Abstract
From 1995 to 2000, a total of 673 Enterococcus faecium and 1,088 Enterococcus faecalis isolates from pigs together with 856 E. faecium isolates from broilers were isolated and tested for susceptibility to four classes of antimicrobial agents used for growth promotion as part of the Danish program of monitoring for antimicrobial resistance. The four antimicrobials were avilamycin, erythromycin, vancomycin, and virginiamycin. Major changes in the use of antimicrobial agents for growth promotion have occurred during the last 6 years in Denmark. The government banned the use of avoparcin in 1995 and of virginiamycin in 1998. Furthermore, the producers have voluntarily stopped all use beginning in 1999. The avoparcin ban in 1995 was followed by a decrease in the occurrence of glycopeptide-resistant E. faecium (GRE) in broilers, from 72.7% in 1995 to 5.8% in 2000. The occurrence of glycopeptide resistance among isolates from pigs remained constant at around 20% from 1995 to 1997. It was shown that, in GRE from pigs, the genes encoding macrolide and glycopeptide resistance were genetically linked and that, following the decrease in the use of tylosin during 1998 and 1999, the occurrence of GRE in pigs decreased to 6.0% in 2000. From 1995 to 1997 the occurrence of erythromycin resistance among E. faecium and E. faecalis isolates from pigs was almost 90%. Use of tylosin decreased considerably during 1998 and 1999, and this decrease was followed by decreases in the occurrence of resistance to 46.7 and 28.1% among E. faecium and E. faecalis isolates from pigs, respectively. Erythromycin resistance among E. faecium isolates from broilers reached a maximum of 76.3% in 1997 but decreased to 12.7% in 2000 concomitantly with more limited use of virginiamycin. Use of virginiamycin increased from 1995 to 1997 and was followed by an increased occurrence of virginiamycin resistance among E. faecium isolates in broilers, from 27.3% in 1995 to 66.2% in 1997. In January 1998 the use of virginiamycin was banned in Denmark, and the occurrence of virginiamycin resistance decreased to 33.9% in 2000. Use of avilamycin increased from 1995 to 1996 and was followed by an increase in avilamycin resistance among E. faecium isolates from broilers, from 63.6% in 1995 to 77.4% in 1996. Since 1996 avilamycin usage has decreased, followed by a decrease in resistance to 4.8% in 2000. Our observations show that it is possible to reduce the occurrence of antimicrobial resistance in a national population of food animals when the selective pressure is removed. Cases in which resistance to vancomycin was linked to resistance to erythromycin were exceptions. In such cases resistance did not decrease until the use of both avoparcin and tylosin was limited.
No acquired resistance genes were demonstrated for bacteria isolated from patients more than 60 years ago (18), but emergence of antimicrobial resistance has invariably followed the introduction of new antimicrobial compounds (25). Multiply resistant pathogenic bacteria have emerged worldwide during the last couple of decades, and multiple antimicrobial resistance is now one of the serious concerns of the new millennium (15, 23, 28). In particular the worldwide emergence of glycopeptide-resistant enterococci and the increase in their occurrence, pose a serious threat to the continued possibility of curing infections in humans (12, 14, 22, 31; D. F. Sahm, Program Abstr. 1st Int. ASM Conf. Enterococci, abstr. addendum, 2000). It is unlikely that new antimicrobial agents can be developed at a rate to adequately combat the increasing number of multiresistant bacteria.
As early as the 1960s, concern over the emergence of resistant bacteria in domestic animals led the Swann Committee (32) to recommend that antimicrobials that were of value for treatment of humans should not be approved for growth promotion in food animals. These guidelines have since been implemented in most European countries. However, the guidelines did not take into account antimicrobials that were of little or no significance in human medicine at the time when they were approved for growth promotion in food animals. Because of the emergence of multiply resistant bacteria causing infections in humans, some of these classes of antimicrobials have become important last resort drugs in the treatment of such infections. Examples include avoparcin, virginiamycin, and avilamycin, which belong to the same classes as the human drugs vancomycin, quinupristin-dalfopristin (Synercid), and evernimicin (Ziracin), respectively. In 2000 the manufacturer of Ziracin suspended its development. European Union rules for approval of feed additives have recently been amended; they now require application for reapproval of all such additives, including antimicrobials used for growth promotion, at fixed intervals.
It has been demonstrated that food animals may serve as a reservoir of resistant bacteria and/or resistance genes that may spread to the human population and thereby limit the medical value of these new antimicrobial classes (1, 3, 36). Thus, interventions reducing this reservoir of resistance genes among food animals may prolong the lifetime of these drugs for human use. For these reasons international public health organizations such as the World Health Organization have recommended the termination or rapid phasing out of the use of antimicrobial growth promoters (AGPs) that are also used for therapy or are under development for therapy (37).
Since 1995, major changes in the consumption of antimicrobial agents used for growth promotion have occurred in Denmark. Avoparcin was banned in May 1995, and virginiamycin was banned in January 1998. Furthermore, the food animal industries decided in 1998 to voluntarily stop all use of antimicrobial agents for growth promotion by the end of 1999. These steps were taken as precautionary measures, to prevent future problems with antimicrobial resistance in human medicine. The ban and voluntary withdrawal were based on the expectation that removal of the selective antimicrobial pressure in animals would reduce the exposure of humans, via food, to resistant bacteria from animals.
Several studies have shown that the occurrence of resistance is closely related to the medical use of a drug, even though the association may be variable (11, 15, 26). This association has also been demonstrated for antimicrobial agents used for growth promotion (4, 6, 9). In contrast, little information is available about the effects of terminating the use of an antimicrobial agent on the occurrence of antimicrobial resistance in large human or animal populations outside hospitals.
Danish food animal production is highly industrialized and well monitored, with the main emphasis on a few categories, such as porkers, broilers, layers, and milk cows, and with limited variation in animal breeds and production methods within each production category. Therefore, it provides a homogenous population suitable for studying changes in bacterial populations living in these reservoirs. A continuous national monitoring program for antimicrobial resistance was established in 1995 (7). This program monitors, for example, the occurrence of resistance among enterococci isolated from healthy animals at slaughter and thereby gives an overview of the general trend of resistance in food animals.
In this study, changes in the occurrence of resistance to glycopeptides, macrolides, oligosaccharides, and streptogramins that have taken place following termination of the use of these antimicrobial agents for growth promotion are reported.
MATERIALS AND METHODS
Details of the sampling scheme and of the isolation, identification, and susceptibility testing of enterococci from broilers and pigs have been published previously (5, 10).
Collection of samples.
All isolates were recovered from cecal samples (pigs) or cloacal swabs (broilers) from healthy animals, and only a single isolate per pig herd or broiler flock was included. The number of samples collected monthly from pigs and weekly from broilers from each slaughterhouse was proportional to the annual number of animals slaughtered. This ensures, as far as possible, that samples are representative of the entire country and that each sample represents a herd or flock.
Isolation, identification, and susceptibility testing.
For broilers, cloacal swabs were enriched in enterococcal broth (Becton Dickinson) overnight at 42°C, followed by subcultivation on Slanetz and Bartley agar (Difco) at 37°C for 18 to 24 h. Cecal samples from pigs were streaked directly onto Slanetz and Bartley agar. Isolates resembling enterococci were subcultured and identified biochemically as Enterococcus faecalis and Enterococcus faecium (5). During 1995 and 1996, susceptibility testing for erythromycin, vancomycin, and virginiamycin was performed by tablet diffusion (5). From 1997 onwards all susceptibility testing was done by determining the MIC using microwell broth dilution (Sensititre; Trek Diagnostic Systems) in Mueller Hinton broth (Difco) according to NCCLS guidelines (27). All susceptibility testing for avilamycin from 1995 to 2000 was performed by determining MICs using agar dilution as previously described (5). In categorizing the results the following breakpoints (5, 27) for resistance were used: for avilamycin, ≥16 μg/ml; for erythromycin, ≥8 μg/ml; for vancomycin, ≥32 μg/ml; and for virginiamycin, ≥8 μg/ml.
Data handling.
Test results were stored in an Oracle, version 7.14, database. Data processing and evaluation were carried out in PC SAS, versions 6.12 and 8.0, and EpiInfo, version 6.02. The 95% confidence interval for the estimated percent resistance was calculated by using a SAS macro according to the work of Blyth (13).
Use of antimicrobial agents for growth promotion.
Manufacturers licensed to produce premixes are obliged to collect data on the use of antimicrobials for growth promotion. These data are requested by The Danish Plant Directorate and published annually. Avilamycin, avoparcin, and virginiamycin have never been used for therapy. Data on the use of antimicrobial agents for therapy are collected by the Danish Medicine Agency. All data are published annually in the Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) report (8). The use of antimicrobial agents for growth promotion in Denmark from 1994 through 2000 is shown in Table 1. The level of use of macrolides and lincosamides for treatment of production animals has been 7 to 8 tons annually in the period 1995 through 1999. Because antimicrobials for food animals are freely available—although therapeutics are available only by prescription—and are competitively priced compared with other European countries, it is generally considered that there is no black market, or only a negligible one, for antimicrobials in food animals. Therefore, the official figures closely represent actual consumption.
TABLE 1.
Use of AGPs in Denmark from 1994 through 2000a
| Growth promoter | Antimicrobial class | Amt used (kg) in:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | ||
| Avilamycin | Oligosaccharide | 433 | 1,665 | 2,740 | 670 | 7 | 91 | 0 |
| Avoparcinb | Glycopeptide | 24,117 | 5,690 | |||||
| Bacitracinc | Polypeptide | 13,689 | 7,910 | 8,399 | 8,544 | 3,945 | 63 | |
| Carbadoxd | Quinoxaline-di-N-oxide | 10,012 | 1,181 | 1,985 | 4,153 | 1,803 | 293 | |
| Flavomycin | Flavophospholipol | 77 | 48 | 18 | 93 | 6 | 665 | 0 |
| Monensin | Ionophor | 4,755 | 5,007 | 4,741 | 3,008 | 935 | 0 | 0 |
| Olaquindoxd | Quinoxaline-di-N-oxide | 22,483 | 16,213 | 13,486 | 17,595 | 28,445 | 9,344 | |
| Salinomycin | Ionophor | 213 | 850 | 759 | 460 | 113 | 0 | 0 |
| Spiramycinc | Macrolide | 95 | 507 | 15 | 3 | 0.3 | 0 | |
| Tylosinc | Macrolide | 37,111 | 52,275 | 68,350 | 62,009 | 13,148 | 1,827 | |
| Virginiamycine | Streptogramin | 2,801 | 2,590 | 5,055 | 10,644 | 892 | 0 | |
| Total | 115,786 | 93,936 | 105,548 | 107,179 | 49,294 | 12,283 | 0 | |
See reference 8.
Banned in Denmark in May 1995; approval suspended in the European Union in 1997.
Approval suspended in the European Union from July 1999.
Banned by the European Union in 1999 because of toxicity.
Banned in Denmark in January 1998; approval suspended in the European Union from July 1999.
Size of the animal population.
Statistics on the production of broilers, cattle, and pigs in Denmark are published annually by Danmarks Statistik (16). The levels of production from 1995 through 1999 are given in Table 2.
TABLE 2.
Production of food animals in Denmark from 1995 through 1999a
| Yr | No. of animals slaughtered (in millions)
|
||
|---|---|---|---|
| Poultry | Cattle | Pigs | |
| 1995 | 113.00 | 19.00 | |
| 1996 | 111.50 | 0.79 | 20.53 |
| 1997 | 130.62 | 0.79 | 21.18 |
| 1998 | 129.33 | 0.73 | 22.87 |
| 1999 | 140.12 | 0.70 | 22.53 |
See reference 16.
RESULTS AND DISCUSSION
The use of antimicrobial agents for growth promotion decreased drastically from 1995 to 2000 (Table 1). In the same period the production of food animals remained constant or even increased (Table 2). Thus, the decreased usage of AGPs does not correlate with major changes in food animal production.
The monitoring of antimicrobial resistance among food animals in Denmark is based on randomly selected bacterial isolates. The sampling strategy used will detect only the most prevalent flora. Thus, resistance may be present at a low level and not be detected by the monitoring system. However, the results can be compared over time and will show changes in the occurrence of resistance.
From 1995 to 2000, a total of 673 E. faecium and 1,088 E. faecalis isolates from pigs, together with 856 E. faecium isolates from broilers, were isolated and tested for antimicrobial susceptibility. The trends in the occurrence of resistance over time and the 95% confidence intervals are given in Table 3. Different numbers of isolates were examined in different years, and during 1995 only a very limited number of isolates were included. This gives very wide 95% confidence intervals for the 1995 results (Table 3).
TABLE 3.
Trends in resistance of E. faecium from broilers and pigs and E. faecalis from pigs in Denmark to selected antimicrobial agents used for growth promotion
| Bacterial species and origin | Yr | No. of isolates | % Resistance (95% confidence interval) to:
|
|||
|---|---|---|---|---|---|---|
| Avilamycin | Erythromycin | Vancomycin | Virginiamycin | |||
| Enterococcus faecium | ||||||
| Broilers | 1995 | 11 | 63.6 (30.8–89.1) | 54.5 (23.4–83.3) | 72.7 (39.0–94.0) | 27.3 (6.0–61.0) |
| 1996 | 106 | 77.4 (68.2–84.9) | 64.2 (54.3–73.2) | 42.5 (32.9–52.4) | 58.5 (48.5–68.0) | |
| 1997 | 207 | 65.2 (58.3–71.7) | 76.3 (69.9–81.9) | 20.8 (15.5–26.9) | 66.2 (59.3–72.6) | |
| 1998 | 154 | 28.6 (21.6–36.4) | 70.8 (62.9–77.8) | 8.4 (4.6–14.0) | 60.4 (52.2–68.2) | |
| 1999 | 189 | 21.2 (15.6–27.7) | 27.5 (21.3–34.5) | 9.0 (5.3–14.0) | 39.2 (32.2–46.5) | |
| 2000 | 189 | 4.8 (2.2–8.8) | 12.7 (8.3–18.3) | 5.8 (2.9–10.2) | 33.9 (27.2–41.1) | |
| Pigs | 1995 | 10 | 0.0 (0.0–30.9) | 80.0 (44.4–97.5) | 20.0 (2.5–55.6) | 60.0 (26.2–87.8) |
| 1996 | 72 | 1.3 (0.0–7.5) | 93.1 (84.5–97.7) | 20.8 (12.2–32.0) | 47.2 (35.3–59.3) | |
| 1997 | 55 | 1.8 (0.1–9.7) | 87.3 (75.5–94.7) | 20.0 (10.4–33.0) | 36.4 (23.8–50.4) | |
| 1998 | 153 | 0.0 (0.0–2.4) | 70.6 (62.7–77.7) | 15.0 (9.8–21.7) | 55.6 (47.3–63.6) | |
| 1999 | 201 | 1.0 (0.1–3.5) | 47.8 (40.7–54.9) | 6.5 (3.5–10.8) | 8.0 (4.6–12.6) | |
| 2000 | 182 | 0.0 (0.0–2.0) | 46.7 (39.3–54.2) | 6.0 (3.1–10.6) | 22.5 (16.7–29.3) | |
| Enterococcus faecalis, pigs | 1995 | 49 | 0.0 (0.0–7.3) | 93.9 (83.1–98.7) | 0.0 (0.0–7.3) | —a |
| 1996 | 259 | 1.9 (0.6–4.4) | 90.7 (86.5–94.0) | 0.0 (0.0–1.4) | — | |
| 1997 | 248 | 3.6 (1.8–6.8) | 91.1 (86.9–94.4) | 0.0 (0.0–1.5) | — | |
| 1998 | 136 | 0.7 (0.0–4.0) | 78.7 (70.8–85.2) | 0.0 (0.0–2.7) | — | |
| 1999 | 200 | 2.5 (0.8–5.7) | 48.0 (40.9–55.2) | 0.0 (0.0–1.8) | — | |
| 2000 | 196 | 0.5 (0.1–2.8) | 28.1 (21.9–34.9) | 0.0 (0.0–1.9) | — | |
—, intrinsically resistant.
Over the entire period only four (0.6%) E. faecium isolates from pigs were resistant to avilamycin. E. faecalis isolates have naturally decreased susceptibility to streptogramin, and thus no results for virginiamycin are given. All E. faecalis isolates were susceptible to vancomycin, and 21 (1.9%) of the isolates from pigs were resistant to avilamycin.
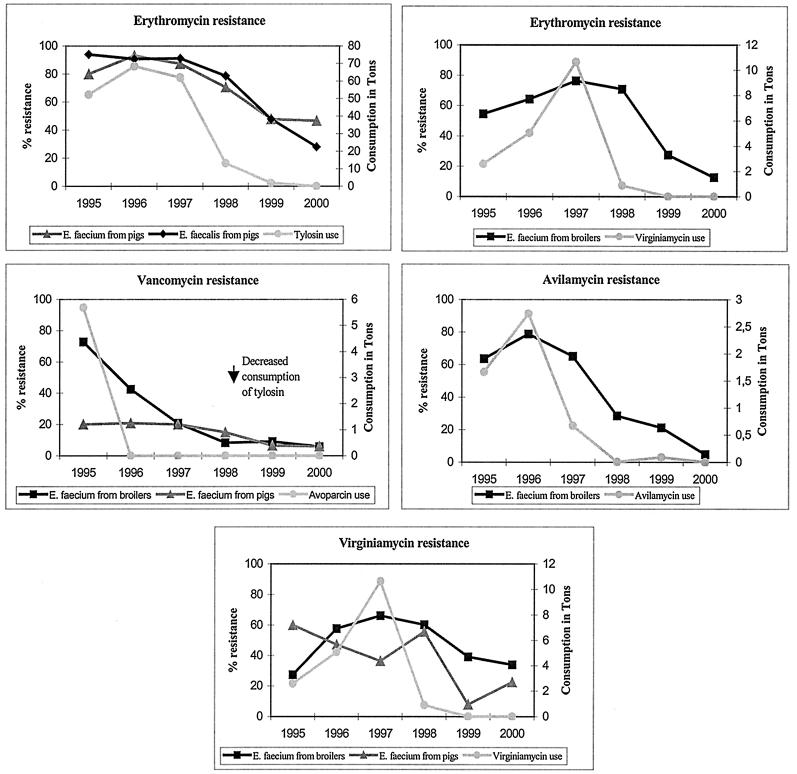
Resistance to antimicrobial agents used for growth promotion was more common in E. faecium than in E. faecalis. The only exception was macrolide resistance, where similar levels were observed. The trends in resistance and the consumption of antimicrobials as growth promoters from 1995 to 2000 are presented in Fig. 1.
FIG. 1.
Consumption of antimicrobial agents for growth promotion and occurrence of antimicrobial resistance in E. faecium and E. faecalis isolates from Danish pigs or broilers from 1995 to 2000.
The total level of consumption of each of the antimicrobial agents used for growth promotion in Denmark is well known, but it is very difficult to obtain exact data on the consumption of antimicrobial agents for growth promotion for the different food animal species. However, the macrolide tylosin is approved only for pigs, and avilamycin has been used primarily for broilers. After the ban on avoparcin in 1995, increased use of tylosin for pigs and of virginiamycin for broilers mainly replaced the use of avoparcin.
The avoparcin ban in 1995 was almost immediately followed by a decrease in the occurrence of glycopeptide-resistant E. faecium (GRE) in broilers (Table 3; Fig. 1). In 1995 72.7% of the isolates were resistant to vancomycin; this decreased to 5.8% in 2000. However, the occurrence of resistance among isolates from pigs remained relatively constant at around 20% during 1995 through 1996. It was shown that all GRE isolates from pigs belonged to the same clone and that genes encoding resistance to glycopeptides and macrolides were located close to each other on the same plasmid (2). Therefore, GRE in pigs may well have been maintained as a consequence of coselection by use of the macrolide tylosin. Indeed, following the decrease in the use of tylosin during 1998 and 1999, the occurrence of GRE in pigs decreased significantly to 6.0% in 2000 (Fig. 1). Nonetheless, almost 6 years after the ban of avoparcin, resistant E. faecium can still be found among broilers and pigs in Denmark, showing that it can take a very long time for resistance to disappear.
For several years the macrolide tylosin was the most widely used AGP for pigs in Denmark. In 1995 the occurrence of resistance among E. faecium isolates from pigs was 80.0%, in 1996 it was 93.1%, and in 1997 it was 87.3%. Among E. faecalis isolates resistance was 93.9% in 1995, 90.7% in 1996, and 91.1% in 1997. The use of tylosin decreased considerably during 1998 and 1999. This was almost immediately followed by a decrease in the occurrence of resistance among both E. faecium and E. faecalis isolates from pigs (Table 3; Fig. 1). Thus, in 1998 resistance among E. faecium isolates had decreased to 70.6%, and that among E. faecalis isolates had decreased to 78.7%. Resistance decreased further, to 47.8 and 48.0%, respectively, in 1999 and to 46.7 and 28.1%, respectively, in 2000. Macrolides are still widely used for treatment of infections in pigs, and thus future trends in the occurrence of resistance cannot yet be predicted.
The occurrence of macrolide resistance among E. faecium isolates from broilers has also been very high, reaching a maximum of 76.3% in 1997. It decreased to 27.5% during 1999 and to 12.7% during 2000, concomitantly with the more-limited use of virginiamycin (Tables 1 and 3; Fig. 1). Relatively minor amounts of the macrolide spiramycin have been used for growth promotion in broilers. However, virginiamycin is a natural combination of two structurally unrelated molecules (groups A and B) (30), and group B has the same mechanism of action as antimicrobials belonging to the macrolides (34). Enzymes methylating the target site of macrolides, lincosamides, and streptogramin B have been observed to be the most common cause of resistance in enterococci (19, 21). In addition, it has recently been shown that some of the genes encoding resistance to group A and group B, respectively, are genetically linked (20, 35). Thus, it seems likely that the use of virginiamycin as a growth promoter for broilers may have selected for macrolide resistance in E. faecium.
The use of virginiamycin increased between 1995 to 1997. The increase was seen mainly in broilers and was associated with an increase in the occurrence of resistance in E. faecium. Thus, virginiamycin resistance among E. faecium isolates from broilers increased from 27.3% in 1995 to 66.2% in 1997. In January 1998, the use of virginiamycin was banned in Denmark, and the occurrence of resistance subsequently decreased to 33.9% in 2000 (Fig. 1). More variation in the occurrence of virginiamycin resistance can be observed among E. faecium isolates from pigs. Thus, resistance decreased from 60.0% in 1995 to 36.4% in 1997, increased in 1998 to 55.6%, decreased again in 1999 to 8.0%, and increased again in 2000 to 22.5% (Table 3). The reason for this variation is not known. However, the increase in resistance during 2000 is mainly due to the emergence of isolates that are simultaneously resistant to erythromycin, kanamycin, penicillin, streptomycin, tetracycline, and virginiamycin (data not shown). These isolates will now be investigated further.
In Denmark, avilamycin has been used primarily for broilers. Use of this oligosaccharide increased from 1995 to 1996, followed by an increase in resistance among E. faecium isolates from 63.6% in 1995 to 77.4% in 1996. Since 1996, the level of use has decreased, almost immediately followed by a decrease in resistance to 4.8% in 2000 (Fig. 1).
The effects of the ban of growth promoters for food animals on the occurrence of antimicrobial resistance among humans have not been determined. However, the GRE carrier rate among healthy humans in Germany decreased from 13% in 1994 to 4% in 1997 (24) following the German ban on avoparcin in 1996. Similarly, a decrease in the occurrence of GRE has been observed among poultry products in Italy during the 18 months following the ban of avoparcin (29) and among humans, broilers, and pigs in The Netherlands from 1997 to 1999 (33) following the ban here.
Our observations show that it is possible to reduce the occurrence of antimicrobial resistance in a national population of food animals when the selective pressure is removed. However, we have also demonstrated that under certain conditions resistance may persist, most likely as a consequence of coselection. It is not possible to foresee whether the occurrence of resistance will decrease to an undetectable level in the future. Vancomycin-resistant E. faecium can still be found among broilers and pigs in Denmark more than 5 years after the ban of avoparcin. In the future, resistant isolates may still persist in low numbers that are not detected by the monitoring methods used. In the case of antimicrobials used in animal production, it is of great importance to prevent increases in the resistance gene reservoir to the extent possible, in particular for agents belonging to classes that are or may become important in human medicine.
The results discussed above represent the first documented effects of large-scale interventions to reduce the occurrence of antimicrobial resistance. They demonstrate that the exposure of humans to bacteria resistant to antimicrobial drugs and to resistance genes through food can be reduced effectively by intervention.
ACKNOWLEDGMENTS
We thank the laboratory technicians of the antimicrobial resistance group for their assistance and Henrik Stryhn to for constructing the SAS macro used for determining the confidence intervals.
This study was part of DANMAP, conducted in collaboration between the Statens Serum Institut, the National Food Agency of Denmark, and the Danish Veterinary Laboratory and funded jointly by the Danish Ministry of Health and the Danish Ministry of Food, Agriculture, and Fisheries.
REFERENCES
- 1.Aarestrup F M. Association between the consumption of antimicrobial agents in animal husbandry and the occurrence of resistant bacteria among food animals. Int J Antimicrob Agents. 1999;12:279–285. doi: 10.1016/s0924-8579(99)90059-6. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup F M. Characterization of glycopeptide-resistant Enterococcus faecium (GRE), from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J Clin Microbiol. 2000;38:2774–2777. doi: 10.1128/jcm.38.7.2774-2777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarestrup F M. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion in Denmark. APMIS. 2000;108(Suppl. 101):3–48. [PubMed] [Google Scholar]
- 4.Aarestrup F M, Carstensen B. Effect of tylosin used as a growth promoter on the occurrence of macrolide resistant enterococci and staphylococci in pigs. Microb Drug Resist. 1998;4:307–312. doi: 10.1089/mdr.1998.4.307. [DOI] [PubMed] [Google Scholar]
- 5.Aarestrup F M, Bager F, Jensen N E, Madsen M, Meyling A, Wegener H C. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS. 1998;106:606–622. doi: 10.1111/j.1699-0463.1998.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 6.Aarestrup F M, Bager F, Andersen J S. The association between the use of avilamycin for growth promotion and the occurrence of resistance among Enterococcus faecium. Microb Drug Resist. 2000;6:71–76. doi: 10.1089/mdr.2000.6.71. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. The Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) APMIS. 1998;106:605. [PubMed] [Google Scholar]
- 8.Anonymous. Consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. DANMAP 99. 2000. Copenhagen, Denmark. [Google Scholar]
- 9.Bager F, Madsen M, Christensen J, Aarestrup F M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med. 1997;31:95–112. doi: 10.1016/s0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 10.Bager F, Aarestrup F M, Madsen M, Wegener H C. Glycopeptide resistance in Enterococcus faecium from broilers and pigs following discontinued use of avoparcin. Microb Drug Resist. 1999;5:53–56. doi: 10.1089/mdr.1999.5.53. [DOI] [PubMed] [Google Scholar]
- 11.Baquero F, Martinez-Beltran J, Loza E. A review of antibiotic resistance patterns of Streptococcus pneumoniae in Europe. J Antimicrob Chemother. 1991;28(Suppl. C):31–83. doi: 10.1093/jac/28.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- 12.Bell J M, Paton J C, Turnigde J. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J Clin Microbiol. 1998;36:2187–2190. doi: 10.1128/jcm.36.8.2187-2190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blyth C R. Approximate binomial confidence limits. J Am Statist Assoc. 1986;81:843–855. [Google Scholar]
- 14.Budavari S M, Saunders G L, Liebowitz L D, Khoosal M, Crewe-Brown H H. Emergence of vancomycin-resistant enterococci in South Africa. S Afr Med J. 1997;87:1557. [PubMed] [Google Scholar]
- 15.Ciba Foundation. Antibiotic resistance: origins, evolution, selection and spread. Chichester, United Kingdom: Wiley and Sons; 1997. [Google Scholar]
- 16.Danmarks Statistik. Statistical yearbook 2000: agricultural statistics. Copenhagen, Denmark: Danmarks, Statistik; 2000. [Google Scholar]
- 17.Hsueh P R, Teng L J, Pan H J, Chen Y C, Wang L H, Chang S C, Ho S W, Luh K T. Emergence of vancomycin-resistant enterococci at a university hospital in Taiwan: persistence of multiple species and multiple clones. Infect Control Hosp Epidemiol. 1999;20:828–833. doi: 10.1086/501592. [DOI] [PubMed] [Google Scholar]
- 18.Hughes V M, Datta N. Conjugative plasmids in bacteria of the ‘pre-antibiotic’ era. Nature. 1983;302:725–726. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- 19.Jensen L B, Frimodt-Moller N, Aarestrup F M. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol Lett. 1999;170:151–158. doi: 10.1111/j.1574-6968.1999.tb13368.x. [DOI] [PubMed] [Google Scholar]
- 20.Jensen L B, Hammerum A M, Aarestrup F M. Linkage of vatE and ermB in streptogramin-resistant Enterococcus faecium isolates from Europe. Antimicrob Agents Chemother. 2000;44:2231–2232. doi: 10.1128/aac.44.8.2231-2232.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenssen W D, Thakker-Varia S, Dubin D T, Weinstein M P. Prevalence of macrolides-lincosamides-streptogramin B resistance and erm gene classes among clinical strains of staphylococci and streptococci. Antimicrob Agents Chemother. 1987;31:883–888. doi: 10.1128/aac.31.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson A P, Warner M, Woodford N, Speller D C, Livermore D M. Antibiotic resistance among enterococci causing endocarditis in the UK: analysis of isolates referred to a reference laboratory. BMJ. 1998;317:629–630. [PMC free article] [PubMed] [Google Scholar]
- 23.Jones R N, Pfaller M A. Bacterial resistance: a worldwide problem. Diagn Microbiol Infect Dis. 1998;31:379–388. doi: 10.1016/s0732-8893(98)00037-6. [DOI] [PubMed] [Google Scholar]
- 24.Klare I, Badstubner D, Konstabel C, Bohme G, Claus H, Witte W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb Drug Resist. 1999;5:45–52. doi: 10.1089/mdr.1999.5.45. [DOI] [PubMed] [Google Scholar]
- 25.Levy S B. Microbial resistance to antibiotics. An evolving and persistent problem. Lancet. 1982;ii:83–88. doi: 10.1016/s0140-6736(82)91701-9. [DOI] [PubMed] [Google Scholar]
- 26.Mouton R P, Hermans J, Simoons-Smit A M, Hoogkamp-Korstanje J A, Degener J E, van Klingeren B. Correlations between consumption of antibiotics and methicillin resistance in coagulase-negative staphylococci. J Antimicrob Chemother. 1990;26:573–583. doi: 10.1093/jac/26.4.573. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 28.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 29.Pantosti A, Del Grosso M, Tagliabue S, Macri A, Caprioli A. Decrease of vancomycin-resistant enterococci in poultry meat after avoparcin ban. Lancet. 1999;354:741–742. doi: 10.1016/S0140-6736(99)02395-8. [DOI] [PubMed] [Google Scholar]
- 30.Pechére J-C. Streptogramins: a unique class of antibiotics. Drugs. 1996;51(Suppl. 1):13–19. doi: 10.2165/00003495-199600511-00005. [DOI] [PubMed] [Google Scholar]
- 31.Schouten M A, Voss A, Hoogkamp-Korstanje J A. Antimicrobial susceptibility patterns of enterococci causing infections in Europe. The European VRE Study Group. Antimicrob Agents Chemother. 1999;43:2542–2546. doi: 10.1128/aac.43.10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swann M M. Joint Committee on the use of Antibiotics in Animal Husbandry and Veterinary Medicine. London, United Kingdom: Her Majesty's Stationery Office; 1969. [Google Scholar]
- 33.van den Bogaard A E, Bruinsma N, Stobberingh E E. The effect of banning avoparcin on VRE carriage in The Netherlands. J Antimicrob Chemother. 2000;46:146–147. doi: 10.1093/jac/46.1.146. [DOI] [PubMed] [Google Scholar]
- 34.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner G, Hildebrandt B, Klare I, Witte W. Linkage of determinants for streptogramin A, macrolide-lincosamide-streptogramin B, and chloramphenicol resistance on a conjugative plasmid in Enterococcus faecium and dissemination of this cluster among streptogramin-resistant enterococci. Int J Med Microbiol. 2000;290:543–548. doi: 10.1016/S1438-4221(00)80020-X. [DOI] [PubMed] [Google Scholar]
- 36.Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. WHO global principles for the containment of antimicrobial resistance in animals intended for food. Report of a WHO consultation. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]