Abstract
Acanthamoeba is a free-living amoeba causing a potentially blinding infection of the cornea. Contact lens wearers are most at risk and account for some 95% of cases. Hydrogen peroxide is used for contact lens disinfection due to its broad antimicrobial activity. Lenses must be neutralized before use to avoid pronounced stinging and possible corneal damage. Neutralization is achieved by adding a catalyst during the disinfection process (one-step) or afterwards (two-step). Here, the activities of commercial peroxide systems and individual solutions against trophozoites and cysts of Acanthamoeba polyphaga were compared. All disinfection systems were active against trophozoites, giving a ≥3-log (99.9%) kill within 1 h. Of the four one-step systems, only one showed some cysticidal activity, giving a 1.28 ± 0.41-log reduction. Both two-step systems were cysticidal, giving a ≥3-log kill at 4 h. All system peroxide solutions were cysticidal, giving a ≥3-log kill by 4 to 6 h. Variation in the cysticidal rate was observed with two solutions that gave a 1.8- to 2.1-log kill at 4 h compared with 3.0 to 4.0 for the rest (P < 0.05). No cysticidal activity was found with the peroxigen sodium perborate or the contact lens protein remover subtilisin A. Two-step systems are cysticidal providing contact times of at least 4 h are employed. Variation in cyst killing occurs between peroxide solutions, possibly due to formulation differences. One-step systems are less effective against Acanthamoeba cysts due to rapid peroxide neutralization. The cysticidal activity of one-step systems could be improved if neutralization rates were retarded.
Acanthamoeba is a genus of free-living amoeba found in most soil and water habitats (21). The organism is characterized by a life cycle of a feeding and dividing trophozoite, which in response to adverse conditions can form a dormant cyst stage (21). Acanthamoeba cysts are resistant to desiccation (16), extremes of temperature from −20 to 56°C (1, 12), and most disinfectants at working concentrations (13).
Acanthamoeba is an opportunistic pathogen, causing fatal encephalitis in the immunocompromised host (19) and, more frequently, a potentially blinding infection of the cornea termed acanthamoeba keratitis (4, 14). Contact lens wearers are most at risk from infection, accounting for approximately 95% of reported cases (22, 23). Infection results from contamination of lens care products, notably the lens storage case, from which the organism adheres to the contact lens and is inoculated onto the cornea (6, 14).
Acanthamoeba keratitis is one of the most difficult ocular infections to treat (4). Therefore, compliance with recommended hygiene procedures for cleaning and disinfecting lenses is fundamental to safe contact lens use and hence the prevention of infection. The two most common methods of contact lens disinfection are the multipurpose solutions in which a single solution is used for disinfecting, cleaning, and storing lenses and hydrogen peroxide-based systems. Hydrogen peroxide is an effective microbial disinfectant, destroying pathogens by oxidation (11). It is active against the resistant cyst form of Acanthamoeba when used at a concentration of 3% with an exposure time of at least 4 to 6 h (5, 14). However, hydrogen peroxide is toxic to the cornea and must be neutralized before lens wear to avoid pronounced stinging, lacrimation, hyperemia, and possible corneal damage (7, 8). One-step hydrogen peroxide systems are available which do not require a separate neutralization step. Here, neutralization is achieved in the storage case during disinfection by using a platinum-coated disk or soluble catalase tablet which catalyzes the decomposition of hydrogen peroxide to water and oxygen. Two-step solutions employ a separate neutralization step through the addition of a catalase or sodium pyruvate solution after a designated disinfection time.
Although previous studies have demonstrated the activity of hydrogen peroxide-based contact lens disinfectants against Acanthamoeba, none have evaluated the relative efficacy of commercially available systems (15, 25). To this end we have compared the activities of one-step and two-step hydrogen peroxide contact lens disinfection systems, their respective solutions, native 3% hydrogen peroxide, the peroxigen sodium perborate, and the lens protein remover subtilisin A against the trophozoites and cysts of Acanthamoeba polyphaga.
MATERIALS AND METHODS
Test strain and culture.
The A. polyphaga Ros strain was used throughout the study. The strain was originally isolated from an unpublished case of acanthamoeba keratitis in the United Kingdom in 1994. Trophozoites were grown in tissue culture flasks at room temperature in a semidefined axenic culture medium. The medium comprised 20.0 g of Biosate (BBL; Becton Dickinson, Oxford, United Kingdom), 5 g of glucose, 0.3 g of KH2PO4, 10 μg of vitamin B12, and 15 mg of l-methionine per liter of deionized water. The pH was adjusted to 6.5 to 6.6 with 1 M NaOH before autoclaving at 121°C for 12 min. Penicillin- streptomycin was added to a final concentration of 150 U/ml before use.
Cysts were prepared from late-log-phase trophozoite cultures using Neff's constant pH encystment medium (20). The trophozoites were washed three times with the encystment medium by centrifugation at 1,000 × g for 10 min. Approximately 107 trophozoites were added to 100 ml of encystment medium in a 175-cm2 tissue culture flask with a filter cap (Nunc, Life Technologies Ltd., Paisley, Scotland) and incubated at 32°C for 8 days, after which microscopic examination showed that >90% of the cells were mature cysts. The cysts were recovered from the flask by gentle rubbing with a cell scraper and centrifuged at 1,000 × g for 10 min. The resulting cyst pellet was washed three times in 1/4 strength Ringer's solution by centrifugation and thereafter stored at 4°C for testing within 14 days.
The cysts were sonicated before use with three pulses at 50% amplitude each for 5 s (Ultrasonic Engineering Ltd., London, England). Previous experiments had shown that this removed cyst clumps without affecting viability. Cell counts were performed with a hemocytometer and adjusted to a concentration of 107 cysts per ml.
Test solutions.
Six commercially available hydrogen peroxide-based contact lens disinfection solutions were studied (Table 1). Chemical solutions of 3% hydrogen peroxide (BDH, Poole, England) and 0.06 or 6% of the peroxigen sodium perborate (Sigma Chemical Company, Poole, England, and Fluka, Steinheim, Germany) were also investigated. The serine protease subtilisin A, from the bacterium Bacillus subtilis, is used for the enzymatic removal of protein from contact lenses (Ultrazyme; Allergan) in the presence of hydrogen peroxide in the Oxysept 1 system. Experiments were conducted to determine whether subtilisin A is cysticidal or if it enhanced the activity of hydrogen peroxide.
TABLE 1.
Hydrogen peroxide systems and solutions studied
| System | Neutralization | Exposure timea |
|---|---|---|
| One-step | ||
| Concerto (Essilor) | Platinum disk | 6 h |
| Oxysept 1 Step (Allergan) | Catalase tablet (0.1 mg) | 6 h |
| Multi (Sauflon) | Platinum disk | 6 h |
| AOSept 1-Step (Ciba Vision) | Platinum disk | 6 h |
| Two-step | ||
| Oxysept 1 (Allergan) | Catalase solution (260 U/ml) | 20 min–overnight |
| 10-10 (Ciba Vision) | Sodium pyruvate solution (0.5%) | 10 min–overnight |
| Other | ||
| Hydrogen peroxide (3%; BDH) | 0.02% catalase | |
| Sodium perborate (6%; Fluka) | 0.02% catalase | |
| Sodium perborate, phosphate-citrate-buffered (0.06%; Sigma) | 0.02% catalase | |
| Subtilisin A (0.4 mg; Ultrazyme; Allergan) | 6 h |
Manufacturer's recommended contact time.
All commercial contact lens disinfection systems were tested according to the manufacturers' recommendations. In separate experiments the efficacies of the hydrogen peroxide solutions in the one- and two-step systems were also compared. With the one-step systems testing was conducted in the contact lens storage cases supplied by the manufacturer. The two-step systems and solutions were tested in sterile 50-ml polypropylene tubes (Becton Dickinson). All experiments were performed in triplicate and repeated on three separate occasions. Control experiments used 1/4 strength Ringer's solution in place of the test solution.
Prior to testing, the pHs of all peroxide solutions were measured using a pH meter (Jenway 3310; Jenway Ltd., Essex, England). The concentration of hydrogen peroxide during the assays was determined using Peroxid test strips (BDH).
Contact lens solution efficacy testing: one-step systems.
Eighty microliters of trophozoites or cysts was inoculated into 8 ml of contact lens disinfection solution in the manufacturers' recommended contact lens storage case to give a final concentration of 105 cells per ml. Immediately, the appropriate neutralizer was added (Table 1). At time intervals of 0, 1, 2, 4, 6, and 8 h the solution was vortexed for 10 s, and, in quadruplet, 20 μl was removed and added to 200 μl of 0.02% bovine liver catalase (Sigma Chemical Company) and left for 5 min to neutralize the peroxide. Following vortexing, serial dilutions of 20 μl in 200 μl of 1/4 strength Ringer's solution were then made across the rows of a 96-well microtiter plate. Finally, 25 μl of Escherichia coli (JM101; optical density at 600 nm of 0.4) was added to each well, and the plate was sealed and incubated at 32°C for up to 7 days. The plates were inspected daily for 7 days for the presence of amoebal growth (excystment and trophozoite replication) at the various dilutions in the wells.
Contact lens solution efficacy testing: two-step systems and hydrogen peroxide solutions.
The two-step peroxide systems, chemical hydrogen peroxide, and sodium perborate were tested. Eight milliliters of test solution was challenged with 80 μl of cysts to give a concentration of 105 per ml. Assays were performed as described for the one-step systems. Subtilisin A (Ultrazyme) was tested either dissolved in 8 ml of 1/4 Ringer's solution, Oxysept 1 Step, or the Oxysept 1 Step system.
Sodium perborate.
The minimum cysticidal concentration (MCC) of sodium perborate was studied. The assay relies on the observation that Acanthamoeba cysts adhere to the bottom of polycarbonate microtiter plate wells and remain attached following drug exposure and removal by washing (13). Twofold dilutions in 1/4 Ringer's solution (100 μl) of 12% sodium perborate or 0.12% buffered sodium perborate were made across the rows of a microtiter plate (Triple Red Laboratory Technology, Oxfordshire, England). An equal volume of 103 cysts was then added to each well, and the plate was sealed and incubated at room temperature overnight. Using a multichannel pipette, the solutions in the wells were removed and replaced with 200 μl of 1/4 strength Ringer's solution and left at room temperature for 15 min. The washing procedure was repeated twice more before finally filling the wells with 100 μl of 1/4 strength Ringer's solution containing live E. coli at an optical density at 540 nm of 0.2. The plates were then sealed and incubated at 32°C for up to 7 days. The MCC was defined as the lowest concentration of sodium perborate solution that resulted in no excystment and trophozoite replication.
Data analysis.
The number of surviving organisms at each time point was determined using Reed and Muench computations (24) as previously described for Acanthamoeba cyst viability enumeration (3). All experiments were performed in triplicate and repeated on three separate occasions. The reduction in viable cysts was plotted as delta logs with standard error of the mean for each time point according to the following formula: log Tn − log T0, where Tn is the viable count at an experimental time point and T0 is the initial viable count at the start of the experiment. Statistical analysis was performed using one-way analysis of variance.
RESULTS
Trophozoites.
All one- and two-step peroxide systems were active against Acanthamoeba trophozoites, giving at least a 3-log reduction (99.9% kill) within the first time point of 1 h (results not shown).
One-step systems.
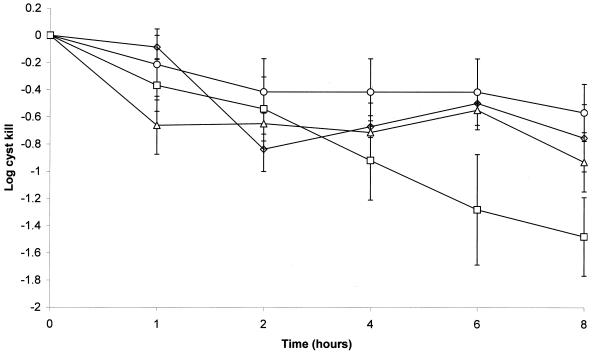
The activities of the commercial one-step disinfection systems against Acanthamoeba cysts are shown in Fig. 1. With the exception of AOSept, the systems gave a <1-log reduction in cyst viability after 8 h of contact time. AOSept gave a 1.28 ± 0.41-log reduction in viability after the manufacturer's recommended contact time of 6 h and a 1.48 ± 0.29-log reduction after 8 h (Fig. 1). However, this observation was not statistically significant (P > 0.05). For all the systems, exposure times of 24 h did not result in further cyst killing beyond that observed at 8 h (results not shown).
FIG. 1.
Activities of one-step hydrogen peroxide contact lens disinfection systems against A. polyphaga cysts. ○, Concerto; ▵, Oxysept 1 Step; □, AOSept 1-Step; ◊, Multi.
Hydrogen peroxide pH and concentration of the solutions during the disinfection-neutralization process are shown in Table 2. All solutions had an initial hydrogen peroxide concentration of 3% that decomposed to 0% within 1 h for Oxysept 1 Step and 4 h for AOSept 1-Step. Both Concerto and Multi showed residual hydrogen peroxide after 6 h of 0.0002 or 0.0005%, respectively. The pH of the peroxide solutions ranged from 3.25 to 6.45 but this had no influence on the relative disinfectant activity (P > 0.05).
TABLE 2.
Hydrogen peroxide concentrations during one- and two-step disinfection and log cyst kill
| System | pH | % Hydrogen peroxide over time (h)
|
Log cyst kill at 6 ha | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 6 | 8 | |||
| One-step | ||||||||
| Concerto | 5.85 | 3.0 | 0.02 | 0.01 | 0.0005 | 0.0002 | 0.00005 | 0.43 ± 0.24 |
| OxySept 1 Step | 3.25 | 3.0 | 0 | 0 | 0 | 0 | 0 | 0.55 ± 0.14 |
| Multi | 6.30 | 3.0 | 0.2 | 0.05 | 0.005 | 0.0005 | 0.0002 | 0.50 ± 0.005 |
| AOSept 1-Step | 6.45 | 3.0 | 0.02 | 0.002 | 0 | 0 | 0 | 1.28 ± 0.41 |
| Two-step | ||||||||
| 10-10 | 3.66 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.41 ± 0.21b |
| Oxysept 1 | 3.35 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.85 ± 0.26b |
| Other | ||||||||
| Hydrogen peroxide (3%) | 5.20 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 4.25 ± 0 |
| Sodium perborate (6%) | 9.5 | 1 | 1 | 1 | 1 | 1 | 1 | 0c |
| Sodium perborate, phosphate-citrate-buffered (0.06%) | 5 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0c |
Manufacturer's recommended disinfection time for one-step systems.
Manufacturer's recommended disinfection times are between 10 or 20 min and overnight.
Exposure, 24 h.
Two-step systems.
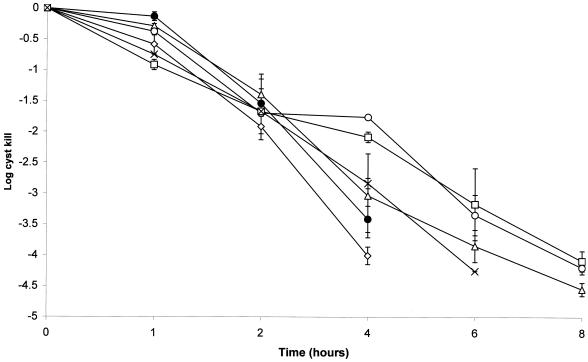
Both the 10-10 and Oxysept 1 systems were cysticidal, giving at least a 3-log reduction in viability after 4 h of contact time prior to neutralization (Fig. 2). The log reductions after 1 h were <1, and they were 1.54 ± 0.39 and 1.39 ± 0.33 after 2 h, respectively. No difference in the rate of killing was found between the two systems (P > 0.05).
FIG. 2.
Activities of hydrogen peroxide contact lens disinfectant solutions against A. polyphaga cysts. The last time point shown indicates the total kill of the challenge inoculum. ○, Concerto; ▵, Oxysept 1; □, AOSept 1-Step; ◊, Multi; ●, 10-10; ×, BDH.
Hydrogen peroxide solutions.
The cysticidal activities of the individual hydrogen peroxide solutions used in the one- and two-step systems are shown in Fig. 2. All solutions were active against Acanthamoeba cysts, giving at least a 3-log reduction in viability after 4 to 6 h of contact time. However, variation in the rate of cyst killing was noted between solutions after 4 h of exposure. Here, Concerto and AOSept gave a 1.76 ± 0.04- and 2.08 ± 0.08-log kill compared with 3.0 to 4.0 for the remainder. This difference is statistically significant (P < 0.05). No significant difference was found between the efficacies of the commercial contact lens peroxide solutions and that of the BDH chemical solution, which gave log reductions of 2.83 ± 0.08 after 4 h and 4.25 after 6 h (Fig. 2).
Concentrations of up to 6% sodium perborate and 0.06% phosphate-citrate-buffered perborate, yielding 1 and 0.02% hydrogen peroxide, respectively, were not cysticidal after 24 h of exposure (Table 2).
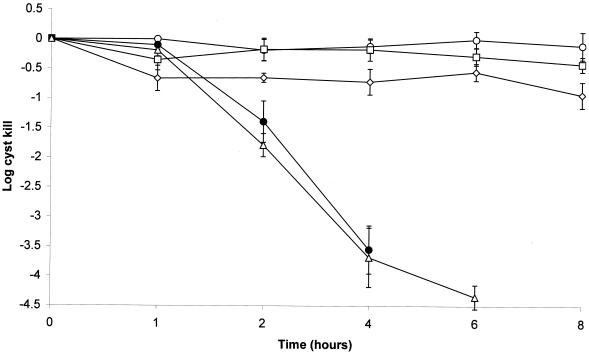
OxySept 1 Step with subtilisin A.
Subtilisin A (Ultrazyme) at 50 μg/ml in 1/4 Ringer's solution showed no cysticidal activity even after 24 h of exposure (Fig. 3). Addition of subtilisin A at 50 μg/ml to Oxysept 1 did not result in enhanced cysticidal activity, giving a 3.55 ± 0.41-log kill after 4 h compared with a 3.68 ± 0.50-log kill with the peroxide solution alone (Fig. 3). Use of the Oxysept 1 Step system with subtilisin A as recommended by the manufacturer did not result in cysticidal activity (Fig. 3).
FIG. 3.
Effect of subtilisin A on the Oxysept 1 Step hydrogen peroxide contact lens disinfection system against A. polyphaga cysts. The last time point shown indicates the total kill of the challenge inoculum. ○, subtilisin A (Ultrazyme); ▵, Oxysept 1 Step; □, Oxysept 1 Step + catalase + subtilisin A; ◊, Oxysept 1 Step + catalase; ●, Oxysept 1 Step + subtilisin A.
DISCUSSION
The findings of this study demonstrate that 3% hydrogen peroxide-based contact lens disinfection systems are effective against Acanthamoeba cysts providing an adequate disinfectant concentration and exposure time are maintained. This requirement is not met by one-step systems that rapidly neutralize the peroxide resulting in no cysticidal activity (5, 15, 25). The possible exception was found with use of the AOSept 1-Step system, which resulted in a 1.28 ± 0.41-log reduction after the manufacturer's recommended contact time of 6 h, compared with a <1-log reduction for the other systems. However, this observation was not found to be statistically significant (P > 0.05). The reasons for the apparent greater activity of the AOSept 1-Step system are unclear as neither the pH nor the rate of peroxide neutralization had a statistically significant effect on the degree of cyst killing. Nor was the peroxide solution of the system any more potent when tested alone against cysts (see below). Possibly, other components of the AOSept peroxide solution, such as the stabilizer, may interact with the platinum catalyst to produce an additive effect.
The pH of the peroxide solutions ranged from 3.25 to 6.45 but this had no effect on the disinfectant activity (P > 0.05), supporting the previous observation with testing against bacteria and fungi (17). The platinum-based systems neutralized the peroxide more slowly than that using catalase, although this did not affect the relative inefficacy of disinfection. Two of the four systems (Concerto and Multi) failed to achieve complete neutralization after the recommended disinfection time of 6 h. However, the residual peroxide levels at this time of 0.0002 to 0.0005% (2 to 5 ppm) are not likely to cause irritation to the eye as 30 ppm has been reported to induce cytotoxicity and 100 ppm has been reported to cause noticeable discomfort (8, 27).
In contrast, both the two-step systems, 10-10 and Oxysept 1, were cysticidal, resulting in at least a 3-log kill after 4 h of contact time and complete kill after 6 h. This observation is in accord with previous studies for two-step systems (5, 13, 15). As expected, the hydrogen peroxide solutions used in the one-step systems were also cysticidal. However, variation in the rate of cyst killing was observed at 4 h with Concerto and AOSept, which gave a 1.8- to 2.1-log kill compared with a 3.0- to 4.0-log kill for the remainder (P < 0.05). Reasons for the difference in activity between the solutions are unclear but would not appear to be due to pH. The pHs of Concerto and AOSept were 5.85 and 6.45, respectively, compared with 3.25 and 6.3 for Oxysept 1 Step and Multi, both of which gave at least a 3-log kill at 4 h. Native 3% hydrogen peroxide (BDH) gave comparable cyst killing to the contact lens disinfecting solutions. However, use of homemade hydrogen peroxide contact lens disinfectant is not recommended as it can contain stabilizers such as phosphoric acid, acetanilide, phenacetin, and sodium stanate.
Unlike the requirements for efficacy testing of contact lens disinfectants against bacteria and fungi, no standard protocol for such testing exists for Acanthamoeba cysts (10). Consequently, a variety of strains, methods for cyst preparation, and assay protocols have been used (2, 3, 5, 9, 15, 25, 26). This has frequently led to conflicting reports on the efficacy of contact lens disinfectants, including hydrogen peroxide (2, 18, 26). Two distinct methods for determining the activity of hydrogen peroxide against Acanthamoeba cysts and trophozoites were used in this study. The microtiter plate assay enables the determination of MCCs and can be used for the screening of new antiacanthamoebal therapeutic and disinfectant agents. Promising compounds can then be investigated further using the time-kill assay (with an appropriate neutralizer) to study the kinetics of cyst killing (3). It is hoped that this will lead to a standardized method for Acanthamoeba disinfectant and drug sensitivity testing.
This study has confirmed the efficacy of two-step commercial hydrogen peroxide contact lens disinfection systems against Acanthamoeba cysts providing a contact time of at least 4 h is used before neutralization (2, 5, 15). The potential disadvantage of hydrogen peroxide disinfection is that the lenses cannot be stored in the solution (25). Therefore, once neutralization is complete there is no residual disinfectant activity for continued antimicrobial protection to prevent contamination by surviving organisms or those introduced from the environment if the case is opened (25). One-step systems offer the convenience of a single disinfection-neutralization process and prevent the painful consequence of inserting nonneutralized lenses into the eye that can occur with two-step systems. However, one-step systems may offer less protection against acanthamoeba keratitis as the neutralization process occurs too rapidly to allow cyst killing to occur. To this end, manufacturers should consider developing one-step systems in which the neutralization process is slowed to provide cysticidal activity.
REFERENCES
- 1.Biddick C J, Rogers L H, Brown T J. Viability of pathogenic and nonpathogenic free-living amoebae in long-term storage at a range of temperatures. Appl Environ Microbiol. 1984;48:859–860. doi: 10.1128/aem.48.4.859-860.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt F H, Ware D A, Visvesvara G S. Viability of Acanthamoeba cysts in ophthalmic solutions. Appl Environ Microbiol. 1989;55:1144–1146. doi: 10.1128/aem.55.5.1144-1146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck S L, Rosenthal R A. A quantitative method to evaluate neutralizer toxicity against Acanthamoeba castellanii. Appl Environ Microbiol. 1996;62:3521–3526. doi: 10.1128/aem.62.9.3521-3526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dart J. Contact lens and prosthesis infections. In: Tasman W, Jaeger E A, editors. Duane's foundations of clinical ophthalmology. New York, N.Y: Lippincott-Raven; 1996. [Google Scholar]
- 5.Davies D J G, Anthony Y, Meakin B J, Kilvington S, Anger C B. Evaluation of the anti-acanthamoebal activity of five contact lens disinfectants. ICLC. 1990;17:14–20. [Google Scholar]
- 6.Gray T B, Cursons R T M, Sherwan J F, Rose P R. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol. 1995;79:601–605. doi: 10.1136/bjo.79.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyulai P, Dziabo A, Kelly W, Kiral R, Hayes C. Relative neutralization ability of six hydrogen peroxide disinfection systems. Contact Lens Spectrum. 1987;2:61–65. [Google Scholar]
- 8.Holden B. A report card on hydrogen peroxide for contact lens disinfection. CLAO J. 1990;16:S61–S64. [PubMed] [Google Scholar]
- 9.Hugo E R, McLaughlin W R, Oh K-H, Tuovinen O H. Quantitative enumeration of Acanthamoeba for evaluation of cyst inactivation in contact lens solutions. Investig Ophthalmol Vis Sci. 1991;32:655–657. [PubMed] [Google Scholar]
- 10.ISO/DIS. Ophthalmic optics—contact lens care products—microbiological requirements and test methods for products and regimens for hygienic management of contact lenses. ISO/DIS 14729. Geneva, Switzerland: ISO; 1999. [Google Scholar]
- 11.Jackett P S, Aber V R, Lowrie D B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium turberculosis. J Gen Microbiol. 1978;104:37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- 12.Kilvington S. Moist-heat disinfection of pathogenic Acanthamoeba cysts. Lett Appl Microbiol. 1989;9:187–189. [Google Scholar]
- 13.Kilvington S. Activity of water biocide chemicals and contact lens disinfectants on pathogenic free-living amoebae. Int Biodeterior. 1990;26:127–138. [Google Scholar]
- 14.Kilvington S, White D G. Acanthamoeba: biology, ecology and human disease. Rev Med Microbiol. 1994;5:12–20. [Google Scholar]
- 15.Kilvington S, Anger C. A comparison of cyst age and assay method on the efficacy of contact lens disinfectants against Acanthamoeba. Br J Ophthalmol. 2001;85:336–340. doi: 10.1136/bjo.85.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingston D, Warhurst D C. Isolation of amoebae from the air. J Med Microbiol. 1969;2:27–36. doi: 10.1099/00222615-2-1-27. [DOI] [PubMed] [Google Scholar]
- 17.Lowe R, Vallas V, Brennan N A. Comparative efficacy of contact lens disinfection solutions. CLAO J. 1992;18:34–40. [PubMed] [Google Scholar]
- 18.Ludwig I H, Meisler D M, Rutherford I, Bican F W, Langston R H S, Visvesvara G S. Susceptibility of Acanthamoeba to soft contact lens disinfection systems. Investig Ophthalmol Vis Sci. 1986;27:626–628. [PubMed] [Google Scholar]
- 19.Martinez A J. Infections of the central nervous system due to Acanthamoeba. Rev Infect Dis. 1991;13:S399–S402. doi: 10.1093/clind/13.supplement_5.s399. [DOI] [PubMed] [Google Scholar]
- 20.Neff R J, Ray S A, Benton W F, Wilborn M. Induction of synchronous encystment (differentiation) in Acanthamoeba sp. Methods Cell Physiol. 1964;1:55–83. [Google Scholar]
- 21.Page F C. A new key to freshwater and soil gymnamoebae. Ambleside, Cumbria, England: Freshwater Biological Association, The Ferry House; 1988. [Google Scholar]
- 22.Radford C, Bacon A S, Dart J K G, Minassian D C. Risk factors for acanthamoeba keratitis in contact lens users: a case control study. Br Med J. 1995;310:1567–1570. doi: 10.1136/bmj.310.6994.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radford C F, Lehmann O J, Dart J K G for the National Acanthamoeba Keratitis Study Group. Acanthamoeba keratitis: multicentre survey in England 1992–6. Br J Ophthalmol. 1998;82:1387–1392. doi: 10.1136/bjo.82.12.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed L J, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 25.Rosenthal R A, Stein J M, McAnally C L, Schlech B A. A comparative study of microbiologic effectiveness of chemical disinfectants and peroxide-neutralizer systems. CLAO J. 1995;21:99–110. [PubMed] [Google Scholar]
- 26.Silvany R E, Dougherty J M, McCulley J P. Effects of contact lens preservatives on Acanthamoeba. Ophthalmology. 1991;98:854–857. doi: 10.1016/s0161-6420(91)32210-3. [DOI] [PubMed] [Google Scholar]
- 27.Tripathi B J, Tripathi R C, Millard C B, Borisuth N S C. Cytotoxicity of hydrogen peroxide to human corneal epithelium in vitro and its clinical implications. Lens Eye Toxicity Res. 1990;7:385–402. [PubMed] [Google Scholar]