both PCoA1 (43.43%) and PCoA2 (22.92%), account for 66.35% of the sample difference. Figure 4b shows a certain distance between the two groups in the first and the second principal coordinates. This infers a potential difference between the microbial composition of Group Prior and Group Post, despite no significant values (P = 0.08) (Fig. 4b). Anosim analysis compares the difference between the groups with the difference within the groups. The calculated R value of 0.017104, indicates that the difference between the groups is greater than the difference within the same group. This suggests that the grouping of our study is significant.
Moreover, the beta analysis of the samples revealed that the EEN renders an influence on the composition of the intestinal flora of the active CD patients.
Analysis of the composition and abundance of bacterial species
In terms of phylum level, the intestinal microbiota of the patients with CD before EEN comprises mainly of Proteobacteria, Firmicutes, Bacteroides, Actinobacteria and Acidobacteria. At the same time, the intestinal microbiota of the fecal samples of Group Post is mainly composed of Proteobacteria, Firmicutes, Bacteroides, Actinobacteria and Verrucomicrobia. Besides, in terms of genus level, Group Prior is dominated by Escherichia-Shigella, Aeromonas, and Proteus. These account for 17.85%, 13.5%, and 11.92% respectively of the total flora. Additionally, the Group Post is dominated by Escherichia-Shigella, Ruminococcus, and Bacteroides, respectively accounting for 13.17%, 9.24% and 7.19% of the entire flora composition (Fig. 5).
Fig. 5.

a, b Pie charts of the abundance ratio of the bacteria in terms of phylum level in Group Prior and Group Post are depicted. c, d Cluster analysis of the relative abundances in Group Prior and Group Post with the Bray–Curtis distance are shown. e, f Bar charts of the relative abundances among all samples with the Bray–Curtis distance are presented. g, h The relationship between the 14 samples and the abundance of the top 30 phyla and genera is presented by the two heatmaps in which the Z value was used to normalize the expression abundance of the same bacteria. The gradient from blue to red reflects the change in abundance from low to high. i, j Circos charts show the correspondence between samples and species from phylum and genus level, reflecting the composition ratio of dominant species in Group Prior and Group Post, and reflecting the distribution ratio of each dominant species among the two groups are depicted
Analysis of the species differences between group prior and group post
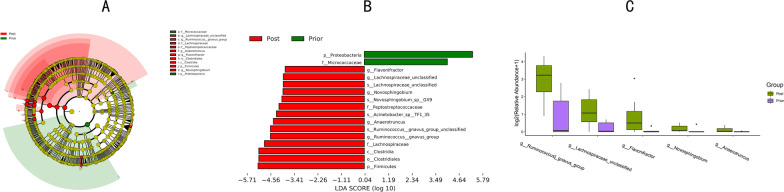
By employing LDA Effect Size analysis (LEfSe difference analysis) of the subjects' samples, we identified and compared the species with significant differences between Group Prior and Group Post. Figure 6a and b indicate that at the phylum level, Proteus has a higher abundance in Group Prior, while Firmicutes has a higher abundance in Group Post. In other words, the 8-week EEN was associated with an increased proportion of Firmicutes and decreased proportion of Proteus in the intestinal flora. In addition, the EEN was corroborated with a higher abundance of Lachnospiraceae, Ruminococcus, Anaerotruncus, Flavonifractor, and Novosphingobium (significant differences between Group Prior and Group Post, with P values of 0.01, 0.02, 0.04, 0.04 and 0.05 respectively). For a better view, we displayed the differences in the fecal flora as a bar plot. (Fig. 6c).
Fig. 6.
a, b LEfSe analysis reveals significant differences in abundance between the two groups. The green node indicates higher species abundancy in group A; the red indicates higher species abundancy in group B. Yellow node reveals no significant difference between the two groups. c The barplot analysis shows the five genera with significant differences between Group Prior and Group Post
Prediction model based on significantly different intestinal flora
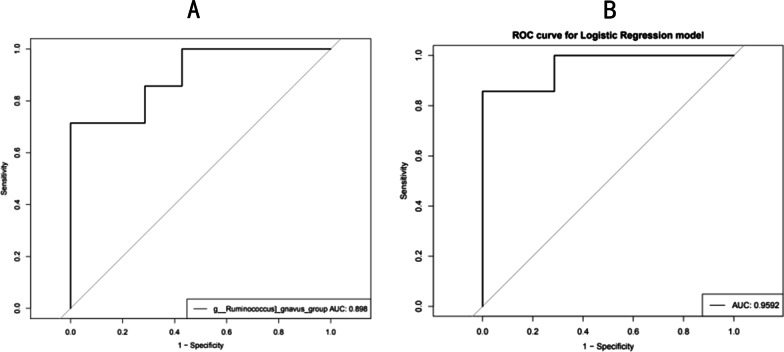
Based on the LDA value of the LEfSe analysis, we selected Lachnospiraceae, Ruminococcus, Anaerotruncus, Flavonifractor, and Novosphingobium as the candidate bacterial markers, and then performed ROC analysis to explore the predictive ability of the intestinal flora before and after treatment of patients with CD. The ROC curve of Ruminococcus suggested this genus as the most reliable when distinguishing patients with CD before and after EEN (AUC = 0.898) (Fig. 7a).
Fig. 7.
a ROC curve of Ruminococcus. b ROC curve that combines the five genus bacterium, Ruminococcus, Lachnospiraceae, Flavonifractor, Novosphingobium and Anaerotruncus
The ROC analysis of five bacteria genera easily allows distinguishing between Group Prior and Group Post (AUC = 0.9592) (Fig. 7b). This helped us to construct a stable and statistically significant optimal prediction model upon a combination of the five bacteria genera Lachnospiraceae, Ruminococcus, Anaerotruncus, Flavonifractor and Novosphingobium.
Analysis of fecal short-chain fatty acids
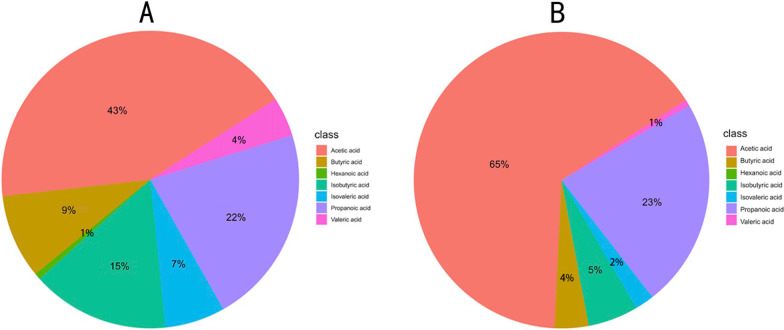
We measured the fecal SCFAs levels of the two groups by GC–MS in order to reveal the potential changes before and after the EEN. In our research, we found that the most abundant SCFA in all seven active CD patients' stool was acetic acid (43%), propanoic acid (22%), butyric acid (9%), isobutyric acid (15%) and isovaleric acid (7%), while valeric acid and caproic acid accounted for only a small ratio of the overall (4% and 1%). After EEN, the composition of SCFAs in the patients’ feces has changed. Here acetic acid had the highest proportion of all SCFAs (65%) (Fig. 8a, b).
Fig. 8.
a Pie chart of the proportion of each SCFA in Group Prior. b Pie chart of the proportion of each SCFA in Group Post
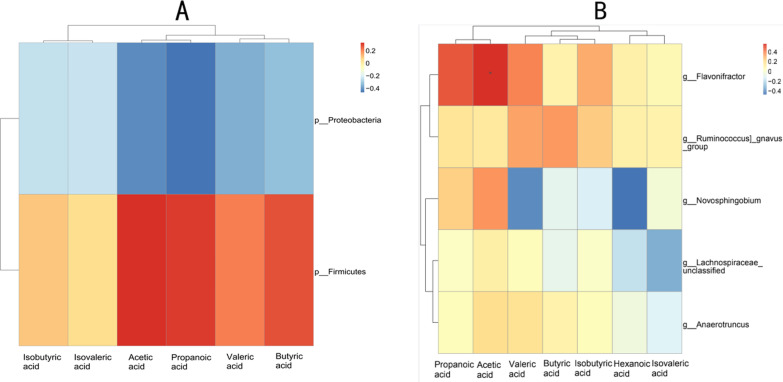
We also combined the abundance of intestinal flora with the level of SCFAs to conduct a Spearman correlation analysis. Here we investigated and represented as a heatmap the correlation between intestinal flora and SCFAs level. Spearman analysis revealed a positive correlation between Firmicutes abundance and the level of fecal SCFAs, especially acetic acid, propanoic acid, butyric acid and valeric acid. Additionally, the analysis indicated a negative correlation between the abundance of Proteobacteria, and the predominant acetic acid and propanoic acid (Fig. 9a). In addition, our data also showed that, in terms of the genus, Flavonifractor and Ruminococcus are positively correlated with all fecal SCFAs and especially with the levels of acetic acid, propanoic acid, and valeric acid. On the other hand, the levels of butyric acid, isobutyric acid and valeric acid are negatively correlated with Novosphingobium (Fig. 9b). Altogether, our results revealed a significant correlation between changes in fecal SCFAs levels and the abundance of intestinal flora.
Fig. 9.
a Spearman correlation heatmap of the relationship between the two significantly different bacterial phyla and the SCFAs. b Spearman correlation heatmap of the relationship between the five significantly different bacterial genera and the SCFAs
Discussion
In the present study, we show that the active condition of all 7 subjects involved, had gained obvious remission after the EEN. This was reflected in the significant reduction of the CDAI, ESR and CRP. Furthermore, our investigations revealed that the subjects' nutrition has been significantly improved after the 8 weeks of treatment. The findings show a change in the composition and the abundance of intestinal flora as well as of SCFAs metabolites' levels after the EEN. In addition, our study delineates a correlation between the changes in the bacterial community and SCFAs. As hypothesized earlier, all of these findings indicate that the EEN may relieve the active state of patients with CD by regulating the composition of the intestinal flora and improving the levels of SCFAs.
Previous studies reported that the intestinal flora of healthy people is mainly composed of several major species such as Firmicutes and Bacteroides [19]. At the same time, the diversity of the intestinal flora of patients with CD is significantly reduced and sometimes, the core flora may further decrease or even disappear. On the other hand, Enterobacterium might significantly increase on the intestinal mucous membrane of these patients [20]. Extensive studies showed that the disorder of the intestinal flora is closely associated with inflammatory bowel disease, including CD. In other words, the intestinal flora of patients with CD is substantially imbalanced [21]. According to a study by Ignacio Catalan-Serra et al. [22], the number of intestinal flora in patients with CD is lower than that documented for healthy individuals. Besides, the changes reflect a decrease in the number of Firmicutes, Bacteroides and other flora, and the overexpression of Enterobacter. Furthermore, Zhang et al. [13] found that the number of opportunistic pathogens, mainly represented by Escherichia coli, in the stool of the patients with active CD was higher than that of patients whose condition was in remission.
Our analysis of fecal bacterium abundance revealed that patients with active CD show an increase of fecal flora composition after EEN compared with that before the treatment. Particularly, the abundance of Firmicutes significantly increased and became the dominant bacteria after the 8-week EEN. At the same time, the abundance of Proteobacteria significantly decreased. The analysis at the genus level revealed that the abundance of Ruminococcus, Novosphingobium, Lachnospiraceae, Anaerotruncus, and Flavonifractor significantly increased after treatment. Matthew et al. [23] found that Ruminococcus can synthesize and secrete a complex glucomannan polysaccharide with a rhamnose backbone and glucose side chains, thereby it can effectively induce dendritic cells to secrete inflammatory cytokine (TNF-α). In addition, Ruminococcus can utilize mucin as a carbon source to directly destroy intestinal barrier function. These both have been associated with the occurrence and development of CD inflammation [23]. Indeed, the findings of our study prove the hypothesis that EEN alleviates the condition of patients with CD by adapting or modulating the structure and composition of the intestinal flora [24].
The analysis of fecal SCFAs levels demonstrated that the level of short-chain fatty acids also changed after EEN. Particularly an increased amount of acetic acid was documented. The Spearman correlation analysis involving two bacterial phyla and 5 bacterial genera indicated significant differences before and after EEN with respect to the fecal SCFAs. Interestingly, the levels of acetic and propanoic acids were positively correlated with the abundance of Firmicutes, but negatively correlated with the abundance of Proteus phylum. In terms of the genus, Flavonifractor and Ruminococcus were positively correlated with all SCFAs in the fecal samples. It means that the effectiveness of EEN is directly proportional to the changes in SCFAs levels. It does suggest that the simultaneous disorder of intestinal flora and the imbalance of SCFAs metabolites represent regulatory factors that relate to the condition of CD. In this way, EEN may alleviate the condition of these patients by ameliorating intestinal flora and metabolism.
Notably, we also selected the five bacterial genera (i.e. Ruminococcus, Lachnospiraceae, Anaerotruncus, Flavonifractor and Novosphingobium) that had significant differences in the changes of abundance during the EEN of CD patients. ROC analysis was employed to distinguish the status before and after treatment based on intestinal flora populations. Our investigations demonstrate that Ruminococcus abundance provides essential information on the state of patients with CD before and after EEN. Furthermore, the combined prediction model of these 5 species of bacteria allows for more accurate prediction.
In conclusion, our study firstly delineated the changes in the composition and abundance of the intestinal flora of patients with active CD after EEN, as well as the changes in the level of their metabolites SCFAs in feces. Secondly, our investigations revealed a direct correlation between the level of SCFAs and changes in bacterial abundance, suggesting a synergistic effect of the two which might be related to the EEN mechanism. Thirdly, the study delineated that levels of acetic acid, propanoic acid, and butyric acid are related to changes in the intestinal flora. As SCFAs members, they may be used as new sensitive indicators to help in better understanding the onset and the development of CD. The last but not least, we successfully designed a reliable and stable prediction model upon the combination of 5 specific bacteria. Altogether, our study demonstrates that the change in the intestinal flora represents a positive and effective basis for CD treatments.
The present study still has some limitations. Firstly, only 7 subjects were successfully followed up. Usually, after patients with CD respond poorly to infliximab (IFX), the processing scheme is increasing the dose of IFX or shortening the interval, for the patients who don’t have high anti-IFX antibodies, or switching to another biological agent. When this study went on, all of the biologics for CD had not been included in the Chinese basic medication insurance, and the patients had to pay for biologics on their own. That is a very large expense and a very important interference factor in making a medication schedule. Just for this reason, the 7 subjects in this study were unable to burden more expensive biologics. So, we selected to conduct EEN only to them. Secondly, the 16S-rDNA prediction function is rather limited if it is compared with the prediction function of metagenomics. Metagenomics can further clarify and delineate the function of flora, revealing the possible mechanism of intestinal flora and SCFAs affecting CD as well as the mechanism behind the EEN. This may further bring insight into Crohn's disease, an illness with unclear pathogenesis to date, and guide the discovery of new therapeutic strategies for CD.
Supplementary Information
Additional file 1: Table S1. The effective sequencing sequence statistics of the Fecal sample.
Acknowledgements
Not applicable.
Abbreviations
- CD
Crohn's disease
- EEN
Exclusive enteral nutrition
- IBD
Inflammatory bowel disease
- SCFA
Short-chain fatty acid
- GC–MS
Gas chromatography–mass spectrometer technology
- 5-ASA
5-Aminosalicylic acid
- IFX
Infliximab
- Pred
Prednisone
- AZA
Azathioprine
- CDAI
Crohn's disease activity index
- ESR
Erythrocyte sedimentation rate
- CRP
C-reactive protein
- BMI
Body mess index
- ALB
Serum albumin
- OUT
Operational Taxonomic Unit
- NMDS
Multidimensional scaling analysis
- PCoA
Principal coordinates analysis
- LEfSe
LDA effect size analysis
Author contributions
JJ and LC designed and carried out the research. JJ and YC screened eligible subjects based on inclusion criterias and exclusion criterias, and collected the subjects’ samples. JJ and LC analyzed the data and prepared the manuscript. HC and YC provided the specialized guidance and support. HC critically revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
The data used and analyzed during the present study are available from the corresponding authors upon reasonable request. The data are not publicly available due to privacy restrictions.
Declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Zhongda Hospital affiliated to Southeast University (Approval Number: 2020ZDSYLL129-P01). All subjects gave informed consent. All the procedures of this study followed the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jingjing Jiang, Email: yc_jjj@126.com.
Lu Chen, Email: 13611569073@163.com.
Yanfang Chen, Email: chenyfmail@163.com.
Hong Chen, Email: njchenhong66@163.com.
References
- 1.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV., Jr Incidence and prevalence of crohn's disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017;15:857–863. doi: 10.1016/j.cgh.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White JR, Jairath V, Moran GW. Evolution of treatment targets in Crohn's disease. Best Pract Res Clin Gastroenterol. 2019;38–39:101599. doi: 10.1016/j.bpg.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Heerasing N, Thompson B, Hendy P, et al. Exclusive enteral nutrition provides an effective bridge to safer interval elective surgery for adults with Crohn's disease. Aliment Pharmacol Ther. 2017;45:660–669. doi: 10.1111/apt.13934. [DOI] [PubMed] [Google Scholar]
- 4.Verburgt CM, Ghiboub M, Benninga MA, de Jonge WJ, Van Limbergen JE. Nutritional therapy strategies in pediatric crohn's disease. Nutrients. 2021;13:212. doi: 10.3390/nu13010212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing) J Dig Dis. 2021;22:298–317. doi: 10.1111/1751-2980.12994. [DOI] [PubMed] [Google Scholar]
- 6.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan E, Gaunt WW, Cardigan T, Garrick V, McGrogan P, Russell RK. The use of exclusive enteral nutrition for induction of remission in children with Crohn's disease demonstrates that disease phenotype does not influence clinical remission. Aliment Pharmacol Ther. 2009;30(5):501–507. doi: 10.1111/j.1365-2036.2009.04067.x. [DOI] [PubMed] [Google Scholar]
- 8.Rubio A, Pigneur B, Garnier-Lengliné H, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn's disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther. 2011;33(12):1332–1339. doi: 10.1111/j.1365-2036.2011.04662.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang Q, Gao X, Chen H, et al. Efficacy of exclusive enteral nutrition in complicated Crohn's disease. Scand J Gastroenterol. 2017;52(9):995–1001. doi: 10.1080/00365521.2017.1335770. [DOI] [PubMed] [Google Scholar]
- 10.Burgess D, Herbison K, Fox J, Collins T, Landorf E, Howley P. Exclusive enteral nutrition in children and adolescents with Crohn disease: dietitian perspectives and practice. J Paediatr Child Health. 2021;57:359–364. doi: 10.1111/jpc.15220. [DOI] [PubMed] [Google Scholar]
- 11.Li G, Wu X, Gao X, et al. Long-term exclusive enteral nutrition remodels the gut microbiota and alleviates TNBS-induced colitis in mice. Food Funct. 2022;13(4):1725–1740. doi: 10.1039/d1fo03579g. [DOI] [PubMed] [Google Scholar]
- 12.Fascì Spurio F, Aratari A, Margagnoni G, Doddato MT, Chiesara F, Papi C. Oral beclomethasone dipropionate: a critical review of its use in the management of ulcerative colitis and Crohn's disease. Curr Clin Pharmacol. 2012;7:131–136. doi: 10.2174/157488412800228875. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Chen SL, Li LB. Correlation between intestinal flora and serum inflammatory factors in patients with Crohn's disease. Eur Rev Med Pharmacol Sci. 2017;21:4913–4917. [PubMed] [Google Scholar]
- 14.Furuhashi T, Sugitate K, Nakai T, Jikumaru Y, Ishihara G. Rapid profiling method for mammalian feces short chain fatty acids by GC–MS. Anal Biochem. 2018;543:51–54. doi: 10.1016/j.ab.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Zhu W. Advances in the role of short-chain fatty acids in Crohn's disease. Parenter Enter Nutr. 2019;26:366–371. [Google Scholar]
- 16.Marques JG, Shokry E, Frivolt K, et al. Metabolomic signatures in pediatric Crohn's disease patients with mild or quiescent disease treated with partial enteral nutrition: a feasibility study. SLAS Technol. 2021;26:165–177. doi: 10.1177/2472630320969147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanes C, Bittinger K, Gao Y, et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29:394–407.e5. doi: 10.1016/j.chom.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clapp M, Aurora N, Herrera L, Bhatia M, Wilen E, Wakefield S. Gut microbiota's effect on mental health: the gut-brain axis. Clin Pract. 2017;7:987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiner P, Neurath MF, Ng SC, et al. Mechanism-based treatment strategies for IBD: cytokines, cell adhesion molecules, JAK inhibitors, gut flora, and more. Inflamm Intest Dis. 2019;4:79–96. doi: 10.1159/000500721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu ZY, Sang LX, Chang B. Isoflavones and inflammatory bowel disease. World J Clin Cases. 2020;8:2081–2091. doi: 10.12998/wjcc.v8.i11.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catalan-Serra I, Brenna Ø. Immunotherapy in inflammatory bowel disease: novel and emerging treatments. Hum Vaccin Immunother. 2018;14:2597–2611. doi: 10.1080/21645515.2018.1461297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke MT, Kenny DJ, Cassilly CD, Vlamakis H, Xavier RJ, Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc Natl Acad Sci USA. 2019;116:12672–12677. doi: 10.1073/pnas.1904099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danilova NA, Abdulkhakov SR, Grigoryeva TV, et al. Markers of dysbiosis in patients with ulcerative colitis and Crohn's disease. Ter Arkh. 2019;91:17–24. doi: 10.26442/00403660.2019.04.000211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The effective sequencing sequence statistics of the Fecal sample.
Data Availability Statement
The data used and analyzed during the present study are available from the corresponding authors upon reasonable request. The data are not publicly available due to privacy restrictions.