Abstract
Metastatic renal cell carcinoma (RCC) remains an incurable malignancy, despite recent advances in systemic therapies. Genetic syndromes associated with kidney cancer account for only 5%–8% of all diagnosed kidney malignancies, and genetic predispositions to kidney cancer predisposition are still being studied. Genomic testing for kidney cancer is useful for disease molecular subtyping but provides minimal therapeutic information. Understanding how aberrations drive RCC development and how their contextual influences, such as chromosome loss, genome instability, and DNA methylation changes, may alter therapeutic response is of importance. We report the case of a 36-yr-old female with aggressive, metastatic RCC and a significant family history of cancer, including RCC. This patient harbors a novel, pathogenic, germline ATM mutation along with a rare germline variant of unknown significance in the BAP1 gene. In addition, somatic loss of heterozygosity (LOH) in BAP1 and ATM genes, somatic mutation and LOH in the VHL gene, copy losses in Chromosomes 9p and 14, and genome instability are also noted in the tumor, potentially dictating this patient's aggressive clinical course. Further investigation is warranted to evaluate the association of ATM and BAP1 germline mutations with increased risk of RCC and if these mutations should lead to enhanced and early screening.
Keywords: clear cell renal cell carcinoma
INTRODUCTION
Renal cancer is the ninth most common cancer and is responsible for around 115,000 deaths per year (Næraa et al. 2019). Patients with small, localized kidney cancers have high survival; in contrast, advanced and metastatic kidney cancer has only a 2-yr survival rate of 20% (Capitanio and Montorsi 2016). Many germline predisposition genes associated with familial kidney cancers have been characterized. These include von Hippel–Lindau (VHL), hereditary leiomyomatosis renal cell carcinoma (HLRCC), Birt–Hogg–Dubé (BHD), hereditary papillary renal carcinoma (HPRCC), succinate dehydrogenase (SDH) deficiency, and tuberous sclerosis complex (TSC) (Guo et al. 2021). von Hippel–Lindau is an autosomal dominant disease, presenting as a result of mutation in the VHL gene (Maher et al. 1991). VHL mutation leads to the development of tumors and cysts in the kidney with high penetrance (Gossage et al. 2015). Chromosome 3 translocation has also been linked to hereditary kidney cancer (Woodward et al. 2010). A balanced translocation between the short arm of Chromosome 3 and the long arm of Chromosome 8 is most common in hereditary kidney cancer and leads to overexpression of the vascular endothelial growth factory (VEGF) (Maher 2018). Germline mutation in the SWI/SNF chromatin remodeling complex, PBRM1, is also tied to a very rare form of hereditary kidney cancer in a dominant transmission pattern (Benusiglio et al. 2015). Hereditary papillary renal cell carcinoma patients are at risk for many microscopic tumors of the kidney, and this syndrome is tied to mutations in the proto-oncogene, tyrosine kinase MET (Schmidt et al. 1997). BHD is a syndrome leading to the development of a variety of types of kidney cancer and is tied to mutation in the FLCN gene (Nickerson et al. 2002). FLCN is in the LKB1/AMPK pathway and leads to chronic activation of mTORC (Woodward et al. 2008). Mutations in fumarate hydratase and succinate dehydrogenase can also lead to hereditary kidney cancer (Baysal et al. 2000). Tuberous sclerosis often leads to renal tumors, particularly benign angiomyolipomas, and is tied to mutations in TSC1 and TSC2, which together activate the mTOR pathway (Peron et al. 2016). Other germline kidney cancer–predisposing genes are BAP1, PTEN, and the SHD family of genes (Haas and Nathanson 2014; Linehan et al. 2019). All of these recorded hereditary genetic cases comprise only 5%–8% of kidney cancers (Haas and Nathanson 2014). There are many other families in which multiple individuals develop kidney cancer without any of the known mutations. In fact, 58% of all kidney cancers are in families that have more than one family member with kidney cancer (Linehan 2012). Although environmental factors are no doubt also important, this suggests some unknown genetic basis of renal cancer. Germline sequencing in patients with known RCC revealed that 5.5% of patients have mutations in RCC-associated genes and 10.5% have mutations in other cancer-associated genes (Carlo et al. 2018). Previous work has implicated DNA repair pathway proteins such as ATM with kidney cancer (Guo et al. 2021). BAP1, a tumor suppressor gene, is also commonly mutated in advanced kidney cancer and a germline mutation is present in some kidney cancers. This data suggests that germline mutations are heavily associated with the development of kidney cancer, but that all of the potential germline risk factors are unknown. Clearly there is an unmet need to uncover other genetic syndromes within RCC that may result in a hereditary predisposition. This case study reveals inherited germline mutations of BAP1 and ATM in a young patient with aggressive, metastatic RCC.
CASE REPORT
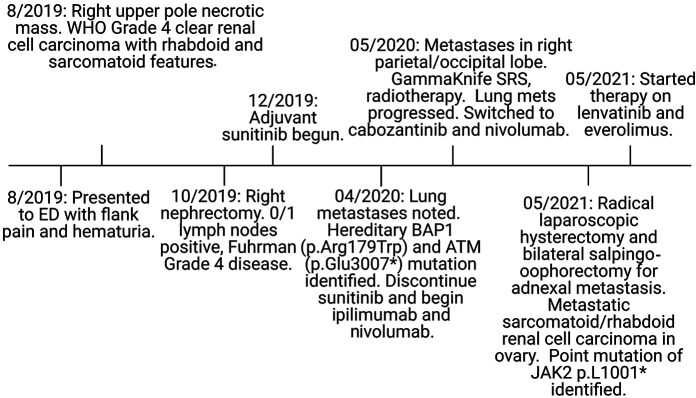
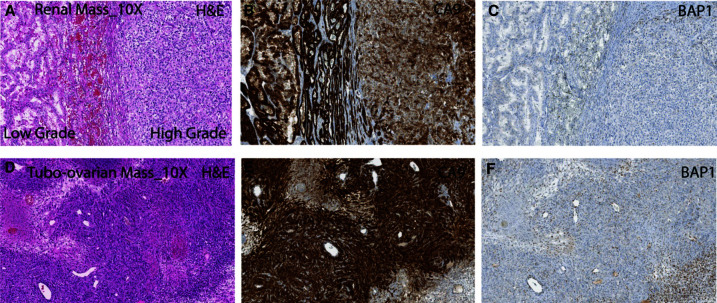
A 36-yr-old female presented to the emergency department with severe right flank pain and gross hematuria (Fig. 1). She reported dysuria, urinary frequency, dizziness, and lightheadedness. Imaging revealed a right upper pole necrotic mass ∼7.5×7.5×8.5 cm. Robotic assisted right nephrectomy occurred on October 1, 2019 with pathology demonstrating pT3 clear cell renal carcinoma, World Health Organization/International Society of Urological Pathology (WHO/ISUP) nuclear grade 4, in concordance with the previously assessed tumor biopsy findings. The tumor was 5.5 cm in size and invading the perirenal adipose tissue. It consisted of 10% sarcomatoid/rhabdoid component; 0/1 lymph nodes were involved. The International Metastatic RCC Database Consortium (IMDC) risk category is intermediate at time of presentation. She was diagnosed with WHO/ISUP grade 4 clear RCC with rhabdoid and sarcomatoid features and with extension into the perirenal soft tissue (pT3a, pN0, cM0) (Fig. 2). Hematoxylin and eosin (H&E) staining of the nephrectomy specimen showed both low- and high-grade areas (Fig. 2A). Carbonic anhydrase 9 (CA9), a characteristic immunohistochemical marker of clear cell RCC (ccRCC) that is not expressed in normal renal tissue, was strongly positive (Fig. 2B). BAP1 expression is extremely low in the tumor region as assessed by immunohistochemistry (Fig. 2C). Adjuvant sunitinib was begun and 4 mo later, lung metastases were noted on imaging performed on April 1, 2020.
Figure 1.
Disease progression from diagnosis to metastasis. A 36-year-old female presented to the emergency department with flank pain and hematuria and was diagnosed with clear cell renal cell carcinoma (ccRCC) in October of 2019. Lung metastases were noted, as well as hereditary mutations in BAP1 and ATM in April of 2020.
Figure 2.
BAP1 staining in primary kidney tumor correlates with grade. (A) Photomicrograph of the right renal mass exhibiting high-grade and low-grade areas of clear cell renal cell carcinoma (hematoxylin and eosin [H&E]; 200×). The corresponding areas showing (B) strong brown membranous staining with carbonic anhydrase IX (CA9; 200×) immunohistochemistry and (C) loss of BRCA1-associated protein 1(BAP1; 200×) immunoexpression in tumor epithelia. (D) The tissue section from the tubo-ovarian metastatic mass shows sarcomatoid differentiation with focal areas of necrosis (H&E; 200×). The corresponding areas (E) exhibiting strong membranous immunoexpression of carbonic anhydrase IX (CA9; 200×) with loss of BRCA1-associated protein 1(BAP1; 200×) immunoexpression in tumor epithelia (F).
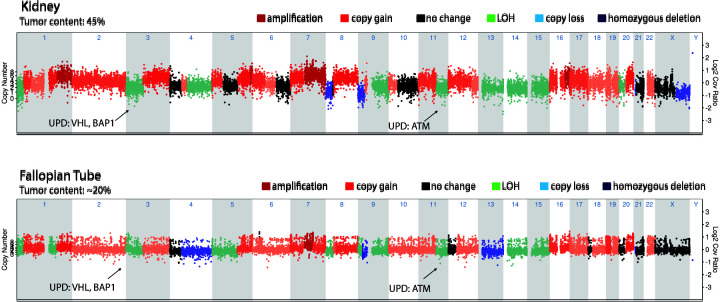
Sequencing of the primary right kidney tumor through MI-ONCOSEQ found a JAK2p.L1001* stop-gain mutation. All sequencing abnormalities are described in Supplemental Table 1. MI-ONCOSEQ uses a targeted panel focusing on protein-coding exons in a 1700-gene set. VHL had a somatic indel, p.Asp150lfsTer9, with an acquired loss of homozygosity by uniparental disomy in the tumor. Sequencing also revealed an acquired uniparental disomy of Chromosome 3p, which contains VHL and BAP1 and uniparental disomy of Chr 11q, which carries ATM (Fig. 3). Outlier gene expression was seen of CA9 and PAX8. CA9 is not expressed in normal kidney tissue, but is expressed in most ccRCCs, secondary to a functional VHL loss in these tumors. Germline testing showed a hereditary BAP1 mutation of unknown significance and a hereditary ATM mutation (Tables 1 and 2; Supplemental Table S1). Somatic mutations in the renal tumor are cataloged in (Tables 1 and 2; Supplemental Table S1). Germline mutation in FANCD2, a key protein in the DNA damage repair pathway, was also noted. The patient was initially started on adjuvant treatment with sunitinib, but new lung metastases were seen ∼4 mo after beginning treatment. Lung biopsy was consistent with metastatic ccRCC. Patient was started on ipilimumab and nivolumab. Within months of therapy, imaging revealed brain metastases in the right parietal/occipital lobe, which were treated with GammaKnife radiosurgery and steroids. She also received palliative radiotherapy to a right calvarial lesion. Progression-free survival on the ipilimumab and nivolumab was only 25 d before progression of disease in lung, neck, and scalp. Lung metastases progressed, and she was switched to cabozantinib and nivolumab. After severe hand-foot syndrome on 60 mg of cabozantinib, the dose was decreased to 40 mg with good tolerability. Progression-free survival on the cabozantinib and nivolumab was 8 mo before imaging showed slight progression of lung nodules, and an adnexal mass was noted to be suspicious for metastatic disease. Pulmonary embolism was also noted in the same time frame.
Figure 3.
Chromosome plot of the primary renal tumor compared to the fallopian tube metastasis. The kidney tumor and the fallopian tube metastatic tumor have very similar genomic landscapes. They both have uniparental disomy of the regions encompassing VHL, BAP1, and ATM.
Table 1.
Germline, kidney, and adnexal mutations
| Germline | Kidney | Adnexal metastasis |
|---|---|---|
| AKT1 p.R406C | AKT1 p.R406C (germline) | AKT1 p.R406C (germline) |
| ATM p.E3007* | ATM p.E3007* (germline) | ATM p.E3007* (germline) |
| BAP1 p.R179W | BAP1 p.R179W (germline) | BAP1 p.R179W (germline) |
| EXT2 p.Y615Ter | EXT2 p.Y615Ter *(germline) | EXT2 p.Y615Ter *(germline) |
| FANCD2 p.N545S | FANCD2 p.N545S (germline) | FANCD2 p.N545S (germline) |
| BAZ1A p.A74Serfs*30 | AFF2 p.S101Y | |
| BTG1 p.P160R | DSP p.K2706del | |
| CDK15 p.S3P | EXT1 p.M100V | |
| DSP p.K2706del | FLT3 p.I538V | |
| EXT1 p.M100V | JAK2 p.L1001* | |
| FANCM p.M1397V | KMT2D p.T1246M | |
| GNA13 p.F245L | KMT2E p.M1115L | |
| JAK2 p.L1001* | LRP5 p.M827I | |
| LRP5 p.M827I | MAP3K14 p.H401Q | |
| MAP3K14 p.H401Q | NF1 p.F23L | |
| NF1 p.F231L | PPP2R2B p.S19delinsRAAA | |
| NR3C1 p.G91V | RYK p.R4fs | |
| NTRK3 p.P738H | SKOR1 p.R799M | |
| NUP214 p.T14494R | VHL p.N150llefsTer9 | |
| TENM2 p.R1017W | ||
| TRIO p.M1000I | ||
| VHL p.N150llefsTer9 | ||
| WNK1 p.E1114D | ||
| XPC p.G217C |
Table 2.
Sequencing quality assessment
| Sample site | Sample quality | Sequencing quality | Library quality | Sample identity (SNP fingerprinting) |
|---|---|---|---|---|
| Fallopian tube | 20% tumor content | Splice junction coverage < 40,000 | Pass | Pass |
| Right kidney | Pass | Pass | Pass | Pass |
(SNP) Single-nucleotide polymorphism.
She underwent a robotic-assisted modified radical laparoscopic hysterectomy and bilateral salpingo-oophorectomy for adnexal metastasis in May of 2021. The uterus showed no endometrial hyperplasia or malignancy, whereas the right fallopian tube and ovary showed metastatic sarcomatoid/rhabdoid RCC in ovary, 9 cm, with 20% punctate geographic necrosis (Fig. 2D–F). She was subsequently treated with lenvatinib and everolimus. Based on immunohistochemical assessment, the adnexal metastasis was strongly positive for CA9. Sequencing showed a somatic point mutation of JAK2 p.L1001* gain of a stop codon (Tables 1 and 3), identical to the one observed in the primary tumor. This JAK2-truncating mutation is associated with resistance to PD1 blockade (Carlo et al. 2018). A somatic indel was also seen in VHL, p.Asp150lfsTer9, with loss of homozygosity by uniparental disomy (Fig. 3). Sequencing also revealed uniparental disomy of Chromosome 3p (VHL, BAP1) and uniparental disomy of Chr 11q (ATM). These mutations were consistent with those noted in original right kidney primary tumor. However, the fallopian tube metastasis had fewer overall somatic mutations than the kidney cancer, which could be due to the lower tumor content noted for this specimen. Again, germline variants of ATM p.Glu3007* stop-gain, with loss of heterozygosity in the tumor, were identified. This ATM variant may lead to eligibility in PARP inhibitors trials. In addition, the BAP1 p.Arg179Trp was again identified, highlighting the need for genetic counseling and the potential role of BAP1 in her disease. Copy-number assessments on specimens from both sites showed overall a heavy copy-number burden involving almost all chromosomes, indicating high genome instability. In addition, loss of Chr 9p and LOH of Chr 14 are notable as these events along with genome instability have been previously associated with poor prognosis (Turajlic et al. 2018) (Fig. 3). Two-month-interval brain magnetic resonance imaging (MRI) after starting everolimus and levatinib showed interval development of four supratentorial enhancing lesions consistent with brain metastasis. Computed tomography (CT) chest/abdomen/pelvis showed interval worsening of mediastinal and bilateral hilar lymphadenopathy. Malignant lymphadenopathy completely surrounded the distal trachea and both mainstem bronchi.
Table 3.
Detailed mutation information
| Gene | Site | Chromosome | HGVS DNA reference | HGVS protein reference | Variant type | Predicted effect | dbSNP/dbVar ID | Allelic fraction |
|---|---|---|---|---|---|---|---|---|
| BAP1 | Germline | Chr 3: 52407219 | NM_004656:c.535C > T | p.Arg179Trp | Missense | Substitution | N/A | Normal allelic fraction: 50.20%; tumor allelic fraction: 78.90% |
| ATM | Germline | Chr 11: 108365356 | NM_000051:c.9019G > T | p.Glu3007Ter | Missense | Substitution | N/A | Normal allelic fraction: 54.10%; tumor allelic fraction: 74.70% |
| EXT2 | Germline | Chr 11: 44232436 | NM_000401:c.1845T > A | p.Tyr615Ter | Missense | Substitution | N/A | Normal allelic fraction: 52.20%; tumor allelic fraction: 54.10% |
A family history of RCC was noted, as her father was diagnosed at age 59 and died of rapidly progressive disease without receiving systemic therapy. Her mother had a basal cell carcinoma, her maternal grandfather multiple myeloma at 73 yr of age, and her maternal uncle had brain cancer. The patient had a benign bone tumor in her right knee as a teenager. Genetic testing at time of diagnosis revealed a germline pathogenic mutation in the ATM gene, c.9019G to T (p.Glu3007*) as well as a variant of unknown significance in the gene BAP1, c.535C to T (p.Arg179Trp). The substitution in ATM induces a nonsense variant, which truncates the final 49-amino acid residues. ATM plays a critical role in DNA repair and response to double-strand breaks (Cremona and Behrens 2014). Twenty percent of metastatic kidney cancers have a mutation in a DNA damage repair protein pathway, and many tumors have ATM mutations (Ged et al. 2020). ATM expression has been reported to be lower in ccRCC than in adjacent normal tissue, and ATM is decreased in tissue from a higher-grade tumor. Low ATM tumors are associated with a lower survival rate, suggesting that ATM expression level could be a factor in ccRCC prognosis (Ren et al. 2019). However, there is no clear association of germline ATMwith hereditary kidney cancer. ATM is associated with increased risk of breast cancer, pancreatic cancer, and prostate cancer, and germline DNA damage repair pathway mutations are seen in 7.3% of kidney cancers (Truong et al. 2021). The germline BAP1, c.535C to T (p.Arg179Trp) mutation is a recurrent somatic mutation in four cases in COSMIC; in RCC, biliary tract cancer, and lung cancer. BAP1 is a tumor suppressor gene involved in DNA damage repair, cell cycle, and cellular differentiation (Peña-Llopis et al. 2012). BAP1 mutation is associated with a syndrome of uveal melanoma, mesothelioma, and ccRCC (Rai et al. 2016). RCC with BAP1 mutation has been reported to have a rapidly progressing aggressive course (Carlo et al. 2019).The germline BAP1 and ATM, coupled with the hereditary predisposition of ccRCC noted, make this case noteworthy.
DISCUSSION
The patient had a strong family history of cancer. Her mother had basal cell carcinoma, her grandfather developed multiple myeloma, and an uncle had brain cancer. Most significantly, her father was diagnosed with aggressive RCC and died from it at the age of 61. Combined with her aggressive disease at a young age, this warranted germline genetic testing. Genetic testing revealed a novel pathogenic ATM mutation that has not been previously associated with RCC and a novel BAP1 mutation. The ATM mutation is associated with increased risk of breast, prostate, and pancreatic cancer (Cremona and Behrens 2014). ATM is a serine/threonine protein kinase that is recruited by double stranded breaks in the DNA. DNA damage repair pathways have been implicated as a frequent pathway mutated in ccRCC. A recent survey of 229 patients revealed that 19% of patients had somatic deleterious DNA damage repair gene alterations, and the most frequently altered genes were CHEK2 (some germline and some somatic) and ATM (all somatic) (Burma et al. 2001). It initiates activation of the DNA damage checkpoint which leads to cell cycle arrest (Ged et al. 2020). This patient also has a germline mutation in FANCD2, a crucial protein in the DNA damage response. This is the first report of this specific germline ATM mutation in a patient with RCC. Further study will be needed to determine c.9019G to T (p.Glu3007*) pathogenicity in renal cancer, and to determine if it leads to higher risk and the potential need for increased surveillance in family members who inherit this gene.
Integrative clinical sequencing of the patient's tumor DNA and germline DNA performed at the University of Michigan Clinical Sequencing Program MIONCOSEQ revealed the driver germline aberrations and the large number of somatic driver insults accumulated in the tumor genome. Besides the ccRCC signature Chromosome 3p loss, losses of 14 and 9p were of interest because of their prognostic association. So, we predict that either Chr 3q, 5q, or 8q may be involved in the Chr 3 translocation in this patient, which, however, can be tested only with whole-genome sequencing data that is currently not available. The high copy-number burden we observed indicates the presence of genome instability in case, which is again associated with poor prognosis.
MIONCOSEQ analysis also revealed several mutations including a mutation in BRCA associated protein 1 (BAP1) (Robinson et al. 2017). BAP1 is a tumor suppressor gene that is commonly mutated in ccRCC. BAP1 germline mutations have been found to segregate with RCC and lead to a substantially increased risk of RCC (Creighton et al. 2013; Popova et al. 2013). BAP1 encodes a nuclear deubiquitinase and requires two hits to eliminate its tumor suppressor function (Dey et al. 2012). BAP1 mutations are associated with high grade clear cell RCC, with renal tumors exhibiting rhabdoid morphology and poor patient outcomes (Peña-Llopis et al. 2012). Low expression of BAP1 is also associated with worse survival. Preclinical studies have suggested that BAP1 mutation may be predictive of sensitivity to both mTOR inhibitors and radiotherapy (Lim et al. 2016). BAP1 mutations are associated with a high Fuhrman grade, coagulative necrosis, and poor outcomes (Brugarolas 2013). This patient has Fuhrman grade 4 disease. Recent reports have generated a description of a hereditary BAP1 tumor syndrome, which involves uveal melanoma, cutaneous melanoma, and mesothelioma (Kobrinski et al. 2019). In addition, BAP1 mutation in ccRCC has been found to be increased in metastatic cases compared to nonmetastatic patients (Meng et al. 2020). Somatic BAP1 mutation at a different highly conserved catalytic residue has been associated in a family with early-onset ccRCC with high Fuhrman grade (Farley et al. 2013). BAP1 and PBRM1 loss is associated with rhabdoid features, and high tumor grade (Peña-Llopis et al. 2012). The variant in this case is reported in ClinVar and is recorded as a variant of uncertain significance by commercial genetics companies Invitae and Ambry. Invitae has seen this particular variant five times, with mixed clinical information. Ambry has seen this variant less than five times. This particular amino acid change from arginine to tryptophan at position 179 is a moderate physicochemical change, and this region is well-conserved, suggesting that this mutation is functional. Interestingly, BAP1 anticorrelates with the other commonly mutated ccRCC gene PBRM1. BAP1 mutation is associated with significantly worse overall survival than PBRM1 mutation ccRCC. Together, this data suggests that BAP1 and PBRM1 mutations like denote two different molecular subtypes with different gene expression patterns and outcomes. Specifically, germline BAP1 mutation c.277A to G (p.Thr93Ala) has been associated with RCC predisposition (Creighton et al. 2013). The variant c.41T to A, p.L14H has also been associated with RCC. The mutation present in the case here has not been reported to be associated with renal cell carcinoma and is currently considered a variant of unknown significance. The patient's family history of RCC, her aggressive clinical course, and negative BAP1 immunohistochemical (IHC) staining data demonstrates that the BAP1 variant reported here is pathogenic and should therefore no longer be considered a variant of unknown significance. Interestingly, in uveal melanoma, concurrent mutation in BAP1 and ATM is correlated with increased tumor stage and increased metastasis risk (Jha et al. 2020). The combination of the ATM and BAP1 mutations is rare, and the double germline occurrence of these mutations is even rarer. The patient's RCC was resistant to contemporary therapies besides the Jak2 mutation noted, which is known to be associated with sarcomatoid RCC (PMID: 30576871) and also may predict for resistance to immune checkpoint inhibitor therapies (Carlo et al. 2018).
Currently, germline BAP1-mutated individuals are recommended to undergo abdominal and respiratory physical examinations starting at 30-yr-old. Ultrasound or MRI surveillance should be considered every 2 years between ages 30 and 55. At age 55, consider a chest/abdomen CT or MRI chest/abdomen with IV contrast every 2 yr (Kobrinski et al. 2019). An annual ophthalmic exam is recommended starting at age 16 consisting of direct and indirect ophthalmoscopy, fundus photography, and ocular ultrasound. Biannual full-skin examinations with dermatologic examination is recommended starting at 18. As for targeted treatment in BAP1-mutated RCC, histone deacetylase inhibitors could be useful in reversing the H2A hyperubiquitination caused by BAP1 loss (Landreville et al. 2012). BAP1 also increases expression of EZH2, so EZH2 inhibitors are another potential targeted therapy to consider (LaFave et al. 2015). Another study showed that the PARP inhibitor rucaparib seems to be selective for BAP1 mutant cell lines (Louie and Kurzrock 2020). JAK2 activation up-regulates PD-L1 and can lead to immune evasion (Gupta et al. 2019). A subset of renal tumors with a sarcomatoid transformation exhibit constitutive PD-L1 overexpression, and these patients should be evaluated for enhanced response to immunotherapy (Gupta et al. 2019). Prioritizing studying the variant identified in this patient, and potential molecular vulnerabilities could open up new classes of targeted therapies.
Conclusion
There is a sizeable proportion of inherited kidney cancer whose genetic origins are unknown. This case suggests pathogenesis of a novel germline ATM mutation and a novel germline BAP1 mutation. BAP1 protein loss of expression in the tumor area as assessed by immunohistochemistry analysis suggests that this BAP1 mutation has functional consequences. The patient also presents with a JAK2 mutation that may predispose to resistance to PD1 blockade. Future work is needed to evaluate whether these individual ATM and BAP1 mutations lead to hereditary predisposition to renal cancer and predict resistance to systemic therapies.
METHODS
IHC CA9 (carbonic anhydrase IX) rabbit polyclonal primary antibody (Cat No. NB100-417, Novus Biologicals) and BAP1 (BRCA1-associated protein 1) mouse monoclonal primary antibody (Cat No. sc-28382, Santa Cruz Biotechnology) was performed on 4 μ formalin-fixed, paraffin-embedded (FFPE) tissue sections. IHC was carried out on the Benchmark XT automated slide staining system using known positive and negative control tissues. For CA9, a strong intensity membranous brown chromogenic staining was taken as positivity. For BAP1, loss of nuclear positivity in the cancer cells was recorded and accompanying non–tumor epithelial cells (immune and stromal cells) exhibiting BAP1 nuclear positivity act as positive internal control. Tumor and normal OncoSeq exome capture libraries and tumor whole transcriptome capture libraries were analyzed for both patients. Quality control for tumor content, sample quality, sequencing quality, library quality, and sample identify with SNP fingerprinting were performed and described in Table 2.
ADDITIONAL INFORMATION
Data Deposition and Access
The novel variants reported have been deposited in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV002107489 for NM_000051.4(ATM):c.9019G>T(p.Glu3007Ter) and SCV002107403 for NM_004656.4(BAP1):c.535C>T(p.Arg179Trp). All sequencing information is in the supplement of this report.
Ethics Statement
We confirm that we obtained written informed consent for research and publication from this patient, including clinical information, sequencing information, and pathological images prior to the submission of this manuscript from the participant. Human investigations were approved by a local Human Investigations Committee and all regulatory and HIPPA guidelines were followed.
Acknowledgments
We thank Drs. Arul Chinnaiyan, Dan Robinson, and Yi-Mi Wu; Ms. Xuhong Cao; members of the MiOncoSeq clinical sequencing team for sequencing; and Marcin Cieslik, Alexander Hopkins, Jamie Estill, and members of the bioinformatics team for processing of the primary data through various analysis pipelines. We also thank MiOncoSeq clinical coordinators Erica Rabban and Janice Bell for coordinating patient consent and sample acquisition.
Author Contributions
D.Z., D.J., and U.V. treated the patient. C.K.-S., R.Ma., Y.Z., R.Me., and S.M.D. performed pathological analysis. H.N.B. and U.V. wrote and edited this manuscript.
Funding
H.N.B. was supported by a T32 training grant (T32-GM008322), a National Institutes of Health (NIH) F30 Predoctoral Fellowship (F30CA257292), and the University of Michigan Systems and Integrative Biology grant.
Competing Interest Statement
The authors have declared no competing interest.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, et al. 2000. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287: 848–851. 10.1126/science.287.5454.848 [DOI] [PubMed] [Google Scholar]
- Benusiglio PR, Couvé S, Gilbert-Dussardier B, Deveaux S, Jeune HL, Costa MD, Fromont G, Memeteau F, Yacoub M, Coupier I, et al. 2015. A germline mutation in PBRM1 predisposes to renal cell carcinoma. J Med Genet 52: 426. 10.1136/jmedgenet-2014-102912 [DOI] [PubMed] [Google Scholar]
- Brugarolas J. 2013. PBRM1 and BAP1 as novel targets for renal cell carcinoma. Cancer J 19: 324–332. 10.1097/ppo.0b013e3182a102d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. 2001. ATM phosphorylates histone H2AX in response to DNA double-strand breaks*. J Biol Chem 276: 42462–42467. 10.1074/jbc.c100466200 [DOI] [PubMed] [Google Scholar]
- Capitanio U, Montorsi F. 2016. Renal cancer. Lancet 387: 894–906. 10.1016/s0140-6736(15)00046-x [DOI] [PubMed] [Google Scholar]
- Carlo MI, Mukherjee S, Mandelker D, Vijai J, Kemel Y, Zhang L, Knezevic A, Patil S, Ceyhan-Birsoy O, Huang K-C, et al. 2018. Prevalence of germline mutations in cancer susceptibility genes in patients with advanced renal cell carcinoma. JAMA Oncol 4: 1228. 10.1001/jamaoncol.2018.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo MI, Hakimi AA, Stewart GD, Bratslavsky G, Brugarolas J, Chen Y-B, Linehan WM, Maher ER, Merino MJ, Offit K, et al. 2019. Familial kidney cancer: implications of new syndromes and molecular insights. Eur Urol 76: 754–764. 10.1016/j.eururo.2019.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton CJ, Morgan M, Gunaratne PH, Wheeler DA, Gibbs RA, Robertson AG, Chu A, Beroukhim R, Cibulskis K, Signoretti S, et al. 2013. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499: 43–49. 10.1038/nature12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremona CA, Behrens A. 2014. ATM signalling and cancer. Oncogene 33: 3351–3360. 10.1038/onc.2013.275 [DOI] [PubMed] [Google Scholar]
- Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, Kirkpatrick DS, Pham VC, Lill JR, Bakalarski CE, et al. 2012. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 337: 1541–1546. 10.1126/science.1221711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MN, Schmidt LS, Mester JL, Peña-Llopis S, Pavia-Jimenez A, Christie A, Vocke CD, Ricketts CJ, Peterson J, Middelton L, et al. 2013. A novel germline mutation in BAP1 predisposes to familial clear-cell renal cell carcinoma. Mol Cancer Res 11: 1061–1071. 10.1158/1541-7786.mcr-13-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ged Y, Chaim JL, DiNatale RG, Knezevic A, Kotecha RR, Carlo MI, Lee C-H, Foster A, Feldman DR, Teo MY, et al. 2020. DNA damage repair pathway alterations in metastatic clear cell renal cell carcinoma and implications on systemic therapy. J Immunother Cancer 8: e000230. 10.1136/jitc-2019-000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossage L, Eisen T, Maher ER. 2015. VHL, the story of a tumour suppressor gene. Nat Rev Cancer 15: 55–64. 10.1038/nrc3844 [DOI] [PubMed] [Google Scholar]
- Guo E, Wu C, Ming J, Zhang W, Zhang L, Hu G. 2021. The clinical significance of DNA damage repair signatures in clear cell renal cell carcinoma. Front Genet 11: 593039. 10.3389/fgene.2020.593039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Cheville JC, Jungbluth AA, Zhang Y, Zhang L, Chen Y-B, Tickoo SK, Fine SW, Gopalan A, Al-Ahmadie HA, et al. 2019. JAK2/PD-L1/PD-L2 (9p24.1) amplifications in renal cell carcinomas with sarcomatoid transformation: implications for clinical management. Mod Pathol 32: 1344–1358. 10.1038/s41379-019-0269-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas NB, Nathanson KL. 2014. Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis 21: 81–90. 10.1053/j.ackd.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha J, Singh MK, Singh L, Pushker N, Bajaj MS, Sen S, Kashyap S. 2020. Expression of BAP1 and ATM proteins: association with AJCC tumor category in uveal melanoma. Ann Diagn Pathol 44: 151432. 10.1016/j.anndiagpath.2019.151432 [DOI] [PubMed] [Google Scholar]
- Kobrinski DA, Yang H, Kittaneh M. 2019. BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res 9: S60–S66. 10.21037/tlcr.2019.11.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFave LM, Béguelin W, Koche R, Teater M, Spitzer B, Chramiec A, Papalexi E, Keller MD, Hricik T, Konstantinoff K, et al. 2015. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 21: 1344–1349. 10.1038/nm.3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, Bowcock AM, Harbour JW. 2012. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 18: 408–416. 10.1158/1078-0432.ccr-11-0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Park HS, Kim S, Kim S, Ali SM, Greenbowe JR, Yang IS, Kwon N-J, Lee JL, Ryu M-H, et al. 2016. Next-generation sequencing reveals somatic mutations that confer exceptional response to everolimus. Oncotarget 7: 10547–10556. 10.18632/oncotarget.7234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan WM. 2012. Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res 22: 2089–2100. 10.1101/gr.131110.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan WM, Schmidt LS, Crooks DR, Wei D, Srinivasan R, Lang M, Ricketts CJ. 2019. The metabolic basis of kidney cancer. Cancer Discov 9: 1006–1021. 10.1158/2159-8290.cd-18-1354 [DOI] [PubMed] [Google Scholar]
- Louie BH, Kurzrock R. 2020. BAP1: not just a BRCA1-associated protein. Cancer Treat Rev 90: 102091. 10.1016/j.ctrv.2020.102091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER. 2018. Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management. World J Urol 36: 1891–1898. 10.1007/s00345-018-2288-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Iselius L, Yates JR, Littler M, Benjamin C, Harris R, Sampson J, Williams A, Ferguson-Smith MA, Morton N. 1991. Von Hippel–Lindau disease: a genetic study. J Med Genet 28: 443. 10.1136/jmg.28.7.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Jiang X, Cui J, Yin G, Shi B, Liu Q, Xuan H, Wang Y. 2020. Genomic analysis reveals novel specific metastatic mutations in Chinese clear cell renal cell carcinoma. BioMed Res Int 2020: 2495157. 10.1155/2020/2495157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Næraa SH, Bersang AB, Dahl C, Subhi Y, Azawi N. 2019. Burden of renal cancer in Nordic countries. Scand J Urol 53: 1–8. 10.1080/21681805.2019.1624610 [DOI] [PubMed] [Google Scholar]
- Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn G, Turner ML, Duray P, Merino M, Choyke P, Pavlovich CP, et al. 2002. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt–Hogg–Dubé syndrome. Cancer Cell 2: 157–164. 10.1016/s1535-6108(02)00104-6 [DOI] [PubMed] [Google Scholar]
- Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, et al. 2012. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 44: 751–759. 10.1038/ng.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron A, Vignoli A, Briola FL, Volpi A, Montanari E, Morenghi E, Ghelma F, Bulfamante G, Cefalo G, Canevini MP. 2016. Do patients with tuberous sclerosis complex have an increased risk for malignancies? Am J Med Genet A 170: 1538–1544. 10.1002/ajmg.a.37644 [DOI] [PubMed] [Google Scholar]
- Popova T, Hebert L, Jacquemin V, Gad S, Caux-Moncoutier V, Dubois-d'Enghien C, Richaudeau B, Renaudin X, Sellers J, Nicolas A, et al. 2013. Germline BAP1 mutations predispose to renal cell carcinomas. Am J Hum Genet 92: 974–980. 10.1016/j.ajhg.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH. 2016. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet 89: 285–294. 10.1111/cge.12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Xue B, Chen M, Liu L, Zu X. 2019. Low expression of ATM indicates a poor prognosis in clear cell renal cell carcinoma. Clin Genitourin Cancer 17: e433–e439. 10.1016/j.clgc.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Robinson DR, Wu Y-M, Lonigro RJ, Vats P, Cobain E, Everett J, Cao X, Rabban E, Kumar-Sinha C, Raymond V, et al. 2017. Integrative clinical genomics of metastatic cancer. Nature 548: 297–303. 10.1038/nature23306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L, Duh F-M, Chen F, Kishida T, Glenn G, Choyke P, Scherer SW, Zhuang Z, Lubensky I, Dean M, et al. 1997. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 16: 68–73. 10.1038/ng0597-68 [DOI] [PubMed] [Google Scholar]
- Turajlic S, Xu H, Litchfield K, Rowan A, Chambers T, Lopez JI, Nicol D, O'Brien T, Larkin J, Horswell S, et al. 2018. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173: 581–594.e12. 10.1016/j.cell.2018.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong H, Sheikh R, Kotecha R, Kemel Y, Reisz PA, Lenis AT, Mehta NN, Khurram A, Joseph V, Mandelker D, et al. 2021. Germline variants identified in patients with early-onset renal cell carcinoma referred for germline genetic testing. Eur Urol Oncol 4: 993–1000. 10.1016/j.euo.2021.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ER, Ricketts C, Killick P, Gad S, Morris MR, Kavalier F, Hodgson SV, Giraud S, Paillerets BB, Chapman C, et al. 2008. Familial non-VHL clear cell (conventional) renal cell carcinoma: clinical features, segregation analysis, and mutation analysis of FLCN. Clin Cancer Res 14: 5925–5930. 10.1158/1078-0432.ccr-08-0608 [DOI] [PubMed] [Google Scholar]
- Woodward ER, Skytte A, Cruger DG, Maher ER. 2010. Population-based survey of cancer risks in Chromosome 3 translocation carriers. Genes Chromosomes Cancer 49: 52–58. 10.1002/gcc.20718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The novel variants reported have been deposited in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV002107489 for NM_000051.4(ATM):c.9019G>T(p.Glu3007Ter) and SCV002107403 for NM_004656.4(BAP1):c.535C>T(p.Arg179Trp). All sequencing information is in the supplement of this report.