Abstract
Although the exact mechanisms have yet to be elucidated, it is clear that cellular immunity plays a role in clearance of human papillomavirus (HPV) infections as it relates to the development of warts. Patients with extensive, recalcitrant, or treatment‐refractory warts may have an underlying immune system impairment at the root of HPV susceptibility. Early recognition of genetic disorders associated with immunologic defects that allow for recalcitrant HPV infection may expedite appropriate treatment for patients. Early recognition is often pivotal in preventing subsequent morbidity and/or mortality that may arise from inborn errors of immunity, such as WHIM (Warts, Hypogammaglobulinemia, Infections, Myelokathexis) syndrome. Among these, cervical cancer is one of the most common malignancies associated with HPV, can be fatal if not treated early, and is seen more frequently in patients with underlying immune deficiencies. A review of diseases with susceptibility to HPV provides clues to understanding the pathophysiology of warts. We also present diagnostic guidance to facilitate the recognition of inborn errors of immunity in patients with extensive and/or recalcitrant HPV infections.
1.
What is already known about this topic?
Warts caused by human papillomavirus often resolve in healthy individuals with or without treatment; however, some patients develop persistent, treatment‐refractory warts.
What does this study add?
Patients with recalcitrant warts may have an underlying immunodeficiency. Early diagnosis and treatment are important to limit progression to more serious morbidities, such as cancer. This review compiles information into one article to help specialists who may encounter patients with recalcitrant warts due to an underlying immunodeficiency.
2. INTRODUCTION
Human papillomavirus (HPV) is a ubiquitous and diverse species of viruses comprising >200 subtypes 1 , 2 , 3 infecting cutaneous and mucosal tissues, leading to warts, papillomas and cancers. 1 , 2 In the United States, >40 million people are estimated to be infected with disease‐associated HPV, with 13 million having acquired a new infection in 2018, 4 leading to high health care system burden ($8 billion in 2010). 5 The prevalence and potential for significant morbidity and mortality from HPV may be higher in patients with underlying immunodeficiencies such as Warts, Hypogammaglobulinemia, Infections, Myelokathexis (WHIM) syndrome. Similarly, patients with undiagnosed inherited or acquired immunodeficiencies may demonstrate refractory or more severe HPV infections. 5
3. AIMS AND METHODS
Herein, we aim to leverage the current literature and our clinical experience evaluating the underlying causes of HPV‐derived recurrent and recalcitrant warts. We conducted a PubMed search for articles containing the search terms ‘primary immunodeficiency’, ‘human papilloma virus (HPV)’ and ‘warts’. The search was performed in June 2021 and included English language results published at any time. Selection criteria for incorporation of the sources found in these searches consisted of relevance to the topic and primacy of the data. We then incorporated the published information on immunodeficiency in HPV infections and developed a proposed algorithm for evaluation and treatment of patients with severe or refractory HPV‐related infections.
4. HPV PATHOPHYSIOLOGY
HPV is divided into alpha, beta, gamma, mu and nu genera based on phylogenetic analysis. 3 The genus gamma is largest (n = 99), followed by alpha (n = 65), beta (n = 54), mu (n = 3) and nu (n = 1). 6 The majority of HPV research focuses on alpha and beta HPV, both of which are associated with cancer. 7 Alpha HPV preferentially infects mucosal epithelia but has been associated with cutaneous warts in normal hosts; a small subset causes anogenital or head and neck cancer. 6 , 7 Beta HPV typically infects cutaneous epithelia such as nail beds and hair follicles, and particularly in combination with ultraviolet (UV) exposure, may lead to skin cancers. 7 While less well understood, current conception of gamma HPV suggests that it rarely causes human disease, though some types may cause transient warts and, as emerging evidence indicates, may interact with tumour suppressor proteins such as p53. 3 Mu and nu subtypes have been associated with warts. 6
Unlike most viruses, multiple HPV strains may be present in a patient 3 , 8 but do not necessarily lead to disease, 9 and the molecular mechanisms of disease are strain specific. Beta and gamma HPV rarely cause disease, but alpha HPV has immunoevasive strategies allowing viruses to persist and form papillomas. 10 Entering a host cell, HPV hijacks host molecular machinery to replicate. 11 Initially, the HPV genome is maintained in the basal epithelium by replicating alongside host cellular DNA. Upon differentiation of basal cells into the suprabasal layers, where host DNA replication machinery is suppressed, HPV‐encoded E6 and E7 proteins enable continued machinery utilisation and can delay epithelial cell differentiation. E6 and E7 combination further facilitates HPV replication by overriding cell cycle checkpoints. 11 Ultimately, HPV speeds up cellular proliferation, induces blood vessel growth, and inhibits major histocompatibility complex expression. 12 These processes induce the clinical features observed in cutaneous and mucosal warts, including hyperkeratosis, small dotted vessels, and disruption of normal skin architecture. 13 , 14 Each HPV strain induces different wart morphologies, leading to clinical manifestations of specific subtypes (Table 1). 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
TABLE 1.
Types of HPV warts and associated strains
| Wart type | HPV strain |
|---|---|
| Common warts (includes filiform warts) (verruca vulgaris) 13 , 15 , 16 , 18 | 1, 2, 3, 4, 7, 27, 29, 57, 75–77 |
| Palmar and plantar warts (includes mosaic warts) 15 , 17 | 1, 2, 4, 27, 57 |
| Flat (plane) warts 13 , 15 | 3, 10, 26–29, 41 |
| Butcher's warts 13 | 7 |
| Cystic warts 13 | 60 |
| Genital warts 20 , 21 | 6, 11, 16, 18, 26, 30, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 85, 89, 97 |
| Ungual warts 19 | 1, 2, 4, 27, 57 |
| Oral warts 14 | 6, 11, 32 |
| Intermediate warts (features of common and flat warts) 14 | 2, 3, 10, 28 |
Note: Various types of warts and the known strains of HPV that are associated with their appearance.
Abbreviation: HPV, human papillomavirus.
Perhaps the most consequential aspect of HPV physiology is its oncogenic potential. In women, high‐risk HPV is associated with cervical intraepithelial neoplasia (CIN), a precursor to invasive cervical cancer if not cleared by the host's immune system. In men, high‐risk HPV is associated with head and neck squamous cell carcinoma and penile cancer. 9 Malignant transformation has been characterised primarily with alpha HPV, split into low and high risk based on propensity for malignancy. 7 , 12 The mechanisms underlying malignant transformation of alpha HPV stem from virally encoded E6 and E7 proteins that target apoptosis and cell cycle regulation via p53 and retinoblastoma tumour suppressor protein (pRb), respectively. 7 E6 and E7 overexpression can lead to incorporation into the host genome, leading to cell immortalisation. 7 Beta HPV is associated with cutaneous squamous cell carcinoma, but carcinogenesis mechanisms differ from high‐risk alpha HPV. 22 While beta HPV likely shares oncogenic similarities with low‐risk alpha HPV, factors such as UV radiation, which can be locally immunosuppressive, may also be involved. 7 , 23 , 24
5. HOST RESPONSE TO HPV
In immunocompetent hosts, HPV infection is eventually recognised and cleared. For subtypes causing genital infections, median HPV infection clearance is 5.9 months, regardless of whether low or high risk, and 75% of patients clear HPV infection within 12 months. 25 HPV persists in the epithelium longer than most viruses, crucial for its infective process. Though mechanisms are being investigated, HPV clearly evades the immune system using several mechanisms, including blocking immune‐related gene expression, immune signalling pathways, and antigen presentation machinery that typically generates epitopes for T cells to identify infected cells. 26 HPV enters through disruptions in the skin barrier—a first line of defence against HPV as evidenced by a higher risk of cutaneous HPV infections in children with atopic dermatitis. 11 , 27 Next, the virus binds via its L1 major capsid protein to heparan sulphate proteoglycans on the epithelial basement membrane and undergoes a change in conformation, exposing the N‐terminus of the L2 minor capsid protein. This prompts L2 proteolysis, which exposes a previously occluded surface of L1 that binds to an unknown receptor on keratinocytes that migrated to close the initial wound, allowing internalisation. 28
Inability to clear HPV may predispose malignant transformation. 7 , 12 Cluster of differentiation 4 (CD4) and CD8 T cells are present in genital warts as they regress, along with adhesion molecule upregulation involved in lymphocyte recruitment. Lymphocytes produce cytokines such as interleukin‐12 (IL‐12), tumour necrosis factor‐alpha (TNFα), and interferon‐gamma (IFNɣ), which are characteristic of a T‐helper 1 (Th1) phenotype, a proinflammatory response leading to cellular immunity. Further evidence for CD4 T‐cell/Th1 response involvement in clearing HPV infections is that individuals with asymptomatic HPV infections have shown strong Th1 response to E2 and E6 HPV proteins, whereas patients with CIN show impaired Th1 response. 29 Additionally, patients with cervical cancer due to HPV infection may have impaired CD8+ T‐cell activation, which leads to a lack of granzyme B and IL‐2R expression. 30
In patients with immunodeficiencies, host recognition and clearance of HPV may be impaired and may perpetuate infection simultaneously with viral immune evasion strategies. 12 , 29 This combination may cause resistant, refractory, or severe warts; progressive papillomas; and eventual malignant transformation. 29 In immunodeficient patients, non–disease‐causing strains may emerge, as evidenced by the recent discovery that beta and gamma HPV cause cutaneous warts. 3 , 29 Understanding dysfunctional immune system components in acquired and inherited immunodeficiencies can reveal immune pathways involved in HPV recognition, disease pathophysiology and host immune response.
6. IMMUNODEFICIENCIES AND HPV SUSCEPTIBILITY
Noninherited causes of immunodeficiency, including Human Immunodeficiency Virus (HIV), malignancy, connective tissue disease, chemotherapy, biologic therapy, and immunosuppressive agents, can lead to symptomatic HPV infections via impairment of several immune system components (Table 2). 2 , 31 , 32 , 33 , 34 , 35 , 36 HIV leads to CD4+ helper T‐cell depletion, and women with HIV are more susceptible to HPV infection, particularly with low CD4+ cell counts. 35 Similarly, altered immune function in diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE) is associated with increased HPV risk. 31 , 36 , 37 SLE is characterised by complex underlying immune dysregulation, including amplification of Th‐type cytokines such as IFN‐γ, IL‐4, IL‐5, IL‐6, and TNF superfamily members mediating increased crosstalk between innate and adaptive immune responses, leading to autoreactive B‐cell activation and persistence. 38 Cytokine milieu alterations may similarly predispose HPV persistence and immortalisation. 39
TABLE 2.
| Acquired | Inherited |
|---|---|
| HIV | WHIM syndrome |
| Malignancy | Epidermodysplasia verruciformis |
| Connective tissue disease | DOCK8 deficiency |
| Chemotherapy | GATA2 deficiency |
| Biologic therapy | LAD1 |
| Immunosuppressive agents | IL2RG/JAK3 deficiency |
| Ataxia telangiectasia | |
| CD28 deficiency | |
| Netherton syndrome | |
| NEMO | |
| SCID | |
| Wiskott–Aldrich syndrome |
Note: The causes of increased susceptibility to recurrent HPV infections, sorted by acquired and inherited causes. Many of the inherited causes are recognised inborn errors of immunity.
Abbreviations: DOCK8, dedicator of cytokinesis 8; GATA2, GATA binding factor 2; HIV, Human Immunodeficiency Virus; HPV, human papillomavirus; LAD1, leucocyte adhesion deficiency 1; NEMO, nuclear factor kappa‐B essential modulator; SCID, severe combined immunodeficiency; WHIM, warts, hypogammaglobulinemia, infections, and myelokathexis.
Many iatrogenic forms of immunosuppression increase HPV risk, including in patients receiving immunosuppression for solid organ transplants and patients receiving TNF inhibitors. 32 , 36 The increased risk of HPV attributable to TNF inhibitors may suggest a particular role for TNF in HPV clearance, evidenced by the capacity for TNFα to stimulate E6 and E7 proteins in HPV‐immortalised keratinocytes. 39
Inherited syndromes and/or immunodeficiencies may also carry greater risk of HPV infections. Diseases such as epidermodysplasia verruciformis (EV), WHIM syndrome, leucocyte adhesion deficiency 1 (LAD1), hyper IgE syndromes caused by dedicator of cytokinesis 8 (DOCK8) mutations, GATA binding protein 2 (GATA2) mutations, interleukin 2 receptor subunit gamma (IL2RG) or Janus kinase 3 (JAK3) deficiency, ataxia telangiectasia (AT), and T‐cell CD28 deficiency can predispose patients to HPV infections in the form of warts and HPV‐related malignancies (Table 2). 29 , 40 , 41 , 42 Integrin subunit β2 (ITGB2), WASP actin nucleation promoting factor (WAS), adenosine deaminase 2 (ADA2), and NFKB inhibitor alpha (NFKBIA) also affect broad elements of the immune system and bring occasional susceptibility to HPV warts. 40 Inherited immune defects found in the aforementioned conditions can provide additional insights into the immune response against HPV infection.
7. EPIDERMODYSPLASIA VERRUCIFORMIS
Epidermodysplasia verruciformis is caused by mutations in EVER1 and EVER2, thought to encode zinc‐transport proteins. 29 , 43 Patients with EV are the archetype of genetic HPV susceptibility. 44 The presence of decreased T lymphocytes and reduced cellular immunity (possibly due to impaired antigen presentation) underscores T‐cell function importance in HPV clearance. 29 However, because patients with EV are not susceptible to other viral pathogens, there may be a specific role of EVER1 and EVER2 in keratinocyte immune response to HPV. 45 In addition, patients with EV are prone to developing squamous cell carcinomas related to HPV infection, further highlighting the interplay between local (keratinocyte) and systemic immune response. 46
Traditionally nonpathogenic strains of HPV (e.g., beta subtype) can cause disease in patients with EV. 29 In EV, beta HPV susceptibility is hypothesised to result from a local TNF signalling defect, 47 zinc transport dysfunction, 48 or increased activity of transcription factors that override E5 protein absence in beta HPVs, which confers resistance in the presence of normal EVER protein function. 49
8. WHIM SYNDROME
Individuals with WHIM syndrome are uniquely susceptible to cutaneous warts associated with gamma HPVs—which generally do not cause disease in immunocompetent individuals. 3 These patients have a C‐X‐C chemokine receptor 4 (CXCR4) defect and experience recurrent bacterial infections due to neutropenia from impaired migration of polymorphonuclear cells to peripheral blood 50 , 51 and may be infected by multiple, simultaneous HPV strains. 3 The broader risk of infection stemming from CXCR4 mutations highlights the role of neutrophils in HPV immunity. 29 , 52 , 53 Lymphopenias may also occur in WHIM syndrome, yet disproportionate susceptibility to HPV versus other lymphopenic disorders may suggest specific susceptibility in WHIM, namely the CXCR4 gain‐of‐function mutation. 54 CXCR4 increases cell proliferation and immortalisation 55 and increases TNFα expression 56 ; both may contribute to HPV immunology. 11 , 29 Importantly, pharmacologic CXCR4 antagonism reduced gamma HPV predominance in patients with WHIM syndrome over time in a clinical trial, a potential target for wart treatment. 3
9. LEUCOCYTE ADHESION DEFICIENCY 1
LAD1 results in frequent skin and mucosal surface infection beginning in infancy and can be fatal. 57 LAD1 is caused by genetic mutations in the common chain (CD18) of β2 integrin that profoundly impair leucocyte mobilisation to inflammation sites. 57 Cutaneous and genital warts have been reported in patients with milder forms of LAD1; more severe forms lack this association due to bone marrow transplantation or death before manifestation. 29
10. DOCK8 MUTATIONS
Mutations underlying hyper IgE syndromes, such as DOCK8, present with a defect in dendritic cell and T‐cell migration, resulting in immunodeficiency characterised by increased cutaneous viral infection susceptibility, including HPV, herpes simplex virus, molluscum contagiosum, and varicella zoster virus. 29 , 58 IgE has an unclear role in HPV but could relate to changes in TNF‐α. 59
11. GATA2 MUTATIONS
Similar to DOCK8, missense or null mutations in GATA2, a transcription factor involved in haematopoiesis and stem cell maintenance, lead to a variety of presentations, with >75% of patients with GATA2 deficiency being infected with HPV. 29 , 60 Importantly, GATA2 mutations typically present in older children or even in adulthood 29 with delayed diagnosis. 60 GATA2 haploinsufficiency can also be attributed to progressive multiple cytopenias, resulting in mycobacterial and fungal infections, and high risk of myelodysplastic syndrome, acute myeloid leukemia, lymphoedema, and pulmonary alveolar proteinosis. Around 50% of patients with GATA2 haploinsufficiency have increased susceptibility to recurrent warts. 40
12. IL2RG OR JAK3 DEFICIENCY
Patients with IL2RG or JAK3 deficiency have a reduced number of natural killer (NK) cells with decreased cytotoxic activity and, presumably, an impaired ability to eliminate virally infected cells. 42 Patients with these deficiencies are known to carry a 50% risk of developing severe cutaneous HPV infections even after haematopoietic stem cell transplantation (HSCT), suggesting that the mutation either carries a defect intrinsic to the skin, or HSCT does not fully replace the faulty immunological component. 40
One patient with a pathogenic germline X‐linked IL2RG mutation was shown to have a second, somatic mutation in IL2RG that reversed the T‐cell deficiency but left the NK cell population affected. The expansion of the HPV skin virome and recalcitrant HPV‐related skin and mucosal warts of this patient support a role for NK cells in HPV susceptibility and clearance. In this case, both benign and malignant HPV‐associated warts cleared with HSCT. 42
13. ATAXIA TELANGIECTASIA
Ataxia telangiectasia (AT), which is caused by biallelic mutations of the ATM gene, affects multiple systems and leads to cerebellar degeneration, telangiectasia, immunodeficiency, and susceptibility to cancer. These mutations result in low B‐cell and naïve CD4+ and CD8+ T‐cell counts and abnormal B‐cell and T‐cell receptor repertoires due to the role of ATM in double‐stranded DNA breaks in V(D)J recombination. 40 The ATM defect ultimately results in impaired antigen recognition, antibody production, and cytotoxic elimination of HPV‐infected cells, resulting in around 20% of patients developing persistent HPV warts. 40 , 61
14. INHERITED T‐CELL CD28 DEFICIENCY
A family with inherited hyperkeratotic cutaneous papillomatosis caused by HPV‐2 infection was identified to have a mutation in CD28. CD28, a costimulatory receptor expressed by T cells, is essential for interactions with antigen‐presenting cells. Patients carrying these mutations exhibit deficient CD4+ immune response against HPV, suggesting a dependence on HLA class II interactions for immunity against some HPV strains. 41
The genetic pathologies listed above that are associated with susceptibility to HPV infection and warts all compromise essential elements of immune function, specifically viral immunity. Impaired T‐cell development, function, and/or mobilisation is present in GATA2, EV, and CD28 deficiency, while defects in antigen presentation are hallmarks of EV, CD28 deficiency, and DOCK8 mutations. 40 , 41 Innate immune cells (e.g., NK cells and neutrophils), which play a role in elimination of virus infection response, are affected in LAD1 deficiency, IL2RG/JAK3 deficiency, and WHIM syndrome. 42 , 50 , 51 , 57 All are essential to mounting an effective immune response to a viral pathogen; however, the very specific susceptibility to HPV and warts suggests a role for these immune elements in the HPV pathogenic mechanism itself. 41
This highlights the importance of recognising potential signs of underlying immunodeficiency, which may include recalcitrant warts. Indeed, genetic mosaicism and incomplete gene penetrance in some immunodeficiencies may complicate diagnosis. 62 Genetic testing has enabled earlier, more accurate diagnosis of suspected immunodeficiencies, 63 , 64 and LAD1 and WHIM syndrome can be confirmed through genetic testing despite heterogeneity in presentation. 50 , 57 Testing for these mutations can confirm diagnosis earlier and lead to a more targeted treatment approach.
15. CHARACTERISATION OF WART RECALCITRANCE, RECURRENCE, AND SEVERITY
With no universally accepted definition of treatment‐refractory, or recalcitrant, warts, a standard approach to evaluating and managing affected patients has been challenging. Leung suggested that warts are recalcitrant if not responding after five treatments over 6 months. 65 This timeline may be too short, as warts often resolve with or without treatment within 18–24 months. 66 , 67 , 68 Leung further estimated that approximately one‐third of common warts become recalcitrant 65 —a staggering number considering the overall prevalence of cutaneous warts. No currently accepted wart severity scale exists, which may be another important factor in understanding underlying immune phenotypes in patients with wart susceptibility. Recalcitrant and recurrent warts must also be distinguished. Recurrent warts resolve with treatment and reappear, suggesting underlying susceptibility to HPV infection as evidenced by HPV persistence and increased cervical cancer risk in certain human leucocyte antigen (HLA) subtypes. 65 , 69 We propose the definitions in Table 3. When observed, recalcitrant, recurrent, and/or severe warts should raise suspicion of underlying causes, specifically of inherited or acquired immunodeficiency. 29 , 70 , 71
TABLE 3.
Proposed definitions
| Definition | |
|---|---|
| Recalcitrant warts | Warts that persist for >18 months despite consistent treatment with ≥2 accepted modalities |
| Recurrent warts | Wart recurrence within 3–6 months of clearance of prior warts on >2 different occasions |
| Wart severity | Based on the number, overall size, thickness and location of warts |
Note: Proposed distinction between recurrent and recalcitrant warts as well as the factors influencing wart severity.
16. CONSIDERING IMMUNODEFICIENCY IN PATIENTS WITH RECALCITRANT WARTS
Prevalence of subclinical immune deficiencies is currently unknown. Nevertheless, in patients with severe, recurrent, or refractory warts, variations in aforementioned gene product expression and resulting immune deficiency may be considered possible components. Including immunodeficiencies in the differential diagnosis of recalcitrant warts may facilitate diagnosis in some patients. Considering current literature and direct experience in treating patients, we propose the following algorithm to aid clinicians in assessment and treatment of patients with recalcitrant warts in whom immunodeficiency should be suspected.
Patients with recalcitrant or recurrent severe warts should be evaluated for evidence of other infections (recurrent bacterial, viral, fungal or otherwise unusual or severe); this may be the first indicator of underlying immunodeficiency. History of recurrent infection, frequent antibiotic use since childhood, or autoimmune disease may raise suspicions of underlying immunity impairment. Additionally, family history of immunodeficiency suggests an inherited cause. With personal or family history suggestive of immunodeficiency or in warts persisting for >2 years, consideration of laboratory testing to exclude acquired and inherited forms of immune deficiency should be performed. Testing should include a complete blood count, comprehensive metabolic panel, HIV test, antinuclear antibody, and quantitative immunoglobulin assessments. If suspicion remains high and diagnosis elusive, genetic screening (first through a panel, and whole exome/genome sequencing if panel results are inconclusive) may confirm diagnosis (Figure 1).
FIGURE 1.
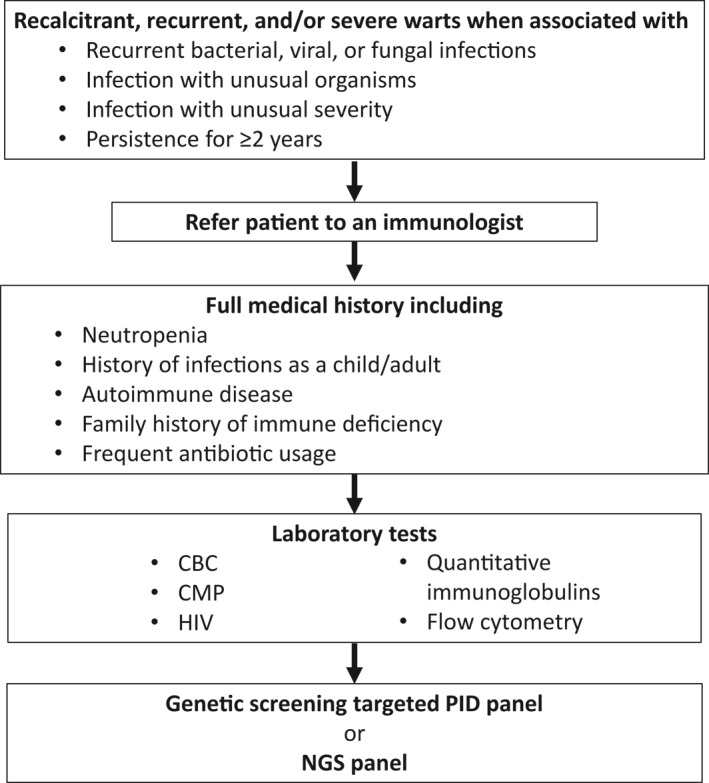
Diagnosis of underlying cause of recalcitrant warts. CBC, complete blood count; CMP, complete metabolic panel; NGS, next‐generation sequencing; PID, primary immunodeficiency
17. TREATMENT OF HPV‐RELATED WARTS AND PAPILLOMAS
The HPV vaccine has led to substantial decrease in incidence and prevalence of HPV‐related cancers and genital warts; despite initial antibody increases, long‐term immunity is not always maintained in people with immunodeficiency. 72 , 73 , 74 , 75 Evidence is emerging on HPV vaccine treatment for common warts and other HPV‐related pathologies, suggesting cross immunogenicity between vaccine HPV subtypes and those causing cutaneous warts. 76 , 77
Many approaches exist to treat warts in healthy patients, although randomised controlled trials have mostly demonstrated equivalent efficacy, and no therapy will be effective in all patients. 78 , 79 , 80 Common treatment approaches, which are summarised in Table 4, include destructive methods, such as salicylic acid or cryotherapy; chemotherapies, such as bleomycin; and immunotherapy, such as contact allergens, intralesional immunotherapy, imiquimod, or interferon. 7 , 12 , 81 , 82 , 83 The significant risk of recurrence attached to both destructive and chemical interventions has resulted in the growing popularity of immunotherapy. 83 Immunotherapy is a targeted approach used for treatment‐refractory warts and is aimed at stimulating the immune system to clear HPV. 83 However, in patients with immunodeficiency, immunotherapies are often less effective. 29 Imiquimod positively modulates the immune response by increasing cellular levels of multiple cytokines such as IFN‐α, IL‐6, and TNF‐α, with resulting antitumour and antiviral effects. 83 Interferon has also shown promise in the treatment of HPV warts, due to its antiviral, antiproliferative, and proimmune effects. In a comprehensive review of 12 studies in patients infected with HPV receiving interferon, five of the studies demonstrated complete response rate in treating genital warts; however, no statistically significant difference was seen in recurrence. While the immune‐stimulatory effects are partially responsible for the success of interferon in otherwise healthy patients, its effectiveness in the treatment of warts in patients with inborn errors of immunity is less clear. 84 Ustekinumab, an antibody against the p40 subunit of IL‐23 and IL‐12, blocks activity by inhibiting IL‐21–dependent production of IL‐17. This therapy was not evaluated for warts specifically, but a patient with LAD1 and intractable inflammatory lesions with dominant IL‐23 and IL‐17 signature saw improvement after 1 year receiving ustekinumab. 85
TABLE 4.
Common treatment approaches for warts
| Therapy type | Examples | Comments |
|---|---|---|
| Destructive | Topical salicylic acid | Patient‐applied salicylic acid and physician‐applied cryotherapy (liquid nitrogen) are the most common treatments for cutaneous warts 81 |
| Cryotherapy | ||
| Trichloroacetic acid | ||
| CO2 laser therapy | ||
| Excision 82 | ||
| Immune modulating 81 , 82 | Intralesional candida antigen | May be better for larger lesions |
| Topical imiquimod | ||
| Th1‐stimulating vaccination 83 | ||
| Interferon | ||
| Antiproliferative | Bleomycin | Target an underlying mechanism of HPV effect on the host genome 7 , 12 |
| Vitamin D analogues | ||
| Podophyllin | ||
| Podophyllotoxin | ||
| 5‐fluorouracil 83 | ||
| Antiviral | Cidofovir 83 | Retinoids offer the advantage of at‐home use 81 |
| Retinoids 82 |
Note: Treatment strategies for warts encompass several categories of therapies based on their mechanisms of action. No single therapy is effective in every patient.
Abbreviations: CO2, carbon dioxide; HPV, human papilloma virus; Th1, T helper type 1.
In conclusion, HPV is a common human‐associated virus typically cleared by the host's immune system. In cases of unusual wart presentation (extensive, recalcitrant or treatment‐refractory), there may be underlying immunodeficiency warranting evaluation that may affect wart prognosis and management. Patients with suspected immunodeficiencies should be referred to an immunologist for evaluation, including genetic testing, as appropriate, to confirm or exclude inborn errors of immunity. For patients affected by such inborn errors, early diagnosis and tailored treatment could profoundly impact outcome, particularly in those patients who may go on to develop HPV‐related malignancies that can be fatal without prompt, targeted intervention.
CONFLICT OF INTEREST
Zampella reports receiving consulting fees from X4 Pharmaceuticals. Cohen has no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
John G Zampella: Conceptualization, Data curation‐Supporting, Formal analysis, Funding acquisition‐Supporting, Investigation, Methodology‐Supporting, Project administration‐Supporting, Resources, Validation, Visualization, Writing ‐ original draft‐Supporting, Writing ‐ review & editing‐Supporting. Bernard Cohen: Conceptualization, Data curation‐Supporting, Formal analysis, Funding acquisition‐Supporting, Investigation, Methodology‐Supporting, Project administration‐Supporting, Resources, Validation, Visualization, Writing ‐ original draft‐Supporting, Writing ‐ review & editing‐Supporting.
ACKNOWLEDGEMENTS
Assistance in the writing of this article was provided by the PRECISIONscientia under the guidance of the authors and funded by X4 Pharmaceuticals.
Zampella J, Cohen B. Consideration of underlying immunodeficiency in refractory or recalcitrant warts: a review of the literature. Skin Health Dis. 2022;2(1):e98. 10.1002/ski2.98
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Cubie HA. Diseases associated with human papillomavirus infection. Virology. 2013;445:21–34. [DOI] [PubMed] [Google Scholar]
- 2. Luria L, Cardoza‐Favarato G. Human papillomavirus. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed 8 Mar, 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448132/ [Google Scholar]
- 3. Pastrana DV, Peretti A, Welch NL, Borgogna C, Olivero C, Badolato R, et al. Metagenomic discovery of 83 new human papillomavirus types in patients with immunodeficiency. mSphere. 2018;3:e00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis RM, Laprise JF, Gargano JW, Unger ER, Querec TD, Chesson HW, et al. Estimated prevalence and incidence of disease‐associated HPV types among 15‐59‐year‐olds in the United States. Sex Transm Dis. 2021;48:273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reusser NM, Downing C, Guidry J, Tyring S. HPV carcinomas in immunocompromised patients. J Clin Med. 2015;4:260–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gheit T. Mucosal and cutaneous human papillomavirus infections and cancer biology. Front Oncol. 2019;9:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomaić V. Functional roles of E6 and E7 oncoproteins in HPV‐induced malignancies at diverse anatomical sites. Cancers. 2016;8:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickson EL, Vogel RI, Bliss RL, Downs LS. Multiple‐type human papillomavirus (HPV) infections: a cross‐sectional analysis of the prevalence of specific types in 309,000 women referred for HPV testing at the time of cervical cytology. Int J Gynecol Cancer. 2013;23:1295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skoulakis A, Fountas S, Mantzana‐Peteinelli M, Pantelidi K, Petinaki E. Prevalence of human papillomavirus and subtype distribution in male partners of women with cervical intraepithelial neoplasia (CIN): a systematic review. BMC Infect Dis. 2019;19:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25:2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moody C. Mechanisms by which HPV induces a replication competent environment in differentiating keratinocytes. Viruses. 2017;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol. 2019;9:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al Aboud A, Nigam P. Wart. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed 8 Mar, 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448132/ [Google Scholar]
- 14. Sterling JC, Handfield‐Jones S, Hudson PM. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144:4–11. [DOI] [PubMed] [Google Scholar]
- 15. Warts. In: Dermatologic disorders. Merck Manuals Professional Edition and Wolters Kluwer; 2021. [Google Scholar]
- 16. Al‐Eitan LN, Tarkhan AH, Alghamdi MA, Al‐Qarqaz FA, Al‐Kofahi HS. Transcriptome analysis of HPV‐induced warts and healthy skin in humans. BMC Med Genom. 2020;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruggink SC, Gussekloo J, de Koning MN, Feltkamp MCW, Bavinck JNB, Quint WGV, et al. HPV type in plantar warts influences natural course and treatment response: secondary analysis of a randomised controlled trial. J Clin Virol. 2013;57:227–32. [DOI] [PubMed] [Google Scholar]
- 18. Gopal V, Shenoy MM, Pinto M. Common warts revisited: a clinical study. Int J Res Derm. 2017;3:261. [Google Scholar]
- 19. Herschthal J, McLeod MP, Zaiac M. Management of ungual warts. Dermatol Ther. 2012;25:545–50. [DOI] [PubMed] [Google Scholar]
- 20. Leslie S, Sajjad H, Kumar S. Genital warts. In: StatPearls [Internet] StatPearls Publishing; 2021. Accessed 8 Mar, 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448132/ [Google Scholar]
- 21. Zhu C, Wang Y, Mao W, Zhang H, Ma J. Prevalence and distribution of HPV types in genital warts in Xi'an, China: a prospective study. BMJ Open. 2019;9:e023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rollison DE, Viarisio D, Amorrortu RP, GheitTommasino T, Tommasino M. An emerging issue in oncogenic virology: the role of beta human papillomavirus types in the development of cutaneous squamous cell carcinoma. J Virol. 2019;93:e01003–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwarz T. Mechanisms of UV‐induced immunosuppression. Keio J Med. 2005;54:165–71. [DOI] [PubMed] [Google Scholar]
- 24. Uberoi A, Yoshida S, Frazer IH, Pitot HC, Lambert PF. Role of ultraviolet radiation in papillomavirus‐induced disease. PLoS Pathog. 2016;12:e1005664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giuliano AR, Lu B, Nielson CM, Flores R, Papenfuss MR, Lee JH, et al. Age‐specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008;198:827–35. [DOI] [PubMed] [Google Scholar]
- 26. Steinbach A, Riemer AB. Immune evasion mechanisms of human papillomavirus: an update. Int J Cancer. 2018;142:224–9. [DOI] [PubMed] [Google Scholar]
- 27. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population‐based study. J Allergy Clin Immunol. 2014;133:1041–7. [DOI] [PubMed] [Google Scholar]
- 28. Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecol Oncol. 2010;118:S12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cromme FV, Walboomers JM, Van Oostveen JW, Stukart MJ, De Gruijl TD, Kummer JA, et al. Lack of granzyme expression in T lymphocytes indicates poor cytotoxic T lymphocyte activation in human papillomavirus‐associated cervical carcinomas. Int J Gynecol Cancer. 1995;5:366–73. [DOI] [PubMed] [Google Scholar]
- 31. Bernatsky SR, Cooper GS, Mill C, Ramsey-Goldman R, Clarke AE, Pineau CA. Cancer screening in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:45–9. [PubMed] [Google Scholar]
- 32. Chin‐Hong PV, Reid GE. Human papillomavirus infection in solid organ transplant recipients: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13590. [DOI] [PubMed] [Google Scholar]
- 33. Flynn JM, Andritsos L, Lucas D, Byrd JC. Second malignancies in B‐cell chronic lymphocytic leukaemia: possible association with human papilloma virus. Br J Haematol. 2010;149:388–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim SC, Schneeweiss S, Liu J, Karlson EW, Katz JN, Feldman S, et al. Biologic disease‐modifying antirheumatic drugs and risk of high‐grade cervical dysplasia and cervical cancer in rheumatoid arthritis: a cohort study. Arthritis Rheumatol. 2016;68:2106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu G, Sharma M, Tan N, Barnabas RV. HIV‐positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wadström H, Frisell T, Sparén P, Askling J. Do RA or TNF inhibitors increase the risk of cervical neoplasia or of recurrence of previous neoplasia? A nationwide study from Sweden. Ann Rheum Dis. 2016;75:1272–8. [DOI] [PubMed] [Google Scholar]
- 37. Bernatsky S, Ramsey‐Goldman R, Gordon C, Joseph L, Boivin JF, Rajan R, et al. Factors associated with abnormal Pap results in systemic lupus erythematosus. Rheumatology (Oxford). 2004;43:1386–9. [DOI] [PubMed] [Google Scholar]
- 38. Lu R, Munroe ME, Guthridge JM, Bean KM, Fife DA, Chen H, et al. Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies. J Autoimmun. 2016;74:182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaiotti D, Chung J, Iglesias M, Nees M, Baker PD, Evans CH, et al. Tumor necrosis factor‐alpha promotes human papillomavirus (HPV) E6/E7 RNA expression and cyclin‐dependent kinase activity in HPV‐immortalized keratinocytes by a ras‐dependent. Mol Carcinog. 2000;27:97–109. [DOI] [PubMed] [Google Scholar]
- 40. Béziat V. Human genetic dissection of papillomavirus‐driven diseases: new insight into their pathogenesis. Hum Genet. 2020;139:919–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Béziat V, Rapaport F, Hu J, Titeux M, Bonnet des Claustres M, Bourgey M, et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell. 2021;184:3812–28.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lisco A, Hsu AP, Dimitrova D, Proctor DM, Mace EM, Ye P, et al. Treatment of relapsing HPV diseases by restored function of natural killer cells. N Engl J Med. 2021;385:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kalińska‐Bienias A, Kowalewski C, Majewski S. The EVER genes – the genetic etiology of carcinogenesis in epidermodysplasia verruciformis and a possible role in non‐epidermodysplasia verruciformis patients. Postepy Dermatol Alergol. 2016;33:75‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Myers DJ, Kwan E, Fillman EP. Epidermodysplasia verruciformis. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021. Accessed 22 Mar, 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534198/ [Google Scholar]
- 45. de Jong SJ, Créquer A, Matos I, Hum D, Gunasekharan V, Lorenzo L, et al. The human CIB1‐EVER1‐EVER2 complex governs keratinocyte‐intrinsic immunity to β‐papillomaviruses. J Exp Med. 2018;15:2289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patel AS, Karagas MR, Pawlita M, Waterboer T, Nelson HH. Cutaneous human papillomavirus infection, the EVER2 gene and incidence of squamous cell carcinoma: a case‐control study. Int J Cancer. 2008;122:2377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaud G, Guillemot D, Jacob Y, Favre M. EVER2 protein binds TRADD to promote TNF‐α‐induced apoptosis. Cell Death Dis. 2013;4:e499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lazarczyk M, Pons C, Mendoza JA, Cassonnet P, Jacob Y, Favre M. Regulation of cellular zinc balance as a potential mechanism of EVER‐mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J Exp Med. 2008;205:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lazarczyk M, Cassonnet P, Pons C, Jacob Y, Favre M. The EVER proteins as a natural barrier against papillomaviruses: a new insight into the pathogenesis of human papillomavirus infections. Microbiol Mol Biol Rev. 2009;73:348–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Badolato R, Donadieu J, WHIM Research Group . How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491–8. [DOI] [PubMed] [Google Scholar]
- 51. Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, et al. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–57. [DOI] [PubMed] [Google Scholar]
- 52. Liu Q, Chen H, Ojode T, Gao X, Anaya‐O'Brien S, Turner NA, et al. WHIM syndrome caused by a single amino acid substitution in the carboxy‐tail of chemokine receptor CXCR4. Blood. 2012;120:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu Q, Pan C, Lopez L, Gao J, Velez D, Anaya‐O’Brien S, et al. WHIM syndrome caused by Waldenström's macroglobulinemia‐associated mutation CXCR4 (L329fs). J Clin Immunol. 2016;36:397–405. [DOI] [PubMed] [Google Scholar]
- 54. Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Curr Opin Hematol. 2009;16:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Meuris F, Carthagena L, Jaracz‐Ros A, Gaudin F, Cutolo P, Deback C, et al. The CXCL12/CXCR4 signaling pathway: a new susceptibility factor in human papillomavirus pathogenesis. PLoS Pathog. 2016;12:e1006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhao C, Lu X, Bu X, Zhang N, Wang W. Involvement of tumor necrosis factor‐alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer. 2010;10:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Etzioni A. Leukocyte adhesion deficiencies: molecular basis, clinical findings, and therapeutic options. Adv Exp Med Biol. 2007;601:51–60. [DOI] [PubMed] [Google Scholar]
- 58. Bergerson JRE, Freeman AF. An update on syndromes with a hyper‐IgE phenotype. Immunol Allergy Clin North Am. 2019;39:49–61. [DOI] [PubMed] [Google Scholar]
- 59. Radi NK, Atiyah IJ, Hamood SSH. Evaluation of serum IgE, TNF alpha at patients with genital warts, Iraq. Eurasia J Biosci. 2020;14:3101–6. [Google Scholar]
- 60. Dorn JM, Patnaik MS, Van Hee M, Smith MJ, Lagerstedt SA, Newman CC, et al. WILD syndrome is GATA2 deficiency: a novel deletion in the GATA2 gene. J Allergy Clin Immunol Pract. 2017;5:1149–52. [DOI] [PubMed] [Google Scholar]
- 61. Driessen GJ, Ijspeert H, Weemaes CM, Haraldsson Á, Trip M, Warris A, et al. Antibody deficiency in patients with ataxia telangiectasia is caused by disturbed B‐ and T‐cell homeostasis and reduced immune repertoire diversity. J Allergy Clin Immunol. 2013;131:1367–75.e9 [DOI] [PubMed] [Google Scholar]
- 62. Chinn IK, Chan AY, Chen K, Chou J, Dorsey MJ, Hajjar J, et al. Diagnostic interpretation of genetic studies in patients with primary immunodeficiency diseases: a working group report of the Primary Immunodeficiency Diseases Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2020;145:46–69. [DOI] [PubMed] [Google Scholar]
- 63. Ameratunga R, Woon ST, Neas K, Love DR. The clinical utility of molecular diagnostic testing for primary immune deficiency disorders: a case based review. Allergy Asthma Clin Immunol. 2010;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chinen J, Lawrence M, Dorsey M, Kobrynski LJ. Practical approach to genetic testing for primary immunodeficiencies. Ann Allergy Asthma Immunol. 2019;123:433–9. [DOI] [PubMed] [Google Scholar]
- 65. Leung L. Recalcitrant nongenital warts. Austr Family Physician. 2011;40:40–2. [PubMed] [Google Scholar]
- 66. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951–5. [DOI] [PubMed] [Google Scholar]
- 67. Kuwabara AM, Rainer BM, Basdag H, Cohen BA. Children with warts: a retrospective study in an outpatient setting. Pediatr Dermatol. 2015;32:679–83. [DOI] [PubMed] [Google Scholar]
- 68. Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leo PJ, Madeleine MM, Wang S, Schwartz SM, Newell F, Pettersson-Kymmer U, et al. Defining the genetic susceptibility to cervical neoplasia‐‐a genome‐wide association study. PLoS Genet. 2017;13:e1006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Abeck D, Tetsch L, Lüftl M, Biedermann T. Extragenital cutaneous warts ‐ clinical presentation, diagnosis and treatment. J Dtsch Dermatol Ges. 2019;17:613–34. [DOI] [PubMed] [Google Scholar]
- 71. Ursini T, Polilli E, Congedo G, Tontodonati M, Di Masi F, Mazzotta E, et al. Complete healing of a giant wart in a severely immune‐compromised patient with HIV infection treated with acupuncture. Case Rep Dermatol. 2011;3:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, et al. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104:444–52. [DOI] [PubMed] [Google Scholar]
- 73. Handisurya A, Schellenbacher C, Reininger B, Koszik F, Vyhnanek P, Heitger A, et al. A quadrivalent HPV vaccine induces humoral and cellular immune responses in WHIM immunodeficiency syndrome. Vaccine. 2010;28:4837–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365:1576–85. [DOI] [PubMed] [Google Scholar]
- 75. Suh DH, Kim M, Lee KH, Eom KY, Kjeldsen MK, Mirza MR, Kim JW. Major clinical research advances in gynecologic cancer in 2017. J Gynecol Oncol. 2018;29:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nofal A, Marei A, Ibrahim AM, Nofal E, Nabil M. Intralesional versus intramuscular bivalent human papillomavirus vaccine in the treatment of recalcitrant common warts. J Am Acad Dermatol. 2020;82:94–100. [DOI] [PubMed] [Google Scholar]
- 77. Pham CT, Juhasz M, Sung CT, Mesinkovska NA. The human papillomavirus vaccine as a treatment for human papillomavirus‐related dysplastic and neoplastic conditions: a literature review. J Am Acad Dermatol. 2020;82:202–12. [DOI] [PubMed] [Google Scholar]
- 78. Cockayne S, Hewitt C, Hicks K, Jayakody S, Kang'ombe AR, Stamuli E, et al. Cryotherapy versus salicylic acid for the treatment of plantar warts (verrucae): a randomised controlled trial. BMJ. 2011;342:d3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. de Haen M, Spigt MG, van Uden CJ, van Neer P, Feron FJM, Knottnerus A. Efficacy of duct tape vs placebo in the treatment of verruca vulgaris (warts) in primary school children. Arch Pediatr Adolesc Med. 2006;160:1121–5. [DOI] [PubMed] [Google Scholar]
- 80. Gilson RJ, Ross J, Maw R, Rowen D, Sonnex C, Lacey CJN. A multicentre, randomised, double‐blind, placebo controlled study of cryotherapy versus cryotherapy and podophyllotoxin cream as treatment for external anogenital warts. Sex Transm Infect. 2009;85:514–19. [DOI] [PubMed] [Google Scholar]
- 81. Bacelieri R, Johnson SM. Cutaneous warts: an evidence‐based approach to therapy. Am Fam Physician. 2005;72:647–52. [PubMed] [Google Scholar]
- 82. Pope M, Kyriakides K, Hoffman C. Treatment of warts in pediatrics: a review. J Fam Med Dis Prev. 2020;6:132. [Google Scholar]
- 83. Thappa DM, Chiramel MJ. Evolving role of immunotherapy in the treatment of refractory warts. Indian Dermatol Online J. 2016;7:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang J, Pu YG, Zeng ZM, Yu Z‐j, Huang N, Deng Q‐w. Interferon for the treatment of genital warts: a systematic review. BMC Infect Dis. 2009;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Moutsopoulos NM, Zerbe CS, Wild T, Dutzan N, Brenchley L, DiPasquale G, et al. Interleukin‐12 and interleukin‐23 blockade in leukocyte adhesion deficiency type 1. N Engl J Med. 2017;376:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


