Abstract
Background
Physical activity and vitamin D receptor (VDR) have been associated with type 2 diabetes mellitus (T2DM). However, the associations of VDR methylation with T2DM and physical activity remained unknown. We aimed to investigate whether VDR methylation was a link between physical activity and T2DM.
Methods
A 1:1 matching case‐control study was designed based on the Henan Rural Cohort Study, including 272 pairs of T2DM patients and nonpatients. Physical activity level was assessed using the International Physical Activity Questionnaire. The high‐resolution melt method was applied to determine the methylation level of the promoter region of VDR. The association between physical activity and T2DM was analyzed with a conditional logistic regression model. The effect modification of VDR methylation levels on the association between physical activity and T2DM was conducted. A multivariate correlation analysis model was applied to investigate correlations of VDR methylation with insulin sensitivity.
Results
Physical activity level was associated with T2DM risk (crude model: odds ratio [OR] 0.611; 95% CI, 0.416‐0.897; adjusted model: OR 0.619; 95% CI, 0.418‐0.917). In effect modification analysis, the effects of physical activity on T2DM were stronger for low VDR methylation levels than for high (P = .025). Moreover, VDR methylation levels were associated with insulin (r = −0.089, P = .039) as well as homeostatic model assessment of insulin resistance (r = −0.098, P = .022).
Conclusions
The methylation status of the VDR promoter is associated with the secretion and sensitivity of insulin. VDR methylation attenuates the association between physical activity and T2DM, indicating that proactively physical activity may reduce the risk of T2DM, especially in people with low VDR methylation level.
Keywords: DNA methylation, insulin resistance, physical activity, type 2 diabetes mellitus, vitamin D receptor
Highlights
Vitamin D receptor (VDR) methylation attenuates the association between physical activity and type 2 diabetes mellitus (T2DM).
The methylation status of the VDR promoter is associated with the secretion and sensitivity of insulin.
A high physical activity level is associated with a decreased risk of T2DM compared to a low physical activity level.
摘要
背景
体力活动和维生素D受体(VDR)与2型糖尿病 (T2DM)有关。然而, VDR甲基化与T2DM和体力活动的关系仍然未知。我们旨在研究VDR甲基化是否是体力活动和T2DM之间的连接。
方法
在河南农村队列研究的基础上设计1:1配对病例对照研究, 包括272对T2DM患者和非糖尿病患者。体力活动水平使用国际体育活动问卷进行评估。应用高分辨率熔融曲线(HRM)测定VDR启动子区的甲基化水平。用条件逻辑回归模型分析体力活动与T2DM的关系。对VDR甲基化水平在体力活动和T2DM之间的联系进行效应修正。应用多元相关分析模型研究VDR甲基化与胰岛素敏感性的相关性。
结果
体力活动水平与T2DM风险相关(原始模型:OR: 0.611, 95%可信区间:0.416‐0.897;校正后模型:OR: 0.619, 95% CI: 0.418‐0.917)。在效应修正分析中, 体力活动对T2DM效应的影响在低VDR甲基化水平的个体强于高VDR甲基化水平(P=0.025)。此外, VDR甲基化水平与胰岛素(r=‐0.089, P=0.039)以及HOMA‐IR(r=‐0.098, P=0.022)相关。
结论
VDR启动子甲基化状态与胰岛素的分泌和敏感性有关。VDR甲基化减弱了体力活动和T2DM之间的联系, 表明积极的体力活动可以降低T2DM风险, 尤其是在VDR甲基化水平较低的人群中。
Keywords: 维生素D受体, DNA甲基化, 2型糖尿病, 胰岛素抵抗, 体力活动
1. INTRODUCTION
Physical activity plays a significant role in prevention of type 2 diabetes mellitus (T2DM). 1 , 2 Many studies have indicated an inverse association between physical activity and the risk of T2DM. 1 , 2 , 3 Increased physical activity could reduce the risk caused by sedentary behavior. 2 , 4 Besides increasing glycolipid uptake and utilization, another beneficial effect of physical activity on T2DM is to regulate expressions of metabolic genes by modulating DNA methylation. 5 Variations of DNA methylation in human islets, skeletal muscle, adipose tissue, and liver were found to be associated with T2DM. 6 Therefore, a better insight into the relationship between physical activity and T2DM could be obtained by investigating DNA methylation caused by physical activity.
The widely known function of vitamin D is to maintain calcium homeostasis and bone metabolism. 7 In the past two decades, accumulative evidence suggested that vitamin D played a role in other biological activities, for example, proliferation, differentiation, apoptosis, and immune response. 8 Thus, vitamin D is associated with many diseases including T2DM. 9 , 10 , 11 More and more studies showed that vitamin D deficiency may be a risk factor of T2DM. 12 , 13 Vitamin D supplementation would benefit prevention and control of T2DM. 14 , 15
The diverse biological functions of vitamin D are accomplished by precise changing of gene expression through the vitamin D receptor (VDR). As the key protein to mediate vitamin D function, VDR may play an important role in the development of T2DM. Meta‐analysis suggested that polymorphism in the VDR gene was associated with T2DM. 16 , 17 Recently, Wei et al reported that VDR as a key modulator of inflammation and β‐cell survival was a potential therapeutic target for T2DM. 18 Thus, aberrant expression of VDR may contribute to the development of T2DM.
DNA methylation as an important epigenetic regulator plays a key role in gene expression. 19 Studies suggested that increased methylation of the VDR promoter was associated with decreased mRNA and VDR protein. 20 , 21 Methylation variation of the VDR promoter had been reported to be associated with cancer. 20 , 22 However, the association between methylation status of the VDR promoter and T2DM remained unknown. Furthermore, whether physical activity is associated with the methylation status of VDR was also unclear.
Therefore, we hypothesized that physical activity could change VDR methylation status and further affect the development of T2DM. This study aimed to examine the relationship between physical activity and methylation variation of the VDR promoter and its effect on T2DM in a case‐control study. The results would shed more light on the role of physical activity in the prevention and control of T2DM.
2. METHODS
2.1. Study subjects
A total of 272 people with T2DM aged 18 to 79 years were from Zhengzhou, Henan province, China. Then 272 individuals without T2DM from the same residential area were selected according to the rule of same gender, same exposure of smoking and drinking, and similar age (less than 3 years difference) to carry out a 1:1 matching case‐control study. The guidelines of the American Diabetes Association (2002) and the criteria of the World Health Organization (1999) were applied to diagnose patients with T2DM. And all the non‐T2DM participants were determined by an oral glucose tolerance test with 75 g glucose.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Life Science Ethics Review Committee of Zhengzhou University. Written informed consent was obtained from all subjects.
2.2. Data collection
The 544 subjects’ information was collected by a standardized questionnaire and physical examination. The questionnaires were completed in face‐to‐face interviews by well‐trained investigators. All the subjects’ weight and height were measured three times, and the averages were used to calculate their body mass index (BMI). All the participants were asked to complete the International Physical Activity Questionnaire‐Short Form (IPAQ‐SF). The IPAQ recorded the different intensity level of physical activity in the last 7 days, including running, brisk walking, hoeing, mountain climbing, jogging, walking, sedentary behavior, etc. The information collected through the IPAQ can be transformed into continuous variables, indicating metabolic equivalents of tasks (MET). According to IPAQ's algorithm, combined with MET and the time and intensity of daily activities, physical activities were divided into three levels: low, moderate, and high. For a detailed scoring protocol, please refer to the website: https://sites.google.com/site/theipaq/scoring-protocol.
2.3. Measurements of glucose and insulin
A kit with the glucose oxidase method was applied for measurement of fasting blood glucose (Kehua Bio‐Engineering Co Ltd, Shanghai, China). The levels of fasting insulin were determined with a radioimmunoassay kit (North Institute of Biotechnology, Beijing, China).
2.3.1. Methylation levels of the VDR promoter region
Peripheral blood DNA was extracted using a whole blood genome DNA isolation kit (Bioteke Co Ltd, Beijing, China) according to the manufacturer's guidelines. The high‐resolution melt (HRM) method was applied to determine the methylation levels of the promoter region of VDR. The target sequence contains 27 CpGs within 300 bp (position from 47 905 396 to 47 905 695, chromosome 12, GRCh38.p12 assembly, National Center for Biotechnology Information [NCBI]). HRM assay was performed on the MeltDoctor HRM platform in the 7500 Fast System (Applied Biosystems, California). The results were processed with the HRM Software in Applied Biosystems.
2.4. Statistical analysis
Categorical variables are described as frequency and percentage and compared by the chi‐square test. Continuous variables with normal distribution are presented as means ± SD and compared with Student's t test. Continuous variables with skew distribution are presented as median (interquartile range) and compared with the Wilcoxon rank sum test.
We analyzed the data with the following strategy. First, we investigated the association between physical activity and T2DM with a conditional logistic regression model in the case‐control study. Second, to identify the combined associations of VDR methylation levels and physical activity with T2DM, VDR methylation levels were grouped according to corresponding medians values, and physical activity was divided into low physical activity and moderate to vigorous physical activity levels according to the criteria of IPAQ. The effect modification on a multiplicative scale was assessed by including the main effect of VDR methylation levels and physical activity and product term in analysis. 23 The P values of the product term were used to identify the effect modification on the multiplicative scale. The effect modification of physical activity on the association between VDR methylation levels and T2DM risk was computed based on the following equation:
where and are the estimates for the two subgroups, and and are their respective standard errors. Finally, a multivariate correlation analysis model was applied to investigate correlations of VDR methylation levels with insulin and homeostatic model assessment of insulin resistance (HOMA‐IR).
All the statistical analyses, except the interaction investigation, were completed with SPSS 21.0 (IBM SPSS, New York). A two‐tailed P value less than .05 was considered as statistically significant.
3. RESULTS
3.1. Subject characteristics
The demographic and biochemical characteristics of the study participants are shown in Table 1. Gender, smoking, and drinking were exactly matched between case and control. There was no significant difference in age between case and control (P = .973). BMI in the case group was higher than in controls (P = .002). Both the level of glucose and insulin in the case group were higher than those in the control (P < .001). There was no significant difference in methylation level of VDR between case and control (P = .673).
TABLE 1.
Demographic and biochemical characteristics of the study participants
| Variables | Control (n = 272) | Case (n = 272) | P | |
|---|---|---|---|---|
| Gender | ‐ | |||
| Male (%) | 101 (37.1) | 101 (37.1) | ||
| Female (%) | 171 (62.9) | 171 (62.9) | ||
| Age (y) | 59.4 ± 12.7 | 59.4 ± 12.6 | .973 | |
| Smoking | ‐ | |||
| Never (%) | 206 (75.7) | 206 (75.7) | ||
| Ever (%) | 19 (7.0) | 19 (7.0) | ||
| Current (%) | 47 (17.3) | 47 (17.3) | ||
| Drinking | ‐ | |||
| Never (%) | 241 (88.6) | 241 (88.6) | ||
| Ever (%) | 11 (4.0) | 11 (4.0) | ||
| Current (%) | 20 (7.4) | 20 (7.4) | ||
| BMI (kg/m2) | 25.5 ± 3.2 | 26.5 ± 3.8 | .002* | |
| Glucose (mmol/L) | 4.7 ± 0.8 | 9.4 ± 3.5 | <.001* | |
| Insulin (mIU/L) | 11.7 ± 6.5 | 14.8 ± 8.1 | <.001* | |
| VDR methylation (%) | 1.24 (1.15, 1.56) | 1.34 (1.15, 1.45) | .673 |
Note: Categorical variables are described as frequency and percentage and compared by a chi‐square test. Continuous variables with normal distribution are presented as means ± SD and compared with Student's t test. Continuous variables with skew distribution are presented as median (interquartile range) and compared with the Wilcoxon rank sum test. “‐” shows exact case‐control matching.
Abbreviations: BMI, body mass index; VDR, vitamin D receptor.
P < .05.
3.2. Association between physical activity and T2DM
The conditional logistic regression model was applied to investigate the association between physical activity and T2DM (Table 2). The result suggests that a high physical activity level is associated with a decreased risk of T2DM compared to a low physical activity level (odds ratio [OR] 0.611; 95% CI, 0.416‐0.897; P = .012). These associations persisted after adjusting for BMI and age (OR 0.619; 95% CI, 0.418‐0.917; P = .017).
TABLE 2.
Associations of physical activity and T2DM
| Physical activity | Control (n = 272) | Case (n = 272) | Crude model | Adjusted model a | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Low | 104 (38.2) | 133 (48.9) | Reference | Reference | ||
| Medium | 54 (19.9) | 49 (18.0) | 0.695 (0.434, 1.113) | .130 | 0.645 (0.398, 1.047) | .076 |
| High | 114 (41.9) | 90 (33.1) | 0.611 (0.416, 0.897) | .012* | 0.619 (0.418, 0.917) | .017* |
Note: The conditional logistic regression model was applied to investigate the association between physical activity and T2DM.
Abbreviations: OR, odds ratio; T2DM, type 2 diabetes mellitus.
Adjusted for age and body mass index.
P value <.05.
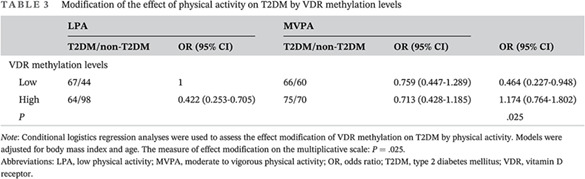
3.3. Effect modification of VDR methylation levels on T2DM by physical activity
The VDR methylation levels were divided into low and high, and according to the criteria of IPAQ, physical activity was divided into low physical activity and moderate to vigorous physical activity level. Effect modification analysis was used to test the association of VDR methylation levels and physical activity with T2DM. The results of effect modification analysis are shown in Table 3. VDR methylation status modified the correlation between physical activity and T2DM. The measure of effect modification on the multiplicative scale is represented by P values (P = .025). In the stratified analysis, the effects of physical activity on T2DM were stronger for low VDR methylation levels than for high (OR 0.464; 95% CI, 0.227‐0.948), which suggests that for people with low VDR methylation level, low physical activity increased the risk of T2DM.
TABLE 3.
Modification of the effect of physical activity on T2DM by VDR methylation levels
| LPA | MVPA | OR (95% CI) | |||
|---|---|---|---|---|---|
| T2DM/non‐T2DM | OR (95% CI) | T2DM/non‐T2DM | OR (95% CI) | ||
| VDR methylation levels | |||||
| Low | 67/44 | 1 | 66/60 | 0.759 (0.447‐1.289) | 0.464 (0.227‐0.948) |
| High | 64/98 | 0.422 (0.253‐0.705) | 75/70 | 0.713 (0.428‐1.185) | 1.174 (0.764‐1.802) |
| P | .025 | ||||
Note: Conditional logistics regression analyses were used to assess the effect modification of VDR methylation on T2DM by physical activity. Models were adjusted for body mass index and age. The measure of effect modification on the multiplicative scale: P = .025.
Abbreviations: LPA, low physical activity; MVPA, moderate to vigorous physical activity; OR, odds ratio; T2DM, type 2 diabetes mellitus; VDR, vitamin D receptor.
3.4. Associations of VDR methylation levels with insulin and HOMA‐IR
A multivariate correlation analysis model was applied to investigate correlations of VDR methylation levels with insulin and HOMA‐IR (Table 4). The results suggest that increased methylation levels of VDR are associated with decreased levels of insulin (r = −0.089, P = .039) as well as decreased HOMA‐IR (r = −0.098, P = .022), adjusted for covariates including physical activity, age, and BMI.
TABLE 4.
Correlations of VDR methylation levels with insulin and HOMA‐IR
| Variables | VDR methylation levels | |
|---|---|---|
| Correlation coefficient | P | |
| Insulin | −0.089 | .039* |
| HOMA‐IR | −0.098 | .022* |
Note: A multivariate correlation analysis model was applied to investigate correlations of VDR methylation levels with insulin and HOMA‐IR. Adjusted for physical activity, age, and body mass index.
Abbreviations: HOMA‐IR, homeostatic model assessment of insulin resistance; VDR, vitamin D receptor.
P value <.05.
4. DISCUSSION
Associations of physical activity with T2DM and VDR methylation were investigated in this study. The results suggest that a high physical activity level is associated with a decreased risk of T2DM compared to a low physical activity level. On the other hand, there was effect modification between VDR methylation status with physical activity and T2DM, which indicated that VDR methylation attenuated the association between physical activity and T2DM. In addition, increased methylation levels of VDR was associated with decreased levels of serum insulin as well as decreased HOMA‐IR.
The islets had a significant expression of the VDR gene. 24 VDR may play a role in maintaining the maturity of β‐cells. 25 Recently, Wei reported that VDR as a key modulator of inflammation and β‐cell survival was a potential therapeutic target for T2DM. 18 Thus, VDR is associated with insulin secretion. Pilon and colleagues reported that VDR gene promoter methylation may play a role in reduced expression of the VDR gene. 20 Meyer found that rs11568820 (AA vs AG/GG) in the VDR gene was associated with both higher VDR promoter methylation and lower VDR mRNA induction, which suggests that there may be an association between VDR promoter methylation and the levels of VDR mRNA. 21 Therefore, an increased methylation level of the VDR promoter may lead to decreased VDR expression and then less secretion of insulin.
Maestro reported that there was a vitamin D response element in the promoter of human insulin receptors, and VDR could specifically recognize the region to regulate the expression of the insulin receptors. 26 In summary, increased methylation levels of the VDR promoter may lead to decreased VDR expression. Decreased VDR levels may cause fewer insulin receptors, which would result in decreased insulin sensitivity. Due to the associations of VDR with insulin secretion and insulin sensitivity, VDR may play a role in development of T2DM. A meta‐analysis conducted by Han indicated that variants of ApaI, BsmI, and FokI in the VDR gene were associated with T2DM. 16 Another meta‐analysis we conducted also suggested that the FokI polymorphism in the VDR gene may be a risk factor of T2DM. 17 All these results demonstrate that VDR may play a role in development of T2DM.
It is worth noting that some studies reported a correlation between VDR methylation status and physical activity, which may be partly responsible for the effect modification, although this association has not been found in this study. Voisin et al reviewed the connection between exercise training and DNA methylation in humans. They found that both acute and chronic exercise could impact DNA methylation in a tissue‐ and gene‐specific manner. 27 Denham and colleagues reported that the sperm methylome could be reprogramed by 3 months of exercise training. 28 Thus, physical activity may decrease the methylation level of the VDR promoter and increase VDR expression. Physical activity may be beneficial for T2DM prevention by improving the biological functions of vitamin D through maintaining low methylation of the VDR promoter.
However, limitations must be acknowledged in the current study. First, the threshold of physical activity or VDR methylation status for T2DM risk could not be determined in the current analysis, which has significant implications for prevention strategies in susceptible populations. Future work will need to focus on the quantitative analysis of this association. Second, the current study could not establish causal associations because of the cross‐sectional design. Prospective, larger sample size research is needed in the future.
In conclusion, the methylation status of the VDR promoter is associated with the secretion and sensitivity of insulin. VDR methylation attenuates the association between physical activity and T2DM, indicating that physical activity should be proactively increased to reduce the risk of T2DM, especially in people with low VDR methylation levels.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure S1. The conceptual mediation model: a, indicates the path from physical activity (Predictor) to VDR methylation (Mediator); b, indicates the path from VDR methylation (Mediator) to T2DM (Outcome); c, indicates the path from physical activity (Predictor) to T2DM (Outcome); c’, indicates the path from physical activity (Predictor) to T2DM (Outcome) when controlled for VDR methylation (Mediator).
Table S1. Mediation analysis of the relationship between physical activity and T2DM by VDR methylation
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (no. 81703270, 81573151, and 81573243), the National Key Research and Development Program “Precision Medicine Initiative” of China (no. 2016YFC0900803), and the China Postdoctoral Science Foundation (no. 2020M672298).
Yu S, Feng Y, Qu C, et al. Vitamin D receptor methylation attenuates the association between physical activity and type 2 diabetes mellitus: A case‐control study. Journal of Diabetes. 2022;14(2):97-103. doi: 10.1111/1753-0407.13239
Funding information China Postdoctoral Science Foundation, Grant/Award Number: 2020M672298; National Natural Science Foundation of China, Grant/Award Numbers: 81573151, 81573243, 81703270; the National Key Research and Development Program “Precision Medicine Initiative” of China, Grant/Award Number: 2016YFC0900803
REFERENCES
- 1. Fan S, Chen J, Huang J, et al. Physical activity level and incident type 2 diabetes among Chinese adults. Med Sci Sports Exerc. 2015;47(4):751‐756. [DOI] [PubMed] [Google Scholar]
- 2. Lee DC, Park I, Jun TW, et al. Physical activity and body mass index and their associations with the development of type 2 diabetes in korean men. Am J Epidemiol. 2012;176(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 3. Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose‐response meta‐analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527‐2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D. Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol. 2019;73(16):2062‐2072. [DOI] [PubMed] [Google Scholar]
- 5. Yang D, Yang Y, Li Y, Han R. Physical exercise as therapy for type 2 diabetes mellitus: from mechanism to orientation. Ann Nutr Metab. 2019;74(4):313‐321. [DOI] [PubMed] [Google Scholar]
- 6. Davegardh C, Garcia‐Calzon S, Bacos K, Ling C. DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol Metab. 2018;14:12‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Driel M, van Leeuwen J. Vitamin D endocrinology of bone mineralization. Mol Cell Endocrinol. 2017;453:46‐51. [DOI] [PubMed] [Google Scholar]
- 8. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76‐89. [DOI] [PubMed] [Google Scholar]
- 9. Muscogiuri G, Altieri B, Annweiler C, et al. Vitamin D and chronic diseases: the current state of the art. Arch Toxicol. 2017;91(1):97‐107. [DOI] [PubMed] [Google Scholar]
- 10. Pittas AG, Dawson‐Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381(6):520‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garbossa SG, Folli F. Vitamin D, sub‐inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord. 2017;18(2):243‐258. [DOI] [PubMed] [Google Scholar]
- 12. Song Y, Wang L, Pittas AG, et al. Blood 25‐hydroxy vitamin D levels and incident type 2 diabetes: a meta‐analysis of prospective studies. Diabetes Care. 2013;36(5):1422‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akter S, Kuwahara K, Matsushita Y, et al. Serum 25‐hydroxyvitamin D3 and risk of type 2 diabetes among Japanese adults: the Hitachi health study. Clin Nutr. 2020;39(4):1218‐1224. [DOI] [PubMed] [Google Scholar]
- 14. Olt S. Relationship between vitamin D and glycemic control in patients with type 2 diabetes mellitus. Int J Clin Exp Med. 2015;8(10):19180‐19183. [PMC free article] [PubMed] [Google Scholar]
- 15. Krul‐Poel YH, Westra S, ten Boekel E, et al. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes (SUNNY trial): a randomized placebo‐controlled trial. Diabetes Care. 2015;38(8):1420‐1426. [DOI] [PubMed] [Google Scholar]
- 16. Han FF, Lv YL, Gong LL, Liu H, Wan ZR, Liu LH. VDR gene variation and insulin resistance related diseases. Lipids Health Dis. 2017;16(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu F, Cui LL, Li X, et al. The genetic polymorphisms in vitamin D receptor and the risk of type 2 diabetes mellitus: an updated meta‐analysis. Asia Pac J Clin Nutr. 2016;25(3):614‐624. [DOI] [PubMed] [Google Scholar]
- 18. Wei Z, Yoshihara E, He N, et al. Vitamin D Switches BAF Complexes to Protect β Cells. Cell. 2018;173(5):1135‐1149.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Law PP, Holland ML. DNA methylation at the crossroads of gene and environment interactions. Essays Biochem. 2019;63(6):717‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pilon C, Rebellato A, Urbanet R, et al. Methylation status of vitamin D receptor gene promoter in benign and malignant adrenal tumors. Int J Endocrinol. 2015;2015:375349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer V, Bornman L. Cdx‐2 polymorphism in the vitamin D receptor gene (VDR) marks VDR expression in monocyte/macrophages through VDR promoter methylation. Immunogenetics. 2018;70(8):523‐532. [DOI] [PubMed] [Google Scholar]
- 22. Castellano‐Castillo D, Morcillo S, Clemente‐Postigo M, et al. Adipose tissue inflammation and VDR expression and methylation in colorectal cancer. Clin Epigenetics. 2018;10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol. 2012;41(2):514‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hummel D, Aggarwal A, Borka K, Bajna E, Kállay E, Horváth HC. The vitamin D system is deregulated in pancreatic diseases. J Steroid Biochem Mol Biol. 2014;144 Pt B:402‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neelankal John A, Jiang FX. An overview of type 2 diabetes and importance of vitamin D3‐vitamin D receptor interaction in pancreatic beta‐cells. J Diabetes Complications. 2018;32(4):429‐443. [DOI] [PubMed] [Google Scholar]
- 26. Maestro B, Davila N, Carranza MC, Calle C. Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84(2–3):223‐230. [DOI] [PubMed] [Google Scholar]
- 27. Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiol. 2015;213(1):39‐59. [DOI] [PubMed] [Google Scholar]
- 28. Denham J, O'Brien BJ, Harvey JT, Charchar FJ. Genome‐wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics. 2015;7(5):717‐731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The conceptual mediation model: a, indicates the path from physical activity (Predictor) to VDR methylation (Mediator); b, indicates the path from VDR methylation (Mediator) to T2DM (Outcome); c, indicates the path from physical activity (Predictor) to T2DM (Outcome); c’, indicates the path from physical activity (Predictor) to T2DM (Outcome) when controlled for VDR methylation (Mediator).
Table S1. Mediation analysis of the relationship between physical activity and T2DM by VDR methylation


