Abstract
Antimicrobial resistance is a world-wide health care crisis. New antimicrobials must both exhibit potency and thwart the ability of bacteria to develop resistance to them. We report the use of synthetic ionophores as a new approach to developing non-resistant antimicrobials and adjuvants. Most studies involving amphiphilic antimicrobials have focused on either developing synthetic amphiphiles that show ion transport, or developing non-cytotoxic analogs of such peptidic amphiphiles as colistin. We have rationally designed, prepared, and evaluated crown ether-based synthetic ionophores (‘hydraphiles’) that show selective ion transport through bilayer membranes and are toxic to bacteria. We report here that hydraphiles exhibit a broad range of antimicrobial properties and that they function as adjuvants in concert with FDA-approved antibiotics against multi-drug resistant (MDR) bacteria. Studies described herein demonstrate that benzyl C14 hydraphile (BC14H) shows high efficacy as an antimicrobial. BC14H, at sub-MIC concentrations, forms aggregates of ∼200 nm that interact with the surface of bacteria. Surface-active BC14H then localizes in the bacterial membranes, which increases their permeability. As a result, antibiotic influx into the bacterial cytosol increases in the presence of BCnHs. Efflux pump inhibition and accumulation of substrate was also observed, likely due to disruption of the cation gradient. As a result, BC14H recovers the activity of norfloxacin by 128-fold against resistant Staphylococcus aureus. BC14H shows extremely low resistance development and is less cytotoxic than colistin. Overall, synthetic ionophores represent a new scaffold for developing efficient and non-resistant antimicrobial-adjuvants.
Antimicrobial resistance is a world-wide health care crisis.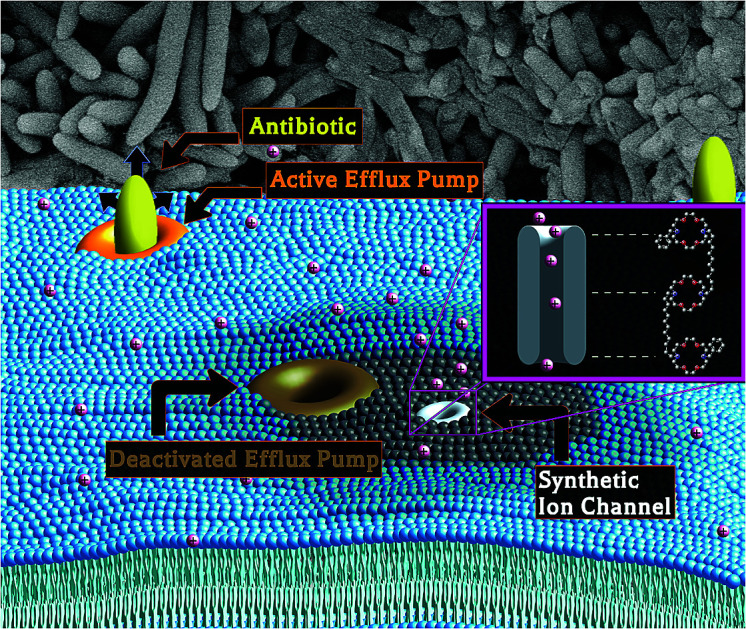
Introduction
The worldwide crisis in bacterial resistance to antibiotics, documented by recent CDC and WHO reports, needs little introduction.1 The problems of antibiotic over-prescription and incomplete regimens are exacerbated by the meager antimicrobial pipeline.1 At present, there is resistance to every FDA-approved antibiotic. The need for new approaches can hardly be overstated.
Two new discovery approaches have recently been reported as a means to obtain antimicrobial peptides from soil microbes. Teixobactin was identified using an iChip that allows culture of previously unculturable bacteria.2 Malacidins were identified using culture-independent studies of DNA samples from soil microbes.3 Notwithstanding these new antibiotics were identified as non-resistant by sequential culturing methods,2,3 gene transfer from the bacteria producing them could eventually lead to resistance. Such discovery methods are critical to address the antibiotic-resistance crisis, but differ fundamentally from the approach presented here.
We have designed and synthesized amphiphiles called hydraphiles that form cation-selective channels in membranes. Some of these compounds exhibit significant antimicrobial properties attributed to the disruption of ion gradients in bacteria.4 Numerous synthetic amphiphiles have been reported, but studies of them were generally limited either to ion transport5 or to a simple survey of antimicrobial potency.6 We report here that hydraphiles form cation selective channels in membranes and recover antimicrobial potency against multidrug resistant (MDR) bacteria by increasing the cytosolic concentration of antibiotics within the pathogen.
Poor membrane permeability and efflux pump function have long been recognized as important contributors to bacterial resistance.7 Efflux pump proteins require a cation gradient or ATP hydrolysis to drive xenobiotics from the bacterial cytosol.8,9 Disruption of these gradients offers a potential mechanism by which resistance may be diminished or eliminated. Antimicrobial resistance in Gram-negative bacteria is typically a combination of second membrane-diminished influx and increased antibiotic efflux.10 Membrane active molecules, such as colistin11 and daptomycin,11 disrupt bacterial membranes and permit increased antimicrobial influx. Sadly, resistance even to these potent antibiotics has recently emerged.12 However, to our knowledge, there is no report of any synthetic adjuvant that can inhibit efflux pump activity and increase membrane permeability to antibiotics. The use of synthetic ion channels as antimicrobial adjuvants not only comprises a new approach, but it also impedes resistance development, as these synthetic amphiphiles are not produced by bacteria.
We report here the application of synthetic amphiphiles (hydraphiles) that penetrate membranes and form cation selective channels. Hydraphiles form aggregates of ∼200 nm that attach to the surface of bacteria. The surface-attached hydraphiles then localize in the Escherichia coli membranes, transport potassium (K+) ions, and selectively increase membrane permeability. The disruption of cation gradients/transport caused by hydraphiles inhibits efflux pump activity, whereas increased membrane permeability allows for higher antibiotic influx. As a result the antibiotic concentration in the cytoplasm of MDR bacteria increases, and its activity is recovered. The amphiphile we call benzyl C14 hydraphile (BC14H, 4) is our most potent adjuvant: it rescues the antibiotic potency in resistant E. coli, MDR Klebsiella pneumoniae, and Staphylococcus aureus. E. coli failed to develop resistance to BC14H over 15 days. BC14H also shows generally lower mammalian cytotoxicity than does colistin.
Experimental section
Compounds used
Synthetic amphiphiles and antibiotics were used for the studies reported here. Synthetic amphiphiles used are called hydraphiles. Hydraphiles were synthesized in the Gokel lab and purity was confirmed by NMR and mass spectrometry. Four hydraphiles differing in its spacer chain lengths were used. We used benzyl C8, C10, C12 and C14 hydraphile. The antibiotics of choice were tetracycline, ampicillin, ciprofloxacin, and colistin. CCCP and reserpine, known efflux pump inhibitors were also used as controls. Gramicidin-D, valinomycin and Triton X-100 were used as ion transport and detergent controls. All the antibiotics, known EPIs and controls were received from Sigma-Aldrich and used as received. Hydraphiles, gramicidin-D, and valinomycin were dissolved in DMSO. All the other compounds were dissolved in autoclaved milli-Q H2O.
Hydraphile syntheses
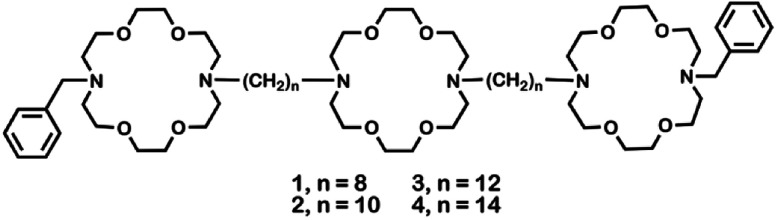
Compounds 1, 2, 3, and 4 are analogs that differ only in the lengths of the polymethylene units connecting the two distal macrocycles to the central ring. Detailed syntheses are reported in N. S. Curvey, S. E. Luderer, J. K. Walker, and G. W. Gokel, Improved Syntheses of Benzyl Hydraphile Synthetic Cation-Conducting Channels, Synthesis, 2014, 46, 2771–2779.
Bacteria used
There were 27 strains of bacteria used for this study. Most of the strains listed in Tables 1 and S1† were cultured in cation adjusted Mueller Hinton II (MHII) media. The S. pneumoniae was cultured in CA-MHB with 5% LHB media. tetRE. coli was prepared by transforming the competent JM109 E. coli with pBR322 plasmid (Carolina Biological). The transformation was performed using heat-shock method as outlined by the manufacturer (Promega). The resulting E. coli cells were tetracycline and ampicillin resistant and designated tetRE. coli. The tetRE. coli expressed tetA efflux pump and β-lactamase enzyme. tetRE. coli was always used in LB media with 100 μg mL−1 ampicillin. The Klebsiella pneumoniae (ATCC BAA 2146™) strain was acquired from ATCC. It is a clinical relevant strain (BSL-2) that was reported to be isolated from a patient's urine sample. The K. pneumoniae is reported to be resistant to more than 30 different antibiotics including classes such as carbapenems, fluoroquinolones, cephalosporins, macrolides and tetracyclines. It expresses efflux pumps from three different classes: RND, ABC and MFS. TetA is the MFS type efflux pump expressed in the K. pneumoniae, which is used for the combination study. The K. pneumoniae strain was used in the MHII media. S. aureus 1199B13 was a gift Dr Glenn Kaatz, it was cultured in MHII media as well.
MIC screening of hydraphiles and antibiotics.
| Strain ID | Antibiotic used | [Antibiotic] (μM) | [Comp. 2] (μM) | [Comp. 4] (μM) |
|---|---|---|---|---|
| S. aureus (control) | Vancomycin | 0.5 | 6 | 1.5 |
| MRSA (USA100) | Vancomycin | 1 | 6 | 1.5 |
| MRSA (USA 300) | Vancomycin | 1 | 6 | 1.5 |
| MRSA (control) | Vancomycin | 1 | 6 | 1.5 |
| E. faecalis (control) | Daptomycin | 2.5 | 6 | 1.5 |
| E. faecalis (VanA) | Daptomycin | 2.5 | 6 | 1.5 |
| E. faecalis (VanB) | Daptomycin | 0.62 | 6 | 1.5 |
| S. pneumoniae (control) | Vancomycin | <0.1 | 6 | 6 |
| S. pneumoniae (R:Pen) | Vancomycin | 0.2 | 6 | 3 |
| S. pneumoniae (R:Levo) | Vancomycin | 0.2 | 6 | 6 |
| K. pneumoniae (ESBL, SHV-12) | Meropenem | 0.65 | 51 | 24 |
| K. pneumoniae (ESBL, FQR) | Meropenem | 0.65 | 26 | 12 |
| K. pneumoniae (KPC-2) | Meropenem | 83 | 51 | 24 |
| A. baumannii (FQR) | Meropenem | 83 | 6.4 | 3 |
| A. baumannii (R:Mero) | Meropenem | 2.6 | 26 | 12 |
| A. baumannii (WT) | Meropenem | 1.3 | 13 | 6 |
| P. aeruginosa (control) | Meropenem | 1.3 | >100 | 94 |
| P. aeruginosa (FQR) | Meropenem | 2.6 | >100 | 94 |
| P. aeruginosa (R:Mero) | Meropenem | >166 | 100 | 47 |
| E. cloacae (FQR) | Meropenem | 1.3 | 26 | 24 |
| E. cloacae (R:Mero) | Meropenem | 42 | 26 | 12 |
| E. aerogenes (R:Cep) | Meropenem | <0.33 | 26 | 12 |
| E. coli (WT) | Meropenem | <0.33 | 13 | 3 |
| E. coli (Tol-) | Meropenem | <0.33 | 13 | 3 |
Minimal inhibitory concentration (MIC)
tetRE. coli was grown in LB Miller media containing 100 μg mL−1 ampicillin. K. pneumoniae was grown in the cation adjusted MHII media. The cells were grown overnight from one colony forming unit (CFU) in 2 mL media. On the day of the experiment, bacteria were knocked back to OD600 = 0.100 and incubated at 37 °C until the OD600 reached 0.500 (4 × 108 CFU mL−1). The cell density was adjusted to 4 × 106 CFU mL−1. The 20 μL diluted cells were added to each well (final volume/well = 200 μL) after the addition of antibiotics or synthetic amphiphiles. The final cell concentration per well was 4 × 105 CFU mL−1.
In a 96-well plate, first the media was added followed by addition of the compounds. The final volume of each well was 200 μL. Compounds were either dissolved in DMSO or dH2O. Compounds were always administered at a constant volume such that the final DMSO concentration in each well was 0.5% volume/volume (1 μL of final 200 μL). For combination studies, after the addition of amphiphiles, antibiotics were added. The compounds or antibiotics that were dissolved in dH2O, 10 μL per well was used. In the case of DMSO alone control, 1 μL of DMSO was added to each well. For dH2O control, 10 μL of dH2O was added to each well. No compound or solvent was added for cells alone and media alone control. Contents of the well were thoroughly mixed by pipetting up and down three times. After mixing, 20 μL cells were added to each well. The plates were incubated at 37 °C for 18-20 hours. Results were collected by determining the OD at λ = 600 nm using a plate reader (BioTek Cytation 3). Each compound was tested in triplicate per plate. Percent inhibition was calculated by comparing to the cell alone control. Growth inhibited of ≥90% was considered as the MIC. The data was reproduced 2 more times on two separate plates.
Checkerboard
In a checkerboard experiment, each column of the plate had different concentration of the amphiphile increasing by serial dilution. Two amphiphiles were tested per plate. Each row of the checkerboard experiment had different concentrations of antibiotics increasing by serial dilutions. The concentrations of amphiphile and antibiotic tested were 1/2, 1/4, 1/8, 1/16 and 1/32 the MIC. E. coli and K. pneumoniae were tested using the checkerboard experiment. The MIC procedure used for the checkerboard experiment was the same as outlined above. Cells and compound alone controls were also used. The checkerboard experiment did not have a biological replicate but the data was reproduced three times before reporting. The data was represented as a heat map.
Growth curve
The growth curve experiment was performed using tetRE. coli. The compounds studied using growth curves were benzyl C14 hydraphile and tetracycline. Here, the E. coli was grown overnight from one CFU in LB Miller media containing 100 μg mL−1 ampicillin. On the day of the experiment the cells were knocked back in LB Miller media containing 100 μg mL−1 ampicillin, to OD600 = 0.550 before use. In a sterile 250 mL flask, 50 mL LB media was added, followed by benzyl C14 hydraphile and tetracycline. The concentrations of benzyl C14 hydraphile tested were 1 and 2 μM. The concentrations of tetracycline tested were 220 and 900 μM. In addition, a growth curve containing a combination of benzyl C14 hydraphile (1 μM) and tetracycline (220 μM) was also tested. Cells only and DMSO (0.5% v/v) alone controls were also performed. Compounds were mixed by swirling the flask. To each flask, knocked back cells were added so that the final cell concentration in each flask was approximately 4 × 105 CFU mL−1. The flasks were incubated at 37 °C and 200 RPM. A 2 mL sample was taken every 30 minutes (for next 24 hours) to determine the OD at λ = 600 nm. A graph was plotted for OD vs. time to generate a growth curve. Average of three trials was used to determine the optical density. Standard deviation in the results was used to plot the error bars.
Localization in bacterial cells
tetRE. coli was grown overnight from one CFU in LB miller media containing 100 μg mL−1 ampicillin. E. coli was knocked back and grown to OD (600 nm) = 1.300 before use. Cells were centrifuged at 6500 RM (3000 × g) for 5 minutes and resuspended in PBS. Dansyl labeled C14 dansyl hydraphile (Cf = 2 μM) was added to 1990 μL of cells. For co-localization study, FM4-64 FX (Cf = 10 μg mL−1) were also added. The cells were incubated for 5 minutes at 200 RPM and 37 °C. Cells were washed again at 6500 RPM (3000 × g) for 5 minutes and re-suspend the cells in fresh PBS. A 20 μL sample was loaded on to clean glass slide and covered with coverslip. The slide was observed using Zeiss LSM 700 microscope.
E. coli membrane permeability using PI and FDA
To test the membrane permeability of the tetRE. coli, the bacteria was first grown overnight from one CFU in media containing 100 μg mL−1 ampicillin at 37 °C and 200RPM. tetRE. coli was then knocked back to OD (600 nm) = 0.550 before use. In a sterile test tube, cells were added followed by either Triton X-100, DMSO or compounds 1–4 at half-MIC concentrations and incubated at 37 °C and 200 RPM. The concentration of DMSO was kept constant at 0.5% by volume in each case. After 30 minutes of incubation, the cells were washed by centrifugation at 3000 × g for 5 minutes and re-suspended in sterile phosphate buffered saline (PBS). Propidium iodide (30 μM, Thermo-Fischer) and fluorescein diacetate (60 μM, Sigma-Aldrich) were added to the tetRE. coli cells in the PBS, mixed by vortexing and incubated at 37 °C and 200 RPM. After 30 minutes, the cells were washed again by centrifugation at 3000 × g for 5 minutes. The pellet was suspended in a fresh PBS, loaded onto a clean glass slide, covered with a cover slip and observed under Zeiss LSM 700 confocal microscope.
Localization and permeability of HEK-293 cells
HEK-293 cells were donated by Dr Michael Nichols. Cell lines were regularly maintained in growth media containing DMEM (ATCC), 10% fetal bovine serum (FBS, ATCC) and 1% penicillin–streptomycin solution (ATCC). Adherent HEK-293 cells were trypsinized using 0.25% (w/v) trypsin–EDTA (Sigma-Aldrich), suspended in a fresh media and diluted to get a concentration of 3 × 105 cells per mL. Cells were seeded in a 96-well plate (100 μL per well) to get 3 × 104 cells per well. The plates were incubated for 24 hours at 5% CO2 and 37 °C to reach a confluency of 80–90%.
In a sterile 1.5 mL micro-centrifuge tube, dansyl C14 hydraphile and dansyl C16 lariat ether (0.5% DMSO) were mixed with PBS. FM4-64 FX, DAPI and PI were also added to PBS for co-localization study. The spent media in the plate was replaced with 100 μL media containing dansyl labeled amphiphiles and other stains. The cells were incubated at 37 °C and 5% CO2 for 30 minutes before observing under Zeiss LSM 700.
For permeability study, the cells were cultured as outlined above. HEK-293 (90% confluent) were then seeded in a 96-well plate to get 30 000 cells per well. After 24 hours of incubation at 37 °C and 5% CO2, the spent media was replaced with media (DMEM and 10% FBS) containing compound 1–4 at 1/2× or 2× MIC. Triton X-100 at 0.1%-by volume (1670 μM) and DMSO 0.5% by volume were also used as controls. After 2 hours of incubation, spent media was replaced with PBS containing propidium iodide (30 μM) and fluorescein diacetate (60 μM) and incubated at 37 °C and 5% CO2. After 2 hour of incubation, the spend media was replaced with fresh PBS and the cells were observed under Zeiss LSM 700 confocal microscope. The images were reported without any alterations. The gain and the intensity in all the images were kept constant.
Scanning electron microscopy
To perform scanning electron microscopy tetRE. coli was grown overnight from one CFU in LB miller media containing 100 μg mL−1 ampicillin. E. coli was knocked back and grown to OD (600 nm) = 1.000 before use. For E. coli alone control, the filter membrane (0.45 μm) was dipped in the E. coli culture. E. coli was treated with various concentration of C8 and C14 hydraphiles for 10 minutes before loading on to filter membrane. The cells were fixed by transferring the filter membrane containing E. coli cells to 2.5% (v/v) glutaraldehyde (stock 25%) for 60 min. Cells were washed for 15 minutes by transferring the membrane in PBS. The cells were stained by transferring the membrane to 1% (v/v) OsO4 for 1 hour. Serial dehydration of the sample was performed with 30%, 50%, 70%, 80%, 90%, 100%, 100% (v/v) ethanol. Sample was dipped in each ethanol concentration for 10 minutes. The cells were critical-point dried and sputter coated with gold before observing under JOEL 6320F SEM. The images were acquired and reports without any modifications.
Potassium transport
The experiment was conducted with tetRE. coli. E. coli was grown overnight from one CFU. Cells were knocked back to OD = 0.600 before use. Cells were centrifuged at 2000 × g and resuspended in sterile PBS. The OD of cells was adjusted to 1.300. To measure the K+ leakage, 1998 μL E. coli suspended in PBS were added to a plastic cup containing magnetic stirrer. The stir plate was turned on to mix the sample and the potassium selective electrode (Orion, Thermo Scientific) was immersed in the sample so that just the membrane was covered in the sample. The voltage (mV) was stabilized for 6–8 minutes. The temperature of the sample was kept constant at 25 °C. The electrode was lifted and 2 μL of the either of the hydraphiles or gramicidin-D were added. The DMSO concentration was kept constant at 0.1% (v/v). The electrode was immersed back in the solution. Multiple concentrations were tested for each compound. The mV reading was recorded every 30 seconds for 15 minutes. The concentration of potassium ion released (mM) and Δ[K+] mM were calculated using the equation from the calibration curve.
To determine the total potassium pool of tetRE. coli, 2 mL of cells were heated at 100 °C for 30 minutes on a heat block. After 30 minutes the sample was cooled down to room temperature for 60 minutes. After 60 minutes the mV reading was recorded and Δ[K+] mM was calculated. The experiment was performed in triplicates and standard deviation in the experiment was calculated.
Efflux pump inhibition
Efflux pump inhibition studies were conducted with S. aureus 1199B in a 96-well microtiter plate with black wells and glass bottom. S. aureus 1199B were grown overnight from one CFU. Cells were knocked back to OD600 = 0.550 before use. In a 1.5 mL micro-centrifuge tube, cell were spun down at 17 000 × g for 3 minutes. Cell were re-suspended in fresh MHII media containing 10 μg mL−1 ethidium bromide and 100 μM CCCP. The OD600 was adjusted to 1.000. The cells were vortexed and incubated at the room temperature for 20 minutes to load cells with ethidium bromide. After 20 minutes, the cells were centrifuged at 17 000 × g for 3 minutes. The supernatant was discarded and the cells were stored on ice. The tubes were warmed to room temperature for 5 minutes and MHII media was added to the tubes and OD600 was adjusted to 0.800. 100 μL of ethidium bromide loaded cells were added to each well containing either C8–C14 hydraphiles, CCCP or reserpine. The contents were mixed by pipetting up and down once and the fluorescence was recorded immediately using Biotek Cytation 3 plate reader. Excitation of 530 nm and emission of 600 nm was used. Reading were collected every minute for 20 minutes. The results were reproduced two more times and standard deviation was calculated. The results were graphed against time.
Ethidium bromide accumulation
Efflux pump inhibition studies were conducted with S. aureus 1199B in a 96-well microtiter plate with black wells and glass bottom. S. aureus 1199B were grown overnight from one CFU. Cells were knocked back to OD600 = 0.550 before use. While the cells grew to the optimal conditions, hydraphiles, CCCP and reserpine stock concentrations were prepared. Mid-log phase cells were centrifuged at 17 000 × g for 3 minutes and re-suspended in fresh MHII media. The OD600 was adjusted to 0.800. In each well 200 μL of cells were added followed by 10 μg mL−1 of ethidium bromide and 1 μL of C8–C14 hydraphiles, reserpine or CCCP. The contents of the well were mixed by pipetting up and down once. Immediately fluorescence was measured using the Biotek Cytation 3 plate reader. Excitation of 530 nm and emission of 600 nm was used. Reading were collected every minute for 20 minutes. The results were reproduced two more times and standard deviation was calculated. The results were graphed against time.
Resistance development to hydraphiles
The ability of tetRE. coli to develop resistance to benzyl C14 hydraphiles and minocycline was determined using sequential culturing method. This method tests the ability of bacteria to develop resistance by inducing constant selective pressure of sub-lethal dose of antibiotics over 15 days. This procedure also accounts for only point mutations. The tetRE. coli was grown overnight from one colony in LB media. E. coli was knocked back to OD 600 nm = 0.100 in a 2 mL LB media and grown to OD = 0.550. LB media was added to the test tubes, followed by the antibiotic and then the E. coli and incubated at 37 °C, 200 RPM for 24 hours. Five different concentrations of hydraphiles and minocycline were set up: 0.25×, 0.5×, 1×, 2×, and 4× MIC. Any cultures that grew at higher than the MIC levels were passaged on antibiotic-free LB Agar plates and the MIC is determined. The samples were also stored for future use. The cells were diluted from the second highest concentration that allowed growth at 1 : 100 in fresh media containing 0.25, 0.5, 1, 2, 2.5 and 4 MIC. Test tubes were incubated at 37 °C, 200 RPM for 24 hours. Any cultures that grew at higher than the MIC levels were passaged on antibiotic-free LB Agar plates and the MIC was determined. The procedure was continued for 15 days. The results are represented in graphical format of MIC vs. days. Average from three trials was used to plot MIC on the graph.
Mammalian cell cytotoxicity
HeLa (ATCC CCL-2) cells were acquired from ATCC. Cos-7 (ATCC CRL-1651) cells were donated by Dr Cynthia Dupureur and HEK-293 cells were donated by Dr Michael Nichols. Cell lines were regularly maintained in growth media containing DMEM (ATCC), 10% fetal bovine serum (FBS, ATCC) and 1% penicillin-streptomycin solution (ATCC). Adherent HEK-293, HeLa and Cos-7 cells were trypsinized using 0.25% (w/v) trypsin–EDTA (Sigma-Aldrich), suspended in a fresh media and diluted to get a concentration of 3 × 105 cells per ml. Cells were seeded in a 96-well plate (100 μL per well) to get 3 × 104 cells per well. The plates were incubated for 24 hours at 5% CO2 and 37 °C to reach a confluency of 80–90%.
In a sterile 1.5 mL micro-centrifuge tube, benzyl C8–C14 hydraphile, CCCP and colistin (0.5% DMSO) were mixed with assay media (DMEM + 10% FBS) and serially diluted by 2-fold each to get MIC, 1/2 MIC and 1/4 MIC concentrations. A control containing 0.5% DMSO was also prepared. After 24 hours, the spent media in the 96-well plate containing HEK-293, HeLa and Cos-7 cells (90% confluency) was replaced with 100 μL media containing the benzyl C8–C14 hydraphile, CCCP and colistin at various concentrations. The cells were incubated at 37 °C and 5% CO2 for 24 hours before performing XTT assay (Sigma-Aldrich). The XTT assay was performed according to the manufacturer's protocol. After 24 hours of treatment with compounds, the media was replaced with PBS and 25 μL XTT was added to each well. The XTT assay works by the reduction of tetrazolium compound by alive cells to the colored soluble formazan product. The absorbance of the product was measured at 450 nm (XTT) and 690 nm (background). Percent survival was calculated by comparing the average absorbance of cells treated with benzyl C8–C14 hydraphile, CCCP and colistin to that of cells alone. Three replicates were performed for each treatment. Average percent survival and standard deviation were calculated and plotted on a graph.
Results and discussion
General
Crown ethers14 form complexes with metallic and organic cations and transport them across various types of membranes.15 Hydraphiles were designed to use crown ethers as a module in a membrane-spanning scaffold to mimic protein channel functions.16 Examples of these synthetic, cation-conducting amphiphiles are illustrated in Fig. 1. Hydraphiles typically possess three diaza-macrocycles connected by alkyl spacer chains and may be represented schematically as R〈crown〉Cn〈crown〉Cn〈crown〉R. Herein, the crowns are 4,13-diaza-18-crown-6 (〈N18N〉) and the connectors (Cn) are polymethylene chains ranging in length from hexylene (C6) to eicosylene (C20).17 The channels that most effectively conduct alkali metal cations14 have Cn chains of 12–16 methylenes.18,19
Fig. 1. Structures of hydraphiles studied.
These bilayer-spanning amphiphiles transport Na+ ions through liposomes16 and exhibit antibacterial properties.4 We previously reported that BC14H, when co-administered at sub-lethal concentrations, enhances the efficacy of erythromycin, kanamycin, rifampicin, and tetracycline against drug-sensitive E. coli, Pseudomonas aeruginosa, and Bacillus subtilis.20,21 Based on the membrane activity of hydraphiles, we initially postulated that efficacy resulted only from a permeability enhancement mechanism. We now report evidence supporting the hypothesis that hydraphiles also inhibit efflux pump function by disrupting bacterial cation gradients.
Hydraphiles 1–4 have N-benzyl groups (R) on the distal macrocycles and linear C8, C10, C12, or C14 spacer chains (Fig. 1).22 Both ion transport and the antimicrobial activity of hydraphiles depend on the overall length of the hydraphiles.4 We expected benzyl C8 and C10 hydraphiles to be controls as they have shown poor ion transport through liposomes.14 The antibiotics studied were tetracycline (5), ciprofloxacin (6), and norfloxacin (7). The membrane disrupter colistin (8), the pore-former gramicidin-D (9),23 the carrier valinomycin (10),24 and the detergent Triton X-100 (11)25 served as controls. Known efflux pump inhibitors such as reserpine (12),21 and carbonyl cyanide 3-chlorophenylhydrazone (CCCP, 13)26 were also used as controls. Structures of controls and other hydraphiles are shown in Fig. S1.†
Minimal inhibitory concentration studies
We determined the MICs of benzyl C10 and benzyl C14 hydraphiles (compounds 2 and 4) as well as those for known antibiotic controls (vancomycin, daptomycin, and meropenem) against 24 bacterial strains. MICs were determined against sensitive and resistant strains of methicillin resistant S. aureus (MRSA), K. pneumoniae, E. coli, Enterococcus faecalis, Streptococcus pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter cloacae, and Enterobacter aerogenes (Table 1). Compound 4 was generally a more potent antimicrobial than 2. This activity correlates with the ability of BC14H (and not BC10H) to span the membrane and to form ion-conducting pores.
It was observed that BC14H was more efficient than current treatment options against highly problematic VRE and MRSA (Gram-positive) strains. The MIC of daptomycin was 2.5 μM against E. faecalis (VanA). The VanA and VanB clusters are responsible for vancomycin resistance in one in three hospital acquired enterococcal infections.1 The MIC for 4 against E. faecalis (VRE/VanA) and MRSA (USA 100, 300) was 1.5 μM. The MIC of vancomycin against MRSA was 1–2 μM. Strains of vancomycin-resistant S. aureus have already been reported and USA 300 and USA 100 are the most common community-and hospital-associated strains. Based on the available treatment options and existing resistance development, BC14H could be considered for antimicrobial monotherapy against E. faecalis.
Synthetic amphiphiles typically show greater antimicrobial activity towards Gram-positive than Gram-negative bacteria.27 Even though the MICs of hydraphiles were higher (less potent) against Gram-negative than Gram-positive bacteria, hydraphiles were more active than controls against A. baumannii (FQR), K. pneumoniae (FQR), and E. cloacae (R:Mero). For example, BC14H was 28-fold, 3-fold and 2-fold more active, respectively, than meropenem against A. baumannii (FQR), K. pneumoniae (KPC2), and E. cloacae (meropenem resistant). The MIC of compound 4 ranged from 3–24 μM against these strains. The CDC has listed these bacteria as serious and urgent threats.1Table 1 shows the results of screening for minimum inhibitory concentrations (MICs) against a variety of microbes.
Adjuvant studies
An urgent need lies with carbapenem resistant Enterobacteriaceae (CRE) and MRSA. It is known that combinations of antibiotics have a lower rate of resistance development than do individual antimicrobials. Our goal is to use hydraphiles as adjuvants to rescue the potency of antimicrobials against bacteria such as CRE and MRSA that are an urgent public health threat.
Typical approaches used to overcome resistance involve either inhibition of the β-lactamase enzyme or a combination of two (or more) different antibiotics. Even though efflux pumps are a major resistance mechanism, at present no efflux pump inhibitor is available for therapeutic use. Hydraphiles 1–4 were assessed for antimicrobial and adjuvant properties against efflux pump expressing MDR bacteria.
The bacteria used for these studies were K. pneumoniae and E. coli (Gram-negative), and S. aureus (Gram-positive). The K. pneumoniae (ATCC BAA 2146™) strain was acquired from ATCC and reported to be isolated from a patient's urine sample. It expresses multiple different efflux pumps (e.g. RND, ABC, TetA).28 This strain is resistant to more than 25 different antibiotics and served in these studies as a clinically relevant control. The tetracycline resistant strain of E. coli (tetRE. coli) was developed by transforming E. coli with the pBR322 plasmid, which contains the tetA and AmpR genes.29 The tetA gene expresses the TetA efflux pump and AmpR gene expresses the β-lactamase enzyme. The TetA efflux pump depends on proton exchange for the transport of tetracycline.8 Since most of the virulent ESKAPE pathogens30 are Gram-negative,31 we assayed E. coli and K. pneumoniae. In addition, S. aureus 1199B overexpresses the NorA efflux pump and is resistant to both norfloxacin and ethidium bromide.13 This Gram-positive strain was tested with 1–4 to further evaluate NorA efflux pump inhibition.32
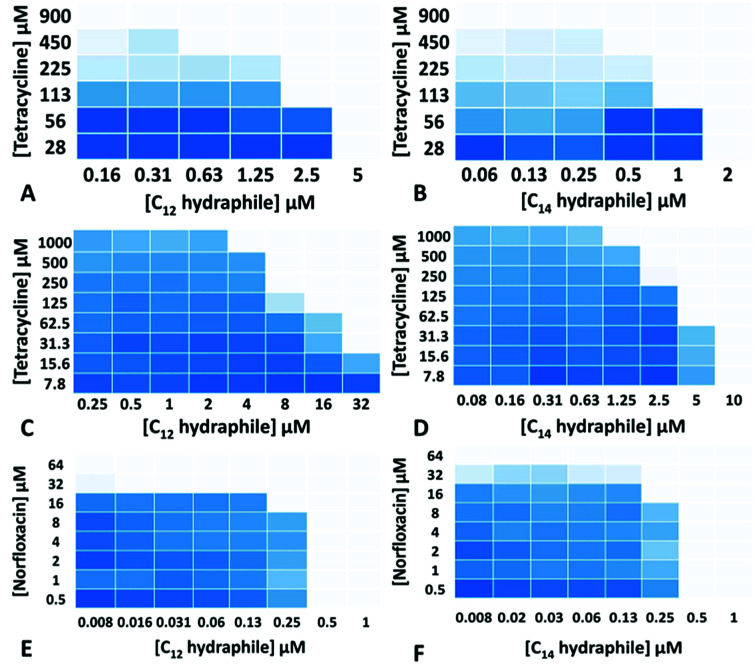
The MICs of compounds 1–4, tetracycline, ciprofloxacin, and norfloxacin were determined against tetRE. coli, K. pneumoniae and S. aureus 1199B (Table S1†). Checkerboard experiments were then conducted with 1–4 to assess tetracycline potency against tetRE. coli and K. pneumoniae and norfloxacin potency against S. aureus 1199B. The concentrations of 3 and 4 ((1/4) − (1/64) of MIC) used in the combination studies are recorded on the abscissas of graphs in Fig. 2. Combination studies with compounds 1 and 2 are shown in Fig. S2.† The ordinates record the concentrations of antibiotics used, also in fractions of MIC. Bacterial growth in the presence of “hydraphile + antibiotic” combinations is indicated by a shade of blue. Uncolored boxes show that the potency (inhibition) of the combination was ≥90%.
Fig. 2. Checkerboard experiments with compounds 3–4 with tetracycline against tetRE. coli (A and B); with tetracycline against K. pneumoniae (C and D) and with norfloxacin against S. aureus 1199B (E and F). Color change from blue (0% inhibition) to white (>90% inhibition) indicates increasing growth inhibition.
As seen in Fig. 2, even though the tetRE. coli and K. pneumoniae were resistant to tetracycline due to active efflux pumps, hydraphiles 3–4 successfully recovered tetracycline efficacy. The extent of tetracycline recovery depended on the concentration and the structure of hydraphile used. At 62 μM (1/4 MIC) and 125 μM (1/2 MIC) of BC8H (1), the MIC of tetracycline was decreased from 900 ± 50 μM to 82 ± 15 μM and 30 ± 8 μM, respectively. This represents an increase in activity of tetracycline by 11-fold and 30-fold, respectively. Similarly, at 1/2 MIC of benzyl C10, C12, and C14 hydraphiles, the activities of tetracycline in tetRE. coli were recovered by 23-, 16-, and 4-fold, respectively. The checkerboard experiments revealed that tetracycline activity was recovered at 8 μM of 1, 2 μM of 2, 625 nM of 3 and 500 nM of 4 against tetRE. coli.
Most of the hydraphile–tetracycline combinations tested against tetRE. coli had fractional inhibitory concentration (FIC) indices33 of ∼0.5. All of the hydraphiles showed some synergy with tetracycline as judged by the FIC < 1 definition. Only BC8H at 1/4 MIC (11-fold recovery) fits the narrower definition of synergy (FIC < 0.5). Growth curves (Fig. S3†) showed that 1–4 did not inhibit bacterial growth at ≤1/2 MIC. We infer that when used in combination, inhibition occurs due to the increased efficacy of tetracycline rather than the additive effect of hydraphiles and tetracycline.
We queried whether hydraphile synergy would be observed with K. pneumoniae and S. aureus, and/or with antibiotics such as the fluoroquinolones. Against K. pneumoniae, which possesses multiple efflux pumps, the activity of tetracycline was recovered by 40-, 8-, 8-, and 16-fold at 1/2 MIC each of 1–4, respectively (Fig. 2c and d, S2c and d†). Benzyl C8–C14 hydraphiles also recovered ciprofloxacin activity against K. pneumoniae (Table S2†). In this case, ciprofloxacin resistance results from both a point mutation and the presence of an efflux pump. Similar results were observed for ciprofloxacin against Gram-positive bacteria. The greatest recovery of antibiotic activity was observed against S. aureus 1199B. At 1/2 MIC of compounds 1–4, the activity of norfloxacin was recovered by 128-fold against NorA efflux pump expressing S. aureus 1199B (Fig. 2e and f, S2e and f†). In the presence of 500 nM of BC14H or BC12H, the MIC of norfloxacin decreased from 64 μM to 500 nM. We speculate that this greater potency enhancement is due to differences in the cytosolic membranes of Gram-negative and Gram-positive bacteria, the latter being more accessible to hydraphiles.
We next compared the activity of hydraphiles against E. coli to that of known ionophores. At 20 μM, a peptide ion channel (gramicidin-D), an ion carrier (valinomycin), and a detergent (Triton X-100) showed tetracycline efficacy enhancements of 1-, 2- and 2-fold, respectively, against tetRE. coli (Table S3†). Lower concentrations showed no recovery of tetracycline activity. No data were obtained for gramicidin-D or valinomycin, both of which were insoluble at >20 μM.
Hydraphile activity was found to be similar to that of CCCP, an efflux pump inhibitor, and to colistin, a membrane disrupter. CCCP dissipates the proton gradient required for antibiotic efflux.25 At 1/2 MIC, CCCP and colistin recovered tetracycline activity by 4-fold and 32-fold, respectively (Table S3†). The FIC index for CCCP was 0.75 and for colistin it was 0.5. Toxicity limits the use of both CCCP and colistin as medicaments.34 Despite recent evidence of resistance, colistin is used as a treatment of last resort for MDR infections. We infer that in efflux pump expressing Gram-negative bacteria, membrane disruption also enhances antimicrobial activity. As such, the result of tetracycline recovery by BC8H is similar to that of colistin, albeit at a much higher concentration (125 μM vs. 125 nM). The activity of efficient ion transporter BC14H is similar to that of CCCP, but hydraphiles may be functioning as membrane disrupters as well as indirect inhibitors of efflux pump activity.
Two conclusions can be drawn from the checkerboard data. First, the presence of hydraphiles 1–4 enhances antibiotic potency irrespective of drug or organism. Second, there is a general trend of higher potency correlated to amphiphile chain length. The efficacy of Na+ ion transport by 1–4 through liposomal bilayers shows a similar trend.15 We infer that the hydraphiles insert in the bacterial membranes, likely enhancing permeability. Compounds 1–4, if of appropriate length, function as non-rectifying ion channels and alter the bacterial ion balance. This results in a deleterious effect on efflux pump function. Results confirming these hypotheses are discussed below.
Membrane localization
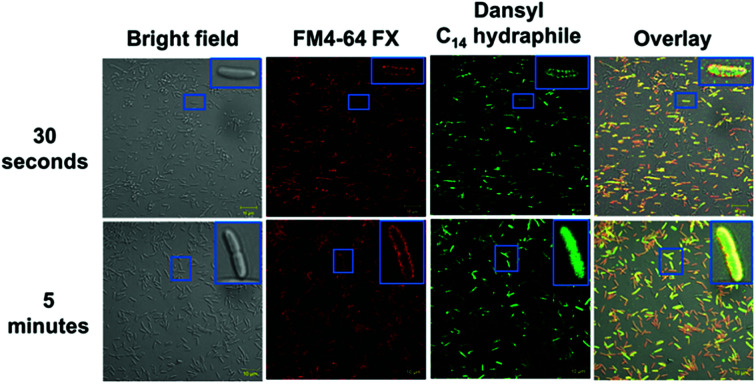
In order for hydraphiles to increase membrane permeability and to form cation selective channels, they must localize in the periphery/membrane of bacteria. Membrane localization of hydraphiles was assessed by using a C14 hydraphile having two fluorescent dansyl groups attached to the distal macrocycles (Fig. S1†).4 The fluorescent dansyl-terminated C14 hydraphile was synthesized to use in tracking its localization in bacteria. Fig. 3 shows confocal images obtained in a study of tetRE. coli with dansyl C14 hydraphile [4 μM] and the fluorescent stain FM4-64 FX [5 μg mL−1], used as a membrane-localizing control. The fluorescence intensity of the diphenylhexatrienyl dye FM4-64 FX increases when it passes from aqueous media into the membrane.35
Fig. 3. Co-localization of FM4-64FX (5 μg mL−1) and dansyl C14 hydraphile in tetRE. coli. Top panel: FM4-64FX was fixed after 5 minutes and dansyl C14 hydraphile was fixed after 30 seconds. Bottom panel: FM4-64FX was fixed after 5 minutes and dansyl C14 hydraphile was fixed after 5 minutes. The scale bars are 10 μm (approximately the width of the smaller boxes).
tetRE. coli cells were fixed with formaldehyde after a 5 minute treatment with FM4-64 FX followed by either a 30 second or 5 minute treatment with dansyl C14 hydraphile. Two data sets resulting from localization studies (0.5 and 5 minute treatments) are shown in Fig. 3. Bacteria were observed to be healthy as judged by the bright field image. The second column shows that FM4-64 FX localized in the bacterial membranes after both 30 seconds and 5 minutes. The FM4-64 FX staining was more visible after 5 minutes than after 30 seconds. Column 3 shows that dansyl C14 hydraphile (dansyl replaces benzyl in 4) localizes in the E. coli membrane within 30 seconds. As seen in the inserts in column 4 of Fig. 3 (30 seconds and 5 minutes), membrane localization of dansyl C14 hydraphile was confirmed in the overlay image of ‘hydraphile (green) + FM4-64 FX (red)’ that appears yellow in the membrane.
Three points were noted after treating E. coli with hydraphiles. First, membrane localization is apparent in the overlay image after only 5 minutes. Second, the cells showed cytoplasmic localization of dansyl C14 hydraphile (Fig. 3, bottom panel). This could result from either cell death or increased membrane permeability; both would permit hydraphile entry into the cytoplasm. Third, it appeared that hydraphile aggregates were present on or within the bacterial membrane. To confirm these observations, scanning electron microscopy (SEM) was conducted on hydraphile-treated E. coli. We also determined if hydraphiles formed aggregates before attaching to the bacterial surface by using SEM and dynamic light scattering (DLS). Images are shown in Fig. 4 and additional images are shown in Fig. S4.†
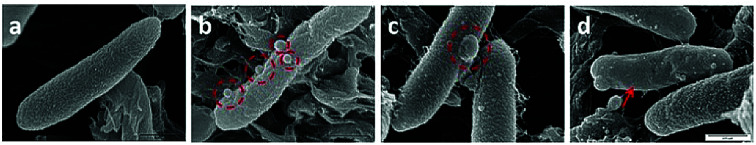
Fig. 4. Scanning electron microscopic images of common traits observed with tetRE. coli when treated with BC14H (4). The image shows the typical corrugated membrane of an untreated E. coli (a), hydraphile aggregates on E. coli surface (b), a membrane blister on the membrane (c), and membrane smoothening (d). The scale for all the images is 0.5 μm (see panel d).
As observed under SEM, the untreated E. coli showed a corrugated surface/membrane (Fig. 4(a)). As a control, hydraphiles alone were observed under SEM. In aqueous media, SEM showed that benzyl C8 and C14 hydraphiles formed aggregates of ∼1–5.5 μm (Fig. S5†). When studied using DLS, BC14H formed aggregates of 300 ± 50 nm in dH2O. The larger aggregates formed by hydraphiles under SEM conditions likely reflects differences in sample preparation. Notwithstanding, aggregates were observed in both cases.
Three observations were made by using SEM concerning E. coli treated with hydraphiles for 5 minutes. (1) Benzyl C8 and C14 hydraphile aggregates of size 226 ± 9 nm and 164 ± 22 nm, respectively, were attached to the E. coli cell surface (Fig. 4(b)). The sizes of aggregates formed in LB Miller media using DLS were found to be 180 nm and 160 nm for BC14H at 7 μM and 3.5 μM, respectively. BC8H at 25 μM in LB Miller media formed aggregates of 227 nm (DLS). (2) Membrane blisters and (3) smooth outer membranes were apparent in the hydraphile-treated E. coli cells (Fig. 4(c) and (d)). Disruption of cytoplasmic membranes is known to cause leakage of cytosolic contents into the periplasmic space, forming ‘membrane blisters’ on the surface of bacteria.36 This could account for the membrane blisters observed in the presence of hydraphiles. The outer membrane of E. coli is corrugated when the bacterium is in a well-balanced ionic condition. Disruption of the internal ion gradient may cause bacterial swelling, making the outer membranes smooth.4 Hydraphile-mediated deregulation of the cation gradient can cause osmotic stress leading to swelling and smoothening of the cellular membrane. The co-localization, DLS, and SEM studies confirm that the hydraphiles form aggregates that attach to the E. coli surface, followed by localization in E. coli membranes. The result is membrane disruption and osmotic stress.
The localization of dansyl C14 hydraphile was also assessed in human embryonic kidney (HEK-293) cells (Fig. S5†), but only at 32 μM in contrast with 4–8 μM in E. coli. Some nuclear staining of HEK-293 cells with dansyl C14 hydraphile was also observed. Thus, hydraphiles localize in the membranes of both mammalian and bacterial cells, but in the former only when the hydraphile concentration is significantly higher. The concentration difference observed between E. coli and HEK-293 cells, while less than 10-fold, suggests that selectivity in treatment is possible.
Membrane permeability
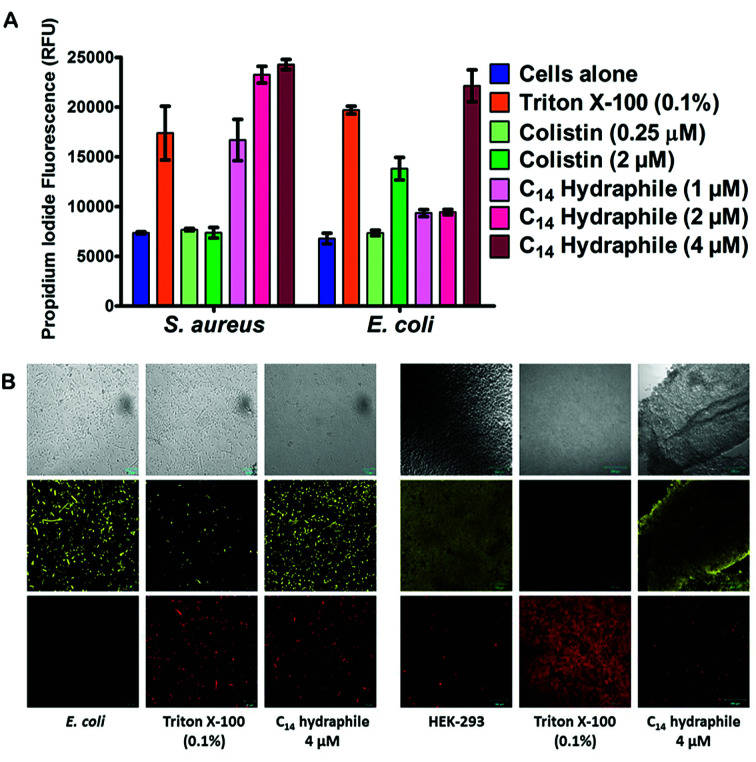
The boundary membrane permeability of tetRE. coli, S. aureus 1199B, and HEK-293 cell membranes were assessed by using propidium iodide (PI). PI is a membrane impermeable stain. If the permeability of the membrane increases, PI passes through the membranes, intercalates into the nucleic acid stack and its fluorescence intensity increases. Triton X-100 and colistin were used as controls. They both disrupt membranes, but Triton X-100 is a well-known detergent whereas colistin is an antibiotic. Compounds 1–4, and controls were tested at half their MICs.
The graph of Fig. 5(A) compares the efficacy of BC14H (4), at 1 μM, 2 μM, and 4 μM. At the concentration of bacteria required to conduct this experiment, the MIC of our most active compound (4) was 8 μM against E. coli. Hence, 1/2 MIC of 4 for this experiment is 4 μM against E. coli and it is 2 μM against S. aureus. As shown in Fig. 5(A), Triton X-100 control at 0.1% (1.6 mM) increases membrane permeability in both S. aureus and E. coli. Colistin specifically targets Gram-negative bacteria; its potency (increase in permeability) against E. coli is ≥2 μM. As was the case with colistin, against E. coli, BC14H (4) increased membrane permeability only at ≥1/2 MIC (4 μM). At 1 μM or 2 μM of 4, the membrane permeability of S. aureus was increased, but that of E. coli was either unaffected or showed only minimal changes. At 2 and 4 μM, compounds 1–3 showed no permeability increase for either S. aureus or E. coli (Fig. S6†). Thus, BC14H (4) was more active than hydraphiles 1–3 against E. coli and 4 was also more active against S. aureus than it was against E. coli.
Fig. 5. (A) Permeability of propidium iodide to E. coli and S. aureus cell membranes mediated by compounds 1–4, Triton X-100, and colistin. The x-axis represents the compounds used whereas the y-axis represents the relative fluorescence unit (RFU). Error bars represent standard deviation in three trials. (B) Benzyl C8–C14 hydraphiles (1/2 MIC) mediated permeability of propidium iodide and fluorescein diacetate in tetRE. coli (scale = 10 μm) and HEK-293 (scale = 100 μm). DMSO (0.5% v/v) and Triton X-100 (0.1% v/v) were used as controls. The top row shows bright field images, the middle row shows FDA fluorescence and the lower row shows propidium iodide (PI) fluorescence.
In the presence of 1/2 MIC of compounds 1–4, the membrane permeability of S. aureus and E. coli were both increased (Fig. S6a and b†). Likewise, at 1/2 MIC concentrations of hydraphiles, antimicrobial potency was increased, but bacterial growth was not inhibited in the absence of antibiotic. Evidence was obtained by using the cell viability stain fluorescein diacetate (FDA). FDA is non-fluorescent until hydrolyzed by endogenous esterases and thus reports cellular vitality.37 The fluorescence of propidium iodide (PI) and FDA in hydraphile-treated E. coli and HEK-293 cells was observed by confocal microscopy.
Fig. 5(B) shows tetRE. coli and HEK-293 cells after treatment with either BC14H or Triton X-100. The images shown in the three left-hand columns of Fig. 5(B) show results for E. coli and the three columns on the right are for HEK-293. In the E. coli and HEK-293 cells treated with BC14H, cellular viability (FDA panel) was comparable to that of the cells alone control. After treatment with compound 4, PI penetration (PI panel) clearly increased in E. coli, indicating enhanced membrane permeability. The increase in PI staining was comparable to that of Triton X-100 at 0.1% (i.e. 1600 μM). Therefore, the increase in bacterial membrane permeability, as observed in Fig. 5(A) and (B), was not caused by cell death.
No increase in mammalian cell (HEK-293) membrane permeability was observed at either 1/2 MIC or 2 MIC concentrations of compound 4 (Fig. 5(B)), which selectively increased the permeability of bacterial cells. Similar results were observed with 1/2 MIC of benzyl C8, C10, and C12 hydraphiles (Fig. S7a†). Compounds 1–3 increased the membrane permeability of E. coli cells without decreasing their cell viability and 1–3 did not increase mammalian cell permeability (Fig. S7b†). We speculate that membrane disruption and permeability increase occur at the site of hydraphile insertion.
Benzyl C8–C14 hydraphiles (1–4) localize in both bacterial and mammalian cell membranes, but are more disruptive of bacterial cell membranes and enhance their permeability to a greater extent. Some selectivity between bacterial and mammalian cells seems likely owing to differences in their structures. The phospholipids such as phosphatidylglycerols found in the bacterial membranes are acidic.38 The lipopolysaccharide of Gram-negative bacteria and teichoic acids of Gram-positive bacteria are anionic in nature.37 The cationic property and amphiphilic nature of antimicrobial peptides such as colistin, allow for interaction with bacterial membranes.39
Mammalian cells, on the other hand, have zwitterionic phosphatidylcholine in the outer leaflet and acidic phospholipids localized in the inner leaflet of membranes.40 Mammalian cell membranes also contain cholesterol. In previous liposomal studies with hydraphiles, addition of cholesterol to liposomes apparently thickened the membranes, shifting peak transport to longer chain lengths. The sensitivity of hydraphiles to membrane thickness suggests that some selectivity between mammalian and bacterial membranes is possible.16 Hydraphile penetration of bacterial membranes suggests that (1–4) could increase membrane permeability and thus the influx of antibiotics into the cells.
Potassium transport
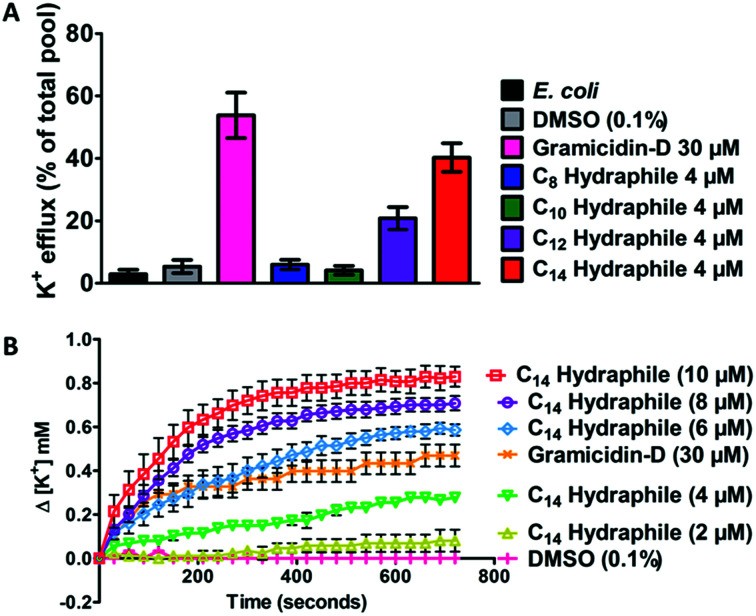
Active efflux requires regulated cation gradients across bacterial membranes. Hydraphiles form cation selective channels in liposomal bilayers that transport sodium and potassium ions, but these channels are non-rectifying. Hydraphiles will transport ions from higher to lower concentrations without regulation. To assess hydraphile-mediated cation transport in bacteria, changes in extracellular potassium ion concentrations were measured after hydraphile addition to tetRE. coli. Total potassium content of the tetRE. coli cells, determined by boiling the bacteria at 100 °C, was set to 100% and potassium cation release is reported on that basis. No change in extracellular [K+] was observed with 0.1% (v/v) DMSO control. The peptide ion channel gramicidin-D [30 μM] fostered K+ efflux of 50–60% in tetRE. coli (Fig. 6(A)). Compounds 1–4 were tested at 4 μM, which is 1/2 the MIC of the most active hydraphile. In the presence of 4 μM of benzyl C8–C14 hydraphiles, 6% (1), 4% (2), 21% (3), and 40% (4) of K+ release was observed (Fig. 6(A)). The potassium release profile 4 > 3 > 2 ≈ 1 correlates directly with the potency enhancement of tetracycline against tetRE. coli (Fig. S8a†).
Fig. 6. (A) Potassium ion release from E. coli cells 10 minutes after treatment with DMSO, gramicidin-D (control) and benzyl C8–C14 hydraphiles (compounds 1–4). The total potassium pool of E. coli was determined by boiling the bacteria at 100 °C for 30 minutes. (B) Change in extracellular potassium concentration over 12 minutes of treatment with various concentrations of benzyl C14 hydraphile (4). Error bars represent standard deviation in three trials.
Loss of internal K+ ion is known to occur when bacterial membrane integrity is compromised. We determined potassium release from E. coli as a function of increasing hydraphile concentration. As seen in Fig. 6(B), increase in the concentration of 4, increases the potassium release from E. coli. The K+ release from E. coli also increased with increasing concentration of 1–3 (Fig. S8b–e†).
The gramicidin-D control showed K+ release to the extent of 50–60% (Fig. 6(A)) when administered at 30 μM. Potassium release by BC14H (4) at 4 μM was ∼40%. When the concentration of 4 was increased from 4 μM to 6 μM, 8 μM, and 10 μM, K+ ion release was enhanced (Fig. 6(B)). Indeed the gramicidin-D release level at 30 μM was exceeded at 6 μM of 4. When the concentration of 4 reached 10 μM, more than 80% of the bacterial pool was released (Fig. 6(B)). No inhibition of E. coli growth was apparent at 1/2 MIC of compound 4. This indicates that at lower concentrations the cation release could be due to the ability of hydraphiles to form channels rather than as a result of membrane disruption or cell death. Membrane penetration and ion transport by hydraphiles is well established, but these two potential contributors have not yet been clearly distinguished. Both processes seem likely as is known for such peptide amphiphiles as colistin and daptomycin.41 Among the compounds tested, the best ion transporters in liposomes15 also showed the highest level of K+ release in E. coli—an interesting parallel considering how different are the boundary membranes.
Efflux pump inhibition
The combination of increased membrane permeability and disruption of cation gradients results in efflux pump inhibition. Efflux pump activity was assayed by evaluating changes in cytoplasmic concentrations of an efflux pump substrate. S. aureus (1199B) over-expresses the NorA efflux pump, for which ethidium bromide (EthBr) and norfloxacin are substrates.30 To the extent EthBr enters the cell cytoplasm, it intercalates with DNA, forming a complex in which fluorescence intensity is dramatically enhanced. Combination studies were first conducted to confirm that activity of either EthBr or norfloxacin could be recovered by compounds 1–4. The benzyl C8–C14 hydraphiles at 1/2 MIC increased the efficacy of EthBr against S. aureus by 2-, 26-, 8-, and 32-fold, respectively (Table S4†). Note that norfloxacin activity against S. aureus 1199B was also recovered by 1–4 as shown above in Fig. 2.
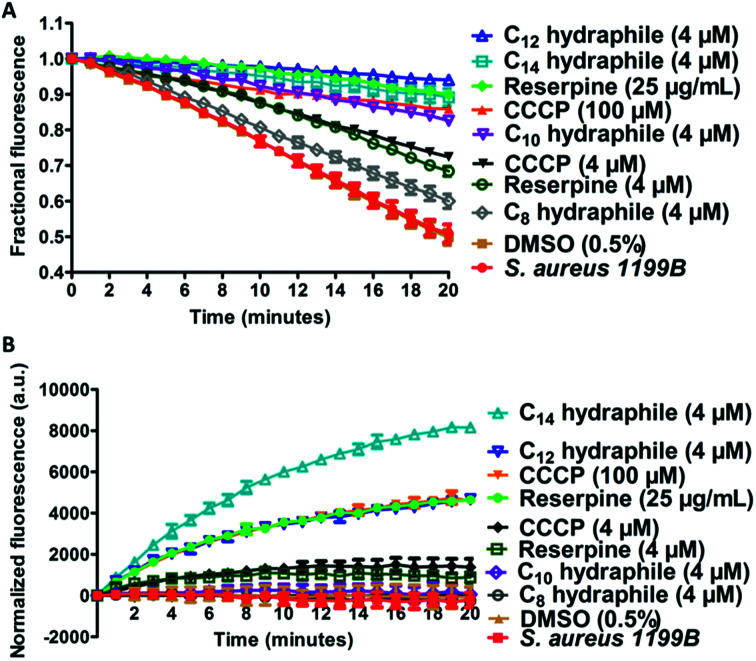
Two studies were conducted to determine the effect of hydraphiles on bacteria expressing NorA efflux pumps. We first determined the ability of the bacterial NorA efflux pump to export EthBr in the presence or absence of hydraphiles (Fig. 7(A)). Thus, S. aureus 1199B cells were preloaded with EthBr by treating S. aureus 1199B with CCCP [100 μM], a known efflux pump inhibitor. Extracellular EthBr and CCCP were removed by washing the cells, followed by addition of 4 μM of 1, 2, 3, or 4. The MIC of BC14H (4) is ∼8 μM against S. aureus (at OD600 ∼0.800), i.e. [4 μM] = 1/2 MIC. The graph of Fig. 7(A) compares the effect on S. aureus 1199B of hydraphiles and known efflux pump inhibitors. EthBr's fluorescent intensity decreased similarly with untreated and 0.5% aq. DMSO-treated S. aureus (Fig. 7(A)). The EthBr fluorescence was little affected by the presence of 4 μM benzyl C10, C12, or C14 hydraphile. Efflux pump function was found to be inhibited to a similar extent by BC14H (4) at 4 μM, CCCP at 100 μM, and reserpine at 41 μM. When the concentrations of CCCP and reserpine were decreased to 4 μM, to match that of BC14H, inhibition of NorA activity was ∼30% lower than for 3 or 4. Benzyl C8 hydraphile at 4 μM showed minimal/no NorA efflux pump inhibition in S. aureus.
Fig. 7. Hydraphiles inhibit the activity of efflux pumps. (A) Release of ethidium bromide from S. aureus 1199B after treatment with reserpine, CCCP, benzyl C8–C14 hydraphiles [4 μM]. The abscissa reports time in minutes and the ordinate reports fractional fluorescence. (B) Accumulation of ethidium bromide in the presence of reserpine, CCCP, benzyl C8–C14 hydraphiles (4 μM) in S. aureus 1199B. The abscissa records time in minutes and the ordinate records normalized fluorescence. The error bars represent the standard deviation in three independent trials.
A complementary experiment was conducted by observing cytoplasmic EthBr accumulation in S. aureus 1199B in the presence of hydraphiles (Fig. 7(B)). EthBr was added to S. aureus 1199B cells followed by one of 1–4 or a control. Changes in fluorescence were monitored during 20 min. The graph of Fig. 7(B) shows that EthBr accumulation was observed following addition of benzyl C12 and C14 hydraphiles, but not the C8 and C10 analogs. Note that the effect of BC12H at 4 μM was similar to that of CCCP at 100 μM and reserpine at 41 μM (=25 μg mL−1). Benzyl C8 and C10 hydraphiles proved ineffective at 4 μM; higher concentrations of these compounds were not tested.
The data obtained with S. aureus 1199B show that hydraphiles inhibit efflux pump activity, causing antibiotics to accumulate in the bacterial cell cytoplasm. Benzyl C12 and C14 hydraphiles are at least comparable to and better, in some cases, than standard efflux pump inhibitors such as CCCP and reserpine. The hydraphiles could mediate both the accumulation of EthBr and inhibition of NorA efflux pump either by disruption of membrane integrity or uncoupling of the efflux pump from the ion gradient, or both.
The data obtained thus far confirm that hydraphiles localize in the membranes of both bacterial and mammalian cells. They affect the membrane permeability of bacterial cells, but they show little effect on mammalian cells. Their presence in bacterial membranes results in potassium efflux from the microbe's cytosol. Hydraphiles also inhibit efflux pump activity and allow for substrate accumulation in the bacterial cytoplasm. As a result, even at ≤1/2 MIC of hydraphiles, the activity of antibiotics were recovered against MDR bacteria. Concerns over mammalian cytotoxicity and resistance development by E. coli to hydraphiles are obviously key questions and are addressed below.
Cytotoxicity and resistance development
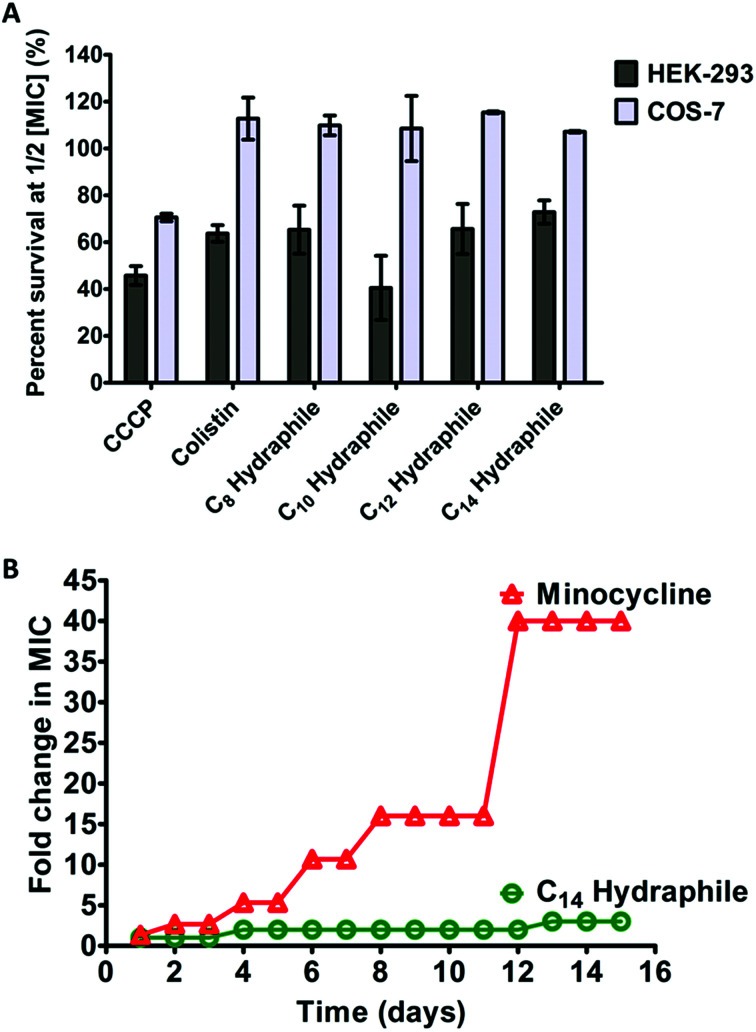
The clinical use of both colistin and CCCP is limited due to cytotoxicity.42 Renal toxicity is a common issue with amphiphilic molecules.34 For a hydraphile to be an effective antibiotic adjuvant, its cytotoxicity to mammalian cells should be low. The cytotoxicity was evaluated for benzyl C8–C14 hydraphiles against two mammalian epithelial cell lines: human embryonic kidney (HEK-293), and simian kidney (Cos-7). Colistin and CCCP served as controls. The viability of the cell lines in the presence of 1/2 MIC of benzyl C8–C14 hydraphiles (1–4), colistin, and CCCP are recorded in Fig. 8(A) below.
Fig. 8. Cytotoxicity and resistance development to benzyl hydraphiles. (A) Cytotoxicity of benzyl C8–C14 hydraphiles and colistin and CCCP at 1/2 MIC concentrations against HEK-293, and Cos-7 cells. The abscissa represents the compounds used and the ordinate represents the percent of cells that survived in the presence of the respective compounds. The error bars represent the standard deviation for three trials. (B) Resistance development by tetRE. coli to BC14H (circles) and minocycline (triangles). The x-axis represents time in days and the y-axis represents increase in MIC (in folds). The data represents the average of three different trials.
CCCP was cytotoxic to all three cell lines. Colistin at 1/2 MIC (0.125 μM) was non-cytotoxic to Cos-7, but HEK-293 showed only about 60% survival. Similar to colistin, compounds 1–4 were completely non-cytotoxic (100% survival) to Cos-7 cell line at 1/2 MIC. Even at MIC concentrations of compounds 1–4, that are twice the concentrations required for adjuvant activity, the survival of Cos-7 was 100% (data not shown). The survival of HEK in the presence of 1/2 MIC of 4 (1 μM) was 70–80%. Hence, the cytotoxicity of BC14H (4) at 1 μM, is lower than that of colistin at 0.125 μM. The survival of HEK-293 cells in the presence of 1/2 MIC of 1, 2, or 3 was 50–70%, comparable to the cytotoxicity of colistin (∼60%).
We determined the median effective concentration (EC50) of compound 4 against human hepatocytes (Fig. S9†). CCCP was used as a control. Survival of human hepatocytes was determined after treating them with 1–100 μM of either compound 4 or CCCP for 72 hours. The EC50 for CCCP was found to be 5 μM. The EC50 for BC14H was 2.1 μM. At 1 μM, 80–100% cell viability was observed for 4. Thus, 4, at concentrations of 500 nM to 1 μM, is non-cytotoxic to HEK-293, Cos-7, and human hepatocytes. At 1 μM 4 recovers antibiotic activity in MDR bacteria (Fig. 2), while being less cytotoxic than colistin. Analogs of 1–4 that show lower cytotoxicity might be required for clinical use. Additional analogs that show better solubility and lower cytotoxicity are under study.
A major concern with any antibiotic is bacterial resistance. Newer antibiotics are often limited in use to conserve their potency. Compounds that target membranes are usually less prone to resistance development by bacteria than those acting exclusively in the cytosol. For example, polymyxins (colistin) have been in clinical use for >50 years, but resistance to polymyxin was identified only recently.12 It is known that developing resistance to a membrane active compound would require multiple changes in membrane composition/synthesis pathways and is energetically unfavorable.2 The data reported here show that hydraphiles are membrane targeting molecules. We therefore exposed tetRE. coli to 4 and to minocycline (positive control) by using the sequential culturing method. E. coli were treated with increasing fractional MIC concentrations of compound 4, for 15 days. The highest concentration of the compound that showed growth was used to determine MICs. Fig. 8(B) shows that tetRE. coli readily developed 40-fold resistance to minocycline (12–15 days). Resistance to 4 did not exceed 1.5- to 2-fold during 15 days. Taken together, at its active concentration, 4 was less cytotoxic than colistin and not susceptible to resistance development in E. coli.
Conclusions
We present the results of a study showing the potential of synthetic ionophores—hydraphiles—as medicaments and as adjuvants. Hydraphiles and other synthetic ionophores have been known for decades. Most of the studies involving such molecules have focused on developing new structures,6 measuring their binding profiles, assessing their effect on ion transport,5 and simple surveys of antibacterial or antifungal properties.26 This is the first report of a synthetic amphiphile that can be used to recover the efficacy of antibiotics against efflux pump expressing multidrug resistant bacteria or ‘superbugs’. The hydraphiles recovered the activity of tetracycline and fluoroquinolones (ciprofloxacin, norfloxacin) against two Gram-negative and one Gram-positive bacteria. Ciprofloxacin and tetracycline activity were recovered by hydraphiles against MDR K. pneumoniae. This demonstrates that hydraphiles can be used to recover antimicrobial potency against MDR bacteria even when resistance arises from multiple resistance mechanisms. BC14H was most effective as an adjuvant against multiple different bacterial strains. Controls indicated that the activity of our synthetic ionophores is similar to that of the efflux pump inhibitor CCCP and to a membrane disrupting peptide such as colistin.
At sub-lethal concentrations, our synthetic ionophores resensitize resistant bacteria, likely by a combination of enhanced membrane permeability and by interrupting ion gradients required for efflux pump function. Studies revealed that hydraphiles form aggregates that attach to the bacterial surface followed by localizing in the cellular membrane. Hydraphiles then selectively increase membrane permeability in bacteria. Efflux pump activity was observed to be inhibited, presumably due to uncoupling of the cation gradient. As a result, more antibiotics pass through the membranes and accumulate in the cytosol. Such function recovers or increases the potency of existing antibiotics. We determined that among the compounds tested, BC14H was the most efficient amphiphile at recovering antimicrobial potency, increasing membrane permeability, cation transport and inhibiting efflux pump activity. BC14H showed greater activity against Gram-positive bacteria, had the lowest mammalian cell cytotoxicity, and E. coli failed to develop resistance against it. Taken together, these novel synthetic amphiphiles hold forth potential as antibiotics per se and as adjuvants to enhance antimicrobial potency and to resensitize resistant pathogens.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank the National Science Foundation (CHE-1307324, 1710549), the National Institutes of Health Preclinical Services Program, and the University of Missouri for partial support of this work. We are also grateful to Dr David Osborn for his assistance with microscopy.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07641c
Notes and references
- (a) Center for Disease Control and Prevention, Antibiotic resistance threats in United States, 2013 [Google Scholar]; (b) World Health Organization, Antimicrobial resistance global report and surveillance, 2014 [Google Scholar]
- Ling L. L. Schneider T. Peoples A. J. Spoering A. L. Engels I. Conlon B. P. Mueller A. Schäberle T. F. Hughes D. E. Epstein S. Jones M. Lazarides L. Steadman V. A. Cohen D. R. Felix C. R. Fetterman K. A. Millett W. P. Nitti A. G. Zullo A. M. Chen C. Lewis K. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hover B. M. Kim S. Katz M. Charlop-Powers Z. Owen J. G. Ternei M. A. Maniko J. Estrela A. B. Molina H. Park S. Perlin D. S. Brady S. F. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat. Microbiol. 2018 doi: 10.1038/s41564-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevy W. M. Donato G. M. Ferdani R. Goldman W. E. Schlesinger P. H. Gokel G. W. Synthetic hydraphile channels of appropriate length kill Escherichia coli. J. Am. Chem. Soc. 2002;124:9022–9023. doi: 10.1021/ja017052o. [DOI] [PubMed] [Google Scholar]
- Cox B. G. and Schneider H., Coordination and Transport Properties of Macrocyclic Compounds in Solution, Elsevier, Amsterdam, 1992, p. 420 [Google Scholar]
- Steed J. W. and Atwood J. L., Supramolecular Chemistry, John Wiley and Sons, Ltd., Chichester, 2009 [Google Scholar]
- Poole K. Efflux mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- McNicholas P. Chopra I. Rothstein D. M. Genetic analysis of the tetA(C) gene on plasmid pBR322. J. Bacteriol. 1992;174:7926–7933. doi: 10.1128/jb.174.24.7926-7933.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Active efflux mechanisms for antimicrobial resistance. Antimicrob. Agents Chemother. 1992;36:695–703. doi: 10.1128/AAC.36.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgurskaya H. I. Lopez C. A. Gnanakaran S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015 doi: 10.1021/acsinfecdis.5b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Fan B. Guan J. Wang X. Cong Y. Activity of Colistin in combination with meropenem, tigecycline, fosfomycin, fusidic acid, rifampicin or sulbactam against extensively drug-resistant Acinetobacter baumannii in a murine thigh-infection model. PLoS One. 2016 doi: 10.1371/journal.pone.0157757. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gould I. M. Miro J. M. Rybak M. J. Daptomycin: The role of high-dose and combination therapy for Gram-positive infections. Int. J. Antimicrob. Agents. 2013;42:202–210. doi: 10.1016/j.ijantimicag.2013.05.005. [DOI] [PubMed] [Google Scholar]
- McGann P. Snesrud E. Maybank R. Corey B. Ong A. C. Clifford R. Hinkle M. Whitman T. Lesho E. Schaecher K. E. Escherichia coli harboring mcr-1 and blaCTX-M on a novel lncF plasmid: first report of mcr-1 in the United States. Antimicrob. Agents Chemother. 2016;60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W. Seo S. M. Ruble C. A. Efflux mediated fluoroquinolone resistance in staphylococcus aureus. Antimicrob. Agents Chemother. 1993;37:1086–1094. doi: 10.1128/AAC.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokel G. W., Crown Ethers and Cryptands, The Royal Society of Chemistry, London, England, 1991, vol. 3, p. 190 [Google Scholar]
- Inoue Y. and Gokel G. W., Cation Binding by Macrocycles, Marcel Dekker, New York, 1990, p. 761 [Google Scholar]
- Gokel G. W. Hydraphiles: Design, Synthesis, and Analysis of a Family of Synthetic, Cation-Conducting Channels. Chem. Commun. 2000:1–9. doi: 10.1039/A903825F. [DOI] [Google Scholar]
- Weber M. E. Schlesinger P. H. Gokel G. W. Dynamic Assessment of Bilayer Thickness by Varying Phospholipid and Hydraphile Synthetic Channel Chain Lengths. J. Am. Chem. Soc. 2005;126:636–642. doi: 10.1021/ja044936+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas G. Lopez C. Klein M. Membrane bound hydraphiles facilitates cation translocation. J. Phys. Chem. B. 2004;108:4231–4235. doi: 10.1021/jp036953b. [DOI] [Google Scholar]
- Skelton A. A. Khedkar V. M. Fried J. R. All-atom molecular dynamics simulations of an artificial sodium channel in a lipid bilayer: the effect of water solvation/desolvation of the sodium ion. J. Biomol. Struct. Dyn. 2016;34:529–539. doi: 10.1080/07391102.2015.1044473. [DOI] [PubMed] [Google Scholar]
- Aktins J. Patel M. B. Cusumano Z. Gokel G. W. Enhancement of antimicrobial activity by synthetic ion channel synergy. Chem. Commun. 2010;46:8166–8167. doi: 10.1039/C0CC03138K. [DOI] [PubMed] [Google Scholar]
- Patel M. B. Garrad E. C. Stavri A. Gokel M. R. Negin S. Meisel J. W. Cusumano Z. Gokel G. W. Hydraphiles enhance antimicrobial potency against Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis. Bioorg. Med. Chem. 2016;24:2864–2870. doi: 10.1016/j.bmc.2016.04.058. [DOI] [PubMed] [Google Scholar]
- Curvey N. S. Luderer S. E. Walker J. K. Gokel G. W. Improved Syntheses of Benzyl Hydraphile Synthetic Cation-conducting Channels. Synthesis. 2014;46:2771–2779. doi: 10.1055/s-0034-1378345. [DOI] [Google Scholar]
- Wallace B. A., Gramicidin and related ion channel-forming peptides, Novartis Foundation, John Wiley & Sons, Ltd., Chichester, 1999, 225, p. 273.ss [Google Scholar]
- Pinkerton M. Steinrauf L. K. Dawkins P. Molecular structure and some transport properties of valinomycin. Biochem. Biophys. Res. Commun. 1969;35:512–518. doi: 10.1016/0006-291X(69)90376-3. [DOI] [PubMed] [Google Scholar]
- (a) Schlieper P. De Robertis E. Triton X-100 as a channel-forming substance in artificial lipid bilayer membranes. Arch. Biochem. Biophys. 1977;184:204–208. doi: 10.1016/0003-9861(77)90343-5. [DOI] [PubMed] [Google Scholar]; (b) Rostovtseva T. K. Bashford C. L. Lev A. A. Pasternak C. A. Triton channels are sensitive to divalent cations and protons. J. Membr. Biol. 1994;141:83–90. doi: 10.1007/BF00232876. [DOI] [PubMed] [Google Scholar]
- Ardebili A. Talebi M. Azimi L. Lari A. R. Effect of efflux pump inhibitor CCCP on the minimal inhibitory concentration of ciprofloxacin in Acinetobacter baumannii clinical isolates. Jundishapur J. Microbiol. 2014;1:8691–8696. doi: 10.5812/jjm.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokel G. W., Negin S. and Cantwell R., Crown Ethers, Comprehensive Supramolecular Chemistry, ed. J. L. Atwood, G. W. Gokel and L. J. Barbour, Elsevier Publishing Company, 2nd edn, 2017, vol. 3, pp. 3–48 [Google Scholar]
- Leski T. Vora G. J. Taitt C. R. Multidrug resistance determinants from NDM-1-producing Klebsiella pneumoniae in the USA. Int. J. Antimicrob. Agents. 2012;40:282–284. doi: 10.1016/j.ijantimicag.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Meisel J. W. Patel M. B. Garrad E. G. Stanton R. Gokel G. W. Reversal of tetracycline resistance in Escherichia coli by noncytotoxic bis(Tryptophan)s. J. Am. Chem. Soc. 2016;138:10571–10577. doi: 10.1021/jacs.6b05578. [DOI] [PubMed] [Google Scholar]
- Boucher H. W. Talbot G. H. Bradley J. S. Edwards J. E. Gilbert D. Rice L. B. Scheld M. Spellberg B. Bartlett J. Bad bugs, no drugs: no ESKAPE! An update from the infectious disease society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barrier and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- Neyfakh A. A. Borsch C. M. Kaatz G. W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 1993;37:128–129. doi: 10.1128/AAC.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum M. C. What is synergy? Pharmacol. Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- Trifi A. Abdellatif S. Daly F. Mahjoub K. Nasri R. Oueslati M. Mannai R. Bouzidi M. Lakhal S. B. Efficacy and toxicity of high-dose colistin in multi-drug resistant Gram-negative Bacilli infection: a comparative study of matched series. Chemotherapy. 2015;16:190–196. doi: 10.1159/000442786. [DOI] [PubMed] [Google Scholar]
- Leevy W. M. Donato G. M. Ferdani R. Goldman W. E. Schlesinger P. H. Gokel G. W. Synthetic hydraphile channels of appropriate length kill Escherichia coli. J. Am. Chem. Soc. 2002;124:9022–9023. doi: 10.1021/ja017052o. [DOI] [PubMed] [Google Scholar]
- Hartmann M. Berditsch M. Hawecker J. Ardakani F. Gerthsen D. Ulrich A. S. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 2010;54:3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubberfield L. C. F. Shaw P. J. A. A comparison of tetrazolium reduction and FDA hydrolysis with other measures of microbial activity. J. Microbiol. Methods. 1990;12:151–162. doi: 10.1016/0167-7012(90)90026-3. [DOI] [Google Scholar]
- Komaratat P. Kates M. The lipid composition of a halotolerant species of Staphylococcus epidermidis. Biochim. Biophys. Acta. 1975;398:464–484. doi: 10.1016/0005-2760(75)90197-6. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta. 2009;1788:1687–1692. doi: 10.1016/j.bbamem.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J. Zwaal R. F. A. Roelofsen B. Comfurius P. Kastelijn D. Deener L. L. M. V. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim. Biophys. Acta. 1973;323:178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- Falagas M. E. Kasiakou S. K. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005;40:1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- Trifi A. Abdellatif S. Daly F. Mahjoub K. Nasri R. Oueslati M. Mannai R. Bouzidi M. Lakhal S. B. Efficacy and toxicity of high-dose colistin in multi-drug resistant Gram-negative Bacilli infection: a comparative study of matched series. Chemotherapy. 2015;16:190–196. doi: 10.1159/000442786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.