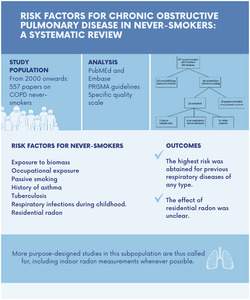
Abstract
Introduction
Relatively little is known about the risk factors for chronic obstructive pulmonary disease (COPD) in never‐smokers, and these factors have not yet been fully characterised. This study therefore sought to analyse COPD risk factors in never‐smokers by conducting a systematic review of the literature on the topic.
Materials and methods
We performed a search in PubMed (Medline) and Embase from 2000 onwards, to locate studies on COPD in never‐smokers. For literature search and evidence synthesis purposes, we used the PRISMA guidelines and drew up a specific quality scale to quantify the evidence of each study included.
Results
The bibliographic search retrieved a total of 557 papers, 20 of which fulfilled the designated inclusion criteria (two case–control studies, four cohort studies and 14 cross‐sectional studies). These studies were undertaken in Europe, the United States, Latin America, Asia and Africa. The risk factors for never‐smokers were varied and ranged from exposure to biomass, occupational exposure and passive smoking to having a history of asthma, tuberculosis or respiratory infections during childhood. The effect of residential radon was unclear. The highest risk was obtained for previous respiratory diseases of any type, with a magnitude much higher than that observed for other risk factors.
Conclusions
There are few studies on COPD risk factors in never‐smokers. More purpose‐designed studies in this subpopulation are thus called for, including well‐designed studies to specifically assess if indoor radon has any role on COPD onset.
Keywords: COPD, epidemiological studies, never‐smoker, systematic review
A sizeable proportion of COPD patients are never‐smokers and many risk factors are implicated in the disease's development, fundamentally exposure to biomass fuel, risk occupations, a history of asthma or tuberculosis, and exposure to passive smoking. The effect of residential radon was unclear. More studies in this subpopulation are thus called for, including indoor radon measurements.
1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is defined as a preventable and treatable disease, characterised by persistence of respiratory symptoms and limitation of airflow due to abnormalities of the respiratory tract and/or alveolar duct, generally caused by exposure to toxic gases or mediated by occupational exposure or of some other kind. 1 It is a disease with a high morbidity and mortality worldwide 2 and ranks as the third leading cause of death in the world. 3 According to the results of the EPISCAN II (Epidemiologic Study of COPD in Spain) study, its prevalence in Spain among the population aged 40 years and over is 11.8% (14.6% in men and 9.4% in women). 4
Smoking has been identified as the principal risk factor for development of COPD. 5 That said, however, 25%–45% of all patients with COPD are never‐smokers, 6 though this prevalence varies significantly depending on the geographical area and on the different epidemiological studies. In Spain, data from the EPISCAN II study show that 27% of patients with COPD are never‐smokers. 4 Yet despite this relatively high incidence of COPD in never‐smokers, few studies have exclusively targeted these subjects, and there is a great degree of ignorance about the specific effect of various risk factors that may have an influence on the appearance of COPD in this subpopulation and the magnitude of such effect. A recent review has indicated that the study of risk factors in never‐smokers is a challenge. 7 Chief among factors other than tobacco associated with a higher risk of COPD are genetic and environmental factors. Among environmental risk factors associated with development of COPD, mention has been made of exposure to biomass fumes, occupational exposure to dust and fumes (in agriculture, animal husbandry, mining, construction, exposure to chemical products in industry), environmental pollution, exposure to passive smoking, chronic asthma and tuberculosis. 8 , 9 Furthermore, there is evidence to show that exposure to residential radon may also be associated with COPD mortality. 10
The high incidence and prevalence of COPD, coupled with the existence of risk factors other than tobacco, make it pertinent to carry out a review and synthesis of existing studies that have analysed the risk factors for development of COPD in never‐smokers, by means of conducting a systematic review of the scientific literature.
2. METHODS
2.1. Data sources and search strategy
A literature search was undertaken in PubMed (Medline) and Embase. The search period covered the preceding 20 years (using ‘publication since 2000’ as the filter), with the last search being made on 1 January 2021. The search was conducted as follows: using the free‐text terms, ‘COPD and (never‐ or non‐smokers)’; applying language filters (English and Spanish); and excluding all communications to congresses, editorials and monographs.
For the literature search and evidence synthesis purposes, we used the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 11
2.2. Inclusion and exclusion criteria
The following inclusion and exclusion criteria were applied to select studies for the systematic review: (a) Study designs could range from cross‐sectional, case–control and cohort studies to systematic reviews and meta‐analyses; (b) to be eligible, studies had to be on human beings, whether in the general population or in a hospital setting; (c) risk factors analysed had to include occupation, passive smoking, infections in childhood, exposure to biomass in cooking/heating, history of tuberculosis, chronic asthma and exposure to residential radon; (d) studies included had to have a minimum of 100 participants, at least 20 of whom had to be never‐smoker patients with COPD; (e) studies that failed to specify the results in never‐smokers were excluded; (f) diagnosis of COPD in patients in the studies included had to be based on self‐reported patient symptoms or spirometric criteria consisting of pre‐bronchodilator values of FEV1/FVC < 70% or post‐bronchodilator values of FEV1/FVC < 70%.
A ‘never‐smoker’ was defined a person who met at least one of the following conditions: anyone who has smoked (a) fewer than 100 cigarettes in his/her lifetime or (b) less than one cigarette per day during a period of no less than 6 months. 12 In any case where this definition did not exist, non‐smokers in the studies included had to be referred to as a ‘never‐smoker’ rather than a ‘non‐smoker’.
2.3. Extraction and synthesis of data from the studies included
Data were extracted homogeneously from the studies included using a purpose‐designed data‐extraction table that listed the author(s), year of publication, sample size, study design, risk factors analysed, results obtained and study quality. We were unable to perform a meta‐analysis due to the high heterogeneity of the studies included. The information on each study is shown in an evidence table, along with a final qualitative conclusion.
2.4. Quality assessment of the studies included
To assess study quality, we drew up a quality scale made up of the following five items: sample size; number of COPD cases in never‐smokers; results adjusted for covariates; study design; and diagnosis of COPD. Each item has a different score, which makes it possible to rate study quality on a scale from 0 to 10 points, with 10 being the maximum score. The scale is shown in Table 1.
TABLE 1.
Quality scale used to assess the included studies
| Item assessed | Characteristic | Weight |
|---|---|---|
| Total sample size | 100–1000 | 0 |
| 1001‐10 000 | 1 | |
| >10 000 | 2 | |
| Number of COPD never‐smoker cases | 20–100 | 0 |
| 101–300 | 1 | |
| >300 | 2 | |
| Covariate adjustment (number) | 2 (age and sex) | 0 |
| >2 | 2 | |
|
Study design |
Cross‐sectional study | 0 |
| Case–control study | 1 | |
| Cohort study | 2 | |
|
COPD diagnosis |
Spirometry not registered or patient‐reported symptoms | 0 |
| Pre‐bronchodilator FEV1/FVC < 70% | 1 | |
| Post‐bronchodilator FEV1/FVC < 70% | 2 | |
| Total | 10 |
3. RESULTS
3.1. Search results
A total of 557 studies were retrieved from the bibliographic search in PubMed and Embase. After perusal of the abstracts, 44 studies were selected for a full‐text reading. Of these, 20 finally met the established inclusion criteria and comprised two case–control studies, four cohort studies and 14 cross‐sectional studies. The studies were undertaken in Europe, the United States, Latin America, Asia and Africa. The most frequent exclusion criteria were sample size of fewer than 20 cases of COPD in never‐smokers and the fact that the analysis of results failed to differentiate never‐smokers. Figure 1 shows a flow chart of the search process.
FIGURE 1.
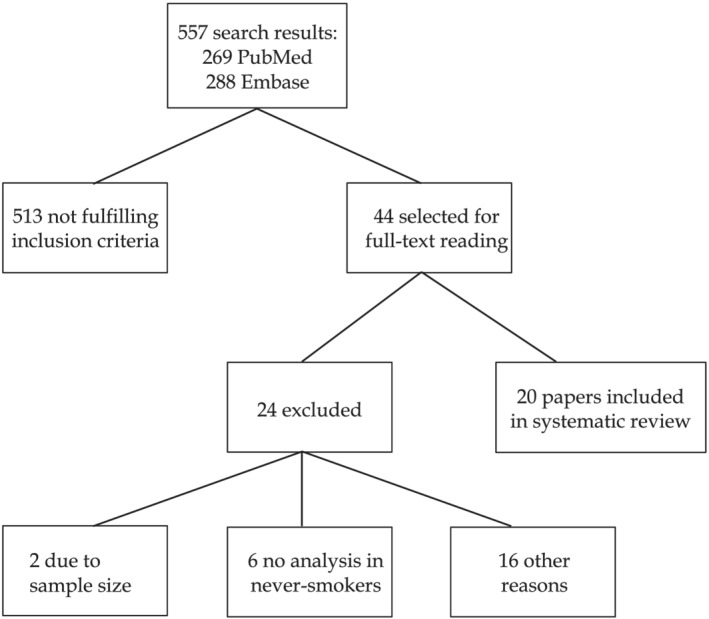
Flow chart showing the inclusion and exclusion process
3.2. Description of studies included
Table 2 gives a description of all the studies included. The mean sample size of the studies included was high, and it was noteworthy that in most of the studies, almost half the sample size corresponded to never‐smokers, and in four of them, all the participants were never‐smokers, meaning that the remaining 16 studies were not designed to assess the role of different risk factors in COPD in never‐smokers.
TABLE 2.
Description of the studies included
| Author and year | Type of study and country | Sample size | No. of never‐smokers (%)/no. of COPD never‐smokers (%) | No. of women (%) | COPD diagnosis | Risk factor analysed | Variable analysed | % increased risk of COPD/risk observed | Score |
|---|---|---|---|---|---|---|---|---|---|
| Orozco‐Levi et al, 20 2006 |
Case–control (hospital) Spain |
120 | 94 (78.3%)/42 (70%) | 120 (100%) | FEV1/FVC < 70% postBD | Biomass | Wood and charcoal | OR 4.5 (95%CI 1.4–14.2; p = 0.01) | 5 |
| Ekici et al, 21 2005 |
Case–control (population) Turkey |
596 | 596 (100%)/140 (100%) | 596 (100%) | FEV1/FVC < 70% preBD | Biomass |
No. of hours cooking × no. of years: ‐Group A: 68.8 h‐years ‐Group B: 68.8–152.4 h‐years ‐Group C: >152.4 h‐years |
23.1% (95% CI 13.4–33.2) ‐Group A: OR 1.7 (95%CI 1.0–3.1; p = 0.04) ‐Group B: OR 2.5 (95%CI 1.4–4.4; p = 0.001) ‐Group C: OR 3.3 (95%CI 1.9–5.7; p = 0.0001) |
5 |
| Regalado et al, 22 2006 |
Cross‐sectional Mexico |
841 | 841 (100%)/111 (100%) | 841 (100%) | FEV1/FVC < 70% preBD | Biomass | PM10 particulate concentration measured by nephelometer for 1 h while cooking |
OR FEV1/FVC < 70% + FEV1 < 80%: 3.9 (95%CI 0.94–16.3; p = 0.06) OR FEV1/FVC < 70%: 1.3 (95%CI 0.7–2.4; p = 0.32) FEV1 decrease 81 mL or 4.7% of that predicted with PM10 > 2.6 mg/m3 |
4 |
| Menezes et al, 19 2005 |
Cross‐sectional Brazil |
999 | 428 (42.9%) never‐smokers/not registered | 558 (55.8%) | FEV1/FVC < 70% postBD |
Occupation Biomass |
Occupation >10 years Exhibition charcoal stove for heating/cooking |
Occupation increases COPD risk 36% (p = 0.08) Coal stove exposure increases COPD risk 40% (p = 0.08) |
4 |
| Tuner et al, 32 2012 |
Cohorts USA |
811 961 | 375 087 (46.21%)/1797 deceased COPD never‐smokers | 449 361 (54.5%) | COPD medical diagnosis | Radon | Radon measurement >100 Bq/m3 | Mortality from COPD 1.03 (95%CI 0.86–1.25) | 6 |
| Lamprecht et al, 31 2011 |
Cross‐sectional China, Turkey, Austria, South Africa, Iceland, Germany, Poland, Norway, Canada, USA, Philippines, Australia, England, Sweden |
10 000 | 4291 (42.9%)/523 (12.2%) | 5231 (52.31%) | FEV1/FVC postBD <70% |
Respiratory infections in childhood Occupation Asthma |
Documented infections in childhood Occupation: Organic powder at least 10 years Asthma |
Infections in childhood: OR females 2.21 (95%CI 0.89–5.47 p = 0.087) and OR males 2.82 (95%CI 0.94–8.51; p = 0.065) Occupation: OR females 1.96 (95%CI 1.2–3.2; p = 0.007), OR males 2.18 (95%CI 0.99–4.8; p = 0.054) Asthma: OR females 4.62 (95%CI 3.04–7.02; p < 0.001) and OR males 4.12 (95%CI 2.06–8.26; p < 0.001) |
7 |
| Perez‐Padilla et al, 29 2012 |
Cross‐sectional Latin America: Chile, Uruguay, Brazil, Venezuela, Mexico |
5315 | 2278 (42.3%)/240 (10.5%) | 3211 (60.41%) | FEV1/FVC < 70% postBD |
Tuberculosis (TBC) Asthma |
Previous diagnosis of tuberculosis or asthma |
TBC: OR 3.66 (95%CI 1.4–9.55; p = 0.01) Asthma: OR 4.24 (95%CI 2.47–7.28; p < 0.001) |
6 |
| Lee et al, 30 2015 |
Cross‐sectional Korea |
5784 | 3473 (57.7%)/258 (31.7%) | 3053 (52.78%) | FEV1/FVC < 70% preBD |
Occupation Tuberculosis |
Occupation: manual labour: construction, mining Previous diagnosis of tuberculosis |
7.6% Occupation: OR 2.6 (95%CI 1.3–5.3; p = 0.007) TBC: OR 4.5 (95%CI 2.3–8.7; p < 0.0001) |
5 |
| Yin et al, 24 2007 |
Cohorts China |
6497 | 6497 (100%)/342 | 5957 (89.6%) | FEV1/FVC < 70% preBD | Passive smoking (home and workplace) | Density and duration (40 h/weeks for >5 years) |
OR home: 1.60 (95%CI 1.23–2.10) OR workplace: 1.50 (95%CI 1.14–1.97; p = 0.002) OR home + workplace: 1.48 (95%CI 1.18–1.85 p = 0.001) |
8 |
| Zhou et al, 25 2009 |
Cross‐sectional China |
20 245 | 12 471 (66.6%)/644 (38.6%) | 10 236 (88.7%) | FEV1/FC < 70% postBD |
Biomass Passive smoking |
Exposure to biomass if used for cooking/heating more than 1 hour Passive smoking: exposure to tobacco at home/workplace |
Biomass cooking: OR 1.31 (95%CI 1.08–1.58; p = 0.006) Biomass heating: OR 1.48 (95%CI 1.07–2.05; p = 0.017) Passive smoking: OR 1.31 (95%CI 1.06–1.61; p = 0.014) |
8 |
| Jordan, 26 2011 |
Cross‐sectional England |
21 104 | 8859 (42%)/not registered | 11 257 (53.3%) | FEV1/FVC < 70% preBD | Passive smoking | Exposure in hours/weeks | OR 1.98 (95%CI 1.03–3.79) if exposure greater than 20 h/week | 5 |
| Hagstad et al, 27 2014 |
Cross‐sectional Sweden |
2118 | 2118 (100%)/140 (6.65%) | 1251 (59.1%) | FEV1/FVC < 70% preBD | Passive smoking | Questionnaire on exposure to tobacco at home and in the workplace |
OR previous home: 2.03 (95%CI 1.23–3.34; p = 0.005) OR all homes: 1.98 (95%CI 1.23–3.18; p = 0.005) OR previous workplace: 2.06 (95%CI 1.30–3.27; p = 0.002) OR all homes and workplaces 3.94 (95%CI 1.41–11; p = 0.009) |
5 |
| Hnizdo, 13 2002 |
Cross‐sectional USA |
9823 | 4369 (44.47%)/106 (15.29%) | 5166 (52.59%) |
FEV1/FVC < 70% preBD+FEV1 < 80% |
Occupation | Occupation: industry and time | COPD fraction attributed to occupation in non‐smokers 31.1% | 5 |
| Blanc et al, 14 2009 |
Cohorts USA |
1504 | 323 (21.47%)/165 (13%) | 876 (58.29%) | FEV1/FVC < 70% preBD | Occupation | Exposure to VGDF and JEM | OR 2 (95%CI 1.28–3.18) | 7 |
| Mehta et al, 15 2012 |
Cohorts Switzerland |
4267 | 1940 (45%)/253 (43.84%) | 2121 (49.7%) | FEV1/FVC < 70% preBD | Occupation |
Biological powder Mineral powder Gases/vapours VGDF |
IRR biological powder: 3.14 (95%CI 0.88–11.24) IRR mineral powder: 3.22 (95%CI 0.84–12.36) IRR gases/vapours: 3.94 (95%CI 1.23–12.58) IRR VGDF: 3.28 (95%CI 1.03–10.41) |
7 |
| Mahmood et al, 23 2017 |
Cross‐sectional India |
200 | 113 (56.5%)/113 (56.5%) | Not registered | FEV1/FVC < 70% postBD |
Biomass Tuberculosis Asthma |
Questionnaire |
Biomass: 53.98%, p = 0.0001 Tuberculosis: 32.74%, p = 0.0001 Asthma: 14.16%; p = 0.003 |
3 |
| Hagstad et al, 16 2015 |
Cross‐sectional Sweden |
1839 | 967 (52.6%)/74 (7.7%) | 870 (47.3%) | FEV1/FVC < 70% postBD | Occupation | Exposure to gas, dust or vapours | OR 1.85 (95%CI 1.03–3‐33) | 5 |
| Tan et al, 28 2015 |
Cross‐sectional Canada |
4893 | 2295 (47%)/147 (6.43%) | 2797 (57.16%) | FEV1/FVC < 70% postBD |
Asthma Childhood infections Passive smoking |
Record of asthma or history of hospitalisation in childhood Passive smoking: partner of a smoker in the preceding 2 weeks |
Asthma: OR 2.23 (1.36–3.66) for mild COPD, OR 4.94 (2.94–8.30) moderate–severe COPD Childhood infections: OR 4.80 (95%CI 2.43–9.46) for COPD moderate–severe Passive smoking: OR 2.60 (1.05–6.43) for mild COPD women |
6 |
| Ramadan, 17 2012 |
Cross‐sectional Egypt |
300 | 120 (40%)/120 (40%) | 70 (23.34%) | FEV1/FVC < 70% postBD |
Occupation Biomass |
Occupation: risk occupation more than 3 months Biomass: cooking exposure/heating for at least 6 months |
Occupation: OR 1.09 (95%CI 0.41–0.78; p < 0.05) Biomass: OR 1.26 (95%CI 0.38–0.82; p < 0.05) |
5 |
| Denguezli et al, 18 2016 | Cross‐sectional. Tunisia | 661 | 485 (73.4%)/28 (4.7%) | 352 (67.2%) | FEV1/FVC < 70% postBD |
Occupation Biomass Asthma Childhood hospitalisation |
Occupation: risk occupation at least 3 months Biomass: exposure at least 6 months Asthma diagnosis Childhood hospitalisation record |
Occupation >10 years: OR 1.87 (95%CI 1140–12.863; p = 0.015) Biomass ≥ 10 years kitchen: OR 1.479 (95%CI 0.3–5.855; p = 0.578) Asthma: OR 10.621 (95%CI 2.897–38.937; p < 0.01) Childhood hospitalisation: OR 3.075 (95%CI 0.35–27.017; p = 0.311) |
4 |
Abbreviations: JEM, job‐exposure matrices; postBD, post‐bronchodilator; preBD, pre‐bronchodilator; TBC, tuberculosis; VGDF, occupational exposure to vapours, gases, dust or fumes.
Table 3 summarises the main findings by the risk factor analysed. The total sample size of the included studies was 909 067, with a median of never‐smokers of 2029 (range 374 993) and a median of COPD never‐smoker of 143.5 (range 1769).
TABLE 3.
Summary by risk factor analysed
| Risk factor analysed | Number studies | OR (maximum) | OR (minimum) | Maximum score | Minimum score |
|---|---|---|---|---|---|
| Occupation | 9 | 4.5 | 1.26 | 8 | 3 |
| Biomass | 8 | 3.94 | 1.09 | 7 | 4 |
| Passive smoking | 5 | 3.94 | 1.31 | 8 | 5 |
| Asthma | 5 | 10.621 | 4.24 | 7 | 3 |
| Tuberculosis | 3 | 4.5 | 3.66 | 6 | 3 |
| Respiratory infections in childhood | 3 | 4.80 | 3.075 | 7 | 4 |
| Radon | 1 | 1.03 | 6 |
An individual analysis of each of the risk factors now follows.
3.3. Occupation
There were nine studies that analysed occupational exposure as a risk factor for development of COPD in never‐smokers. Of these, two were conducted in the United States 13 , 14 : The first study 13 found a fraction of COPD attributable to work in never‐smokers of 31.1%; and the second 14 showed an increase in risk (OR 1.98, 95%CI 1.26–3.09) with occupational exposure to vapours, gases, dust or fumes (VGDF) and with job‐exposure matrices. A study conducted in Switzerland 15 found evidence of an increased risk of COPD in never‐smokers with occupational exposure to biological powder (OR 3.14, 95%CI 0.88–11.24), mineral powder (OR 3.22, 95%CI 0.84–12.36), exposure to gas and vapours (OR 3.94, 95%CI 1.23–12.58) and VGDF (OR 3.28, 95%CI 1.03–10.41). Another study undertaken in Sweden 16 obtained an OR of 1.85 (95%CI 1.03–3.33) for development of COPD with exposure to gas, dust or vapours. Two studies 17 , 18 showed a significant association between development of COPD in never‐smokers and holding a risk occupation for at least 3 months. Lastly, a study undertaken in Brazil 19 established that occupation increases the risk of developing COPD by 36% (p = 0.08).
3.4. Biomass
There were eight studies included that analysed biomass as a risk factor for development of COPD. Three of these exclusively included women never‐smokers and analysed the effect of biomass as a risk factor for development of COPD. 20 , 21 , 22 A Spanish study 20 concluded that the combined use of wood and coal significantly raised the risk of COPD (OR 4.5, 95%CI 1.4–14.2). A study conducted in Turkey 21 found that 23.1% of COPD cases could be attributed to exposure to biomass, with risk rising in response to greater exposure measured in hours per year. In Mexico, Regalado et al 22 concluded that women who use biomass for cooking have a reduction in pulmonary function as compared with those who cook with gas. The study conducted by Mahmood et al 23 in India showed exposure to a biomass source as being the principal risk factor for developing COPD, with a 54% increase in risk (p = 0.0001).
3.5. Passive smoking
The review included five studies that analysed the association between passive smoking and development of COPD in never‐smokers. Two of these were undertaken in China. 24 , 25 The first was a cohort study that included 6497 non‐smokers, 342 of whom had a diagnosis of COPD. It found an association between passive smoking in the home and workplace and an OR of 1.48 (95%CI 1.18–1.85) for a high level of exposure consisting of 40 h per week over the course of at least 5 years. The second reported a prevalence of COPD in never‐smokers of 5.2%, and an association with exposure to passive smoking in the home and in the workplace, with an OR of 1.31 (95%CI 1.06–1.61). Jordan 26 conducted a study in the United Kingdom with the aim of analysing passive smoking as a risk factor for COPD: Among never‐smokers, the risk was 1.98 (95%CI 1.03–3.79) for an exposure of more than 20 h per week. In Sweden, Hagstad et al 27 found an association between COPD and exposure to passive smoking in the home and the workplace, with an OR of 3.94 (95%CI 1.41–11, p = 0.009). A study undertaken in Canada 28 showed a prevalence of COPD in never‐smokers of 6.4% (27% of all COPD) and analysed passive smoking as a risk factor, reporting an OR of 2.6 (95%CI 1.05–6.43) for women with mild COPD.
3.6. Asthma
There were five studies that analysed asthma as a risk factor for development of COPD in never‐smokers, and all found a significant association. The strongest association was reported in a study conducted in Tunisia by Denguezli et al, 18 with an OR of 10.62 (95%CI 2.90–38.94, p < 0.01). The study undertaken in India 23 reflected a 14.16% increased risk of COPD in subjects with a previous diagnosis of asthma.
3.7. Tuberculosis
The review included three studies that analysed previous diagnosis of tuberculosis as a risk factor for development of COPD. Perez‐Padilla et al 29 conducted a study in Latin America that included 2278 never‐smokers, 240 of whom presented with COPD and were never‐smokers, and observed that the OR for development of COPD with previous diagnosis of tuberculosis was 3.66 (95%CI 1.4–9.55). A cross‐sectional study undertaken in Korea, 30 which included 258 COPD never‐smokers, found an OR of 4.5 (95%CI 2.3–8.7) in subjects who had a previous diagnosis of tuberculosis. Similarly, the third study, conducted in India, 23 reported a 32.74% increased risk of COPD in subjects with a history of tuberculosis.
3.8. Respiratory infections
There were three studies that analysed the role of childhood respiratory infections in the development of COPD. Lamprecht et al 31 included 523 never‐smokers with diagnosis of COPD and found evidence to show that, in those with a history of respiratory infections in childhood, the OR was 2.21 (95%CI 0.89–5.47) in women and 2.82 (95%CI 0.94–8.41) in men. A study carried out in Canada 28 showed an OR for moderate‐to‐severe COPD of 4.8 (95%CI 2.43–9.46). Lastly, a study conducted in Tunisia by Denguezli et al 18 reported a threefold higher risk of developing COPD (OR 3.075, 95%CI 0.35–27.02) in subjects with a record of hospitalisation due to childhood respiratory infections.
3.9. Radon
The review included one study that analysed the association between radon and COPD. Turner et al 32 carried out a cohort study in the United States with a large sample size and observed an association between radon concentration > 100 Bq/m3 and COPD mortality, which, in the case of never‐smokers, was 1.03 (95%CI 0.86–1.25). Furthermore, they found evidence of a significant positive linear trend in COPD mortality with increasing categories of radon concentrations (p = 0.05).
3.10. Quality of studies included
The quality of the studies reviewed ranged from 3 to 8 points, with a mean score of 5.5 points.
4. DISCUSSION
The studies reviewed show a significant association between incidence of COPD in never‐smoker patients and occupational exposure, exposure to biomass, passive smoking and having previously suffered from asthma, tuberculosis or respiratory infections during childhood. In addition, they suggest that residential radon could increase COPD mortality, though more research is needed to confirm this finding. In most of the studies and for most of the exposures analysed, the association observed was statistically significant. It should be noted that, among the risk factors, the effect is most pronounced for previous respiratory diseases (asthma, tuberculosis) or having presented with respiratory infections compared with exposures of an occupational or environmental nature.
More than 2800 million persons commonly use biomass fuels for cooking. 33 The percentage varies widely among countries and regions and ranges from 30% to 75% in rural areas 34 , 35 , 36 : For instance, in countries such as India, biomass fuels are used for cooking and heating in almost 90% of rural homes and a third of urban dwellings. Indeed, every year, over 1.5 million persons around the world die of pneumonia, chronic respiratory diseases and lung cancer, due to indoor air pollution caused by biomass fuel used in cooking. 37 Biomass fuels account for 2.9% of all deaths worldwide and 3.7% of the total morbidity and mortality burden in developing countries. 34 This systematic review included studies that analysed biomass used for cooking and heating as a risk factor for development of COPD in never‐smokers in different geographical areas (Mexico, Turkey, Brazil, China, India, Egypt and Spain) and found an increased risk of COPD of 4.5 in a case–control study undertaken in Spain. 20 Furthermore, this risk is observed to increase with the number of hours engaged in cooking.
Some studies on occupations that entail exposure to toxic gases in the workplace, 38 grain dust on farms 39 and fumes and dust in factories, have observed a strong association with development of COPD. 40 The fraction attributable to occupation‐related COPD ranges from 9% to 31%, 41 but the real attributable risk is unclear due to the fact that the definition of COPD is not standardised in epidemiological studies, particularly those carried out in developing countries. The studies with a cross‐sectional design included in this systematic review describe an association between occupation and development of COPD in never‐smokers. Hnizdo 13 estimates a work attributable fraction for development of COPD in never‐smokers of 31.1%, with the main occupations being associated with the transport, stocking and handling of materials used in processing and construction. Other studies having a better design but smaller sample size than the above, conducted in the United States 14 and Switzerland, 15 also analysed occupation as a risk factor for development of COPD. Blanc et al 14 found that exposure to VGDF was associated with double the risk of COPD being developed by never‐smokers, and Mehta et al 15 described how exposure by an adult Swiss population to biological powder, mineral powder, gas/vapours and VGDF was associated with COPD, at least to a moderate degree, with the highest risk being posed by exposure to gas and vapours (OR 3.94).
An association has been reported for passive smoking as a potential risk factor for development of respiratory diseases, and the studies reviewed show a uniform increase in risk. The biological mechanism would presumably be the same as that observed in active smokers, though the inflammatory component and time of induction might possibly be less for never‐smokers than for active smokers. One of the studies found a stronger association with a higher number of weekly hours of exposure to active smoking habit 26 or in cases of simultaneous exposure in the home and in the workplace. 27
It has been reported that chronic inflammation of the respiratory tract and chronic airflow obstruction in asthma sufferers could cause remodelling due to thickening and fibrosis of the airways 42 and that this remodelling could be progressive and irreversible, giving rise to development of COPD. There are similar mechanisms between development of chronic asthma and COPD, with an increase in neutrophils, proteases and oxidative stress. Moreover, and especially in the case of developing countries, one should bear in mind that inappropriate treatment of chronic asthma or severe asthma without inhaled corticosteroids could contribute to development of COPD. A longitudinal study 43 conducted a follow‐up across 15 years and found that subjects with self‐reported diagnosis of asthma presented with a greater decline in FEV1, something that could be related with a baseline reduction in FEV1 and an increase in impaired lung function that is characteristic of COPD.
Pulmonary tuberculosis is associated with chronic airflow obstruction during diagnosis, treatment and years after undergoing treatment. 44 , 45 The degree of airflow obstruction is linked to disease spread, and the prevalence of obstruction varies between 28% and 68% of patients with tuberculosis. Patients with pulmonary tuberculosis generally develop a maximum loss of lung function within the 6 months following diagnosis and stabilise at 18 months of completing the treatment. 46 , 47 The biological mechanism responsible for this chronic obstruction of the respiratory tract might be the fibrosis of the airways caused by tuberculosis, as well as the immune response to mycobacteria that may cause inflammation of the respiratory tract, likewise characteristic of COPD. Furthermore, the degree of bronchial obstruction is related to disease severity as measured by radiological extension.
Indoor air pollution by biomass fuel is a factor that influences the development of respiratory infections during childhood and is an important cause of childhood mortality in developing countries, particularly across Asia and Africa. 48 The survivors of these respiratory infections could present with factors that might predispose them to COPD in adult life. Other factors such as poverty, low socio‐economic level and malnutrition could contribute to the increase in respiratory infections during childhood. Several studies 49 , 50 , 51 have shown that, after controlling for confounding factors such as smoking habit, persons who suffered from respiratory infections during childhood displayed lower FEV1 and FVC values, suggesting poor lung development. Another possible hypothesis is that there may be genetic factors that predispose persons to respiratory infections during childhood, as well as a lower FEV1 in adult life, though this hypothesis may imply that the alteration in pulmonary growth might precede infection of the respiratory tract. Bacterial infection due to Streptococcus pneumoniae and Haemophilus influenzae is frequently cited as the aetiology of severe pneumonia in children 52 so that the impact of such infections on the prevalence of COPD in developing countries is likely to be higher due to their inappropriate treatment. The effect of having suffered from asthma, tuberculosis or previous respiratory infections on development of COPD in never‐smokers is considerably greater than that of other exposures.
Radon is the most important source of ionising radiation of natural origin for human beings. 53 When it is inhaled, the solid particles into which the gas decays are retained in the lungs and irradiate alpha particles to the cells lining the lungs, bringing about molecular changes and possibly damaging DNA. In 2020, Conde‐Sampayo 54 conducted a systematic review of exposure to residential radon and COPD and reported a possible trend towards the existence of this association, though no definitive conclusion could be reached. This implies that the effect of radon on COPD is unknown. Apart from the Cancer Prevention Study II, which observed an association between radon and COPD mortality, there is another ecological study undertaken in Galicia, but not included in this review, which did find evidence of an association between radon concentration and hospital admissions due to COPD. 55 Another recent study, albeit conducted on smokers, has reported that radon increases risk of COPD in smokers. 56 In light of these findings, studies are called for to analyse this association.
This review has some strengths, the most important of which is having been based on the PRISMA guidelines and, by extension, having used a rigorous method. Furthermore, a specific quality assessment scale was developed, which indicates that the studies are of medium quality. External validity is also high, in that the studies reviewed were conducted in different geographical areas.
Conversely, this review also has some limitations. The main limitation lies in the heterogeneity of the methodology used by the different studies, something that made it impossible to perform a meta‐analysis, overall or individual, for any of the risk factors analysed. The studies included also displayed differences in the definition of COPD so that, in some studies, only airflow obstruction was considered, without taking the reported symptomatology into account. We have not formally measured the risk of bias, because this study is not a meta‐analysis, but bias may be present due to different reasons in the included study (retrospective design, accuracy on measuring different risk factors [i.e. passive smoking or exposure to VGDF]). A further limitation is the use of a non‐validated scale to assess the quality of the included studies, though similar scales have been used by our group in other systematic reviews. 57 , 58 , 59
In conclusion, a sizeable proportion of patients with COPD are never‐smokers, and many risk factors are implicated in the disease's development, fundamentally exposure to biomass fuel, risk occupations, a history of asthma or tuberculosis and exposure to passive smoking. The available literature points to evidence of an association between exposure to residential radon and COPD mortality. The absence of a greater number of studies specifically conducted on never‐smokers is extremely noteworthy, particularly when smoking in the most developed countries is progressively decreasing and the percentage of COPD in never‐smokers will necessarily be gradually increasing. The need for more research on this topic is evident. These studies should exclusively include never‐smokers and assess all the potential risk factors of COPD. The sample size should be high, at least 500 participants with more than 200 COPD never‐smokers to obtain reliable effect estimations. Strategies should be drawn up by the authorities, which are designed to reduce the risk of development of COPD in never‐smokers through lowering the exposure to toxic substances at home. Similarly, there is a need for more studies on never‐smokers, so as to allow for the possible role played by exposure to residential radon to be elucidated.
CONFLICT OF INTEREST
None.
ETHICS STATEMENT
Due to the nature of this study, Ethics Committee approval was not required.
AUTHOR CONTRIBUTIONS
ARR conceived the idea and designed the methodology and the inclusion and exclusion criteria. APS did the bibliographic search. CCP, CRG, RG and CRR extracted the data. All authors have read and provided intellectual input to the manuscript. All authors have approved the final version of the manuscript and take public responsibility of its content.
ACKNOWLEDGEMENTS
This study was partially funded by a Carlos III Health Institute (ISCIII) grant (PI20/00476), with co‐funding from the European Regional Development Fund (ERDF).
Pando‐Sandoval A, Ruano‐Ravina A, Candal‐Pedreira C, et al. Risk factors for chronic obstructive pulmonary disease in never‐smokers: A systematic review. Clin Respir J. 2022;16(4):261-275. doi: 10.1111/crj.13479
This paper forms part of the work leading to Ana Pando Sandoval's PhD degree.
Funding information Carlos III Health Institute grant, Grant/Award Number: PI20/00476; European Regional Development Fund
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Global Initiative for Chronic Obstructive Lung Disease . Global strategy for the diagnosis, management and prevention of chronic obstructive lung disease (2021 report). https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 2. Montserrat‐Capdevila J, Godoy P, Marsal JR, Barbé‐Illa F. Factores asociados a la mortalidad global en los pacientes diagnosticados de enfermedad pulmonar obstructiva crónica. Atención Primaria. 2015;47(8):498‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. López‐Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14‐23. [DOI] [PubMed] [Google Scholar]
- 4. Soriano JB, Alfageme I, Miravitlles M, et al. Prevalence and determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. 2021;57(1):61‐69. [DOI] [PubMed] [Google Scholar]
- 5. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. The Lancet. 2007;370(9589):765‐773. [DOI] [PubMed] [Google Scholar]
- 6. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non‐smokers. The Lancet. 2009;374(9691):733‐743. [DOI] [PubMed] [Google Scholar]
- 7. Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248‐1256. [DOI] [PubMed] [Google Scholar]
- 8. Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693‐718. [DOI] [PubMed] [Google Scholar]
- 9. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. The Lancet. 2015;385(9971):899‐909. [DOI] [PubMed] [Google Scholar]
- 10. Barbosa‐Lorenzo R, Ruano‐Ravina A, Fernández‐Villar A, López‐Pardo E, Carballeira‐Roca C, Barros‐Dios JM. COPD prevalence and hospital admissions in Galicia (Spain). An analysis using the potential of new health information systems. Pulmonology. 2018;24(6):323‐329. [DOI] [PubMed] [Google Scholar]
- 11. Urrútia G, Bonfill X. Declaración PRISMA: una propuesta para mejorar la publicación de revisiones sistemáticas y metaanálisis. Med Clin. 2010;135(11):507‐511. [DOI] [PubMed] [Google Scholar]
- 12. Pérez‐Ríos M, Schiaffino A, López MJ, et al. Questionnaire‐based second‐hand smoke assessment in adults. Eur J Public Health. 2013;23(5):763‐767. [DOI] [PubMed] [Google Scholar]
- 13. Hnizdo E. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the third national health and nutrition examination survey. Am J Epidemiol. 2002;156(8):738‐746. [DOI] [PubMed] [Google Scholar]
- 14. Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2008;64(1):6‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta AJ, Miedinger D, Keidel D, et al. Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss cohort study on air pollution and lung and heart diseases in adults. Am J Respir Crit Care Med. 2012;185(12):1292‐1300. [DOI] [PubMed] [Google Scholar]
- 16. Hagstad S, Backman H, Bjerg A, et al. Prevalence and risk factors of COPD among never‐smokers in two areas of Sweden – occupational exposure to gas, dust or fumes is an important risk factor. Respir Med. 2015;109(11):1439‐1445. [DOI] [PubMed] [Google Scholar]
- 17. Ramadan MB, Elmahallawy II. Prevalence characteristics of COPD in never smokers. Egypt J Chest Dis Tuberc. 2012;61:59‐65. [Google Scholar]
- 18. Denguezli M, Daldoul H, Harrabi I, et al. COPD in nonsmokers: reports from the Tunisian population‐based burden of obstructive lung disease study. PLOS One. 2016;11(3):e0151981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menezes AMB, Jardim JR, Pérez‐Padilla R, et al. Prevalence of chronic obstructive pulmonary disease and associated factors: the PLATINO study in São Paulo. Brazil Cadernos de Saúde Pública. 2005;21(5):1565‐1573. [DOI] [PubMed] [Google Scholar]
- 20. Orozco‐Levi M. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(3):542‐546. [DOI] [PubMed] [Google Scholar]
- 21. Ekici A, Ekici M, Kurtipek E, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99(1):93‐98. [DOI] [PubMed] [Google Scholar]
- 22. Regalado J, Pérez‐Padilla R, Sansores R, et al. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med. 2006;174(8):901‐905. [DOI] [PubMed] [Google Scholar]
- 23. Mahmood T, Singh R, Kant S, Shukla A, Chandra A, Srivastava R. Prevalence and etiological profile of chronic obstructive pulmonary disease in nonsmokers. Lung India. 2017;34(2):122‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yin P, Jiang C, Cheng K, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou biobank cohort study. The Lancet. 2007;370(9589):751‐757. [DOI] [PubMed] [Google Scholar]
- 25. Zhou Y, Wang C, Yao W, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509‐518. [DOI] [PubMed] [Google Scholar]
- 26. Jordan RE, Cheng KK, Miller MR, Adab P. Passive smoking and chronic obstructive pulmonary disease: cross‐sectional analysis of data from the health survey for England. BMJ Open. 2011;1(2):e000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hagstad S, Bjerg A, Ekerljung L, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298‐1304. [DOI] [PubMed] [Google Scholar]
- 28. Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never‐smokers and ever‐smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822‐829. [DOI] [PubMed] [Google Scholar]
- 29. Perez‐Padilla R, Fernandez R, Lopez Varela MV, et al. Airflow obstruction in never smokers in five Latin American cities: the PLATINO study. Arch Med Res. 2012;43(2):159‐165. [DOI] [PubMed] [Google Scholar]
- 30. Lee SJ, Chang JH, Kim SW, Kong KA, Ryu YJ, Lee JH. Risk factors for chronic obstructive pulmonary disease among never‐smokers in Korea. Int J Chron Obstruct Pulmon Dis. 2015;10:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers. Chest. 2011;139(4):752‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turner MC, Krewski D, Chen Y, Pope CA, Gapstur SM, Thun MJ. Radon and COPD mortality in the American Cancer Society cohort. Eur Respir J. 2012;39(5):1113‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonjour S, Adair‐Rohani H, Wolf J, et al. Solid fuel use for household cooking: country and regional estimates for 1980–2010. Environ Health Perspect. 2013;121(7):784‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruce N, Perez‐Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ. 2000;78:1078‐1092. [PMC free article] [PubMed] [Google Scholar]
- 35. Smith KR. National burden of disease in India from indoor air pollution. Proc Natl Acad Sci. 2000;97(24):13286‐13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith K, Mehta S, Maeusezahl‐Feuz M. Indoor air pollution from household use of solid fuels: comparative quantification of health risks. In: Ezzati M, Lopez A, Rodgers A, Murray CJL, eds. Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. World Health Organization; 2004:1435‐1493. [Google Scholar]
- 37. Torres‐Duque C, Maldonado D, Perez‐Padilla R, Ezzati M, Viegi G. Biomass fuels and respiratory diseases: a review of the evidence. Proc am Thorac Soc. 2008;5(5):577‐590. [DOI] [PubMed] [Google Scholar]
- 38. Chester EH, Gillespie DG, Krause FD. The prevalence of chronic obstructive pulmonary disease in chlorine gas workers. Am Rev Respir Dis. 1969;99:365‐373. [DOI] [PubMed] [Google Scholar]
- 39. Husman K, Koskenvuo M, Kaprio J. Role of environment in the development of chronic bronchitis. Eur J Respir Dis Suppl. 1987;152:57‐63. [PubMed] [Google Scholar]
- 40. Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989;140(3_pt_2):S85‐S91. [DOI] [PubMed] [Google Scholar]
- 41. Trupin L, Earnest G, San Pedro M, et al. The occupational burden of chronic obstructive pulmonary disease. Eur Respir J. 2003;22(3):462‐469. [DOI] [PubMed] [Google Scholar]
- 42. Vignola AM, Kips J, Bousquet J. Tissue remodeling as a feature of persistent asthma. J Allergy Clin Immunol. 2000;105(6):1041‐1053. [DOI] [PubMed] [Google Scholar]
- 43. Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15‐year follow‐up study of ventilatory function in adults with asthma. New England Journal of Medicine. 1998;339(17):1194‐1200. [DOI] [PubMed] [Google Scholar]
- 44. Birath G, Caro J, Malmberg R. Airways obstruction in pulmonary tuberculosis. Scand J Respir Dis. 1966;47:27‐36. [PubMed] [Google Scholar]
- 45. Snider GL, Doctor L, Demas TA. Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis. 1971;103:625‐640. [DOI] [PubMed] [Google Scholar]
- 46. Plit ML, Anderson R, van Rensburg CEJ, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro‐inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998;12(2):351‐356. [DOI] [PubMed] [Google Scholar]
- 47. Chung KP, Chen JY, Lee CH, et al. Trends and predictors of changes in pulmonary function after treatment for pulmonary tuberculosis. Clinics. 2011;66(4):549‐556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: global burden of disease study. The Lancet. 1997;349(9063):1436‐1442. [DOI] [PubMed] [Google Scholar]
- 49. Shaheen SO, Barker DJ, Shiell AW, Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med. 1994;149(3):616‐619. [DOI] [PubMed] [Google Scholar]
- 50. Johnston IDA, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998;338(9):581‐587. [DOI] [PubMed] [Google Scholar]
- 51. Shaheen SO, Sterne JAC, Tucker JS, Florey CDV. Birth weight, childhood lower respiratory tract infection, and adult lung function. Thorax. 1998;53(7):549‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vuori E, Peltola H, Kallio MJT, Leinonen M, Hedman K. Etiology of pneumonia and other common childhood infections requiring hospitalization and parenteral antimicrobial therapy. Clin Infect Dis. 1998;27(3):566‐572. [DOI] [PubMed] [Google Scholar]
- 53. Ruano‐Ravina A, Wakeford R. The increasing exposure of the global population to ionizing radiation. Epidemiology. 2020;31(2):155‐159. [DOI] [PubMed] [Google Scholar]
- 54. Conde‐Sampayo A, Lorenzo‐González M, Fernández‐Villar A, Barros‐Dios JM, Ruano‐Ravina A. Exposure to residential radon and COPD: a systematic review. Int J Chron Obstruct Pulmon Dis. 2020;15:939‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barbosa‐Lorenzo R, Ruano‐Ravina A, Ramis R, et al. Residential radon and COPD. An ecological study in Galicia, Spain. Int J Radiat Biol. 2017;93(2):222‐230. [DOI] [PubMed] [Google Scholar]
- 56. Ruano‐Ravina A, Cameselle‐Lago C, Torres‐Durán M, et al. Indoor radon exposure and COPD, synergic sssociation? A multicentric, hospital‐based case–control study in a radon‐prone area. Arch Bronconeumol. 2020;S0300‐2896(20):30539–1. [DOI] [PubMed] [Google Scholar]
- 57. Torres‐Durán M, Barros‐Dios JM, Fernández‐Villar A, Ruano‐Ravina A. Residential radon and lung cancer in never smokers. A systematic review. Cancer Letters. 2014;345(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 58. Rodríguez‐Martínez Á, Torres‐Durán M, Barros‐Dios JM, Ruano‐Ravina A. Residential radon and small cell lung cancer. A systematic review. Cancer Lett. 2018;426:57‐62. [DOI] [PubMed] [Google Scholar]
- 59. Casal‐Mouriño A, Valdés L, Barros‐Dios JM, Ruano‐Ravina A. Lung cancer survival among never smokers. Cancer Lett. 2019;451:142‐149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


