Abstract
Background
Merkel cell carcinoma (MCC), a rare cutaneous neuroendocrine endocrine tumour is increasing in incidence, and continues to carry a poor prognosis.
Objectives
The objectives of this study were to examine all Irish cases of MCC from 1 January 1994 over 2 decades, focusing on gender and organ transplantation recipients (OTRs). Cases were identified from the National Cancer Registry of Ireland. Covariates of interest included age, body site, period of diagnosis, deprivation‐status and history of non‐melanoma skin cancer (NMSC).
Results
In total 314 MCC cases were identified. A female predominance was noted (53.8%). Comparison between age‐standardised rates between the earliest period (1994–1996) with the latest period (2012–2014) showed an increase of 105% in total. The trend in age‐standardised incidence rates were noted to be increasing significantly (p = 0.0004). Average age at diagnosis was 77.6 years (male 75.1 years, female 79.7 years). Overall, the majority of MCC cases presented on the head and neck (n = 170, 54.1%). Differences in anatomical location of MCCs were noted between genders. Males were found to be more likely to have a history of previous NMSCs (males n = 73 [57.9%], females n = 53 [42.1%]). Thirty‐one percentage of patients died from MCC, average survival was 3.5 years in those who died of this malignancy. Ten organ transplant recipients developed MCC. OTR who developed MCC were diagnosed at a younger average age of 65.1 years. Standardized incidence ratio for MCC in OTR was 59.96. A higher proportion of OTR died from MCC (70%), with a shorter median survival of 0.14 years. In competing risks regression, gender was not significantly associated with risk of dying, females having a non‐significantly higher hazard of dying. Organ transplant recipients and patients from less deprived areas were at greater risk of dying from MCC.
Conclusions
This population based study provides epidemiological, clinical and outcome data for MCC over a 20‐year period.

What's already known about this topic?
Merkel cell carcinoma (MCC) is an aggressive cutaneous neuroendocrine skin cancer, most likely arising from basal stem cells that show neuroendocrine differentiation.
While rare, it is increasing in incidence.
MCC portends a poor prognosis.
The pathophysiology is incompletely understood, however photo damage and Merkel cell polyoma virus play a role. Immunosuppressed patients are at increased risk from MCC.
What does this study add?
This study examined MCC in the Irish population over a 20‐year period from 1994 to 2014, identifying 314 cases from the National Cancer Registry.
Females developed MCC at an older age, on sites with less sun exposure, are less likely to have had prior non‐melanoma skin cancers, and have a longer survival time than their male counterparts.
In multivariate competing risks survival analysis, organ transplant recipients and patients from less deprived areas were at greater risk of dying from MCC.
1. INTRODUCTION
Merkel cell carcinoma (MCC) is an aggressive cutaneous neuroendocrine skin carcinoma, most likely arising from basal stem cells that show neuroendocrine differentiation. 1 While MCC is rare, with an annual incidence rate of 0.7 per 100 000 persons, 2 numerous population based studies demonstrate increasing incidence. 2 , 3 Previous research has shown MCC has lower cumulative overall average survival rates compared with melanoma from 12 months extending to 10 years post diagnosis. 4
The pathophysiology of MCC remains incompletely understood. It is recognized that Merkel cell polyoma virus (MCPyV) is integrated into the host genome in a monoclonal pattern in approximately 80% of MCC. In contrast, MCPyV negative tumours demonstrate signature ultraviolet radiation mutations with a much higher mutational burden. 5 Recent data on survival suggests better outcomes for women than men diagnosed with MCC. 6 In contrast, there is a marked increased risk of MCC in immunosuppressed individuals, with aggressive tumour behaviour, increased risk of metastases and poorer disease specific survival. 7
This study examined epidemiological, clinical and outcome data for MCC within the Irish population, including organ transplant recipients (OTRs) over a 20‐year period, focusing on the effect of gender and chronic immunosuppression on diagnosis and prognosis.
2. METHODS
All cases of MCC in Ireland are reported to the National Cancer Registry Ireland (NCRI), a national body that collects data on cancer diagnosis and survival in all patients in the Republic of Ireland. It has prospectively collected data since 1 January 1994. Cases of MCC between 1994 and 2014 were identified. Covariates of interest including age, anatomical location of MCC, period of diagnosis, deprivation status, and risk of non‐melanoma skin cancers (NMSCs) were examined relative to gender and compared in OTR with non‐transplanted patients (Table 1). Chi‐Square tests were used to compare differences in co‐variates by sex and transplant status (Tables 1 and 2). Deprivation was measured based on electoral division (ED) of residence. Patients were assigned a deprivation score on the basis of their Electoral District of residence. The index of deprivation used is based on information collected on education, unemployment and other socioeconomic factors. The level of deprivation in each ED was measured and the population was split into quintiles with the 20% least deprived in quintile 1 and the 20% most deprived in quintile 5. Age‐standardised incidence rates were calculated using the 1976 European Standard Population and the standardized incidence ratio (SIR) was calculated for MCC in OTR as compared to the general population, as previously described. 8
TABLE 1.
Patient co‐variates of interest and outcomes relative to gender
| n (%) | Males n = 145 | Females n = 169 | Chi‐square p‐value |
|---|---|---|---|
| Age groups (years) | |||
| <65 | 23 (15.9) | 12 (7.1) | ‐ |
| 65–79 | 60 (41.4) | 60 (35.5) | ‐ |
| 80+ | 62 (42.8) | 97 (57.4) | 0.009 |
| Average age at diagnosis (years) | 75.07 | 79.69 | ‐ |
| NMSC | |||
| No history of NMSC | 72 (49.7) | 116 (68.6) | ‐ |
| 1 NMSC | 45 (31.0) | 40 (23.7) | ‐ |
| 2 NMSC | 27 (18.6) | 13 (7.7) | ‐ |
| 3 NMSC | 1 (0.7) | 0 (0.0) | 0.002 |
| Deprivation | |||
| 1 – Least deprived | 22 (15.2) | 35 (20.7) | ‐ |
| 2 | 13 (9.0) | 16 (9.5) | ‐ |
| 3 | 24 (16.5) | 17 (10.1) | ‐ |
| 4 | 23 (15.9) | 30 (17.7) | ‐ |
| 5 – Most deprived | 52 (35.9) | 62 (36.7) | ‐ |
| Missing | 11 (7.6) | 9 (5.3) | 0.458 |
| Overall survival at 5 years | |||
| Alive | 65 (44.8) | 64 (37.9) | ‐ |
| Died from MCC | 45 (31.0) | 52 (30.8) | ‐ |
| Died from other cause | 35 (24.1) | 53 (31.4) | 0.305 |
| Period of diagnosis | |||
| 1994–2003 | 44 (30.3) | 59 (34.9) | ‐ |
| 2004–2014 | 101 (69.7) | 110 (65.1) | 0.390 |
Abbreviations: MCC, Merkel cell carcinoma; NMSC, non‐melanoma skin cancer.
TABLE 2.
Patient co‐variates of interest and outcomes relative to transplant status
| n (%) | MCC in the general population | MCC in OTR | Fisher's exact p‐value |
|---|---|---|---|
| n = 304 | n = 10 | ||
| Gender | |||
| Male | 135 (44.4) | 10 (100) | ‐ |
| Female | 169 (55.6) | 0 (0) | <0.001 |
| Age groups (years) | |||
| <65 | 31 (10.2) | 4 (40) | ‐ |
| 65–79 | 114 (37.5) | 6 (60) | ‐ |
| 80+ | 159 (52.3) | 0 (0) | <0.001 |
| Location of MCC | |||
| Head and neck | 163 (53.6) | 7 (70) | ‐ |
| Trunk | 11 (3.6) | 1 (10) | ‐ |
| Upper limb | 36 (11.8) | 1 (10) | ‐ |
| Lower limb | 68 (22.4) | 0 (0) | ‐ |
| Other | 26 (8.5) | 1 (10) | 0.237 |
| NMSC | |||
| No history of NMSC | 188 (61.8) | 0 (0) | ‐ |
| 1 NMSC | 82 (27.0) | 3 (30) | ‐ |
| 2 NMSC | 33 (10.9) | 7 (70) | ‐ |
| 3 NMSC | 1 (0.3) | 0 (0) | <0.001 |
| Deprivation | |||
| 1 – Least deprived | 57 (18.8) | 0 (0) | ‐ |
| 2 | 29 (9.5) | 0 (0) | ‐ |
| 3 | 40 (13.2) | 1 (10) | ‐ |
| 4 | 51 (16.8) | 2 (20) | ‐ |
| 5 – Most deprived | 107 (35.2) | 7 (70) | ‐ |
| Missing | 20 (6.6) | 0 (0) | 0.335 |
| Overall survival at 5 years | |||
| Alive | 126 (41.4) | 3 (30) | ‐ |
| Died from MCC | 90 (29.6) | 7 (70) | ‐ |
| Died from other cause | 88 (28.9) | 0 (0) | 0.013 |
| Period of diagnosis | |||
| 1994–2003 | 100 (32.9) | 3 (30) | ‐ |
| 2004–2014 | 204 (67.1) | 7 (70) | 1.000 |
Abbreviations: MCC, Merkel cell carcinoma; NMSC, non‐melanoma skin cancer; OTR, organ transplantation recipient.
Competing risks analysis was performed with patients recorded as either alive, having died from MCC or from other causes 5‐year‐post diagnosis. Death due to MCC was the event of interest and death due to other causes was the competing risk. Fine‐Grey competing risks regression models were used to test whether gender was associated with the hazard of dying from MCC. All covariates of interest were included in the initial model. Gender was included in the final models, which were adjusted for the covariates of interest, with variables significant at 5% included in the final model. The proportional hazards assumption was tested by examining whether the interactions between survival time and the co‐variates were significant. This was done by the inclusion of time varying co‐variates in the models.
STATA SE (version 15.1 StataCorp) was used for the data analysis.
3. RESULTS
In total 314 cases of MCC were identified between 1994 and 2014. Comparison between age‐standardised rates between the earliest period (1994–1996) with the latest period (2012–2014) showed an increase of 105% in total (Figure 1). Age‐standardised rates for the population increased significantly (p‐value = 0.0004). When comparing the trends between males and females, there was no significant difference. Over the 20‐year period there were greater numbers of females than males diagnosed with MCC (females, n = 169 [53.8%], males n = 145 [46.2%]). The average age at diagnosis was 77.6 years (males 75.1 years vs. females 79.7 years) (Table 1). Male patients were more likely to present under the age of 65 years (males n = 23 [15.9%] vs. females n = 12 [7.1%]). While female patients were more likely to present over 80 years of age (females n = 97 [57.3%] vs. males n = 62 [42.8%]). One patient developed MCC under the age of 20 years, with two patients diagnosed in the third decade of life and 2 further patients diagnosed in their fourth decade. Of note, no patient was diagnosed with more than one MCC.
FIGURE 1.
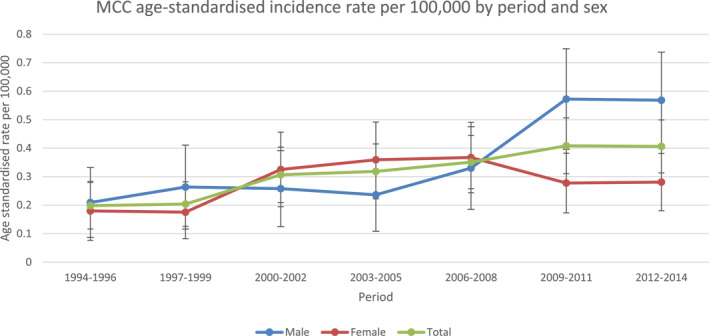
Age standardized incidence rate
Differences in anatomical location of MCCs were noted between genders. Overall, the majority of MCC cases presented on the head and neck (n = 170, 54.1%). Males were more likely to present with MCC in this location (males n = 190 [60.7%] vs. females n = 152 [48.5%]). The lower limb was the second most common anatomical site for MCC development (n = 68, 21.7%). Almost three times as many females developed MCC in this site compared with their male counterparts (Figure 2).
FIGURE 2.
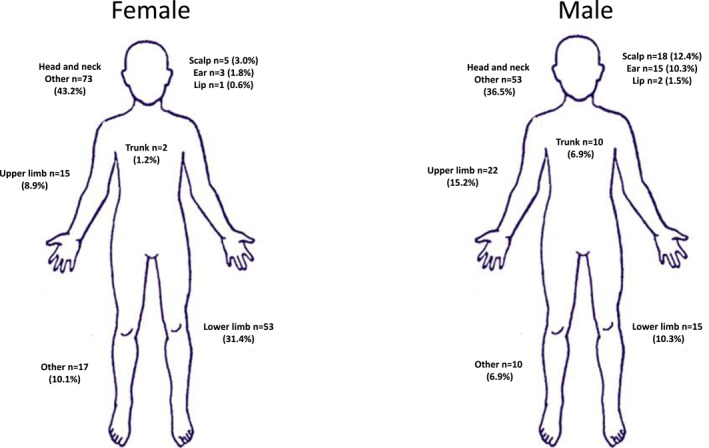
Variation in anatomical location of Merkel cell carcinoma by gender
In total, 188 patients (59.9%) diagnosed with MCC had no history of NMSC. While 126 patients (40.1%) had a history of NMSCs (males n = 73 [57.9%], females n = 53 [42.1%] [p‐value is 0.002]). Malignant melanoma was diagnosed in 4 (1.3%) of MCC patients. MCC was commonest in the two most deprived groups, n = 167 (53.2%), compared with 86 cases (27.4%) occurring in the two least deprived group. For both males and females, over half of cases occurred in the two most deprived groups (males n = 75, 51.7% vs. females n = 92, 54.4%).
At the censoring date (31 December 2014), 106 (33.6%) patients were alive. Two hundred and eight patients had died, 100 (31.8%) died from MCC. Of these, 46 were male and 54 were female. Of those who died from MCC, average survival time was 3.5 years (males 3.4 years, females 3.5 years). Of those diagnosed with MCC on the head & neck, 35% died of their disease, while 44% of those with MCC in other anatomical sites died of MCC.
Ten MCC cases were identified in OTR (nine renal transplant recipients, one heart transplant recipient) (Table 2). There were 3346 solid organ transplants recorded in the Irish national transplant registries over the period included in this study. 9 In keeping with overall changes MCC case number, the number of MCC cases diagnosed in OTR more than doubled between the first and second periods examined in this study. The SIR for MCC in OTR was 59.96 (C.I. 19.3, 139). All OTR diagnosed with MCC were male. The average age at diagnosis in OTR was 65.1 years compared to 79.0 years in the non‐transplanted patients. The average time from transplantation to the development of MCC was 14.1 years. In OTR, the majority of MCCs developed on the head and neck (n = 7,70%). All of the OTRs in this study also had been diagnosed with an NMSC, with (n = 7, 70%) of OTR having had two or more NMSCs. All but one of the MCC cases in OTR (n = 9, 90%) were diagnosed in the two most deprived groups. Of all OTR diagnosed with MCC, the majority (n = 7, 70%) died from this disease. Median survival for OTR who died from MCC was 0.14 years.
Two‐hundred‐fifty‐one patients (79.9%) underwent surgical intervention, 106 patients (33.8%) received radiation treatment while 28 patients (8.9%) received chemotherapy.
In competing risks regression, gender was not significantly associated with the risk of dying from MCC, with females having a non‐significantly higher hazard of dying from MCC. OTR and less deprived patients were at significantly increased risk of dying from MCC (Table 3). The proportional hazards assumption was not violated in the cause specific survival analysis.
TABLE 3.
Competing risks regression analysis up to 5 years post diagnosis for MCC deaths
| SHR | p‐value | 95% LL | 95% UL | Wald test p‐value | |
|---|---|---|---|---|---|
| Gender | |||||
| M | 1 | ‐ | ‐ | ‐ | ‐ |
| F | 1.05 | 0.830 | 0.68 | 1.61 | 0.830 |
| Patients | |||||
| Patients without organ transplant | 1 | ‐ | ‐ | ‐ | ‐ |
| OTR patients | 4.97 | 0.002 | 1.78 | 13.83 | 0.002 |
| Deprivation | |||||
| 1 – Least deprived | 1 | ‐ | ‐ | ‐ | ‐ |
| 2 | 1.61 | 0.201 | 0.78 | 3.32 | ‐ |
| 3 | 0.84 | 0.627 | 0.41 | 1.72 | ‐ |
| 4 | 0.48 | 0.049 | 0.24 | 1.00 | ‐ |
| 5 – Most deprived | 0.66 | 0.153 | 0.37 | 1.17 | ‐ |
| Missing | 0.48 | 0.159 | 0.17 | 1.34 | 0.033 |
Abbreviations: LL, lower limit; MCC, Merkel cell carcinoma; OTR, organ transplantation recipient; SHR, sub hazard ratio; UL, upper limit.
4. DISCUSSION
This study which examined national registry data over 2 decades, supports epidemiological trends around increasing MCC incidence but also identified some gender related patterns that warrant further investigation. We demonstrated more than a 100% increase in the number of MCC cases diagnosed in Ireland between 2004 and 2014 compared with the previous decade (Table 1). The age standardized incidence rate trended significantly upwards over the period. As the ICD‐10 code for MCC was first coined in 2009, and CK‐20 staining for MCC became available in 1995, the years reviewed in this paper reflect a gradual transition to more accurate registration of cases. Similarly, between 2000 and 2013, a 95% increase in MCC incidence was noted in the United States, higher than increases seen in other UV associated skin malignancies, such as melanoma (57% increase). 2 It has been suggested that the rapid increase in incidence rates for melanoma may be in part explained by increased diagnostic scrutiny and overdiagnosis. 10 MCC, however, does not have a benign counterpart or precursor lesion. Confirmatory immunohistochemistry also allows for greater pathological diagnostic certainty.
Of note in this Irish, largely fair‐skinned homogenous population, increasing incidence of MCC over the periods examined was seen particularly in older patients and in men, with a less substantial increase in female patients (Figure 1). MCC is known to be largely a disease of the elderly. Indeed, in this cohort of patients the average age at diagnosis was 77.6 years of age with <3% of cases diagnosed in patients under 50 years of age.
MCC is rare, typically described as occurring in fair‐skinned, elderly male patients on sun exposed skin, but in our study a female preponderance (53.8%) in MCC was noted. While MCC is traditionally thought be commoner in males, 11 , 12 , 13 , 14 , 15 there are regional variations with a number of reviews of MCC in Northern Europe also demonstrating a female predominance. 16 , 17 , 18 , 19 , 20 , 21 This incidence varied with age, with males more likely to develop MCC under the age of 65 years, while females were more likely to present over 80 years of age. A review from Queensland, Australia revealed the highest incidence rate of MCC internationally (1.6 per 100 000), 12 supporting the link between high rates of UV exposure and development of MCC. The anatomical location of MCC development in this review supports this concept. Overall, the head and neck was the most common site. MCC developed more commonly on the ears and scalp in males where there is likely to be less hair cover. Forty percentage of MCC patients in the Irish cohort also had a history of NMSCs. There is a significant difference in NMSC history by sex, with females less likely to have NMSC history. Polyoma virus negative MCCs are shown more likely to occur in sun exposed regions, and have a higher mutational burden including UV light signature mutations. 22 Poor survival rates from MCC have been demonstrated in New Zealand which is thought be linked to high UV exposure, fair skin type and lower rates of MCPyV positivity in this region. 23 Interestingly, the lower limbs were noted overall to be the second most common location for MCC development. MCC numbers in this location were three times more common in females. While squamous cell carcinoma in situ and melanoma are recognized as commonly occurring on the lower limbs of females, a propensity of MCC for this site has not previously been described.
Although female MCC patients presented at an older age, likely with an increased burden of co‐morbidities, they had longer average survival times. A recent study demonstrated improved survival in females with MCC. 6 The authors suggest this may be due to inherent immunological differences between genders. In competing risks regression in this study, gender was not statistically significantly associated with the risk of dying from MCC, with females having a non‐significantly higher hazard of dying from MCC.
The NCRI has demonstrated higher overall cancer incidence in the most deprived patients in Ireland compared with the least deprived. 24 The opposite pattern has been observed in both MCC and malignant melanoma. 24 , 25 This study demonstrated that patients from a less deprived socioeconomic group were at increased risk of dying from MCC. Other studies have noted patients from lower socioeconomic groups tend to have higher rates of MCC in the head and neck region and advanced disease 26 and may have poorer survival rates than patients with MCC in other sites. 27 This may be in part have been related to more conservative surgical approaches and a higher propensity for metastatic spread. This is in contrast to our review, in which a lower percentage of patients with MCC located on the head and neck died of their disease, compared with cases of MCC located on other anatomical locations. In this review a high proportion (90%) of OTR with MCC were in the two most deprived groups. It has been shown however that deprivation in itself is associated with a poor prognosis in renal disease. 28
Our group recently documented increasing MCC numbers in renal transplant recipients as the mean age at transplantation increases 29 Our findings paralleled other studies that have shown skin cancers occur an average of 30 years earlier in OTR than those in the general population. 30 The almost 60‐fold increase in relative risk for MCC within our OTR population is even greater than that shown in a review of 189 498 solid OTR in the United States demonstrating an overall 23.8‐fold increased risk for MCC. The authors suggest that immunosuppressive medications may act synergistically with UV radiation to increase MCC risk 31 and potentiate metastatic spread as indicated in the very short median survival time of 0.225 years for OTR cohort who died from MCC.
There are a number of limitations regarding this analysis. The number of OTRs in this cohort is proportionally small when compared with the general population. The NCRI does not record subtype of keratinocyte cancer as a cause of death. While this is a limitation of this study, it is assumed, those diagnosed with MCC died from this malignancy rather than another NMSC. Polyoma virus status was not available on the cases in this review.
This population based study provides epidemiological, clinical and outcome data for MCC over a 20‐year period. Females were shown to develop MCC at an older age, more frequently on sites less typically sun exposed, are less likely to have had prior NMSCs, and have a longer survival time than their male counterparts. In contrast, male patients with MCC were on average younger than female counterparts with MCC more likely to occur on the head and neck and in association with a prior history of NMSC. Further studies correlating MCPyV status and UV mutational load of tumours in these different groups are required. The role of a functioning immune system in preventing MCC was highlighted in our cohort of OTR who had a significantly increased incidence of MCC in male OTR, with an earlier age at presentation, association with other NMSC and significantly reduced median survival.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
E. Keeling: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing. E. O'Leary: Data curation; Formal analysis; Investigation; Methodology; Writing – review & editing. S. Deady: Data curation; Formal analysis; Investigation; Methodology. J. P. O Neill: Formal analysis; Investigation; Supervision; Writing – review & editing. P. J. Conlon: Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Writing – review & editing. F. J. Moloney: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – review & editing.
ACKNOWLEDGEMENT
The authors received no financial support for the research, authorship, and/or publication of this article.
Open access funding enabled and organized by Projekt DEAL.
Keeling E, O'Leary E, Deady S, O Neill JP, Conlon PJ, Moloney FJ. Gender and immunosuppression impact on Merkel cell carcinoma diagnosis and prognosis. A population based cohort study. Skin Health Dis. 2022;2(1):e80. 10.1002/ski2.80
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paulson KG, Park SY, Vandeven NA, Lachance K, Thomas H, Chapuis AG, et al. Merkel cell carcinoma: current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78:457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaar O, Gillstedt M, Lindelof B, Wennberg‐Larkö A‐M, Paoli J. Merkel cell carcinoma incidence is increasing in Sweden. J Eur Acad Dermatol Venereol. 2016;30:1708–13. [DOI] [PubMed] [Google Scholar]
- 4. Grabowski J, Saltzstein SL, Sadler GR, Tahir Z, Blair S. A comparison of Merkel cell carcinoma and melanoma: results from the California cancer registry. Clin Med Oncol. 2008;2:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tam M, Luu M, Barker CA, Gharavi NM, Hamid O, Shiao SL, et al. Improved survival in women versus men with Merkel cell carcinoma. J Am Acad Dermatol. 2021. Feb;84(2):321–9. [DOI] [PubMed] [Google Scholar]
- 7. Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma‐specific survival independent of stage. J Invest Dermatol. 2013;133:642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menzies S, O'Leary E, Callaghan G, Galligan M, Deady S, Gadallah B, et al. Declining incidence of keratinocyte carcinoma in organ transplant recipients. Br J Dermatol. 2019. Nov;181(5):983–91. [DOI] [PubMed] [Google Scholar]
- 9. O'Neill JP, Sexton DJ, O'Leary E, O'Kelly P, Murray S, Deady S, et al. Post‐transplant malignancy in solid organ transplant recipients in Ireland, The Irish Transplant Cancer Group. Clin Transplant. 2019. Oct;33(10):e13669. [DOI] [PubMed] [Google Scholar]
- 10. Welch HG, Mazer BL, Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384(1):72–9. [DOI] [PubMed] [Google Scholar]
- 11. Girschik J, Thorn K, Beer TW, Heenan PJ, Fritschi L. Merkel cell carcinoma in Western Australia: a population‐based study of incidence and survival. Br J Dermatol. 2011;165:1051–7. [DOI] [PubMed] [Google Scholar]
- 12. Youlden DR, Soyer HP, Youl PH, Fritschi L, Baade PD. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Dermatol. 2014;150:864–72. [DOI] [PubMed] [Google Scholar]
- 13. Fitzgerald TL, Dennis S, Kachare SD, Vohra NA, Wong JH, Zervos EE. Dramatic increase in the incidence and mortality from Merkel cell carcinoma in the United States. Am Surg. 2015;81:802–6. [DOI] [PubMed] [Google Scholar]
- 14. Reichgelt BA, Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population‐based study of 808 cases in 1993–2007. Eur J Cancer. 2011;47:579–85. [DOI] [PubMed] [Google Scholar]
- 15. Albores‐Saavedra J, Batich K, Chable‐Montero F, Sagy N, Schwartz AM, Henson DE. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study. J Cutan Pathol. 2010;37:20–7. [DOI] [PubMed] [Google Scholar]
- 16. Hussain SK, Sundquist J, Hemminki K. Incidence trends of squamous cell and rare skin cancers in the Swedish national cancer registry point to calendar year and age‐dependent increases. J Invest Dermatol. 2010;130:1323–8. [DOI] [PubMed] [Google Scholar]
- 17. Kukko H, Bohling T, Koljonen V, Tukiainen E, Haglund C, Pokhrel A, et al. Merkel cell carcinoma – a population‐based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737–42. [DOI] [PubMed] [Google Scholar]
- 18. Uitentuis SE, Louwman MWJ, van Akkooi ACJ, Bekkenk MW. Treatment and survival of Merkel cell carcinoma since 1993: a population‐based cohort study in The Netherlands. J Am Acad Dermatol. 2019;81(4):977–83. [DOI] [PubMed] [Google Scholar]
- 19. Kieny A, Cribier B, Meyer N, Velten M, Jégu J, Lipsker D. Epidemiology of Merkel cell carcinoma. A population‐based study from 1985 to 2013, in northeastern of France. Int J Cancer. 2019;144(4):741–5. [DOI] [PubMed] [Google Scholar]
- 20. Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, Wohlfahrt J, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793–801. [DOI] [PubMed] [Google Scholar]
- 21. Fondain M, Dereure O, Uhry Z, Guizard AV, Woronoff AS, Colonna M, et al. Merkel cell carcinoma in France: a registries‐based, comprehensive epidemiological survey. J Eur Acad Dermatol Venereol. 2018;32(8):1292–6. [DOI] [PubMed] [Google Scholar]
- 22. Wong SQ, Waldeck K, Vergara IA, Schroder J, Madore J, Wilmott JS, et al. UV‐associated mutations underlie the etiology of MCV‐negative Merkel cell carcinomas. Cancer Res. 2015;75(24):5228–34. [DOI] [PubMed] [Google Scholar]
- 23. Lee Y, Chao P, Coomarasamy C, Mathy JA. Epidemiology and survival of Merkel cell carcinoma in New Zealand: a population‐based study between 2000 and 2015 with international comparison. Australas J Dermatol. 2019;60(4):e284–91. [DOI] [PubMed] [Google Scholar]
- 24. National Cancer Registry/Northern Ireland Cancer Registry All‐Ireland Cancer Atlas 1995–2007. Cork/Belfast; 2011. [Google Scholar]
- 25. Ezaldein HH, Ventura A, DeRuyter NP, Yin ES, Giunta A. Understanding the influence of patient demographics on disease severity, treatment strategy, and survival outcomes in Merkel cell carcinoma: a surveillance, epidemiology, and end‐results study. Oncoscience. 2017;4(7–8):106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madankumar R, Criscito MC, Martires KJ, Stein JA. A population‐based cohort study of the influence of socioeconomic factors and race on survival in Merkel cell carcinoma. J Am Acad Dermatol. 2017;76(1):166–7. [DOI] [PubMed] [Google Scholar]
- 27. Morand GB, Madana J, Da Silva SD, Hier MP, Mlynarek AM, Black MJ. Merkel cell carcinoma of the head and neck: poorer prognosis than non‐head and neck sites. J Laryngol Otol. 2016;130(4):393–7. [DOI] [PubMed] [Google Scholar]
- 28. Guthrie GD, Bell S. Deprivation and kidney disease—a predictor of poor outcomes. Clin Kidney J. 2019;13(2):128–32. 10.1093/ckj/sfz151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keeling E, Murray SL, Williams Y, Sexton DJ, O'Kelly P, Deady S, et al. Merkel cell carcinoma in kidney transplant recipients in Ireland 1964‐2018. Br J Dermatol. 2019;181(6):1314–15. 10.1111/bjd.18218 [DOI] [PubMed] [Google Scholar]
- 30. Mullen DL, Silberberg SG, Penn I, Hammond WS. Squamous cell carcinoma of the skin and lip in renal homograft recipients. Cancer. 1976;37:729–34. [DOI] [PubMed] [Google Scholar]
- 31. Clarke CA, Robbins HA, Tatalovich Z, Lynch CF, Pawlish KS, Finch JL, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015. Jan 8;107(2):dju382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


