Abstract
Background
Bullous pemphigoid (BP) is the most frequent autoimmune blistering disease of the skin affecting the elderly. BP is immunopathologically characterized by autoantibodies against BP180 and BP230. With the growing evidence of cell‐mediated autoimmunity in the pathogenesis of BP, it still remains unclear whether mast cells (MCs) are involved, due to conflicting data obtained from Kit‐dependent MC‐deficient mouse models.
Objectives
To clarify the role of MCs in experimental BP; the dynamics in cutaneous MC numbers, associated immune cells and the development of disease in Kit‐independent MC‐deficient mouse model.
Methods
Employing a recently established murine adult passive transfer model of BP induced by the transfer of pathogenic immunoglobulin G (IgG), lesional skin biopsies were investigated histologically and immunohistochemically for the time‐dependent MC accumulation and dermal infiltration.
Results
The numbers of cutaneous MCs increased following the induction of BP, in part, maintained by MC proliferation. Numbers of T cells, neutrophils and eosinophils in the skin also increased after BP induction, with eosinophils showing a preferential co‐localization with MCs. Furthermore, clinical disease manifestation in MC‐deficient Mcpt5Cre/Dicer fl/fl mice remained unchanged compared to MC‐sufficient Dicer fl/fl mice. The composition of the immune cell infiltration including as T cells, neutrophils and eosinophils was largely unaffected by the absence of MCs.
Conclusion
MCs do not play a pivotal role in the pathogenesis of passive IgG‐transfer mediated BP model. Their increase in number may be a bystander effect following tissue injury. We therefore suggest caution regarding the selection of MCs as sole targets for the development of novel drugs for BP.
1.
What is already known about this topic?
There is much controversy surrounding the role of mast cells in bullous pemphigoid. Previous studies have employed the widely used Kit‐dependent mast cell‐deficient mouse strains, which are associated with several limitations.
What does this study add?
We report for the first time the activity of mast cells in experimental bullous pemphigoid, using a Kit‐independent mast cell‐deficient mouse model.
We describe the clinical inflammatory milieu orchestrated by dermal infiltrates in a time‐dependent manner, providing a better understanding of the disease pathogenesis.
2. INTRODUCTION
Bullous pemphigoid (BP) is an autoimmune skin blistering disease of the elderly, characterized by autoantibodies against two hemidesmosomal antigens BP180 and BP230 that adhere the epidermis to the dermis. 1 , 2 These autoantibodies cause loss of cell‐to‐matrix adhesion, resulting in subepidermal blistering. 2 , 3 , 4
While anti‐BP180 autoantibodies have been noted to directly remodel hemidesmosomal function, 5 skin pathology in BP is mostly driven by the induction of inflammation following the formation of immune complexes at the dermal‐epidermal junction (DEJ). 6 Cell‐mediated autoimmunity is a significant contributor to the pathogenesis of BP. 7 , 8 Numerous studies have demonstrated the potential role of mast cells (MCs) 9 , 10 , 11 , 12 , 13 , 14 as well as the involvement of infiltrating cells including neutrophils, 8 eosinophils 15 and basophils 16 , 17 in the pathogenesis of BP. The accumulation of these cellular infiltrates in the skin and subsequent production of deleterious substances, such as reactive oxygen species and matrix metalloproteinases among others, compromise the function of the dermal‐epidermal adhesion complex, leading to the separation of DEJ. 18 , 19 However, the mechanisms that govern the infiltration and kinetics of inflammatory cells in tissue destruction are complex and have only lately begun to be more readily understood.
Research into the role of such cellular infiltrates has explored the function of MCs in the immunopathogenesis of BP. Since early observations of MCs within the lesional skin biopsies of BP patients, 9 , 11 disease model‐based studies of their functional role in BP and epidermolysis bullosa acquisita (EBA), 13 , 20 have led to conflicting results. Whether MCs are functionally involved in the pathogenesis of BP has remained unclear.
Until recently, studies investigating the function of MCs in various disease models have used Kit‐dependent MC‐deficient mouse strains. 13 These strains, however, face some limitations such as anaemia and neutropenia in Kit W/Wv mice or neutrophilia and thrombocytosis in Kit W‐sh/W‐sh mice. 21 , 22 To overcome limitations associated with Kit‐dependent MC‐deficient mouse models, recent advances have led to the development of MC‐deficient mouse models independent of Kit 23 , 24 , 25 , 26 , 27 that, besides MC deficiency, have no other immune abnormalities, thus allowing the relevance of skin MCs to be studied.
To decipher the functional relevance of MCs in BP, we used a recently established a murine adult passive transfer model of BP 28 and assessed cutaneous infiltration of MCs in conjunction with other immune cells. Moreover, we studied the development of experimental BP in Kit‐independent MC‐deficient Mcpt5Cre/Dicer fl/fl mice. 27
3. MATERIALS AND METHODS
3.1. Mice
We used WT C57BL/6J mice 28 and heterozygous Mcpt5Cre mice, 23 which express Cre recombinase under control of the Mcpt5 promoter, that were crossed with Dicer fl/fl C57BL/6J mice. 29 As we and others have previously shown, Mcpt5Cre/Dicer fl/fl mice are characterized by selective constitutive ablation of connective tissue‐type MCs in various tissues including the back skin. 25 , 27 C57BL/6J mice were purchased from Janvier Labs (Le Genest‐Saint‐Isle, France). A total of 100 mice (43 females, 57 male) were used, all of which were aged between 6 and 13 weeks when used for experiments.
Mice were housed and bred at 22°C and 55% humidity, with a light‐dark cycle of 12 h, in specific pathogen‐free conditions and with ad libitum supply of food and water at the animal facility of the University of Luebeck, Luebeck, Germany. The experiments were performed in accordance with institutional and state guidelines on animal welfare, approved by the respective governmental administration and carried out by certified personnel.
3.2. Generation of anti‐COL17 IgG
The non‐collagenous region NC15A of murine type XVII collagen (COL17) was expressed as glutathione‐S‐transferase (GST) fusion protein, purified by affinity chromatography, 7 and the generation of pathogenic anti‐COL17 immunoglobulin G (IgG) was performed as described. 30 In brief, New Zealand white rabbits were immunized with recombinant GST‐tagged NC15A domain of murine COL17, the rabbit serum was purified using protein G, and the reactivity of IgG fractions was analyzed by indirect immunofluorescence microscopy of murine skin. 28 Normal rabbit serum was obtained from C.C.Pro (Oberdorla, Germany).
3.3. Adult passive transfer BP mouse model
Rabbit anti‐COL17 IgG or normal rabbit IgG was injected s.c. at doses of 20 mg per injection into the neck of WT and Mcpt5Cre/Dicer fl/fl mice every second day over the course of 12 days (6 × 20 mg in total). Skin lesions (erythema, erosions and crusts) were assessed on days 0, 2, 4, 8 and 12. The percentage of the total body surface affected by lesions was calculated as described (ears, 5%; eyes, 1%; snout, 2.5%; forelegs, 10%; hind legs, 20%; tail, 10%; trunk, 40%; oral mucosa, 2.5%; and head and neck, 9%). At different time points, we obtained skin biopsies from the neck region for histological and immunohistochemical analyses.
3.4. Histology and immunohistochemistry
For the assessment of BP features, paraffin‐embedded sections from skin biopsies (5‐μm‐thick) were stained with haematoxylin and eosin. Demonstration of IgG deposition at the basement membrane was performed on frozen sections of lesional skin biopsies using immunofluorescence staining with fluorescein isothiocyanate (FITC)‐conjugated AffiniPure Donkey anti‐rabbit IgG (1:100; Jackson Immuno Research), incubated for 1 h at room temperature and washed three times for 5 min in phosphate‐buffered saline. Toluidine blue staining was performed on paraffin‐embedded lesional skin for the immunohistochemical identification of MCs, whilst the visualization of MCs with immunoflorescence used avidin (dilution 1:200; BioLegend), blocked with 3% bovine serum albumin (Carl Roth, Karlsruhe, Germany) in Tris‐buffered saline (TBS). To enumerate T cells, neutrophils and eosinophils, we stained sections with rat anti‐CD3 (clone CD3‐12, dilution 1:100; Abcam), anti‐Ly6G (clone 1A8, dilution 1:100; Abcam) and anti‐major basic protein (MBP) (dilution 1:250; purchased from Jacobsen Laboratory, Division of Allergy, Asthma and Clinical Immunology, Mayo Clinic), respectively. To assess proliferation of MCs, sections were double stained with anti‐Ki67 (clone 16A8, dilution 1:100; BioLegend). Prior to incubation with antibodies, we performed antigen retrieval using citrate buffer at pH 6.0 (anti‐CD3 and anti‐Ki67) or enzymatic digestion (anti‐Ly6G and anti‐MBP; Digest‐All Pepsin; Life Technologies). All primary antibodies were diluted in TBS with normal goat serum (2.5%) and applied overnight. Corresponding isotype controls (rat IgG1, IgG2a; BioLegend) for all primary antibodies were applied in relation to their respective dilutions as indicated above. Alexa Fluor® 594‐coupled goat anti‐rat antibody was used as secondary antibody (dilution 1:200; Jackson ImmunoResearch). Histologic and immunoflorescence analyses were performed at 200× magnification in five high power fields. Counts were used to calculate the mean number of cells per mm2 using a BZ‐9000E series Keyence microscope with BZ Analyzer software (Keyence, Neu‐Isenburg) and Image J software 1.52q (National Institute of Health). The degranulation of MCs was assessed semiquantitatively according to Wershil et al. 31 with slight modifications. MCs were classified as not degranulated (intact) and degranulated (>10% of the granules exhibiting fusion or discharge).
3.5. Statistical analysis
Statistical analysis was performed, as indicated in the figure legends, with one‐way analysis of variance (ANOVA) with Dunn's multiple comparisons test, two‐way ANOVA with Sidak's and Tukey's multiple comparisons test as well as with Welch's t‐test using GraphPad Prism 8 (GraphPad Software). In all tests, a p‐value of 0.05 was considered to be statistically significant.
4. RESULTS
4.1. Passive transfer of anti‐COL17 IgG in adult mice produces clinical and immunopathological features of the human disease
In line with previous results, 28 , 32 , 33 we observed the induction of experimental BP by repetitive injections of anti‐COL17 pathogenic IgG into WT C57BL/6J mice (Figure 1). During BP induction, mice developed erythema and erosions on the ears, the neck, limbs, trunk, and around the eyes (Figure 1b). The disease severity in BP mice clearly increased with the progression of the disease (Figure 1e). Clinical disease manifestation was associated with IgG deposition along the DEJ (Figure 1c), subepidermal blistering, as well as a dermal leukocyte infiltration (Figure 1d).
FIGURE 1.
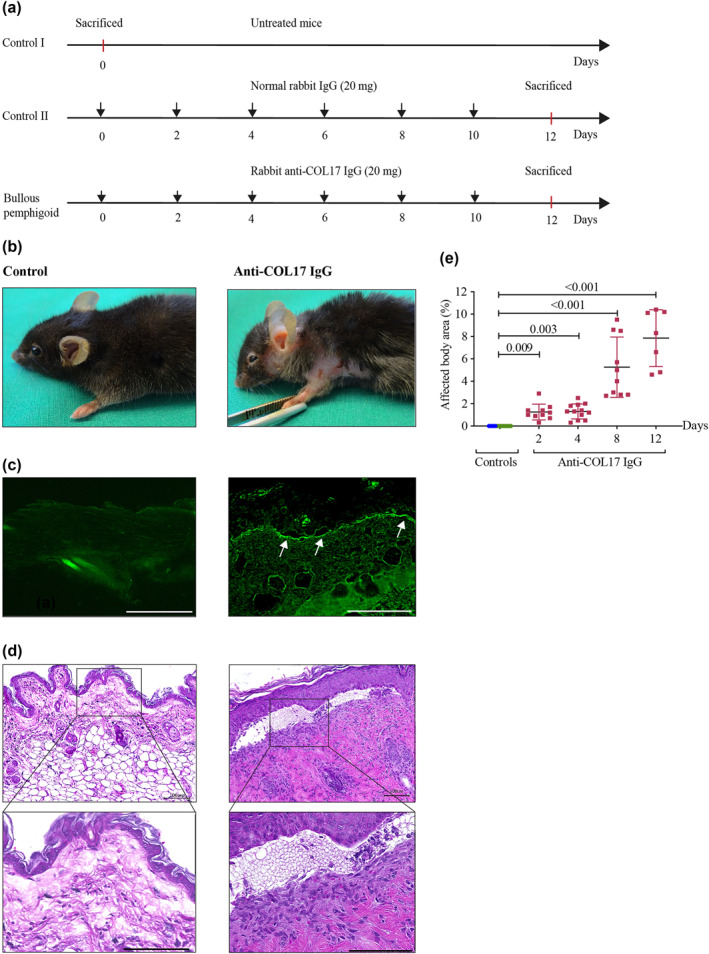
Experimental set‐up of passive transfer mouse model of bullous pemphigoid (BP) in adult mice. (a) Experimental BP was induced by s.c. injections of anti‐COL17 immunoglobulin G (IgG) on every second day over the course of 12 days (lower line; BP). Control mice were either not treated (upper line; control I) or treated with normal rabbit IgG over the course of 12 days (mid line; control II). (b–d) Representative photographs of the BP mouse phenotype. (b) Skin phenotypes on day 12 (left panel: control II; right panel: anti‐COL17 IgG‐treated mouse). Lesions developing in anti‐COL17 IgG‐treated mice are characterized by erythema, erosions and crusts. (c) Immunofluorescence staining with fluorescein isothiocyanate‐conjugated AffiniPure Donkey anti‐rabbit IgG of the dermal‐epidermal junction (DEJ) in normal rabbit IgG‐treated mouse (left panel) and lesional skin biopsy from anti‐COL17 IgG‐treated mouse (right panel) on day 4 (magnification: 400×, scale bar: 100 μm). White arrows indicate the deposition of IgG autoantibodies at the DEJ. (d) Haematoxylin and eosin staining of a skin biopsy obtained from control II (left photograph) and a lesional skin biopsy from anti‐COL17 IgG‐treated mouse on day 12 (right photograph). The photographs show the separation of the DEJ, thickened epidermis and dermal infiltration (upper panels, magnification: 200×, scale bar: 100 μm; lower panels, magnification: 400×, scale bar: 100 μm). (e) Time‐dependent development of BP lesions was scored by calculating the affected body surface as described. 28 Control mice (controls) included control I (n = 12, green dots) and control II (n = 6, blue dots). Statistical analysis was performed using one‐way analysis of variance with Dunn's multiple comparisons test. Data are presented as a scatter diagram with mean ± SD
4.2. Numbers of MCs are increased in experimental BP skin lesions
Following the induction of BP, we observed a significant increase in MC numbers as early as day 2 (Figure 2). Increased MC numbers remained elevated over the entire course of the experiment (Figure 2a,b). MCs were found to cluster particularly beneath the separated DEJ (Figure 2c). Despite the increase in MC numbers on day 2 after the initial injection of anti‐COL17 IgG and the persistence of elevated numbers of MCs with increasing disease activity, no correlation was observed between the number of MCs and disease activity (data not shown). This suggests that MCs are not the main drivers of the disease. The progressive increase of MCs and their preferential localization beneath the separated DEJ could be a bystander effect.
FIGURE 2.
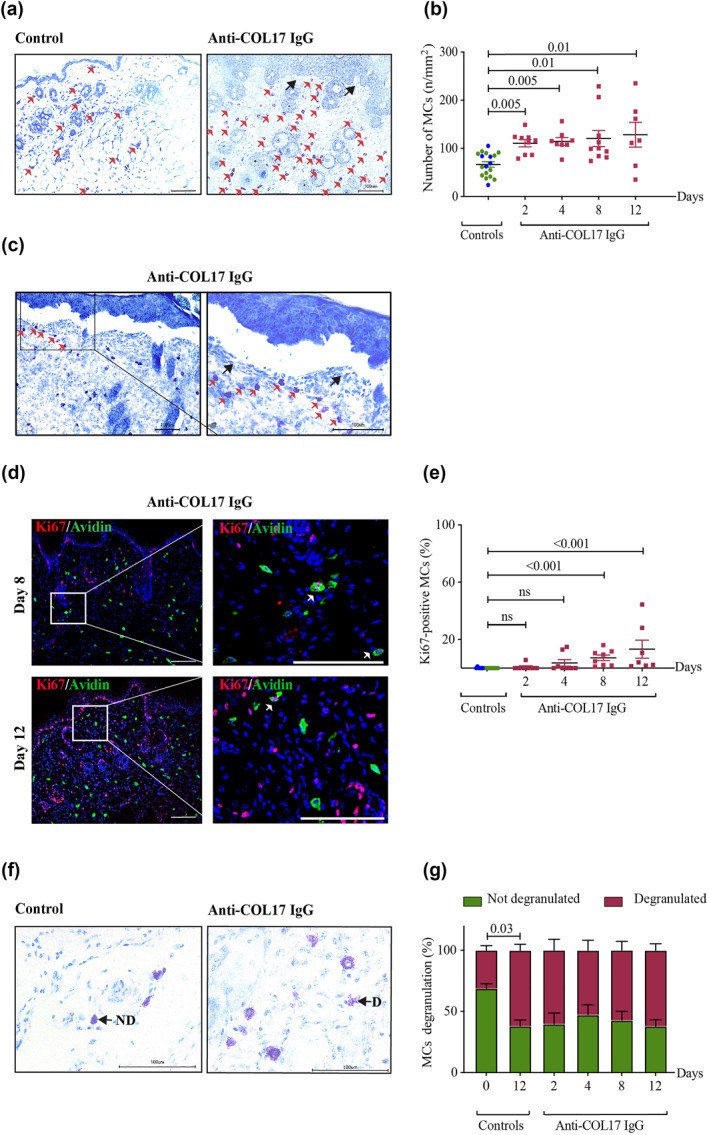
Cutaneous mast cells (MCs) increase in number and proliferate during experimental bullous pemphigoid (BP). (a–c) Increase in MC numbers as well as their preferential localization in experimental BP. (a, c) Red arrows indicate MCs and black arrows indicate separation of the dermal‐epidermal junction (a, magnification: 200×, scale bar: 100 μm; c, left image, magnification: 200×, scale bar: 100 μm; right image, magnification: 400×, scale bar: 100 μm). (d, e) Proliferation of MCs in experimental BP. (d) White arrows indicate double‐positive proliferating MCs (left panel, magnification: 200×, scale bar: 100 μm; right panel, magnification: 600×, scale bar: 100 μm). MCs were visualized using double immunofluorescence staining with avidin and anti‐Ki67. (f, g) Degranulation of MCs in experimental BP. (f) Black arrows indicate not degranulated and degranulated (d) MCs (magnification: 600×, scale bar: 100 μm). (a, c, f) MCs were visualized using toluidine blue staining. (b, e, g) Statistical analyses were performed using (b, e) one‐way analysis of variance (ANOVA) with Dunn's multiple comparisons tests and (g) two‐way ANOVA with Tukey's multiple comparisons test. Data are presented as (b, e) scatter diagrams with mean ± SEM and (e) as bar graph with mean ± standard error of mean (SEM). Control mice (controls) include control I (n = 12, green dots) and control II (n = 6, blue dots). (g) At each time point, six to eight mice were investigated
4.3. Proliferation of MCs is increased in late experimental BP
To explore the origin of increased MC numbers, we performed double immunofluorescence staining of MCs with avidin‐FITC and Ki67‐specific antibodies (Figure 2d,e). There were only few proliferating MCs detectable in the BP mouse skin samples on day 2, comparable to the numbers we detected in the samples of our controls. This indicates that mechanisms other than proliferation, such as extravasation or tissue redistribution, may primarily account for the early increase of MCs. On the other hand, pronounced proliferation could be observed on days 8 and 12, after multiple injections of pathogenic anti‐COL17 IgG. This suggests that proliferation is involved in maintaining increased MC numbers at later stages of the BP disease process.
4.4. MC degranulation is not specific to anti‐COL17 IgG treatment
We detected MC degranulation in anti‐COL17 IgG‐treated mice in situ upon injection of antibodies (Figure 2f,g). However, there was no significant difference between anti‐COL17 IgG‐treated mice and control mice which received normal rabbit IgG (Figure 2g). This suggests that MC degranulation is not specific to anti‐COL17 IgG but could rather be induced by repeated s.c. injections or provoked by scratching. Such mechanically induced MC degranulation has been reported by Xanthos et al. 34
4.5. Numbers of T cells, neutrophils and eosinophils are increased in experimental BP skin lesions
To explore the involvement of other inflammatory cells during the development of experimental BP, we next determined the numbers of T cells, neutrophils and eosinophils in the skin of WT C57BL/6J mice during the development of the BP phenotype over 12 days (Figure 3). By comparison of anti‐COL17 IgG‐treated mice with the controls, we found significantly higher numbers of T cells (Figure 3a,b), neutrophils (Figure 3c,d) and eosinophils (Figure 3e,f) in anti‐COL17 IgG‐treated mice, which confirms previous observations in humans 6 as well as this and other mouse models of BP, 4 , 13 , 28 , 33 indicating that the passive transfer model used in this study is suitable for the investigation of immune cells in BP. The numbers of neutrophils and eosinophils both increased at the beginning and in the late phase of disease (Figure 3c,e), possibly induced by the repeated injections of anti‐COL17 IgG, which may represent acute and chronic stages of inflammation. Interestingly, double staining with avidin‐positive MCs revealed that eosinophils (Figure 3f), unlike neutrophils (Figure 3d), often co‐localize with MCs, which suggests a possible cross‐talk between these two cell types.
FIGURE 3.
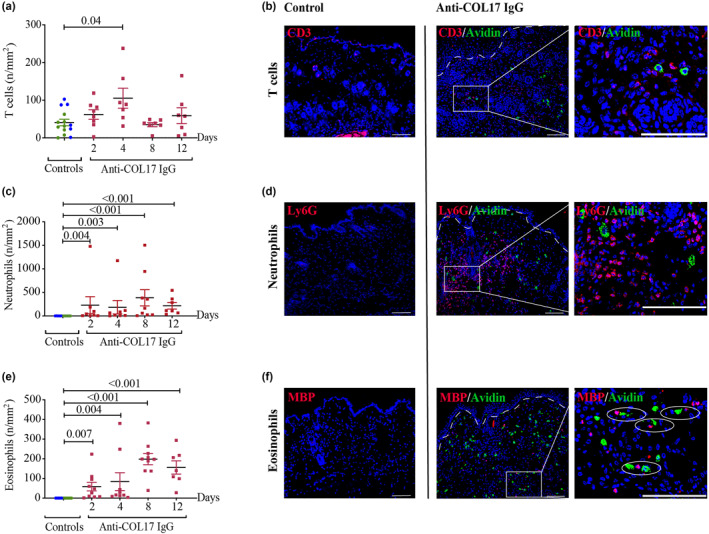
Numbers of T cells, neutrophils and eosinophils are increased in experimental bullous pemphigoid (BP) skin lesions. Lesional skin biopsies of anti‐COL17 immunoglobulin G‐treated mice were analyzed for (a, b) CD3‐positive T cells, (c, d) Ly6G‐positive neutrophils and (e, f) major basic protein‐positive eosinophils using immunofluorescence staining. (a, c, e) Statistical analysis was performed using one‐way analysis of variance with Dunn's multiple comparisons test. Data are presented as scatter diagrams with mean ± standard error of mean. Control mice (controls) include control I (n = 8, green dots) and control II (n = 6, blue dots). (b, d, f) Infiltrating of T cells on day 4, neutrophils on day 8 and eosinophils on day 12 are depicted using double immunofluorescence staining with avidin‐positive mast cells (MCs; middle panels, magnification: 200×, scale bar: 100 μm; right panels, magnification: 600×, scale bar: 100 μm). (f) Circles (right photograph) indicate co‐localization of eosinophils and MCs
4.6. Constitutive deficiency of MCs in Mcpt5Cre/Dicer fl/fl mice does not alter the development of experimental BP
Prompted by the above findings, we explored whether MCs play an active functional role in BP. For this purpose, we induced experimental BP in constitutively MC‐deficient Mcpt5Cre/Dicer fl/fl mice (Figure 4). Since repopulation of MCs has been reported in Kit W‐sh/W‐sh mice during inflammation, 12 , 21 we assessed MC numbers at different time points over the course of the experiment and were able to exclude repopulation of MCs in Mcpt5Cre/Dicer fl/fl mice on days 8 and 12 (Figure 4a; data not shown). Following the induction of BP, we found no significant difference in disease severity between Mcpt5Cre/Dicer fl/fl and Dicer fl/fl mice (Figure 4b). Both strains exhibited comparable clinical and histologic features, such as blisters, separation of DEJ, thickened epidermis as well as a dense cellular infiltrate in the upper dermis (Figure 4c,d), despite the near complete absence of MCs in Mcpt5Cre/Dicer fl/fl mice.
FIGURE 4.
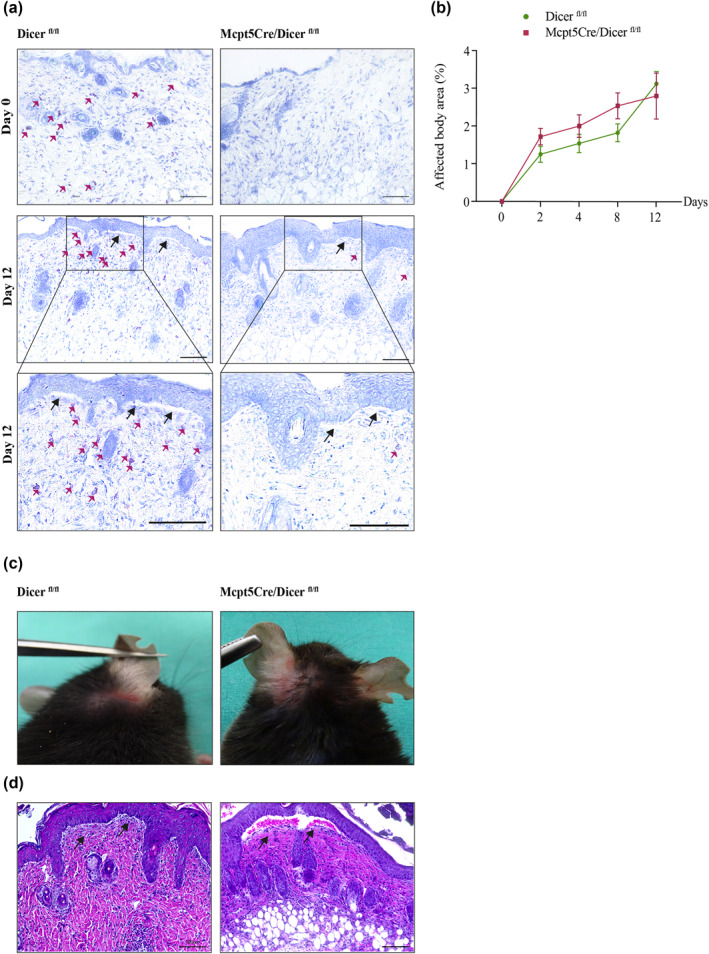
Constitutive deficiency of mast cells (MCs) in Mcpt5Cre/Dicer fl/fl mice does not alter the development of experimental bullous pemphigoid (BP). (a–d) Development of the BP phenotype in MC‐deficient Mcpt5Cre/Dicer fl/fl and MC‐sufficient Dicer fl/fl control mice. (a) To confirm the absence of MCs in Mcpt5Cre/Dicer fl/fl mice, histological staining of MCs with toluidine blue before and after the induction of BP was performed. Red arrows indicate MCs (upper and middle images, magnification: 200×, scale bar: 100 μm; lower images, magnification: 400×, scale bar: 100 μm). Black arrows indicate the separation of the dermal‐epidermal junction (DEJ). (b) Severity of the BP phenotype in Mcpt5Cre/Dicer fl/fl and Dicer fl/fl mice was assessed at different time points by calculating the affected body surface. At each time point, 6–19 mice were examined. Data were analyzed with two‐way analysis of variance with Sidak's multiple comparisons test. Data are presented as a curve diagram with mean ± standard error of mean. (c) Clinical manifestation of BP in Mcpt5Cre/Dicer fl/fl mice and Dicer fl/fl mice. (d) Representative photographs of histological staining (haematoxylin and eosin) of the head and neck lesional skin in MC‐deficient Mcpt5Cre/Dicer fl/fl and MC‐sufficient Dicer fl/fl control mice. Black arrows indicate the separation of the DEJ (magnification: 200×, scale bar: 100 μm)
4.7. Constitutive deficiency of MCs in Mcpt5Cre/Dicer fl/fl mice does not affect numbers of T cells, neutrophils and eosinophils
Considering the importance of cell‐mediated autoimmunity to the pathogenesis of BP, 7 , 8 it is believed that some of these cellular infiltrates, such as neutrophils, depend on MCs for their recruitment. 13 In order to investigate whether MC deficiency in experimental BP affects other immune cells, we evaluated the numbers of T cells, neutrophils and eosinophils in Mcpt5Cre/Dicer fl/fl and Dicer fl/fl mice on day 12 of our experiment (Figure 5). There were no significant differences in T cell (Figure 5a,b), neutrophil (Figure 5c,d) and eosinophil numbers (Figure 5e,f). This observation supports the notion that the absence of MCs in experimental BP is perhaps compensated by other immune cells with overlapping roles, highlighting that the recruitment of neutrophils in experimental BP can occur in a MC‐independent manner.
FIGURE 5.
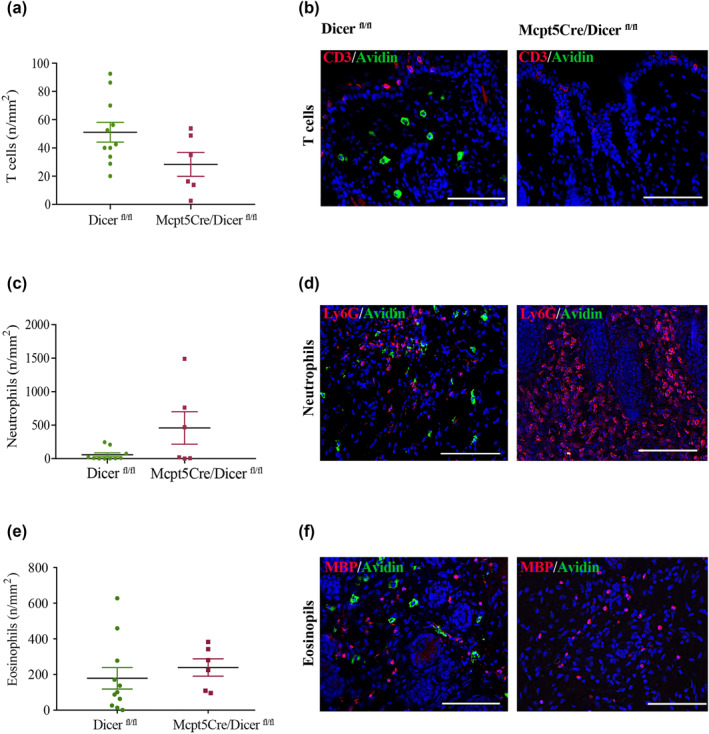
Constitutive deficiency of mast cells (MCs) in Mcpt5Cre/Dicer fl/fl mice does not alter the numbers of T cells, neutrophils and eosinophils in experimental bullous pemphigoid (BP). Lesional skin biopsies of anti‐COL17 immunoglobulin G‐treated MC‐deficient Mcpt5Cre/Dicer fl/fl mice and MC‐sufficient Dicer fl/fl control mice were analyzed for (a, b) CD3‐positive T cells, (c, d) Ly6G‐positive neutrophils and (e, f) major basic protein‐positive eosinophils using immunofluorescence staining on day 12. (a, c, e) Statistical analysis was performed with Welch's t‐test (no statistically significant differences were observed). Data are presented as scatter diagrams with mean ± standard error of mean. (b, d, f) Infiltrates of T cells, neutrophils and eosinophils on day 12 are depicted using double immunofluorescence staining with avidin‐positive MCs (magnification: 400×, scale bar: 100 μm)
5. DISCUSSION
The potential role of MCs in the pathogenesis of BP has been the subject of debate in recent times. Although increased numbers of MCs have been reported in a variety of human pathologies 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 and disease models, 44 , 45 , 46 , 47 , 48 in BP, the concept of increased MC numbers and MC degranulation was first reported as early as 1978 by Wintroub and colleagues. 9 To this day, however, the functional relevance of MCs in the pathogenesis of BP has remained controversial. This comes after one of the first murine studies using Kit‐dependent MC‐deficient mice reported protection from BP in these mice, 13 but later work by Kasprick et al., which employed the Kit‐independent Mcpt5Cre/iDTR fl/fl strain in their study of MCs in the a model of EBA contradicted this finding. 20 Yet, while both EBA and BP are often placed in the same context, they are not only different in regard to target autoantigens, 1 , 49 but also with respect to their different pathologies and prognosis.
To address the role of MC in BP, we herein specifically selected Mcpt5Cre/Dicer fl/fl mice because this strain holds several advantages compared to other MC‐deficient Mcpt5Cre/iDTR fl/fl mice. The Mcpt5Cre/iDTR mouse strain relies on selective expression of a high‐affinity receptor for diphtheria toxin (DT) in MCs and its targeting by DT treatment. 23 , 50 Previous studies investigating other inflammatory disease models in Mcpt5Cre/iDTR fl/fl mice have shown some repopulation of cutaneous and peritoneal MCs about 3 weeks after the last DT treatment, 51 , 52 which raises concern on the sufficiency of MC depletion for the study of autoimmune blistering diseases. Furthermore, 4 weeks of DT pretreatment, followed by 12 additional days of passive anti‐COL17 IgG injection into Mcpt5Cre/iDTR fl/fl mice is a lengthy experimental time that may put mice under stress, which in turn, influences MCs. 53 For this reason, Mcpt5Cre/Dicer fl/fl mice were employed to overcome these limitations.
Interestingly, in our Kit‐independent MC‐deficient Mcpt5Cre/Dicer fl/fl mice, experimental BP developed independently of MCs. Following the induction of BP, we observed a separation of the DEJ regardless of the absence of MCs. This finding is in contrast to previous studies reporting on protection from BP in Kit‐dependent MC‐deficient Kit W/Wv and Mgf Sl/Sl‐d mice. 13 , 21 These previous studies, however, employed a passive transfer model of BP in neonatal mice lasting only for 12–24 h, which limits the comparability to our results. However, our study ties in with recent reports on unaltered tumour growth 54 and wound healing 55 in Kit‐independent MC‐deficient mice, despite pronounced MC infiltration. Furthermore, in our Mcpt5Cre/Dicer fl/fl mice, the composition of the immune cell infiltrate in the skin turned out to be largely unaffected by the absence of MCs. This implies that the absence or presence of MCs in experimental murine BP is not essential to drive skin pathology. The recruitment of neutrophils can be mediated in a MC‐independent manner. 14 Again, these findings contrast with previous observations made in Kit‐dependent MC‐deficient mice. For example, Chen et al. found that in Kit‐mutant mice, anti‐BP180 antibody‐induced neutrophil infiltration depends mainly on MCs. 13 Thus, our results, contradicting previous observations both on BP development as well as MC‐dependent immune cell infiltration in Kit‐dependent MC‐deficient mice, highlight the importance of a careful interpretation of data relying on Kit‐dependent MC‐deficient mouse models.
CONFLICT OF INTEREST
The authors confirm that no conflict of interest to declare.
ACKNOWLEDGEMENTS
The authors are pleased to acknowledge Dr. Alexander Tarakhovsky (Rockefeller University, NY, USA), who originally provided Dicer fl/fl mice. Mcpt5Cre/Dicer fl/fl mice were kindly provided by Dr. med. M. Worm, Charité, Berlin, Germany, who had received the mouse strain earlier from Prof. Dr. med. K. Hartmann, University of Cologne, Cologne, Germany. This research was funded by Deutsche Forschungsgemeinschaft, RTG 1727.
Open access funding enabled and organized by Projekt DEAL.
Nsiah‐Dosu S, Scholz C, Orinska Z, Sadik CD, Ludwig RJ, Schmidt E, et al. Mast cell‐deficient mice Mcpt5Cre/Dicer fl/fl redefine the role of mast cells in experimental bullous pemphigoid. Skin Health Dis. 2022;2(1):e70. 10.1002/ski2.70
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–32. [DOI] [PubMed] [Google Scholar]
- 2. Nishie W, Shimizu H. Doxycycline‐inducible autoimmune blistering skin disease model. In: Nakao K, Minato N, Uemoto S, editors. Innovative medicine: basic research and development. Tokyo: Springer; 2015. p. 109–17. [PubMed] [Google Scholar]
- 3. Schmidt‐Ullrich B, Rule A, Schaumburg‐Lever G, Leblanc C. Ultrastructural localization of in vivo‐bound complement in bullous pemphigoid. J Invest Dermatol. 1975;65(2):217–9. [DOI] [PubMed] [Google Scholar]
- 4. Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ‐specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92(5):2480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kitajima Y, Hirako Y, Owaribe K, Yaoita H. A possible cell‐biologic mechanism involved in blister formation of bullous pemphigoid: anti‐180‐kD BPA antibody is an initiator. Dermatology. 1994;189(Suppl. 1):46–9. [DOI] [PubMed] [Google Scholar]
- 6. Ambach A, Zillikens D, Klingert B, Hartmann AA, Burg G. Immune phenotyping of mononuclear infiltrate in bullous pemphigoid. Hautarzt. 1992;43(2):81–5. [PubMed] [Google Scholar]
- 7. Hirose M, Recke A, Beckmann T, Shimizu A, Ishiko A, Bieber K, et al. Repetitive immunization breaks tolerance to type XVII collagen and leads to bullous pemphigoid in mice. J Immunol. 2011;187(3):1176–83. [DOI] [PubMed] [Google Scholar]
- 8. Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, et al. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100(5):1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wintroub BU, Mihm MC Jr., Goetzl EJ, Soter NA, Austen KF. Morphologic and functional evidence for release of mast‐cell products in bullous pemphigoid. N Engl J Med. 1978;298(8):417–21. [DOI] [PubMed] [Google Scholar]
- 10. Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol. 2007;127(11):2605–11. [DOI] [PubMed] [Google Scholar]
- 11. Kaminska R, Helisalmi P, Harvima RJ, Naukkarinen A, Horsmanheimo M, Harvima IT. Focal dermal‐epidermal separation and fibronectin cleavage in basement membrane by human mast cell tryptase. J Invest Dermatol. 1999;113(4):567–73. [DOI] [PubMed] [Google Scholar]
- 12. Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol. 2013;13(5):362–75. [DOI] [PubMed] [Google Scholar]
- 13. Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108(8):1151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen R, Fairley JA, Zhao ML, Giudice GJ, Zillikens D, Diaz LA, et al. Macrophages, but not T and B lymphocytes, are critical for subepidermal blister formation in experimental bullous pemphigoid: macrophage‐mediated neutrophil infiltration depends on mast cell activation. J Immunol. 2002;169(7):3987–92. [DOI] [PubMed] [Google Scholar]
- 15. Lin L, Hwang BJ, Culton DA, Li N, Burette S, Koller BH, et al. Eosinophils mediate tissue injury in the autoimmune skin disease bullous pemphigoid. J Invest Dermatol. 2018;138(5):1032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66(8):1107–13. [DOI] [PubMed] [Google Scholar]
- 17. Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, et al. Identification of a potential effector function for IgE autoantibodies in the organ‐specific autoimmune disease bullous pemphigoid. J Invest Dermatol. 2003;120(5):784–8. [DOI] [PubMed] [Google Scholar]
- 18. Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, et al. Gelatinase B‐deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188(3):475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Z, Shapiro SD, Zhou X, Twining SS, Senior RM, Giudice GJ, et al. A critical role for neutrophil elastase in experimental bullous pemphigoid. J Clin Invest. 2000;105(1):113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kasprick A, Yu X, Scholten J, Hartmann K, Pas HH, Zillikens D, et al. Conditional depletion of mast cells has no impact on the severity of experimental epidermolysis bullosa acquisita. Eur J Immunol. 2015;45(5):1462–70. [DOI] [PubMed] [Google Scholar]
- 21. Yu X, Kasprick A, Hartmann K, Petersen F. The role of mast cells in autoimmune bullous dermatoses. Front Immunol. 2018;9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitamura Y, Go S, Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978;52(2):447–52. [PubMed] [Google Scholar]
- 23. Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, et al. Mast cell‐specific Cre/loxP‐mediated recombination in vivo. Transgenic Res. 2008;17(2):307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dahdah A, Gautier G, Attout T, Fiore F, Lebourdais E, Msallam R, et al. Mast cells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. J Clin Invest. 2014;124(10):4577–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oh SY, Brandal S, Kapur R, Zhu Z, Takemoto CM. Global microRNA expression is essential for murine mast cell development in vivo. Exp Hematol. 2014;42(10):919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forster A, Blissenbach B, Machova A, Leja S, Rabenhorst A, Wilmschen S, et al. Dicer is indispensable for the development of murine mast cells. J Allergy Clin Immunol. 2015;135(4):1077–80. [DOI] [PubMed] [Google Scholar]
- 28. Schulze FS, Beckmann T, Nimmerjahn F, Ishiko A, Collin M, Kohl J, et al. Fcγ receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am J Pathol. 2014;184(8):2185–96. [DOI] [PubMed] [Google Scholar]
- 29. Yi R, O'Carroll D, Pasolli HA, Zhang Z, Dietrich FS, Tarakhovsky A, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet. 2006;38(3):356–62. [DOI] [PubMed] [Google Scholar]
- 30. Sitaru C, Mihai S, Otto C, Chiriac MT, Hausser I, Dotterweich B, et al. Induction of dermal‐epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115(4):870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE‐dependent cutaneous late phase reactions in the mouse is mast cell‐dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor‐alpha. J Clin Invest. 1991;87(2):446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karsten CM, Beckmann T, Holtsche MM, Tillmann J, Tofern S, Schulze FS, et al. Tissue destruction in bullous pemphigoid can be complement independent and may be mitigated by C5aR2. Front Immunol. 2018;9(9):488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chakievska L, Holtsche MM, Kunstner A, Goletz S, Petersen BS, Thaci D, et al. IL‐17A is functionally relevant and a potential therapeutic target in bullous pemphigoid. J Autoimmun. 2019;96:104–12. [DOI] [PubMed] [Google Scholar]
- 34. Xanthos DN, Gaderer S, Drdla R, Nuro E, Abramova A, Ellmeier W, et al. Central nervous system mast cells in peripheral inflammatory nociception. Mol Pain. 2011;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patella V, Marino I, Arbustini E, Lamparter‐Schummert B, Verga L, Adt M, et al. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97(10):971–8. [DOI] [PubMed] [Google Scholar]
- 36. Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33(6):961–6. [DOI] [PubMed] [Google Scholar]
- 37. Molin D, Edstrom A, Glimelius I, Glimelius B, Nilsson G, Sundstrom C, et al. Mast cell infiltration correlates with poor prognosis in Hodgkin's lymphoma. Br J Haematol. 2002;119(1):122–4. [DOI] [PubMed] [Google Scholar]
- 38. Ribatti D, Vacca A, Marzullo A, Nico B, Ria R, Roncali L, et al. Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B‐cell non‐Hodgkin's lymphomas. Int J Cancer. 2000;85(2):171–5. [PubMed] [Google Scholar]
- 39. O'Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, et al. Increased mast cells in the irritable bowel syndrome. Neuro Gastroenterol Motil. 2000;12(5):449–57. [DOI] [PubMed] [Google Scholar]
- 40. Ammit AJ, Bekir SS, Johnson PR, Hughes JM, Armour CL, Black JL. Mast cell numbers are increased in the smooth muscle of human sensitized isolated bronchi. Am J Respir Crit Care Med. 1997;155(3):1123–9. [DOI] [PubMed] [Google Scholar]
- 41. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast‐cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–705. [DOI] [PubMed] [Google Scholar]
- 42. Sugamata M, Ihara T, Uchiide I. Increase of activated mast cells in human endometriosis. Am J Reprod Immunol. 2005;53(3):120–5. [DOI] [PubMed] [Google Scholar]
- 43. Nakajima S, Krishnan B, Ota H, Segura AM, Hattori T, Graham DY, et al. Mast cell involvement in gastritis with or without Helicobacter pylori infection. Gastroenterology. 1997;113(3):746–54. [DOI] [PubMed] [Google Scholar]
- 44. Dahlin JS, Feinstein R, Cui Y, Heyman B, Hallgren J. CD11c+ cells are required for antigen‐induced increase of mast cells in the lung. J Immunol. 2012;189(8):3869–77. [DOI] [PubMed] [Google Scholar]
- 45. Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116(6):1633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, et al. Pulmonary CXCR2 regulates VCAM‐1 and antigen‐induced recruitment of mast cell progenitors. Proc Natl Acad Sci U S A. 2007;104(51):20478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kakizoe E, Li SH, Kobayashi Y, Nishikori Y, Dekio S, Okunishi H. Increases in mast cells and chymase in fibroproliferative paws of collagen‐induced arthritic mice. Inflamm Res. 1999;48(6):318–24. [DOI] [PubMed] [Google Scholar]
- 48. Chang DZ, Ma Y, Ji B, Wang H, Deng D, Liu Y, et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2011;17(22):7015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Woodley DT, Briggaman RA, O'Keefe EJ, Inman AO, Queen LL, Gammon WR. Identification of the skin basement‐membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med. 1984;310(16):1007–13. [DOI] [PubMed] [Google Scholar]
- 50. Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, et al. A Cre‐inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2(6):419–26. [DOI] [PubMed] [Google Scholar]
- 51. Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34(6):973–84. [DOI] [PubMed] [Google Scholar]
- 52. Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33(12):613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, et al. Mast cells in stress, pain, blood‐brain barrier, neuroinflammation and Alzheimer's disease. Front Cell Neurosci. 2019;13:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghouse SM, Polikarpova A, Muhandes L, Dudeck J, Tantcheva‐Poor I, Hartmann K, et al. Although abundant in tumor tissue, mast cells have no effect on immunological micro‐milieu or growth of HPV‐induced or transplanted tumors. Cell Rep. 2018;22(1):27–35. [DOI] [PubMed] [Google Scholar]
- 55. Willenborg S, Eckes B, Brinckmann J, Krieg T, Waisman A, Hartmann K, et al. Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin‐induced fibrosis. J Invest Dermatol. 2014;134(7):2005–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


