Abstract
Purpose
To describe trends in the use of corneal topography and tomography for the management of keratoconus patients at a single academic institution and to identify how these trends may be associated with different procedural interventions.
Patients and Methods
In this retrospective cohort study, keratoconus patients treated from 2012 to 2019 were identified. The electronic health record was reviewed for the presence of corneal topography or tomography imaging completed within seven days of the first visit and the highest level of intervention at the first and most recent visit. Patients were grouped as pediatric (<18 years) or adult (≥18 years). Chi-square tests and linear regressions were used to evaluate trends and to determine which factors were predictive for receiving corneal collagen cross-linking (CXL) versus other surgical interventions (intrastromal corneal ring segments, lamellar keratoplasty, or penetrating keratoplasty) by the most recent visit.
Results
A total of 873 keratoconus patients met inclusion criteria. The use of corneal topography at the first visit remained relatively consistent from 2012 to 2019, while corneal tomography usage at the first visit increased from 3.9% in 2015, when corneal tomography was introduced, to 52.8% in 2019. Each year was associated with an 11.2% ± 1.9% increase in the use of corneal tomography at the first visit in pediatric patients and 6.7% ± 0.5% in adult patients. Use of corneal tomography at the first visit was a significant predictor for receiving CXL procedures (P < 0.001) and a negative predictor for requiring other surgical interventions (P = 0.032) when controlling for the year of the first visit.
Conclusion
Obtaining corneal tomography at the first visit has become the standard of care in keratoconus, especially for pediatric patients. Moreover, the increase in corneal tomography imaging has coincided with an increase in CXL procedures and decrease in other surgical interventions.
Keywords: keratoconus, topography, tomography, corneal collagen cross-linking, keratoplasty
Introduction
Keratoconus is a bilateral, progressive corneal ectasia which can result in irregular astigmatism and vision loss.1,2 The prevalence of keratoconus has been estimated to be between 0.17 per 1000 in the United States Medicare population to 1.38 per 1000 in the total global population,3,4 although additional epidemiological studies have demonstrated that the prevalence may be even greater than these estimates.5,6 Keratoconus commonly develops in the second or third decade of life, and associated risk factors include atopic disease, family history of keratoconus, and eye rubbing.4,7,8 Some have suggested that keratoconus is more prevalent in males,9 but this is not consistent across all studies.3,6 Furthermore, socioeconomic status has been shown to be a significant predictor of keratoconus severity and progression.10
Early diagnosis and management of keratoconus is imperative to prevent vision loss and improve visual outcomes. Current diagnostic imaging modalities include corneal topography and tomography, which are used in tandem with corneal biomechanical measurements and epithelial thickness mapping.11 Corneal topography uses Placido disc reflection to identify anterior corneal curvature.12 Corneal tomography uses either Scheimpflug imaging or optical coherence tomography (OCT) to measure both the anterior and posterior elevation, as well as corneal thickness,12 which allows for improved detection of mild ectasia.13,14 To our knowledge, no prior studies have indicated the prevalence of corneal topography and tomography usage in ophthalmology practices with keratoconus patients, nor the outcomes associated with using corneal tomography. In this study, we describe the prevalence of corneal topography and tomography usage and the associated patient outcomes, namely the need for corneal collagen cross-linking (CXL) or other surgical interventions (intrastromal corneal ring segments, lamellar keratoplasty, or penetrating keratoplasty), at a single academic institution.
Methods
This study was approved by the Institutional Review Board at the University of California San Francisco (UCSF) and was conducted in accordance with the Declaration of Helsinki. All accessed data remained in compliance with data protection and privacy regulations. All patients with an International Classification of Diseases (ICD) diagnosis of keratoconus (ICD-9 code 371.6, ICD-10 code H18.609) were retrospectively identified and screened for eligibility. Clinical documentation of keratoconus and complete data on one or more of the study outcomes were required for inclusion. The retrospective period dated from 2012, when Epic (Verona, Wisconsin) was implemented as the electronic health record at UCSF, to 2019. The electronic health record was reviewed for clinical characteristics including age, sex, race, and underlying developmental disability. All characteristics were collected from the first visit for each patient. Age was categorized into pediatric patients (age <18 years) and adults (age ≥18 years).
The primary outcome was defined as the presence of corneal topography (Tomey TMS-4, Phoenix, AZ) and/or tomography (Oculus Pentacam, Wetzlar, Germany) imaging within ±7 days of the first and most recent visits. The decision over which corneal imaging modality was chosen in the evaluation of keratoconus patients was per the attending physician’s preference. Interventions for keratoconus were defined as no specific intervention, rigid gas permeable (RGP) or scleral lenses, corneal collagen cross-linking (CXL), intrastromal corneal ring segments (ICRS), lamellar keratoplasty, and penetrating keratoplasty. The interventions were grouped as nonprocedural (no specific interventions and RGP or scleral lenses), CXL, and other surgical interventions (ICRS, lamellar keratoplasty, and penetrating keratoplasty) for statistical analysis.
Statistical analyses were performed using a Chi-square test to evaluate differences between categorical variables, univariate linear regression to evaluate the association between predictor variables and corneal imaging modality, and multivariate analysis to identify differences in interventions. Significance was defined as P<0.05. Analyses were performed using R software version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
1746 eyes from 1038 individuals were identified with an ICD-9 or ICD-10 diagnosis of keratoconus. Of these individuals, 873 (84.1%) had clinical documentation confirming the diagnosis of keratoconus and a documented first visit date. In this cohort, 791 (90.6%) were adults and 82 (9.4%) were pediatric patients at the time of their first visit. Fifty-seven (6.5%) had a clinical diagnosis of some form of developmental disability, 55 of whom (96.5%) were adults at the time of their first visit. Additional demographic information is included in Table 1.
Table 1.
Clinical and Demographic Characteristics of Keratoconus Patients
| Parameters | Total KCN Patients (N=873) | Adult KCN Patients (N=791) | Pediatric KCN Patients (N=82) |
|---|---|---|---|
| Diagnosis age (years ± SD) | 40.3 ± 17.1 | 43.0 ± 15.7 | 14.9 ± 2.5 |
| Female (n, %) | 295 (33.8) | 266 (33.6) | 29 (35.4) |
| Race (n, %) | |||
| Caucasian | 360 (41.2) | 343 (43.4) | 17 (20.7) |
| Black | 77 (8.8) | 74 (9.4) | 3 (3.7) |
| Asian | 67 (7.7) | 57 (7.2) | 10 (12.2) |
| Other | 244 (27.9) | 206 (26.0) | 38 (46.3) |
| Unknown/declined | 125 (14.3) | 111 (14.0) | 14 (17.1) |
| Developmental disability (n, %) | 57 (6.5) | 55 (7.0) | 2 (2.4) |
| Tomography first visit (n, %) | 210 (24.1) | 178 (22.5) | 32 (39.0) |
| Topography first visit (n, %) | 450 (51.5) | 408 (51.6) | 42 (51.2) |
Abbreviation: KCN, keratoconus.
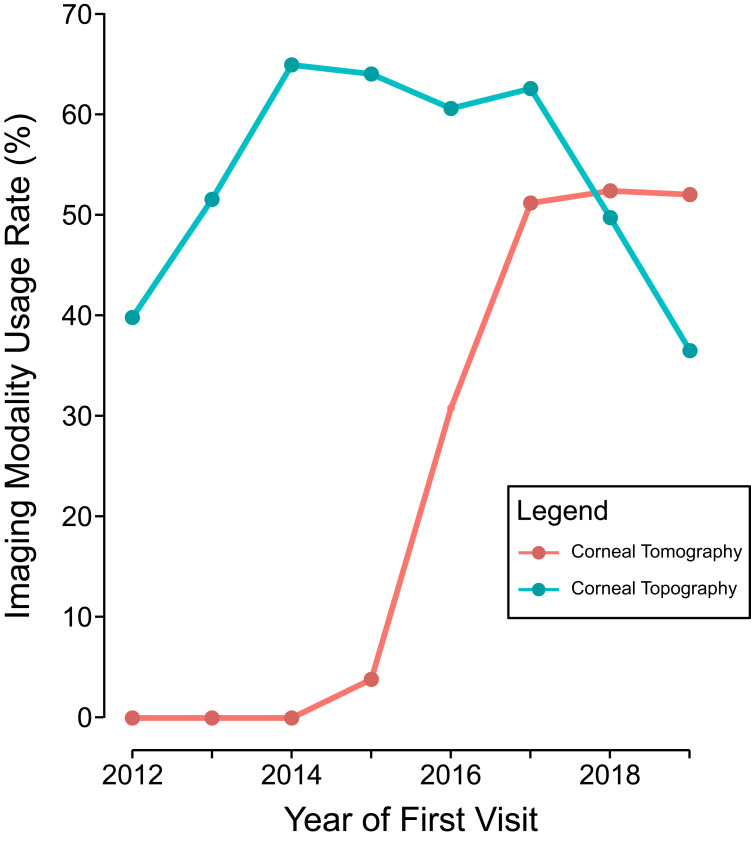
Corneal tomography was introduced at this institution in February 2015. The percentage of patients receiving corneal tomography at the first visit increased from 3.9% in 2015 to 52.8% by the year 2019, whereas the rate of corneal topography usage at the first visit remained steady (40.4% in 2012 and 37.0% in 2019, see Figure 1). In total, 39.0% of pediatric patients received corneal tomography imaging within 7 days of the first visit compared to 22.5% of adult patients (P=0.001). The use of corneal topography imaging at the first visit was more similar between pediatric and adult patients at 51.2% and 51.6% (P=1.00) respectively. Between 2012 and 2019, there was an 11.2% ± 1.9% increase in corneal tomography usage per year (P<0.001) for pediatric patients. In adults, corneal tomography usage at the first visit increased by 6.7% ± 0.5% per year (P<0.001). When comparing rates of imaging in patients with developmental disabilities, 17.5% had corneal tomography at the first visit compared to 24.6% of those without developmental disabilities (P=0.30). 50.9% of patients with developmental disabilities had corneal topography, and 51.5% of patients without developmental disabilities had corneal topography (P=1.00). In patients with developmental disabilities, corneal tomography at the first visit increased by 7.5% ± 1.8% per year (P<0.001), compared to 7.0% ± 4.6% growth in usage in patients without developmental disabilities (P<0.001).
Figure 1.
Change in the rates of corneal topography and corneal tomography usage near the first visit over time. Corneal topography usage at the first visit stayed relatively constant from 2012 to 2019, whereas corneal tomography usage increased significantly since it was introduced in February 2015. Imaging near the first visit was defined as having either corneal topography or tomography done ±7 days from the first visit.
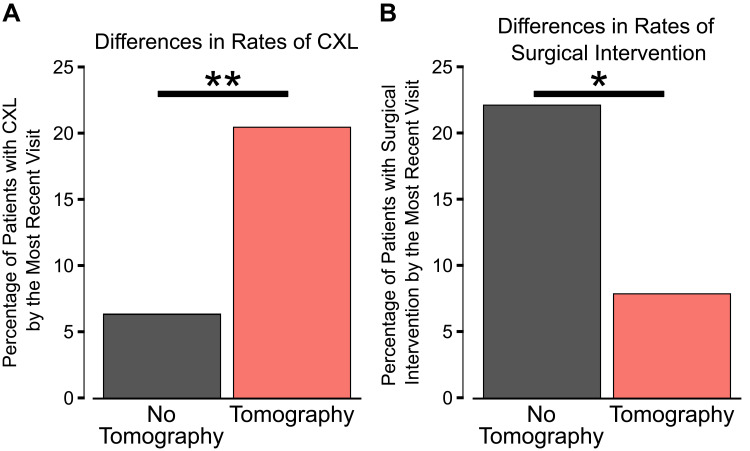
Of the 1746 eyes that met our inclusion criteria, 405 (23.2%) had corneal tomography completed on the first visit. Multivariate analysis showed that corneal tomography at the first visit was a significant predictor for receiving CXL (β=9.9%, Standard error 1.8%, P<0.001), while corneal topography was a significant negative predictor for CXL (β=−2.7%, 1.4%, P=0.047) when controlling for the year of the first visit (Figure 2A and Table 2). Corneal tomography at the first visit also a significant negative predictor for requiring other surgical interventions (β=−5.4%, 2.5%, P=0.032), while corneal topography at the first visit was not significantly associated with a change in the rate of requiring other surgical interventions (β=−3.2%, 1.9%, P=0.088) when controlling for the year of the first visit (Figure 2B and Table 2).
Figure 2.
Differences in interventions by the most recent visit for patients who did receive corneal tomography near the first visit compared to those who did not. (A) The percentage of patients with corneal collagen cross-linking (CXL) was significantly greater in those who did receive corneal tomography imaging near the first visit (P<0.001). (B) In contrast, the percentage of patients requiring another surgical intervention for keratoconus (defined as having ICRS, lamellar keratoplasty, or penetrating keratoplasty) was significantly lower in patients who did have corneal tomography near the first visit compared to patients without tomography (P=0.048). Statistics: Multivariate linear regression controlling for year of the first visit. *P<0.05, **P<0.001.
Table 2.
Association of Imaging Modality and Intervention
| Variable | CXL (β Coefficient, %) | SE (%) | P value |
|---|---|---|---|
| Topography first visit | −2.7 | 1.4 | 0.047* |
| Tomography first visit | 9.9 | 1.8 | <0.001** |
| Pediatric patient | 23.5 | 2.3 | <0.001** |
| Developmental disability | 6.2 | 2.7 | 0.022* |
| Year of first visit | 0.004 | 0.001 | <0.001** |
| Variable | Surgical Intervention† (β Coefficient, %) | SE (%) | P value |
| Topography first visit | −3.2 | 1.9 | 0.088 |
| Tomography first visit | −5.4 | 2.5 | 0.032* |
| Pediatric patient | −11.9 | 3.2 | <0.001** |
| Developmental disability | 0.6 | 3.7 | 0.862 |
| Year of first visit | −0.006 | 0.001 | <0.001** |
Notes: *P<0.05; **P<0.001. †Surgical interventions are defined as intrastromal corneal ring segments, lamellar keratoplasty, or penetrating keratoplasty.
Abbreviation: CXL, corneal collagen cross-linking.
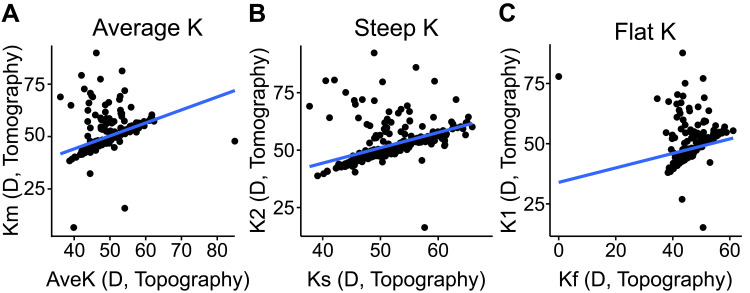
When considering keratometry for patients who had corneal topography and corneal tomography within 30 days from one another for the first visit (690 eyes, 39.5%), the Km from corneal tomography had a significant correlation with the AveK from corneal topography with an adjusted R2 = 0.16 (P<0.001, Figure 3A). Likewise, the steep K and flat K from corneal topography also correlated with the corneal tomography from the K2 (adjusted R2 = 0.21, P<0.001) and K1 (adjusted R2 = 0.04, P<0.001), respectively (Figures 3B and C).
Figure 3.
Correlation between keratometry measurements from corneal topography and corneal tomography for patients at their first visit. Patients with corneal topography and tomography within 30 days from each other were included (N=690 eyes). (A) The correlation between the average keratometry from corneal topography (AveK) and tomography (Km) had an adjusted R2 of 0.16 (P<0.001). (B) The adjusted R2 value for the correlation between the steep K from corneal topography (Ks) and tomography (K2) was 0.21 (P<0.001). (C) The adjusted R2 value for correlation between the flat K from corneal topography (Kf) and tomography (K1) was 0.04 (P<0.001). All K values are in diopters (D).
Discussion
Corneal topography is a mainstay in the evaluation of keratoconus with nearly half the patients in this study receiving one at their first visit and no significant change in its usage over time. However, the use of corneal tomography at a keratoconus patient’s initial visit has increased dramatically since 2015, when it was acquired at this academic institution. Interestingly, we have found that pediatric patients are more likely to receive corneal tomography imaging at their first visit than adult patients. Corneal tomography usage is also predictive of receiving CXL and for not receiving other surgical interventions needed to treat keratoconus.
Corneal tomography employs Scheimpflug imaging to create a three-dimensional structure of the cornea, which allows providers to evaluate the posterior surface and corneal pachymetry in addition to the anterior surface. This gives providers a more adept detection of even mild corneal ectasia.13,14 In addition, the inclusion of the Belin-Ambrosio enhanced ectasia display on the Oculus Pentacam has also been shown to be more sensitive at detecting early keratoconus and mild progression.15,16 While prospective, longitudinal studies are required to fully evaluate the long-term outcomes of using corneal tomography, this study suggests that corneal tomography can be used to provide earlier CXL intervention to minimize keratoconus progression and later need for more invasive surgical intervention.
This study also demonstrates a good correlation between corneal topography and tomography measurements. Although the usage of Placido disc corneal topography generates a more accurate topographical representation of the anterior surface of the cornea,12,17 our study demonstrated a significant correlation between the average keratometry measures taken by corneal topography (AveK) versus tomography (Km) and between the steep and flat keratometry readings across both imaging modalities. A previous study showed good agreement between corneal topography and tomography, although topography tended to underestimate the steep K, flat K, and the AveK in eyes with keratoconus.18 Currently, one of the most widely used keratoconus grading systems, the Amsler-Krumeich classification system,19 relies solely on corneal topography. Newer proposed grading systems, such as the ABCD from Belin & Duncan,16,20 take into account best spectacle corrected visual acuity, both the anterior and posterior corneal surfaces, and corneal pachymetry, which can only be evaluated using corneal tomography. Thus, further work is required to better define keratoconus classification that incorporates advances in technology as well as limitations of both corneal topography and tomography.
At our institution, both corneal topography and tomography are billed under the same Current Procedural Treatment (CPT) code (92025). Therefore, disparities in healthcare coverage and fees did not contribute to the choice of one imaging modality over the other. However, these socioeconomic factors could prevent some patients from receiving timely diagnosis, which may result in delayed keratoconus treatment.10 As such, future studies should compare the cost-benefit of receiving corneal topography versus tomography.
One limitation in our study is that it describes the trends at only a single academic institution, limiting its generalizability. Furthermore, the data were collected retrospectively, which makes it difficult to fully appreciate changes in patients over time, as we have only two time points (first and last visit), and some patients were lost to follow up. In addition, the retrospective nature of this study limits our ability to make conclusive causal-effect relationships between imaging modalities and patient outcomes. We found patients who received corneal tomography imaging were more likely to undergo corneal collagen cross-linking, which may have contributed to the lower need for keratoplasties in this group.21–24 We believe this is because corneal tomography incorporates software to facilitate with earlier decision making for intervention, such as the Belin-Ambrosio enhanced ectasia display.16 However, the positive change in attitude towards CXL over the last decade, especially with shown benefit for early intervention in pediatric and developmentally delayed populations may have also contributed to this association.25,26 Longitudinal studies may evaluate whether improved corneal imaging provided by corneal tomography has allowed for quicker access to CXL.
In summary, corneal tomography has increasingly become the standard of care for keratoconus patients. We demonstrate that use of corneal tomography was associated with fewer invasive surgical interventions. Even though CXL usage also gained in popularity during this the study period due to evidence of its efficacy and safety, we also show a correlation between increased corneal tomography and CXL procedure. Finally, we highlight the correlation between metrics obtained by corneal topography and tomography to illustrate that future efforts to re-classify and define keratoconus should consider the strengths and limitations of both imaging modalities, as well as the cost to patients, to accurately diagnose keratoconus and to initiate proper treatment plans.
Acknowledgments
This project received funding from the University of California San Francisco (UCSF) Inquiry Funding Office and was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR001872. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This project was also made possible in part by unrestricted grant funding from the All May SeeTM Foundation (San Francisco, CA) and Research to Prevent Blindness (New York, NY) to the University of California San Francisco, Department of Ophthalmology.
Disclosure
Dr Neel D Pasricha reports consulting fees from iota Biosciences, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297–319. doi: 10.1016/s0039-6257(97 [DOI] [PubMed] [Google Scholar]
- 2.Andreanos KD, Hashemi K, Petrelli M, Droutsas K, Georgalas I, Kymionis GD. Keratoconus Treatment Algorithm. Ophthalmol Ther. 2017;6(2):245–262. doi: 10.1007/s40123-017-0099-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reeves SW, Ellwein LB, Kim T, Constantine R, Lee PP. Keratoconus in the Medicare population. Cornea. 2009;28(1):40–42. doi: 10.1097/ICO.0b013e3181839b06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi H, Heydarian S, Hooshmand E, et al. The Prevalence and Risk Factors for Keratoconus: a Systematic Review and Meta-Analysis. Cornea. 2020;39(2):263–270. doi: 10.1097/ICO.0000000000002150 [DOI] [PubMed] [Google Scholar]
- 5.Godefrooij DA, de Wit GA, Uiterwaal CS, Imhof SM, Wisse RPL. Age-specific Incidence and Prevalence of Keratoconus: a Nationwide Registration Study. Am J Ophthalmol. 2017;175:169–172. doi: 10.1016/j.ajo.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 6.Chan E, Chong EW, Lingham G, et al. Prevalence of Keratoconus Based on Scheimpflug Imaging: the Raine Study. Ophthalmology. 2021;128(4):515–521. doi: 10.1016/j.ophtha.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 7.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293–322. doi: 10.1016/0039-6257(84 [DOI] [PubMed] [Google Scholar]
- 8.Sahebjada S, Al-Mahrouqi HH, Moshegov S, et al. Eye rubbing in the aetiology of keratoconus: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2021;259(8):2057–2067. doi: 10.1007/s00417-021-05081-8 [DOI] [PubMed] [Google Scholar]
- 9.Woodward MA, Blachley TS, Stein JD. The Association Between Sociodemographic Factors, Common Systemic Diseases, and Keratoconus: an Analysis of a Nationwide Healthcare Claims Database. Ophthalmology. 2016;123(3):457–465.e2. doi: 10.1016/j.ophtha.2015.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad TR, Kong AW, Turner ML, et al. Socioeconomic Correlates of Keratoconus Severity and Progression. Cornea. 2022;1:34. doi: 10.1097/ICO.0000000000002993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanellopoulos AJ, Asimellis G. OCT corneal epithelial topographic asymmetry as a sensitive diagnostic tool for early and advancing keratoconus. Clin Ophthalmol Auckl NZ. 2014;8:2277–2287. doi: 10.2147/OPTH.S67902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan R, Chan TC, Prakash G, Jhanji V. Applications of corneal topography and tomography: a review. Clin Experiment Ophthalmol. 2018;46(2):133–146. doi: 10.1111/ceo.13136 [DOI] [PubMed] [Google Scholar]
- 13.Chan TCY, Biswas S, Yu M, Jhanji V. Comparison of corneal measurements in keratoconus using swept-source optical coherence tomography and combined Placido-Scheimpflug imaging. Acta Ophthalmol. 2017;95(6):e486–e494. doi: 10.1111/aos.13298 [DOI] [PubMed] [Google Scholar]
- 14.Ambrósio R, Valbon BF, Faria-Correia F, Ramos I, Luz A. Scheimpflug imaging for laser refractive surgery. Curr Opin Ophthalmol. 2013;24(4):310–320. doi: 10.1097/ICU.0b013e3283622a94 [DOI] [PubMed] [Google Scholar]
- 15.Belin MW, Ambrósio R. Scheimpflug imaging for keratoconus and ectatic disease. Indian J Ophthalmol. 2013;61(8):401–406. doi: 10.4103/0301-4738.116059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belin MW, Duncan JK. Keratoconus: the ABCD Grading System. Klin Monatsbl Augenheilkd. 2016;233(6):701–707. doi: 10.1055/s-0042-100626 [DOI] [PubMed] [Google Scholar]
- 17.Kanclerz P, Khoramnia R, Wang X. Current Developments in Corneal Topography and Tomography. Diagnostics. 2021;11(8):1466. doi: 10.3390/diagnostics11081466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penna RR, de Sanctis U, Catalano M, Brusasco L, Grignolo FM. Placido disk-based topography versus high-resolution rotating Scheimpflug camera for corneal power measurements in keratoconic and post-LASIK eyes: reliability and agreement. Int J Ophthalmol. 2017;10(3):453–460. doi: 10.18240/ijo.2017.03.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amsler M. [The “forme fruste” of keratoconus]. Wien Klin Wochenschr. 1961;73:842–843. German. [PubMed] [Google Scholar]
- 20.Belin MW, Kundu G, Shetty N, Gupta K, Mullick R, Thakur P. ABCD: a new classification for keratoconus. Indian J Ophthalmol. 2020;68(12):2831–2834. doi: 10.4103/ijo.IJO_2078_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37(1):149–160. doi: 10.1016/j.jcrs.2010.07.030 [DOI] [PubMed] [Google Scholar]
- 22.Wollensak G. Corneal collagen crosslinking: new horizons. Expert Rev Ophthalmol. 2010;5(2):201–215. doi: 10.1586/eop.10.7 [DOI] [Google Scholar]
- 23.Godefrooij DA, Gans R, Imhof SM, Wisse RPL. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94(7):675–678. doi: 10.1111/aos.13095 [DOI] [PubMed] [Google Scholar]
- 24.Sandvik GF, Thorsrud A, Råen M, Østern AE, Sæthre M, Drolsum L. Does Corneal Collagen Cross-linking Reduce the Need for Keratoplasties in Patients With Keratoconus? Cornea. 2015;34(9):991–995. doi: 10.1097/ICO.0000000000000460 [DOI] [PubMed] [Google Scholar]
- 25.Alipour F, Ansari S, Dadman N, Hafezi F. Accelerated Corneal Collagen Cross-Linking in Pediatric Keratoconus. J Curr Ophthalmol. 2021;33(3):285–290. doi: 10.4103/joco.joco_163_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad TR, Pasricha ND, Rose-Nussbaumer J. Corneal Collagen Cross-Linking Under General Anesthesia for Pediatric Patients With Keratoconus and Developmental Delay. Cornea. 2020;39(5):546–551. doi: 10.1097/ICO.0000000000002197 [DOI] [PubMed] [Google Scholar]