Abstract
The primary function of the gut is to procure nutrients. Synchronized mechanical activities underlie nearly all its endeavours. Coordination of mechanical activities depends on sensing of the mechanical forces, in a process called mechanosensation. The gut has a range of mechanosensory cells. They function either as specialized mechanoreceptors, which convert mechanical stimuli into coordinated physiological responses at the organ level, or as non-specialized mechanosensory cells that adjust their function based on the mechanical state of their environment. All major cell types in the gastrointestinal tract contain subpopulations that act as specialized mechanoreceptors: epithelia, smooth muscle, neurons, immune cells, and others. These cells are tuned to the physical properties of the surrounding tissue, so they can discriminate mechanical stimuli from the baseline mechanical state. The importance of gastrointestinal mechanosensation has long been recognized, but the latest discoveries of molecular identities of mechanosensors and technical advances that resolve the relevant circuitry have poised the field to make important intellectual leaps. This Review describes the mechanical factors relevant for normal function, as well as the molecules, cells and circuits involved in gastrointestinal mechanosensing. It concludes by outlining important unanswered questions in gastrointestinal mechanosensing.
Mechanical manipulation of luminal contents is required for nearly all gut functions. The gut generates a range of well-orchestrated mechanical activities that require the sensation of mechanical forces for feedback and coordination. Indeed, the importance of mechanical factors in gut function is explored in classic texts1,2. When Bayliss and Starling described the “law of the intestine” in 1899, they stated that the “most effective method of stimulating the intestine is the mechanical method”3. The gut detects forces in time and space by the process of mechanosensation. Mechanosensation relies on specialized mechanosensory cells (mechanoreceptors) that use mechanosensors to convert force into electrochemical signals. The responses detected by mechanosensors are amplified (signal amplification) and coordinated by mechanotransducers, in a process called mechanotransduction4. The specialized sensory cells, such as mechanosensory neurons, relay the transduced signals to effectors such as smooth muscle cells (SMCs) that drive physiological responses such as the peristaltic reflex. As the gut is a mechanically active organ, all cells experience and adjust their functions based on the surrounding mechanical forces. We increasingly recognize that, in mechanical organs, cells whose primary function is not sensation of force, such as SMCs, rely on force sensing for their normal operation. In other words, such cells adjust their primary functions in response to the mechanical cues that surround them. Thus, to comprehend mechanosensing by the gut, we need to define the mechanical environment of the gut and understand how both specialized and non-specialized mechanosensory cells work. By the end of this Review, we aim to have the reader appreciate the gut’s crucial physical properties; the mechanical functions that it performs and the forces that it generates in the process; the known cells, molecules and circuits involved in gut mechanosensation; the limitations in translatability of discoveries from animal model studies; and the exciting new areas of research that focus on discovering unknown gut mechanosensors, defining mechanical landscapes and developing tools that probe physiological mechanical cues in animal models and humans and human diseases.
Mechanosensors.
Proteins directly involved in converting a mechanical stimulus into an intracellular electrochemical signal, for example, mechanogated ion channels that convert mechanical forces into an ionic flow.
Signal amplification.
Intracellular signalling steps between the primary signal generated by the mechanosensor and the final cellular output.
Mechanotransducers.
Proteins that amplify the signals generated by the mechanosensors and conduct the downstream cellular signalling in response to force.
Non-specialized mechanosensory cells.
Cells with a given non-mechanosensory primary function that use mechanosensors to sense mechanical stimuli and tune their own function in response to the physical state of their environment.
Physical properties and activities
To begin thinking about how the gut senses mechanical forces, we must first consider the physical properties of the gut. These are detailed in other reviews and texts5. Here, we focus on aspects crucial for mechanosensing.
Physical properties
The luminal gut is mainly a tubular structure approximately 9 m long in humans6. Compartment transitions are demarcated by sphincters, areas of local muscle thickening that function as imperfect one-way valves. The tube changes dimensions in its cross-section and length to disassemble, reassemble and propel luminal contents6. The wall of the tube is a composite, or laminated, material, made of layers with different physical properties. The layers are mucosa, submucosa, tunica muscularis and serosa7–10 (FIG. 1). The resting physical properties of these layers, such as stiffness, resting tension and Poisson ratio, vary along the length of the gastrointestinal tract11–14 (BOX 1). For example, mucosa and submucosa are the softest layers in the small bowel, but in the stomach, they define wall stiffness14. The resting mechanical properties of the layers and their arrangement define the local mechanical environment of a mechanoreceptor. Furthermore, like most soft tissues, the gut wall is viscoelastic (BOX 1), which makes the forces felt by mechanoreceptors vary over time.
Fig. 1 |. Static mechanical properties of the gut wall.
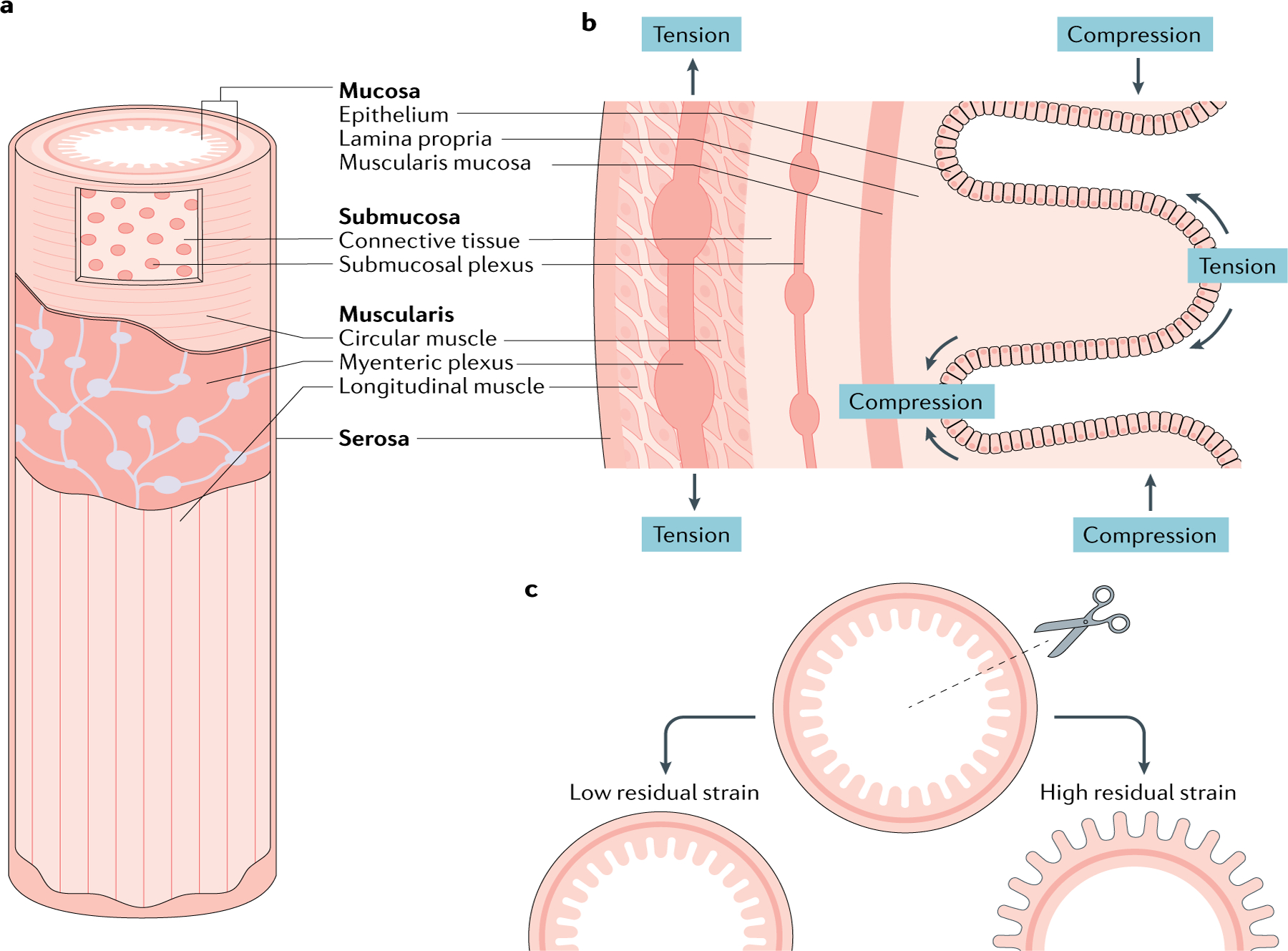
a | An oblique view to show the layers needed to understand the composite nature of the wall. b | Longitudinal cross-section showing a different view of the laminated structure and enteric nervous system organization. The layers closest to the lumen are under a constant state of compression, whereas those further away are in a state of tension (stretch). The four layers of the gut wall are identified in both cross-sections for orientation. c | Experiments to determine residual strain (BOX 3) and other passive mechanical properties of the gut wall. A radial cut on a gut segment causes it to open. Some segments have small opening angles (left) and others turn completely ‘inside out’ (right). The opening angle correlates with the balance of compression and tension between the tissue layers.
Box 1 |. Physical properties of the intestine.
Viscoelasticity
Property describing a material that exhibits viscous and elastic qualities. Pure elastic objects behave like a spring: force and deformation are linearly related and the release of force returns the object to its original shape. In viscoelastic materials, the viscous components, such as interstitial liquids in the gut wall, make it such that parts of the material ‘flow’. Consequently, the gut shows stress relaxation: force dissipates over time when tissue remains deformed for a period of time.
Compression
Application of an inwardly directed force on an object, colloquially known as ‘pushing’ on the object.
Tension
Application of an outwardly directed force on an object, colloquially known as ‘pulling’ on the object. It can be considered the opposite of compression.
Strain
Upon application of force, the resulting change in length of an object normalized to its original length.
Poisson ratio
A dimensionless quantity that describes how much sideways expansion an object that is being squeezed undergoes. Calculated as a change in object length in directions that are perpendicular to the direction of applied force.
Residual strain
- Baseline strain of tissue when the gastrointestinal lumen is empty and no external forces are acting on the tissue. Residual strain can be quantified as the deformation when all intrinsic forces acting on the tissue are removed. Gregersen and Kassab168, and others8,11:
- pig duodenum, inner surface: compression (−0.13 to −0.06)
- pig duodenum, outer surface: tension (0.05–0.14)
- guinea pig jejunum with villi, inner surface: compression (−0.33 ± 0.06)
- guinea pig jejunum without villi, inner surface: compression (−0.05 ± 0.030)
- rat colon and rectum, serosa: tension (0.1–0.25, 0.07–0.16)
- rat colon and rectum, mucosa: compression (−0.06 to −0.1 and −0.16 to −0.09)
- For comparison, Vaishnav and Vossoughi169 used a similar method to determine residual strains in the pig aortic segments:
- intima: compression (−0.117 to −0.057)
- adventitia: tension (0.056–0.120)
In addition to the layered structure, connections between the layers have a range of physical properties. The submucosa is fluid filled and links to the muscularis layer by a fibrous connection15. The flowing property of the viscoelastic gut wall is due in part to this fluid layer. The high water content also makes the submucosa resistant to compressive forces16, allowing the submucosal layer to take on the role of shock absorber, dampening forces originating from the lumen before they reach the muscle. The fluidity of the submucosal layer also allows it to function as a lubricant coat between the two adjacent layers; therefore, the mucosa could also slide over the muscle and expose intrinsic mechanoreceptors to stretch from shear forces16. The extent of the relative movement of mucosa over the muscularis remains poorly understood. With its hydration-dependent roles as shock absorber and lubricant, the submucosa could have a major role in detecting and dissipating light mechanical stimuli, according to experiments on the pig, guinea pig and human colon samples16,17. Similar to mucosa and submucosa, the tunica muscularis is layered, with circular and longitudinal layers not only having different physical properties but also allowing for sliding relative to each other2. Knowledge of the serosal contribution to static mechanical properties of the gastrointestinal wall is very limited. In all, the differences in physical properties and laminated arrangement lead to non-homogeneous force distributions across the gut wall in space and time. Consequently, mechanosensors in the different layers of the gut wall have different mechanical thresholds18.
Smooth muscle, the contractile component of the muscular layers, can be approximated as a spring. At rest, smooth muscle is like a loaded spring owing to SMCs being in a state of partial contraction, which leads to a resting tone13. Mechanoreceptors located in this layer can be tuned to a reference point that accounts for baseline tension in the tissue. For example, mechanoreceptors in the muscular layers have higher activation thresholds than those in the mucosa18. As the physical parameters of tunica muscularis are fairly homogeneous, it is an optimal location to integrate length and tension sensors that report the overall state of volume and contractility of the organ19. An analogy can be drawn to skeletal muscle. The complementary mechanosensory functions of length and tension sensing are accomplished in the muscularis by mechanosensors that behave similarly to muscle spindles and Golgi tendon organs in skeletal muscle20. Like the Golgi tendons, the muscularis in-series receptors are tension sensors that report on smooth muscle tone21: they are responsive to distension, especially if rapid. Muscularis in-parallel sensors are like muscle spindles and primarily detect stretching or lengthening (FIG. 2).
Fig. 2 |. Dynamic activities of the gut wall.
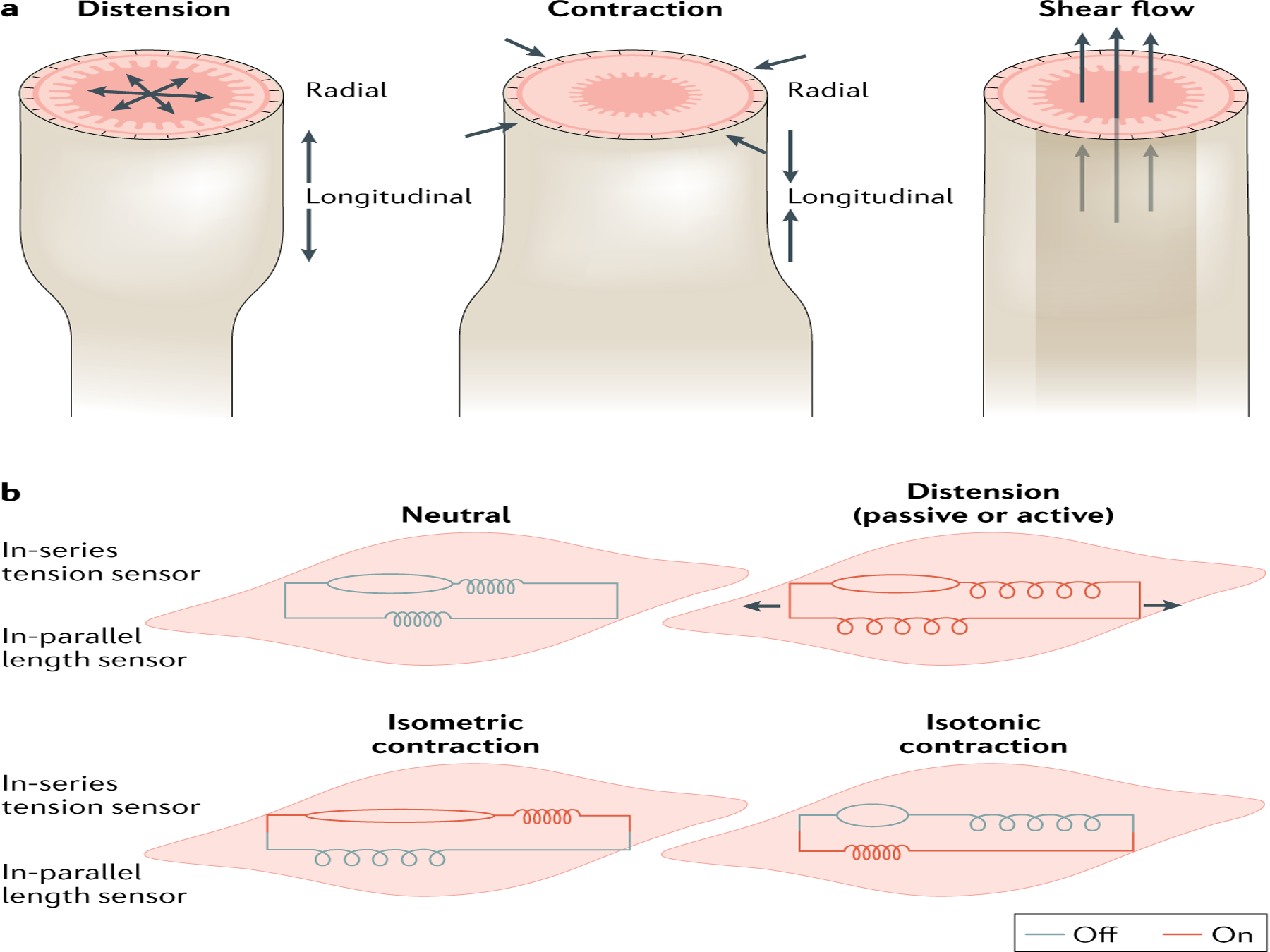
a | Mechanosensation by gut wall tissues enables the organ to respond to three types of physical activity: distension, contraction and flow (shear). Distension and contraction have longitudinal and radial components. b | Inseries and in-parallel mechanoreceptors are differentially activated depending on the gut activity. Red colour means that the mechanoreceptors are engaged as a result of the activity; grey colour means that they are not engaged.
In all, the layered structure of the gut wall with layers of varying physical properties enables adaptations in mechanoreceptors to detect a wide range of the intrinsic and extrinsic mechanical forces in time and space.
Mechanosensory activities
Force sensing is crucial for regulating mechanical programmes active during both digestion and the inter-digestive periods1,22. The mechanoreceptors encounter a balance of forces from a combination of active and passive distension balanced with wall contraction. Thus, mechanoreceptors detect the resulting wall tension due to tone, displacement or both, as well as shear from relative sliding of the layers and luminal flow generated by these activities23 (FIG. 2).
Distension.
The fundamental aspect of Bayliss and Starling’s peristaltic reflex is that it is initiated most effectively by local distension3. Mechanical stimuli, such as distension, initiate several types of contractile programmes. Distension can be passive (organ filling) or active (fundic relaxation and rectoanal inhibitory reflex (RAIR))5,24. The gut distends, either passively or actively, as the lumen is filled by ingested and secreted materials or by gas produced via microbial fermentation. Luminal filling leads to an increase in cross-section and length, but a decrease in wall thickness5. The layers experience these effects non-uniformly because of differences in their Poisson ratios (BOX 1). The intestinal epithelium and mucosa experience apical compression, whereas the basal region of the mucosa stretches. In response to luminal mechanical stimuli, the physiology and shape of the mucosa are controlled through mechanosensing and mechanical activity by the muscularis mucosa25,26. In the locations where the tunica muscularis is the stiffest of the layers, the luminal filling should lead to small changes in circumference. In these locations, muscularis is best suited for the sensation of higher force thresholds, as higher forces are required to achieve a given displacement.
Contraction.
Gut smooth muscle, independently of innervation, contracts in response to stretch. This response is called the myogenic reflex27,28. It is particularly important for the function of sphincters, for which smooth muscle mechanosensation likely plays a crucial part. Wall stretch also initiates peristaltic contractions. The pathways that result in oral contraction and aboral relaxation are part of a broader, complex, coordinated motor programme that some experts term peristalsis and not a ‘peristaltic reflex’29. Peristalsis depends on neuronal mechanisms and is one of the principal contractile programmes that advance intraluminal contents over large distances. As already described, mechanoreceptors in the tunica muscularis are classified as in-parallel length sensors and in-series tension sensors (FIG. 2). Both play a part in peristalsis. In the small intestine, segmentation contractions mix intraluminal contents with intestinal secretions5. This contractile programme typically involves the circular muscle, as it does not serve the purpose of advancing contents through the gut tube. Segmentation is best understood as a very local response to distension. It can be evoked in a ring of circular muscle roughly a centimetre long5. These contractions occur in the bulging region roughly at the midpoint between two previous contractions. However, segmentation can cover large portions of the small intestine by an unknown mechanism30. A major limitation to progress in our understanding of these important motor activities is that the molecular identity of the mechanosensors that initiate and coordinate peristalsis and segmentation is currently unknown.
Flow.
Intraluminal pressure in a closed volume leads to the flow of contents along the pressure gradient. Even though the mechanical activities of the gastrointestinal tract move material in all directions, they ultimately result in the bulk transfer of ingesta aborally because of the contractile gradient. The characteristics of the intraluminal contents are not constant throughout the tract. We can generalize that the upper gastrointestinal tract encounters mostly solids, the middle mostly liquids and the lower tract encounters solids again. Sensing the physical state of the intraluminal contents would be useful, and some evidence suggests that it happens in vivo. In rats, the proximal colon will produce antiperistaltic waves if it receives soft and moist material31. In humans, solid diet components such as bran accelerate small bowel transit32. Mechanosensation should have a role in distinguishing the physical state of intraluminal contents. Solids can generate distension, and liquids are more suitable for generating shear. Finer particles and low-viscosity fluids generate light mechanical stimuli. Mucosal mechanoreceptors would be better suited for light touch sensation owing to force dissipation along the laminated tube wall. Conversely, bigger solid particles and highly viscous fluids can be aptly sensed by deeper tube layers, since the force magnitude may be large enough to penetrate through the tissue.
Mechanosensory cells that detect forces
Non-specialized mechanosensory cells
Given the non-stop mechanical activity of the gut, all cells in the gut wall and luminal residents, perhaps even the microbiota33, might encounter persistent mechanical stimuli.
Syncytium.
As all cells in the gastrointestinal tract live in the mechanical environment, they have developed mechanisms to sense and adjust their functions in response to mechanical forces. In the gastrointestinal tract, the most numerous cell type is the SMC. SMCs are mechanosensitive and would be logical sensory elements to coordinate the myogenic reflex mentioned earlier in this Review. However, we still know little about the mechanosensitivity of SMCs. For example, are all gastrointestinal SMCs mechanosensitive or only subpopulations, as might be the case for vascular SMCs34? SMC express various mechanosensitive elements, such as mechanosensitive ion channels35,36, but the mechanosensitive elements directly responsible for gastrointestinal myogenic reflex are not firmly established. Interstitial cells of Cajal (ICCs) closely associate with SMCs (FIG. 3) and coordinate the cyclical contractile activity of SMCs by generating and propagating electrical slow waves that depolarize SMCs37. ICCs set the frequency of gastric slow waves, the frequency of which is responsive to force, suggesting ICC mechanosensitivity at least in this organ37. Similar subpopulations of mechanosensitive ICCs can be speculated in other organs. Indeed, human jejunal ICCs express mechanosensitive ion channels38. There are several types of ICC, classified by their location in the layered structure of the gut wall39: some generate slow waves, while others are involved in signal transduction, such as neuromuscular signalling. We do not yet know if all types are mechanosensitive, and if they are, whether they participate in mechanosensing in collaboration with SMCs and neurons40. In addition to SMCs and ICCs, fibroblast-like cells (PDGFR+) have important roles in regulating motility, likely through purinergic signalling41,42, but nothing is known about their responses to force.
Fig. 3 |. Circuits, cells and molecules involved in gut mechanosensation.
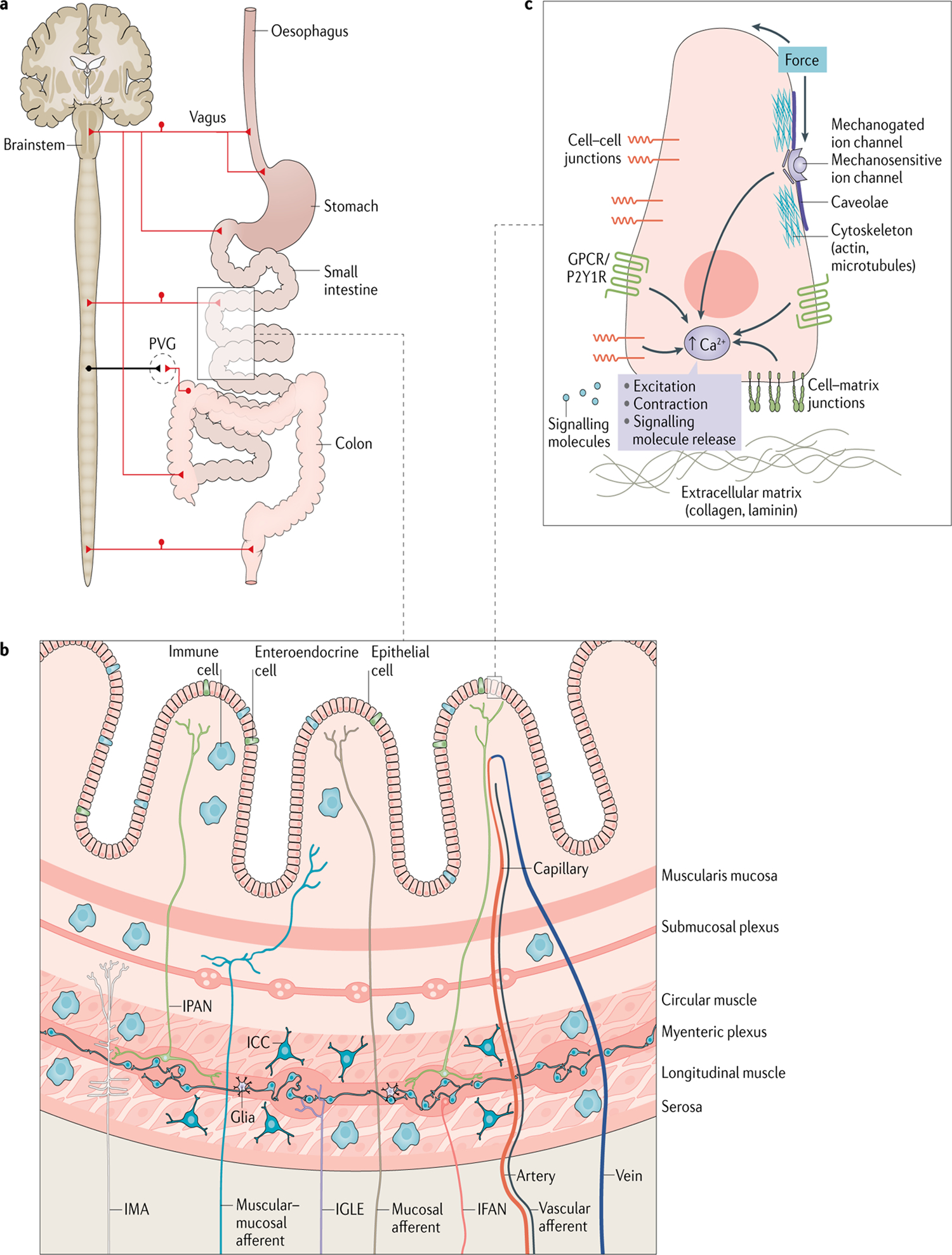
a | Extrinsic innervation of the gastrointestinal tract. From the vantage of the central nervous system, vagal, thoracolumbar and lumbosacral pathways innervate distinct regions of the gastrointestinal tract. There are three prevertebral ganglia (PVGs): coeliac, superior mesenteric and inferior mesenteric. b | Constituent cells of mechanosensory circuits in a section of the gut wall. All highlighted cell types are discussed in the main text. c | Mechanosensors in a generic mechanoreceptor. Mechanical force gets transduced by several proteins in the mechanoreceptor, including mechanogated ion channels, integrins and cytoskeletal filaments, among others. Mechanotransducers such as purine receptor P2Y1R and mechanosensitive G-protein-coupled receptors (GPCRs) amplify pathways initiated by mechanosensors. Mechanotransducers carry out their amplification role by activating secondary messengers such as intracellular calcium (Ca2+). ICC, interstitial cell of Cajal; IFAN, intestinofugal neuron; IGLE, intraganglionic laminar ending; IMA, intramuscular array; IPAN, intrinsic primary afferent neuron.
Glia.
Glia are abundant and have a diverse range of physiological functions in the gut (reviewed elsewhere43). Although studies show that glia are mechanosensitive44, very little is known about their mechanosensory mechanisms and functions. For instance, in vivo studies in mice and humans have implicated glia in linking immune cells and enteric neurons in the development of visceral hypersensitivity45.
Immune cells.
There is three-way communication between the microbiota, immune cells and components that endow sensorimotor function, neurons46 and myocytes47,48 (FIG. 3). Some general immune functions, such as antigen recognition and T cell receptor (TCR) triggering, are recognized to depend on mechanical sensing49,50 (reviewed elsewhere51–53), but immune cell mechanosensitivity of specific gastrointestinal immune components could also have direct physiological consequences. Studies of immune cell mechanosensitivity have gained traction in organs with mechanical function, but little is known about this topic in the gut. For example, in mouse lung macrophages, cyclic pressure regulates inflammation due to infection or fibrosis50. The mechanosensitive channel transient receptor potential vanilloid-type 4 (TRPV4) presumably has a central role in mechanosensation by cells of the innate immune system. TRPV4 has been implicated in neutrophil chemotaxis, macrophage phagocytosis and the formation of reactive oxygen species54. At the adaptive level, mechanical forces are also crucial regulators of TCR activation, which is essential for T cell activation49,55. Despite these advances, the field of immune mechanosensing is still in its infancy: the mechanosensitivity of and its roles for most immune cells are simply unknown. Gastrointestinal-specific advances can shed light on crucial questions, such as does immune cell mechanosensitivity contribute to the well-established concept of post-inflammatory and/or infectious mechanical hypersensitivity and dysmotility?
Non-sensory enteric nerves.
Enteric neurons form interconnected plexuses: Meissner’s in the submucosa, and Auerbach’s, sandwiched between the circular and longitudinal layers of muscularis externa (FIG. 1). The beautiful arrangement of myenteric neurons into a polygonal mesh has mechanical implications: accommodation of axial or radial change by compensating in the other direction prevents axonal tearing during gastrointestinal function5. Auerbach’s plexus neurons ‘float’ in loose collagen stroma, which allows them to glide during muscle activity56. The mechanosensitivity of neurons in the myenteric plexus of the cat small intestine was described by Mayer and Wood in 1975 based on electrical responses57. Using mechanical filaments or intraganglionic fluid injections and imaging of intact plexuses, about a quarter of myenteric neurons were found to be mechanosensitive in the stomach58, small bowel56,59 and colon56 of guinea pigs and mice. The highest fractions of mechanosensitive neurons were motor neurons and interneurons, which do not have an assigned mechanosensory function56,59. Most were rapidly adapting, but some neurons were slowly, and even ultra-slowly adapting, suggesting that myenteric neurons might be intrinsically sensitive to movement and distension56. Mechanosensitivity of the soma does not have a clear role. In loosely dissociated cultured guinea pig and human myenteric neurons, about half responded to mechanical deformation of the neurites, some responded to soma deformation, and they showed a range of adaptation to force, from rapid to ultra-slow. These proportions matched the findings from intact tissues, but it was unclear which subtype of enteric neuron was studied60. Furthermore, we still need to understand whether it is mechanosensitivity of the soma and/or the processes that drives physiological function. This process is invariably dictated by the expression of mechanosensitive proteins in distinct cellular sublocations. The hunt for mechanoreceptors and mechanotransducers in enteric nerves is ongoing. Mechanogated channel PIEZO1 is expressed in soma and neurites of human submucosal and myenteric neurons, although there is as yet no evidence that it functions as a primary mechanoreceptor in these cells61. Mechanosensitive channel TRPV2 expressed in gastric inhibitory motor neurons is necessary for adaptive relaxation in mice62. Blocking the sodium/calcium exchanger NCX1 attenuates mechanically induced Ca2+ transients in rat and human oesophageal myenteric neurons63. Future studies will have to match the spatial localization of relevant molecular targets with mechanical responses.
Specialized mechanoreceptors
The primary role of specialized mechanoreceptors is to detect mechanical stimuli and convert them into physiological responses. These cells form afferent limbs of sensory circuits. Their function is to inform the effector limbs of the circuits, which carry out the physiological end function.
Specialized mechanoreceptors.
Cells whose primary function is the conversion of a mechanical stimulus into a physiological signal that influences the behaviour of other cells.
Epithelial touch sensors.
Mucosal pressure leads to a large outflow of serotonin (5-HT), suggesting that enterochromaffin (EC) cells that produce 5-HT are mechanosensitive in mice64. Interestingly, EC cells have developmental and functional similarities to the skin’s light touch sensors, Merkel cells65. The mucosal layer is best suited for mechanosensing light stimuli that originate intraluminally. In both Merkel and EC cells, the mechanosensitive ion channel PIEZO2 serves as the primary mechanosensor66,67. EC cells are a subtype of a broad population of epithelial sensory cells called enteroendocrine cells (EECs), and several subpopulations express PIEZO2 (REF.68). The mechanosensitivity of channels such as PIEZO2 is affected by their localization in microdomains within the plasma membrane and cytoskeleton69. The rapid mechanosensitive receptor current generated by PIEZO2 leads to longer-lasting Ca2+ signalling and 5-HT release by mechanisms that require further elucidation in tissue cultures70. Additionally, mechanical stimulation of EC cell models leads to activation of G-protein-coupled receptors71 that, in turn, leads to purine release and autocrine 5-HT release69. Intriguingly, both Merkel and EC cells also express the P2Y1 receptor, which contributes to touch-induced impulse generation and EC cell mechanosensitivity69. Mechanosensitive EECs participate in ‘gut touch’, which is an intrinsic mechanosensory function of the gastrointestinal mucosa that enables the intestines to sense physical properties of luminal contents, such as particulate size, similar to size and texture sensing by the fingertips and oral mucosa72.
Receptor current.
Ionic current generated during sensory transduction, such as the opening of the mechanogated ion channels.
Generator potentials.
Transmembrane electrical potentials or voltage shifts due to receptor current that engage voltage-sensing transducer amplification elements.
Intrinsic primary afferent neurons.
The gut is unique compared with all other organs because it has an extensive built-in intrinsic, or enteric, nervous system (ENS), which has been covered in excellent previous reviews73,74. Some call the ENS the ‘second’ or ‘little’ brain, but others have argued that it might evolutionarily predate the central nervous system (CNS)75. Within the ENS, Dogiel type II intrinsic primary afferent neurons (IPANs) have cell bodies in the submucosal and myenteric plexuses along the entire length of the gastrointestinal tract. IPANs are intrinsic sensory neurons that process signals from the lumen, immune system and from within the gut wall itself76. In the mouse small intestine, they can be identified by the expression of calcitonin gene-related peptide (CGRP), 145 kDa intermediate molecular weight neurofilament (NF145kDa) and advillin56,77. IPANs are involved in responses of the gastrointestinal tissue to stretch56,78,79. They are not the only mechanosensitive intrinsic neurons (mechanosensitivity of the ENS has been excellently covered in detail elsewhere80). IPANs are specialized mechanoreceptors in the ENS because they have been formally proved with guinea pigs to initiate neural reflexes in response to physical deformation77. Conversely, the role of other intrinsic mechanosensitive neurons in mechanically induced reflexes needs further investigation80. When their processes are stretched, myenteric IPANs produce generator potentials and alter excitability79,81. Single-cell sequencing efforts in IPANs are beginning to identify potential mechanosensors, such as PIEZO2 (REF.82).
Interestingly, although distortion of IPAN processes leads to excitation, forces applied to cell bodies lead to inhibition. These findings suggest that the mechanical properties of resident cells are heterogeneous79, just like the mechanical properties of macroscopic tissue. The molecules and mechanisms of IPAN mechanosensitivity are unclear. It is appealing to surmise that sensory cells tune the mechanosensory properties of their parts to the mechanical properties of their tissues. Examples in the interplay between static physical and mechanosensory properties are emerging, such as the necessity for a baseline cell membrane tension in mechanosensory neurons83.
Extrinsic sensory neurons.
Extensive extrinsic sensory neuronal innervation links the gut and the brain (FIG. 3). Extrinsic innervation is discussed in detail in several excellent reviews84,85, so we focus on mechanosensing by the extrinsic sensory neurons. The abdominal and pelvic viscera are innervated by vagal, splanchnic and pelvic nerves, which originate either from the brainstem (vagal) or from the spinal cord (splanchnic and pelvic)84 (FIG. 3). These nerves have characteristic anatomical, physiological and molecular features86. From the vantage of the CNS, the extrinsic gut innervation uses three anatomically distinct sensory pathways — vagal, thoracolumbar and lumbosacral. From the vantage of the gut wall, five structurally distinct types of sensory afferent ending are strategically positioned to determine the mechanical state of the gut wall: intraganglionic laminar endings (IGLEs) that surround the myenteric plexus; intramuscular arrays (IMAs); mucosal; muscular–mucosal; and vascular endings that run alongside vessel branch points18,84 (FIG. 3). The latest discoveries point to impressive complexity and diversity of afferent ending structures. An individual extrinsic axon can have multiple sensory endings, and interestingly, individual axons can terminate as different morphological endings in different layers of the colon, including novel endings such as intraganglionic varicose endings87,88. Each morphological ending might correspond to a distinct mechanosensory function, but we await functional demonstration of how this structure influences mechanosensory function.
The landscape of molecular mechanosensors and mechanotransducers is not as neatly defined for extrinsic innervation of the gastrointestinal tract. It might be that a handful of molecules when placed in these specialized sensory endings can be positioned to detect unique mechanical signals. Or it could be that an equally large number of protein families is necessary to sense mechanical stimuli by each ending type. The identities of mechanosensitive proteins in extrinsic neurons remain largely unknown, although progress is ongoing. For example, extrinsic fibres projecting to the colon and originating in the nodose and dorsal root ganglia express members of the mechanosensitive acid-sensing ion channel (ASIC) family89,90. In mice, ASIC subtypes contribute differentially to mechanosensation by neuronal afferents depending on the target organ: Asic1 mutations increase mechanosensation by stomach and colon afferents, whereas Asic2 mutations increase mechanosensation by gastro-oesophageal mucosal endings91. As highlighted in the Future directions section of this Review, molecular candidates for mechanoreceptors and mechanotransducers in this neuronal population can be drawn from peripheral sensory neurons, particularly those that participate in pain signalling (reviewed elsewhere92).
The vagus nerve is an information super-highway between the gut and the brain, informing both about the interoceptive state of the body. Filling of luminal organs, such as the stomach and small intestine93 regulates immediate satiation of hunger94 and even thirst94,95. Stomach filling regulates feeding, and studies in cats show that stretch activates vagal projecting neurons96. Mechanical distension of the gastric wall, as would happen during feeding, is reported by vagal IGLEs94,97. Although the stomach is a logical location to sense filling due to food, in mice, there is a subset of vagal nerve projections that form IGLEs in the small intestine, which could play a dominant part in the regulation of food intake by sensing intestinal filling93. There appears to be a pathway that integrates mechanosensory information on instantaneous volume changes in the upper gastrointestinal tract, transmits it to the parabrachial nucleus in the brainstem and translates it into appetite-suppressing signals98. Although the identities of the mechanoreceptors in this and many other pathways remain elusive, we describe in the Future directions section of this Review the exciting advances that are poised to identify the molecular identities of the mechanosensors involved.
IGLEs lie near the myenteric plexus and, therefore, near enteric mechanosensory neurons. The basic IGLE consists of a large diameter parent axon that bifurcates upon entering the ganglion and arborizes its laminar endings in close contact with the connective tissue capsule99. In the guinea pig oesophagus, IGLEs are proposed to be intrinsically mechanosensitive based on response kinetics, but mechanically induced responses were not inhibited by traditional stretch-activated ion channel blockers97. If intrinsically mechanosensitive, IGLEs have characteristics of in-series tension mechanoreceptors, on the basis of their location and function. Another sensory structure is the IMA, which has features of an in-parallel length mechanoreceptor100 (FIG. 2). Vagal101 and spinal intramuscular afferents project their fibres into the syncytium throughout the length of the gastrointestinal tract. They might be important sensory structures at two ends of the gastrointestinal tract: the oesophageal and anal sphincters84. They frequently run close to the ICCs, which may be mechanosensitive37. The arrangement of ICCs and intramuscular afferents is reminiscent of muscle spindle arrangement, as opposed to IGLEs, which are more reminiscent of Golgi tendon organs21.
Mucosally projecting endings from the vagus and spinal nerves are involved in sensing light mechanical distortion, either by themselves102 or in collaboration with mechanosensitive EECs that release ATP69 and 5-HT in response to force103. This system has similarities to somatosensory touch, a two-mechanoreceptor system composed of a mechanosensitive epithelial Merkel cell104 with some releasing 5-HT105 and a mechanosensitive sensory neuron106,107. Another intriguing parallel between gut mechanosensing and the sensory system of the skin is the intestinal presence of structures similar to Meissner corpuscles, which are receptive to light touch in the skin108. There are also combined ‘muscular–mucosal’ afferents. These are low-threshold, distension-sensitive intraganglionic mechanoreceptors also activated by light mucosal distortion, according to evidence in the ferret oesophagus and stomach109. We do not currently know whether these sensory fibres use branching endings to detect mechanical inputs from both muscle and mucosa, or if they are strategically positioned in a subepithelial plexus that can report physical activity from both locations.
The spinal vascular afferents, which make up one-third to a half of all lumbosacral afferents to the gut18, run along with the branch points of mesenteric vessels110 into the submucosa111. Anatomically, they are divided into ‘mesenteric’ and ‘serosal’, depending on the location of their mechanosensitive sites112. Firm local compression of the mesentery or the gut wall activates these fibres in mice18. The vascular afferents are also sensitive to the dynamic phase of distension, for example, during contraction and distension during peristaltic activity110. These endings might be nociceptors. For example, as their position is on vessels, they report on the mechanical aspects of blood flow, being explicitly activated in rats by a decrease in blood vessel wall tension113. There are two mechanosensitive populations: one with relatively high thresholds to distension plus slow firing rates, and another with lower thresholds and higher excitability114. The vascular afferents could serve a dual function by also controlling mechanically induced vasodilation, thereby coordinating mesenteric blood flow with changes in gut motility115. Some vascular afferent endings are structurally like the classic Pacinian corpuscles that detect vibration in the somatosensory system116. Indeed, these mechanoreceptors respond to phasic movements during the dynamic phase of distension. As the gut seldom encounters such high-frequency stimuli, they are tuned to lower frequencies110.
Intestinofugal afferent neurons.
Intestinofugal afferent neurons (IFANs)117 straddle the divide between intrinsic and extrinsic innervation of the gastrointestinal tract: IFANs have cell bodies in the myenteric ganglia and project out of the gut to prevertebral ganglia118, where they stimulate sympathetic outflow. IFANs are arranged in parallel with circular muscle. They are active during distension but are ‘silent’ during contraction. Thus, IFANs are volume sensors that detect intestinal stretch and help maintain the wall in a relaxed condition during filling by providing a counterbalance to the myogenic reflex117. IFANs have been postulated as mechanoreceptors that integrate mechanical signals at the level of the prevertebral ganglia, but as they make extensive synaptic contacts, we do not definitively know that they are directly mechanosensitive117,119. It is worth noting that the proportion of mechanosensitive cells and neuronal endings is not fixed. So-called silent afferents are mechanically insensitive until they are ‘sensitized’, presumably by inflammatory mediators120 or by yet to be discovered signalling cues.
Mechanosensory circuits
Spatial and temporal integration of mechanosensory information is crucial for organizing normal physiological functions such as the well-coordinated peristaltic reflex. The mechanosensory cells discussed already assemble to form the afferent limbs of mechanosensing circuits. Specialized mechanoreceptors are at the centre of the circuits that influence physiological function. Non-specialized mechanosensory cells use mechanical feedback to regulate their function, but they likely also have auxiliary roles in dedicated mechanosensory circuits24. The gut is unique compared with other organs that rely exclusively on extrinsic sensory innervation; in the gut, all regions contain dual innervation from the intrinsic and extrinsic nervous systems84.
Neuroepithelial.
The gastrointestinal lumen communicates with the outside environment. As such, the gut possesses mechanosensory circuits similar to those in the skin to detect luminal mechanical stimuli. For example, similar to the oral cavity and skin, which detect texture and stiffness of food, the luminal gut senses the physical properties of luminal contents32,72,121. Intriguingly, structures similar to Meissner and Pacinian corpuscles, which are specialized mechanosensory endings in the skin, are found in the gut108,110,116. Although structural similarities suggest that these might respond to light touch and high-frequency stimuli, respectively, the roles of these structures are currently unclear.
The gut also has a neuroepithelial connection akin to the Merkel cell — a sensory neuron connection involved in sensing skin light touch122. Mechanical stimulation of epithelial EC cells, which are similar to Merkel cells, leads to activation of IPANs123. This neuroepithelial connection regulates local secretory66,124 and extrinsic sensory neurons94, which inform the gut–brain axis. In light touch sensation in the mouse skin, neuroepithelial mechanosensory circuits rely on synaptic transmission105,122. In the mouse gut, neuroepithelial sensory connections couple epithelial mechanosensitive EECs with neurons, either modulating94 or directly activating the latter66. There has been major progress in elucidating neuroepithelial synaptic communication between a group of EECs called neuropods and extrinsic sensory neurons125, which can mediate sensory transduction for direct input to the CNS in mice126. It remains unknown whether mechanotransduction takes the same route or requires refinement before relay to the CNS (discussed later). Moreover, the proportion of EECs that participate in such synaptic communication with extrinsic neurons is unclear. With respect to intrinsic neurons, the mode of communication between EECs and IPANs remains unresolved65,123. Notably, there is no distinct requirement for the neurons synapsing with epithelial mechanoreceptors to be mechanoreceptors themselves. Although peristalsis can be initiated in animal models by stroking the mucosa127,128, peristalsis-like activity can also be elicited in mice by mechanical stimulation of the tunica muscularis129. Thus, mechanosensitivity of both mucosa and tunica muscularis are likely involved in peristalsis. This observation is reminiscent of the two-receptor arrangement in skin touch sensing107, in which both the epithelial and neuronal cells are mechanosensitive, and, a similar two-receptor arrangement is possible in gastrointestinal neuroepithelial circuits.
Interstitial or glial–neuron.
An important intrinsic mechanosensory reflex is the RAIR, which is used in the diagnosis of Hirschsprung disease (BOX 2). RAIR is a nitric oxide-mediated relaxation of the anal canal in response to even small mechanical stimuli in the rectum24. Interestingly, ICCs have been implicated in RAIR mechanotransduction24, suggesting an ICC–neuron communication in mechanotransduction. Even though subpopulations of ICCs elsewhere are mechanosensitive, we do not know whether in RAIR ICCs are mechanoreceptors or downstream recipients of signals generated by bona fide mechanoreceptors. In the stomach, ICCs reside very close to another presumably mechanosensitive structure, the IMA of the vagal projections40. But, as discussed, although structure suggests function, we do not currently know whether ICC–IMA connections are functional mechanoreceptors or indeed how they work. Glia mechanosensitivity in the gut remains understudied44. Nevertheless, glia also reside near enteric and extrinsic neurons, and ample communication between glia and neurons in the gut has been demonstrated in vivo130, suggesting the possibility of glia–neuron mechanosensing units. There is a growing possibility that mechanosensing by immune cells such as macrophages might control physiological gut function, specifically through their connections with neurons46,130, smooth muscle48 and ICCs131.
Box 2 |. Pathologies with known or suspected disruption of gastrointestinal mechanosensation.
Hirschsprung disease
Irritable bowel syndrome
Functional dyspepsia
Diverticula
Ulcerative colitis
Nausea
Obesity
Centrally mediated abdominal pain syndrome
Dyssynergic defaecation
Achalasia
Functional dysphagia
Crohn’s disease
Visceral hypersensitivity
Neuron–neuron.
There are several types of mechanosensitive neuron in the gut wall, including IPANs, extrinsic sensory neurons, interneurons, muscle motor and secretomotor neurons. Structurally, these neurons are often near each other, such as the IGLEs formed by extrinsic neurons that serve as mechanosensory endings93. We still do not know how the ENS and extrinsic neurons communicate: is there a role for ENS modulation of mechanosensory signals carried by extrinsic primary afferents? The discovery of mechanosensitive interneurons and other intrinsic neurons raises the possibility, especially given that there is already bidirectional flow of information between the ENS and CNS132. Interestingly, IGLEs signal during both distension and contraction133, suggesting structural and functional specialization. The spatial arrangement is suggestive of interaction, and indeed neuron–neuron mechanosensory connections might give the ENS the ability to refine mechanosensory responses before sending signals to the CNS.
Future directions
Impressive progress has been made in our understanding of mechanosensation in the gut. We propose that many more exciting discoveries lie ahead. Here, we focus on some emerging areas, both technical and intellectual, that will bear fruit in our understanding of gut mechanosensation. These areas include discoveries of mechanosensors, definition of the landscape of mechanical energy distribution in the gut wall and development of novel approaches to test gut mechanosensation.
First, it is worth emphasizing that many of the studies referenced up to now have used a variety of model organisms. We have attempted to highlight the organisms on which gastrointestinal mechanosensation has been studied because interspecies developmental and dietary differences pose important confounders. Future efforts to understand gut mechanosensation in humans will need to include some validation that the discoveries made in these other species translate to human physiology. A 2020 study that identified mechanosensitive neurons in the porcine submucosal plexus validated its animal observations with human studies16. The common features of mechanosensitive neurons were interpreted as evidence of conserved mechanosensory pathways and strengthened the use of this animal model, which has been proposed to share important anatomical, nutritional and physiological similarities with humans16. Tools such as voltage and calcium dyes, as well as ultrafast neuroimaging techniques, allow researchers to study neurons in large animals and in fields with more cells16. Although labour-intensive, challenged by the availability of human samples, and limited by lack of refined diagnostic techniques to study mechanosensing in humans (when compared with fields such as neurology), these types of comparative studies are more necessary than ever as mechanosensation becomes clinically recognized for its role in human disease (BOX 2).
Identifying mechanosensors and pathways
Acute cellular mechanosensation depends on mechanosensors, which are molecules that convert mechanical stimuli into chemical and electrical signals. Cytoskeletal proteins134–136, cell–cell junctions137 and cell–extracellular matrix junctions138–140 are mechanosensors potentially expressed in cells of the gastrointestinal tract. These proteins vary in response kinetics to mechanical stimuli. An important type of mechanosensor that allows rapid force sensing is the mechanosensitive ion channel. Previous reviews have aptly described the mechanosensitive ion channels broadly141, in the gut142,143 and in nociception92. To be considered a mechanosensor channel, the transmembrane protein must fulfil four requirements144 (BOX 3). Drawing conclusions on ion channels being mechanoreceptive without all criteria being fulfilled can lead to misidentification. For example, transient receptor potential (TRP) proteins comprise a large family of channels that are gated by a range of chemical and physical stimuli, including mechanical force. Several TRP channels, such as TRPA1 (REF.145), were proposed to be mechanosensitive. Nevertheless, rigorous testing146 ruled out the majority, and now only some remain viable candidates. For example, TRPV2 is demonstrably mechanosensitive, expressed in inhibitory motor neurons of the gastric myenteric plexus and seems to have a role in gastric adaptive relaxation in mice62. Another noteworthy example is the search for mechanosensors in the IGLEs. If IGLEs are mechanosensitive, their mechanosensors remain unknown. As mechanosensors can be lowly expressed but have major downstream effects, their discovery is akin to looking for a small needle in a haystack. Often, promising molecular candidates are involved in signal transduction instead of in sensing primary stimuli. For example, ASICs, candidate mechanosensors, are expressed by IGLEs, yet stomach stretch responses were reduced but not eliminated in individual Asic2 and Asic3 knockout mice. Interestingly, mechanosensitivity was increased in Asic1 knockouts91. The incomplete elimination of mechanosensitivity in these cases suggests that TRP channels and ASICs might not be mechanosensors, but could instead play a part in mechanotransduction. Force might be the first stimulus that membrane-residing proteins sense147. We are discovering that many ion channels gated by stimuli other than force, such as voltage, are frequently mechanosensitive35,36,148. These channels can contribute substantially to cell mechanosensation in vitro, especially in electrically excitable cells149,150. However, we still do not know whether the voltage-gated ion channels serve as mechanosensors or mechanotransducers. In addition to mechanosensitive ion channels, mechanosensitive G-protein-coupled receptors are emerging as sensors of acute forces in endothelial cells and SMCs151,152. It will be crucial for future research to determine which proteins serve as mechanosensors and which as mechanotransducers. The identity of the primary mechanosensors has not been established in most established mechanoreceptors in the gut. As these molecules are attractive drug targets153, there is an ongoing intense hunt for their identities.
Box 3 |. Four criteria of a mechanosensitive channel.
Spatial and temporal expression in a mechanosensory organ.
Must be required for the response to mechanical stimuli. Hence, channel removal must eliminate the response to a mechanical stimulus.
Molecular alteration of the channel must change the nature of the mechanical response.
-
Heterologous channel expression must demonstrate mechanical gating.
According to REF.144
Technical advances are poised to answer these questions. Circuit tracing using viruses125,154 and transgenic animal models, single-cell RNA sequencing (RNA-seq)155 and connecting omics with function using techniques such as patch-seq156,157 will drive functional dissection of enteric circuitry and provide molecular resolution in the hunt for mechanoreceptors and mechanotransducers. For example, traced and examined at the single-cell level, colon-projecting sensory neurons from the thoracic and pelvic levels form seven clusters, with multiple of these clusters expressing the mechanogated ion channel PIEZO2 (REF.155). Efforts to decode the human ENS at single-cell resolution are already under way158.
Visualizing forces
Distension and contraction powerfully activate visceral pain pathways84. The arrangement of mechanoreceptors in series or in parallel allows the tissue to sense both contraction and distension. Yet, at the molecular level, mechanosensors can only detect force through displacement. Visceral sensory mechanisms are studied by isolating mechanoreceptors, applying different kinds of stimuli and studying the resulting responses. The responses might be conscious visceral sensations, physiological reflexes and electrophysiological responses. A problem that arises from this approach is that common forms of stimuli, such as organ filling and peristaltic movement, have the potential to activate more than one cell112. Another challenge is that mechanosensory signals are likely integrated in brain–gut signalling. A single cell type can be activated by what we perceive as different stimuli. Leek112 drew an analogy to visual sensory systems: detection of the colour ‘yellow’ follows from the stimulation of red and green retinal cones, and detection of the colour ‘magenta’ follows from the stimulation of red cones again, but this time in conjunction with blue cones. Similarly, he posited, what we perceive as ‘fullness’ may follow from the stimulation of tension and serosal mechanoreceptors, whereas ‘flow’ may follow from the stimulation of serosal mechanoreceptors in conjunction with mucosal ones112. Studies connecting structure and function in real time, therefore, are highly revealing. For example, connecting mechanical stimulation of the gut wall with a recording of electrical activity and mechanoreceptor visualization, although technically demanding, is poised to bear fruit111. Intravital colonic imaging has seen major progress in mice159. It might one day be possible to simultaneously and directly observe how multiple mechanoreceptors are activated in response to various stimuli. For example, intravital calcium imaging of traced neurons with reporters of historical activity, such as CaMPARI160,161, could shed light on neuronal reflexes initiated by luminal distension. To determine how forces activate mechanosensory elements, we need to visualize the force distribution in cells162 and tissues163. This spatial information needs to be related to direct measurements of mechanosensor activation.
New and relevant techniques
Mechanosensation is a tissue property tightly controlled in space and time. To effectively study mechanosensitivity, stimuli must be as physiological as possible. Reductionist approaches and highly reproducible mechanical stimuli applied to cells and tissues, such as poking and shear stress in cells, infusing fluids into ganglia59, and von Frey hairs and intraluminal balloons in tissues, are crucial to determining the molecular and cellular basis of mechanosensation. Indeed, understanding gut mechanosensing at the cellular and molecular levels is unambiguous and poised to provide molecular targets. However, given the importance of mechanosensation by the gut, the gut’s mechanosensory system is highly redundant. The result, per Bozler, is that “variability in the responses obtained is partly due to the use of abnormal, crude stimuli”164, suggesting that, even in the golden age of physiology more than 70 years ago, more physiologically relevant mechanical stimuli were required. Innovative technical approaches to model mechanical stimuli in the gut will be mechanistically revealing. For example, varying the physical properties of intraluminal contents to deliver physiologically relevant mechanical stimuli in vivo could be highly revealing32,121,165, especially as the physical properties of food still serve as some of the best therapies for gastrointestinal sensorimotor disorders, such as gastroparesis and constipation165. An additional consideration is that mechanoreceptors do not live in isolation. Some of them, such as subpopulations of EECs, multitask by having dual roles as mechanosensors and chemosensors166,167. Further improvements in technical approaches to best reflect physiological stimuli are required.
Conclusions
Mechanosensing is important for normal gut function, yet it remains superficially understood. In this Review, we focus on the physical properties of the gut, and on the molecules, cells and circuits involved in gut mechanosensing. Mechanosensation depends on the ability of the gut to feel mechanical stimuli, as determined by its mechanical properties and as elicited by activity during digestion. Given the vibrant mechanical world in the gut, normal function requires the engagement of specialized mechanoreceptors that are arranged in mechanosensing circuits, as well as of non-specialized mechanosensory cells that tune their behaviour in response to surrounding forces. The mechanosensing and mechanotransduction mechanisms, which vary in time and space, are accomplished by a range of molecules (mechanosensors and mechanotransducers) in these cells, but their identities remain mostly undiscovered. Technological advances and discoveries of novel mechanosensors are poised to substantially advance this field.
Key points.
Mechanosensation is the ability to sense mechanical forces and transduce them into physiological responses.
The gut is a mechanically active organ in which all cells must sense the forces emanating from the digestion of intraluminal contents and organ activity, such as motility.
All cells reside in tissue at a baseline mechanical state; the gastrointestinal tract is a layered (composite) organ in which the baseline mechanical state varies by spatial localization.
Non-specialized mechanosensory cells sense force to adjust their function; specialized mechanoreceptors are mechanosensory cells that guide physiological organ responses to mechanical stimuli.
Gastrointestinal mechanoreceptors share similarities with mechanoreceptors in other sensory and non-sensory organs; leveraging these similarities helps in understanding the purpose and function of mechanoreceptors in the gastrointestinal tract.
Mechanosensory circuits built into the gastrointestinal wall allow for spatial and temporal integration of mechanical stimuli into a coordinated physiological response (for example, peristaltic reflex), and connections to the extrinsic mechanosensory circuits are crucial for brain–gut communication (for example, sense of fullness).
Areas with research potential include the discovery of unknown mechanosensors, quantification of the baseline mechanical state of the gastrointestinal wall, and the development of novel tests for gut-specific mechanosensation.
Acknowledgements
The authors thank the members of the Mayo Clinic Enteric NeuroScience Program (ENSP) group (G. Farrugia, J. H. Szurszewski, S. J. Gibbons and D. R. Linden), P. Gottlieb (SUNY, Buffalo, NJ, USA) for their constructive feedback, and L. Busby for administrative assistance. NIH support is acknowledged for GM065841, DK128913 (A.M.-P.), and DK052766, DK106456, DK100223 (A.B.).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Cannon WB The Mechanical Factors of Digestion (Longmans, Green & Co., 1911). [Google Scholar]
- 2.Alvarez WC The Mechanics of the Digestive Tract: An Introduction to Gastroenterology 2nd edn (Hoeber, 1928). [Google Scholar]
- 3.Bayliss WM & Starling EH The movements and innervation of the small intestine. J. Physiol 24, 99–143 (1899). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingber DE Cellular mechanotransduction: putting all the pieces together again. FASEB J 20, 811–827 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Gregersen H Biomechanics of the Gastrointestinal Tract: New Perspectives in Motility Research and Diagnostics (Springer, 2003). [Google Scholar]
- 6.Stevens CE & Hume ID Comparative Physiology of the Vertebrate Digestive System 2nd edn (Cambridge Univ. Press, 2004). [Google Scholar]
- 7.Liao D, Zhao J & Gregersen H Three-dimensional geometry analysis of the stomach in type II diabetic GK rats. Diabetes Res. Clin. Pract 71, 1–13 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhao J & Gregersen H The villi contribute to the mechanics in the guinea pig small intestine. J. Biomech 41, 806–812 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Zhao J, Nakaguchi T & Gregersen H Biomechanical and histomorphometric colon remodelling in STZ-induced diabetic rats. Dig. Dis. Sci 54, 1636–1642 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Dinning PG, Arkwright JW, Gregersen H, O’Grady G & Scott SM Technical advances in monitoring human motility patterns. Neurogastroenterol. Motil 22, 366–380 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Gao C & Gregersen H Biomechanical and morphological properties in rat large intestine. J. Biomech 33, 1089–1097 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Gao C, Zhao J & Gregersen H Histomorphometry and strain distribution in pig duodenum with reference to zero-stress state. Dig. Dis. Sci 45, 1500–1508 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhao J, Liao D & Gregersen H Effect of smooth muscle tone on morphometry and residual strain in rat duodenum, jejunum and ileum. J. Biomech 41, 2667–2672 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Liao D, Chen P, Kunwald P & Gregersen H Stomach stress and strain depend on location, direction and the layered structure. J. Biomech 41, 3441–3447 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Benias PC et al. Structure and distribution of an unrecognized interstitium in human tissues. Sci. Rep 8, 4947 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filzmayer AK et al. Compression and stretch sensitive submucosal neurons of the porcine and human colon. Sci. Rep 10, 13791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frieling T, Wood JD & Cooke HJ Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am. J. Physiol 263, G91–G96 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Brierley SM, Jones RC 3rd, Gebhart GF & Blackshaw LA Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Corsetti M, Gevers AM, Caenepeel P & Tack J The role of tension receptors in colonic mechanosensitivity in humans. Gut 53, 1787–1793 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver KM et al. Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat. Commun 12, 1451 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iggo A Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q. J. Exp. Physiol. Cogn. Med. Sci 42, 130–143 (1957). [DOI] [PubMed] [Google Scholar]
- 22.Cannon WB Peristalsis, segmentation, and the myenteric reflex. Am. J. Physiol 30, 114–128 (1912). [Google Scholar]
- 23.Beyder A In pursuit of the epithelial mechanosensitivity mechanisms. Front. Endocrinol 9, 804 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lorijn F et al. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut 54, 1107–1113 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synnerstad I, Ekblad E, Sundler F & Holm L Gastric mucosal smooth muscles may explain oscillations in glandular pressure: role of vasoactive intestinal peptide. Gastroenterology 114, 284–294 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Choe K et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J. Clin. Invest 125, 4042–4052 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvarez WC The myogenic nature of the rhythmic contractions of the intestine. Am. J. Physiol. Leg. Content 59, 421–430 (1922). [Google Scholar]
- 28.Dinning PG, Costa M, Brookes SJ & Spencer NJ Neurogenic and myogenic motor patterns of rabbit proximal, mid, and distal colon. Am. J. Physiol. Gastrointest. Liver Physiol 303, G83–G92 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Dinning PG et al. Neural mechanisms of peristalsis in the isolated rabbit distal colon: a neuromechanical loop hypothesis. Front. Neurosci 8, 75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huizinga JD et al. The origin of segmentation motor activity in the intestine. Nat. Commun 5, 3326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon WB The relation of tonus to antiperistalsis in the colon. Am. J. Physiol. Leg. Cont 29, 238–249 (1911). [Google Scholar]
- 32.McIntyre A, Vincent RM, Perkins AC & Spiller RC Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut 40, 223–227 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HJ, Li H, Collins JJ & Ingber DE Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl Acad. Sci. USA 113, E7–E15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobnikar L et al. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun 9, 4567 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrugia G et al. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology 117, 900–905 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Beyder A et al. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol 588, 4969–4985 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Won KJ, Sanders KM & Ward SM Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc. Natl Acad. Sci. USA 102, 14913–14918 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strege PR et al. Sodium current in human intestinal interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol 285, G1111–G1121 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Komuro T Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J. Physiol 576, 653–658 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powley TL et al. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil 20, 69–79 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Sanders KM, Koh SD, Ro S & Ward SM Regulation of gastrointestinal motility — insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol 9, 633–645 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurahashi M et al. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J. Physiol 589, 697–710 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seguella L & Gulbransen BD Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol 18, 571–587 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liñán-Rico A et al. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype. Inflamm. Bowel Dis 22, 1812–1834 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grubisic V et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep 32, 108100 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller PA et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kraichely RE, Strege PR, Sarr MG, Kendrick ML & Farrugia G Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am. J. Physiol. Gastrointest. Liver Physiol 296, G833–G839 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo J et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity 49, 107–119.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng Y et al. Mechanosensing drives acuity of alphabeta T-cell recognition. Proc. Natl Acad. Sci. USA 114, E8204–E8213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Solis AG et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 573, 69–74 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X et al. Unraveling the mechanobiology of immune cells. Curr. Opin. Biotechnol 66, 236–245 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Upadhyaya A Mechanosensing in the immune response. Semin. Cell Dev. Biol 71, 137–145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu C, Chen W, Lou J, Rittase W & Li K Mechanosensing through immunoreceptors. Nat. Immunol 20, 1269–1278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michalick L & Kuebler WM TRPV4-A missing link between mechanosensation and immunity. Front. Immunol 11, 413 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu P et al. Mechano-regulation of peptide-MHC class I conformations determines TCR antigen recognition. Mol. Cell 73, 1015–1027 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzuoli G & Schemann M Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS ONE 7, e39887 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer CJ & Wood JD Properties of mechanosensitive neurons within Auerbach’s plexus of the small intestine of the cat. Pflug. Arch 357, 35–49 (1975). [DOI] [PubMed] [Google Scholar]
- 58.Mazzuoli-Weber G & Schemann M Mechanosensitive enteric neurons in the guinea pig gastric corpus. Front. Cell. Neurosci 9, 430 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mazzuoli G & Schemann M Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. J. Physiol 587, 4681–4694 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kugler EM et al. Mechanical stress activates neurites and somata of myenteric neurons. Front. Cell Neurosci 9, 342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzuoli-Weber G et al. Piezo proteins: incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res 375, 605–618 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Mihara H, Suzuki N, Yamawaki H, Tominaga M & Sugiyama T TRPV2 ion channels expressed in inhibitory motor neurons of gastric myenteric plexus contribute to gastric adaptive relaxation and gastric emptying in mice. Am. J. Physiol. Gastrointest. Liver Physiol 304, G235–G240 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Dong H, Tang B, Jiang Y & Mittal RK Na+/Ca2+ exchanger 1 is a key mechanosensitive molecule of the esophageal myenteric neurons. Acta Physiol 225, e13223 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Bulbring E & Crema A The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J. Physiol 146, 18–28 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treichel AJ, Farrugia G & Beyder A The touchy business of gastrointestinal (GI) mechanosensitivity. Brain Res 1693, 197–200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alcaino C et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl Acad. Sci. USA 115, E7632–E7641 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol 595, 79–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Billing LJ et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice — identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol. Metab 29, 158–169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linan-Rico A et al. Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front. Neurosci 10, 564 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertrand PP Real-time measurement of serotonin release and motility in guinea pig ileum. J. Physiol 577, 689–704 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chin A et al. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am. J. Physiol. Gastrointest. Liver Physiol 302, G397–G405 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treichel AJ et al. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology 10.1053/j.gastro.2021.10.026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spencer NJ & Hu H Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol 17, 338–351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furness JB The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol 9, 286–294 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Furness JB & Stebbing MJ The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol. Motil 10.1111/nmo.13234 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Furness JB, Jones C, Nurgali K & Clerc N Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol 72, 143–164 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Melo CGS et al. Identification of intrinsic primary afferent neurons in mouse jejunum. Neurogastroenterol. Motil 32, e13989 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kunze WA, Clerc N, Bertrand PP & Furness JB Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J. Physiol 517, 547–561 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kunze WA, Clerc N, Furness JB & Gola M The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J. Physiol 526, 375–385 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzuoli-Weber G & Schemann M Mechanosensitivity in the enteric nervous system. Front. Cell. Neurosci 9, 408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mao Y, Wang B & Kunze W Characterization of myenteric sensory neurons in the mouse small intestine. J. Neurophysiol 96, 998–1010 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Morarach K et al. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci 24, 34–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qi Y et al. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat. Commun 6, 8512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brookes SJ, Spencer NJ, Costa M & Zagorodnyuk VP Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol 10, 286–296 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Furness JB, Rivera LR, Cho H-J, Bravo DM & Callaghan B The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol 10, 729–740 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Smith-Edwards KM et al. Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology 157, 522–536 e522 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spencer NJ, Kyloh MA, Travis L & Dodds KN Sensory nerve endings arising from single spinal afferent neurons that innervate both circular muscle and myenteric ganglia in mouse colon: colon–brain axis. Cell Tissue Res 381, 25–34 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Spencer NJ, Melinda A, Kyloh MA, Travis L & Dodds KN Identification of spinal afferent nerve endings in the colonic mucosa and submucosa that communicate directly with the spinal cord: the gut–brain axis. J. Comp. Neurol 528, 1742–1753 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Hughes PA, Brierley SM, Young RL & Blackshaw LA Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J. Comp. Neurol 500, 863–875 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Holzer P Acid-sensing ion channels in gastrointestinal function. Neuropharmacology 94, 72–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Page AJ et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54, 1408–1415 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharif-Naeini R Role of mechanosensitive ion channels in the sensation of pain. J. Neural Transm 127, 407–414 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Bai L et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 e1123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams EK et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zimmerman CA et al. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 568, 98–102 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paintal AS A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J. Physiol 126, 255–270 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zagorodnyuk VP, Chen BN & Brookes SJ Intraganglionic laminar endings are mechanotransduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol 534, 255–268 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim DY et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Berthoud HR, Patterson LM, Neumann F & Neuhuber WL Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat. Embryol 195, 183–191 (1997). [DOI] [PubMed] [Google Scholar]
- 100.Phillips RJ & Powley TL Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Rev 34, 1–26 (2000). [DOI] [PubMed] [Google Scholar]
- 101.Berthoud HR & Powley TL Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol 319, 261–276 (1992). [DOI] [PubMed] [Google Scholar]
- 102.Grundy D, Blackshaw LA & Hillsley K Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity. Dig. Dis. Sci 39, 44S–47S (1994). [DOI] [PubMed] [Google Scholar]
- 103.Kirchgessner AL, Tamir H & Gershon MD Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J. Neurosci 12, 235–248 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maksimovic S et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chang W et al. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc. Natl Acad. Sci. USA 113, E5491–E5500 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ranade SS et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Woo SH, Lumpkin EA & Patapoutian A Merkel cells and neurons keep in touch. Trends Cell Biol 25, 74–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huber AR, Agostini-Vulaj D, Drage MG & Lemmon JW Tactile corpuscle-like bodies (Wagner–Meissner corpuscles) of the colorectum: a series of 5 cases. Int. J. Surg. Pathol 25, 684–687 (2017). [DOI] [PubMed] [Google Scholar]
- 109.Page AJ & Blackshaw LA An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J. Physiol 512, 907–916 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bessou P & Perl ER A movement receptor of the small intestine. J. Physiol 182, 404–426 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song X et al. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 137, 274–284, e271 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leek BF Abdominal and pelvic visceral receptors. Br. Med. Bull 33, 163–168 (1977). [DOI] [PubMed] [Google Scholar]
- 113.Brunsden AM, Brookes SJ, Bardhan KD & Grundy D Mechanisms underlying mechanosensitivity of mesenteric afferent fibers to vascular flow. Am. J. Physiol. Gastrointest. Liver Physiol 293, G422–G428 (2007). [DOI] [PubMed] [Google Scholar]
- 114.Malin SA, Christianson JA, Bielefeldt K & Davis BM TPRV1 expression defines functionally distinct pelvic colon afferents. J. Neurosci 29, 743–752 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meehan AG & Kreulen DL A capsaicin-sensitive inhibitory reflex from the colon to mesenteric arteries in the guinea-pig. J. Physiol 448, 153–159 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sheehan D The afferent nerve supply of the mesentery and its significance in the causation of abdominal pain. J. Anat 67, 233–249 (1933). [PMC free article] [PubMed] [Google Scholar]
- 117.Szurszewski JH, Ermilov LG & Miller SM Prevertebral ganglia and intestinofugal afferent neurones. Gut 51 (Suppl. 1), i6–i10 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Weems WA & Szurszewski JH An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J. Neurophysiol 41, 305–321 (1978). [DOI] [PubMed] [Google Scholar]
- 119.Hibberd TJ, Zagorodnyuk VP, Spencer NJ & Brookes SJ Identification and mechanosensitivity of viscerofugal neurons. Neuroscience 225, 118–129 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Gold MS & Gebhart GF Nociceptor sensitization in pain pathogenesis. Nat. Med 16, 1248–1257 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hebden JM, Blackshaw PE, Perkins AC, D’Amato M & Spiller RC Small bowel transit of a bran meal residue in humans: sieving of solids from liquids and response to feeding. Gut 42, 685–689 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoffman BU et al. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 100, 1401–1413 e1406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pan H & Gershon MD Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J. Neurosci 20, 3295–3309 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cooke HJ, Sidhu M & Wang YZ 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol. Motil 9, 181–186 (1997). [DOI] [PubMed] [Google Scholar]
- 125.Kaelberer MM et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaelberer MM, Rupprecht LE, Liu WW, Weng P & Bohorquez DV Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu. Rev. Neurosci 43, 337–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raiford T & Mulinos MG The myenteric reflex as exhibited by the exteriorized colon of the dog. Am. J. Physiol. Leg. Content 110, 129–136 (1934). [Google Scholar]
- 128.Smith TK & Furness JB Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J. Autonomic Nerv. Syst 25, 205–218 (1988). [DOI] [PubMed] [Google Scholar]
- 129.Spencer NJ et al. By what mechanism does ondansetron inhibit colonic migrating motor complexes: does it require endogenous serotonin in the gut wall? Neurogastroenterol. Motil 25, 677–685 (2013). [DOI] [PubMed] [Google Scholar]
- 130.Delvalle NM et al. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell Mol. Gastroenterol. Hepatol 6, 321–344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cipriani G et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 154, 2122–2136 e2112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furness JB, Callaghan BP, Rivera LR & Cho HJ The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol 817, 39–71 (2014). [DOI] [PubMed] [Google Scholar]
- 133.Lynn P, Zagorodnyuk V, Hennig G, Costa M & Brookes S Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J. Physiol 564, 589–601 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Romero S, Le Clainche C & Gautreau AM Actin polymerization downstream of integrins: signaling pathways and mechanotransduction. Biochem. J 477, 1–21 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Zimmermann D & Kovar DR Feeling the force: formin’s role in mechanotransduction. Curr. Opin. Cell Biol 56, 130–140 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Fujiwara S, Matsui TS, Ohashi K, Mizuno K & Deguchi S Keratin-binding ability of the N-terminal Solo domain of Solo is critical for its function in cellular mechanotransduction. Genes. Cell 24, 390–402 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Schwayer C et al. Mechanosensation of tight junctions depends on ZO-1 phase separation and flow. Cell 179, 937–952 e918 (2019). [DOI] [PubMed] [Google Scholar]
- 138.Kechagia JZ, Ivaska J & Roca-Cusachs P Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol 20, 457–473 (2019). [DOI] [PubMed] [Google Scholar]
- 139.Potla R et al. Molecular mapping of transmembrane mechanotransduction through the β1 integrin–CD98hc–TRPV4 axis. J. Cell Sci 133, jcs248823 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bachmann M, Kukkurainen S, Hytonen VP & Wehrle-Haller B Cell adhesion by integrins. Physiol. Rev 99, 1655–1699 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Ranade SS, Syeda R & Patapoutian A Mechanically activated ion channels. Neuron 87, 1162–1179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brierley SM Molecular basis of mechanosensitivity. Auton. Neurosci 153, 58–68 (2010). [DOI] [PubMed] [Google Scholar]
- 143.Kraichely RE & Farrugia G Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol. Motil 19, 245–252 (2007). [DOI] [PubMed] [Google Scholar]
- 144.Arnadottir J & Chalfie M Eukaryotic mechanosensitive channels. Annu. Rev. Biophys 39, 111–137 (2010). [DOI] [PubMed] [Google Scholar]
- 145.Corey D et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432, 723–730 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Gottlieb P et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflug. Arch 455, 1097–1103 (2008). [DOI] [PubMed] [Google Scholar]
- 147.Anishkin A, Loukin SH, Teng J & Kung C Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl Acad. Sci. USA 111, 7898–7905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lyford GL et al. alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am. J. Physiol. Cell Physiol 283, C1001–C1008 (2002). [DOI] [PubMed] [Google Scholar]
- 149.Hao J et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron 77, 899–914 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Neshatian L et al. Ranolazine inhibits voltage-gated mechanosensitive sodium channels in human colon circular smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol 309, G506–G512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xu J GPR68 senses flow and is essential for vascular physiology. Cell 173, 762–775.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mederos y Schnitzler M et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27, 3092–3103 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bagal SK et al. Ion channels as therapeutic targets: a drug discovery perspective. J. Med. Chem 56, 593–624 (2013). [DOI] [PubMed] [Google Scholar]
- 154.Bohorquez DV et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest 125, 782–786 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hockley JRF et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Camunas-Soler J et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab 31, 1017–1031 e1014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cadwell CR et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol 34, 199–203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Drokhlyansky E et al. The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622 e1623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rakhilin N et al. An intravital window to image the colon in real time. Nat. Commun 10, 5647 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fosque BF et al. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760 (2015). [DOI] [PubMed] [Google Scholar]
- 161.Moeyaert B et al. Improved methods for marking active neuron populations. Nat. Commun 9, 4440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guo J, Wang Y, Sachs F & Meng F Actin stress in cell reprogramming. Proc. Natl Acad. Sci. USA 111, E5252–E5261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eisenstein M Mechanobiology: a measure of molecular muscle. Nature 544, 255–257 (2017). [DOI] [PubMed] [Google Scholar]
- 164.Bozler E Myenteric reflex. Am. J. Physiol 157, 329–337 (1949). [DOI] [PubMed] [Google Scholar]
- 165.Major G et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 152, 124–133 e122 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Bellono NW et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198 e116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schemann M & Mazzuoli G Multifunctional mechanosensitive neurons in the enteric nervous system. Auton. Neurosci 153, 21–25 (2010). [DOI] [PubMed] [Google Scholar]
- 168.Gregersen H & Kassab G Biomechanics of the gastrointestinal tract. Neurogastroenterol. Mot 8, 277–297 (1996). [DOI] [PubMed] [Google Scholar]
- 169.Vaishnav RN & Vossoughi J Residual stress and strain in aortic segments. J. Biomech 20, 235–239 (1987). [DOI] [PubMed] [Google Scholar]


