Abstract
Famciclovir (FCV) is efficacious in the treatment of acute herpes zoster and recurrent genital infections but has not been used to treat ocular herpes simplex virus (HSV) infections. We evaluated the efficacy of orally administered FCV in treating HSV-1 epithelial keratitis and determined its effects on the establishment of latency and subsequent reactivation. Rabbits were inoculated with HSV-1 strain 17 syn+ and treated twice daily with increasing concentrations of FCV (60 to 500 mg/kg of body weight). This resulted in a significant, dose-dependent improvement in keratitis scores, as well as prolonged survival. Regardless of the dose of drug used, all groups exhibited the high rates of spontaneous and induced reactivation characteristic of 17syn+. The efficacy of 250 mg of FCV per kg was also compared to topical treatment with 1% trifluorothymidine (TFT). Although TFT treatment was more effective at reducing eye disease, FCV-treated rabbits had a better survival rate. Real-time quantitative PCR analysis of rabbit trigeminal ganglia (TG) demonstrated that FCV significantly reduced the HSV-1 copy number compared to that after treatment with TFT or the placebo but not in a dose-dependent manner. In summary, oral FCV treatment significantly reduces the severity of corneal lesions, reduces the number of HSV-1 genomes in the TG, improves survival, and therefore may be beneficial in reducing the morbidity of HSV keratitis in the clinic.
Herpes simplex virus type 1 (HSV-1) typically infects mucosal surfaces or the skin and manifests as herpes labialis, stomatitis, or keratitis. The virus travels by retrograde axonal transport through the neurons innervating these areas to infect the sensory ganglia and establish a latent infection. The virus can also travel to the brain, causing encephalitis, a life-threatening infection. HSV-1 typically remains latent; however, certain stimuli, such as stress, fever, UV irradiation, and immunosuppression, cause the virus to reactivate and reappear at the original site of infection or at any other site innervated by the ganglion (53). Recurrent herpes keratitis causes significant morbidity, corneal scarring, reduced visual acuity, and eventual blindness. Although disease severity is most often associated with recurrence, primary infection of the eye can be equally detrimental. The only available “cure” for severe recurrent herpes keratitis is corneal transplantation; however, transplant recipients are still susceptible to recurrent disease, as the virus has not been eliminated from the latent reservoir (28, 42).
Since the advent of the first antiviral, idoxuridine, in 1962 (25; see also references 26 and 27), research has focused on new therapies to prevent recurrent herpes virus infections. Treatment for HSV-1 infection is dependent on the site of the acute or recurrent lesion. For example, orofacial lesions can be treated with a topical cream of penciclovir (PCV) or acyclovir (ACV), encephalitis is treated with systemic ACV, and herpes keratitis is treated with topical drops of trifluorothymidine (trifluridine) (TFT) (7). The difficulty with treatment arises from the ability of the herpes virus to establish and maintain a latent state within the neuron. Current therapies act only at the site of viral replication and do not counteract the latent virus in a nonreactivating neuron or prevent neuronal reactivation. Clinical trials of prophylactic treatment have been conducted in an attempt to prevent or reduce neuronal reactivation. One such study conducted by the Herpetic Eye Disease Study Group indicates that patients with a history of ocular HSV disease and given oral ACV (400 mg/kg) twice daily (b.i.d.) for 1 year had a 50% decrease in the number of episodes of stromal keratitis from the number experienced by the placebo-treated group (16). The data collected from this group were also analyzed in regard to individual herpetic eye diseases (17). They found that prophylactic ACV treatment reduced all herpetic eye disease by about half compared to what occurred with the placebo. ACV treatment was also beneficial for superficial disease such as blepharitis and epithelial keratitis, as well as deeper forms such as stromal keratitis and keratouveitis (17). At the time this clinical study was initiated (September 1992), ACV was the only commercially available oral antiherpetic drug. The efficacy of valaciclovir (VCV), the oral prodrug of ACV, was tested in latently infected rabbits that underwent excimer laser keratectomy to determine if HSV-1 shedding could be prevented following the photoablative procedure (10). Latently infected rabbits given either 100 or 150 mg of VCV per kg of body weight per day intraperitoneally for 7 days beginning 1 day before the procedure were found to have significantly fewer shedding days than the placebo-treated rabbits. Prophylactic treatment with VCV may be beneficial to patients with a history of ocular HSV that are undergoing this procedure.
Famciclovir (FCV), the oral prodrug of PCV, has increased oral bioavailability, and the active triphosphate form persists in the infected cell longer than ACV (36). FCV is currently approved for the treatment of herpes zoster and the treatment and suppression of genital herpes (7, 9). FCV has also been used in clinical trials for herpes labialis (2, 8, 11, 39, 41, 43, 44). A clinical trial by Spruance et al. (44) demonstrated that oral FCV decreased the median lesion size in a dose-dependent manner and that, at higher doses, it decreased the time to healing following UV radiation-induced HSV labialis. Those investigators obtained similar results with this model when oral FCV was combined with a topical corticosteroid (43). FCV prophylaxis was effective in preventing HSV recurrences following laser skin resurfacing (2). These studies indicate that oral FCV prophylaxis may benefit patients that suffer from numerous HSV recurrences.
We report the use of orally administered FCV as a therapy for ocular herpes infection in rabbits. We found a significant dose-dependent effect on the slit lamp examination (SLE) scores for the eyes of rabbits acutely infected with HSV-1 strain 17syn+. Comparisons of oral FCV to topical TFT (the treatment of choice for herpes keratitis) indicated that TFT was more effective in reducing HSV-1 lesions; however, FCV-treated rabbits had a significantly lower HSV-1 genome copy number in their trigeminal ganglia (TG) relative to that of control or TFT-treated rabbits. Therefore, oral FCV significantly reduced the severity of corneal lesions and may be beneficial in reducing morbidity in HSV keratitis patients.
MATERIALS AND METHODS
Virus and cells.
The virus used in this study was HSV-1 strain 17syn+ (received from the laboratory of Jack Stevens, University of California at Los Angeles). The virus was propagated on primary rabbit kidney (PRK) cells in Earle's minimal essential medium (Gibco Life Technologies, Gaithersburg, Md.) with 10% fetal bovine serum. Plaque assays were performed on CV-1 (African green monkey) cells. The presence of infectious virus in the reactivation experiments was assessed by placing tear film swabs in a tube containing a confluent monolayer of PRK cells. The swabs were removed after 48 h, and the monolayers were examined for cytopathic effect (CPE) and determined to be either positive or negative (4, 18, 24).
Drug experiments.
Several antiviral experiments were conducted to determine the efficacies of the therapies against acute ocular herpes keratitis. The experimental design was as follows.
(i) Oral gavage of FCV b.i.d.
The antiviral efficacy of oral FCV on acute ocular herpes was determined in rabbits inoculated with HSV-1 strain 17syn+. Corneas were examined for lesions by slit lamp microscopy with 0.1% fluorescein to visualize the corneal epithelium. The SLE score scale was as follows: 0, no corneal involvement; 0.25, punctate lesions; 0.5, multiple punctate and/or small dendritic lesions; 1.0, large dendritic lesions; 1.5, dendritic lesions with mild stromal involvement; 2.0, stromal involvement with the pupillary iris visible; 3.0, severe stromal involvement with the pupillary iris not visible; and 4.0, severe stromal keratitis with the peripheral iris not visible. The acute corneal lesions were initially assessed on postinoculation (p.i.) day 3, and the rabbits were divided into five treatment groups based on SLE scores to allow for an even distribution of high and low scores. Treatment groups consisted of rabbits administered a placebo (H2O) or 60, 120, 250, or 500 mg of FCV (provided as a powder from SmithKline Beecham Pharmaceuticals, Collegeville, Pa.) per kg of body weight dissolved in deionized water. The drug was delivered b.i.d. by gastric gavage using an 18-gauge feeding tube (Sherwood Medical, St. Louis, Mo.). The drug was flushed from the tube using 3.5 ml of water to ensure complete drug delivery. Some precipitation of the FCV in the 500-mg/kg solutions was noted during the gavage period, which could have resulted in the rabbits receiving a lower actual dose. The treatment period consisted of five consecutive days starting on p.i. day 3. Each of the FCV groups contained 5 rabbits, and the placebo group contained 10 rabbits. A second, similar experiment was conducted with treatment beginning on p.i. day 3 and continuing for eight consecutive days with 12 rabbits per group.
(ii) Comparisons of oral FCV to the standard therapy, TFT, for herpes keratitis.
The effect of oral FCV was also compared to the drug of choice in the treatment of herpes keratitis, TFT (Viroptic; GlaxoWellcome, Research Triangle Park, N.C.). Rabbits were inoculated with HSV-1 strain 17syn+, subjected to SLE on the appropriate day, and randomly assigned to treatment groups based on SLE scores. The treatment groups consisted of rabbits administered (i) 250 mg of FCV per kg b.i.d., (ii) a placebo (H2O) b.i.d., (iii) topical drops of TFT (1 drop given 5 times per day), or (iv) topical drops of balanced salt solution (BSS; 1 drop given 5 times per day). FCV and the placebo were given b.i.d. as described above, while the TFT and BSS drops were topically administered five times daily for eight consecutive days. Each treatment group, including the placebo group, contained 10 rabbits. The rationale for the start of treatment at 72 h is that it closely mimics the initiation of treatment in a clinical setting. In another group of rabbits, treatment with FCV and TFT was initiated 24 h after inoculation to assess the outcome of the treatment on herpetic keratitis and viral DNA load in the TG (see below).
Inoculation of rabbit eyes with HSV-1 strain 17syn+.
New Zealand White rabbits (1 to 1.5 kg; McNeil Rabbitry, Carriere, Miss.) were inoculated with 5 × 105 PFU of HSV-1 strain 17syn+ in each eye following light scarification. Each eye was anesthetized with a drop of 0.5% proparacaine HCl (Alcaine; Alcon, Humacao, Puerto Rico) and scarified with a 30-gauge needle in a 2 by 2 crosshatch pattern covering the center of the cornea. The viral suspension was placed in the lower cul-de-sac, and the eyelid was closed and gently massaged for 30 s. The acute infection was monitored by slit lamp microscopy starting on p.i. day 3. The SLE was done daily from p.i. days 3 through 10 and again on p.i. days 12 and 14. The rabbits were examined again on p.i. day 20 to assess the recovery of the corneal epithelium from the herpes keratitis. All rabbits exhibiting acute lesions with subsequent recovery were considered latently infected (24).
Acute viral titers were determined on the tear film samples collected from three eyes from different rabbits in each group. Prior to the start of daily treatments with FCV and TFT, a tear film sample was collected by placing a Dacron-tipped swab (Puritan; Hardwood Products Co., Guilford, Maine) in the lower cul-de-sac of the eye, with care being taken to avoid the cornea. The tears were allowed to absorb for 10 s, and the swab was removed and placed in 1 ml of Earle's minimal essential medium with 2% fetal calf serum. The tubes containing the swabs were agitated at 37°C for 1 h, the swabs were removed, and the eluate was used in plaque assays. The plaque assays were performed on CV-1 cells without an overlay and stopped 36 h after the addition of the eluate dilution by adding 5% buffered formalin. Plaques were visualized with crystal violet staining.
Spontaneous reactivation of latently infected rabbits that received FCV treatment during the acute infection.
Spontaneous reactivation was monitored for 20 continuous days starting on p.i. day 21 to determine the presence of virus. A Dacron swab was placed in the lower cul-de-sac of each eye and then passed gently over the conjunctiva and cornea to collect the tear film. The swab was placed in a tube containing a confluent PRK monolayer and monitored for CPE as described above.
Transcorneal epinephrine iontophoresis of latently infected rabbits that received FCV treatment during acute infection.
Prior to iontophoresis, each rabbit eye was again examined by slit lamp microscopy to determine the integrity of the corneal epithelium following spontaneous reactivation. Each rabbit eye that did not have corneal scarring or neovascularization underwent induced reactivation by transcorneal iontophoresis of 0.015% epinephrine (0.8 mA, 8 min) to induce viral shedding (24). Iontophoresis was performed for three consecutive days (22, 31). Ocular swabbing was conducted prior to iontophoresis each day and for 5 additional days (total of 7 days after the first iontophoresis procedure) as described above.
Collection of TG samples from the rabbits following each experiment.
The rabbits were handled and maintained in accordance with the guidelines of the Association for Research in Vision and Ophthalmology in its Resolution on the Use of Animals in Research. The TG were harvested and snap frozen in liquid nitrogen for later analysis (24). Rabbits were sacrificed by an intravenous injection of sodium pentobarbital (Sigma, St. Louis, Mo.), the hair and skin were removed from the skull area, and the skullcap was removed. The brain was removed, and the TG were exposed. The bony processes were removed, and the TG were excised by severing the connection at the optic chiasm at the lateral cranial nerve pairs and at the dorsal root entry zone of the brain.
Real-time quantitative PCR analysis of DNA extracted from the TG from rabbits infected with HSV-1 strain 17syn+.
To quantitate the viral load during latency, DNA was isolated from rabbit TG using a commercial extraction method (QIAamp tissue kit; Qiagen, Valencia, Calif.). Real-time quantitative PCRs were performed in 50-μl volumes containing 2× TaqMan Universal PCR Master mix (Perkin-Elmer, Norwalk, Conn.) and 100 ng of DNA for the detection of viral load. Reaction mixtures contained 200 nM concentrations of TaqMan primers and a 200 nM concentration of the TaqMan probe. Primer pairs and probes are described in Table 1 and were designed using Primer Express software (Perkin-Elmer). Probes were labeled at the 5′ end with the fluorescent reporter dye Fam and at the 3′ end with the fluorescent quencher dye Tamra (Synthegen, Houston, Tex.) to allow direct detection of the PCR product. Amplification and detection were performed using an ABI7700 sequence detector (PE Biosystems, Norwalk, Conn.) Relative copy number was calculated using a standard curve generated from purified HSV-1 (SC-16) viral DNA that had been serially diluted in 10 ng of rabbit genomic DNA (Clontech, Palo Alto, Calif.) per μl. Viral DNA was diluted to contain from 1 copy to 1 million copies in 2 μl and subjected to TaqMan PCR with each primer set to generate standard curves and evaluate relative primer sensitivity (40).
TABLE 1.
Primer sequences and probes used in real-time quantitative PCRs
| Primer | Sequence |
|---|---|
| gC (UL44) | |
| Forward | 5′-GATGCCGGTTTCGGAATTC-3′ |
| Reverse | 5′-CCCATGGAGTAACGCCATATCT-3′ |
| Probe | Fam-ACCCGCATGGAGTTCCGCCTC-Tamra |
| ICP27 (UL54) | |
| Forward | 5′-CGCCAAGAAAATTTCATCGAG-3′ |
| Reverse | 5′-ACATCTTGCACCACGCCAG-3′ |
| Probe | Fam-CTGGCCTCCGCCGACGAGAC-Tamra |
| β-Actin | |
| Forward | 5′-CCCCCTGAACCCCAAGG-3′ |
| Reverse | 5′-CCGGCGTGTTGAACGTCT-3′ |
| Probe | Fam-CAACCGCGAGAAGATGACCCAGATCA-Tamra |
Statistical analysis.
The statistical analysis of data was conducted as follows.
(i) SLE score analysis.
The SLE scores were analyzed using a nested design by analysis of variance (34). In this model, the rabbits were considered the nested effect within the treatments (drug or placebo). The main effects assessed in the model were treatments, days of SLE, and the interaction of treatments and days. The effects of the treatment and day were assessed individually using the nested effect rather than the overall error. Each treatment mean on each day was tested for statistical significance using a protected t test of least-square means (34).
(ii) Reactivation frequency analysis.
The ratios of the number of positive swabs for each rabbit to the total number of swabs collected for each FCV treatment group in both the spontaneous and epinephrine-induced reactivation experiments were analyzed using logistic regression analysis. In this analysis, the outcome was the frequency of positive swabs per rabbit compared to the number of total swabs and the dependent variable was the FCV concentration, with the placebo being assigned a concentration of 0 mg/kg (1).
(iii) HSV-1 genome copy number analysis.
HSV-1 genome copy number was analyzed using a one-way analysis of variance, with copy number as the outcome and treatment (drug or placebo at 24 or 72 h) as the dependent variable. We conducted individual comparisons of the results of protected t tests of least-square means (34, 51).
RESULTS
Dose-dependent effect of oral FCV on herpetic keratitis SLE scores.
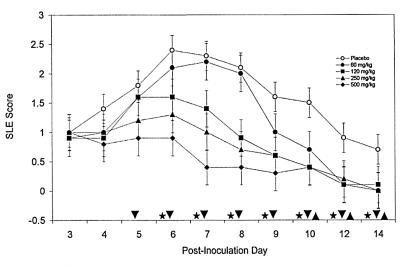
To determine the efficacy of oral FCV, rabbits were inoculated with 17syn+ and treated b.i.d. with various doses of oral FCV from p.i. days 3 through 7. The upper dose was selected in part based on the maximum amount of FCV we could dissolve (500 mg/kg); the lowest dose (60 mg/kg) was shown to be effective based on data obtained with murine HSV infection models (reviewed in references 13 and 45). Treatment with oral FCV demonstrated a statistically significant, dose-dependent improvement in keratitis SLE scores (Fig. 1). Statistical analysis indicates that the SLE scores of animals treated with the oral FCV doses of 120, 250, and 500 mg/kg were significantly lower than the SLE scores of animals treaetd with the placebo beginning on p.i. day 6 (third day of treatment; P < 0.05). The 60-mg/kg dose of FCV did not have a significant effect relative to that of the placebo on p.i. days 3 through 8. However, the SLE scores of this group became statistically less than those of the placebo group on p.i. days 9, 12, and 14. This significant difference was maintained in all the FCV treatment groups to the end of the evaluation period. The data were reproduced in a second independent experiment with treatment starting on p.i. day 3 and continuing through p.i. day 10 (data not shown).
FIG. 1.
Reduction in herpes keratitis SLE scores due to oral FCV therapy. Groups of five rabbits each were inoculated with 2 × 105 PFU of HSV-1 strain 17syn+ and treated b.i.d. with various doses of FCV by oral gavage. The keratitis was monitored by slit lamp microscopy from p.i. day 3 through day 14. The FCV doses were 60, 120, 250, and 500 mg/kg, and the placebo was water. SLE scores after FCV treatment that are significantly different from SLE scores after administration of the placebo (P = 0.0001) are represented by the symbols on the x axis, where ▾ is 250 and 500 mg of FCV per kg, ★ is 120 mg of FCV per kg, and ▴ is 60 mg of FCV per kg. The error bars represent the standard errors of the least-square means.
Efficacy of oral FCV compared to that of topical drops of TFT.
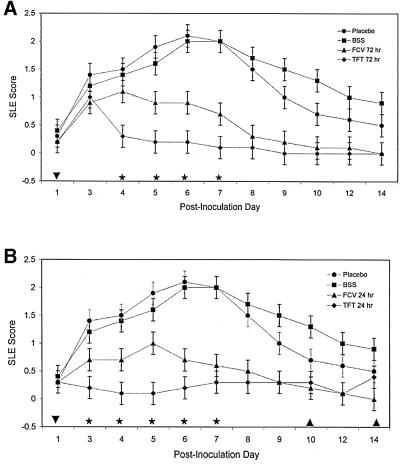
To determine if oral FCV was as effective as the current treatment regimen for herpetic keratitis, topical drops of TFT, rabbits were inoculated with 17syn+ and treated with either oral FCV at 250 mg/kg b.i.d. or topical drops of 1% TFT five times a day. There was a statistically significant improvement in the herpetic keratitis scores for both FCV and TFT compared to the score for no drug when treatment was initiated at 72 h p.i. (Fig. 2A). Topical TFT was significantly better than FCV during p.i. days 4 through 7; however, by p.i. day 8, the sixth day of oral FCV, there was no difference in the SLE scores for the two drugs. When treatment was begun at 24 h p.i., both FCV and TFT were more efficacious than the placebos in reducing the SLE scores in herpetic keratitis (Fig. 2B), similar to what occurred when treatment started at 72 h p.i. Up to p.i. day 6, TFT was significantly more effective in reducing SLE scores than FCV. Both drugs appeared to have equal efficacies from p.i. day 7 through 14. The initiation of FCV treatment 24 h after inoculation did not decrease the interval of time for FCV to equal TFT in the SLE scores (6 days of treatment).
FIG. 2.
Reduction in keratitis SLE scores due to oral FCV or topical TFT. Groups of 10 rabbits each were inoculated with 2 × 105 PFU of HSV-1 strain 17syn+ and treated with oral FCV at 250 mg/kg given b.i.d. by oral gavage or with topical drops of TFT given five times a day. The treatments were started at either 72 h (A) or 24 h (B) and continued for eight consecutive days. The keratitis was monitored by slit lamp microscopy, as indicated on the x axis of each graph. The placebo was water given orally, while BSS was given as topical drops. TFT SLE scores that are significantly different from FCV SLE scores (★; P = 0.0001), TFT and FCV SLE scores that exhibit no difference (▾), and TFT, FCV, and BSS SLE scores that exhibit no difference (▴) are noted on the x axis. The error bars represent the standard errors of the least-square means.
Effects of oral FCV and topical TFT on the acute viral titers in the tear film.
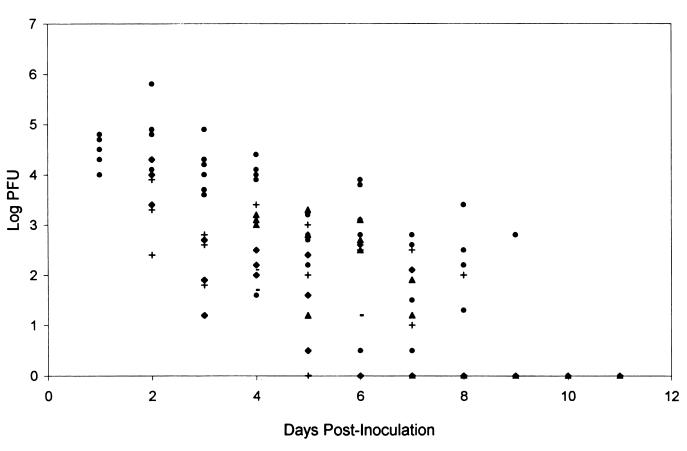
The effects of oral FCV and topical TFT were also evaluated by determining the acute viral titers present in the tears of rabbits receiving antiviral therapy or no drug. Acute viral titers were determined for each group prior to the start of daily therapy. Linear-regression analysis of the scatter plot (Fig. 3) indicates that there was no significant difference in the amounts of virus present in the tears of the rabbits in the antiviral groups compared to that present in the tears of the rabbits in the placebo group. TFT-treated rabbits did clear the virus more quickly than FCV- or placebo-treated rabbits. No infectious virus was detected after 4 days of treatment regardless of when treatment was initiated (24 or 72 h p.i.). It took at least 5 days of treatment with FCV before no infectious virus was detected. By p.i. day 10, rabbits in all groups had no detectable infectious virus in their tear films.
FIG. 3.
Scatter plot of acute viral titers in the tear film samples of rabbits receiving the placebo or antiviral therapy. Eyes from three randomly selected rabbits were swabbed to collect tear film samples, which were assessed for infectious viral titer by plaque assay. The swabs were collected prior to treatment with oral FCV at 250 mg/kg given b.i.d. by oral gavage or with topical drops of TFT given five times a day. The treatments were started at either 24 or 72 h and continued for eight consecutive days. Treatment groups consisted of rabbits that received no drug (●), TFT started at 24 h (⧫), TFT started at 72 h (−), FCV started at 24 h (+), and FCV started at 72 h (▴).
Effect of oral FCV given during acute infection on reactivation.
Rabbits previously treated during acute infection with various doses of FCV were assessed for spontaneous viral shedding in their tear films following the establishment of latency. A high frequency of spontaneous shedding was observed in all treatment groups (Tables 2 to 4). Statistical analysis by logistic regression indicates that there is no significant difference between results for the FCV-treated groups and the placebo group (overall model P value = 0.7308). We also examined the induced-reactivation frequencies following transcorneal iontophoresis of epinephrine (Tables 2, 3, and 5). Statistical analysis by logistic regression indicated that FCV treatment during acute infection did not affect induced reactivation (overall model P value = 0.3141).
TABLE 2.
Spontaneous and induced reactivation frequencies in latently infected rabbits after oral FCV treatment during acute infection
| FCV treatment (mg/kg)c | Spontaneous reactivationa
|
Induced reactivationb
|
||||
|---|---|---|---|---|---|---|
| Total no. of rabbitsd | % of positive swabs/rabbit (mean ± SE)e | Total no. of swabsf | Total no. of rabbits | % of positive swabs/rabbit (mean ± SE) | Total no. of swabs | |
| 0 (water) | 6 | 26 ± 7 | 239 | 5 | 47 ± 10 | 70 |
| 60 | 4 | 30 ± 9 | 159 | 3 | 36 ± 20 | 42 |
| 120 | 5g | 24 ± 9 | 182 | 4h | 16 ± 8 | 49 |
| 250 | 5 | 24 ± 9 | 200 | 5 | 23 ± 9 | 70 |
| 500 | 4 | 28 ± 8 | 159 | 4 | 36 ± 7 | 56 |
Spontaneous reactivation indicates that virus was shed without an induction stimulus.
In induced reactivation, rabbits underwent transcorneal iontophoresis with 0.015% epinephrine.
Treatment the rabbits received during acute infection; see Materials and Methods.
Number of rabbits included in each group.
The means and standard errors of the means are calculated from results for multiple rabbits in each group from one experiment. The spontaneous and induced reactivation experiments were conducted on the same set of rabbits.
Total number of swabs collected over the duration of the study; see Materials and Methods.
One rabbit died during the spontaneous swabbing experiment.
One rabbit had only one eye iontophoresed due to corneal scarring.
TABLE 4.
Selected data from rabbits undergoing spontaneous reactivation
| Rabbit | Treatment | Eyea | Day p.i.
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | |||
| J2 | Placebo (water) | OD | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| OS | − | − | − | − | − | − | − | − | − | − | + | + | + | − | − | − | − | − | − | − | ||
| J9 | OD | − | − | + | − | − | − | + | − | + | + | + | + | + | + | + | + | + | + | + | + | |
| OS | − | − | + | + | + | + | − | − | − | − | − | − | − | − | − | + | + | + | + | − | ||
| J3 | 60 mg of FCV per kg | OD | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| OS | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| J13 | OD | − | − | − | − | − | − | + | + | − | + | + | + | + | + | − | − | − | − | − | − | |
| OS | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| J6 | 120 mg of FCV per kg | OD | − | − | − | + | + | + | + | + | + | + | + | NDb | ND | ND | ND | ND | ND | ND | ND | ND |
| OS | − | − | − | + | + | + | + | + | + | + | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| J22 | OD | − | − | − | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | |
| OS | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| J12 | 250 mg of FCV per kg | OD | − | − | + | − | − | − | − | + | + | + | + | + | + | + | + | − | − | − | − | − |
| OS | − | − | + | − | + | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | ||
| J16 | OD | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | + | + | + | + | − | |
| OS | − | − | + | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| J11 | 500 mg of FCV per kg | OD | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| OS | − | − | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | + | ||
| J26 | OD | − | − | + | + | + | + | + | + | + | + | − | − | − | − | − | − | − | − | − | − | |
| OS | − | − | + | − | + | − | + | + | + | + | + | + | + | − | − | − | − | − | − | − | ||
OD, right eye; OS, left eye.
ND, not done due to death of the rabbit.
TABLE 3.
Spontaneous- and induced-reactivation summarya
| Rabbit | Spontaneous sheddingb after administration of FCV at:
|
Induced reactivationc after administration of FCV at:
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 mg/kg
|
60 mg/kg
|
120 mg/kg
|
250 mg/kg
|
500 mg/kg
|
0 mg/kg
|
60 mg/kg
|
120 mg/kg
|
250 mg/kg
|
500 mg/kg
|
|||||||||||
| OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | OD | |
| 1 | 3 | 0 | 0 | 13 | 8d | 8d | 4 | 3 | 4 | 1 | 6 | 6 | 1 | NDe | ND | ND | 1 | 5 | 1 | 4 |
| 2 | 8 | 14 | 3f | 3 | 2 | 3 | 1 | 0 | 1f | 12 | 2 | 2 | 5 | 6 | 1 | 4 | 0 | 0 | 2 | 1 |
| 3 | 10 | 12 | 0 | 7 | 0 | 0 | 10 | 9 | 9 | 1 | 2 | 0 | ND | 0 | 2 | 0 | 0 | 3 | 4 | 3 |
| 4 | 6 | 2f | 11 | 11 | 1 | 9 | 3 | 5 | 9 | 8 | 6 | ND | 3 | 0 | 0 | 1 | 4 | 0 | 4 | 1 |
| 5 | 1 | 1 | 2 | 2 | 10 | 3 | 1 | ND | ND | 0 | 3 | 0 | ||||||||
| 6 | 2 | 1 | 4 | 2 | ||||||||||||||||
Data are presented for each rabbit eye used in the spontaneous- and induced-reactivation experiments. OS, left eye; OD, right eye.
Spontaneous reactivation indicates that virus was shed without an induction stimulus; data are for 20 days per eye unless noted otherwise.
In induced reactivation, rabbits underwent transcorneal iontophoresis with 0.015% epinephrine; data are for 7 days per eye.
One rabbit died during spontaneous swabbing, and only 11 swabs per eye were collected.
ND, not done due to corneal scarring.
One sample was contaminated and not counted in the summary; data are for 19 days per eye.
TABLE 5.
Selected data from rabbits undergoing epinephrine-induced reactivation
| Rabbit | Treatment | Eyea | Day p.i.
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | |||
| J2 | Placebo (water) | OD | − | − | + | + | + | + | + | + |
| OS | − | − | + | + | + | + | + | + | ||
| J9 | OD | − | − | − | − | − | − | + | + | |
| OS | − | − | + | − | − | − | − | + | ||
| J3 | 60 mg of FCV per kg | OD | NDb | ND | ND | ND | ND | ND | ND | ND |
| OS | − | − | − | − | − | − | − | + | ||
| J13 | OD | − | − | − | − | − | − | − | − | |
| OS | ND | ND | ND | ND | ND | ND | ND | ND | ||
| J7 | 120 mg of FCV per kg | OD | − | − | − | − | + | + | + | + |
| OS | − | − | − | − | − | + | − | − | ||
| J22 | OD | − | − | − | − | − | − | − | + | |
| OS | − | − | − | − | − | − | − | − | ||
| J12 | 250 mg of FCV per kg | OD | − | − | + | + | + | − | − | − |
| OS | − | − | − | − | − | − | − | − | ||
| J16 | OD | − | − | − | − | − | − | − | − | |
| OS | − | − | − | − | + | + | + | + | ||
| J11 | 500 mg of FCV per kg | OD | − | − | − | − | + | + | + | + |
| OS | − | − | − | + | − | − | − | − | ||
| J26 | OD | − | − | − | − | − | − | − | + | |
| OS | − | − | + | − | + | + | − | + | ||
OD, right eye; OS, left eye.
ND, not done due to corneal scarring.
Effect of oral FCV or topical TFT therapy on the establishment of latency in TG.
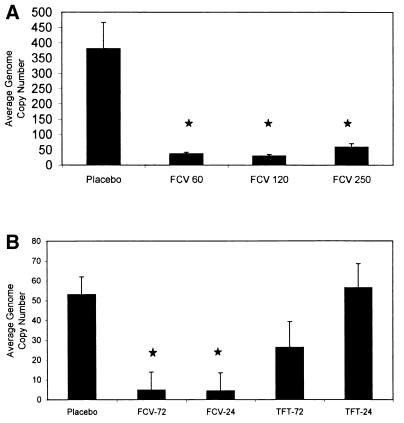
Real-time quantitative PCR was used to determine the effect of oral FCV on the establishment of latency in TG. The relative HSV-1 genome copy numbers in TG were significantly lower in the FCV-treated rabbits than in the placebo-treated rabbits (Fig. 4A and Table 6). Statistical analysis indicated that oral FCV, regardless of the dose, significantly reduced the copy number of the viral gene for gC (Fig. 4A) (P < 0.05). The copy number was verified with a second primer pair for ICP27 (Table 6). There was not a linear relationship between DNA copy number and FCV dose as was seen in the SLE scores.
FIG. 4.
Quantitation of the HSV-1 genome copy number in the TG of latently infected rabbits previously treated with antiviral therapy or placebo. The TG were analyzed using real-time quantitative PCR with primer pairs and a probe for glycoprotein C using 100 ng of total TG DNA collected. (A) Average HSV-1 genome copy numbers in the TG of latently infected rabbits previously treated with no drug or 60, 120, or 250 mg of oral FCV per kg; (B) average HSV-1 genome copy numbers in the TG of latently infected rabbits previously treated with no drug, oral FCV, or topical TFT started at 24 or 72 h p.i. The error bars represent the standard errors of the least-square means, and the ★ indicates a P value of 0.0001.
TABLE 6.
Quantitative analysis of HSV-1 DNA and cellular DNA of individual rabbit TG with and without FCV treatmenta
| Rabbit | No. of copies of gC gene after treatment with:
|
No. of copies of ICP27 gene after treatment with:
|
No. of copies of β-actin (104) after treatment with:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | FCV at:
|
Placebo | FCV at:
|
Placebo | FCV at:
|
|||||||
| 60 mg/kg | 120 mg/kg | 250 mg/kg | 60 mg/kg | 120 mg/kg | 250 mg/kg | 60 mg/kg | 120 mg/kg | 250 mg/kg | ||||
| 1 | 132.8 | 42.2 | 12.5 | 69.7 | 74.9 | 11.3 | 7.7 | 60.8 | 21.5 | 23.0 | 23.0 | 22.4 |
| 2 | 276.4 | 34.9 | 22.1 | 27.8 | 151.3 | 18.3 | 18.4 | 14.4 | 21.6 | 23.6 | 22.7 | 22.6 |
| 3 | 137.4 | 32.2 | 42.0 | 30.3 | 28.5 | 11.2 | 20.0 | 14.2 | 22.1 | 23.8 | 23.3 | 22.6 |
| 4 | 673.4 | 46.3 | 30.3 | 48.6 | 401.5 | 17.8 | 19.5 | 23.2 | 20.1 | 23.6 | 22.6 | 22.2 |
| 5 | 569.5 | 35.0 | 22.9 | 56.2 | 91.0 | 5.5 | 18.6 | 18.7 | 22.0 | 22.9 | 23.1 | 21.1 |
| 6 | 350.3 | 46.9 | 21.1 | 63.6 | 72.4 | 33.3 | 6.5 | 13.0 | 22.0 | 22.2 | 23.0 | 21.3 |
| 7 | 219.7 | 45.1 | 15.3 | 73.4 | 41.9 | 32.6 | 5.8 | 31.1 | 22.2 | 23.9 | 22.6 | 22.1 |
| 8 | 84.4 | 10.4 | 29.8 | 152.6 | 18.9 | 8.8 | 8.9 | 21.6 | 22.0 | 23.1 | 21.8 | 22.2 |
| 9 | 919.1 | 60.2 | 57.8 | 21.5 | 492.3 | 34.0 | 36.7 | 8.3 | 21.9 | 22.7 | 22.0 | 20.5 |
| 10 | 450.0 | 22.0 | 48.6 | 48.9 | 158.1 | 16.5 | 41.3 | 7.8 | 22.2 | 22.6 | 23.1 | 22.0 |
| Mean | 381.3 | 37.5 | 30.2 | 59.3 | 153.1 | 18.9 | 18.3 | 21.3 | 21.8 | 23.2 | 22.7 | 21.9 |
| SE | 85.8 | 4.4 | 4.7 | 11.8 | 51.6 | 3.4 | 3.9 | 4.9 | 0.2 | 0.2 | 0.2 | 0.2 |
One hundred nanograms of TG DNA was subjected to quantitative PCR, and copy numbers relative to those of a standard curve are shown. The means and standard errors of the means are calculated from results for multiple rabbits in each group from one experiment.
Since TFT was effective in reducing both SLE scores and acute viral titers, we compared the HSV-1 viral DNA loads in the TG of latently infected rabbits that received either TFT or FCV by real-time PCR. The HSV-1 genome copy numbers when primers for gC were used were significantly reduced in rabbits receiving the oral dose of FCV, regardless of the initiation time (24 or 72 h p.i.) (P value = 0.0003), compared to those in rabbits receiving no treatment or topical TFT at both 24 and 72 h (Fig. 4B). There was no significant difference between copy numbers in TFT-treated and untreated TG (P values = 0.8320 and 0.0787 for 24 and 72 h p.i., respectively). The copy numbers were verified with a second primer pair for ICP27 (data not shown).
Effects of FCV and TFT therapy on rabbit survival rates.
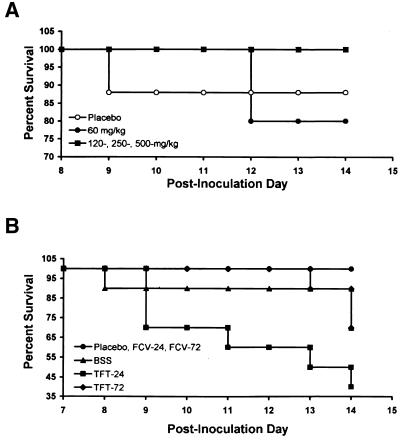
To determine the effects of FCV and TFT on the survival rates of rabbits during the acute infection, we monitored the rabbits for 2 weeks following inoculation. Rabbits receiving oral FCV b.i.d. (120, 250, and 500 mg/kg) had a significantly higher rate of survival than that of the placebo controls (Fig. 5A) (P = 0.0001) as determined by the Wilcoxon equality-over-stratum analysis (33). While topical TFT can reduce the severity of herpetic keratitis and viral titers in the tear film, it is unable to protect the animal from the morbidity of herpes encephalitis as determined by seizures, head tilt, lateral recumbancy, and death. FCV-treated rabbits had a significantly higher rate of survival than TFT-treated rabbits (Fig. 5B) (P = 0.0001).
FIG. 5.
Trend for an increase in rates of survival of rabbits receiving oral FCV therapy. Rabbits were inoculated with 17syn+ and then treated with either oral FCV or topical TFT drops. (A) The rate of survival of rabbits that received FCV was higher than that of rabbits that received the placebo (water) or 60 mg of FCV per kg (P = 0.0001). (B) The rates of survival of the rabbits that were treated with either TFT or FCV show that FCV protects the animal better than TFT does (P = 0.0001). BSS was delivered as topical drops.
DISCUSSION
We used HSV-1 infection of rabbit corneas to determine the efficacy of oral FCV on ocular herpes infection and the establishment of latency. An advantage of performing such a study with rabbits is that it is possible to assess the effect of antiviral therapy on spontaneous and induced HSV-1 reactivation. HSV-1 strain 17syn+ has been used in the Hill laboratory in numerous reactivation studies because of its high frequency of reactivation in rabbits (5, 9, 19–21, 23). The use of this virus allows us to assess any changes in the pathogenesis of the infection caused by the antiviral therapy. Our results indicate that oral FCV administered b.i.d. can, in a dose-dependent manner, significantly improve the herpetic keratitis SLE scores. While oral FCV was not as effective as topical drops of TFT in reducing acute ocular infection or viral titers in the tear film, it was significantly better than the placebo, regardless of whether therapy was started at 24 or 72 h p.i. In addition, although therapeutic doses of FCV did not prevent the establishment of latency or decrease the spontaneous- and induced-reactivation frequencies compared to what occurred with the placebo, there was a significant reduction in the genome copy number in the TG. This reduction was not dose dependent. Rabbits that received FCV also had a significant increase in the rate of survival compared to that of rabbits that received TFT.
Many antiviral efficacy studies are conducted with mice because they are less expensive, easier to house, and require much less drug. Brandt et al. (6) used mice to demonstrate that TFT at various topical doses could improve SLE scores and reduce viral titers in the eye and TG, as well as reduce reactivation in TG explant cultures. LeBlanc et al. (32) also used mice to determine the efficacies of FCV and VCV and reported that the two drugs were equally effective in reducing viral titers in the eye and mortality rates; however, VCV was better than FCV at decreasing the viral load in the TG of latently infected mice. Placebo-treated mice did not survive, so the overall effect of viral load reduction could not be assessed. The lower HSV genome copy numbers, however, did not affect reactivation rates either in TG explant cultures or following UV irradiation of the cornea (32). Kaufman et al. (30) have demonstrated, with the rabbit eye model, that a topical ointment of PCV, the parent drug of FCV, while not as efficacious as TFT, may prove beneficial in the treatment of epithelial keratitis in a clinical venue. Neither TFT nor PCV had an effect on spontaneous recurrences after therapy was stopped. While we did not assess the effect of TFT on induced reactivation in our study, we found no difference between the viral genome copy number in TFT-treated rabbits and that in the placebo-treated group. We predict that reactivation frequencies in the TFT group would be similar to those in the untreated group.
Other routes of HSV inoculation have also been investigated to determine the efficacies of antiviral agents. The mouse footpad was used to determine the efficacy of ACV given intraperitoneally as well as in drinking water; no evidence of acute viral replication was seen in the TG 4 days p.i., and there was a significant reduction in the number of neurons harboring latent virus as determined with the reporter gene for β-galactosidase (12). Ashton et al. (3) demonstrated that FCV was better than ACV in reducing titers in peritoneal washings from mice infected intraperitoneally with HSV-1 strain SC16. This difference was suggested to be due to the increase in the intracellular half-life of the active PCV triphosphate compared to that of the ACV triphosphate. Kaufman et al. (29) examined spontaneous HSV-1 shedding in rabbits receiving 1 mg of ACV per ml in drinking water. They found that continuous therapy did not prevent recurrent shedding even though the serum drug concentration was 1 to 2 μmol/liter (29). Nesburn et al. (35) used the same dose of ACV in rabbits undergoing epinephrine-induced reactivation and found that they could prevent viral shedding following iontophoresis. No serum drug value was reported. HSV-1 latently infected rabbits undergoing excimer laser keratectomy were given intraperitoneal doses of VCV to determine if reactivation could be prevented. Intraperitoneal doses of 50, 100, and 150 mg/kg/day resulted in levels in serum of 5.8, 9.85, and 16.3 μg/h/ml, similar to levels in human serum following a single dose of 500 or 750 mg (10). Only the two higher doses prevented HSV reactivation following the laser surgery.
In a series of reports, researchers (14, 46, 48, 50) using the mouse ear demonstrated that FCV is better than VCV in clearing infectious virus from tissues (ear and brain stem), reducing inflammation in the ear, restoring weight gain, and preventing reactivation in TG cocultures. They found that treatment with either drug could reduce the neuronal latency but could not completely prevent the establishment of latent virus in the innervating ganglion (15, 48). FCV significantly reduced the number of latent neurons compared to the number reduced by VCV or no treatment as detected by in situ hybridization, regardless of when therapy was initiated. Despite finding latently infected neurons, explant cultures from mice treated with FCV or VCV (starting either 1 day before or 1 day after inoculation) were negative (15, 47, 49). There is a poor correlation between the number of positive latent neurons and the number of explant cultures that yield virus.
Thackray and Field concluded that the ocular route of inoculation used by LeBlanc may lead to the uptake of the virus by the nerve endings and axonal transport for the unamplified colonization of the TG (49). The use of high inoculation titers may allow for the direct transport of virus to the TG, but as little as 60 mg of FCV per kg b.i.d. prevented the amplification of the genome in the TG. FCV treatment during an acute infection caused a nonlinear reduction in HSV-1 genome copy number in the TG without affecting the frequency of spontaneous viral shedding or epinephrine-induced reactivation compared to what occurred in placebo-treated rabbits. In contrast, acute viral tear film titers of FCV-treated animals were not significantly different relative to those of placebo-treated rabbits. FCV-treated animals cleared virus from the tear film in 5 to 6 days, whereas placebo-treated rabbits required 10 days. The variability of this finding may be due to the testing of three random eyes from each group per day rather than the same three eyes throughout the entire acute phase. It appears that FCV can affect HSV copy number at a lower concentration than the concentration needed to have an effect on corneal infection. However, the minimum dose of FCV required to decrease genome copy number in the TG has not been established in our study.
Treatment with topical TFT, the treatment of choice in clinics, inhibited viral replication at the corneal surface, as observed by decreased virus titer in tear film samples after 4 days of treatment. However, TFT did not prevent viral replication in TG, since genome copy numbers detected by real-time PCR were similar to those detected in TG obtained from placebo-treated animals (regardless of the time of initiation of treatment). Moreover, treatment with TFT had no effect on the incidence of encephalitis-induced mortality. Rabbits treated with FCV had a significantly higher survival rate than either TFT-treated or untreated animals.
Extrapolation from results of rabbit studies to determine efficacy in humans is difficult. An in vitro study using liver extracts of the enzyme aldehyde oxidase indicates that the rabbit enzyme metabolizes FCV differently than the human enzyme does (38). Differences in the metabolism of FCV and PCV were also seen in guinea pigs and rats (38, 52). Healthy individuals were administered a range of therapeutic doses of FCV (125, 250, 500, and 750 mg) as a single dose. Plasma PCV levels increased in a linear manner with the increasing FCV dose (37). No plasma PCV levels were determined in our study. However, we have demonstrated the activity of FCV in doses as low as 60 mg/kg b.i.d. in reducing viral load in TG and decreases in the severity of corneal lesions in a dose-dependent manner. These data demonstrate the effective conversion of FCV to PCV. While the doses used in this study are quite high, clinical trials using from 125 to 2,250 mg of FCV daily to treat herpes zoster or genital herpes have been reported with very few adverse effects (36).
Clinical trials have shown that FCV is effective in the treatment of acute herpes labialis and genital herpes (39, 41) and as a prophylactic treatment of UV-induced herpes labialis, excimer laser skin resurfacing, and genital herpes (2, 11, 43, 44). We report that FCV may be beneficial in reducing corneal morbidity in patients with recurrent herpes keratitis. Therapeutic doses of FCV improved the SLE scores in rabbits acutely infected with HSV-1 and resulted in a significant decrease in the HSV-1 genome copy number in the TG of latently infected rabbits. The use of FCV during an episode of recurrent keratitis may reduce the amount of acute virus in the TG, thereby reducing the establishment of new latent foci. Further studies need to be conducted to determine the efficacy of prophylactic FCV treatment for patients with recurrent herpes keratitis.
ACKNOWLEDGMENTS
This work was supported in part by U.S. Public Health Service grants EY06311 (J.M.H.), EY06996 (J.M.L.), and EY06986 (M.E.M.) from the National Eye Institute, National Institutes of Health, and an unrestricted departmental award to the LSU Eye Center from Research to Prevent Blindness, Inc., New York, N.Y. J.M.H. is supported by a 2001 Senior Scientific Investigator award from Research to Prevent Blindness, Inc.
We gratefully acknowledge the technical expertise of Maxine Simpson-Evans and the statistical analysis by Hilary W. Thompson. We also acknowledge Rhonda Gagnard, Marianne Mullens, Arnab Ray, Kristi App, and Kristina Braud for their assistance with the animals.
REFERENCES
- 1.Agresti A. Categorical data analysis. New York, N.Y: Wiley; 1990. [Google Scholar]
- 2.Alster T S, Nanni C A. Famciclovir prophylaxis of herpes simplex virus reactivation after laser skin resurfacing. Dermatol Surg. 1999;25:242–246. doi: 10.1046/j.1524-4725.1999.08197.x. [DOI] [PubMed] [Google Scholar]
- 3.Ashton R J, Abbott K H, Smith G M, Sutton D. Antiviral activity of famciclovir and acyclovir in mice infected intraperitoneally with herpes simplex virus type 1 SC16. J Antimicrob Chemother. 1994;34:287–290. doi: 10.1093/jac/34.2.287. [DOI] [PubMed] [Google Scholar]
- 4.Berman E J, Hill J M. Spontaneous ocular shedding of HSV-1 in latently infected rabbits. Investig Ophthalmol Vis Sci. 1985;26:587–590. [PubMed] [Google Scholar]
- 5.Bloom D C, Hill J M, Devi-Rao G, Wagner E K, Feldman L T, Stevens J G. A 348-base-pair region in the latency-associated transcript facilitates herpes simplex virus type 1 reactivation. J Virol. 1996;70:2449–2459. doi: 10.1128/jvi.70.4.2449-2459.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt C R, Coakley L M, Grau D R. A murine model of herpes simplex virus-induced ocular disease for antiviral drug testing. J Virol Methods. 1992;36:209–222. doi: 10.1016/0166-0934(92)90052-f. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E. Antivirals for the treatment of herpesvirus infections. J Antimicrob Chemother. 1993;32(Suppl. A):121–132. doi: 10.1093/jac/32.suppl_a.121. [DOI] [PubMed] [Google Scholar]
- 8.Degreef H the Famciclovir Herpes Zoster Clinical Study Group. Famciclovir, a new oral antiherpes drug: results of the first controlled clinical study demonstrating its efficacy and safety in the treatment of uncomplicated herpes zoster in immunocompetent patients. Int J Antimicrob Agents. 1994;4:241–246. doi: 10.1016/0924-8579(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 9.Devi-Rao G B, Aguilar J S, Rice M K, Garza H H, Jr, Bloom D C, Hill J M, Wagner E K. Herpes simplex virus genome replication and transcription during induced reactivation in the rabbit eye. J Virol. 1997;71:7039–7047. doi: 10.1128/jvi.71.9.7039-7047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhaliwal D K, Romanowski E G, Yates K A, Barnhorst D A, Goldstein M, Deeter R G, Fish D N, Gordon Y J. Valacyclovir inhibits recovery of ocular HSV-1 after experimental reactivation by excimer laser keratectomy. Cornea. 1999;18:693–699. doi: 10.1097/00003226-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Mitoma F, Sibbald R G, Shafran S D, Boon R, Saltzman R L. Oral famciclovir for the suppression of recurrent genital herpes: a randomized controlled trial. Collaborative Famciclovir Genital Herpes Research Group. JAMA. 1998;280:887–892. doi: 10.1001/jama.280.10.887. [DOI] [PubMed] [Google Scholar]
- 12.Dobson A T, Little B B, Scott L L. Prevention of herpes simplex virus infection and latency by prophylactic treatment with acyclovir in a weanling mouse model. Am J Obstet Gynecol. 1998;179:527–532. doi: 10.1016/s0002-9378(98)70390-4. [DOI] [PubMed] [Google Scholar]
- 13.Field H J. Famciclovir—origins, progress and prospects. Exp Opin Investig Drugs. 1996;5:925–938. [Google Scholar]
- 14.Field H J, Tewari D, Sutton D, Thackray A M. Comparison of efficacies of famciclovir and valaciclovir against herpes simplex virus type 1 in a murine immunosuppression model. Antimicrob Agents Chemother. 1995;39:1114–1119. doi: 10.1128/aac.39.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Field H J, Thackray A M. Early therapy with valaciclovir or famciclovir reduces but does not abrogate herpes simplex virus neuronal latency. Nucleosides Nucleotides Nucleic Acids. 2000;19:461–470. doi: 10.1080/15257770008033021. [DOI] [PubMed] [Google Scholar]
- 16.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 17.Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease—effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol. 2000;118:1030–1036. [PubMed] [Google Scholar]
- 18.Hill J M, Dudley J B, Shimomura Y, Kaufman H E. Quantitation and kinetics of adrenergic induced HSV-1 ocular shedding. Curr Eye Res. 1986;5:241–246. doi: 10.3109/02713688609020049. [DOI] [PubMed] [Google Scholar]
- 19.Hill J M, Garza H H, Jr, Su Y H, Meegalla R, Hanna L A, Loutsch J M, Thompson H W, Varnell E D, Bloom D C, Block T M. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J Virol. 1997;71:6555–6559. doi: 10.1128/jvi.71.9.6555-6559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill J M, Gebhardt B M, Wen R, Bouterie A M, Thompson H W, O'Callaghan R J, Halford W P, Kaufman H E. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J Virol. 1996;70:3137–3141. doi: 10.1128/jvi.70.5.3137-3141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J M, Maggioncalda J B, Garza H H, Jr, Su Y H, Fraser N W, Block T M. In vivo epinephrine reactivation of ocular herpes simplex virus type 1 in the rabbit is correlated to a 370-base-pair region located between the promoter and the 5′ end of the 2.0-kilobase latency-associated transcript. J Virol. 1996;70:7270–7274. doi: 10.1128/jvi.70.10.7270-7274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J M, O'Callaghan R J, Hobden J A. Ocular iontophoresis. In: Mitra A K, editor. Ophthalmic drug delivery system. New York, N.Y: Marcel Dekker, Inc; 1993. pp. 331–354. [Google Scholar]
- 23.Hill J M, Rayfield M A, Haruta Y. Strain specificity of spontaneous and adrenergically induced HSV-1 ocular reactivation in latently infected rabbits. Curr Eye Res. 1987;6:91–97. doi: 10.3109/02713688709020074. [DOI] [PubMed] [Google Scholar]
- 24.Hill J M, Wen R, Halford W P. Pathogenesis and molecular biology of ocular HSV in the rabbit. In: Brown M S, MacLean A R, editors. Herpes simplex virus protocols. Totowa, N.J: Humana Press/Wiley; 1998. pp. 291–315. [Google Scholar]
- 25.Kaufman H E. Clinical cure of herpes simplex keratitis by 5-iodo-2′-deoxyuridine. Proc Soc Exp Biol Med. 1962;109:251–252. doi: 10.3181/00379727-109-27169. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman H E. The first effective antiviral. In: Adams J, Merluzzi V J, editors. The search for antiviral drugs. Boston, Mass: Birkhauser; 1993. pp. 1–21. [Google Scholar]
- 27.Kaufman H E, Martola E-L, Dohlman C H. Use of 5-iodo-2′-deoxyuridine (IDU) in treatment of herpes simplex kertitis. Arch Ophthalmol. 1962;68:235–239. doi: 10.1001/archopht.1962.00960030239015. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman H E, Rayfield M A, Gebhardt B M. Herpes simplex viral infections. In: Kaufman H E, Barron B A, McDonald M B, editors. The cornea. Newport, Mass: Butterworth-Heinemann; 1998. pp. 247–277. [Google Scholar]
- 29.Kaufman H E, Varnell E D, Centifanto-Fitzgerald Y M, DeClercq E, Kissling G E. Oral antiviral drugs in experimental herpes simplex keratitis. Antimicrob Agents Chemother. 1983;24:888–891. doi: 10.1128/aac.24.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman H E, Varnell E D, Thompson H W. Trifluridine, cidofovir, and penciclovir in the treatment of experimental herpetic keratitis. Arch Ophthalmol. 1998;116:777–780. doi: 10.1001/archopht.116.6.777. [DOI] [PubMed] [Google Scholar]
- 31.Kwon B S, Gangarosa L P, Burch K D, deBack J, Hill J M. Induction of ocular herpes simplex virus shedding by iontophoresis of epinephrine into rabbit cornea. Investig Ophthalmol Vis Sci. 1981;21:442–449. [PubMed] [Google Scholar]
- 32.LeBlanc R A, Pesnicak L, Godleski M, Straus S E. The comparative effects of famciclovir and valaciclovir on herpes simplex virus type 1 infection, latency, and reactivation in mice. J Infect Dis. 1999;180:594–599. doi: 10.1086/314962. [DOI] [PubMed] [Google Scholar]
- 33.Mera R, Thompson H W, Prasad C. Analyzing data from experiments in which the outcome is time to an event. Nutr Neurosci. 1998;1:319–326. doi: 10.1080/1028415X.1998.11747242. [DOI] [PubMed] [Google Scholar]
- 34.Milliken G A, Johnson D E. Analysis of messy data. New York, N.Y: Van Nostrand Reinhold; 1984. [Google Scholar]
- 35.Nesburn A B, Willey D E, Trousdale M D. Effect of intensive acyclovir therapy during artificial reactivation of latent herpes simplex virus (41563) Proc Soc Exp Biol Med. 1983;172:316–323. doi: 10.3181/00379727-172-41563. [DOI] [PubMed] [Google Scholar]
- 36.Perry C M, Wagstaff A J. Famciclovir. A review of its pharmacological properties and therapeutic efficacy in herpesvirus infections. Drugs. 1995;50:396–415. doi: 10.2165/00003495-199550020-00011. [DOI] [PubMed] [Google Scholar]
- 37.Pue M A, Pratt S K, Fairless A J, Fowles S, Laroche J, Georgiou P, Prince W. Linear pharmacokinetics of penciclovir following administration of oral doses of famciclovir 125, 250, 500 and 750 mg to healthy volunteers. J Antimicrob Chemother. 1994;333:119–127. doi: 10.1093/jac/33.1.119. [DOI] [PubMed] [Google Scholar]
- 38.Rashidi M R, Smith J A, Clarke S E, Beedham C. In vitro oxidation of famciclovir and 6-deoxypenciclovir by aldehyde oxidase from human, guinea pig, rabbit and rat liver. Drug Metab Dispos. 1997;25:805–813. [PubMed] [Google Scholar]
- 39.Romanowski B, Aoki F Y, Martel A Y, Lavender E A, Parsons J E, Saltzman R L. Efficacy and safety of famciclovir for treating mucocutaneous herpes simplex infection in HIV-infected individuals. Collaborative Famciclovir HIV Study Group. AIDS. 2000;14:1211–1217. doi: 10.1097/00002030-200006160-00019. [DOI] [PubMed] [Google Scholar]
- 40.Saldanha C E, Lubinski J, Martin C, Nagashunmugam T, Wang L, Van Der Keyl H, Tal-Singer R, Friedman H M. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J Virol. 2000;74:6712–6719. doi: 10.1128/jvi.74.15.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schacker T, Hu H, Koelle D M, Zeh J, Saltzman R, Boon R, Shaughnessy M, Barnum G, Corey L. Famciclovir for the suppression of symptomatic and asymptomatic herpes simplex virus reactivation in HIV-infected persons. A double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:21–28. doi: 10.7326/0003-4819-128-1-199801010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Shuttleworth G, Shimeld C, Easty D. Viral disease of the eye. In: Richman D D, Whitley R J, Hayden F G, editors. Clinical virology. New York, N.Y: Churchill Livingstone; 1997. pp. 159–184. [Google Scholar]
- 43.Spruance S L, McKeough M B. Combination treatment with famciclovir and a topical corticosteroid gel versus famciclovir alone for experimental ultraviolet radiation-induced herpes simplex labialis: a pilot study. J Infect Dis. 2000;181:1906–1910. doi: 10.1086/315528. [DOI] [PubMed] [Google Scholar]
- 44.Spruance S L, Rowe N H, Raborn G W, Thibodeau E A, D'Ambrosio J A, Bernstein D I. Peroral famciclovir in the treatment of experimental ultraviolet radiation-induced herpes simplex labialis: a double-blind, dose-ranging, placebo-controlled, multicenter trial. J Infect Dis. 1999;179:303–310. doi: 10.1086/314605. [DOI] [PubMed] [Google Scholar]
- 45.Sutton D, Kern E R. Activity of famciclovir and penciclovir in HSV-infected animals: a review. Antivir Chem Chemother. 1993;4(Suppl. 1):37–46. [Google Scholar]
- 46.Thackray A M, Field H J. Comparison of effects of famciclovir and valaciclovir on pathogenesis of herpes simplex virus type 2 in a murine infection model. Antimicrob Agents Chemother. 1996;40:846–851. doi: 10.1128/aac.40.4.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thackray A M, Field H J. Differential effects of famciclovir and valaciclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency. J Infect Dis. 1996;173:291–299. doi: 10.1093/infdis/173.2.291. [DOI] [PubMed] [Google Scholar]
- 48.Thackray A M, Field H J. Famciclovir and valaciclovir differ in the prevention of herpes simplex virus type 1 latency in mice: a quantitative study. Antimicrob Agents Chemother. 1998;42:1555–1562. doi: 10.1128/aac.42.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thackray A M, Field H J. Further evidence from a murine infection model that famciclovir interferes with the establishment of HSV-1 latent infections. J Antimicrob Chemother. 2000;45:825–833. doi: 10.1093/jac/45.6.825. [DOI] [PubMed] [Google Scholar]
- 50.Thackray A M, Field H J. Persistence of infectious herpes simplex virus type 2 in the nervous system in mice after antiviral chemotherapy. Antimicrob Agents Chemother. 2000;44:97–102. doi: 10.1128/aac.44.1.97-102.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson H W, Mera R, Prasad C. The analysis of variance (ANOVA) Nutr Neurosci. 1999;2:43–55. doi: 10.1080/1028415X.1999.11747262. [DOI] [PubMed] [Google Scholar]
- 52.Vere Hodge R A, Sutton D, Boyd M R, Harnden M R, Jarvest R L. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 [9-(4-hydroxy-3-hydroxymethylbut-1-yl)gaunine; penciclovir] Antimicrob Agents Chemother. 1989;33:1765–1773. doi: 10.1128/aac.33.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner E K, Bloom D C. Experimental investigation of herpes simplex virus latency. Clin Microbiol Rev. 1997;10:419–443. doi: 10.1128/cmr.10.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]