Abstract
The Fitzpatrick scale has been in use for skin colour typing according to the tanning potential of skin since its inception in 1975–1976. Thomas Fitzpatrick developed the scale to classify persons with ‘white skin’ in order to select the correct amount of UVA in Joules/cm2 for PUVA treatment for psoriasis. Since then, it has been widely used in Dermatology to gauge the skin's reaction to UV exposure, tanning potential, assessment of sunburn risk and amount of sun protection required for individual patients. However, the use of this scale has been of limited utility because of different self‐perception in different areas of the world, particularly among those with skin of colour. Skin cancer risk is loosely inversely correlated with the initial genetic/inherent amount of melanin (most research has focused on eumelanin) present in the skin, although the pattern of exposure and amount of UV radiation required causing DNA damage varies widely according to different cancers. In this review, we have shown that the Fitzpatrick scale is neither correct nor adequate to reflect sunburn and tanning risk for skin of colour. Therefore, it may give both patients and physicians a false sense of security that there is little risk that people of colour can develop skin cancers. We have reviewed the small but not insignificant risk of skin of colour developing skin cancers and emphasise that there remains much research that needs to be done in this field.
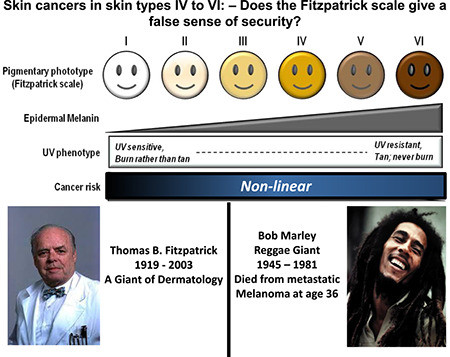
Fitzpatrick scale skin type IV to VI does not adequately nor accurately define these types, and gives the mistaken impression that skin cancers are not relevant. Skin cancers do occur in these skin types, although at a lower frequency, and more needs to be done in terms of targeting and advising BAME communities for better skin health and sun protection.
1.
What is already known about this topic?
The Fitzpatrick scale was initially developed for typing skin types in order to protect them from UV damage from UVA phototherapy
Fitzpatrick types VI to VI do not adequately nor accurately portray the wide range of skin tone and types that are normally classified into these 3 categories
What does this study add?
The published evidence demonstrates clearly that skin cancers do occur in skin types VI‐VI, but at a lower level
BAME communities do not have adequate levels of skin health promotional advice, and sun protection advice
There is a dearth of dermatology research on skin of colour
Abbreviations
- Asian
persons tracing their origins to primarily Asian origins
- Black
non‐Hispanic persons of primarily African origins
- Hispanic
Persons tracing their origins to primarily Central or South American origins
- White
non‐Hispanic persons of primarily European Caucasian origins
2.
Thomas Fitzpatrick MD (Harvard) developed his eponymously named skin type classification in 1975, as an aid to skin research into the tanning potential of human skin. 1 As Dr Fitzpatrick himself attested, it was developed to classify persons with white skin in order to select the correct amount of UVA in Joules/cm2 for PUVA treatment for psoriasis. 2 The scale was initially developed with eye and skin colour as the major parameters, but these were misleading, so the ability to tan when exposed to ultra‐violet (UV) radiation was then used as the major parameter. 3 This scale has largely superseded Von Luschan's chromatic scale, which was widely used in the beginning of the 20th century for anthropometry and racial studies. 4 This contained 36 different colour variants for racial typing, and has been abandoned by most, in part due to the inaccuracies and inefficiencies inherent in such a user‐dependant system for discriminatory racial profiling (for selective reproduction and sterilisations); see Table 1.
TABLE 1.
Current Fitzpatrick's type classification based on sun‐tanning potential compared to Von Luschan's numerical classification based on skin colour
| Fitzpatrick type | Von Luschan's chromatic scale | Correlation with tanning potential |
|---|---|---|
| I | 0–6 | Always burns, never tans |
| II | 7–13 | Usually burns, minimal tanning |
| III | 14–20 | Occasionally burns, usually tans uniformly |
| IV | 21–27 | Rarely burns, always tans well |
| V | 28–34 | Very rarely burns, tans very easily |
| VI | 35–36 | Never burns, always tans |
Although Fitzpatrick's scale is widely used in Dermatology to help classify the natural pigmentation of human skin and the effects thereon after UV exposure, it is not without its problems. The correlation of skin pigmentation (defined as variable skin tone ranging from very light to very dark) with susceptibility to development of skin cancers is not linear and therefore a six‐category scale is not appropriate for risk assessment as to skin oncogenic potential (likelihood of developing skin cancers) when stated outcomes such as ‘never burns’ or ‘very rarely burns’ are patently not true. Numerous clinical observational studies and epidemiological studies have shown that darker skin has a much reduced risk of developing skin cancer, both melanoma or non‐melanoma skin cancers (NMSC). This is partly due to the photoprotective effect of UV filtering by the increased melanin found in the epidermis. Kaidbey et al. 5 found that black skin filtered out five times as much UV radiation compared to Caucasian skin so that black skin has been estimated to have an intrinsic SPF of 13.4, in contrast to light skin with an SPF of 3.3. However, it is also very clear that there is no single shade of pigmentation for black skin and there is a wide biological range from light to very dark and any estimates of intrinsic SPF must be taken in this context.
The Fitzpatrick scale has been described as ‘Anglo‐Irish centric’ or ‘simply irrelevant’ when used for skin of colour or outside the Western World. In Europe, Fitzpatrick I–IV may be used to describe the various skin shades common to those with mainly European ancestry, with IV, V and VI also used for brown to black skin, from either central Africa, parts of South America, the South Pacific and Australia and much of the Caribbean including those of mixed ancestry living in other parts of the world. Type V may then be applied to people from many parts of Asia, North Africa and South African, Central and Southern America. However, the scale may be used differently by people from other parts of the world: for example, those in living in parts of Asia or North Africa may consider that the local population has mainly type III or IV skin, and self‐reporting of skin colour has been shown to be influenced by the community that person lives in. 6 , 7 Self‐reporting of skin colour using the scale is also known to be less accurate than a trained dermatologist's assessment, 8 and found to exclude the majority of Black people who self‐report. 9 The scale is often used wrongly, is not used consistently and therefore is of limited utility.
One of the main problems with the scale is that it reports that type V and VI skin ‘usually or always tans, never or rarely burns’. These categorisations are wholly inaccurate, and reflect attitudes prevalent in the 1960s and 1970s amongst Dermatologists who originated predominantly from Europe or North America. There are studies that show that sunburn amongst darker skin is much more common than previously thought. 10 , 11 There has been an epidemic of skin cancer rising in prevalence and incidence amongst the lighter skinned populations in the Western world and almost certainly a slower concomitant rise amongst darker skinned populations as well but this is not reflected in skin cancer registries which are nearly non‐existent in less‐developed countries.
If there has been a rise in skin cancers amongst people of darker skin types, then this should be reflected in skin cancer registries from Asian countries such as Singapore, which have highly developed and modern societies with diverse populations of mainly type III to VI skin types. Indeed, publications such as Sng et al. 12 document increasing rates of NMSC amongst their population, with the elderly Chinese (type III–IV skin) exhibiting threefold the incidence rates compared to the Malays and Indians who have typically type IV–VI skin. The decreasing rates of skin cancers in skin of increasing pigmentation is confirmed. 13 We suggest that there is a likely concomitant undocumented rise amongst populations in Africa (particularly Southern Africa where there were waves of migration from Europe and Asia, and consequently a highly mixed population) and the Indian Subcontinent (where the upper castes commonly had lighter coloured skin compared to the lower castes (farmers, labourers, manual workers, etc.).
The commonest skin cancers in people of colour are the NMSCs of which Squamous Cell Carcinomas (SCCs) and Basal Cell Carcinomas (BCCs) comprise the majority. SCCs are the most common skin cancers in people of African and Asian Indian descent, with BCCs next most frequent. 14 , 15 Risk factors for NMSC have been studied extensively but nearly all conducted in populations with predominantly less pigmented skin. Cumulative UV exposure is the most important risk factor for NMSC in lighter coloured skin, which also include elderly age, chronic scarring processes, ionising radiation, inflammatory conditions, HPV infection, immunosuppression, genodermatoses such as albinism, xeroderma pigmentosum and so forth. 15 , 16 , 17 Tadokoro et al. 18 studied melanin content and degree of UVA and B induced DNA damage in normal skin of various ethnic groups. They found that baseline skin eumelanin content was inversely correlated with extent of DNA damage and damage was appreciable in all skin types, even at low levels of exposure. Interestingly, skin of colour was able to more efficiently repair solar‐induced DNA damage than skin from those with light skin. Therefore, the depth of colour of skin (darker skin colour) may be a non‐linear marker indicating more efficient molecular repair processes that help protect skin cell DNA from ionising radiation.
Melanoma incidence was found not to correlate with increased residential UV exposure in skin of colour (Black, Hispanic, Asian and Native Americans). 19 In contrast, Hu et al. 20 discovered a positive correlation of melanoma incidence with UV exposure in White, Hispanic and Black people, implying that high intermittent exposure to UV of skin of colour was important in the development of melanoma. The tendency for melanomas and SCCs to develop in UV‐protected areas in skin of colour implies that UV radiation may not be significant for these two skin cancers, in contrast to BCCs (which do develop predominantly in sun‐exposed areas). 21 These contradictory findings confirm that UV significance in the development of skin cancers in skin of colour remains much under‐researched.
There is solid evidence for a causative role of sunlight (UV radiation) exposure in melanoma oncogenesis, although the relationship between sunlight and melanoma is complex. 22 Eighty percent of melanomas develop in regions of the world where most light‐skinned people get intermittent intense sun exposure, often during short holidays. Intermittent strong sun exposure and sunburn history, especially in childhood, have been identified as strong risk factors for melanoma. 23 UV exposure in childhood appears to be a strong driver risk for induction of mutations in the ‘melanocytic system’ and the development of melanocytic naevi. Length of sun exposure, high UV indices and light skin are strongly associated with the development of melanocytic naevi in childhood. 24 , 25 , 26 In adulthood, sunburn and increased sun exposure are associated with the development of solar lentigines and lentigo maligna in sun‐exposed areas. 27 , 28
Latitude studies amongst populations with relatively low migration in Europe demonstrate a strong correlation with fatal melanoma incidence in less sunny climates, which in turn, indirectly correlates with skin pigmentation. 22 By contrast, studies in the US showed that in non‐Hispanic whites, where there has been considerable migration over the last 300 years, there was strong correlation between melanoma incidence and lower latitudes and higher mean UV indices. This effect was also shown in Hispanics and Blacks; thereby strongly suggesting that these risk factors are involved in the same mechanistic pathways towards development of melanoma in all humans. 19 , 29 Further epidemiological data demonstrating that intermittent exposure to high doses of UV sufficient to cause sunburn comes from childhood studies on sunburn 23 and the greatest increase in melanoma incidence appear to be on the torso in men, and legs in women, which are areas of the body exposed to intermittent sun exposure. 30 , 31 , 32
There is much underestimation of the risks of skin cancer in skin of colour, both amongst the general population, and in the communities of people of colour. 11 , 33 , 34 People of colour are also less likely to use sunscreen, and less likely to report sunburn, and have much less familiarity with skin examinations or the need to ask for such examinations. Although the risks of developing skin cancers are lower relative to white populations, these skin cancers tend to be associated with higher morbidity and mortality due to later presentation/delayed diagnoses, and larger tumour volumes. 9 , 16 , 21
In conclusion, we strongly suggest that the Fitzpatrick scale is not appropriate when used for phototyping skin colour in relation to skin cancer risks, especially since tanning and sunburning are not envisaged for people of colour. It has and will continue to give a false sense of security to both physicians and patients. We advocate against its use for people of colour, instead digital photography with standard lighting and the use of artificial intelligence with alternative skin typing systems 35 , 36 , 37 may be better suited to help visualise and objectively categorise skin of colour in relation to UV radiation damage, photoageing and skin cancers. We reiterate that there is a severe lack of data for people of colour developing skin cancers, and that this must be a priority for dermatological research, especially here in the UK, which has an increasingly diverse population in the 21st century.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Goon P, Banfield C, Bello O, Levell NJ. Skin cancers in skin types IV–VI: does the Fitzpatrick scale give a false sense of security? Skin Health Dis. 2021;1(3):e40. 10.1002/ski2.40
Peter Goon and Nick J. Levell are joint senior authors.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study
REFERENCES
- 1. Fitzpatrick TB. Soleil et peau [Sun and skin]. J Méd Esthétique. 1975;2:33–4. [Google Scholar]
- 2. Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. N Engl J Med. 1974;291:1207–11. [DOI] [PubMed] [Google Scholar]
- 3. Fitzpatrick TB. The validity and practicality of sun‐reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. [DOI] [PubMed] [Google Scholar]
- 4. Jablonski NG. The evolution of human skin and skin color. Annu Rev Anthropol. 2004;33:585–623. [Google Scholar]
- 5. Kaidbey KH, Agin PP, Sayre RM, Kligman AM. Photoprotection by melanin—a comparison of black and Caucasian skin. J Am Acad Dermatol. 1979;1:249–60. [DOI] [PubMed] [Google Scholar]
- 6. Chan JL, Ehrlich A, Lawrence RC, Moshell AN, Turner ML, Kimball AB. Assessing the role of race in quantitative measures of skin pigmentation and clinical assessments of photosensitivity. J Am Acad Dermatol. 2005;52:609–15. [DOI] [PubMed] [Google Scholar]
- 7. He SY, McCulloch CE, Boscardin WJ, Chren M‐M, Linos E, Arron ST. Self‐reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eilers S, Bach DQ, Gaber R, Blatt H, Guevara Y, Nitsche K, et al. Accuracy of self‐report in assessing Fitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289–94. [DOI] [PubMed] [Google Scholar]
- 9. Pichon LC, Landrine H, Corral I, Hao Y, Mayer JA, Hoerster KD. Measuring skin cancer risk in African Americans: is the Fitzpatrick skin type classification scale culturally sensitive? Ethn Dis. 2010;20:174–9. [PubMed] [Google Scholar]
- 10. Diffey BL, Fajuyigbe D, Wright CY. Sunburn and sun protection in black skin. Int J Dermatol. 2019;58:1053–5. [DOI] [PubMed] [Google Scholar]
- 11. Kim M, Boone SL, West DP, Rademaker AW, Liu D, Kundu RV. Perception of skin cancer risk by those with ethnic skin. Arch Dermatol. 2009;145:207–8. [DOI] [PubMed] [Google Scholar]
- 12. Sng J, Koh D, Siong WC, Choo TB. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61:426–32. [DOI] [PubMed] [Google Scholar]
- 13. Koh D, Wang H, Lee J, Chia KS, Lee HP, Goh CL. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: analysis of the Singapore Cancer Registry data 1968‐97. Br J Dermatol. 2003;148:1161–6. [DOI] [PubMed] [Google Scholar]
- 14. Gloster HM, Jr , Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741–60. [DOI] [PubMed] [Google Scholar]
- 15. Higgins S, Nazemi A, Chow M, Wysong A. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Ds. 2018;44:903–10. [DOI] [PubMed] [Google Scholar]
- 16. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748–62. [DOI] [PubMed] [Google Scholar]
- 17. Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519–26. [DOI] [PubMed] [Google Scholar]
- 18. Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, et al. UV‐induced DNA damage and melanin content in human skin differing in racial/ethnic origin. Faseb J. 2003;17:1177–9. [DOI] [PubMed] [Google Scholar]
- 19. Eide MJ, Weinstock MA. Association of UV Index, latitude, and melanoma incidence in nonwhite populations‐‐US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol. 2005;141:477–81. [DOI] [PubMed] [Google Scholar]
- 20. Hu S, Parmet Y, Allen G, Parker DF, Ma F, Rouhani P, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369–74. [DOI] [PubMed] [Google Scholar]
- 21. Battie C, Gohara M, Verschoore M, Roberts W. Skin cancer in skin of color: an update on current facts, trends, and misconceptions. J Drugs Dermatol JDD. 2013;12:194–8. [PubMed] [Google Scholar]
- 22. Shipman AR, Clark AB, Levell NJ. Sunnier European countries have lower melanoma mortality. Clin Exp Dermatol. 2011;36:544–7. [DOI] [PubMed] [Google Scholar]
- 23. Autier P, Dore JF, Doré J, Gefeller O, Cesarini J, Lejeune F, et al. Melanoma risk and residence in sunny areas. Br J Canc. 1997;76:1521–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bauer J, Büttner P, Wiecker TS, Luther H, Garbe C. Interventional study in 1,232 young German children to prevent the development of melanocytic nevi failed to change sun exposure and sun protective behavior. Int J Canc. 2005;116:755–61. [DOI] [PubMed] [Google Scholar]
- 25. Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Effect of sunscreen and clothing on the number of melanocytic nevi in 1,812 German children attending day care. Am J Epidemiol. 2005;161:620–7. [DOI] [PubMed] [Google Scholar]
- 26. Wiecker TS, Luther H, Buettner P, Bauer J, Garbe C. Moderate sun exposure and nevus counts in parents are associated with development of melanocytic nevi in childhood. Cancer. 2003;97:628–38. [DOI] [PubMed] [Google Scholar]
- 27. Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Canc. 1997;73:198–203. [DOI] [PubMed] [Google Scholar]
- 28. Garbe C, Krüger S, Orfanos CE, Büttner P, Weiß J, Soyer HP, et al. Associated factors in the prevalence of more than 50 common melanocytic nevi, atypical melanocytic nevi, and actinic lentigines: multicenter case‐control study of the central malignant melanoma registry of the German Dermatolgocial Society. J Invest Dermatol. 1994;102:700–5. [DOI] [PubMed] [Google Scholar]
- 29. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Canc. 1999;80:827–41. [DOI] [PubMed] [Google Scholar]
- 30. Mackie RM, Marks R, Green A. The melanoma epidemic. BMJ. 1996;312:1362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marks R. Epidemiology of melanoma. Clin Exp Dermatol. 2000;25:459–63. [DOI] [PubMed] [Google Scholar]
- 32. Rigel DS, Carucci JA. Malignant melanoma: prevention, early detection, and treatment in the 21st century. CA A Cancer J Clin. 2000;50:215–36. [DOI] [PubMed] [Google Scholar]
- 33. Halder RM, Bridgeman‐Shah S. Skin cancer in African Americans. Cancer. 1995;75:667–73. [DOI] [PubMed] [Google Scholar]
- 34. Pichon LC, Corral I, Landrine H, Mayer JA, Adams‐Simms D. Perceived skin cancer risk and sunscreen use among African American adults. J Health Psychol. 2010;15:1181–9. [DOI] [PubMed] [Google Scholar]
- 35. Chardon A, Cretois I, Hourseau C. Skin colour typology and suntanning pathways. Int J Cosmet Sci. 1991;13:191–208. [DOI] [PubMed] [Google Scholar]
- 36. De Rigal J, Abella M‐L, Giron F, Caisey L, Lefebvre MA. Development and validation of a new skin color chart. Skin Res Technol. 2007;13:101–9. [DOI] [PubMed] [Google Scholar]
- 37. Del Bino S, Bernerd F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br J Dermatol. 2013;169 (suppl 3):33–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study


