Abstract
Background
Diabetes is a cardiometabolic comorbidity that may predispose COVID‐19 patients to worse clinical outcomes. This study sought to determine the prevalence of diabetes in hospitalized COVID‐19 patients and investigate the association of diabetes severe COVID‐19, rate of acute respiratory distress syndrome (ARDS), mortality, and need for mechanical ventilation by performing a systematic review and meta‐analysis.
Methods
Individual studies were selected using a defined search strategy, including results up until July 2021 from PubMed, Embase, and Cochrane Central Register of Controlled Trials. A random‐effects meta‐analysis was performed to estimate the proportions and level of association of diabetes with clinical outcomes in hospitalized COVID‐19 patients. Forest plots were generated to retrieve the odds ratios (OR), and the quality and risk assessment was performed for all studies included in the meta‐analysis.
Results
The total number of patients included in this study was 10 648, of whom 3112 had diabetes (29.23%). The overall pooled estimate of prevalence of diabetes in the meta‐analysis cohort was 31% (95% CI, 0.25‐0.38; z = 16.09, P < .0001). Diabetes significantly increased the odds of severe COVID‐19 (OR 3.39; 95% CI, 2.14‐5.37; P < .0001), ARDS (OR 2.55; 95% CI, 1.74‐3.75; P = <.0001), in‐hospital mortality (OR 2.44; 95% CI, 1.93‐3.09; P < .0001), and mechanical ventilation (OR 3.03; 95% CI, 2.17‐4.22; P < .0001).
Conclusions
Our meta‐analysis demonstrates that diabetes is significantly associated with increased odds of severe COVID‐19, increased ARDS rate, mortality, and need for mechanical ventilation in hospitalized patients. We also estimated an overall pooled prevalence of diabetes of 31% in hospitalized COVID‐19 patients.
Keywords: COVID‐19, diabetes, meta‐analysis, mortality, prevalence, SARS‐CoV‐2, ventilation
Highlights
Diabetes may predispose COVID‐19 hospitalized patients to worse clinical outcomes.
Our findings indicate that diabetes is significantly associated with increased odds of severe COVID‐19, acute respiratory distress syndrome, mortality, and need for mechanical ventilation in hospitalized patients.
COVID‐19 patients with diabetes are at high risk of morbidity and mortality.
摘要
背景
糖尿病是心脏代谢性伴随疾病, 可能使新型冠状病毒肺炎患者的临床结局更差。本研究旨在通过系统回顾和meta分析, 确定住院新型冠状病毒肺炎患者中糖尿病的患病率, 并调查糖尿病与严重新型冠状病毒肺炎疾病, 急性呼吸窘迫综合征(ARDS)发生率, 死亡率和机械通气需求之间的关系。
方法
从PubMed, Embase和Cochrane Central Register of Control Trials中选择个别研究, 采用定义的搜索策略, 包括截至2021年7月的结果。对住院的新型冠状病毒肺炎患者进行随机效应meta分析, 以评估糖尿病与临床结果的比例和水平。生成森林图以检索优势比, 并对meta分析中包括的所有研究进行质量和风险评估。
结果
本研究共纳入患者10648例, 其中糖尿病患者3112例(29.23%)。Meta分析队列中糖尿病总体合并估计的患病率为31%(95% CI 0.25‐0.38, z=16.09 p<0.0001)。糖尿病显著增加严重新型冠状病毒肺炎严重程度(OR 3.39, 95% CI 2.14~5.37, p<0.0001), 急性呼吸窘迫综合征(OR 2.55, 95% CI 1.74~3.75, p=<0.0001), 住院死亡率(OR 2.44, 95% CI 1.93~3.09, p<0.0001)和机械通气(OR 3.03, 95%CI 2.17~4.22, p<0.0001)。
结论
我们的meta分析显示糖尿病与住院患者严重新型冠状病毒肺炎, 急性呼吸窘迫综合征的发生率, 死亡率和需要机械通气的增加显著相关。我们还估计在住院的新型冠状病毒肺炎患者中糖尿病的总体合并患病率为31%。
Keywords: SARS‐CoV‐2, 新型冠状病毒肺炎, 糖尿病, meta分析, 患病率, 死亡率, 通气
1. INTRODUCTION
The COVID‐19 pandemic is an ongoing public health crisis, with over 245 373 039 confirmed cases globally, including 4 979 421 deaths as of 29 October 2021 (according to the World Health Organization [WHO]). 1 Factors associated with poor outcomes, including in‐hospital morbidity and mortality, in COVID‐19 hospitalized patients are of considerable interest. 2 More specifically, health comorbidities may predispose patients to an increased risk of poor outcomes following COVID‐19 infection. 2 , 3 , 4 Previous studies have indicated a relatively higher prevalence of diabetes in COVID‐19 patients compared to those without diabetes. 5 Moreover, previous meta‐analyses have indicated that diabetes is associated with increased risk of severe COVID‐19, acute respiratory distress syndrome (ARDS), and in‐hospital mortality. 6 , 7 , 8 , 9 , 10 , 11 However, several of these studies included patients under 18 years of age or from the community setting.
It is of clinical and public health interest to understand how diabetes mediates outcomes in COVID‐19 hospitalized patients. The current study sought to determine in‐hospital prevalence of diabetes in COVID‐19 patients and investigate the association of diabetes with COVID‐19 severity, ARDS rate, mortality, and the need for mechanical ventilation in hospitalized COVID‐19 adult patients by performing a systematic review and meta‐analysis.
With this study, we wanted to investigate the following questions:
What is the pooled prevalence of diabetes in hospitalized COVID‐19 patients drawn from the meta‐analysis?
Is diabetes associated with COVID‐19 severity, increased rate of ARDS, need for mechanical ventilation, and in‐hospital mortality?
2. METHODS
2.1. Literature search: Identification and selection of studies
Relevant articles were retrieved from the following databases until July 2021: PubMed, Embase, and Cochrane Central Register of Controlled Trials. The search terms used included diabetes or diabetic or t1dm or t2dm or diabetes mellitus and covid‐19 or covid or coronavirus or severe acute respiratory syndrome coronavirus 2 or SARS‐CoV‐2. Nonhuman studies and articles not written in English were excluded by applying filters. The detailed search strategy is provided in the Supplementary Information. The Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) flowchart was used to present the search strategy and studies included in the meta‐analysis (Figure 1). The PRISMA 2020 (Supplemental Table S6), Meta‐analysis of Observational Studies in Epidemiology (MOOSE) (Supplemental Table S5), and Standards for Reporting of Diagnostic Accuracy Studies (STARD 2015) checklists (Supplemental Table S7) were also adhered to for reporting.
FIGURE 1.
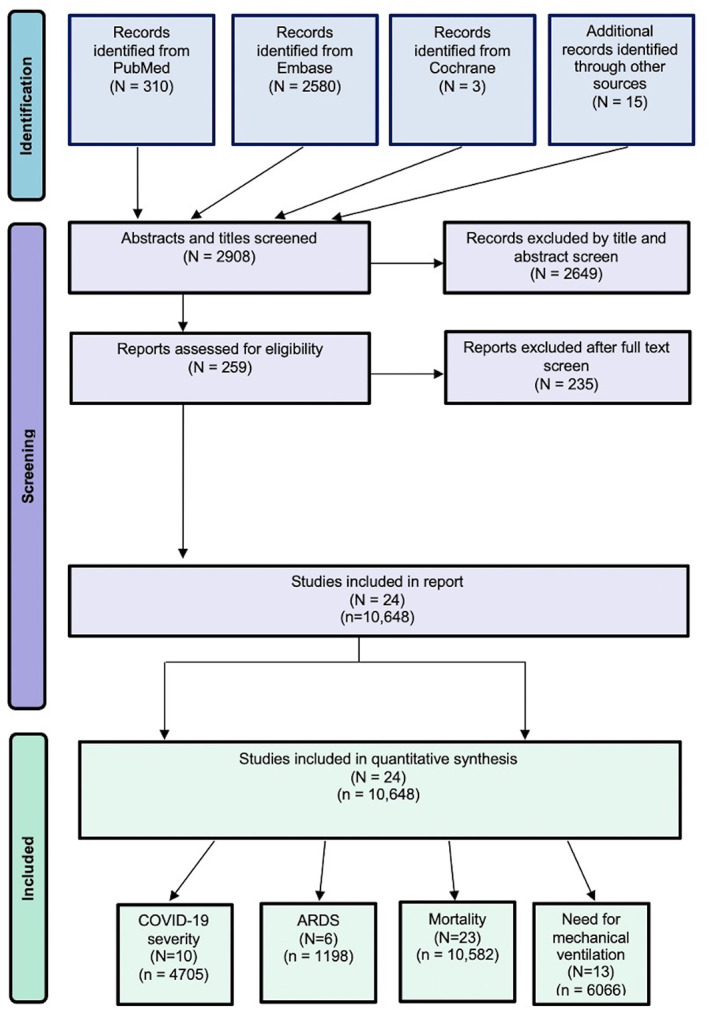
The PRISMA flowchart showing the studies included in the meta‐analysis. ARDS, acute respiratory distress syndrome; n, cohort size; N, number of studies
2.2. Inclusion and exclusion criteria
For studies to be included, the following inclusion criteria were applied: (a) age ≥ 18 years, (b) availability of comparative data between diabetes and non‐diabetes groups, (c) patients with a confirmed diagnosis of COVID‐19 in a hospital setting, and (d) studies with good methodological design (with appropriate sample size deemed to be >20 patients in each group). Besides, the following criteria were applied to exclude studies: (a) nonhuman/animal studies; (b) duplicated publications; (c) nonavailability of full‐text articles; (d) case reports, case series, letters, systematic reviews, and meta‐analyses; and (e) studies provided in abstract form that did not have relevant data on diabetes or relevant clinical outcomes were not reported.
2.3. Data extraction
The title and abstracts were reviewed on Endnote (X9.3.3; Clarivate) first to rule out that the articles did not meet the eligibility criteria. The remaining articles were examined thoroughly to determine whether they should be included in the systematic review or meta‐analysis according to the eligibility criteria. Reference lists of articles included were also reviewed for possible inclusion. The screening was conducted independently by two authors experienced in meta‐analysis. Any disagreements were discussed, and final decisions were reached by consensus. The data from each study/trial were extracted independently using a data extraction sheet to obtain the following information: (a) baseline demographics (author, country, and year of publication), (b) study population (age of patients, sample size, characteristics of COVID‐19 patients, and presence or absence of diabetes), and (c) outcome measures (severity of COVID‐19, in‐hospital ARDS, mortality, and need for mechanical ventilation). The outcome measures were tabulated for the diabetes and non‐diabetes groups. The definition of severe disease differed between studies, with the presence of respiratory distress or admission to the intensive care unit as common criteria. We dichotomized severity groups into severe and non‐severe. Where studies had separate groups for severe and critical cases, these two groups were combined for the purpose of this meta‐analysis. ARDS was defined according to the Berlin definition in two studies and undefined in two other studies. 12 Mortality was defined as in‐hospital death within the study period for all studies. Mechanical ventilation was defined as invasive mechanical ventilation in five studies, intubation in two studies, and ventilation in one study.
2.4. Quality assessment of included studies
The modified Jadad analysis scale was independently used by two researchers to assess the methodological quality of each study. 13 The bias due to funding was also evaluated, independent of the quality assessment, based on the declaration of funding sources and conflicts of interest. 14
2.5. Statistical analysis
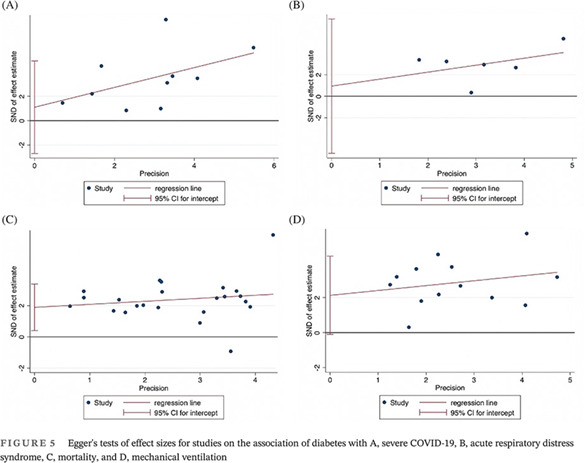
All statistical analyses were performed using STATA (Version 13.0, StataCorp LLC, College Station, Texas). The baseline characteristics of patient populations were synthesized from all included studies. The crude prevalence of diabetes within the overall cohort was determined. Where applicable, median, and interquartile ranges were converted to mean and SD using the method described by Wan et al 15 In this study, the association of diabetes with severity of COVID‐19, odds of ARDS, mortality, and need for mechanical ventilation was investigated by performing the DerSimonian‐Laird random‐effects meta‐analysis. Summary effects and heterogeneity obtained from the meta‐analysis were tabulated. Forest plots were generated to present the odds ratios (OR) (effects measure), 95% CI, percentage weight, and the heterogeneity between studies included in the meta‐analysis. When applicable, tests of overall effect, vis a vis P value, and Z‐test were also reported. Egger's tests of effect sizes corresponding to each clinical outcome were performed (Figure 2 and Supplemental Table S3). To assess the heterogeneity between the studies, I2 statistics and P values were used (<40% = low, 30%‐60% = moderate, 50%‐90% = substantial, and 75%‐100% = considerable). 16 Other heterogeneity parameters including Cochran's Q, H‐Test (relative excess in Cochran's Q over its degrees of freedom), and tau (for heterogeneity variance estimates) were also analyzed. The regression‐based Egger's test for small‐study effects was also used to evaluate funnel plot asymmetry quantitatively (Figure 5). The effects of an individual study on the overall meta‐analysis were also evaluated, for stability and sensitivity, using STATA's “metaninf” package (Supplemental Figure S1). Effect size analyses for the association of diabetes with severity, ARDS, mortality and mechanical ventilation are shown in Supplemental Figure S2. A Begg's funnel plot was used to visually detect the presence of publication bias, identified by the presence of asymmetry on the funnel plot (Supplemental Figure S3). P values <.05 indicated statistically significant association.
FIGURE 2.
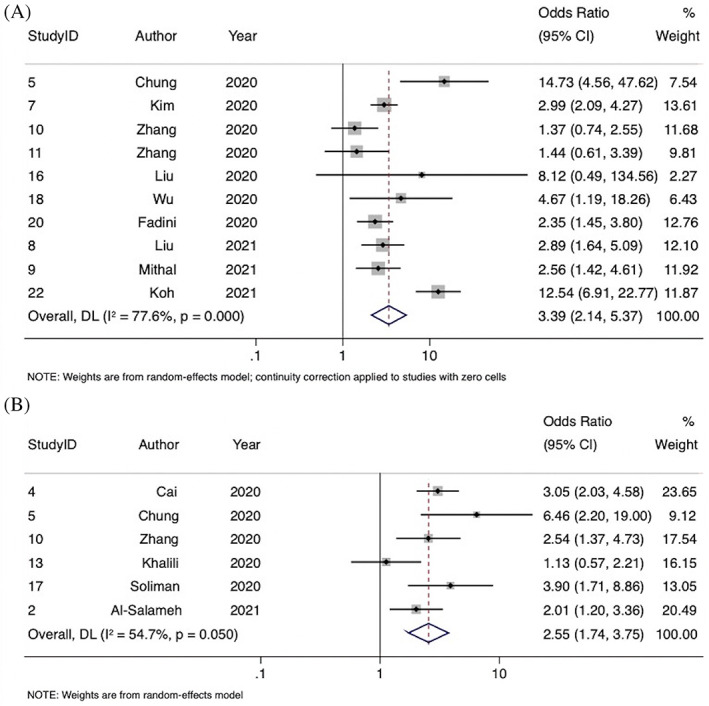
Forest plot showing the association of diabetes with A, severe COVID‐19 and B, acute respiratory distress syndrome
For estimating prevalence, the “metaprop” STATA command was used to pool proportions by performing a DerSimonian‐Laird random‐effects meta‐analysis of proportions obtained from the individual studies. 17 To stabilize the variances, Freeman‐Tukey double arcsine transformation was also applied to stabilize the variances in order to calculate the pooled estimates. For prevalence meta‐analysis, the heterogeneity was estimated from the inverse‐variance fixed‐effect model as well as by using the I2 measure. Forest plots were created to report the overall estimates of the meta‐analysis.
3. RESULTS
3.1. Description of included studies
There were 24 included studies, with a total cohort of 10 648 patients, of whom 3112 had diabetes (29.23%). The mean age ± SD of all included studies was 49.87 ± 32.32 years, and there were 5953 males overall (55.91%). COVID‐19 diagnosis was defined as a positive reverse transcription polymerase chain reaction (RT‐PCR) test in 21 studies; as history, fever or respiratory symptoms, CT imaging abnormalities, and a positive RT‐PCR in 1 study; and was not defined in 2 studies. Three studies specifically investigated type 2 diabetes only, with the remainder not distinguishing between type 1 and 2. Data on diabetes severity or duration were not provided in any studies. Although the overall crude prevalence rate of diabetes was 29.23%, our meta‐analysis demonstrated that the overall pooled estimated prevalence rate was 31% (95% CI, 0.25‐0.38; z = 16.09, P < .0001). A forest plot showing the prevalence of diabetes in COVID‐19 hospitalized patients is included in Figure 3. Supplemental Table S4 shows the full results of the random‐effects meta‐analysis of proportions obtained from individual studies. Considerable heterogeneity was found between the studies (I2 = 98.04%, P < .0001). A description of the clinical characteristics of the included studies can be found in Table 1. Summary effects and heterogeneity from the meta‐analysis on the association of diabetes are provided in Table 2. Supplemental Table S1 provides a summary of significant meta‐analysis outcomes. Summary data and performance estimates for sensitivity and specificity analysis are also provided in Supplemental Table S2. The results of methodological quality, risk of bias, and funding bias assessment of included studies are provided in Supplemental Table S3. One study demonstrated a moderate potential for funding bias. Effect size analyses for severe COVID‐19, ARDS, mortality, and mechanical ventilation are also presented in Supplemental Figure S2.
FIGURE 3.
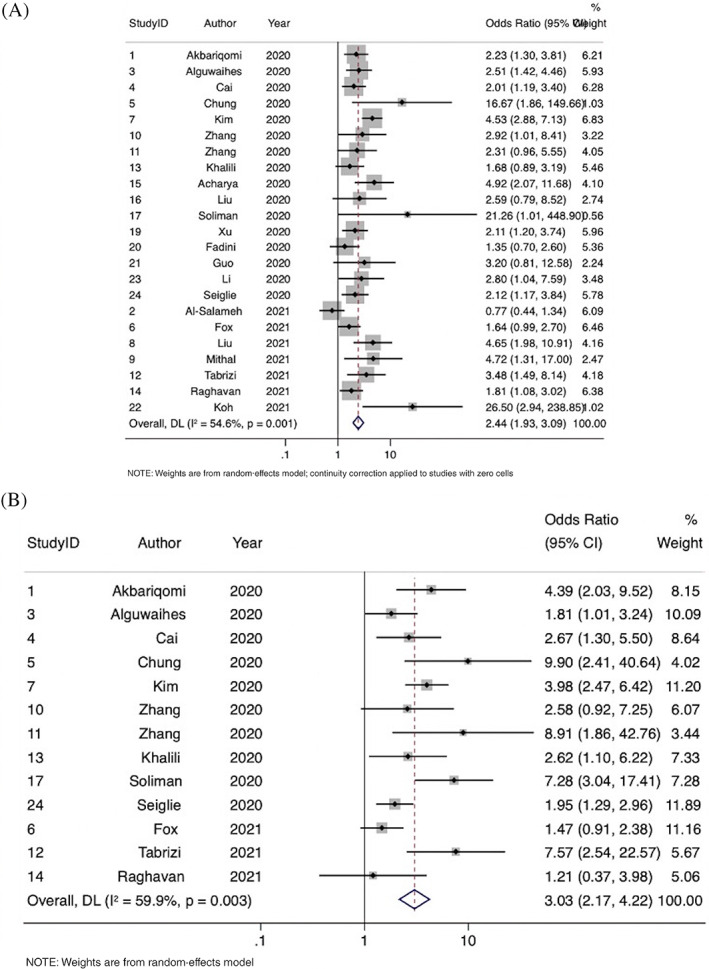
Forest plots showing the association of diabetes with (A) mortality and (B) use of mechanical ventilation
TABLE 1.
Clinical characteristics and clinical outcomes of studies included in the meta‐analysis
| Study ID | Author | Year | Country | Study type | Cohort | Age (mean ± SD) | Male (%) | Severity definition | ARDS definition | Mechanical ventilation definition | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | DM | nDM | Overall | DM | nDM | |||||||||||
| 1 |
Akbariqomi 28 |
2020 | Iran | Retrospective | 595 | 54.33 ± 13.38 | 53.33 ± 14.97 | 57.3 ± 16.36 | 67.39 | 66.89 | 67.56 | National Health Committee of China criteria | NR | Invasive mechanical ventilation | ||
| 2 | Al‐Salameh 2 | 2021 | France | Retrospective | 432 | 72.61 ± 15.78 | 72 ± 18.62 | 72.83 ± 14.64 | 55.09 | 52.05 | 63.48 | Berlin definition | ||||
| 3 | Alguwaihes 32 | 2020 | Saudi Arabia | Retrospective | 439 | 55.2 ± 13.74 | NR | NR | 68.34 | 66.67 | 71.94 | Required intubation | ||||
| 4 |
Cai 33 |
2020 | China | Retrospective | 941 | 57.02 ± 12.46 | 68.41 ± 14 | 56 ± 12.15 | 48.25 | 49.59 | 48.04 | NR | Invasive mechanical ventilation | |||
| 5 |
Chung 36 |
2020 | South Korea | Retrospective | 110 | 56.9 ± 17 | 66.3 ± 8.9 | 53.5 ± 17.9 | 43.64 | 48.28 | 41.98 | Chinese Center for Disease Control and Prevention criteria | Berlin definition | Invasive mechanical ventilation | ||
| 6 | Fox 34 | 2021 | USA | Retrospective | 355 | 66.21 ± 14.21 | 66.42 ± 12.67 | 66.03 ± 15.46 | 49.01 | 51.81 | 46.56 | Need for intubation | ||||
| 7 | Kim 39 | 2020 | South Korea | Retrospective | 1082 | 56.06 ± 17.55 | 68.3 ± 11.9 | 56.5 ± 18 | 35.49 | 45.11 | 32.82 | Severe disease defined as at least one of: ICU care, high‐flow O2 nasal cannulae, mechanical ventilation, CRRT, or ECMO | Mechanical ventilation | |||
| 8 | Liu 31 | 2021 | China | Retrospective | 233 | 62.33 ± 14.17 | 65.33 ± 10.7 | 63.5 ± 19.09 | 49.36 | 51.25 | 48.37 | National Health Committee of China criteria | ||||
| 9 | Mithal 35 | 2021 | India | Prospective | 401 | 54.06 ± 12.35 | 59.8 ± 12.1 | 47.7 ± 16.5 | 68.83 | 74.6 | 63.68 | WHO Ordinal Scale 5 and above | ||||
| 10 | Zhang 40 | 2020 | China | Retrospective | 258 | 63.33 ± 10.44 | 64.33 ± 10.62 | 63 ± 11.2 | 53.49 | 60.32 | 51.28 | National Health Committee of China criteria | Berlin definition | Invasive mechanical ventilation | ||
| 11 | Zhang 38 | 2020 | China | Retrospective | 166 | 62.7 ± 14.2 | 65.6 ± 11.4 | 61.04 ± 15.34 | 51.2 | 54.1 | 49.52 | National Health Committee of China Criteria | Invasive mechanical ventilation | |||
| 12 | Tabrizi 41 | 2021 | Iran | Retrospective | 268 | 57.33 ± 17.14 | 63.67 ± 12.75 | 50 ± 17.23 | 53.36 | 53.54 | 53.19 | Invasive mechanical ventilation | ||||
| 13 | Khalili 42 | 2020 | Iran | Retrospective | 254 | 65.7 ± 12.51 | 66.28 ± 12.51 | 65.03 ± 12.53 | 55.91 | 55.91 | 55.91 | Berlin definition | Invasive mechanical ventilation | |||
| 14 | Raghavan 43 | 2021 | India | Retrospective | 845 | 55.51 ± 15.78 | 51 ± 17 | 60 ± 13 | 65.44 | 67.54 | 63.36 | Need for intubation | ||||
| 15 | Acharya 37 | 2020 | South Korea | Retrospective | 324 | 55 ± 21.4 | 69.8 ± 13.5 | 51.9 ± 21.4 | 41.67 | 36.36 | 42.75 | |||||
| 16 | Liu 44 | 2020 | China | Retrospective | 934 | 13.96 ± 62.03 | 64.5 ± 10 | 61.6 ± 14.5 | 48.61 | 47.48 | 48.81 | NR | ||||
| 17 | Soliman 45 | 2020 | Qatar | Retrospective | 303 | 13.25 ± 39.31 | 52.1 ± 12.67 | 36.22 ± 11.43 | NR | NR | Mechanical ventilation | |||||
| 18 | Wu 46 | 2020 | China | Retrospective | 66 | 14.71 ± 49.5 | 52.55 ± 13.7 | 47.98 ± 15.11 | 66.67 | 72.73 | 63.64 | National Health Committee of China Criteria | ||||
| 19 | Xu 47 | 2020 | China | Retrospective | 364 | 64.33 ± 13.4 | 65.33 ± 12.01 | 63 ± 15.66 | 56.59 | 54.39 | 57.6 | |||||
| 20 | Fadini 48 | 2020 | Italy | Retrospective | 413 | 64.9 ± 15.4 | 69.7 ± 13.8 | 63.3 ± 15.5 | 59.32 | 65.42 | 57.19 | Composite of admission to the ICU (including all subjects needing mechanical ventilation) or death | ||||
| 21 | Guo 49 | 2020 | China | Retrospective | 174 | 58.33 ± 13.46 | 61.67 ± 10.8 | 57 ± 14.24 | 43.68 | 54.05 | 40.88 | |||||
| 22 | Koh 50 | 2021 | Singapore | Retrospective | 1042 | 39 ± 11 | 48 ± 13 | 36.82 ± 10.19 | 95.39 | 92.14 | 95.9 | WHO criteria | ||||
| 23 | Li 51 | 2020 | China | Retrospective | 199 | 62.67 ± 18.67 | 68.67 ± 10.8 | 57 ± 14.24 | 44.72 | 65.79 | 31.71 | |||||
| 24 | Seiglie 52 | 2020 | USA | Retrospective | 450 | 17.29 ± 63.62 | 66.7 ± 14.2 | 61.6 ± 18.8 | 57.56 | 61.8 | 54.78 | |||||
| Study ID | Smoking (current or past), n (%) | Obesity, n (%) | Hypertension, n (%) | CVD, n (%) | Pulmonary disease, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | DM | nDM | Overall | DM | nDM | Overall | DM | nDM | Overall | DM | nDM | Overall | DM | nDM | ||
| 1 | 40 (6.7) | 9 (6) | 31 (6.9) | 176 (29.6) | 48 (32.4) | 128 (28.6) | 172 (28.9) | 72 (48.6) | 100 (22.3) | 112 (18.8) | 40 (27) | 72 (16.1) | 87 (14.6) | 24 (16.2) | 63 (14.09) | |
| 2 | 105 (35.84) | 28 (35.4) | 77 (36.0) | 124 (37.7) | 37 (32.2) | 87 (27.4) | 255 (59.2) | 91 (79.1) | 164 (51.7) | 148 (34.3) | 49 (42.6) | 99 (31.2) | 39 (9.0) | 6 (5.2) | 33 (10.4) | |
| 3 | 9 (2.6) | 178 (42.2) | 187 (42.6) | 44 (10.0) | ||||||||||||
| 4 | 272 (28.9) | 66 (53.7) | 206 (25.2) | 35 (3.7) | 6 (4.9) | 29 (3.5) | ||||||||||
| 5 | 16 (14.5) | 7 (24.1) | 9 (11.) | 37 (33.6) | 16 (55.2) | 21 (25.9) | 10 (9.1) | 5 (17.2) | 5 (6.2) | 4 (3.6) | 2 (6.9) | 2 (2.5) | ||||
| 6 | 272 (76.6) | 151 (90.9) | 121 (64.0) | 45 (12.7) | 22 (13.3) | 23 (12.2) | ||||||||||
| 7 | 374 (34.6) | 147 (62.6) | 227 (26.8) | 72 (6.7) | 16 (6.8) | 56 (6.6) | ||||||||||
| 8 | 90 (38.6) | 43 (53.8) | 47 (30.7) | 20 (8.6) | 8 (10.0) | 12 (7.8) | ||||||||||
| 9 | 164 (40.9) | 111 (58.7) | 53 (25.0) | 24 (6.0) | 10 (5.3) | 14 (6.6) | ||||||||||
| 10 | 98 (38.0) | 29 (46.0) | 69 (35.4) | 39 (15.1) | 15 (23.8) | 24 (12.3) | 9 (3.5) | 2 (3.2) | 7 (3.6) | |||||||
| 11 | 31 (18.7) | 12 (19.7) | 19 (18.1) | 91 (54.8) | 37 (60.7) | 54 (51.4) | 76 (45.8) | 35 (56.4) | 41 (39.0) | 30 (18.1) | 16 (26.2) | 14 (13.3) | 19 (11.4) | 9 (14.7) | 10 (9.5) | |
| 12 | 85 (32.7) | 63 (50.8) | 22 (16.2) | 110 (41.0) | 75 (59.5) | 35 (25.0) | 27 (10.0) | 14 (11.0) | 13 (9.3) | |||||||
| 13 | 14 (5.5) | 8 (6.3) | 6 (4.7) | 109 (42.9) | 68 (53.5) | 41 (32.3) | 7 (2.8) | 4 (3.1) | 3 (2.4) | |||||||
| 14 | 352 (41.9) | 247 (58.4) | 105 (24.9) | |||||||||||||
| 15 | 55 (17.0) | 11 (20.0) | 44 (16.4) | 80 (24.7) | 32 (58.2) | 48 (17.8) | 19 (5.9) | 8 (14.6) | 11 (4.1) | |||||||
| 16 | ||||||||||||||||
| 17 | 46 (15.2) | 31 (52.5) | 15 (6.1) | |||||||||||||
| 18 | 27 (40.9) | 12 (44.4) | 15 (55.6) | |||||||||||||
| 19 | ||||||||||||||||
| 20 | 53 (27.7) | 15 (28.3) | 38 (27.5) | 212 (51.3) | 73 (68.2) | 139 (45.4) | 72 (18.0) | 27 (26.0) | 45 (15.3) | |||||||
| 21 | 43 (24.7) | 10 (27.0) | 33 (24.1) | 32 (18.4) | 12 (32.4) | 20 (14.6) | 14 (9.7) | 2 (5.4) | 12 (8.7) | |||||||
| 22 | ||||||||||||||||
| 23 | ||||||||||||||||
| 24 | 191 (42.4) | 91 (51.4) | 100 (36.8) | 178 (39.6) | 134 (75.3) | 44 (24.7) | 110 (24.4) | 46 (25.8) | 66 (24.3) | |||||||
| Study ID | Severity, n (%) | ARDS, n (%) | Mortality, n (%) | Mechanical ventilation, n (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM severe | nDM severe | DM | nDM | DM | nDM | DM | nDM | |||||||||
| 1 | 36 (24.32) | 55 (12.3) | 26 (17.57) | 39 (8.72) | 16 (10.81) | 12 (2.68) | ||||||||||
| 2 | 31 (26.96) | 49 (15.51) | 20 (17.39) | 68 (21.45) | ||||||||||||
| 3 | 60 (20.48) | 17 (9.29) | 60 (20.27) | 17 (12.32) | ||||||||||||
| 4 | 47 (38.21) | 138 (16.87) | 21 (17.07) | 76 (9.29) | 11 (8.94) | 29 (3.55) | ||||||||||
| 5 | 10 (43.48) | 68 (91.89) | 11 (37.93) | 7 (8.64) | 5 (17.24) | 1 (1.23) | 8 (27.59) | 3 (3.7) | ||||||||
| 6 | 45 (27.11) | 35 (18.52) | 48 (28.92) | 41 (21.69) | ||||||||||||
| 7 | 170 (18.78) | 65 (40.88) | 44 (18.72) | 41 (4.84) | 37 (15.74) | 38 (4.49) | ||||||||||
| 8 | 26 (32.5) | 89 (58.17) | 18 (22.5) | 9 (5.88) | ||||||||||||
| 9 | 151 (79.89) | 193 (91.04) | 12 (6.35) | 3 (1.42) | ||||||||||||
| 10 | 18 (28.57) | 69 (35.38) | 24 (38.10) | 38 (19.49) | 7 (11.11) | 8 (4.1) | 7 (11.11) | 9 (4.62) | ||||||||
| 11 | 9 (14.75) | 21 (20) | 13 (21.31) | 11 (10.48) | 9 (14.75) | 2 (1.9) | ||||||||||
| 12 | 22 (17.32) | 8 (5.67) | 23 (18.11) | 4 (2.84) | ||||||||||||
| 13 | 21 (16.54) | 19 (14.96) | 29 (22.83) | 19 (14.96) | 19 (14.96) | 8 (6.3) | ||||||||||
| 14 | 43 (10.19) | 25 (5.91) | 6 (1.42) | 5 (1.18) | ||||||||||||
| 15 | 11 (20) | 13 (4.83) | ||||||||||||||
| 16 | 139 (100) | 773 (97.23) | 4 (2.88) | 9 (1.13) | ||||||||||||
| 17 | 14 (23.73) | 10 (4.1) | ||||||||||||||
| 18 | 7 (31.82) | 4 (9.09) | ||||||||||||||
| 19 | 27 (23.68) | 32 (12.8) | ||||||||||||||
| 20 | 40 (37.38) | 62 (20.26) | 15 (14.02) | 33 (10.78) | ||||||||||||
| 21 | 4 (10.81) | 5 (3.65) | ||||||||||||||
| 22 | 31 (22.14) | 20 (2.22) | 4 (2.86) | 1 (0.11) | ||||||||||||
| 23 | 11 (14.47) | 7 (3.14) | ||||||||||||||
| 24 | 28 (15.73) | 22 (8.09) | 66 (37.08) | 63 (23.16) | ||||||||||||
Abbreviations: ARDS, acute respiratory distress syndrome; CRRT, continuous renal replacement therapy; CVD, cardiovascular disease; DM, diabetes mellitus; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; nDM, no diabetes mellitus; NR, not reported; WHO, World Health Organization.
TABLE 2.
Summary effects and heterogeneity obtained from the meta‐analysis of the association of diabetes with clinical outcomes in hospitalized COVID‐19 patients
| Outcome | Summary effects | |||||||
|---|---|---|---|---|---|---|---|---|
| REDL | Heterogeneity a | Heterogeneity variance estimates | ||||||
| Effect measure | OR (95% CI) | Tests of overall effect | Cochran's Q | H | I2 b | P value | tau≤ c | |
| COVID‐19 severity | OR | 3.39 (2.14‐5.37) | P < .0001, z = 5.206 | 40.21 | 2.11 (95% CI, 1.48‐2.71) | 77.6% (95% CI, 54.2%‐86.4%) | P < .0001 | 0.3706 |
| ARDS | OR | 2.55 (1.74‐3.75) | P < .0001, z = 4.772 | 11.05 | 1.49 (95% CI, 1.00‐2.42) | 54.7% (95% CI, 0%‐79.9%) | P = .05 | 0.1200 |
| Mortality | OR | 2.44 (1.93‐3.09) | P < .0001, z = 7.425 | 48.47 | 1.48 (95% CI, 1.111‐1.845) | 54.6% (95% CI, 19%‐70.6%) | P = .001 | 0.1586 |
| Need for mechanical ventilation | OR | 3.03 (2.17‐4.22) | P < .0001, z = 6.524 | 29.93 | 1.58 (95% CI, 1.06‐2.07) | 59.9% (95% CI, 11.3%‐76.7%) | P = .003 | 0.1974 |
Abbreviations: ARDS, acute respiratory distress syndrome; H, relative excess in Cochran's Q over its degrees of freedom; I2, proportion of total variation in effect estimate due to between‐study heterogeneity (based on Cochran Q); OR, odds ratio; Q, heterogeneity measures were calculated from the data with CI based on noncentral chi‐square (common effect) distribution for Cochran Q test; REDL, DerSimonian‐Laird random‐effects method.
Heterogeneity measures were calculated from the data with 95% CI based on gamma (random effects) distribution for Q.
Values of l2 are percentages.
Heterogeneity variance estimates (tau≤) were derived from REDL.
3.2. Association of diabetes with severe COVID‐19
Overall, 10 studies were included in the meta‐analysis of the association of diabetes with severe COVID‐19, comprising 4705 patients. Severity of disease definitions varied between studies and included criteria such as admission to the intensive care unit, oxygen stats, and need for intubation. Diabetes was associated with significantly increased odds of severe COVID‐19 (OR 3.39; 95% CI, 2.14‐5.37; P < .0001, z = 5.206) (Figure 4 and Table 2). Substantial heterogeneity was found between the studies (I2 = 77.6% [95% CI, 2.7%‐90.4%], P < .0001). There was some evidence of publication bias from visualization of the funnel plot (Supplemental Figure S3).
FIGURE 4.
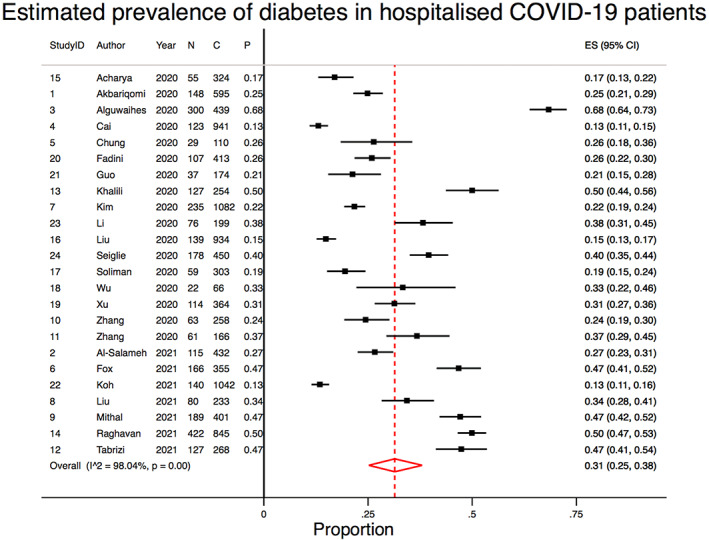
Forest plots showing the pooled prevalence of diabetes in COVID‐19 hospitalised patients. N, number of patients with diabetes; C, number of patients with COVID‐19; P, proportion of COVID‐19 patients with diabetes
3.3. Association of diabetes with ARDS
Six studies were included in the meta‐analysis of the association of diabetes with in‐hospital ARDS, comprising 2298 patients. ARDS was defined by the Berlin definition in four studies and undefined in two. Diabetes was associated with significantly increased odds of ARDS (OR 2.55; 95% CI, 1.74‐3.75; P < .0001, z = 4.772) (Figure 4). Moderate‐to‐substantial heterogeneity was found between the studies (I2 = 54.7% [95% CI, 0%‐82.9%], P = .05). There was some evidence of publication bias observed by visual inspection of the funnel plot (Supplemental Figure S3).
3.4. Association of diabetes with in‐hospital mortality
Overall, 23 studies were included in the meta‐analysis of the association of diabetes with mortality, comprising 10 582 patients. Mortality was defined as the death of a patient within the follow‐up period of the study in all included studies. Diabetes was significantly associated with increased odds of in‐hospital mortality (OR 2.44; 95% CI, 1.93‐3.09; P < .0001, z = 7.425) (Figure 5). Moderate‐to‐substantial heterogeneity was found between the studies (I2 = 54.6% [95% CI, 3.8%‐73.7%], P = .001). No evidence of publication bias was observed by visual inspection of the funnel plot (Supplemental Figure S3).
FIGURE 5.
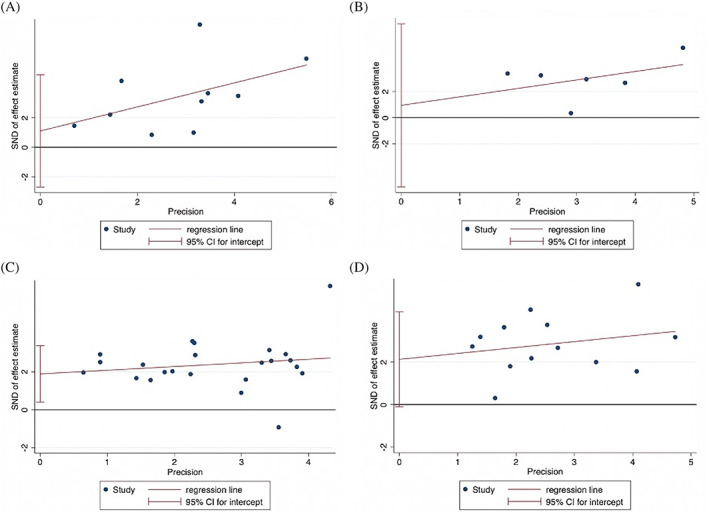
Egger's tests of effect sizes for studies on the association of diabetes with A, severe COVID‐19, B, acute respiratory distress syndrome, C, mortality, and D, mechanical ventilation
3.5. Association of diabetes with the need for mechanical ventilation
Overall, 13 studies were included in the meta‐analysis of the association of diabetes with the requirement of mechanical ventilation, comprising 6066 patients. Mechanical ventilation was defined as invasive mechanical ventilation in seven studies, need for intubation in two studies, and mechanical ventilation in three studies. Diabetes was associated with significantly increased odds of need of mechanical ventilation (OR 3.03; 95% CI, 2.17‐4.22; P < .0001, z = 6.524) (Figure 5). Moderate‐to‐substantial heterogeneity was found between the studies (I2 = 59.9% [95% CI, 0%‐80.5%], P = .003). There was some evidence of publication bias observed by visual inspection of the funnel plot (Supplemental Figure S3).
4. DISCUSSION
Our study clearly demonstrates that diabetes in COVID‐19 hospitalizedpatients is associated with poor clinical outcomes. Diabetes was significantly associated with increased odds of severe COVID‐19, need for mechanical ventilation, ARDS, and in‐hospital mortality. This is consistent with previous studies investigating the association of diabetes with certain clinical outcomes; however, several key clinical endpoints were not reported. 6 , 7 , 8 , 9 , 10 , 11 Therefore, our study provides evidence on how diabetes mediates outcomes in hospitalized COVID‐19 adult patients. The crude prevalence of diabetes in hospitalized patients varied from 15% to 40% in various studies. 18 , 19 , 20 Our meta‐analysis estimated an overall pooled prevalence of 31% of diabetes in hospitalized COVID‐19 patients.
Various mechanisms have been implicated in the poor clinical trajectory due to COVID‐19 in diabetes patients. Diabetes‐induced hyperglycemia leads to several issues, including chronic inflammation and impairment of the immune system through oxidative stress and diminished functioning of antibodies, macrophages, and chemokines. 21 , 22 It is known that COVID‐19 further exacerbates inflammatory responses, potentially resulting in cytokine storms. 22 It also contributes to diabetes‐related issues such as endothelial dysfunction and coagulation‐related issues. 23 It has also been postulated that diabetes‐related alveolar microangiopathy in the lungs could be contributing to worsened clinical outcomes. 24 These complications may lead to the increased severity, acute respiratory distress, and mortality seen in diabetes patients. 25
Several factors may contribute to the clinical outcomes of COVID‐19 in patients with diabetes. 26 , 27 There is a close relationship between diabetes and cardiovascular complications such as obesity and hypertension. 28 , 29 , 30 , 31 After controlling for these conditions and other comorbidities, some studies included in this meta‐analysis found that there was no significant association between diabetes and mortality from COVID‐19. 2 , 32 , 33 , 34 Hypertension itself is an important risk factor within COVID‐19 patients leading to worsened outcome. 35 Other important risk factors include disease duration, age, and fasting blood glucose (FBG). 28 , 36 , 37 Cai et al 33 found a significant relationship between higher FBG levels and mortality but not between diabetes and mortality. Similarly, Zhang et al 38 found a significant association between higher FBG and poor clinical outcomes. This highlights the importance of glucose testing of COVID‐19 patients, and not only relying on previous history of diabetes. Given the known severity of COVID‐19 in elderly patients, age should also be closely considered. In a retrospective study, Kim et al 39 found that there was a significant association between diabetes and mortality in older COVID‐19 patients, but not in younger.
There are several limitations for this meta‐analysis. Out of the included studies, only Mithal et al 35 was prospective in design. This means that the criteria for inclusion in the diabetes group relied largely on previous clinical history and would have excluded some new diabetes cases. Consequently, there may be some patients with undiagnosed diabetes included in the control group. The data on body mass index, diabetes duration, and glycosylated hemoglobin levels are not available for all studies to allow a meta‐analysis to be performed. Detailed information on the diabetes profile, such as its duration, have not been reported. The effect of these factors, including impact of poor glycemic control, on COVID‐19 severity and outcomes in COVID‐19 patients with diabetes are subject of future investigation. Another major limitation is the variation in outcome definitions. In particular, the outcomes of severity and need for mechanical ventilation had varied definitions (Table 1). Findings should be interpreted on methodological design and the study population. However, given that we performed a random‐effects model, some of these variabilities and heterogeneities will be covered. Future studies on long‐term impact of COVID‐19 on patients with diabetes would provide insights on the optimal management strategies.
In conclusion, diabetes is an important clinical consideration in the current COVID‐19 pandemic. Our meta‐analysis clearly demonstrates that diabetes is associated with poor outcomes such as severe disease, need for mechanical ventilation, ARDS, and mortality. This has implications for the importance of tailored strategies for management of COVID‐19 patients with diabetes.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Sonu M. M. Bhaskar conceived the study; contributed to the planning, methodology, draft, and revision of the manuscript; and supervised the students. Sonu M. M. Bhaskar encouraged Sian A. Bradley to investigate and supervised the findings of this work. Sian A. Bradley and Sonu M. M. Bhaskar wrote the first draft of this paper, and Sian A. Bradley was involved in the investigation, data collection, data curation, and analysis. All authors contributed to the writing and revision of the manuscript. All authors approved the final draft of the manuscript.
AVAILABILITY OF DATA AND MATERIALS
The original contributions presented in the study are included in the article and online Supplementary Information. Further inquiries can be directed to the corresponding author.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
Funding for the NSW Brain Clot Bank (Chief Investigator: Dr Bhaskar) from the NSW Ministry of Health (2019‐2022) is acknowledged. The funding body has no role in the study design, data collection, analysis, interpretation of findings, and manuscript preparation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the affiliated/funding organization/s.
Bradley SA, Banach M, Alvarado N, Smokovski I, Bhaskar SMM. Prevalence and impact of diabetes in hospitalized COVID‐19 patients: A systematic review and meta‐analysis. Journal of Diabetes. 2022;14(2):144-157. doi: 10.1111/1753-0407.13243
Funding information NSW Ministry of Health, Grant/Award Number: NSW Brain Clot Bank [2019‐2022]
REFERENCES
- 1. World Health Organisation . WHO Coronavirus (COVID‐19) Dashboard. Updated 14 July. Accessed October 29, 2021. https://covid19.who.int/
- 2. Al‐Salameh A, Lanoix JP, Bennis Y, et al. Characteristics and outcomes of COVID‐19 in hospitalized patients with and without diabetes. Diabetes Metab Res Rev. 2021;37(3):e3388. doi: 10.1002/dmrr.3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh AK, Gillies CL, Singh R, et al. Prevalence of co‐morbidities and their association with mortality in patients with COVID‐19: a systematic review and meta‐analysis. Diabetes Obes Metab. 2020;22(10):1915‐1924. doi: 10.1111/dom.14124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhaskar S, Jovanovic S, Katyal A, Namboodiri NK, Chatzis D, Banach M. Is COVID‐19 another case of obesity paradox? ‐ results from an international ecological study on behalf of the REPROGRAM consortium obesity study group. Arch Med Sci. 2021. doi: 10.5114/aoms/136447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hussain S, Baxi H, Chand Jamali M, Nisar N, Hussain MS. Burden of diabetes mellitus and its impact on COVID‐19 patients: a meta‐analysis of real‐world evidence. Diabetes Metab Syndr. 2020;14(6):1595‐1602. doi: 10.1016/j.dsx.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo L, Shi Z, Zhang Y, et al. Comorbid diabetes and the risk of disease severity or death among 8807 COVID‐19 patients in China: a meta‐analysis. Diabetes Res Clin Pract. 2020;166:108346. doi: 10.1016/j.diabres.2020.108346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression: diabetes and COVID‐19. Diabetes Metab Syndr. 2020;14(4):395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaminska H, Szarpak L, Kosior D, et al. Impact of diabetes mellitus on in‐hospital mortality in adult patients with COVID‐19: a systematic review and meta‐analysis. Acta Diabetol. 2021;58(8):1101‐1110. doi: 10.1007/s00592-021-01701-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID‐19? A meta‐analysis. Diabetes Metab Syndr. 2020;14(4):535‐545. doi: 10.1016/j.dsx.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aggarwal G, Lippi G, Lavie CJ, Henry BM, Sanchis‐Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. J Diabetes. 2020;12(11):851‐855. doi: 10.1111/1753-0407.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corona G, Pizzocaro A, Vena W, et al. Diabetes is most important cause for mortality in COVID‐19 hospitalized patients: systematic review and meta‐analysis. Rev Endocr Metab Disord. 2021;22(2):275‐296. doi: 10.1007/s11154-021-09630-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526‐2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 13. Katyal A, Calic Z, Killingsworth M, Bhaskar SMM. Diagnostic and prognostic utility of computed tomography perfusion imaging in posterior circulation acute ischemic stroke: a systematic review and meta‐analysis. Eur J Neurol. 2021;28(8):2657‐2668. doi: 10.1111/ene.14934 [DOI] [PubMed] [Google Scholar]
- 14. Faggion CM Jr, Atieh M, Zanicotti DG. Reporting of sources of funding in systematic reviews in periodontology and implant dentistry. Br Dent J. 2014;216(3):109‐112. doi: 10.1038/sj.bdj.2014.47 [DOI] [PubMed] [Google Scholar]
- 15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta‐analyses. In: Higgins JP, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 62. Cochrane; Chichester, UK: John Wiley & Sons; 2021. [Google Scholar]
- 17. Higgs M, Sim J, Traynor V. Incidence and risk factors for new‐onset atrial fibrillation following coronary artery bypass grafting: a systematic review and meta‐analysis. Intensive Crit Care Nurs. 2020;60:102897. doi: 10.1016/j.iccn.2020.102897 [DOI] [PubMed] [Google Scholar]
- 18. Bach LA, Ekinci EI, Engler D, et al. The high burden of inpatient diabetes mellitus: the Melbourne public hospitals diabetes inpatient audit. Med J Aust. 2014;201(6):334‐338. doi: 10.5694/mja13.00104 [DOI] [PubMed] [Google Scholar]
- 19. Lan NSR, Li C, Fegan PG. Diabetes prevalence is high in hospital patients: a Western Australia perspective. Intern Med J. 2019;49(4):551‐552. doi: 10.1111/imj.14247 [DOI] [PubMed] [Google Scholar]
- 20. Comino EJ, Harris MF, Islam MD, et al. Impact of diabetes on hospital admission and length of stay among a general population aged 45 year or more: a record linkage study. BMC Health Serv Res. 2015;15(1):12. doi: 10.1186/s12913-014-0666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barber TM. COVID‐19 and diabetes mellitus: implications for prognosis and clinical management. Review. Expert Rev Endocrinol Metab. 2020;15(4):227‐236. doi: 10.1080/17446651.2020.1774360 [DOI] [PubMed] [Google Scholar]
- 22. Buicu AL, Cernea S, Benedek I, Buicu CF, Benedek T. Systemic inflammation and COVID‐19 mortality in patients with major noncommunicable diseases: chronic coronary syndromes, diabetes and obesity. Review. J Clin Med. 2021;10(8):1545. doi: 10.3390/jcm10081545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drucker DJ. Diabetes, obesity, metabolism, and SARS‐CoV‐2 infection: the end of the beginning review. Cell Metab. 2021;33(3):479‐498. doi: 10.1016/j.cmet.2021.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazucanti CH, Egan JM. SARS‐CoV‐2 disease severity and diabetes: why the connection and what is to be done? Review. Immun Ageing. 2020;17(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holly JMP, Biernacka K, Maskell N, Perks CM. Obesity, diabetes and COVID‐19: an infectious disease spreading from the east collides with the consequences of an unhealthy Western lifestyle. Review. Front Endocrinol (Lausanne). 2020;11:582870. doi: 10.3389/fendo.2020.582870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhaskar S, Bradley S, Chattu VK, et al. Telemedicine as the new outpatient clinic gone digital: position paper from the pandemic health system REsilience PROGRAM (REPROGRAM) international consortium (part 2). Front Public Health. 2020;8:410. doi: 10.3389/fpubh.2020.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhaskar S, Rastogi A, Chattu VK, et al. Key strategies for clinical management and improvement of healthcare Services for Cardiovascular Disease and Diabetes Patients in the coronavirus (COVID‐19) settings: recommendations from the REPROGRAM consortium. Front Cardiovasc Med. 2020;7:112. doi: 10.3389/fcvm.2020.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akbariqomi M, Hosseini MS, Rashidiani J, et al. Clinical characteristics and outcome of hospitalized COVID‐19 patients with diabetes: a single‐center, retrospective study in Iran. Diabetes Res Clin Pract. 2020;169:108467. doi: 10.1016/j.diabres.2020.108467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Q, Xu L, Dai Y, et al. Cardiovascular manifestations in severe and critical patients with COVID‐19. Clinl Cardiol. 2020;43(7):796‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID‐19 in association with glucose‐lowering medication. Diabetes Care. 2020;43(7):1399‐1407. doi: 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Lu R, Wang J, et al. Diabetes, even newly defined by HbA1c testing, is associated with an increased risk of in‐hospital death in adults with COVID‐19. BMC Endocr Disord. 2021;21(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alguwaihes AM, Al‐Sofiani ME, Megdad M, et al. Diabetes and Covid‐19 among hospitalized patients in Saudi Arabia: a single‐Centre retrospective study. Cardiovasc Diabetol. 2020;19(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cai Y, Shi S, Yang F, et al. Fasting blood glucose level is a predictor of mortality in patients with COVID‐19 independent of diabetes history. Diabetes Res Clin Pract. 2020;169:108437. doi: 10.1016/j.diabres.2020.108437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fox T, Ruddiman K, Lo KB, et al. The relationship between diabetes and clinical outcomes in COVID‐19: a single‐center retrospective analysis. Acta Diabetol. 2021;58(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mithal A, Jevalikar G, Sharma R, et al. High prevalence of diabetes and other comorbidities in hospitalized patients with COVID‐19 in Delhi, India, and their association with outcomes. Diabetes Metab Syndr. 2021;15(1):169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung SM, Lee YY, Ha E, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. 2020;44(3):405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Acharya D, Lee K, Lee DS, Lee YS, Moon SS. Mortality rate and predictors of mortality in hospitalized COVID‐19 patients with diabetes. Healthcare (Basel). 2020;8(3):338. doi: 10.3390/healthcare8030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Li H, Zhang J, et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: a single‐Centre, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020;22(8):1443‐1454. doi: 10.1111/dom.14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim MK, Jeon JH, Kim SW, et al. The clinical characteristics and outcomes of patients with moderate‐to‐severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J. 2020;44(4):602‐613. doi: 10.4093/dmj.2020.0146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Cui Y, Shen M, et al. Association of diabetes mellitus with disease severity and prognosis in COVID‐19: a retrospective cohort study. Diabetes Res Clin Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moghaddam Tabrizi F, Rasmi Y, Hosseinzadeh E, et al. Diabetes is associated with higher mortality and severity in hospitalized patients with COVID‐19. EXCLI J. 2021;20:444‐453. doi: 10.17179/excli2021-3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khalili S, Moradi O, Kharazmi AB, Raoufi M, Sistanizad M, Shariat M. Comparison of mortality rate and severity of pulmonary involvement in coronavirus Disease‐2019 adult patients with and without type 2 diabetes: a cohort study. Can J Diabetes. 2021;45(6):524‐530. doi: 10.1016/j.jcjd.2020.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raghavan A, Nanditha A, Satheesh K, et al. Profile and prognosis of patients hospitalized for COVID‐19 virus infection with and without diabetes ‐ an observational study from South India. Diabetes Metab Syndr. 2021;15(4):102143. doi: 10.1016/j.dsx.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Z, Li J, Huang J, et al. Association between diabetes and COVID‐19: a retrospective observational study with a large sample of 1,880 cases in Leishenshan hospital, Wuhan. Front Endocrinol. 2020;11:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soliman AT, Prabhakaran Nair A, Al Masalamani MS, et al. Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus versus nondiabetic symptomatic patients with COVID‐19: a comparative study. Acta Biomed. 2020;91(3):e2020010. doi: 10.23750/abm.v91i3.10214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu J, Zhang J, Sun X, et al. Influence of diabetes mellitus on the severity and fatality of SARS‐CoV‐2 (COVID‐19) infection. Diabetes Obes Metab. 2020;22(10):1907‐1914. doi: 10.1111/dom.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu Z, Wang Z, Wang S, et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID‐19. J Diabetes. 2020;12(12):909‐918. doi: 10.1111/1753-0407.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fadini GP, Morieri ML, Boscari F, et al. Newly‐diagnosed diabetes and admission hyperglycemia predict COVID‐19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. doi: 10.1016/j.diabres.2020.108374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;36(7):e3319. doi: 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koh H, Moh AMC, Yeoh E, et al. Diabetes predicts severity of COVID‐19 infection in a retrospective cohort: a mediatory role of the inflammatory biomarker C‐reactive protein. J Med Virol. 2021;93(5):3023‐3032. doi: 10.1002/jmv.26837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li G, Deng Q, Feng J, Li F, Xiong N, He Q. Clinical characteristics of diabetic patients with COVID‐19. J Diabetes Res. 2020;2020:1652403. doi: 10.1155/2020/1652403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seiglie J, Platt J, Cromer SJ, et al. Diabetes as a risk factor for poor early outcomes in patients hospitalized with covid‐19. Diabetes Care. 2020;43(12):2938‐2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The original contributions presented in the study are included in the article and online Supplementary Information. Further inquiries can be directed to the corresponding author.


