Abstract
To investigate the effects of resveratrol on the drug resistance of 5-FU in the colon cancer chemotherapy, an MTT assay was used to detect the effects of 5-FU and resveratrol combined with 5-FU on the proliferation of the LoVo and SW480 cell lines. Flow cytometry was used to detect the effect of 5-FU combined with resveratrol on the survival rate of the LoVo and SW480 cells. A western blot was used to detect the expression levels of the proteins associated with colon cancer. After flow sorting, the percentage of the SW480 and the LoVo cell line CD133+ was 97.5% and 95.8%, respectively. The cells cultured in vitro showed more rapid cell proliferation and differentiation. The MTT assay showed that as compared with the survival rate of the blank group LoVo and CD133+ LoVo cells, the survival rate of the cells containing the 5-FU group was lower (P < 0.05). When 5-FU was used in combination with different concentrations of resveratrol, the abovementioned phenomenon was more prominent. The sorted colon cancer cells have dry stem cells, and the sorted CD133+ cells are more resistant to drugs; the combination of resveratrol and 5-FU has the best effect on the colon cancer cells. Preliminary studies on the mechanism of action of the drug show that a combination of 5-FU and resveratrol regulates apoptosis in CD133+ colon cancer stem cells by regulating the BAX gene; however, more complex mechanisms may also be involved.
To investigate the effects of resveratrol on the drug resistance of 5-FU in the colon cancer chemotherapy, an MTT assay was used to detect the effects of 5-FU and resveratrol combined with 5-FU on the proliferation of the LoVo and SW480 cell lines.
Introduction
Over the past 30 years, the incidence of colon cancer has increased in most countries and regions, including China.1–3 The main treatment for colon cancer is currently based on chemotherapeutic drugs;4 however, the disease is prone to relapse. This may be related to the tumor stem cells, which are similar to the normal stem cells. Tumor stem cells possess a strong ability to regenerate, repair DNA,5 and expel the chemotherapeutic drugs out of the cell.6 Therefore, study of the resistance of stem cells to chemotherapeutic agents and how this resistance can be overcome is a topic of significant interest in this field. Yang Yu7 reported that cancer stem cells play a role in maintaining the potential for tissue differentiation in recurring solid tumors. Youzhi Li8 reported that pancreatic cancer recurrence and metastasis were inhibited by inhibiting the stem cells. Sheung Tat Fan9 reported the recurrence of hepatocellular carcinoma by the circulating cancer stem cells released from primary tumors. Thomas Dittmar10 reported that the cause of cancer recurrence was the integration of stem cells in tumors. In addition, cancer stem cells are resistant to drugs. Xiaoxiang Chen11 reported that cancer stem cells control the epithelial–mesenchymal transition and thus control the resistance of high-grade ovarian serous tumors. Cristian Tomasetti12 reported the positive role of stem cell symmetry and asymmetric divisions in the development of cancer resistance.
Resveratrol (RES) is a natural active compound13 mainly extracted from peanuts, grapes, Polygonum cuspidatum, and other plants.14 It is a non-flavonoid polyphenol, insoluble in water, but soluble in organic solvents such as ethanol and acetone. It exists in the free state and forms glycosides under natural conditions. 5-FU is a commonly used chemotherapy drug for colorectal cancer treatment. As its dose increases, the side effects of the drug also increase and resistance to the drug develops frequently. Therefore, its clinical application is limited.15 Some studies have shown that a combination of chemotherapeutic drugs and traditional Chinese medicine not only can reduce adverse drug reactions but can also produce synergies between drugs and promote better efficacy.16 Resveratrol has been reported to exhibit cellular anticancer activity in breast cancer,17 skin cancer,18 and liver cancer.19 More importantly, it has been found that resveratrol can be combined with chemotherapeutics to reduce drug resistance during some cancer treatments. Laura H. Engelke20 reported the effects of ellagic acid and resveratrol on the epithelial ovarian cancer cell line A2780. Sin Ho Kweon21 reported that resveratrol-mediated downregulation of MRP1 was associated with the reversal of resistance to doxorubicin in acute leukemia cells. Xu22 reported that resveratrol inhibited the epithelial–mesenchymal transitions by regulating the PTEN–Akt signaling pathway in gastric cancer. Jie Meng23 reported the use of liposome-encapsulated resveratrol and paclitaxel in the treatment of breast cancer cell resistance reversal.
In summary, resveratrol demonstrates good resistance to drugs in the treatment of multiple cancers with chemotherapeutic agents. However, its effects have not been reported in colon cancer cells. In this study, the effect of resveratrol combined with 5-FU has been investigated on colon cancer cells and stem cells. We have confirmed the effect of resveratrol on drug resistance in colon cancer cells and determined its mechanism of action at the gene level.
Results and discussion
Cancer stem cell selection and separation
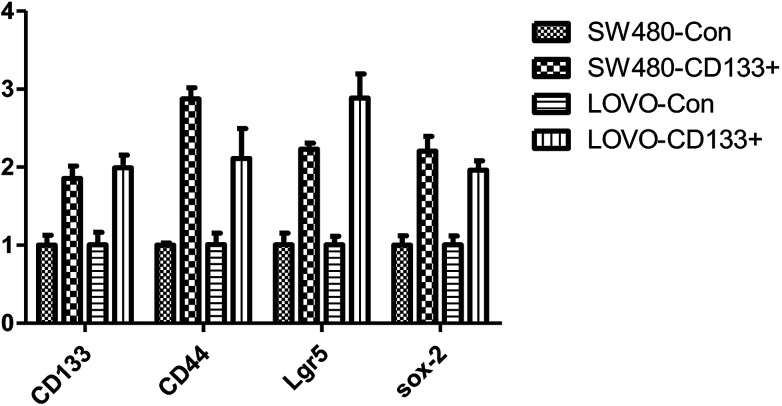
CD133, CD44, Lgr5, and Sox-2 are tumor marker genes.24 The results of the cancer stem cell sorting (Fig. 1) show that the expression levels of these four tumor markers are significantly higher in cancer stem cells (P < 0.01) than those in the normal cells. The isolated stem cells were confirmed to be exact.
Fig. 1. Expression data of CD133+ and other stem cell markers in colon cancer cell.
Flow cytometry verification
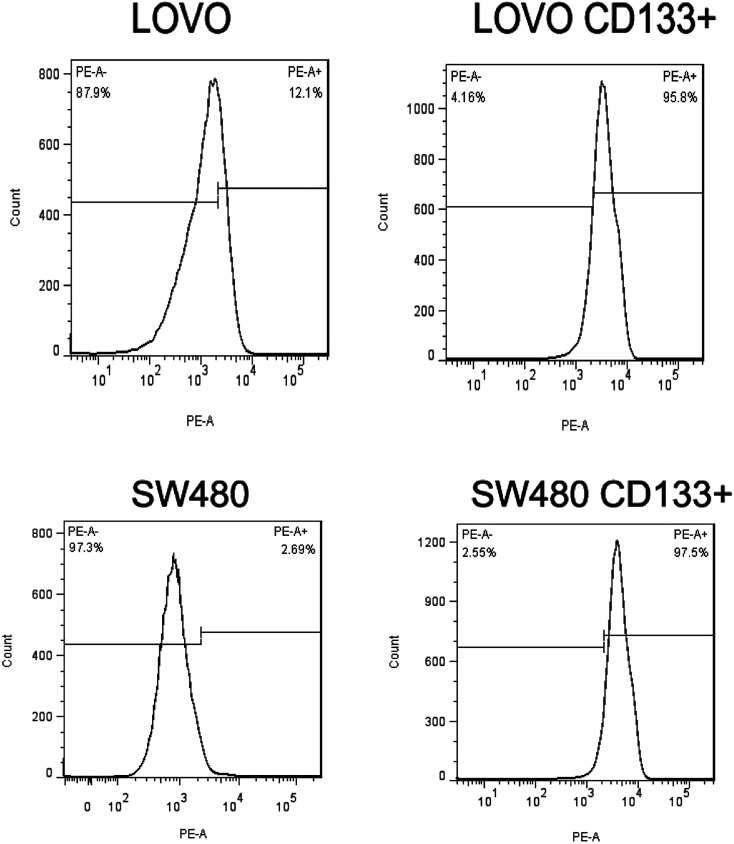
Flow cytometry results (Fig. 2) showed that the CD133+ cells reached 95.8% and 97.5% in colorectal cancer LoVo and SW480 cells after tumor cell sorting, respectively. Experimental results also confirmed that the isolated stem cells were exact.
Fig. 2. Flow detection of the results of cell separation.
In vitro ball validation results
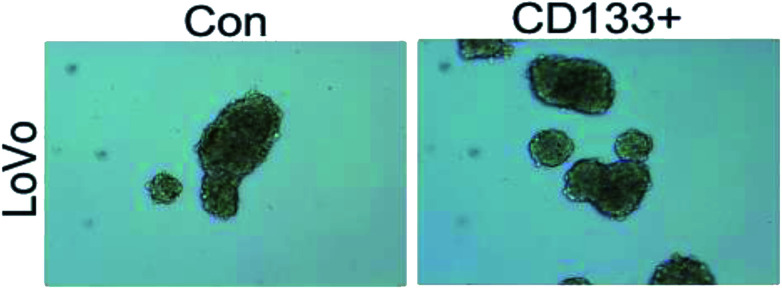
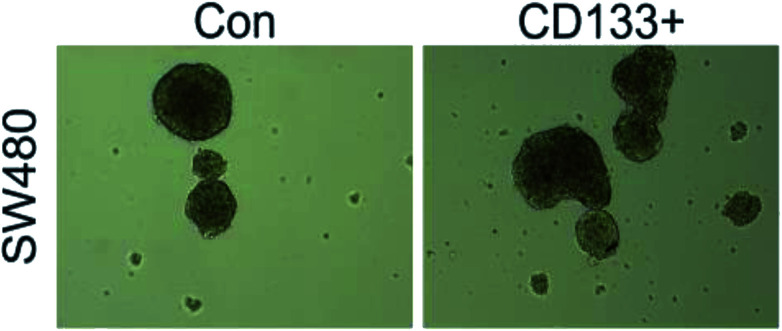
As shown in Fig. 3 and 4, the LoVo and SW480 cells are more capable of reproduction after sorting and have more ability to differentiate than the unsorted stem cells.
Fig. 3. LoVo balling in vitro before and after separation.
Fig. 4. SW480 balling in vitro before and after separation.
MTT assay validates the effect of resveratrol on the drug resistance of SW480 stem cells
The experimental results obtained via the MTT assays when 5-FU at different concentrations was reacted with resveratrol are shown in Table 1. The experimental results obtained when the same concentration of 5-FU was combined with different concentrations of resveratrol according to the previous method are shown in Table 2.
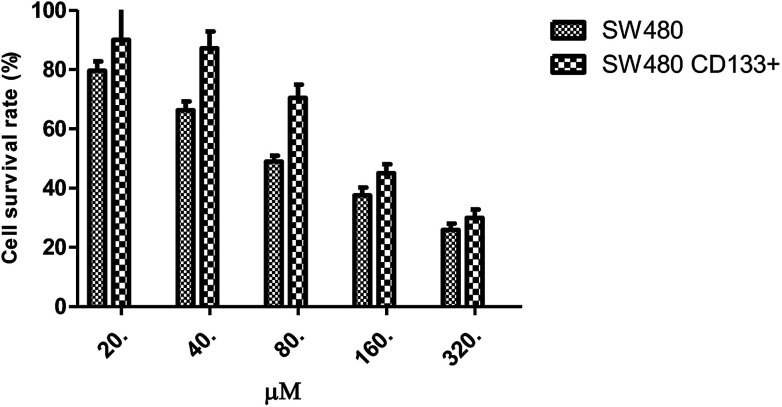
Effects of 5-FU at different concentrations on the survival rate of SW480 cells and SW480 tumorigenic stem cells.
| 0 μM | 20 μM | 40 μM | 80 μM | 160 μM | 320 μM | |
|---|---|---|---|---|---|---|
| SW480 | 1.210 ± 0.082 | 0.964 ± 0.038 | 0.802 ± 0.037 | 0.592 ± 0.024 | 0.454 ± 0.032 | 0.313 ± 0.026 |
| SW480 CD133+ | 1.318 ± 0.092 | 1.187 ± 0.136 | 1.149 ± 0.075 | 0.929 ± 0.059 | 0.594 ± 0.040 | 0.395 ± 0.037 |
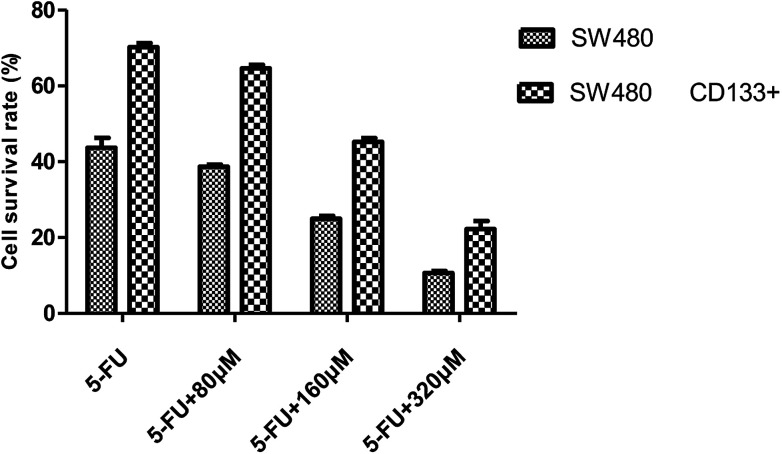
Effects of 5-Fu at the same concentration and resveratrol at different concentrations on the survival rate of SW480 cells and SW480 tumorigenic stem cells.
| 0 μM | 5-FU | 5-FU + 80 μM | 5-FU + 160 μM | 5FU + 320 μM | |
|---|---|---|---|---|---|
| SW480 | 1.324 ± 0.023 | 0.723 ± 0.021 | 0.683 ± 0.020 | 0.441 ± 0.028 | 0.188 ± 0.020 |
| SW480 CD133+ | 1.495 ± 0.014 | 1.050 ± 0.036 | 0.966 ± 0.030 | 0.676 ± 0.035 | 0.332 ± 0.070 |
Fig. 5 shows that the CD133+ SW480 cells were added to 5-FU after being compared with the parental cells. The cell survival rate of CD133+ SW480 cells decreased by 25.852% from 79.656%, and that of the parental cells decreased by 29.94% from 90.076%. The CD133+ SW480 cells were therefore less sensitive to 5-FU as compared to the parental cells. 5-FU combined with resveratrol acted on the colon cancer cell line SW480 and on the sorted CD133+ SW480 stem cells (Fig. 6). Preliminary results showed that with an increase in the resveratrol concentration, the survival rate of the SW480 cells decreased from 43.68% to 10.634% and the survival rate of the CD133+ SW480 cells decreased from 70.234% to 22.232%. The reason for the decrease in the survival rate is that resveratrol promotes apoptosis of tumor stem cells and tumor cells.25 A decrease in the sensitivity of 5-FU to resveratrol indicates that resveratrol confers drug resistance, and it can be seen in Fig. 6 that the effect of drug resistance is positively correlated with the dose of resveratrol. The next step is to explore the mechanism of resveratrol in this process from the aspects of genetic pathway screening and in vivo research.
Fig. 5. Effect of FU on the survival rate of SW480 cells and SW480 tumorigenic stem cells.
Fig. 6. Bar graph of survival rate of SW480 cells and SW480 CD133+ cells.
MTT assay validates the effect of resveratrol on the drug resistance of LoVo stem cells
Table 3 shows the results of the reaction of different concentrations of 5-FU with the same concentration of resveratrol according to the MTT assays. Table 4 shows the results of the reaction of the same concentration of 5-FU with different concentrations of resveratrol according to the MTT assays.
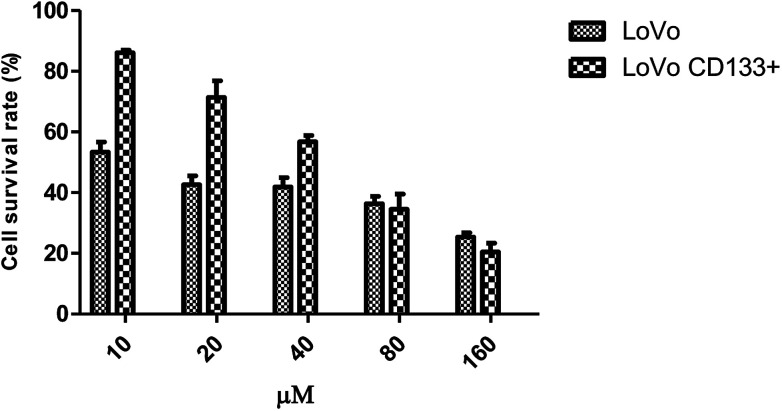
Effects of 5-FU at different concentrations on the survival rate of LoVo cells and LoVo CD133+ tumorigenic stem cells.
| 0 μM | 10 μM | 20 μM | 40 μM | 80 μM | 160 μM | |
|---|---|---|---|---|---|---|
| LoVo | 0.476 ± 0.040 | 0.254 ± 0.016 | 0.203 ± 0.014 | 0.199 ± 0.015 | 0.173 ± 0.012 | 0.121 ± 0.007 |
| LoVo CD133+ | 1.129 ± 0.323 | 1.041 ± 0.009 | 0.870 ± 0.067 | 0.692 ± 0.026 | 0.421 ± 0.061 | 0.250 ± 0.035 |
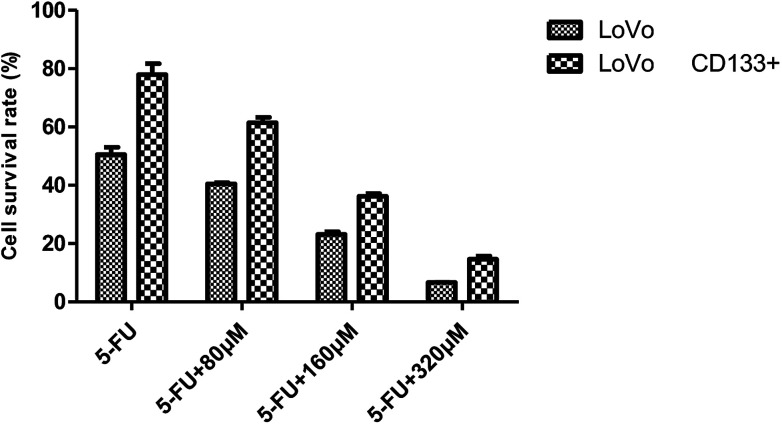
Effects of 5-Fu at the same concentration and resveratrol at different concentrations on the survival rate of LoVo cells and LoVo CD133+ tumorigenic stem cells.
| 0 μM | 10 μM | 20 μM | 40 μM | 80 μM | 160 μM | |
|---|---|---|---|---|---|---|
| LoVo | 0.476 ± 0.040 | 0.254 ± 0.016 | 0.203 ± 0.014 | 0.199 ± 0.015 | 0.173 ± 0.012 | 0.121 ± 0.007 |
| LoVo CD133+ | 1.129 ± 0.323 | 1.041 ± 0.009 | 0.870 ± 0.067 | 0.692 ± 0.026 | 0.421 ± 0.061 | 0.250 ± 0.035 |
As shown in Fig. 7, the concentration of 10, 20, 40, 80, and 160 μM of 5-FU acts on the LoVo CD133+ and LoVo colon cancer cells; the LoVo CD133+ cells have a higher survival rate than the LoVo cells. In a certain range, the same concentration of 5-FU acts on CD133+ LoVo and LoVo colon cancer cells; CD133+ LoVo cells have a higher survival rate than the LoVo cells; therefore, CD133+ LoVo cells are more resistant to drugs than the parental cells. The calculated IC50 of 5-FU for the LoVo cells is 13 μM and that for the CD133+ LoVo cells is 48 μM. This indicates that the CD133+ LoVo cells are more resistant to drugs than the parental cells.
Fig. 7. Effect of FU on the survival rate of LoVo cells and LoVo tumorigenic stem cells.
As shown in Fig. 8, it was found that 5-FU combined with resveratrol acted on LoVo colon cancer cells and the isolated CD133+ LoVo colon cancer stem cells. With an increase in the resveratrol concentration, the LoVo cell survival rate decreased from 50.512% to 6.622% and the CD133+ LoVo cell survival rate decreased to 14.604% from 77.954%. The reduced sensitivity of 5-FU to resveratrol indicates that resveratrol confers drug resistance, and it can be seen from Fig. 8 that the effect of drug resistance is positively correlated with the resveratrol dose.
Fig. 8. Bar graph of survival rate of LoVo cells and LoVo CD133+ cells with 5-Fu + resveratrol.
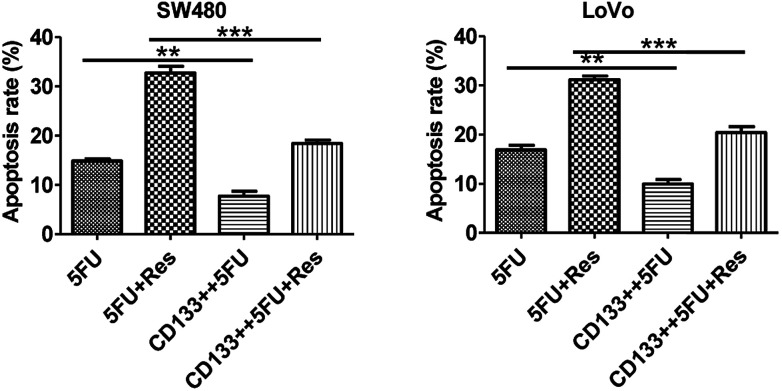
From the data presented in Fig. 9, it can be concluded that 5-FU alone acts on CD133+ SW480 colon cancer stem cells, and the apoptosis rate is reduced from 15% to 10%. When 5-FU and resveratrol acted on SW480 CD133+, the apoptosis rate decreased from 30% to 18%, which was significantly different from that of 5-FU only (P < 0.05). The same results can be obtained when 5-FU alone and 5-FU combined with resveratrol act on the CD133+ LoVo cells. In addition, both the colon cancer stem cells (CD133+) and common colon cancer cells have a large difference in apoptosis rates when 5-FU combined with resveratrol is used and when 5-FU is used alone.
Fig. 9. SW480 cell and LoVo cells' flow apoptotic data histogram (** indicates significant difference; *** indicates a very significant difference).
Many studies have been reported on the inhibitory activity of resveratrol and the mechanism of its effect on tumor cells.26,27 We also studied the effect of resveratrol on the survival rate of LoVo cells and LoVo CD133+ cells in the early stage. The results showed that resveratrol has a certain effect of inhibiting the survival on the above two kinds of cells, and this effect is dose-related; we believe that the effect of resveratrol combined with 5-FU is better than that of only the latter mainly due to the synergistic inhibition by the former.
Resveratrol inhibits the regulation of cancer stem cell resistance by gene selection
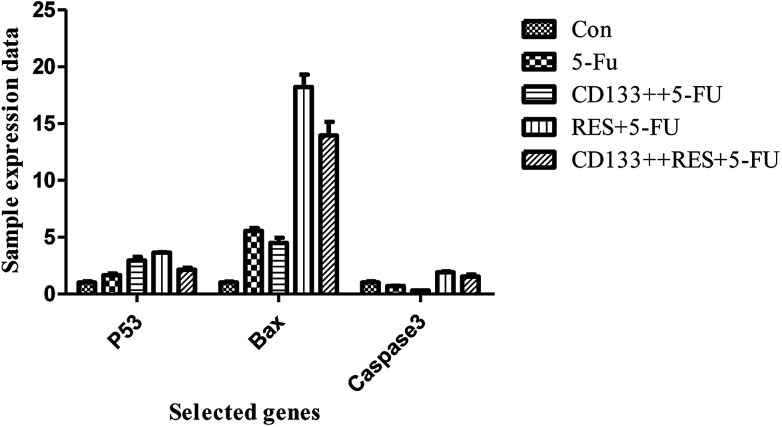
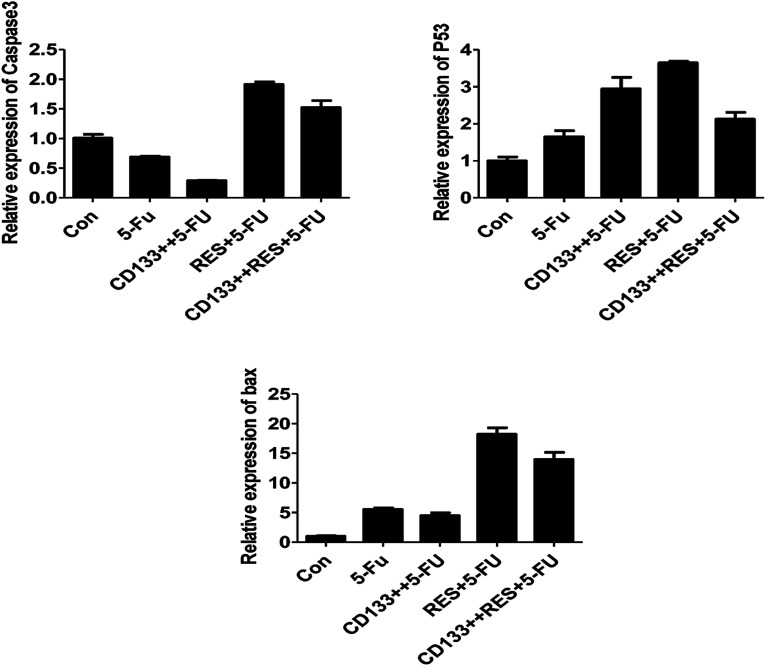
As shown in Fig. 10, all blank and CD133+ cells were treated with 5-FU, and the expression of apoptosis-related genes differed (excluding CASP3) (P < 0.05); this indicated that the CD133+ group had lower resistance than the blank cells. Moreover, three selected genes were treated with 5-FU and resveratrol. Only the expression of the BAX gene was significantly increased (P < 0.01); this indicated that the gene and resveratrol inhibited the resistance of colon cancer cells to 5-FU. As can be seen from Fig. 11, the normal cells and cancer stem cells in the BAX gene were treated by resveratrol combined with 5-FU and 5-FU alone, and when 5-FU (conventional cell chemotherapy group), CD133+ + 5-FU (stem cell chemotherapy group), RES + 5-FU (conventional cell chemotherapy + resveratrol group), and CD133+ + RES + 5-FU (stem cell chemotherapy + resveratrol group) were compared, the results showed that resveratrol might regulate stem cell resistance to 5-FU by regulating apoptosis via the BAX gene.
Fig. 10. Expression data of p53, BAX and other genes in LoVo cell line samples.
Fig. 11. p53, BAX, CASP3 gene content in LoVo cell line samples.
Conclusion
The rate of apoptosis is an important indicator of the efficacy of chemotherapeutic drugs. The present study shows that regulation of resveratrol activity can affect the apoptosis induced by chemotherapeutic drugs in tumor cells. Resveratrol has been found to reduce the rate of apoptosis induced by chemotherapeutic agents in breast cancer28 although the mechanism of its action is unknown. 5-FU is a basic chemotherapeutic drug used to treat colorectal cancer. Apoptosis is an important indicator of its efficacy. In the present study, resveratrol combined with 5-FU acts on the SW480 and LoVo colon cancer cells, significantly inhibiting the apoptosis induced by 5-FU.
We found that after sorting, the colon cancer stem cells were more resistant to chemotherapeutic drugs. A possible reason for this is that CD133+ cells can escape innate immunity and adaptive immune surveillance, participate in the formation of tumor blood vessels, and activate stem cell-related signaling pathways. The secretion of IL-4 mediates drug resistance and prevents tumor cell apoptosis; this indicates that IL-4 can be used as a marker to identify colorectal cancer stem cells and perform interventional therapy.29,30 However, the specific mechanism of its action needs further study.
Resisting or escaping chemotherapy-induced apoptosis is one of the most important mechanisms of chemotherapy resistance in tumors. The results of our experiment show that the inhibition of resveratrol activity reduces the induction of apoptosis in 5-FU cells, and the BAX gene participates in this process. Reports suggest that BAX regulation is involved in the mechanism. BAX is an important suppressor gene in the apoptosis signaling pathway and plays a crucial role in the regulation of apoptosis31via a combination of resveratrol and the p53 and CASP3 genes. 5-FU cell apoptosis experiments show significant differences only regarding the BAX gene, which confirm this theory.
Our experimental results showed that the effects of resveratrol on apoptosis induced by 5-FU in SW480 and LoVo cells were approximately the same. The effect of resveratrol combined with 5-FU on apoptosis in SW480 and LoVo cells and expression of the BAX gene were analyzed. We found that apoptosis in SW480 cells and LoVo cells induced by resveratrol combined with 5-FU was still significant. Resveratrol regulates apoptosis via a combination of 5-FU-regulated BAX genes and is important in the drug tolerance mechanism, but there may also be more complex mechanisms involved.
Based on the abovementioned analysis, we conclude that the apoptosis induced in colon cancer cells by resveratrol is caused by (1) decreased apoptosis resistance; (2) CD133+ cells escaping the innate immunity and adaptive immune surveillance and participating in tumor vasculature, and (3) resveratrol and 5-FU combining to regulate the BAX gene to regulate stem cell apoptosis.
Methods
Materials
Resveratrol (homemade, 99%); 5-FU (99%, HARVEY USA); colon cancer SW480 and LoVo cell line (provided by the Hunan Aijia Biotechnology Company).
Instruments and reagents
CO2 cell incubator (ThermoFisher Scientific, USA); biosafety cabinet (Heal Force, BIOsafe12); inverted microscope (Motic, AE200); flow cytometer (BD, FACSCanto II); 60 mm cell culture dish (Corning, 430166); nucleic acid quantifier, mRNA reverse transcription kit, (ThermoFisher Scientific, USA); pancreatic digestion solution (Auragene Corporation), PBS (Auragene Corporation); CD133 MicroBead Kit-Tumor Tissue (MACS Miltenyi Biotec); fetal bovine serum (Gemini).
Selection and isolation of cancer stem cells
CD133+ is a surface-specific marker molecule for stem cells and cancer stem cells.32 Therefore, the cancer stem cells used for the study were sorted from normal cells according to the presence of CD133+. Magnetic CD133+ stem cells were selected by magnetic sorting.
Flow detection assays
Each group of cells was digested with an EDTA-free trypsin digestion solution, washed twice with PBS (2000 rpm, centrifuged for 5 min), and obtained. Then, 500 μL binding buffer suspension cells and 5 μL Annexin V-FITC were added, and the solution was mixed. After this, 5 μL of propidium iodide was added, and the solution was mixed. The reaction was carried out at room temperature in the dark for 5–15 min. Flow cytometry analysis and detection were performed within 1 h.
Cell balling assays
The CD133+, LoVo, and SW480 cells were cultured in a cell incubator for 4 h at 37 °C and 5% CO2, then replaced with a spheronization medium and incubated for further 48 h. Individual conglomerate cells were obtained separately and digested with a 0.05% trypsin digestion solution. A single cell suspension with a percentage of single cells above 95% was created with the culture medium. Cells were counted, and the cell concentration was diluted to 1 × 103 with a spheronizing medium. Then, 1 mL of single cell suspension was kept as a spare. 1 μL single cell suspension was added to each well of a 96-well ultra-low adsorption plate (containing 200 μL stem cell medium per well), normal cells and 3 stem cells, each cell total 180 wells (200 μL PBS in 96-well plate edge wells). The cells were observed using a microscope, and any wells containing no or few cells were removed. The cells were observed daily using the microscope for approximately 6–10 days, and the number and size of the tumors formed was determined.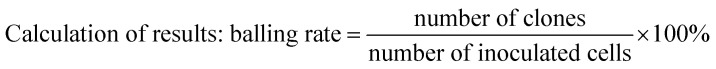
SW480 stem cell assays
Logarithmic phase SW480 and its CD133+ stem cells were obtained by trypsinization by adjusting the cell suspension concentration of each group to 5 × 104 cells per mL, then adding 100 μL cell suspension to each well of a 96-well plate, and plating the cells for measurement. For 2 × 104 cells per well (peripheral wells were filled with sterile PBS to eliminate edge effects), 5 replicate wells were set in each group. The SW480 parental cell group was first plated and then dosed. The SW480 CD133+ cell group was plated the day before and was given the dose the next day. The 5-FU concentrations were 20, 40, 80, 160, and 320 μM, which were spread the day before and the medicine was added the next day. The concentration of 5-FU was 15 μM, the concentrations of febutriterol (5-FU) were 80, 160, and 320 μM; these were incubated overnight at 37 °C in a 5% CO2 incubator. The 96-well plate was removed after every 24 h. The medium was removed, 100 μL 10% MTT medium was added to each well, and incubated in the cell incubator for 4 h. The medium in the well was carefully aspirated, and 100 μL DMSO was added to each well to fully dissolve the crystals. Within 10 min of adding DMSO, the enzyme standard was used. The instrument was tested for absorbance at 570 nm in each well, and GraphPad data was used. The cell viability and the cell growth inhibition rate were calculated according to the following formulae: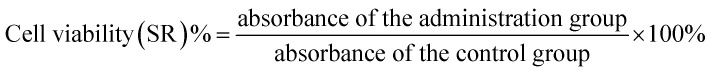
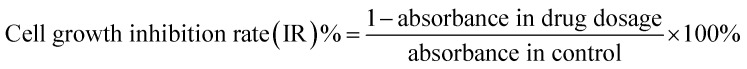
LoVo stem cell assays
Log phase LoVo cells and LoVo CD133+ stem cells were obtained by trypsin digestion, and the cell suspension concentration was adjusted to 5 × 104 cells per mL in each group. Then, 100 μL of cell suspension was added to each well of a 96-well plate. The cells were measured at 2 × 104 cells per well (peripheral wells were filled with sterile PBS to eliminate the edge effects), and 5 replicates were set in each group. The SW480 parental cell group was first plated and then dosed. The LoVo CD133+ cell group was plated the day before and was given the next day. 5-FU concentrations were: 0 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM; 5-FU concentrations were: 15 μM. These were incubated overnight at 37 °C in a 5% CO2 incubator. The 96-well plate was removed after every 24 h. The medium was removed, 100 μL of 10% MTT medium was added to each well, and incubation was carried out in the cell incubator for 4 h. The medium in the well was carefully aspirated, and 100 μL DMSO was added to each well to fully dissolve the crystals. Within 10 min after adding DMSO, the enzyme standard was used. The instrument was tested for absorbance at 570 nm in each well using GraphPad data. The cell viability and the cell growth inhibition rate were calculated according to the following formulae: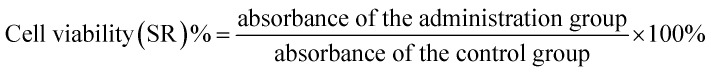
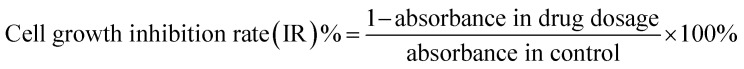
Flow cytometry assays
After sorting with CD133+ magnetic beads, the cells were counted and inoculated into 6-well cell culture plates. The next day, flow cytometry was conducted for different concentrations of drugs (5-FU 15 μM, RES 80 μM). Each group of cells was digested with EDTA-free trypsin digestion solution and washed twice with PBS (2000 rpm, centrifuged for 5 min) to obtain the cells. Then, 500 μL binding buffer suspension cells and 5 μL Annexin V-FITC were added, and the solution was mixed well. After this, 5 μL of propidium iodide was added, and mixing was continued. The reaction continued for 5–15 min at room temperature in the dark. Flow cytometric observation and detection was performed within 1 h.
Real-time PCR analysis
Real-time PCR was used to detect the expression of p53, BAX, and CASP3 genes in the LoVo cell line samples. The selected genes are all reported to be closely related to colon cancer.33,34
Cell lines provided by the cell platform were selected for PCR verification. Herein, 2 μg of total RNA from each sample was reverse transcribed to cDNA. The 20 μL RT-PCR reaction mixture was as follows: 10 μL 2× SYBR Green QPCR mix, 1 μL each of upstream and downstream primers, 1 μL cDNA, and 7 μL water; reaction conditions: pre-denaturation at 95 °C for 3 min; subsequent denaturation at each step of 95 °C, 10 s, p53, BAX, CASP3, primer annealing at 60 °C extended for 30 s for a total of 40 cycles each time in the extension phase to read the fluorescence value.
Statistical analysis
The experimental data were statistically analyzed using the SPSS V17.0 software. The experimental results are expressed as the mean ± standard deviation. Statistical analysis was performed using the t-test to analyze the differences between the groups. The values of P < 0.05 were considered statistically significant.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This work was supported by National Key R&D Program of China (2016YFD0600805).
References
- Whittemore A. S. Colorectal cancer incidence among Chinese in North America and the People's Republic of China: variation with sex, age and anatomical site. Health Policy. 1989;14(2):563–568. doi: 10.1093/ije/18.3.563. [DOI] [PubMed] [Google Scholar]
- Parkin D. M. Pisani P. Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int. J. Cancer. 1993;54(4):594. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- Pathy S. Lambert R. Sauvaget C. et al., The incidence and survival rates of colorectal cancer in India remain low compared with rising rates in East Asia. Dis. Colon Rectum. 2012;55(8):900–906. doi: 10.1097/DCR.0b013e31825afc4e. [DOI] [PubMed] [Google Scholar]
- Modest D. P. Denecke T. Pratschke J. et al., Surgical treatment options following chemotherapy plus cetuximab or bevacizumab in metastatic colorectal cancer-central evaluation of FIRE-3. Eur. J. Cancer. 2018;88:77–86. doi: 10.1016/j.ejca.2017.10.028. [DOI] [PubMed] [Google Scholar]
- Huo J. S. Baylin S. B. Zambidis E. T. Cancer-like epigenetic derangements of human pluripotent stem cells and their impact on applications in regeneration and repair. Curr. Opin. Genet. Dev. 2014;28(28):43–49. doi: 10.1016/j.gde.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmink B. L. Verheem A. Van Houdt W. J. et al., The secretome of colon cancer stem cells contains drug-metabolizing enzymes. J. Proteomics. 2013;91:84. doi: 10.1016/j.jprot.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Yu Y. Ramena G. Elble R. C. The role of cancer stem cells in relapse of solid tumors. Front. Biosci. 2011;4:1528–1541. doi: 10.2741/e478. [DOI] [PubMed] [Google Scholar]
- Li Y. Rogoff H. A. Keates S. et al., Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. U. S. A. 2015;112(6):1839. doi: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S. T. Yang Z. F. Ho D. W. et al., Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann. Surg. 2011;254(4):569–576. doi: 10.1097/SLA.0b013e3182300a1d. [DOI] [PubMed] [Google Scholar]
- Dittmar T. Nagler C. Schwitalla S. et al., Recurrence cancer stem cells – made by cell fusion? Med. Hypotheses. 2009;73(4):542–547. doi: 10.1016/j.mehy.2009.05.044. [DOI] [PubMed] [Google Scholar]
- Chen X. Zhang J. Zhang Z. et al., Cancer stem cells, epithelial-mesenchymal transition, and drug resistance in high-grade ovarian serous carcinoma. Hum. Pathol. 2013;44(11):2373–2384. doi: 10.1016/j.humpath.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C. Levy D. Role of symmetric and asymmetric division of stem cells in developing drug resistance. Proc. Natl. Acad. Sci. U. S. A. 2010;107(39):16766–16771. doi: 10.1073/pnas.1007726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. J. Hu L. Xia Y. P. et al., Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J. Neuroinflammation. 2016;13(1):84. doi: 10.1186/s12974-016-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frémont L. Biological effects of resveratrol. Life Sci. 2000;66(8):663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Katona C. Kralovánszky J. Rosta A. et al., Putative role of dihydropyrimidine dehydrogenase in the toxic side effect of 5-fluorouracil in colorectal cancer patients. Oncology. 1998;55(5):468. doi: 10.1159/000011897. [DOI] [PubMed] [Google Scholar]
- Chen Y. Z. Li Z. D. Gao F. et al., Effects of combined Chinese drugs and chemotherapy in treating advanced non-small cell lung cancer. Chin. J. Integr. Med. 2009;(6):415–419. doi: 10.1007/s11655-009-0415-2. [DOI] [PubMed] [Google Scholar]
- Chottanapund S. Van Duursen M. B. Navasumrit P. et al., Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol. In Vitro. 2014;28(7):1215–1221. doi: 10.1016/j.tiv.2014.05.015. [DOI] [PubMed] [Google Scholar]
- Junco J. J. Mancha A. Malik G. et al., Resveratrol and P-glycoprotein inhibitors enhance the anti-skin cancer effects of ursolic acid. Mol. Cancer Res. 2013;11(12):1521. doi: 10.1158/1541-7786.MCR-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri F. Zarnani A. H. Zand H. et al., Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur. J. Pharmacol. 2013;718(1–3):34–40. doi: 10.1016/j.ejphar.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Engelke L. H. Hamacher A. Proksch P. et al., Ellagic Acid and Resveratrol Prevent the Development of Cisplatin Resistance in the Epithelial Ovarian Cancer Cell Line A2780. J. Cancer. 2016;7(4):353. doi: 10.7150/jca.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon S. H. Song J. H. Kim T. S. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Commun. 2010;395(1):104–110. doi: 10.1016/j.bbrc.2010.03.147. [DOI] [PubMed] [Google Scholar]
- Xu J. Liu D. Niu H. et al., Resveratrol reverses doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2017;36(1):19. doi: 10.1186/s13046-016-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie M. Guo F. Xu H. et al., Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells In Vivo. Sci. Rep. 2016;6:22390. doi: 10.1038/srep22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atashpour S. Fouladdel S. Movahhed T. K. et al., Quercetin induces cell cycle arrest and apoptosis in CD133+ cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015;18(7):635–643. [PMC free article] [PubMed] [Google Scholar]
- Li Y. BäCkesjö C. M. Haldosén L.-A. et al., Resveratrol inhibits proliferation and promotes apoptosis of osteosarcoma cells. Eur. J. Pharmacol. 2009;609(1–3):13–18. doi: 10.1016/j.ejphar.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Mahyar-Roemer M. Katsen A. Mestres P. et al., Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer. 2001;94(5):615–622. doi: 10.1002/ijc.1516. [DOI] [PubMed] [Google Scholar]
- Aires V. Brassart B. Carlier A. et al., A role for peroxisome proliferator-activated receptor gamma in resveratrol-induced colon cancer cell apoptosis. Mol. Nutr. Food Res. 2014;58(9):1785–1794. doi: 10.1002/mnfr.201300962. [DOI] [PubMed] [Google Scholar]
- Tili E. Michaille J. J. Alder H. et al., Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 2010;80(12):2057–2065. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro M. Alea M. P. Di S. A. et al., Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1(4):389. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Francipane M. G. Alea M. P. Lombardo Y. et al., Crucial role of interleukin-4 in the survival of colon cancer stem cells. Cancer Res. 2008;68(11):4022. doi: 10.1158/0008-5472.CAN-07-6874. [DOI] [PubMed] [Google Scholar]
- Xiong H. Y. Guo X. L. Bu X. X. et al., Autophagic cell death induced by 5-FU in Bax or PUMA deficient human colon cancer cell. Cancer Lett. 2010;288(1):68–74. doi: 10.1016/j.canlet.2009.06.039. [DOI] [PubMed] [Google Scholar]
- Jing F. Kim H. J. Kim C. H. et al., Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int. J. Oncol. 2015;46(4):1582. doi: 10.3892/ijo.2015.2844. [DOI] [PubMed] [Google Scholar]
- Huerta S. Gao X. Dineen S. et al., Role of p53, Bax, p21, and DNA-PKcs in radiation sensitivity of HCT-116 cells and xenografts. Surgery. 2013;154(2):143–151. doi: 10.1016/j.surg.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Chakraborty S. Mazumdar M. Mukherjee S. et al., Restoration of p53/miR-34a regulatory axis decreases survival advantage and ensures Bax-dependent apoptosis of non-small cell lung carcinoma cells. FEBS Lett. 2014;588(4):549–559. doi: 10.1016/j.febslet.2013.11.040. [DOI] [PubMed] [Google Scholar]