Abstract
Carbamazepine (CBZ)-induced Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are strongly associated with the HLA-B*15:02 allele. Screening HLA-B*15:02 before CBZ administration might prevent CBZ-induced SJS/TEN by enabling clinicians to prescribe alternative therapy for positive patients. Similar to other Southeastern Asian countries, HLA-B*15:02 is highly prevalent in Indonesia. Therefore, we assessed the economic value of HLA-B*15:02 screening before CBZ prescription to patients with epilepsy in Indonesia. A generic cost-effectiveness model and decision support tool, developed to enable users to perform an initial cost-effectiveness analysis from a healthcare provider/payer perspective, were used to assess the value of HLA-B*15:02 genotyping. The incremental cost-effectiveness ratio of adopting universal HLA-B*15:02 screening was 656 444 671 Indonesian Rupiah (IDR)/quality-adjusted life year (QALY) gained for patients compared with 2 634 975 574 IDR/QALY gained for providing valproic acid (alternative drug) without screening. Thus, neither HLA-B*15:02 screening nor substitution with VPA meets the Indonesian threshold for cost effectiveness. However, the improved outcomes with this test in other Asian countries may inform the desirability of implementation in Indonesia even with suboptimal cost-effectiveness.
Keywords: cost utility analysis, SJS/TEN, HLA-B*15:02, Indonesian population, generic model
Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening acute inflammatory skin conditions that are among the most severe adverse drug reactions (ADRs) [1,2]. Approximately 80% of SJS/TEN cases are caused by medications, with the remainder caused by chemical exposure, mycoplasma pneumonia, viral infection, or immunization [3,4]. The clinical presentation of SJS/TEN includes a rash with target-like lesions and mucositis involving ocular, oropharyngeal, and genital surfaces. Patients are systemically unwell and experience fever and malaise [5]. The mortality rate of patients with SJS/TEN is 5%–50%, and 30%–70% of surviving patients suffer from long-term sequelae such as severe dry eye syndrome and trichiasis [2,6,7].
Carbamazepine (CBZ), a drug prescribed for the treatment of seizure disorders, bipolar disorder, trigeminal neuralgia, and chronic pain, is one of the drugs most frequently linked to SJS/TEN and other cutaneous eruptions including maculopapular exanthema (MPE) and Drug Rash with Eosinophilia and Systemic Symptoms Syndrome (DRESS Syndrome) [8–13]. Previous studies have shown that the development of SJS/TEN induced by CBZ also has a genetic basis [11], and a strong association has been identified between the HLA-B*15:02 allele and risk for CBZ-induced SJS/TEN among Han Chinese [13–14] and in Southeast Asian countries, including Thailand [15], India [16], and Malaysia [17]. However, the allele is rare in populations of European descent [11]. Evidence for the association between HLA-B*15:02 and MPE was observed in the Thai population [12], but subsequent studies have disputed the association. DRESS does not appear to be associated with HLA-B*15:02, but is associated with another allele, HLA-B*58:01 [13].
Screening for HLA-B*15:02 in individuals with Southeast Asian ancestry before initiating CBZ treatment was found to decrease the incidence of SJS/TEN in Taiwan [18], Thailand [19], and Singapore [20]. This practice is also recommended by drug regulatory agencies such as the U.S. Food and Drug Administration [21], UK Medicines and Healthcare Products Regulatory Agency [22], and the European Medicines Agency [23]. However, it has not been implemented in most Asian countries owing to limited data regarding the contribution of HLA-B*15:02 to drug-induced SJS/TEN and the cost effectiveness of genotype-guided approaches, especially across Southeast Asian countries.
Indonesia is among the Southeast Asian countries that has not adopted HLA-B*15:02 screening. Previously, we described the high frequency of the HLA-B*15:02 allele in healthy Javanese and Sundanese populations [24,25]. Additionally, our recent study revealed a significant association between the HLA-B*15:02 allele and the risk of CBZ-induced SJS/TEN in the Javanese–Sundanese population in Indonesia [26]. These data suggest that the Indonesian population may especially benefit from genetic screening prior to CBZ treatment by identifying the risk of developing SJS/TEN.
In Indonesia, CBZ is the first-line drug for the treatment of psychiatric and neurologic diseases owing to its affordability and efficacy. However, owing to concerns regarding the development of SJS/TEN, some clinicians prefer to prescribe alternative drugs, such as valproic acid (VPA). The implementation of HLA-B*15:02 screening for patients with newly diagnosed epilepsy and neuropathic pain would allow the prescription of alternative medications (e.g., VPA) to patients testing positive for the allele and CBZ for those with a negative result. However, the cost effectiveness of implementing HLA-B*15:02 screening prior to CBZ treatment should be analyzed relative to the current practice of prescribing CBZ universally without screening or the prescription of VPA or other drugs universally without screening to influence Indonesia health care policy decisions, especially the Indonesian Ministry of Health decisions. The present study was performed to analyze the cost effectiveness of screening adult patients newly diagnosed with epilepsy for HLA-B*15:02 to prevent CBZ-induced SJS/TEN.
Materials and Methods
Model structure
We utilized a generic cost-effectiveness model and a decision support tool developed to enable users to perform an initial cost-effectiveness analysis from a healthcare provider/payer perspective (only direct medical costs were used) to assess the value of HLA-B*15:02 genotyping for adult patients newly diagnosed with epilepsy to prevent CBZ-induced SJS/TEN [27]. We enrolled adult patients (aged 40 years or older, based on the model assumption of the average age of epilepsy onset = 40 years and life expectancy = 60 years [28]) with newly diagnosed epilepsy in Indonesia for whom CBZ was considered suitable as first-line therapy, by providing requested input values that are specific to Indonesia. The model assumed that VPA has efficacy and safety profiles comparable with those of CBZ but without the risk of SJS/TEN [29]. The model also assumed that neither CBZ nor VPA induces other ADRs; that the probability of VPA-induced SJS/TEN is zero; and that the probability of CBZ-induced SJS/TEN in an HLA-B*15:02 negative population is zero [27]. The lifetime was defined as a length of 1 year. The primary outcomes were lifetime costs, quality-adjusted life-years (QALYs) gained, and incremental cost-effectiveness ratio (ICER) in Indonesian Rupiah (IDR) per QALY gained.
The decision tree for the generic model (Fig. 1) consisted of three potential strategies for patients requiring epilepsy treatment: first, administering CBZ to all patients without HLA-B*15:02 screening (reflecting the current practice in Indonesia) and second, testing patients for the HLA-B*15:02 allele and prescribing VPA for those that tested positive and CBZ for those that tested negative. The latter group was further divided into patients with a true negative and a false negative result. A false negative result of the HLA-B*15:02 screening test indicates that a patient does not have the allele while in fact, the patient possesses the allele. In the third strategy, patients did not undergo HLA-B*15:02 screening and were treated with VPA. There were two potential initial outcomes for all patients: developed or did not develop SJS/TEN.
Fig 1.
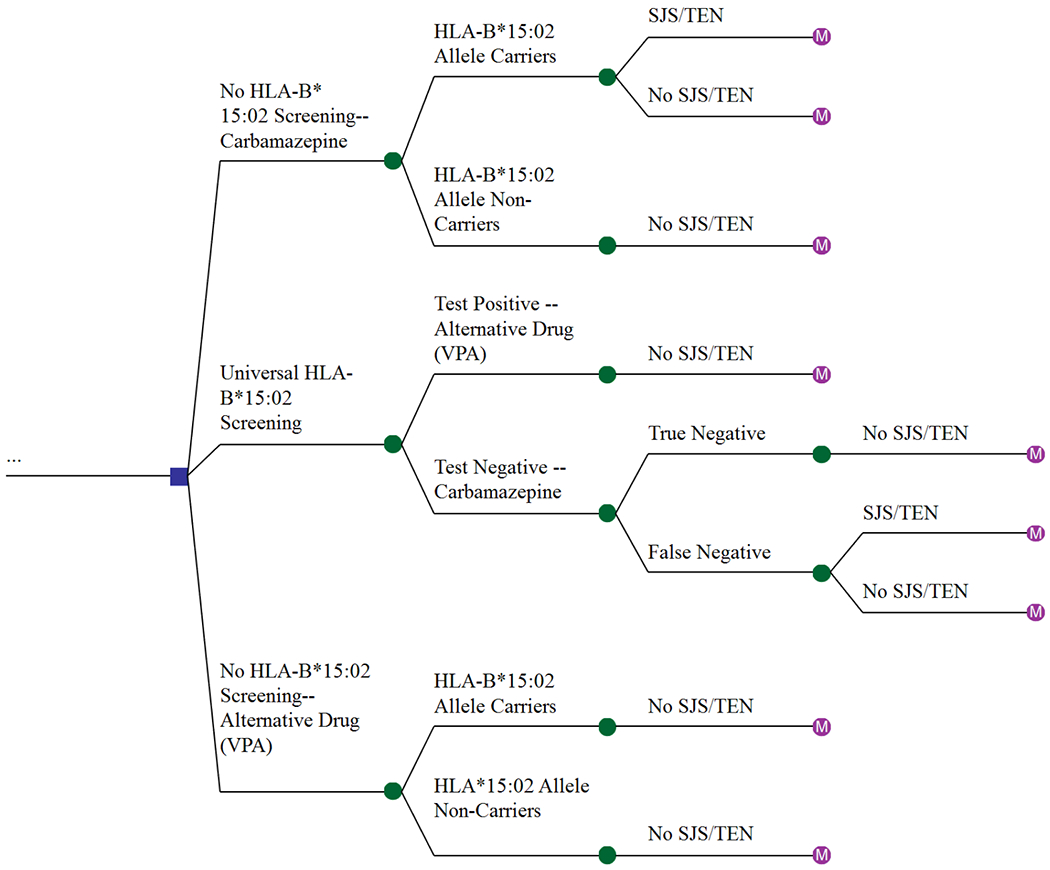
Decision tree model showing the three potential strategies for the treatment of patients with epilepsy.
A Markov model (Fig. 2) was used to generate the estimates of the lifetime effect of CBZ or VPA. There were three potential outcomes for patients that developed SJS/TEN and were hospitalized for treatment: (a) recovery; (b) recovery with long-term sequelae; or (c) death (Fig. 2a). The remaining patients did not develop SJS/TEN (Fig. 2b) and died from other causes. The parameters used in the analysis are presented in Table 1 and are discussed in detail below.
Fig 2. Markov model showing the three potential outcomes for patients treated with CBZ or VPA.
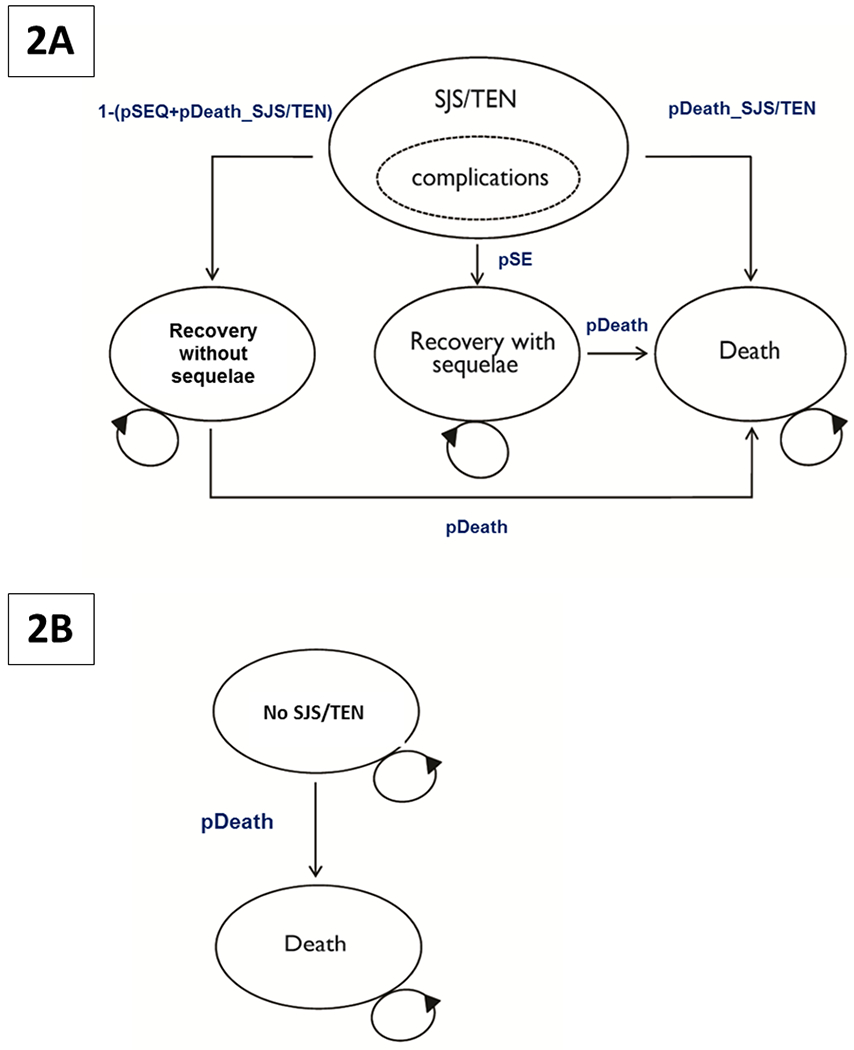
A. Patients that developed SJS/TEN following CBZ treatment
B. Patients that did not develop SJS/TEN following treatment with CBZ or VPA
Abbreviations: SJS: Steven–Johnson Syndrome; TEN: toxic epidermal necrolysis; CBZ: carbamazepine; VPA: valproic acid.
Table 1:
Input parameters used in the generic model analysis
| Variable | Distribution | Mean | Reference |
|---|---|---|---|
| Required input variable | |||
| pHLA (Prevalence of HLA-B*15:02 allele (carrier status) in population) | Beta | 0.208 | [24] |
| pSTpos (Probability of CBZ-induced SJS/TEN in HLA-B*15:02-positive patients) | Beta | 0.01139 | [26, 30,31] |
| Cost parameters (2015 Indonesian Rupiah (IDR) values) | |||
| cHLA-B (Cost of HLA-B*1502 screening test (includes all costs related to test)) | Gamma | 1,000,000 | Input by user |
| DC_SJS (Annual direct medical cost of CBZ-induced SJS/TEN) | Gamma | 5,026,302 | Input by user |
| DC_SEQ (Annual direct medical cost of sequelae (base-case value assumes dry eye syndrome)) | Gamma | 4,425,000 | Input by user |
| DC_Epi (Annual direct medical cost of epilepsy treatment with CBZ) | Gamma | 1,064,909 | Input by user |
| DC_Alt_Epi (Annual direct medical cost of epilepsy treatment with VPA) | Gamma | 2,457,384 | Input by user |
| Optional input variables | |||
| uSEQ (Utility score of patients who experience SJS/TEN sequelae (assumes dry eye syndrome)) | Beta | 0.68 | [27] |
| uEpi (Utility score of patients with epilepsy) | Beta | 0.85 | [27] |
| Epi_Trt_duration (Treatment duration of epilepsy) | Uniform | 30 | [27] |
| cDC (Discount Rate for costs) | 0.3 | [27] | |
| cDO (Discount Rate for outcomes) | 0.3 | [27] | |
Model parameters
Table 1 shows the input parameters requested in the generic model. A complete list of parameters, including default parameter values, are documented in the generic model presented in supplementary materials PHG500725sm_1.docx, Appendix A [27].
Predictive value of HLA-B*15:02 screening
The prevalence of HLA-B*15:02 carriers in the study population was 20.8% [24]. This was estimated from 237 unrelated individuals of Javanese or Sundanese–Javanese ethnicity from the general population in the western part of Java Island, Indonesia. Subjects were interviewed to derive their ethnics background, dating back three generations. The probability of CBZ-induced SJS/TEN in HLA-B*15:02-positive patients in Indonesia was assumed to be 1.1% [26, 30, 31].
Costs and utilities
We calculated the direct medical costs incurred by patients for the treatment of CBZ-induced SJS/TEN based on information provided by experts and the Indonesia Case Base Groups (INA-CBGs) system, a prospective payment system formulated by the Social Security Institution of Health that sets the diagnosis and procedure grouping, without considering its type and the amount of health service provided. INA-CBGs data are representative of all Indonesians. The price per unit of the antiepileptic drug in 2015 was obtained from the INA-CBGs data. All costs are reported in IDR in 2015.
Cost-effectiveness analysis
Cost effectiveness was determined as an incremental cost per QALY gained for HLA-B*15:02 screening versus no HLA-B*15:02 screening before CBZ administration. The total costs and QALYs associated with each treatment strategy were calculated over the span of a lifetime.
Sensitivity analysis
A probabilistic sensitivity analysis was carried out to estimate the impact of the uncertainty using probability distributions relative to base-case values for some parameters to determine the ICER. A cost-effectiveness ceiling threshold of 150 000 000 IDR per QALY gained was used.
Results
Base-case cost-effectiveness analysis
Table 2 shows the base-case cost effectiveness of HLA-B*15:02 screening, presented in IDR and QALYs. Compared with the current practice of “no screening” and the prescription of CBZ, HLA-B*15:02 screening and the prescription of VPA to patients tested positive for the allele resulted in an improvement in QALYs by 0.011 at a marginal increase in cost of IDR 6 951 845. Using these estimates, the ICER was identified as IDR 656 444 671 per QALY gained. Further, the prescription of VPA for all patients who were not screened was dominated by the testing arm as it yielded the same QALYs as the screening strategy but at a higher cost of IDR 49 610 785.
Table 2:
Cost-effectiveness of three strategies for patients with epilepsy treated by CBZ in Indonesia (base-case)*
| Strategy | Cost (IDR) | Incremental cost (IDR) | QALYs | Incremental QALYs | ICER (IDR/QALY gained) |
|---|---|---|---|---|---|
| Current practice of no HLA-B*15:02 screening and CBZ treatment | 21,678,072 | 0 | 16.98 | 0 | 0 |
| HLA-B*15:02 screening, with positive and negative patients treated with VPA and CBZ, respectively | 28,629,918 | 6,951,845 | 16.99 | 0.011 | 656,444,671 |
| No HLA-B*15:02 screening and VPA treatment | 49,610,785 | 27,932,713 | 16.99 | 0.011 | 2,634,975,574 |
IDR, Indonesia Rupiah 2015 value; QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio.
Values are rounded to the nearest whole number.
Sensitivity analysis
For the sensitivity analysis, we calculated the probability that the three treatment strategies were cost effective at different ceiling ratios (Fig. 3). As a result, the Indonesian cost-effectiveness threshold (estimated as three times gross domestic product per capita = IDR 150 000 000) was derived. Compared with HLA-B*15:02 screening and VPA treatment, the current practice of treating patients with CBZ had the highest probability of being cost effective. In fact, a negative correlation was found for the probability of cost effectiveness for the current practice and the threshold value. As the cost-effectiveness probability of the screening strategy was positively correlated with the ceiling threshold, it was more likely to be cost effective than “no screening” and the prescription of either CBZ or VPA above a threshold value of IDR 500 000 000.
Fig 3.
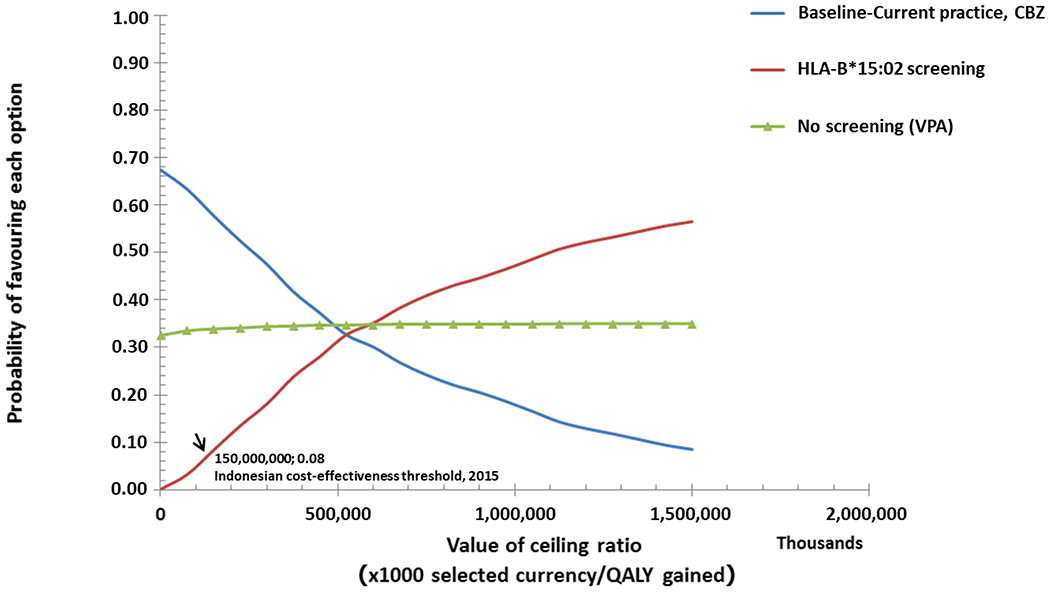
Cost-effectiveness acceptability curves: the likelihood that each strategy will be acceptable to a decision maker, ultimately influencing their willingness to pay.
The cost effectiveness of universal HLA- B*15:02 screening was sensitive to the probability of CBZ-induced SJS/TEN occurring in a patient that tested positive for HLA-B*15:02. Thus, a threshold analysis was carried out to determine the ICER for the range of CBZ-induced SJS/TEN prevalence in the Indonesian population. The probabilistic sensitivity analysis revealed that screening with genetic testing increased costs and QALY in all the iterations. Furthermore, an analysis of the number needed to screen revealed that 423 patients with epilepsy should be screened for the HLA-B*15:02 allele to prevent one case of SJS/TEN.
Discussion
Pharmacogenomics plays an increasingly important role in identifying genetic markers of drug response, including the risk for drug hypersensitivity. Pharmacogenomics seeks to enable healthcare systems to deliver optimal evidence-based and efficient therapy and avoid adverse events in patients. Policies for the adoption of a pharmacogenomic test should thus rely on rigorously validated evidence of a relationship between genotype and drug response (i.e., clinical validity). Additionally, a clear course of action should be derived for disease treatment based on genotype results [32].
Previous studies have consistently demonstrated that the development of CBZ-induced SJS/TEN is influenced by genetics [11]. Additionally, a strong association was identified between the HLA-B*15:02 allele and the risk for CBZ-induced SJS/TEN among Han Chinese [13–14] and in Southeast Asian countries, including Thailand [15], India [16], and Malaysia [17]. Similar results were also found in an Indonesian study [26]. It was recently reported that HLA-B*15:02 allele was not specific for carbamazepine-induced SJS/TEN only, but was also significantly associated with carbamazepine-induced MPE in the Thai population [12]. However, no association of HLA-B*15:02 with MPE was observed in several other studies [13, 33, 34, 35]. In contrast to SJS/TENS, data for the model parameters is lacking for MPE. Prior models have not included MPE, and the generic model used also did not include this condition. Therefore, MPE was not included in the analysis. Further study is needed to confirm the relationship between MPE and HLA-B*15:02 in other populations and to develop the parameters needed to perform an analysis using the model.
With the increasing cost of health care, it has become increasingly important to weigh the cost versus benefit of genotyping to guide therapeutic decisions. Cost utility and decision analyses are required not only to guide clinicians in health care decisions but also to facilitate the safe and efficient delivery of health care. As a result, costs and the allocation of resources can play a major role in clinician decision making. In this study, we used a previously validated generic model populated with values based on country-specific data (i.e. carrier prevalence, costs of care, and the probability of adverse events in HLA-B*15:02 positive patients) and using evidence-based assumptions that reflect the Indonesian perspective. We observed that screening for the CBZ-induced SJS/TEN susceptibility allele, HLA-B*15:02, or the prescribing of alternative drugs, such as VPA, for all patients decreased the number of CBZ-induced SJS/TEN cases, but with a cost effectiveness that exceeds the current societal thresholds. Comparing the two alternative strategies, HLA-B*15:02 screening was identified to be the preferred choice relative to alternative drug treatment as it was less expensive, despite the similar health outcomes achieved with both the strategies. The use of a generic model significantly reduces the time and expertise needed to perform the analysis. There are other alternatives to CBZ besides VPA for the Indonesian population; however, because the model employed in this study used VPA for the analysis, we opted to collect information regarding VPA alone. Moreover, because the model assumed equivalent efficacy and zero adverse events, more expensive medications than VPA would be less cost effective in clinical practice.
A recent study in Indonesia [26] and the Philippines [36] revealed that the B75 serotype plays an important role in these populations. The HLA-B75 serotype includes HLA-B*15:02, HLA-B*15:08, HLA-B*15:11, HLA-B*15:15, HLA-B*15:21, and HLA- B*15:31 [37]. The B-75 serotype allele has been reported in the populations of several Asian countries; these include HLA-B*15:11 in Japan [38]; HLA-B*15:08 in India [36]; and HLA-B*15:21 in Thailand [15], Indonesia [26], and the Philippines [36]. As the B75 serotype allele was observed in several populations, Yuliwulandari et al (2017) [26] suggested assessing the performance of a B75 serotype allele screening in Asian countries, in addition to HLA-B*15:02 screening. However, the currently available data are insufficiently robust to inform assumptions for the model; therefore, the analysis was restricted to HLA-B*15:02. As more data accumulate, a further study should be conducted to derive and evaluate a model for the cost effectiveness of B75 although, given the results of the sensitivity analysis, it is unlikely to result in the cost effectiveness of the intervention. In this study, a previously developed and validated cost-effectiveness model was used. Changing model parameters would have necessitated the reconstruction of the model. The current generic model could be repurposed for such a study, avoiding the effort to create a model de novo. However, it is important to recognize that in scenarios where evidence is accumulating rapidly, models cannot remain static, as was highlighted in a decision-analysis to inform a Lynch screening implementation program [39].
This study had some limitations. First, as SJS/TEN is a rare condition in the Indonesian population, very few patients with SJS/TEN were enrolled, which may affect the accuracy of the cost and the utility estimates. Second, as an active surveillance system was not implemented to measure the prevalence of CBZ-induced SJS/TEN in the Indonesian population, we assumed that it would be similar to that reported in other countries, especially in the case of data reported from the Thai population [19]. Therefore, the availability of prevalence data from Indonesia may improve the parameter estimates used in the model. The sensitivity analysis shows that the model is relatively insensitive to population prevalence, in so far as achieving a cost-effectiveness threshold is concerned. Other severe cutaneous adverse events, such as drug-induced hypersensitivity syndrome (DiHS or DRESS), can occur and could potentially affect the model. Based on informal communication with authors of previously published work, it appears that DiHS was implicitly included in prior models; therefore, it is not expected to affect the results. The model also assumes no adverse events in non-carriers of HLA-B *15:02 and in those treated with VPA. While the risk is likely not zero, published evidence is sufficient to indicate that the risk in these populations is extremely low, such that the use of zero in the assumption should not unduly bias the result. Any value greater than zero would shift the result to even less favorable cost-effectiveness, and therefore, the conclusion would not be altered. These limitations can be mitigated via a sensitivity analysis, which enables analysis over a wide range of cost and utility estimates.
Collectively, our findings suggest that from the Indonesian societal perspective, it is not cost effective to carry out HLA-B *15:02 screening for patients who would often be administered CBZ. However, these findings could demonstrate to policymakers, such as the Ministry of Health, the effect of implementing a pharmacogenomic-guided medical intervention in the Indonesian population. Such implementation could reduce the economic impact of this adverse drug reaction and would be similar to the genome-guided warfarin treatment in Croatia, which involves the reimbursement of pharmacogenomic testing costs [40]. Additionally, given the improvements in clinical outcomes with HLA-B*15:02 screening in Taiwan [18], Thailand [19], and Singapore [20] and the availability of more effective alternative drugs, implementing HLA-B*15:02 testing to predict the risk for CBZ-induced SJS/TEN may become an effective and desirable strategy in Indonesia even if cost-effectiveness is less than the predicted value.
Acknowledgments
This project was funded by a grant from the U.S. National Institutes of Health/National Human Genome Research Institute (no. U01HG007269) and Directorate of Higher Education, Ministry of Research, Technology and Higher Education of the Republic of Indonesia by World Class Professor Program (no 123.56/D2.3/KP/2018).
We thank the YARSI collaborative hospitals and participants for their contribution to this study. We also thank to Dr. Rizaldy T Pinzon, Dr. Syukrini Bahri, Dr. Isa M Noor and Ms. Ratih Puspita who supported in sample and data collection.
Footnotes
Conflicts of Interests
The authors declare no conflict of interest regarding the information presented in this manuscript.
Supplementary information is available at The Pharmacogenomics Journal’s website.
References
- [1].Kumar A, Sarkar S, Praharaj S, Akhtar SK, Diwakar M. Stevens-Johnson syndrome progressing to toxic epidermal necrolysis with haloperidol and carbamazepine combination. Ind Psychiatry J 2011; 20: 131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gerull R, Nelle M, Schaible T. Toxic epidermal necrolysis and Stevens–Johnson syndrome: a review. Crit Care Med 2011; 39: 1521–1532. [DOI] [PubMed] [Google Scholar]
- [3].Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med 1994; 331: 1272–1285. [DOI] [PubMed] [Google Scholar]
- [4].Roujeau J, Allanore L, Liss Y, Mockenhaupt M. Severe cutaneous adverse reactions to drugs (SCAR): definitions, diagnostic criteria, genetic predisposition. Dermatol Sin 2009; 27: 203–209. [Google Scholar]
- [5].Fernando SL. Severe cutaneous adverse reactions. In: Khopkar U (ed). Skin Biopsy - Diagnosis and Treatment. IntechCopen: London, 2013, pp 45–103. [Google Scholar]
- [6].Yip LW, Thong BY, Lim J, Tan AW, Wong HB, Handa S et al. Ocular manifestations and complications of Stevens–Johnson syndrome and toxic epidermal necrolysis: an Asian series. Allergy 2007; 62: 527–531. [DOI] [PubMed] [Google Scholar]
- [7].Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis 2010; 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rzany B, Correia O, Kelly JP, Naldi L, Auquier A, Stern R. Risk of Stevens–Johnson syndrome and toxic epidermal necrolysis during first weeks of antiepileptic therapy: A case-control study. Study Group of the International Case Control Study on Severe Cutaneous Adverse Reactions. Lancet 1999; 353: 2190–2194. [DOI] [PubMed] [Google Scholar]
- [9].Ferrell PBJ, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 2008; 9: 1543–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tennis P, Stern RS. Risk of serious cutaneous disorders after initiation of use of phenytoin, carbamazepine, or sodium valproate: a record linkage study. Neurology 1997; 49: 542–546. [DOI] [PubMed] [Google Scholar]
- [11].Lonjou C, Thomas L, Borot N, Ledger N, de Toma C, LeLouet H et al. A marker for Stevens–Johnson syndrome: ethnicity matters. Pharmacogenomics J 2006; 6: 265–268. [DOI] [PubMed] [Google Scholar]
- [12].Sukasem C, Chaichan C, Nakkrut T, Satapornpong P, Jaruthamsophon K, Jantararoungtong T et al. Association between HLA-B alleles and carbamazepine-induced maculopapular exanthema and severe cutaneous reactions in Thai patients. J Immunol Res 2018; 2018: 2780272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006; 16: 297–306. [DOI] [PubMed] [Google Scholar]
- [14].Zhang Y, Wang J, Zhao LM, Peng W, Shen GQ, Xue L et al. Strong association between HLA-B*1502 and carbamazepine-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in mainland Han Chinese patients. Eur J Clin Pharmacol 2011; 67: 885–887. [DOI] [PubMed] [Google Scholar]
- [15].Tassaneeyakul W, Tiamkao S, Jantararoungtong T, Chen P, Lin SY, Chen WH et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia 2010; 51: 926–930. [DOI] [PubMed] [Google Scholar]
- [16].Mehta TY, Prajapati LM, Mittal B, Joshi CG, Sheth JJ, Patel DB et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol 2009; 75: 579–582. [DOI] [PubMed] [Google Scholar]
- [17].Chang CC, Too CL, Murad S, Hussein SH. Association of HLA-B * 1502 allele with carbamazepine- induced toxic epidermal necrolysis and Stevens-Johnson syndrome in the multi-ethnic Malaysian population. Int J Dermatol 2011; 50: 221–224. [DOI] [PubMed] [Google Scholar]
- [18].Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med 2011; 364: 1126–1133. [DOI] [PubMed] [Google Scholar]
- [19].Rattanavipapong W, Koopitakkajorn T, Praditsitthikorn N, Mahasirimongkol S, Teerawattananon Y. Economic evaluation of HLA-B*15:02 screening for carbamazepine- induced severe adverse drug reactions in Thailand. Epilepsia 2013; 54: 1628–1638. [DOI] [PubMed] [Google Scholar]
- [20].Dong D, Sung C, Finkelstein EA. Cost-effectiveness of HLA-B*1502 genotyping in adult patients with newly diagnosed epilepsy in Singapore. Neurology 2012; 79: 1259–1267. [DOI] [PubMed] [Google Scholar]
- [21].US Food and Drug Administration, 2007. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketdrugsafetyinformationforPatientsandProviders/ucm124718.htm. Accessed February 27, 2017.
- [22].UK Medicines and Healthcare Products Regulatory Agency, 2012. Available at: http://www.mhra.gov.uk/Safetyinformation/DrugSafety Update/CON084888. Accessed February 27, 2017.
- [23].European Medicines Agency, 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/07/WC500130391.pdf. Accessed February 27, 2017.
- [24].Yuliwulandari R, Kashiwase K, Nakajima H, Uddin J, Susmiarsih TP, Sofro ASM et al. Polymorphisms of HLA genes in Western Javanese (Indonesia): close affinities to Southeast Asian populations. Tissue Antigens 2009; 73: 46–53. [DOI] [PubMed] [Google Scholar]
- [25].Yuliwulandari R, Sachrowardi Q, Nakajima H, Kashiwase K, Hirayasu K, Mabuchi A et al. Association of HLA-A, -B, and -DRB1 with pulmonary tuberculosis in western Javanese Indonesia. Hum Immunol 2010; 71: 697–701. [DOI] [PubMed] [Google Scholar]
- [26].Yuliwulandari R, Kristin E, Prayuni K, Sachrowardi Q, Suyatna FD, Menaldi SL et al. Association of the HLA-B alleles with carbamazepine-induced Stevens – Johnson syndrome / toxic epidermal necrolysis in the Javanese and Sundanese population of Indonesia : the important role of the HLA-B75 serotype. Pharmacogenomics 2017; 18: 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Snyder SR, Hao J, Cavallari LH, Geng Z, Elsey A, Johnson JA et al. Generic cost-effectiveness models: A proof of concept of a tool for informed decision-making for public health precision medicine. Public Health Genomics 2018; 21: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gaitatzis A, Johnson AL, Chadwick DW, Shorvon SD, Sander JW. Life expectancy in people with newly diagnosed epilepsy. Brain 2004; 127: 2427–2432. [DOI] [PubMed] [Google Scholar]
- [29].Marson AG, Williamson PP, Hutton JL, Clough HE, Chadwick DW. Carbamazepine versus valproate monotherapy for epilepsy. Cochrane Database Syst Rev 2000; 3: CD001030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen Z, Liew D, Kwan P. Real-world efficiency of pharmacogenetic screening for carbamazepine-induced severe cutaneous adverse reactions. PLoS One 2014; 9: e96990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cheung YK, Cheng SH, Chan EJ, Lo SV, Ng MHL, Kwan P. HLA-B alleles associated with severe cutaneous reactions to antiepileptic drugs in Han Chinese. Epilepsia 2013; 54: 1307–1314. [DOI] [PubMed] [Google Scholar]
- [32].Snyder SR, Mitropoulou C, Patrinos GP, Williams MS. Economic evaluation of pharmacogenomics: A value-based approach to pragmatic decision making in the face of complexity. Public Health Genomics 2014; 17: 256–264. [DOI] [PubMed] [Google Scholar]
- [33].Wu XT, Hu FY, An DM, Yan B, Jiang X, Kwan P et al. Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsi Behav. 2010; 19: 405–408. [DOI] [PubMed] [Google Scholar]
- [34].Wang Q, Zhou J-q, Zhou L-m, Chen Z-y, Fang Z-y, Chen S-d et al. Association between HLA-B*1502 allele and carbamazepine-induced severe cutaneous adverse reactions in Han people of southern China mainland. Seizure. 2011; 20: 446–448. [DOI] [PubMed] [Google Scholar]
- [35].Locharernkul C, Loplumlert J, Limotai C, Korkij W, Desudchit T, Tongkobpetch S et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008; 49: 2087–2091. [DOI] [PubMed] [Google Scholar]
- [36].Capule F, Tragulpiankit P, Mahasirimongkol S, Jittikoon J, Wichukchinda N, Alentajan-Aleta LT et al. Association of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis with the HLA-B75 serotype or HLA-B*15:21 allele in Filipino patients. Pharmacogenomics J 2020; e-pub ahead of print 3 January 2020; doi: 10.1038/s41397-019-0143-8 [DOI] [PubMed] [Google Scholar]
- [37].Marsh SGE, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA et al. Nomenclature for factors of the HLA system, 2010. Tissue Antigens 2010; 75: 291–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M, Kurose K et al. HLA-B*1511 is a risk factor for carbamazepine-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Epilepsia 2010; 51: 2461–2465. [DOI] [PubMed] [Google Scholar]
- [39].Gudgeon JM, Williams JL, Burt RW, Samowitz WS, Snow GL, Williams MS. Lynch syndrome screening implementation: Business analysis by a healthcare system. Am J Manag Care 2011; 17: e288–300. [PubMed] [Google Scholar]
- [40].Mitropoulou C, Fragoulakis V, Bozina N, Vozikis A, Supe S, Bozina T et al. Economic evaluation of pharmacogenomic-guided warfarin treatment for elderly Croatian atrial fibrillation patients with ischemic stroke. Pharmacogenomics 2015; 16: 137–148. [DOI] [PubMed] [Google Scholar]


