Abstract
Human epidermal growth factor receptor 2 (HER2) positive breast cancer accounts for about 20% of all breast cancers and this subtype has been historically associated with worse prognosis. Margetuximab is a chimeric and Fc-engineered monoclonal antibody directed to HER2 that can enhance the activation of the innate and adaptive immune responses while maintaining trastuzumab’s antiproliferative effects. Margetuximab in combination with chemotherapy was approved by the US FDA in December 2020 for patients with metastatic HER2+ breast cancer who have received two or more HER2-targeted regimens. This approval was based on the results of the SOPHIA trial that showed a modest improvement in progression-free survival with margetuximab and chemotherapy compared to trastuzumab and chemotherapy. Ongoing studies are assessing the role of margetuximab in other settings and diseases such as early stage breast cancer and gastrointestinal malignancies. Here we review the rationale for the development of margetuximab, previous and ongoing clinical trials and current role in clinical practice.
Keywords: margetuximab, metastatic breast cancer, HER2-positive
Introduction
Breast cancer is the leading cancer and the second leading cause of cancer-related deaths in the US.1 Human epidermal growth factor receptor 2 (HER2/neu or ERRB2) positive breast cancer accounts for around 20% of all breast cancers and this subtype was historically associated with worse prognosis.2,3 With the development of targeted therapies, the prognosis of HER2+ breast cancer improved dramatically.4 Since the approval of trastuzumab in 1998 significant advances have been made in drug development and in the understanding of the immune mechanisms of the action of monoclonal antibodies (mABs).4–7
The current standard first line of treatment for advanced HER2+ breast cancer is a taxane, trastuzumab, and pertuzumab (THP) based on the findings of the CLEOPATRA study.7,8 Over the past years, four HER2-targeted agents have been approved for the treatment of metastatic HER2+ breast cancer. Until recently, the antibody-drug conjugate (ADC) ado-trastuzumab emtansine (TDM1) was the standard second-line therapy based on the EMILIA study.9,10 However, based on impressive findings of the Phase 3 DESTINY Breast-03 study, the ADC fam-trastuzumab deruxtecan is now the preferred second-line treatment.11,33 The tyrosine kinase inhibitors (TKI) neratinib and tucatinib (in combination with capecitabine and/or trastuzumab) were approved based on the NALA and HER2CLIMB studies, respectively.6,12 Finally, the Fc-engineered immune activating anti-ERBB2 IgG1 immunoglobulin margetuximab was approved by the US Food and Drug Administration (FDA) in 2020 based on the SOPHIA study.13 Currently, for patients with heavily pretreated HER2 metastatic disease who remain candidates for systemic therapies, options include at least one HER2-targeted agent with chemotherapy and are often selected based on previous treatments, location of metastatic disease, comorbidities, as well as physician and patient’s choice. Here we review the rationale for the development of margetuximab, previous and ongoing clinical trials and current role in clinical practice.
Pharmacology
Basic Pharmacology
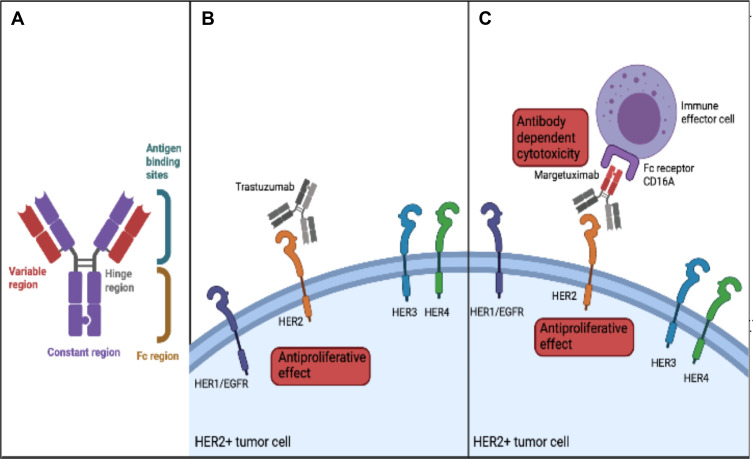
Margetuximab (MGAH22, margetuximab-cmkb) is a human/mouse chimeric and Fc-engineered mAB directed to HER2.14 Margetuximab and trastuzumab bind the same epitope of the HER2 receptor with different affinities.14 The main difference between these drugs is a five amino acid substitution in the IgG1 Fc domain (Figure 1A). This leads to increased binding capacity to CD16A (FcγRIIIA) and reduced binding capacity to CD32B (FcγRIIB) which, as discussed below, leads to improved ADCC.14–16 In this way, while maintaining trastuzumab’s antiproliferative effects, margetuximab can enhance the activation of the innate and adaptive immune responses13–16 (Figure 1B and C).
Figure 1.
Mechanism of action of margetuximab. (A) Structure of margetuximab (B). Mechanism of action of trastuzumab (C). Mechanism of action of margetuximab.
Margetuximab is administered as an intravenous infusion. The recommended dose for the treatment of patients with metastatic HER2-positive disease is 15 mg/kg every three weeks.17 The initial dose is administered over two hours and the subsequent infusions are administered over 30 minutes.17 There are no dose reductions recommended for patients with mild to moderate renal or hepatic impairment.17 The exposure-response of margetuximab remains unknown. Like other mAB, margetuximab is metabolized into smaller peptides by various proteases.17
Fc Receptor
The Fcγ receptor family consists of one inhibitory and several activating receptors that are expressed through the hematopoietic system.16 Activating receptors on effector cells, such as natural killer cells or macrophages, release inflammatory cytokines and initiate antibody dependent cytotoxicity (ADCC).16,18 Margetuximab has increased binding capacity to the activating receptor CD16A (FcγRIIIA) and decreased binding to CD32B (FcγRIIB), an inhibitory receptor in hematopoietic cells, with the goal to optimize ADCC.15,16
Single nucleotide polymorphisms (SNPs) in the coding regions of Fcγ receptor genes have been associated with differential affinities that can affect the immune and clinical response to several IgG1 mAB, including trastuzumab and margetuximab.14,18 CD16A is encoded by two alleles that are known to affect IgG binding.18,19 For example, a variant in the extracellular domain of FcγRIIIa can affect binding capacity with a high affinity valine variant (V158) and a lower affinity phenylalanine (F158) variant- of note, around 85% of the population are F158 carriers.18,19 Another example is in the FcγRIIa extracellular domain in which the histidine (H131) variant confers higher affinity than the arginine (R131) variant.18
In vitro studies have demonstrated that trastuzumab-induced ADCC was higher in mononuclear cells homozygous for V158 relative to other genotypes; aditionally, a correlation between Fcγ SNPs and clinical response to trastuzumab has also been reported.20,21 A study of 54 patients with HER2+ metastatic breast cancer treated with trastuzumab and a taxane, revealed that those homozygous for the V158 genotype had improved objective response rate and progression-free survival (PFS) when compared to other genotypes.21 Another study included 35 patients receiving trastuzumab containing regimens in the neoadjuvant or metastatic settings showed that patients homozygous for the H131 allele had better pathologic and objective responses relative to other genotypes.22
Allelic variations of the Fcγ receptor have been assessed as secondary endpoints of some clinical trials with margetuximab (Table 1). Preclinical data showed that margetuximab led to better ADCC in the low-binding allele F158.14 The Phase 3 SOPHIA trial compared margetuximab plus chemotherapy to trastuzumab plus chemotherapy in patients with metastatic HER2+ breast cancer.13 An exploratory analysis of SOPHIA assessed allelic variations of the Fcγ receptor and showed that the presence of an F158 allele may predict response to margetuximab. In addition, these patients were also found to have a numerical improvement in overall survival (OS) and PFS with margetuximab relative to trastuzumab. Conversely, margetuximab provided no clinical benefit for patients homozygous for V158, which is opposite to that seen in the trastuzumab studies. Although pharmacogenomic testing is not done in the current clinical practice, one should consider these variants when designing therapies enhancing immune response, such as margetuximab.
Table 1.
Published and Ongoing Clinical Trials with Margetuximab (Clinicaltrials.gov as of December 8, 2021)
| Study Name (NCT Identifier) | Phase | Sample Size | Design | Objectives | Status |
|---|---|---|---|---|---|
| Breast cancer | |||||
| Margetuximab or trastuzumab (MARGOT- NCT04425018) | Phase 2 | 171 | Randomized study comparing neoadjuvant paclitaxel and pertuzumab in combination with margetuximab or trastuzumab for stage II–III HER2+ breast cancer | pCR, residual cancer burden, radiologic response rate, adverse events, EFS, RFI, OS | Recruiting |
| Study to evaluate pharmacokinetics of margetuximab in Chinese patients with HER2+ metastatic breast cancer (NCT04398108) | Phase 1 | 16 | Single-arm study of margetuximab in combination of capecitabine, vinorelbine, gemcitabine in metastatic HER2+ breast cancer | Pharmacokinetic studies (maximal concentration, time of maximal concentration, area under the curve and half-life) | Completed |
| Study to evaluate efficacy and safety of margetuximab in Chinese patients with HER2+ metastatic breast cancer (NCT04262804) | Phase 2 | 120 | Randomized trial comparing trastuzumab plus chemotherapy (capecitabine, vinorelbine, gemcitabine) vs margetuximab plus chemotherapy in metastatic HER2+ breast cancer | PFS, OS, ORR, DoR, CBR, allelic variation of Fc-receptor | Recruiting |
| Margetuximab in relapsed or refractory advanced breast cancer (NCT01828021) | Phase 2 | 25 | Single arm assessing margetuximab as monotherapy in advanced HER2+ breast cancer | Best overall response, response rate | Completed |
| Margetuximab plus chemotherapy vs trastuzumab plus chemotherapy in the treatment of HER2+ metastatic breast cancer (SOPHIA - NCT02492711) | Phase 3 | 624 | Randomized trial comparing trastuzumab plus chemotherapy (capecitabine, vinorelbine, gemcitabine, eribulin) vs margetuximab plus chemotherapy in metastatic HER2+ breast cancer | PFS, OS, incidence of infusion reactions | Active, not recruiting |
| Other malignancies | |||||
| Margetuximab in HER2+ carcinomas (NCT01148849) | Phase 1 | 66 | Single arm assessing margetuximab as monotherapy in HER2+ breast and gastric cancer | Adverse events, maximum tolerated dose | Active, not recruiting |
| MGD013 in patients with unresectable metastatic neoplasms (NCT03219268) | Phase 1 | 353 | Dose escalation and cohort expansion study assessing MGD013 (PD-L1 and LAG3 DART molecule) in advanced solid tumors. Will include a cohort of 99 patients with HER2+ tumors (including breast and gastric) |
Adverse events, maximum tolerated dose, pharmacokinetic studies, percentage of patients with anti-drug antibody, OS | Active, not recruiting |
| Margetuximab and pembrolizumab for advances HER2+ gastric or GEJ cancer (NCT02689284) | Phase 1/2 | 95 | Single-arm study with margetuximab and pembrolizumab | Maximal tolerated dose of margetuximab when given with pembrolizumab, investigate anti-tumor activity per RECIST and ORR | Completed |
| Margetuximab in combination with INCMGA00012 and chemotherapy or MGD013 and chemotherapy in metastatic HER2+ Gastric or GEJ Cancer (MAHOGANY— NCT04082364) | Phase 2/3 | 860 | Randomized study with 5 arms: (1) margetuximab plus INCMGA00012 (anti-PD-L1) (2) margetuximab, INCMGA00012 plus standard chemotherapya (3) margetuximab plus MGD013 (PD-L1 and LAG3 DART molecule) (4) margetuximab, MGD013 plus standard chemotherapy (5) Trastuzumab plus standard chemotherapy |
Adverse events, ORR, OS, PFS, DoR, DCR, patient reported quality of life | Recruiting |
Note: aXELOX and mFOLFOX.
Abbreviations: HER2, human epidermal growth factor receptor 2; PCR, pathologic complete response; EFS, event free survival; RFI, recurrence-free interval; PFS, progression-free survival; OS, overall survival, ORR, objective response rate; DoR, duration of response; CBR, clinical benefit rate; GEJ, gastroesophageal junction; RECIST, response evaluation criteria in solid tumors; PD-L1, program cell death ligand 1.
It remains unclear why patients with higher affinity variants did not benefit from margetuximab. One possible explanation is that the poor prognosis features were not balanced between the groups. For example, a higher percentage of patients in the V158 homozygous group had brain metastases, similarly, patients older than 60 years and with two or more prior lines of therapy were more likely to be V158 homozygous. Allelic variation testing for CD16A is not commercially available and it was not an integral biomarker in the design of the study that led to the FDA approval of margetuximab. Therefore, it is not recommended to test patients prior to initiation of therapy. Ongoing studies such as the MARGOT trial (NCT04425018) are selecting patients with low affinity alleles to receive margetuximab or trastuzumab and are likely to provide more information about the role for upfront allelic variation testing.
Drug Development
Animal models revealed that margetuximab lead to enhanced ADCC against HER2+ tumor cells when compared to trastuzumab, including cells resistant to trastuzumab in mice transgenic for human CD16A-F158.14 In addition, studies in monkeys revealed that margetuximab was well tolerated and had similar pharmacokinetic parameters to other HER2-targeted mABs.14
A first in-human, multicenter, Phase 1 (NCT01148849) dose escalation and dose expansion study enrolled 66 patients (including 27 with breast cancer) with HER2+ advanced solid tumors.23 In this study two regimens with different schedules of single agent margetuximab were administered: (1) 0.3, 1.0, 3.0, and 6.0 mg/kg weekly or (2) 10, 15, 18 mg/kg every three weeks. A maximum tolerated dose was not reached in either group. Among 24 patients with breast cancer evaluable for response, 11 (48%) experienced tumor reduction with confirmed responses in four patients (17%) and three patients continue on monotherapy margetuximab for four years.24 Treatment was overall well tolerated with mostly grade 1–2 adverse events (AE). The only grade 3 or higher AEs noted were infusion reaction (n=1), lymphopenia (n=2), increase in lipase (n=1), amylase (n=2) and alkaline phosphatase (n=1). No events of cardiomyopathy were observed. Given the high rate of infusion reactions, it is recommended to consider premedications with antihistamines, corticosteroids and antipyretics for patients with mild to moderate infusion-related reactions.17 In the menitioned study, peripheral blood mononuclear cells were isolated from patients treated with the weekly regimen prior to the fourth infusion. Margetuximab ADCC activity was compared with trastuzumab ex vivo and margetuximab demonstrated greater cytotoxicity which persisted after therapy; this was measured by ex vivo isolation of peripheral blood mononuclear cells at baseline and at day 22. Aditionally, asingle arm, open label, Phase 2 (NCT01828021) study is ongoing to assess the efficacy of margetuximab in patients with pretreated advanced HER2+ breast cancer.25 The results of this study are awaited.
The Phase 3 SOPHIA (NCT02492711) study randomized 536 patients with pretreated advanced HER2+ breast cancer to receive margetuximab with chemotherapy or trastuzumab plus chemotherapy.13 Patients treated with margetuximab had an improvement in PFS when compared to trastuzumab (5.8 vs 4.9 months, respectively, HR: 0.76, 95%CI: 0.59–0.98, p=0.03). Although statistically significant, the PFS improvement was modest, particularly when compared to other novel agents which have shown to be more effective in patients with heavily pretreated HER2+ disease. In SOPHIA, there was also a numerical improvement in OS, with a median OS of 21.6 months with margetuximab and 19.8 with trastuzumab (HR: 0.89, 95%CI: 0.69–1.13, p=0.33). Patients treated with margetuximab had a numerically higher objective response rate and clinical benefit ratio when compared to trastuzumab. The central blinded objective response rate was 58% in the margetuximab arm and 42% in the trastuzumab arm (p=0.0597). The clinical benefit ratio was 95% and 65%, respectively (p=0.0026). The duration of response was similar between the arms (6.1 vs 6.0 months). In terms of toxicity, infusion reactions were more common in the margetuximab arm 13.3 vs 3.4%, with 1.5% being grade 3 or higher. Grade 3 decrease in left ventricular ejection fraction (LVEF) occurred in 3% of each arm. Only patients with low-affinity CD16A-F158 derived benefit from margetuximab. The SOPHIA trial lead to the approval of margetuximab by the US FDA in December of 2020; margetuximab was approved in combination with chemotherapy for patients with metastatic HER2+ breast cancer who have received two or more HER2-targeted regimens, including at least one for metastatic disease (Figure 2). A recent presentation of the final OS analysis, revealed that the median OS in the intention to treat population was not statistically significantly different between the margetuximab and trastuzumab arms (21.6 vs 21.9 months, HR: 0.95, 95%CI: 0.77–1.17, p=0.62).26 Amongst patients with a CD16A-158F low affinity allele, margetuximab prolonged median OS compared to trastuzumab (23.3 vs 20.8 months, HR: 0.86, 95%CI: 0.69–1.08, p=0.19). Patients homozygous for the F allele had a greater benefit with margetuximab with a median OS of 23.6 vs 19.2 months (HR: 0.72, 95% CI: p=0.05). Similar to what was reported before, patients that were VV homozygotes had a median OS of 31.1 months with trastuzumab and 22 months with margetuximab (HR: 1.77, 95%CI: 101–3.12, p=0.04). It remains unclear if these findings will lead to modifications in the current indications of margetuximab from the regulatory standpoint.
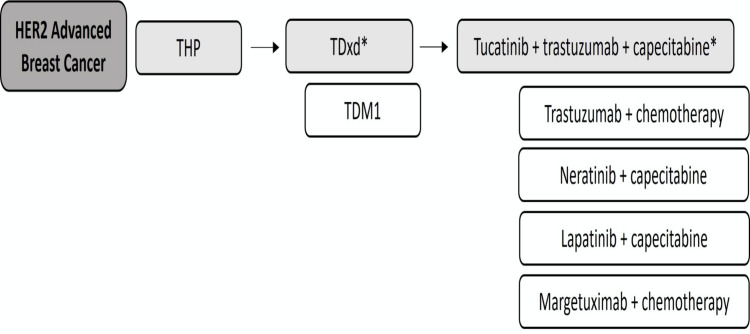
Figure 2.
Proposed treatment algorithm for patients with metastatic HER2-positive breast cancer.
Notes: *Preferred. Patients with CNS involvement must be evaluated for local therapy. TDxd and tucatinib+trastuzumab+capecitabine are preferred agents when CNS penetration of a systemic drug is desirable.
Abbreviations: THP, taxane, trastuzumab and pertuzumab; TDM1, ado-trastuzumab emtansine; TDxd, fam-trastuzumab deruxtecan.
Margetuximab in Other HER2-positive Malignancies
There is growing interest in assessing the role of margetuximab in several HER2+ malignancies. The aforementioned Phase 1 study with margetuximab monotherapy included patients with HER2+ gastroesophageal, colorectal, gall bladder and lung cancer.23 A single arm, Phase 1/2 study (CP-MGAH22-05, NCT02689284) evaluated the safety and efficacy of margetuximab in combination with pembrolizumab in patients with previously treated HER2+ gastroesophageal adenocarcinoma.27 In the expansion phase, 86 patients received margetuximab 15 mg/kg plus pembrolizumab 200 mg every three weeks. At a median follow-up of 19.9 months, the combination showed acceptable safety and tolerability. The PFS was 2.7 months and the median OS 12.5 months; no significant differences were seen in subgroup analyses by program cell death ligand 1 (PD-L1) status or CD16A genotype. Objective responses were seen in 18% of patients and 53% of patients achieved disease control. When assessing AE, 44% of patients developed grade 3, 4% grade 4 toxicities, and there were 3 (3%) deaths reported. The most common grade 3–4 AEs were anemia (18%) and vomiting (5%). Finally, the ongoing MAHOGANY study (NCT04082364) is a Phase 2/3 assessing the role of margetuximab in combination with several novel immunotherapy agents with or without chemotherapy in advanced gastric or gastroesophageal junction adenocarcinoma.28 Margetuximab has been shown to enhance in vitro immune effector cell activation and LAG-3, PD-1 and PD-L1 expression. This led to an expansion cohort of patients with advanced HER2-positive tumors that were treated with margetuximab and MGD013, a bispecific DART molecule binding PD-1 and LAG3.29 The MAHOGANY trial will provide additional information regarding the potential synergistic effect of immune checkpoint inhibitors and margetuximab in HER2+ gastrointestinal malignancies.27
Clinical Applications and Ongoing Trials
The MARGOT trial (NCT04425018) is investigating the role of margetuximab, pertuzumab and a taxane vs trastuzumab, pertuzumab and a taxane for 12 weeks in the neoadjuvant setting in stage II–III HER2+ breast cancer followed by margetuximab or trastuzumab with pertuzumab in the adjuvant setting to complete a total of one year. The MARGOT trial is limited to patients with CD16A F158 genotype. This study will provide information about de-escalation of therapy in early stage disease.13
There is growing interest in understanding the role of immune checkpoint inhibitors (ICIs) in HER2+ breast cancer. The single arm, Phase 1/2 PANACEA study assessed the role of pembrolizumab plus trastuzumab in trastuzumab resistant advanced HER2+ breast cancer. This study showed safety and durable responses only in the subgroup with PD-L1+ disease.30 Similarly, the Phase 2 KATE2 study revealed that only patients with PD-L1+ disease derived benefit from atezolizumab and ado-trastuzumab emtansine.31 Conversely the IMpassion050, a Phase 3 study that assessed HER2 targeted therapy, chemotherapy with or without atezolizumab in the neoadjuvant setting, showed no increase of pathologic complete response with the addition of atezolizumab, regardless of the PD-L1 status.32 There are currently no ICIs approved for the treatment of HER2+ breast cancer, although there are ongoing studies assessing the role of ICIs in selected HER2+ and PD-L1+ tumors. The combination of margetuximab with ICIs represents an attractive option to enhance immunity of tumors traditionally considered immunologically quiescent especially since safety data are available for this combination in gastrointestinal malignancies.27
Several molecules are currently being investigated in HER2 positive breast cancer, including novel antibody drug conjugates (ADC) and tyrosine kinase inhibitors (TKI), CDK4/6 inhibitors and alpha-specific PI3K inhibitors. There may be an opportunity for clinical trials exploring novel therapeutic combinations of margetuximab with some of these agents.
Conclusion
Margetuximab is a chimeric and Fc-optimized mAB directed to HER2 currently approved in combination with chemotherapy for the treatment of advanced HER2+ breast cancer. This drug represents another option for the treatment of patients with metastatic disease. Ongoing studies will help determine the role of margetuximab in the treatment of breast cancer, including in the neoadjuvant setting, and in combination with other agents beyond chemotherapy.
Disclosure
Dr Filipa Lynce reports personal fees from AstraZeneca, Daiichi, ION, and BMS, outside the submitted work. The authors report no other potential conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 3.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):63–69. doi: 10.3816/CBC.2004.n.011 [DOI] [PubMed] [Google Scholar]
- 4.Vogel C, Cobleigh MA, Tripathy D, et al. First-line, single-agent Herceptin (R) (trastuzumab) in metastatic breast cancer. Eur J Cancer. 2001;37(Suppl 1):25–29. doi: 10.1016/S0959-8049(00)00405-6 [DOI] [PubMed] [Google Scholar]
- 5.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2019, 382:610–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi: 10.1056/NEJMoa1914609 [DOI] [PubMed] [Google Scholar]
- 7.Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0 [DOI] [PubMed] [Google Scholar]
- 8.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. doi: 10.1093/annonc/mdu486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortés JKS, Chung W, Im S, Park YH. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized Phase III DESTINY-Breast03 study. Ann Oncol. 2021;32(suppl_5):S1283–S1346. doi: 10.1016/j.annonc.2021.08.2087 [DOI] [Google Scholar]
- 12.Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in her2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020; 38. 3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rugo HS, Im SA, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:573. doi: 10.1001/jamaoncol.2020.7932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13(6):R123. doi: 10.1186/bcr3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67(18):8882–8890. doi: 10.1158/0008-5472.CAN-07-0696 [DOI] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206 [DOI] [PubMed] [Google Scholar]
- 17.MARGENZA. MARGENZA (Margetuximab-Cmkb) [Package Insert]. Rockville, MD: MacroGenics Inc; 2020. [Google Scholar]
- 18.Musolino A, Naldi N, Dieci MV, et al. Immunoglobulin G fragment C receptor polymorphisms and efficacy of preoperative chemotherapy plus trastuzumab and lapatinib in HER2-positive breast cancer. Pharmacogenomics J. 2016;16(5):472–477. doi: 10.1038/tpj.2016.51 [DOI] [PubMed] [Google Scholar]
- 19.Lehrnbecher T, Foster CB, Zhu S, et al. Variant genotypes of the low-affinity fcgamma receptors in two control populations and a review of low-affinity fcgamma receptor polymorphisms in control and disease populations. Blood. 1999;94(12):4220–4232. doi: 10.1182/blood.V94.12.4220.424k08_4220_4232 [DOI] [PubMed] [Google Scholar]
- 20.Koene HR, Kleijer M, Algra J, Roos D. von Dem Borne AE, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–1114. [PubMed] [Google Scholar]
- 21.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957 [DOI] [PubMed] [Google Scholar]
- 22.Tamura K, Shimizu C, Hojo T, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22(6):1302–1307. doi: 10.1093/annonc/mdq585 [DOI] [PubMed] [Google Scholar]
- 23.Bang YJ, Giaccone G, Im SA, et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann Oncol. 2017;28(4):855–861. doi: 10.1093/annonc/mdx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im S-A, Bang Y-J, Oh D-Y, et al. Abstract P6-18-11: long-term responders to single-agent margetuximab, an Fc-modified anti-HER2 monoclonal antibody, in metastatic HER2+ breast cancer patients with prior anti-HER2 therapy. Cancer Res. 2019;79(4 Supplement):P6–18–1–P6–1. [Google Scholar]
- 25.Pegram MD, Miller K, Tan-Chiu E, et al. A single-arm, open-label, phase 2 study of MGAH22 (margetuximab) [fc-optimized chimeric anti-HER2 monoclonal antibody (mAb)] in patients with relapsed or refractory advanced breast cancer whose tumors express HER2 at the 2+ level by immunohistochemistry and lack evidence of HER2 gene amplification by FISH. J Clin Oncol. 2014;32(15_suppl):TPS671–TPS671. [Google Scholar]
- 26.Rugo H, Cardoso F, Cortes J, Curigliano G. Phase 3 SOPHIA study of margetuximab (M) + chemotherapy (CTX) vs trastuzumab (T) + CTX in patients (pts) with HER2+ metastatic breast cancer (MBC) after prior anti-HER2 therapies: final overall survival (OS) analysis. SABCS; 2021.
- 27.Catenacci DVT, Kang YK, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21(8):1066–1076. doi: 10.1016/S1470-2045(20)30326-0 [DOI] [PubMed] [Google Scholar]
- 28.Catenacci DV, Rosales M, Chung HC, et al. MAHOGANY: margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17(10):1155–1164. doi: 10.2217/fon-2020-1007 [DOI] [PubMed] [Google Scholar]
- 29.Luke JJ, Patel MR, Hamilton EP, et al. A Phase I, first-in-human, open-label, dose-escalation study of MGD013, a bispecific DART molecule binding PD-1 and LAG-3, in patients with unresectable or metastatic neoplasms. J Clin Oncol. 2020;38(15_suppl):3004. doi: 10.1200/JCO.2020.38.15_suppl.3004 [DOI] [Google Scholar]
- 30.Loi S, Giobbie-Hurder A, Gombos A, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019;20(3):371–382. doi: 10.1016/S1470-2045(18)30812-X [DOI] [PubMed] [Google Scholar]
- 31.Emens LA, Esteva FJ, Beresford M, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21(10):1283–1295. doi: 10.1016/S1470-2045(20)30465-4 [DOI] [PubMed] [Google Scholar]
- 32.Huober BC, Niilkura N, Niikura N, et al. VP6-2021: iMpassion050: a phase III study of neoadjuvant atezolizumab + pertuzumab + trastuzumab + chemotherapy (neoadj A + PH + CT) in high-risk, HER2-positive early breast cancer (EBC). Ann Oncol. 2021;32(8):1061–1062. doi: 10.1016/j.annonc.2021.05.800 [DOI] [Google Scholar]
- 33.Cortés J, Kim SB, Chung WP, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022. 386(12):1143–1154. doi: 10.1056/NEJMoa2115022 [DOI] [PubMed] [Google Scholar]