Abstract
Background
The coronavirus disease 2019 (COVID‐19) is a newly recognized illness that has spread rapidly all over the world. More and more reports highlight the risk of venous thromboembolism (VTE) in COVID‐19. Our study aims to identify in‐hospital VTE risk and bleeding risk in COVID‐19 patients.
Methods
We retrospectively studied 138 consecutively enrolled patients with COVID‐19 and identified in‐hospital VTE and bleeding risk by Padua Prediction Score and Improve bleed risk assessment model. The clinical data and features were analyzed in VTE patients.
Results
Our findings identified that 23 (16.7%) patients with COVID‐19 were at high risk for VTE according to Padua prediction score and 9 (6.5%) patients were at high risk of bleeding for VTE prophylaxis according to Improve prediction score. Fifteen critically ill patients faced double high risk from thrombosis (Padua score more than 4 points in all 15 [100%] patients) and hemorrhage (Improve score more than 7 points in 9 [60.0%] patients). Thrombotic events were identified in four patients (2.9%) of all COVID‐19 patients. All of them were diagnosed with deep vein thrombosis by ultrasound 3 to 18 days after admission. Three (75.0%) were critically ill patients, which means that the incidence of VTE among critically ill patients was 20%. One major hemorrhage happened in critically ill patients during VTE treatment.
Conclusion
Critically ill patients with COVID‐19 suffered both a high risk of thrombosis and bleeding risks. More effective VTE prevention strategies based on an individual assessment of bleeding risks were necessary for critically ill patients with COVID‐19.
Keywords: coronavirus disease 2019, deep vein thrombosis, improve bleed risk assessment model, Padua prediction score, venous thromboembolism
Abbreviations
- COVID‐19
coronavirus disease 2019
- DVT
deep vein thrombosis
- ECMO
extracorporeal membrane oxygenation
- FiO2
fraction of inspired oxygen
- GFR
glomerular filtration rate
- ICU
intensive care unit
- IPC
intermittent pneumatic compression
- LMWH
low molecular weight heparin
- PTE
pulmonary thromboembolism
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- VTE
venous thromboembolism
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) is a newly recognized illness that has spread rapidly around the world. 1 , 2 , 3 , 4 The clinical spectrum of COVID‐19 ranges from mild to critically ill cases. Previous studies have mentioned old age, and those with coexisting medical conditions were more likely to have a poor prognosis. 5 These factors, as well as infection, bedridden, and respiratory failure are all risk factors for venous thromboembolism (VTE). 6 , 7 Once COVID‐19 is complicated with deep vein thrombosis (DVT) or fatal pulmonary thromboembolism (PTE), the treatment will be challenging, and patients with VTE may have a worse clinical outcome.
In this study, we assessed the risk of VTE and bleeding and compared the risks between critically ill patients and those of non‐critically ill in hospitalized patients with COVID‐19 from the Shanghai Public Health Clinical Center. We also report our experience with four patients that suffered clinically striking thrombotic events with COVID‐19.
2. METHODS
2.1. Study design and participants
This single‐center, retrospective, observational study was done at Shanghai Public Health Clinical Center (Shanghai, China), which is a designated hospital to treat patients with COVID‐19. We retrospectively analyzed patients from January 21, 2020, to February 21, 2020, who had been diagnosed with COVID‐19, according to WHO interim guidance. 8 Laboratory confirmation of COVID‐19 infection was performed by the local health authority. 1 , 2 Critically ill patients were defined as those admitted to the intensive care unit (ICU) who required mechanical ventilation or had a fraction of inspired oxygen (FiO2) of at least 60% or more. 9 Identification of critically ill patients was achieved by reviewing and analyzing admission logs and histories from all available electronic medical records and patient care resources. The Ethics Commission of Shanghai Public Health Clinical Center approved this study. Written informed consent was waived due to the rapid emergence of this infectious disease.
2.2. Data collection and definition of terms
Variables that were collected from the patients' electronic medical records and completed from the medical files included demographic, anthropometric, and clinical variables that are components of the Padua prediction score and Improve bleeding risk prediction score.
The Padua prediction score was calculated, for each patient at study entry, according to the weight and number of the following risk factors; active cancer (3 point), previous VTE (3 points), reduced mobility (3 points), already known thrombophilic condition (3 points), recent (≤month) trauma and/or surgery (2 points), elderly age (≥70 years) (1 point), heart and/or respiratory failure (1 point), acute myocardial infarction or ischemic stroke (1 point), acute infection and/or rheumatologic disorder (1 point), ongoing hormonal therapy (1 point), and obesity (body mass index [BMI] ≥30 kg/m2) (1 point). A high risk of VTE is defined as a cumulative score ≥4 and a low risk as one of <4. 10
The Improve bleeding risk prediction score was calculated according to the weight and number of the following risk factors; active gastroduodenal ulcer (4.5 points), bleeding within past 3 months (4 points), admission platelets <50 × 109 cells/L (4 points), hepatic failure (2.5 points), ICU/CCU stay (2.5 points), central venous catheter (2 points), rheumatic disease (2 points), active malignancy (2 points), age 40–80 (1.5 points), age ≥85 (3.5 points), renal disease: glomerular filtration rate (GFR) 30–59 ml/min (1 point), GFR < 30 ml/min (2.5 points). A high risk of bleeding is defined as a cumulative score ≥7 and a low risk as one of <7. 11
The risk factors were evaluated on admission. Dynamic evaluations were further conducted if the patient's situation changed depending on the VTE and bleeding risk. Routine thromboprophylaxis was provided to patients whose Padua score more than four points or according to the clinicians' decision based on clinical presentation and D‐dimer levels, even in patients with low Padua score. For those with Improve score more than 7, intermittent pneumatic compression (IPC) or low intensive thromboprophylaxis was suggested. Lower extremity compression ultrasound (CUS) was performed for all critically ill patients and those with a high risk of VTE and a high level of D‐dimer. If possible, these patients received computed tomography pulmonary angiogram (CTPA). VTE and bleeding complications and their management were recorded.
2.3. Statistical analysis
Statistical calculations were done with the software package Statistical Package for the Social Sciences version 21 (IBM SPSS Statistics; Armonk, NY, USA). We expressed descriptive data as mean (standard deviation) or median (interquartile range) for continuous variables and number (%) for categorical variables. We assessed differences between critically ill patients and non‐critically ill ones using a two‐sample t test or Wilcoxon rank‐sum test depending on parametric or nonparametric data for continuous variables and Fisher's exact test for categorical variables. Tests were two‐sided with significance set at α less than 0.05.
3. RESULTS
3.1. Demographic and clinical characteristics
The study population included 138 hospitalized patients with confirmed COVID‐19 in Shanghai Public Health Clinical Center, of whom 15 (10.9%) were critically ill. Eighty‐one (58.7%) patients were male. The average age was 52.43 ± 16.68 years. Of the 138 patients, 56 (40.6%) had one or more coexisting medical conditions. Hypertension (39 [28.3%]) and diabetes (16 [11.6%]) were the most common coexisting conditions. Compared with non‐critically ill patients (n = 123), critically ill patients were significantly older (60.07 ± 14.25 years vs. 50.52 ± 15.97 years; p < 0.01) and were more likely to have underlying comorbidities, including atrial fibrillation (3 [20.0%] vs. 3 [2.4%]), hypertension (8 [53.3%] vs. 31 [25.2%]), and stroke (4 [26.7%] vs. 0 [0%]). Furthermore, critically ill patients had abnormally elevated D‐dimer levels (normal value: 0 ~ 0.5 μg/ml) on baseline, which was significantly higher than non‐critically ill patients (0.7 [0.4,1.4] vs. 0.4 [0.3,0.8], p < 0.01). There were no significant differences in gender, weight, and other complications between critically ill and non‐critically ill patients.
3.2. Risk prediction of VTE in COVID‐19
Padua score in COVID‐19 patients ranged from 1 point to 9 points and the median risk score was 1 point. Overall, 115 (83.3%) patients had Padua score <4 (low risk for VTE), and 23 (16.7%) patients had Padua score ≥4 (high risk for VTE). The presence of high risk for VTE was more common among patients with critically ill than those with non‐critically ill patients (15 [100%] vs. 7 [6.5], p < 0.01), as illustrated in Table 1 and Figure 1. The most common Padua risk parameters involved in COVID‐19 were acute infection (138 [100%]), heart failure or respiratory failure (55 [39.9%]), reduced mobility (21 [15.2%]), and elderly age (17 [12.3%]). Compared with non‐critically ill patients, critically ill patients were more likely to have the following VTE risk factors, including reduced mobility(15 [100.0%] vs. 6 [5.0%], p < 0.001), elderly age (7 [46.7%] vs. 10 [8.3%], p < 0.001), respiratory failure (11 [73.3] vs. 44 [36.4%], p < 0.01) and obesity (2 [13.3%] vs. 0 [0%], p = 0.01), as shown in Table 2. All patients at high VTE risk received adequate prophylaxis.
TABLE 1.
Demographics and baseline characteristics of patients with COVID‐19
| All patients (n = 138) | Critically ill (n = 15) | Non‐critically ill (n = 123) | p value | |
|---|---|---|---|---|
| Characteristics | ||||
| Age, years | 52.4 ± 16.7 | 60.1 ± 14.3 | 50.5 ± 16.0 | <0.01 |
| Men, n (%) | 81 (58.70) | 12 (80.00) | 69 (56.10) | 0.06 |
| Weight, kg | 68.75 ± 13.71 | 74.33 ± 15.51 | 68.07 ± 13.40 | 0.08 |
| D‐dimer a , μg/ml | 0.43 (0.30,0.89) | 0.74 (0.44,1.35) | 0.39 (0.29,0.83) | <0.01 |
| Comorbidity, n (%) | ||||
| Malignancy | 4 (2.9) | 1 (6.7) | 3 (2.4) | 0.37 |
| Obesity | 1 (0.7) | 1 (6.7) | 0 (0) | 0.11 |
| Liver disease | 2 (1.4) | 0 (0) | 2 (1.6) | 0.79 |
| DM | 16 (11.6) | 4 (26.7) | 12 (9.8) | 0.08 |
| Kidney disease | 3 (2.2) | 1 (6.7) | 2 (1.6) | 0.29 |
| AF | 6 (4.3) | 3 (20.0) | 3 (2.4) | 0.02 |
| CHD | 7 (5.1) | 2 (13.3) | 5 (4.1) | 0.17 |
| Hypertension | 39 (28.2) | 8 (53.3) | 31 (25.2) | 0.03 |
| Stroke | 4 (2.9) | 4 (26.7) | 0 (0) | <0.001 |
| Padua score | <0.001 | |||
| <4, n (%) | 115 (83.3) | 0 (0) | 115 (93.5) | |
| ≥4, n (%) | 23 (16.7) | 15 (100.0) | 7 (6.5) | |
| Improve score | <0.001 | |||
| <7, n (%) | 129 (93.5) | 6 (40.0) | 123 (100.0) | |
| ≥7, n (%) | 9 (6.5) | 9 (60.0) | 0 (0) | |
| Confirmed VTE, n (%) | 4 (2.9) | 3 (26.7) | 1 (0) | <0.001 |
| Prophylaxis, n (%) | 41 (30.1) | 15 (100.0) | 26 (21.5) | <0.001 |
Abbreviations: AF, atrial fibrillation; CHD, coronary heart disease.
The levels of D‐dimer were obtained on admission.
FIGURE 1.
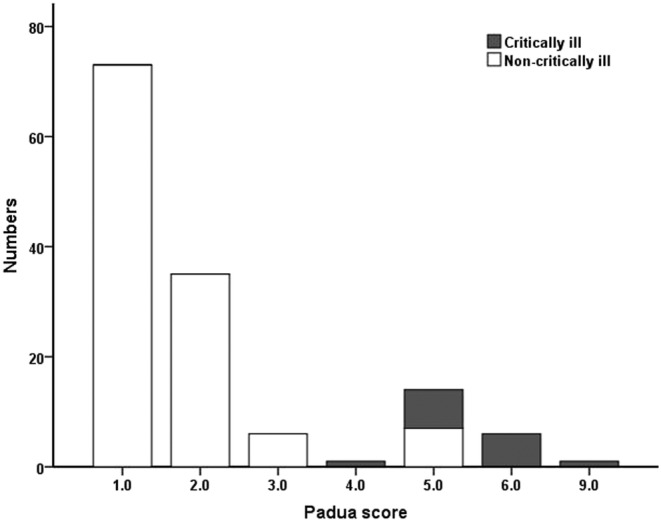
Distribution of patients and Padua scores
TABLE 2.
Padua prediction score component in patients with COVID‐19 on admission
| All patients (n = 138) | Critically ill (n = 15) | Non‐critically ill (n = 123) | p value | |
|---|---|---|---|---|
| Active cancer | 1 (0.7) | 1 (6.7) | 0 (0) | 0.11 |
| Previous VTE | 1 (0.7) | 15 (100.0) | 1 (0.8) | 0.89 |
| Reduced mobility | 21 (15.2) | 15 (100.0) | 6 (6.0) | <0.001 |
| Known thrombophilic condition | 0 (0) | 0 (0) | 0 (0) | N |
| Recent trauma or/and surgery | 0 (0) | 0 (0) | 0 (0) | N |
| Age ≥70 years | 17 (12.3) | 7 (46.7) | 10 (8.3) | <0.001 |
| Heart and/or respiratory failure | 55 (39.9) | 11 (73.3) | 44 (36.4) | 0.006 |
| Acute myocardial infarction or stroke | 1 (0.7) | 1 (6.7) | 0 (0) | 0.11 |
| Acute infection and/or rheumatologic disorder | 138 (100.0) | 15 (100.0) | 123 (100.0) | N |
| Obesity | 2 (1.4) | 2 (13.3) | 0 (0) | 0.01 |
| Hormonal treatment | 0 (0) | 0 (0) | 0 (0) | N |
Note: Categorical variables are summarized with numbers and percentages.
Abbreviations: N, not applicable; VTE, venous thromboembolism.
3.3. Bleeding risk prediction for VTE prophylaxis in COVID‐19
Improve score in COVID‐19 patients ranged from 0 point to 12.5 points and the median risk score was 1.5 point. Overall, 129 (93.5%) patients had Improve score <7 (low risk for bleeding), and 9 (6.5%) patients had Improve score ≥7 (high risk for bleeding). The presence of a high risk for bleeding was obviously more common among patients with critically ill than those with non‐critically ill patients (9 [60.0%] vs. 0 [0%], p < 0.01), as illustrated in Table 1 and Figure 2. The most common Improve risk parameters involved in COVID‐19 were age between 40 and 84 years (95 [68.8%]), ICU stay (15 [10.9%]), and central venous catheter (14 [10.1%]). Compared with non‐critically ill patients, critically ill patients were more likely to have the following bleeding risk factors, including bleeding with past 3 months (5 [33.3%] vs. 1 [0.8%], p < 0.001), hepatic failure (3 [20.0%] vs. 2 [1.6%], p = 0.011), ICU stay (15 [100.0%] vs. 0 [0%], p < 0.001), central venous catheter (13 [86.7%] vs. 1 [0.8%], p < 0.001), age between 40 and 84 years (14 [93.3%] vs. 81 [65.9%], p = 0.023), and GFR less than 30 ml/min(2 [13.3%] vs. 0 [0%], p = 0.011). For patients both at high risk for VTE and bleeding, most patients accepted half‐dose heparin or low molecular weight heparin (LMWH) and two patients applied IPC. A total of 6 (4.3%) patients who were all at high bleeding risk experienced bleeding event after anticoagulant therapy, including mild hematuria or microscopic hematuria (3 [2.2%]), mild gastrointestinal bleeding (1 [0.7%]), moderate nosebleed (1 [0.7%]), and severe hemothorax (1 [0.7%]) (Table 3).
FIGURE 2.
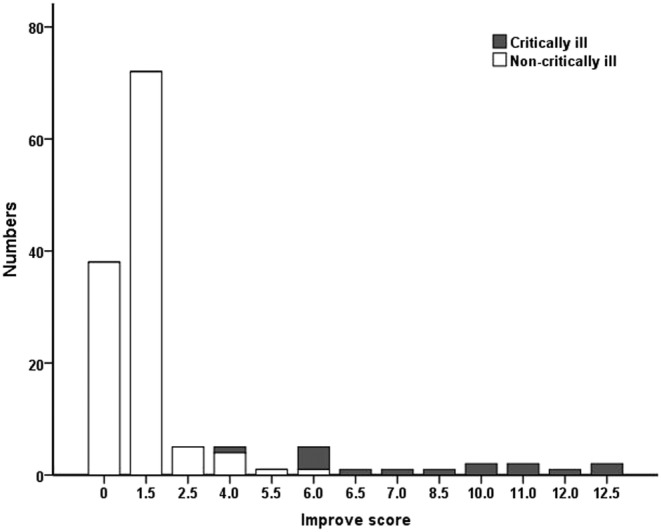
Distribution of patients and Improve scores
TABLE 3.
Improve prediction score component in patients with COVID‐19
| All patients (n = 138) | Critically ill (n = 15) | Non‐critically ill (n = 123) | p value | |
|---|---|---|---|---|
| Active gastroduodenal ulcer | 0 (0) | 0 (0) | 0 (0) | N |
| Bleeding within past 3 month | 6 (4.3) | 5 (33.3) | 1 (0.8) | <0.01 |
| Admission platelets <50 × 109 cells/L | 2 (1.4) | 2 (13.3) | 0 (0) | 0.01 |
| Hepatic failure | 5 (3.6) | 3 (20.0) | 2 (1.6) | 0.01 |
| ICU/CCU stay | 15 (10.9) | 15 (100.0) | 0 (0) | <0.01 |
| Femoral catheters | 14 (10.1) | 13 (86.7) | 1 (0.8) | <0.01 |
| Rheumatic disease | 0 (0) | 0 (0) | 0 (0) | N |
| Active malignancy | 1 (0.7) | 1 (6.7) | 0 (0) | 0.11 |
| Age | ||||
| 40–80 | 95 (68.8) | 14 (93.3) | 81 (65.9) | 0.02 |
| ≥85 | 0 (0) | 0 (0) | 0 (0) | N |
| Renal disease | ||||
| GFR 30–59 ml/min | 4 (2.9) | 2 (13.3) | 2 (1.6) | 0.06 |
| GFR <30 ml/min | 2 (1.4) | 2 (13.3) | 0 (0) | 0.01 |
Note: Categorical variables are summarized with numbers and percentages.
Abbreviations: GFR, glomerular filtration rate; N, not applicable.
3.4. Thrombotic complications in COVID‐19
Thrombotic events were identified in four patients (2.9%) of all COVID‐19 patients. All of them were diagnosed with DVT by ultrasound on the 3rd to 18th day of admission. Three (75.0%) were critically ill patients, which means that the incidence of VTE among critically ill patients was up to 20%. All four patients were male whose ages ranged from 25 to 70 years and had one or more coexisting medical conditions. Baseline D‐dimer was elevated, but to varying degrees in three patients. The other patient had a normal D‐dimer level on the baseline which increase to 3.9 μg/ml at 14 days after admission. Three of these patients received mechanical ventilation and central venous catheter treatment; one of whom used continuous renal replacement therapies (CRRT); and two had extracorporeal membrane oxygenation (ECMO) treatment. All patients accepted anticoagulant therapy with LMWH or heparin. One patient with a high risk of bleeding had severe hemothorax. All patients survived except one died of respiration failure (Table 4).
TABLE 4.
Thrombotic complications in our patients with COVID‐19
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age | 70 | 25 | 64 | 64 |
| Sex | Male | Male | Male | Male |
| Comorbidity | DM, HTN | Obesity | HTN | HTN |
| D‐dimer a , μg/ml | 1.39 | 0.39 | 0.54 | 1.00 |
| Ventilation | Y | Y | Y | N |
| CRRT | Y | N | N | N |
| ECMO | N | Y | Y | N |
| Venous catheterization | Y | Y | Y | N |
| Padua score | 6 | 6 | 5 | 5 |
| Improve score | 5 | 7.5 | 5 | 1.5 |
| VTE complication | DVT | DVT | DVT | DVT |
| VTE diagnosis day | 3 | 18 | 8 | 10 |
| Prophylaxis | LMWH | Heparin | LMWH heparin | LMWH |
| Bleeding event after anticoagulant therapy | None | Hemothorax | None | None |
| Outcome | Survived | Deceased | Survived | Survived |
Abbreviations: CRRT, continuous renal replacement therapies; ECMO, extracorporeal membrane oxygenation; VTE, venous thromboembolism.
The levels of D‐dimer were obtained on admission.
4. DISCUSSION
Our findings identified that 16.67% of patients with COVID‐19 were at high risk for VTE according to the Padua prediction score, and 6.52% of patients were at high risk of bleeding for VTE prophylaxis according to Improve prediction score. The prediction risk of VTE (6.5%), as well as the incidence of VTE (0.8%), was low in non‐critically patients. However, critically ill patients faced double high risk from thrombosis (Padua score more than 4 points in 100% of critically ill patients) and hemorrhage (Improve score more than 7 points in 60.0% of critically ill patients). Furthermore, we identified a high incidence of VTE (20.0%) in critically ill patients with COVID‐19, despite the use of universal, guideline‐recommended thromboprophylaxis. Critically ill patients suffered a marked incidence of bleeding (26.7%), which suggested a complicated situation in VTE prophylaxis to COVID‐19.
As described, there are several reasons for the high risk of VTE in critically ill patients with COVID‐19. On the one hand, the critically ill population in our findings had three qualities in physiology, including venous stasis due to sedation or bedridden, hypercoagulability caused by glucocorticoid and immunoglobulins, and endothelial damage from venous catheterization and/or ECMO. On the other hand, a significant number of COVID‐19 patients, especially critically ill ones, were the aged 1 who were easy to complicate with VTE high‐risk factors, such as heart failure, stroke, cancer, and diabetes. In addition, critically ill patients had a higher level of D‐dimer compared to non‐critically one, 5 which might be associated with hypercoagulability induced by coronavirus. All these factors increased the risk of developing potentially deadly blood clots.
More interestingly, the national study in China of COVID‐19 patients had found 407 (40%) among total of 1026 patients were at high risk of VTE, which was much higher than our study. 12 The difference might be due to two reasons. First of all, we evaluated the Padua prediction score mostly during the first week of admission. Some risk factors may be changed after 1 week, such as respiratory failure and reduced mobility. Second, the risk of reduced mobility, which had 3 points in the Padua prediction score, was low in our study. Most non‐critically ill patients had no or slight limitation during ordinary activity and were not encouraged to bed rest. No matter the different rates of COVID‐19 patients with high VTE risk, more and more attention has been paid to the role of VTE in COVID‐19. 13 , 14 , 15
The COVID‐19 patients, especially critically ill ones, should pay attention to the high risk of bleeding during thromboprophylaxis. Older age is the high‐risk factor of both thrombosis and hemorrhage. 11 , 16 Nearly 70% of patients in our study had age‐related bleeding risk. Besides age, coexisting medical conditions, including tumors, renal or liver failure, hypertension, and diabetes, brought the risk of bleeding in our patients. Moreover, some invasive treatments increased the bleeding risk, especially ECMO, which is widely used in critically ill patients. 17
Our findings confirmed four patients with VTE complications. The VTE rate was 2.9% in all COVID‐19 patients. Recent small‐scale investigations have shown that the incidence of VTE for COVID‐19 patients in ICU was 25% to 31%, which is much higher than the rate in our study. However, there have been few reports of VTE complicated in patients with non‐critically ill, and the total incidences of thrombotic events in COVID‐19 patients is uncertain. The recent study from Milan showed that the rate of VTE was 4.1% in COVID‐19 patients, which was similar to our study. Furthermore, the number did not mean the rate of VTE complications occurring in COVID‐19 patients was low. More likely, it was the consequence of effective thromboprophylaxis in patients classified as being at high risk of thrombosis. The high incidence of VTE in critically ill patients of COVID‐19 despite the universal use of guideline‐recommended VTE prophylaxis was similar to sepsis 18 but markedly higher than published reports in critically ill patients without sepsis, 19 , 20 suggesting that dysregulated hemostasis and coagulation in severe COVID‐19.
Notably, both VTE complications and major bleeding events occurred in critically ill patients. Hence, routine thromboprophylaxis was provided to critically ill patients based on an individual assessment of their thrombosis and bleeding risks in our study. For critically ill patients with extremely high levels of D‐dimer and Fibrinogen degradation product (FDP) associated with pulmonary microthrombosis, heparin was recommended. For those at a very high risk of bleeding, mechanical prophylaxis was instituted. 21 For those who used ECMO, better control of the aPTT (through better control of either coagulopathy or anticoagulation) was essential. 22
This study has several limitations. First, a small population was included in this study. We hope that the findings presented here will encourage a larger cohort study. Second, this is a retrospective study. The data in this study permit a preliminary assessment of VTE and the bleeding risk of patients with COVID‐19. Further prospective studies need to determine the exact incidence of VTE among these patients and focus on hemorrhage complications during thromboprophylaxis.
In conclusion, critically ill patients with COVID‐19 suffered both a high risk of thrombosis and bleeding risks. However, the prediction risk of VTE and major bleeding was low in non‐critically patients. More effective VTE prevention strategies based on an individual assessment of bleeding risks were necessary for critically ill patients with COVID‐19.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICS STATEMENT
The Ethics Commission of Shanghai Public Health Clinical Center approved this study. Written informed consent was waived due to the rapid emergence of this infectious disease.
AUTHOR CONTRIBUTIONS
Lan Wang designed the study and drafted the manuscript. Lan Zhao and Feng Li collected the data and co‐drafted the manuscript. Jinmin Liu and Jin‐fu Xu co‐designed the study and characterized patients. Ji Liu, Qiuhong Li, Jin Gu, Shuo Liang, and Qinhua Zhao characterized patients, interpreted data, and helped writing the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank all patients involved in the study.
The work was funded by the Program of National Natural Science Foundation of Shanghai (18ZR1431500).
Wang L, Zhao L, Li F, et al. Risk assessment of venous thromboembolism and bleeding in COVID‐19 patients. Clin Respir J. 2022;16(3):182-189. doi: 10.1111/crj.13467
Drs. Wang, Zhao and Li contributed equally to this work and are joint first authors.
Funding information Program of National Natural Science Foundation of Shanghai, Grant/Award Number: 18ZR1431500
Contributor Information
Jinmin Liu, Email: jinmingliu@tongji.edu.cn.
Jin‐fu Xu, Email: jfxucn@163.com.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet. Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Streiff MB, Agnelli G, Connors JM, et al. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J Thromb Thrombolysis. 2016;41(1):32‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I9‐I16. [DOI] [PubMed] [Google Scholar]
- 8. WHO . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. 2020. Jan 11, 2020.
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450‐2457. [DOI] [PubMed] [Google Scholar]
- 11. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69‐79. [DOI] [PubMed] [Google Scholar]
- 12. Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID‐19. Lancet Haematol. 2020;7(5):e362‐e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng DX, Xu JL, Mao QX, et al. Association of Padua prediction score with in‐hospital prognosis in COVID‐19 patients. QJM. 2020;113(11):789‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klok FA, Barco S, Turpie AGG, et al. Predictive value of venous thromboembolism (VTE)‐BLEED to predict major bleeding and other adverse events in a practice‐based cohort of patients with VTE: results of the XALIA study. Br J Haematol. 2018;183(3):457‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965‐1975. [DOI] [PubMed] [Google Scholar]
- 18. Kaplan D, Casper TC, Elliott CG, et al. VTE incidence and risk factors in patients with severe sepsis and septic shock. Chest. 2015;148(5):1224‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M. Venous thromboembolic disease: an observational study in medical‐surgical intensive care unit patients. J Crit Care. 2000;15(4):127‐132. [DOI] [PubMed] [Google Scholar]
- 20. Cook D, Crowther M, Meade M, et al. Deep venous thrombosis in medical‐surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33(7):1565‐1571. [DOI] [PubMed] [Google Scholar]
- 21. Wells PS, Forgie MA, Rodger MA. Treatment of venous thromboembolism. Jama. 2014;311(7):717‐728. [DOI] [PubMed] [Google Scholar]
- 22. Sy E, Sklar MC, Lequier L, Fan E, Kanji HD. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: a systematic review and meta‐analysis. J Crit Care. 2017;39:87‐96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


