An EUS-guided fine-needle aspiration/biopsy (EUS-FNAB) is indispensable for the pathological evaluation of subepithelial and pancreatobiliary lesions.[1,2,3] To perform a successful EUS-FNAB, it is important to create sharp EUS images of the lesion, especially to clarify the margins of the lesion; therefore, a water immersion-assisted EUS is applied.[4] However, water immersion is sometimes ineffective in the second part of the duodenum for anatomical reasons. We herein report a case in which a gel immersion-assisted EUS-FNAB was effective for ampullary lesions even though conventional water immersion was ineffective. A 54-year-old man developed acute pancreatitis. ERCP revealed stenosis of the main pancreatic duct and an elevated subepithelial-like lesion surrounding the ampulla [Figure 1], which was evaluated by EUS and an EUS-FNAB. Although a water immersion-assisted EUS/EUS-FNAB was initially attempted, it was difficult to obtain adequate EUS images since the injected water flowed quickly from the duodenum to the small intestine [Figure 2 and Video 1]. We, therefore, injected 60 ml of OS-1 gel (originally developed and used for oral treatment of dehydration; Otsuka Pharmaceutical Factory, Tokushima, Japan) instead of water through the working channel of the echoendoscope [Figure 3]. The injected OS-1 gel remained in the duodenum for a much longer time than the water had due to its viscosity [Video 2]. OS-1 gel immersion helped to obtain sharp EUS images with a clear lesion margin, revealing that the lesion was arising from the second layer of the duodenum [Figure 4]. The OS-1 gel did not interfere with the EUS-FNAB itself, and the procedure was successfully performed without any complications [Video 3]. After the biopsy, most of the gel was sucked from the duodenum. A pathological analysis of the specimen obtained by the EUS-FNAB revealed the lesion to be caused by inflammatory changes. This gel immersion EUS/EUS-FNAB is a simple and new option for the evaluation of ampullary lesions that can be performed at any facility.
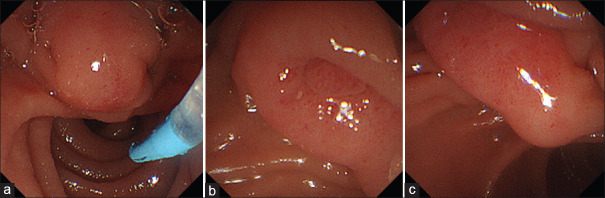
Figure 1.
The elevated subepithelial-like lesion spreading to the anal side of the ampulla subjected to an EUS/EUS-FNAB. (a) Oral side of the ampulla. (b) Ampulla. (c) Anal side of the ampulla
Figure 2.
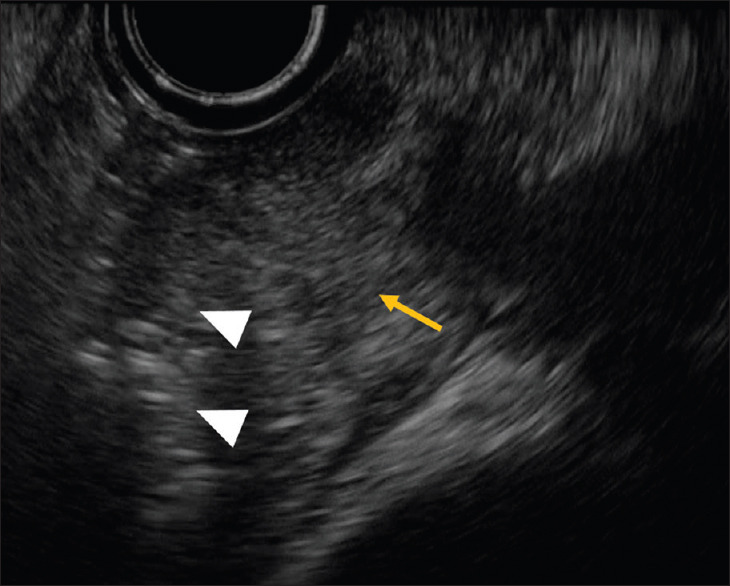
Water immersion-assisted EUS for ampullary lesions. A representative image of ampullary lesions obtained by water immersion-assisted EUS is shown. Sharp EUS images could not be obtained due to artifacts caused by the presence of air (triangle) close to the ampulla (yellow arrow) in the second duodenal portion
Figure 3.
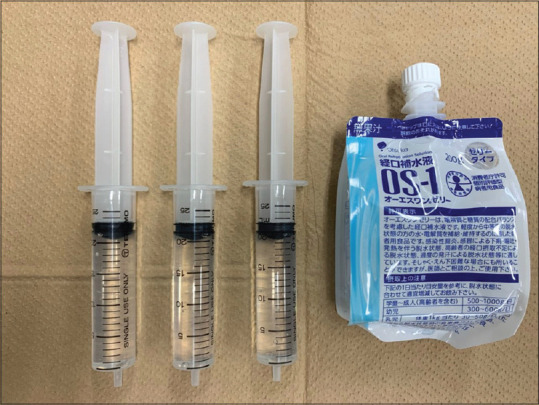
The OS-1 gel and syringes used for the gel immersion-assisted EUS/EUS-FNAB. The OS-1 gel was injected into the duodenum through the working channel of the echoendoscope, and a gel immersion-assisted EUS/EUS-FNAB was performed
Figure 4.
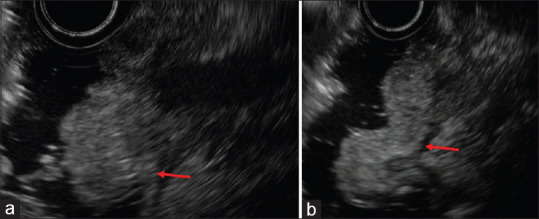
Gel immersion-assisted EUS for ampullary lesions. (a and b) Representative images of ampullary lesions obtained by the gel immersion-assisted EUS are shown. The injected OS-1 gel remained in the duodenum for much longer than the water had due to its viscosity. Sharp EUS images with a clear lesion margin were obtained not only for the ampulla (a) but also for the anal side of the ampulla (b). The lesion was arising from the second layer of the duodenum
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his names and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.eusjournal.com
REFERENCES
- 1.Facciorusso A, Wani S, Triantafyllou K, et al. Comparative accuracy of needle sizes and designs for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest endosc. 2019;90:893–903.e897. doi: 10.1016/j.gie.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Naveed M, Siddiqui AA, Kowalski TE, et al. A Multicenter comparative trial of a novel EUS-guided core biopsy needle (SharkCore™) with the 22-gauge needle in patients with solid pancreatic mass lesions. Endosc Ultrasound. 2018;7:34–40. doi: 10.4103/eus.eus_27_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osoegawa T, Minoda Y, Ihara E, et al. Mucosal incision-assisted biopsy versus endoscopic ultrasound-guided fine-needle aspiration with a rapid on-site evaluation for gastric subepithelial lesions: A randomized cross-over study. Dig Endosc. 2019;31:413–21. doi: 10.1111/den.13367. [DOI] [PubMed] [Google Scholar]
- 4.Baysal B, Masri OA, Eloubeidi MA, et al. The role of EUS and EUS-guided FNA in the management of subepithelial lesions of the esophagus: A large, single-center experience. Endosc ultrasound. 2017;6:308–16. doi: 10.4103/2303-9027.155772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.