Abstract
Background
Clinical practice guidelines for schizophrenia and major depressive disorder have been published. However, these have not had sufficient penetration in clinical settings. We developed the Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE) project as a dissemination and education programme for psychiatrists.
Aims
The aim of this study is to assess the effectiveness of the EGUIDE project on the subjective clinical behaviour of psychiatrists in accordance with clinical practice guidelines before and 1 and 2 years after participation in the programmes.
Method
A total of 607 psychiatrists participated in this study during October 2016 and March 2019. They attended both 1-day educational programmes based on the clinical practice guidelines for schizophrenia and major depressive disorder, and answered web questionnaires about their clinical behaviours before and 1 and 2 years after attending the programmes. We evaluated the changes in clinical behaviours in accordance with the clinical practice guidelines between before and 2 years after the programme.
Results
All of the scores for clinical behaviours in accordance with clinical practice guidelines were significantly improved after 1 and 2 years compared with before attending the programmes. There were no significant changes in any of the scores between 1 and 2 years after attending.
Conclusions
All clinical behaviours in accordance with clinical practice guidelines improved after attending the EGUIDE programme, and were maintained for at least 2 years. The EGUIDE project could contribute to improved guideline-based clinical behaviour among psychiatrists.
Keywords: Clinical practice guidelines, educational programme, schizophrenia, major depressive disorder, EGUIDE project
Clinical practice guidlines for psychiatric disorders
Clinical practice guidelines provide recommendations for optimising patient treatment, and are based on a systematic review of evidence and an assessment of the advantages and disadvantages of alternative care options and standard tools for clinical decision-making. Various guidelines for the clinical practice of psychiatric disorders have been published,1–8 and in many countries, psychiatrists commonly make clinical decisions based on clinical practice guidelines. In Japan, clinical practice guidelines for psychiatric disorders were only published 9 years ago, and Japanese psychiatrists usually make clinical decisions based on their own experience or knowledge and not based on clinical practice guidelines. As a result, pharmacotherapy for psychiatric disorders in Japan has been different from that recommended in clinical practice guidelines in other countries.9–14 To change this situation, the Japanese Society of Neuropsychopharmacology published the ‘Guideline for Pharmacological Therapy of Schizophrenia’ (clinical practice guideline for schizophrenia) in 2015,15 and the Japanese Society of Mood Disorders published the ‘Treatment Guideline: Major Depressive Disorder’ in 2012,16 which was revised to the ‘Treatment Guideline II: Major Depressive Disorder’ (clinical practice guideline for major depressive disorder) in 2016.17
Dissemination of clinical practice guidelines in Japan
Although the clinical practice guidelines of schizophrenia and major depressive disorder have been published, pharmacotherapy for these disorders has not undergone sufficient transformation in Japan.9,10 For example, our project previously showed that for patients with schizophrenia, 57.1% were prescribed antipsychotic monotherapy, 15.5% were prescribed antipsychotic monotherapy without any other psychotropics and 31.7% received no prescription of anxiolytics or hypnotics. In addition, at 84 institutions before doctors participated in the educational programmes, 58.6% of patients with depression undergoing in-patient treatment were prescribed antidepressant monotherapy and 25.1% received no prescription of anxiolytics or hypnotics.18,19 To improve these statistics, dissemination of and education on the guidelines for Japanese psychiatrists was needed. Thus, we launched the Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE) project in 2016. The purpose of the EGUIDE project is to disseminate the guidelines by conducting educational programmes on the clinical practice guidelines for schizophrenia and major depressive disorder for psychiatrists, and to standardise medical practices in accordance with the clinical practice guidelines. We have already reported on the educational method and the effectiveness of the EGUIDE project in improving knowledge of the clinical practice guidelines in psychiatrists.20 Additionally, the effectiveness of the educational programmes was investigated by evaluating psychiatrists’ clinical behaviours in accordance with the clinical practice guidelines.
The aim of this research is to assess the efficacy of the EGUIDE project in changing clinical behaviours in accordance with the clinical practice guidelines in psychiatrists before and 1 and 2 years after they attend the programmes.
Method
Design and participants
The EGUIDE project recruited psychiatrists from >100 medical institutions in Japan, who volunteered to participate in study during October 2016 and March 2019. All participants signed informed written consent forms. This study was approved by the ethics committee at the National Center of Neurology and Psychiatry (approval number A2017-105) and each of the participating universities, hospitals and clinics. The procedures were carried out in accordance with the Helsinki Declaration. The study protocol was registered in the University Hospital Medical Information Network registry (identifier UMIN000022645). The participants attended a 1-day educational programme on schizophrenia and depression based on the clinical practice guideline for schizophrenia (published by the Japanese Society of Neuropsychopharmacology) and the clinical practice guideline for major depressive disorder (published by the Japanese Society of Mood Disorders). We conducted lectures on the guidelines and discussions using two clinical cases to describe the guidelines and how to apply them in practice. The participants received emails including URLs for self-administered web questionnaires. Using the questionnaires, they retrospectively rated their clinical behaviours in accordance with the clinical practice guidelines in the 6 months before the programme they attended, and they again rated themselves each year thereafter, for 2 years. The effectiveness of each programme was assessed based on changes in the scores of the self-administered questionnaires before and after the programmes.
Assessment measures
To assess participants’ clinical behaviours with respect to general use of the clinical guidelines, a self-administered questionnaire was created consisting of six items rated on a five-point Likert scale (Supplementary Table 1 available at https://doi.org/10.1192/bjo.2022.44). Participants assessed their clinical behaviours in accordance with the clinical practice guidelines for schizophrenia and major depressive disorder by creating self-administered questionnaires, each consisting of 14 items rated on a five-point Likert scale (Supplementary Tables 2 and 3). The evaluations of clinical behaviours were divided into the following six stages, according to the degree of achievement: not achieved (0–20% achieved), slightly achieved (21–40% achieved), approximately half achieved (41–60% achieved), moderately achieved (61–80% achieved), almost achieved (81–100% achieved) and no opportunity.
Statistical analysis
As the representative value for each of the five achievement levels, an intermediate value was used: 10 for ‘not achieved’, 30 for ‘slightly achieved’, 50 for ‘about half achieved’, 70 for ‘moderately achieved’ and 90 for ‘almost achieved’. Therefore, there were five scores, and the scores ranged from 10 to 90. We excluded ‘no opportunity’ from the analysis. We used the Kolmogorov–Smirnov test to evaluate data normality in the scores for the clinical behaviours. We assessed the homoscedasticity of variance with Levine's test. Data were analysed by Kruskal–Wallis test with statistical significance, because the Kolmogorov–Smirnov test did not show normal distribution or homoscedasticity. To compare the changes in the scores for the clinical behaviours between before and 1 and 2 years after attending the programme, the Bonferroni correction was applied for multiple testing when the Kruskal–Wallis test was statistically significant. A significance threshold of 0.05 was applied for multiple testing. The data were initially imputed in Microsoft Excel for Mac (Microsoft Corp, 2010), and then analysed with SPSS version 24.0 for Mac (IBM SPSS Inc, 2016).
Results
Demographics of the participants
A total of 607 psychiatrists from 134 medical institutions participated in educational programmes on the clinical guidelines for treatment of schizophrenia and depression during the 3 years of the EGUIDE project recruitment (October 2016 to March 2019). All of the participants attended a 1-day educational programme on schizophrenia and depression based on the clinical practice guidelines. Of these, 594 participants joined the email-based survey, 425 responded to the web questionnaires at baseline, 270 responded at 1 year and 140 responded at 2 years. Some participants have dropped out during each follow-up period. The numbers of participants who answered web questionnaires, excluding those who dropped out and those who reported ‘no opportunity’ for general use of the clinical guidelines at baseline and after the programmes, are shown in Supplementary Tables 4–6. They were aged 26–70 years, with an average age of 34.4 ± 7.6 years; 369 (72.5%) were male. Years of professional experience ranged from 1 to 35 years, with an average of 5.7 ± 6.7 years. A total of 463 belonged to university hospitals, 20 belonged to psychiatric hospitals, 25 belonged to general hospitals and one belonged to a clinic when attending the programme.
Changes in the scores for clinical behaviours in accordance with the pre- and post-programme
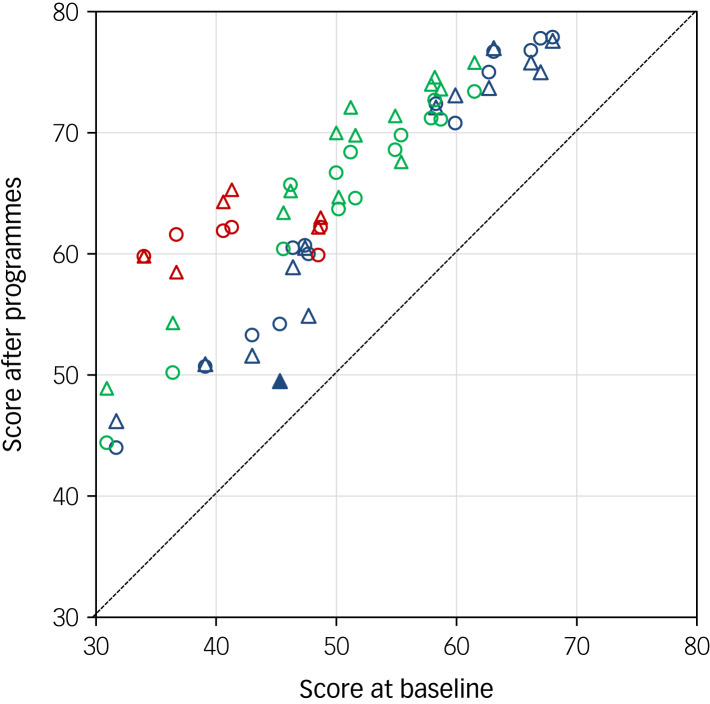
A comparison of all clinical behaviour scores in accordance with the guidelines at baseline and after the clinical practice guideline programmes is shown in Fig. 1. All of the mean scores had increased significantly at 1 year after attending the programme. Furthermore, the clinical behaviours of participants were still significantly higher at 2 years compared with the baseline, with the exception of ‘Choosing treatment with modified electroconvulsive therapy for patients with treatment-resistant schizophrenia’.
Fig. 1.
Comparison of clinical behaviour scores for the use of clinical guidelines at baseline and after the ‘Guideline for Pharmacological Therapy of Schizophrenia’ and ‘Treatment Guideline II: Major Depressive Disorder’ programmes. The x- and y-axes indicate the score for each question at baseline and the score for each question after programme participation, respectively. Details of each score are shown in Tables 1–3. Blue circles indicate clinical behaviour scores that increased significantly 1 year after attending the ‘Guideline for Pharmacological Therapy of Schizophrenia’ programme, compared with before the course (S1–S14). Blue triangles indicate clinical behaviour scores that increased significantly 2 years after attending the ‘Guideline for Pharmacological Therapy of Schizophrenia’ programme, compared with before the course (S1–S7, S9–S14). Green circles indicate clinical behaviour scores that increased significantly 1 year after attending the ‘Treatment Guideline II: Major Depressive Disorder’ programme, compared with before the course (D1–D14). Green triangles indicate clinical behaviour scores that increased significantly 2 years after attending the ‘Treatment Guideline II: Major Depressive Disorder’ programme, compared with before the course (D1–D14). Red circles indicate clinical behaviour scores that increased significantly 1 year after attending the ‘Guideline for Pharmacological Therapy of Schizophrenia’ and ‘Treatment Guideline II: Major Depressive Disorder’ programmes, compared with before the course (G1–G6). Red triangles indicate clinical behaviour scores that increased significantly 2 years after attending the ‘Guideline for Pharmacological Therapy of Schizophrenia’ and ‘Treatment Guideline II: Major Depressive Disorder’ programmes, compared with before the course (G1–G6). Solid blue triangles indicate clinical behaviour scores that were not significantly elevated 2 years after attending the ‘Guidelines for the Pharmacotherapy of Schizophrenia’ programme compared with before the programme (S8).
The mean and statistical results of the clinical behaviours in accordance with the general use of the guidelines for before and 2 years after programme participation are shown in Table 1, and the data of multiple comparisons are shown in Supplementary Table 7. In all subclasses of clinical behaviours consistent with the general use of the clinical guidelines, the mean scores increased significantly after attending the programme. Furthermore, the clinical behaviours of participants were maintained for 2 years, as the mean score was not significantly different between 1 and 2 years after the programme. One year after attending the programme, a large and significant change was observed in ‘Using treatment guidelines when deciding on the treatment policy in discussions with patients and family’ (Z = 12.75, P = 3.2 × 10−37, r = 0.50). Additionally, moderate and significant changes were seen in ‘Trying to treat patients in accordance with guidelines if their previous treatments are not in accordance with guidelines’ (Z = 12.81, P = 1.4 × 10−37, r = 0.50), ‘Recommending pharmacotherapy for schizophrenia to fellow doctors in accordance with the guideline’ (Z = 9.94, P = 2.9 × 10−23, r = 0.39) and ‘Recommending treatment for depression to fellow doctors in accordance with the guideline’ (Z = 9.83, P = 8.7 × 10−23, r = 0.39).
Table 1.
Comparison of clinical behaviour scores for the general use of clinical guidelines at baseline and after the programmes
| Baseline | One year later | Two years later | Statisticsa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | H | P-value | ||
| G1 | Using treatment guidelines when deciding on the treatment policy in discussions with patients and family | 34.0 | ±22.2 | 59.8b | ±21.3 | 59.8c | ±21.0 | 204.97 | 3.1 × 10−45 |
| G2 | Trying to treat patients in accordance with the guidelines if their previous treatments are not in accordance with guidelines | 36.7 | ±21.8 | 61.6b | ±20.0 | 58.5c | ±21.1 | 192.27 | 1.8 × 10−42 |
| G3 | Pharmacotherapy for schizophrenia in your hospital/clinic is in accordance with the guideline | 48.5 | ±22.4 | 59.9b | ±21.7 | 62.2c | ±21.4 | 62.40 | 2.8 × 10−14 |
| G4 | Recommending pharmacotherapy for schizophrenia to fellow doctors in accordance with the guideline | 40.6 | ±24.5 | 61.9b | ±23.2 | 64.3c | ±21.4 | 138.20 | 9.8 × 10−31 |
| G5 | Treatment for depression in your hospital/clinic is in accordance with the guideline | 48.7 | ±23.4 | 62.2b | ±20.0 | 63.0c | ±21.5 | 73.49 | 1.1 × 10−16 |
| G6 | Recommending treatment for depression to fellow doctors in accordance with the guideline | 41.3 | ±24.3 | 62.2b | ±23.5 | 65.3c | ±20.8 | 138.38 | 8.9 × 10−31 |
The complete questions are noted in Supplementary Table 1. An intermediate value was used as the representative value for each of the five achievement levels: 0–20, 21–40, 41–60, 61–80 and 81–100. The scores ranged from 10 to 90.
The Kruskal–Wallis test was used for the statistical analysis as the Kolmogorov–Smirnov test did not indicate normal distribution of clinical behaviour scores at baseline or 1 or 2 years after the programme. The significance level was set at <0.05.
The mean scores of clinical behaviours increased significantly 1 year after attending the programme compared with baseline.
The mean scores of clinical behaviours increased significantly 2 years after attending the programme compared with baseline.
Table 2 shows the changes in clinical behaviour scores before and after attending the educational programme for the clinical practice guideline for schizophrenia. Supplementary Table 8 shows the multiple comparison of clinical behaviour scores at baseline and 1 and 2 years after the programmes. The mean scores of all of the subclasses increased significantly after the programme. The clinical behaviours of participants, except ‘Choosing treatment with modified electroconvulsive therapy for patients with treatment-resistant schizophrenia’, were maintained for 2 years. A moderate and significant change was observed in ‘Choosing antipsychotic monotherapy but not a combination of antipsychotics’ (Z = 8.04, P = 9.0 × 10−16, r = 0.31) 1 year after attending the programme.
Table 2.
Comparison of clinical behaviour scores at baseline and after the ‘Guideline for Pharmacological Therapy of Schizophrenia’ programme
| Baseline | One year later | Two years later | Statisticsa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | H | P-value | ||
| S1 | Choosing antipsychotic monotherapy but not a combination of antipsychotics | 46.4 | ±22.9 | 60.5b | ±19.4 | 58.9c | ±20.3 | 75.74 | 3.6 × 10−17 |
| S2 | Refraining from using psychotropic drugs other than antipsychotics | 31.7 | ±20.9 | 44.0b | ±23.8 | 46.2c | ±21.4 | 68.10 | 1.6 × 10−15 |
| S3 | Providing continuous guidance on the daily administration of antipsychotics | 66.2 | ±23.7 | 76.8b | ±17.4 | 75.8c | ±18.9 | 40.39 | 1.7 × 10−9 |
| S4 | Ensuring the appropriate dose and timing of pharmacological treatment and the extent of medication adherence in treatment for recurrence or relapse of schizophrenia | 62.7 | ±23.5 | 75.0b | ±18.1 | 73.7c | ±20.3 | 56.86 | 4.5 × 10−13 |
| S5 | Choosing medication considering the response to medications in the past in treatment for recurrence or relapse of schizophrenia | 63.1 | ±23.3 | 76.7b | ±17.1 | 77.0c | ±17.4 | 78.02 | 1.1 × 10−17 |
| S6 | Defining those with treatment-resistant schizophrenia as patients with schizophrenia who, despite taking at least two antipsychotics with adequate doses and timing, have persistent symptoms | 58.3 | ±26.9 | 72.4b | ±20.7 | 72.1c | ±21.4 | 54.31 | 1.6 × 10−12 |
| S7 | Choosing treatment with clozapine for patients with treatment-resistant schizophrenia | 43.0 | ±27.5 | 53.3b | ±27.9 | 51.6c | ±26.4 | 15.75 | 3.8 × 10−4 |
| S8 | Choosing treatment with modified electroconvulsive therapy for patients with treatment-resistant schizophrenia | 45.3 | ±25.9 | 54.2b | ±26.1 | 49.5 | ±27.5 | 12.35 | 2.1 × 10−3 |
| S9 | Continuing administration of antipsychotics for at least 1 year for first episode psychosis for relapse prevention | 67.0 | ±23.8 | 77.8b | ±17.6 | 75.0c | ±19.5 | 36.14 | 1.4 × 10−8 |
| S10 | Choosing long-acting injection antipsychotics for patients whose relapse is due to low medication adherence | 47.7 | ±23.8 | 60.0b | ±24.1 | 54.9c | ±25.6 | 36.13 | 1.4 × 10−8 |
| S11 | For recovery from cognitive impairment in schizophrenia, refraining from using anticholinergics | 47.4 | ±24.5 | 60.7b | ±22.9 | 60.5c | ±21.2 | 57.31 | 3.6 × 10−13 |
| S12 | For recovering from cognitive impairment in schizophrenia, refraining from using benzodiazepines | 39.1 | ±23.3 | 50.7b | ±24.3 | 50.9c | ±24.1 | 46.84 | 6.7 × 10−11 |
| S13 | Choosing second-generation antipsychotics to decrease the possibility of extrapyramidal adverse effects | 68.0 | ±21.9 | 77.9b | ±16.5 | 77.6c | ±16.9 | 46.33 | 8.7 × 10−11 |
| S14 | Choosing oral medication for the management of psychomotor agitation if possible | 59.9 | ±23.1 | 70.8b | ±20.0 | 73.1c | ±19.4 | 55.92 | 7.2 × 10−13 |
The complete questions are noted in Supplementary Table 2. An intermediate value was used as the representative value for each of the five achievement levels: 0–20, 21–40, 41–60, 61–80 and 81–100. The scores ranged from 10 to 90.
The Kruskal–Wallis test was used for the statistical analysis as the Kolmogorov–Smirnov test did not indicate normal distribution of clinical behaviour scores at baseline or 1 or 2 years after the programme. The significance level was set at <0.05.
The mean scores of clinical behaviours increased significantly 1 year after attending the programme compared with baseline.
The mean scores of clinical behaviours increased significantly 2 years after attending the programme compared with baseline.
The following are notable points about several general clinical behaviours whose results differed from those previously reported in other countries. The mean score of ‘Choosing antipsychotic monotherapy but not a combination of antipsychotics’ increased after the programme, and a significant change was observed (H = 75.74, P = 3.5 × 10−17): the score was approximately 60 after the programme (46.4 at baseline, 60.5 at 1 year after the programme and 58.9 at 2 years after the programme). In addition, the mean score for ‘Refraining from using psychotropic drugs other than antipsychotics’ also increased significantly after the programme (H = 68.10, P = 1.6 × 10−15), but the score was still <50 after the programme (31.7 at baseline, 44.1 at 1 year after the programme and 46.2 at 2 years after the programme). Although the score increased throughout the programme for ‘For recovery from cognitive impairment in schizophrenia, refraining from using benzodiazepines’ (H = 46.84, P = 6.7 × 10−11), the score at 2 years after the programme was 50.9.
Table 3 shows the mean values and the statistical results of the clinical behaviours before and after the programme for the clinical practice guideline for major depressive disorder. The multiple comparisons of the clinical behaviour scores at baseline and 1 and 2 years after the programme are shown in Supplementary Table 9. In all subclasses, the mean scores increased significantly after attending the programme, and the clinical behaviours were maintained for 2 years. One year after attending programme, moderate changes were observed in ‘Diagnosing depression, including the classification of the severity, based on the DSM-5’ (Z = 9.66, P = 4.6 × 10−22, r = 0.37), ‘In diagnosis, assessing information from any person other than the patient and functional impairments before the onset’ (Z = 8.27, P = 1.4 × 10−16, r = 0.32), ‘When the treatment does not work well, reassessing the diagnosis, pharmacotherapy and environmental management’ (Z = 8.65, P = 5.2 × 10−18, r = 0.33), ‘For mild depression, adding cognitive–behavioural therapy and new-generation antidepressants to fundamental intervention if necessary’ (Z = 9.23, P = 2.7 × 10−10, r = 0.36) and ‘For sleep disorders, providing sleep hygiene instructions before pharmacotherapy’ (Z = 8.72, P = 2.7 × 10−18, r = 0.34).
Table 3.
Comparison of clinical behaviour scores at baseline and after the ‘Treatment Guideline II: Major Depressive Disorder’ programme
| Baseline | One year later | Two years later | Statisticsa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | H | P-value | ||
| D1 | Diagnosing depression, including the classification of the severity, based on the DSM-5 | 46.2 | ±25.3 | 65.7b | ±22.8 | 65.2c | ±21.1 | 116.36 | 5.4 × 10−26 |
| D2 | In diagnosis, assessing information from any person other than the patient and functional impairments before the onset | 55.4 | ±22.6 | 69.8b | ±18.8 | 67.6c | ±20.2 | 79.14 | 6.5 × 10−18 |
| D3 | Focusing on empathic or supportive care and performing fundamental interventions such as psychological education first | 61.5 | ±22.6 | 73.4b | ±18.8 | 75.8c | ±18.1 | 73.62 | 1.0 × 10−16 |
| D4 | When the treatment does not work well, reassessing the diagnosis, pharmacotherapy and environmental management | 58.2 | ±22.4 | 72.7b | ±18.5 | 74.6c | ±16.9 | 101.99 | 7.1 × 10−23 |
| D5 | For mild depression, adding cognitive–behavioural therapy and new-generation antidepressants to fundamental intervention if necessary | 50.0 | ±22.6 | 66.7b | ±20.1 | 70.0c | ±21.0 | 120.00 | 8.7 × 10−27 |
| D6 | For moderate/severe depression, using antidepressant monotherapy with adequate doses and timing and considering modified electroconvulsive therapy if necessary | 58.7 | ±23.5 | 71.1b | ±20.3 | 73.6c | ±19.8 | 69.70 | 7.3 × 10−16 |
| D7 | For moderate/severe depression, if antidepressants are effective but not enough, treating with lithium or antipsychotics or T3/T4 as augmentation therapy | 51.6 | ±25.1 | 64.6b | ±24.2 | 69.8c | ±22.0 | 67.84 | 1.9 × 10−15 |
| D8 | Refraining from using long-term administration of anxiolytics | 36.4 | ±22.0 | 50.2b | ±24.4 | 54.3c | ±23.0 | 81.05 | 2.5 × 10−18 |
| D9 | Refraining from using long-term administration of hypnotics | 30.9 | ±21.0 | 44.4b | ±23.3 | 48.9c | ±23.8 | 87.01 | 1.3 × 10−19 |
| D10 | For psychotic depression, using a combination of antidepressants and antipsychotics | 57.9 | ±25.3 | 71.2b | ±20.3 | 74.0c | ±17.1 | 62.49 | 2.7 × 10−14 |
| D11 | For psychotic depression, using modified electroconvulsive therapy | 50.2 | ±25.5 | 63.7b | ±23.8 | 64.7c | ±23.5 | 42.44 | 6.1 × 10−10 |
| D12 | For depression in children and adolescents, providing environmental management, psychological education, supportive intervention and family support before pharmacotherapy | 54.9 | ±25.5 | 68.6b | ±22.1 | 71.4c | ±20.2 | 52.48 | 4.0 × 10−12 |
| D13 | For sleep disorders, considering differential diagnosis of primary sleep disorders such as obstructive sleep apnoea syndrome first | 45.6 | ±25.2 | 60.4b | ±24.9 | 63.4c | ±23.4 | 74.39 | 7.0 × 10−17 |
| D14 | For sleep disorders, providing sleep hygiene instructions before pharmacotherapy | 51.2 | ±24.8 | 68.4b | ±22.6 | 72.1c | ±19.7 | 112.55 | 3.6 × 10−25 |
The complete questions are noted in Supplementary Table 3. An intermediate value was used as the representative value for each of the five achievement levels: 0–20, 21–40, 41–60, 61–80 and 81–100. The scores ranged from 10 to 90.
The Kruskal–Wallis test was used for the statistical analysis as the Kolmogorov–Smirnov test did not indicate normal distribution of the clinical behaviour scores at baseline or 1 or 2 years after the programme. The significance level was set at <0.05.
The mean scores of clinical behaviours increased significantly 1 year after attending the programme compared with baseline.
The mean scores of clinical behaviours increased significantly 2 years after attending the programme compared with baseline.
The following are notable points about several general clinical behaviours whose results differed from those previously reported in other countries. The score for the clinical behaviour ‘Refraining from using long-term administration of anxiolytics’ increased significantly throughout the programme (H = 81.05, P = 2.5 × 10−18), but it was as low as approximately 50 after attending the programme (36.4 at baseline, 50.2 at 1 year after the programme and 54.3 at 2 years after the programme). Although a significant change was also observed in ‘Refraining from using long-term administration of hypnotics’ (H = 87.01, P = 1.2 × 10−19), the score was <50 after the programme (30.9 at baseline, 44.4 at 1 year after the programme and 48.9 at 2 years after the programme).
Discussion
This is the first study to investigate the effectiveness of the educational programme in the improvement of psychiatrists’ clinical behaviours in accordance with the clinical practice guidelines.
A total of 607 psychiatrists from 134 medical institutions participated in educational programmes on the clinical guidelines for treatment of schizophrenia and depression during the 3 years of the EGUIDE project recruitment, and all clinical behaviours in accordance with clinical practice guidelines improved after attending the EGUIDE programme and were maintained for at least 2 years. These results suggest that our educational programmes could help to improve psychiatrists’ clinical behaviours in accordance with the guidelines. To the best of our knowledge, no study has shown that educational programmes for clinical practice guideline have led to sustained improvements in clinical behaviour over years.
Previous studies have indicated that there could be a large gap between the development of evidence-based guidelines and their implementation in clinical settings,18,19,21–23 and that a combination of several guideline dissemination and implementation strategies aimed at healthcare professionals has failed to reduce antipsychotic polypharmacy for schizophrenia out-patients.24 The pathway from evidence to guidelines is highly developed, but the development of guideline implementation strategies has been insufficient and examined in only a few studies.22,25–27 Although the barriers to improving guideline adherence have not yet been generalised, some reports suggest that low awareness and dissemination of guidelines, as well as inadequate supply systems, could affect their implementation.22,26 In this regard, the EGUIDE project has set up a supply system and provides the opportunity to learn about and become proficient in the guidelines.
The number of psychiatrists in Japan is almost 16 000, and approximately 1000 psychiatrists have already participated in the education programmes in the past 5 years. In 2020, only 6.3% of psychiatrists had completed these programmes, but the EGUIDE project estimates that 2000 psychiatrists will finish the programmes in the next 5 years. If >10% of psychiatrists in Japan achieve improvements in clinical behaviour in accordance with the clinical practice guidelines, treatment for schizophrenia and depression could change, which could lead to an improvement in quality of life for patients in Japan.
To better disseminate the clinical practice guidelines, the education programmes of the EGUIDE project need further improvement, and we seek to improve the delivery method annually. We revised the lecture materials associated with items for which knowledge was considered insufficient, and reported results suggesting that the revision of the lecture materials may have improved the degree of understanding of the clinical practice guidelines.28
This study has several limitations that should be taken into account when interpreting the results. First, because this study was performed in a single-arm design without a control group, it was difficult to assess the precise effectiveness of the programme despite it being a before-and-after comparison study. Second, since the questionnaires used to evaluate clinical behaviours in accordance with clinical practice guidelines were not validated, and we used a subjective method of assessment, it was unclear whether the questionnaire could adequately assess whether clinical behaviours were in accordance with clinical practice guidelines. Third, because of the lack of background information on the participants, we presumed that there might be many potential confounding factors related to the improvement of clinical behaviours. Fourth, although an annual web questionnaire survey works well as a reminder for past participants to recall the content of the educational programmes and make them aware of whether clinical behaviours are in accordance with clinical practice guidelines, this evaluation is subjective. To assess the precise effect of the education programme, it is necessary to assess changes in quality indicators, such as the prescriptions issued by participants. The results for the improvement of clinical knowledge of the clinical practice guidelines have already been published,20 and in-patient prescribing behaviour will be the object of another paper.
Despite these limitations, the educational programme of the EGUIDE project is considered to be an effective means of guideline dissemination and education. Further dissemination of clinical practice guidelines for schizophrenia and major depression in a variety of clinical settings is needed.
In conclusion, the EGUIDE project, as a dissemination and education programme for the clinical practice guidelines for schizophrenia and major depressive disorder, has the potential to contribute to improvement in clinical behaviours of psychiatrists in accordance with the guidelines.
Further research is needed to clarify the effectiveness of the EGUIDE project for the improvement of quality indicators in clinical situations.
Acknowledgements
We thank all of the participants who contributed to this study.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) under grant numbers JP18dk0307060, JP19dk0307083 and JP20dk0307081; the Health and Labor Sciences research grants (H29-Seishin-Ippan-001, 19GC1201); the Japanese Society of Neuropsychopharmacology and the Japanese Society of Mood Disorders. K.W., N.S., R.H., T.T. and H.H. were funded by AMED (grant number JP18dk0307060). H.H., K. Inada, S.N. and H. Yamada were funded by AMED (grant numbers JP19dk0307083 and JP20dk0307081). H.H., K. Inada, Y.T. and Y.Y. were funded by the Health and Labor Sciences research grants (grant number 19GC1201). The funders had no involvement in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
H. Yamada and M. Motoyama were critically involved in collecting and analysing the data, and wrote the first draft of the manuscript. K. Inada and K.W. were critically involved in the design of the study and contributed to the interpretation of the data and writing of the manuscript. N. Hasegawa, K.M., J.M., K. Ohi, N.Y-F., N.S., T.T., K. Ichihashi, N. Hashimoto, Y.T., J.I., H.H., K.F., K. Ogasawara and H.I. were involved in analysing the data and contributed to the interpretation of the data and the writing of the manuscript. S.N., M.T., T. Nagasawa, C.K., K.A., T.I., T.O., H.K., A.H., M.F., T. Nakamura, K.N., R.F., S.Y., H. Yamagata, E.K., A.M., S.O., M. Makinodan, M.K., T.K., Y.Y., M.U. and T.S. were involved in and contributed to the participant recruitment process and data collection, and the data interpretation. R.H. supervised the entire project, and was critically involved in data collection, design, analysis and interpretation of the data. All authors approved the final version of the manuscript and agree to be accountable for all aspects of this work.
Data availability
The data are not for public use due to privacy and ethical restrictions (informed consent has not been obtained for the public availability of raw data).
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1192/bjo.2022.44.
click here to view supplementary material
Declaration of interest
None.
References
- 1.American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia, Third Edition. American Psychiatric Association, 2016. [Google Scholar]
- 2.Keating D, McWilliams S, Schneider I, Hynes C, Cousins S, Strawbridge J, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open 2017; 7: e013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer M, Severus E, Möller H-J, Young AH; WFSBP Task Force on Unipolar Depressive Disorders. Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract 2017; 21: 166–76. [DOI] [PubMed] [Google Scholar]
- 4.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthøj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia - a short version for primary care. Int J Psychiatry Clin Pract 2017; 21: 82–90. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry 2016; 61: 540–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuipers E, Yesufu-Udechuku A, Taylor C, Kendall T. Management of psychosis and schizophrenia in adults: summary of updated NICE guidance. BMJ 2014; 348: g1173. [DOI] [PubMed] [Google Scholar]
- 7.Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry 2017; 62: 604–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, Maric NP, Salokangas RKR, Riecher-Rössler A, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry 2015; 30: 388–404. [DOI] [PubMed] [Google Scholar]
- 9.Huang C-Y, Yang S-Y, Mojtabai R, Lin S-K, He Y-L, Chong M-Y, et al. Trends of polypharmacy and prescription patterns of antidepressants in Asia. J Clin Psychopharmacol 2018; 38: 598–603. [DOI] [PubMed] [Google Scholar]
- 10.Yang S-Y, Chen L-Y, Najoan E, Kallivayalil RA, Viboonma K, Jamaluddin R, et al. Polypharmacy and psychotropic drug loading in patients with schizophrenia in Asian countries: fourth survey of research on Asian prescription patterns on antipsychotics. Psychiatry Clin Neurosci 2018; 72: 572–9. [DOI] [PubMed] [Google Scholar]
- 11.Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res 2012; 138: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa A, Williams A, Sado M, Oguchi Y, Mischoulon D, Smith F, et al. Comparison of treatment selections by Japanese and US psychiatrists for major depressive disorder: a case vignette study. Psychiatry Clin Neurosci 2015; 69: 553–62. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Koyama A, Higuchi T. Polypharmacy and excessive dosing: psychiatrists’ perceptions of antipsychotic drug prescription. Br J Psychiatry 2005; 187: 243–7. [DOI] [PubMed] [Google Scholar]
- 14.Uchida H, Suzuki T, Mamo DC, Mulsant BH, Tsunoda K, Takeuchi H, et al. Survey of benzodiazepine and antidepressant use in outpatients with mood disorders in Japan. Psychiatry Clin Neurosci 2009; 63: 244–6. [DOI] [PubMed] [Google Scholar]
- 15.Japanese Society of Neuropsychopharmacology. Japanese society of neuropsychopharmacology: “guideline for pharmacological therapy of schizophrenia”. Neuropsychopharmacol Rep 2021; 41: 266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japanese Society of Mood Disorders. Treatment Guideline: Major Depressive Disorder. Igakusyoin, 2012. [PubMed] [Google Scholar]
- 17.Japanese Society of Mood Disorders. Treatment Guideline II: Major Depressive Disorder. Igakusyoin, 2016. [Google Scholar]
- 18.Iida H, Iga J, Hasegawa N, Yasuda Y, Yamamoto T, Miura K, et al. Unmet needs of patients with major depressive disorder - findings from the ‘Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)’ project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci 2020; 74: 667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichihashi K, Hori H, Hasegawa N, Yasuda Y, Yamamoto T, Tsuboi T, et al. Prescription patterns in patients with schizophrenia in Japan: first-quality indicator data from the survey of “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project. Neuropsychopharmacol 2020; 40: 281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takaesu Y, Watanabe K, Numata S, Iwata M, Kudo N, Oishi S, et al. Improvement of psychiatrists’ clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project: a nationwide dissemination, education and evaluation study. Psychiatry Clin Neurosci 2019; 73: 642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S-C, Jang EY, Xiang Y-T, Kanba S, Kato TA, Chong M-Y, et al. Network analysis of the depressive symptom profiles in Asian patients with depressive disorders: findings from the research on Asian psychotropic prescription patterns for antidepressants (REAP-AD). Psychiatry Clin Neurosci 2020; 74: 344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoong SL, Hall A, Stacey F, Grady A, Sutherland R, Wyse R, et al. Nudge strategies to improve healthcare providers’ implementation of evidence-based guidelines, policies and practices: a systematic review of trials included within Cochrane systematic reviews. Implement Sci 2020; 15: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto N, Yasui-Furukori N, Hasegawa N, Ishikawa S, Numata S, Hori H, et al. Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: real-world evidence from the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) psychiatric treatment project. Asian J Psychiatry 2021; 63: 102744. [DOI] [PubMed] [Google Scholar]
- 24.Bighelli I, Ostuzzi G, Girlanda F, Cipriani A, Becker T, Koesters M, et al. Implementation of treatment guidelines for specialist mental health care. Cochrane Database Syst Rev 2016; 12: CD009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 282: 1458–65. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Han C, Yoon H-K, Pae C-U, Kim M-J, Park S-Y, et al. Experiences and barriers to implementation of clinical practice guideline for depression in Korea. BMC Psychiatry 2013; 13: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzog DP, Wagner S, Ruckes C, Tadic A, Roll SC, Härter M, et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur Arch Psychiatry Clin Neurosci 2017; 267: 711–21. [DOI] [PubMed] [Google Scholar]
- 28.Numata S, Nakataki M, Hasegawa N, Takaesu Y, Takeshima M, Onitsuka T, et al. Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep 2021; 41: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://doi.org/10.1192/bjo.2022.44.
click here to view supplementary material
Data Availability Statement
The data are not for public use due to privacy and ethical restrictions (informed consent has not been obtained for the public availability of raw data).