Abstract
Background
Androgenetic alopecia (AGA) is the most common form of non‐scarring alopecia in humans. Several studies have used different laboratory models to study the pathogenesis and interventions for AGA. These study models have proved beneficial and have led to the approval of two drugs. However, the need to build on existing knowledge remains by examining the relevance of study models to the disease.
Objective
We sought to appraise laboratory or pre‐clinical models of AGA.
Method
We searched through databases (PubMed, ScienceDirect, Web of Science, World CAT, Scopus and Google Scholar) for articles on AGA‐related studies from 1942 to March 2019 with a focus on study models.
Results
The search rendered 101 studies after screening and deduplication. Several studies (70) used in vitro models, mostly consisting of two‐dimensional monolayer cells for experiments involving the characterization of androgen and 5‐alpha reductase (5AR) and inhibition thereof, the effects of dihydrotestosterone (DHT) and biomarker(s) of AGA. Twenty‐seven studies used in vivo models of mice and monkeys to investigate DHT synthesis, the expression and inhibition of 5AR and hair growth. Only four studies used AGA‐related or healthy excisional/punch biopsy explants as ex vivo models to study the action of 5AR inhibitors and AGA‐associated genes. No study used three‐dimensional [3‐D] organoids or organotypic human skin culture models.
Conclusion
We recommend clinically relevant laboratory models like human or patient‐derived 3‐D organoids or organotypic skin in AGA‐related studies. These models are closer to human scalp tissue and minimize the use of laboratory animals and could ultimately facilitate novel therapeutics.

The current laboratory models of Androgenetic alopecia (AGA) are not representative of the disorder. Thus, this study evaluated all the laboratory models of AGA used in the last seven decades.This article recommends the use of patient‐derived 3‐D skin/ scalp cells or tissue culture models for future research studies on AGA instead of non‐representative 2‐D human and animal models.
1.
What's already known about this topic
Laboratory models are used to study the aetiology, pathogenesis and the efficacy of therapeutic interventions for AGA. These models, though beneficial, have only resulted in two FDA‐approved drugs for the treatment of AGA, which may be partly due to the lack of disease‐related models.
What's new or what does this study add?
This systematic review summarizes published evidence of laboratory models used in AGA‐related research and revealed the scarcity of studies that use human or patient‐derived 3‐D tissue culture models. These models are more suitable and more physiologically representative than 2‐D cell culture and animal models for pre‐clinical research. Thus, this article could guide the selection of appropriate study models for future research, and thus facilitate research translation.
2. BACKGROUND
Androgenetic alopecia (AGA) is the most common form of non‐scarring hair loss in humans. 1 , 2 , 3 The prevalence of AGA varies between races and ethnicities. 1 This disparity is attributed to the different methods of measuring prevalence, making it difficult to compare studies. 1 , 2 Nonetheless, about 50% of men of European descent are affected by the age of 50 years; this proportion increases to 90% with age. 1 , 4 , 5 Furthermore, AGA is estimated to affect about 19% of women of European ancestry, while the prevalence and severity of AGA are considered low in Asian and African men. 1 , 4 , 6
In the early 1940s, Hamilton proved that genetic predisposition and male hormone stimulation are prerequisites in AGA development. 7 After this discovery, several AGA models were created to delineate the pathophysiology and evaluate the effectiveness of novel therapeutics using both laboratory (in vitro, in vivo and ex vivo) and non‐laboratory models. These models, though beneficial, however, have limitations since most are not fully representative of AGA. Also, available information on the molecular pathology and mechanism of AGA action is modest and thus hampers the progression of potential new treatments to human clinical trials. This problem may explain why only two drugs (Minoxidil, a vasodilator, and Finasteride, an alpha two reductase inhibitor) are currently approved and available for AGA treatment. Therefore, it is imperative to build upon existing models of AGA to facilitate the discovery of novel therapeutics.
This systematic review aims to appraise all AGA‐related study models published to date for the testing of AGA interventions. However, not intended to evaluate the efficacy of AGA treatments.
3. METHODS
The protocol for this study was registered and published in the International Prospective Register of Systematic Reviews (reg. number CRD42018107182; http://www.crd.york.ac.uk/PROSPERO).
3.1. Search strategy
The Medical Search Headings terms used for the search were Androgenetic alopecia, Androgenic Alopecia, Hair Loss, and Male pattern baldness. Other search terms included In vitro, In vivo, Ex vivo, Finasteride, Minoxidil, Proliferation, Hair loss culture models and Xenografts, Tissue culture, Plant extracts, Hair shedding, Hair scalp biopsies, Induced alopecia, Cell differentiation, Cell lines, Primary cells, 2‐D Models, 3‐D Models, and Significant hair growth. The ‘NOT’ Boolean operator was used to exclude reviews, ‘transplant studies’, ‘human studies’, and ‘clinical studies’ (Table S1).
3.2. Information sources
There was a search for articles on publication databases (PubMed, ScienceDirect, Web of Science, World CAT, Scopus and Google Scholar) from January 1942 until March 2019. A search strategy was developed for PubMed and adapted as per the requirements of other databases. A hand search in Google Scholar and Science Direct supplemented the search. The review also looked at unpublished articles from conferences and libraries (dissertations).
3.3. Inclusion criteria
The inclusion criteria for the articles selected are as follows: preclinical laboratory studies and models (in vitro, in vivo and ex vivo) published in the English language. Our search excluded all articles involving human, clinical, diagnostic, drugs/phytochemicals efficacy and hair or cell transplant studies. Search results were filtered to include the research studies relevant to this review as per the pre‐decided inclusion criteria.
3.4. Data extraction and synthesis
We used a predesigned template adapted from Cochrane to populate the data extracted from the selected studies (Table S2). We analysed these data based on certain variables: the type of model (i.e., in vitro, ex vivo and in vivo), cell‐types and techniques. We grouped research articles with similar variables.
3.5. Quality assessment and risk of bias
The selected articles' quality was assessed by two authors (SN and AA) using a predesigned template validated by three independent assessors (Table S3). The quality assessors scored 10 similar questions, compared scoring results and any disagreements resolved through consensus. The articles were assessed against three questions, as follows:
Is the model used in the study appropriate or relevant (i.e., the use of patients' or control direct tissues or biopsies, patient‐derived primary or immortalized two dimensional [2‐D] or three dimensional [3‐D] cells/cell lines)? Studies that used patient‐related or human tissue instead of animal tissue as models had high scores.
Do the techniques and methods of analysis used in the study adequately address the research question?
Are the results from the research study validated by more than one research technique and are the results reproducible (i.e., the number of independent experiments)? Articles with results confirmed by three or more techniques, and a minimum of three independent experiments, scored higher than studies that used two or one methods.
The maximum score per question was five points.
4. RESULTS
4.1. Study selection
Our initial search rendered 1228 related articles after removing duplicates. A further review of the full‐text articles based on set eligibility criteria excluded 1128 and an additional two with the reason (spheroids but unrelated to AGA). Three more research papers retrieved from an updated hand search supplement the remaining 98 articles, thus bringing the entire selected research articles to 101. These articles included studies involving 70 in vitro, 4 ex vivo and 27 in vivo models (Figure 1).
FIGURE 1.

A PRISMA diagram illustrating the search, screening and assessment strategy of articles reviewed, and qualitative synthesis of the selected articles
4.2. Data extraction and synthesis of included studies
A summary of the study models from the included studies (i.e., in vitro, ex vivo and in vivo) is illustrated (Figure 2) and grouped under the following headings (Table 1): Study objective(s), Models, Techniques and Outcomes. From the 101 articles, 15 demonstrated the requirement of androgens for AGA development using in vitro and ex vivo models with different cell‐based experimental techniques. These techniques include cell proliferation assays involving androgen‐induced overgrowth and androgen receptor antagonism. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 Twenty‐four studies investigated gene expression profiling 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 and markers for AGA. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Fourteen studies focused on the action of 5‐alpha reductase (5AR), which converts the androgen (testosterone) into dihydrotestosterone (DHT) and the inhibition of this enzyme 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 using enzymatic, radiochemical and cell proliferation assays. Thirteen studies used the same techniques to investigate the role of other enzymes in the mediation of AGA. 4 , 6 , 10 , 43 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 To find alternative treatments for AGA, four studies investigated the effect of plant extracts and phytocompounds on the inhibition of 5AR type 2 using the dermal papilla cells (DPCs) proliferation assay. 43 , 45 , 48 , 53 Only three studies investigated efficient drug delivery methods, such as nanotechnology. 66 , 67 , 68 In contrast, 27 articles used mice/mice xenografts, rats and monkeys as in vivo models to study the efficacy of Finasteride, Minoxidil and plant extracts/compounds in the treatment of AGA. Different assays were used, including 5AR enzymatic action on androgens, DHT effect on hair and hair appendages growth experiments. 10 , 21 , 46 , 50 , 51 , 52 , 64 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92
FIGURE 2.
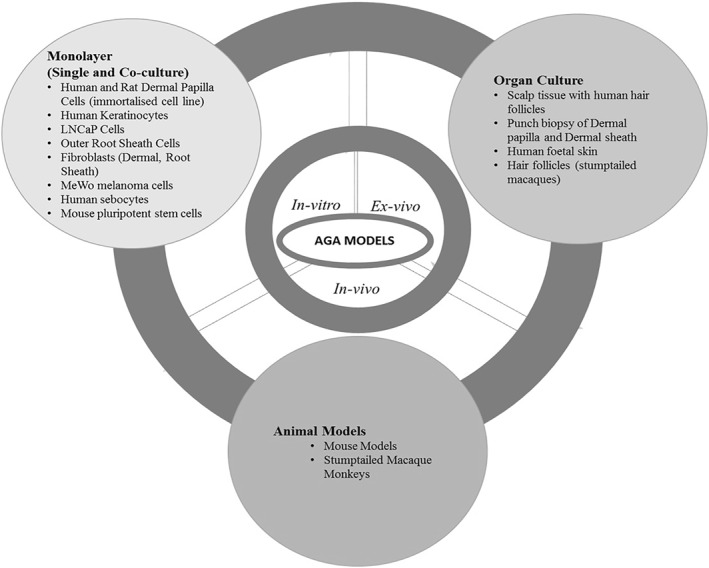
An overview of the in vitro, ex vivo and in vivo model of androgenetic alopecia (AGA) included in the review
TABLE 1.
A summary of the extracted data from the selected and reviewed research articles
| Study objective(s) | Models | Techniques | Outcomes | References | |
|---|---|---|---|---|---|
| No. studies | |||||
| In vitro | Androgens are required for AGA mediation |
|
|
The models proved that androgens are required for the mediation of AGA | |
|
The role of phytocompounds |
|
|
The phytochemical compounds tested inhibited the action of 5AR‐ type 2 and regulated multiple genes associated with AGA (IGF‐1, DKK‐1, TGF‐β1, IL‐1α, VEGF) | ||
| In vitro and Ex vivo | The action of 5 α‐reductase and inhibitors |
|
|
5AR concentrated in DPC changes testosterone into DHT in the cytoplasm | |
| Enzymes responsible for the intracellular activation and inactivation of androgens showed mRNA expression patterns that correlated with the enzyme activities with and without use of selective enzyme inhibitors respectively. | |||||
|
|
|
|
||
| Efficient delivery methods of drugs |
|
|
The resultant PLGA nanoparticles were an efficient encapsulation system for FNS. No toxic effects observed from the S. cerevisiae model | ||
| In vivo | The use of finasteride, minoxidil, plant extracts and plant compounds for treatment of AGA |
|
|
|
Abbreviations: AGA, androgenetic alopecia; DKK‐1, Dickkopf WNT signalling pathway inhibitor 1; DPC, dermal papilla cells; FNS, finasteride; GEG, gene expression graphs; HaCaTs cells, human keratinocytes; HFSC, hair follicle stem cells; IGF‐1, insulin‐like growth factor‐1; IL‐1α, interleukin‐1; LNCaP cells, prostate adenocarcinoma cells; LSESr, lipidosterolic extract of Serenoa repens; MeWo, melanoma cells: human melanoma cell line; MTS, (3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium); PC3 cells, human prostate cancer cell line; PHFCs, primary hair follicle fibroblast cells; SLN, solid lipid nanoparticles; SZ95 sebocytes, immortalized human sebaceous gland cell lines; TGF‐β1, transforming growth factor beta1; VEGF, vascular endothelial growth factor.
4.3. Quality assessment of included studies
The overall results show a relatively high percentage (62%) of articles with a middle‐risk score (Figure 3). The evaluation of these articles revealed that 75% used appropriate models, while 25% of the studies having a high‐risk score on the reliability of the models. The models used are monolayer (single or co‐culture) of immortalized cell lines, human skin tissue, or mice/mice xenografts, or stump‐tailed macaques. The articles had clear objectives or research questions, with methods and procedures that precisely addressed these objectives. About 38% of the selected studies had not validated experimental results with more than one technique.
FIGURE 3.

A quality assessment chart indicating the risk bias contained in the studies of the selected articles. Q1–Q3 = Assessment questions. Q1 = Is the model used in the study appropriate or relevant (i.e., the use of patients' or control direct tissues or biopsies, patient‐derived primary or immortalized 2‐D or 3‐D cells/cell lines)? Q2 = Do the techniques and methods of analysis used in the study adequately address the research question? Q3 = Are the results from the research study validated by more than one research technique, and are the results reproducible (i.e., the number of independent experiments)?
5. DISCUSSION
This systematic review, which sought to appraise models used in preclinical AGA research, rendered three groups of models: in vitro (n = 70), ex vivo (n = 4) and in vivo (n = 27).
5.1. In vitro 2‐D models
The use of in vitro models is fundamental to all biomedical studies. The advancements made in studying cells, bacteria and viruses are often the first strides into understanding in vivo conditions. The in vitro models from included studies were 2‐D monolayer (single or co‐culture cells) of immortalized or primary epithelial and mesenchymal cell lines. Immortalized cell lines are often used in research in the place of primary cells because they are cost‐effective, easy to use and provide an unlimited supply of material. These cells can also grow indefinitely in culture and bypass ethical concerns associated with animal and human tissue use. However, immortalized cell lines undergo significant mutations to become immortal, which can alter cellular physiology. Also, genetic changes can occur over multiple passages, leading to phenotypic differences among isolates and unreproducible experimental data.
Examples of immortalized cells used in the reviewed articles were human prostate cancer (PC3) cells, human keratinocytes (HaCaTs), prostate adenocarcinoma (LNCaP) cells, human sebaceous gland (SZ95 sebocytes) cells, human melanoma cells, human embryonic kidney 293 cells and the Shionogi carcinoma‐115 cells. 4 , 6 , 10 , 43 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65
Although the use of these epithelial cells provided useful data to study potential AGA therapeutics, they are not related to this disorder. Therefore, some studies used DPCs. 43 , 45 , 48 , 53 Scalp DPCs are specialized mesenchymal cells of tissues affected by AGA that exist in the dermal papilla located at the basal layer of hair follicles. These cells play a pivotal role in the hair cycle. These cells were used as 2‐D in vitro models in androgen‐based and 5AR kinetics and inhibition assays to study the paradox of DHT, gene expression and AGA markers in two culture systems. The first system involved introducing growth factors (from keratinocytes‐conditioned growth medium with or without medium containing fibroblast growth factor) to cultured DPCs. The second system included the co‐culture of DPCs with effector cells (e.g., HaCaTs). Although these 2‐D models are instrumental in enhancing the understanding of AGA's molecular pathology and mechanism, there have been limitations associated with these 2‐D models. For instance, 2‐D models do not accurately recapitulate the tissue architecture and cellular mechanisms present in a whole skin, impeding advancement towards effective AGA therapeutics.
5.2. In vivo models
Twenty‐seven studies used either mice or rats and monkeys as in vivo models, the most common being C57BL/6, Kunming mice and male Sprague Dawley rats, male Wister/ST rats, C57BL/6NCrSlc strain mice, C3H/He strain mice, B6CBACF1/J female mice, male Swiss albino and human scalp hair grafted into nude mice (i.e., human scalp hair xenografts). 21 , 46 , 50 , 51 , 52 , 64 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 91 , 92 These rodents are used in general as models in medical research due to genetic, biological and behavioural similarities to humans. More so, they are useful in replicating many human disorders.
The stump‐tailed macaque monkey was the only primate‐related in vivo model used in the reviewed studies. 10 , 88 , 90 Stump‐tailed macaque was the preferred choice because they possess hereditary balding characteristics similar, in many respects, to those of AGA in humans. However, macaques are found only in Asia, and the cost, risk and near extinction compromise the use of this model. Hence, the number of studies using these monkeys has diminished over the years. Animal models are considered suitable for studying human diseases as their biological milieu resembles human homoeostatic conditions. However, the use of animals in research is subject to strict ethical guidelines and often laden by the high human translational failures, perhaps due to inadequate mimicry of human pathophysiology. Thus, 3‐D ex vivo models are fast becoming more prominent in understanding AGA.
5.3. Ex vivo models
Only four of the included studies were ex vivo, using either diseased (AGA) or normal excisional and punch biopsy explants (whole skin organ cultures). 6 , 23 , 60 , 67 These models were used to study the action of 5AR inhibitors and the role of genes in AGA. Skin explants can maintain the cutaneous structure of the skin and, hence, allowing for studies, for example, that evaluate the effects of 5AR inhibitors on tissue morphology and gene expression. Although ex vivo organ culture is an easy‐to‐use and relatively cheap model, its use may be hampered by human ethical consideration and the availability of skin organ donors leading to insufficient skin specimens.
5.4. Risk of bias
A validity assessment evaluated the risk of bias in the models and experimental techniques used in the included studies. The overall results show a relatively high percentage of articles with a middle‐risk score. This observation may result from the low scores in the reliability of methods used and validation of results (we recommend a minimum of three techniques for validation). Result validation is a critical component in method evaluation because it reveals the specificity, accuracy, precision and, ultimately, the data's reliability. Validation is also essential to demonstrate that the model and technique used is suitable for the intended use. Many of the included studies (76%) were not exhaustive, with only 24% using three or more techniques to validate their results. Therefore, there is a need for continuity in work already done and the development of models with more relevant technologies.
Assessing the risk of bias of studies contained in the body of evidence is foundational and central to all systematic reviews to improve the transparency, consistency and scientific rigour of the research. Bias refers to factors that can confound the overall observations and conclusions of a study, which may lead to inappropriate recommendations. These discrepancies can result in wasted resources and loss of opportunities to discover effective therapies.
6. LIMITATIONS
None of the included studies used 3‐D models of AGA. The use of 3‐D AGA models (e.g., organ culture from either punch biopsies, skin tissues from patients [healthy or diseased] or cells isolated from tissue samples) has an advantage over 2‐D models. These models allow experimentation with human diseased tissues under controlled conditions than would be challenging to achieve in 2‐D models, which may prove unachievable with in vivo models, thus allowing for a more detailed cellular and molecular characterization. Interestingly, 2‐D models can be used to create 3‐D models. The 3‐D models can either be organoid cultures or be organotypic skin cultures. Organoids are in vitro‐derived 3‐D cell aggregates derived from primary tissue, which possess similar composition and architecture to primary tissue, and exhibit organ functionality. On the other hand, organotypic skin cultures use primary human cells, and cell culture inserts to recapitulate the stratified epidermal architecture of the skin to replicate the normal anatomy and physiology. This approach, especially with whole skin punch biopsies, allows for the in vitro maintenance of skin appendages, for example, hair follicles. Therefore, organotypic skin cultures may be instrumental in preclinical AGA research to validate the mechanisms of diseases and test the novel therapeutics.
7. CONCLUSION
There is substantial evidence on the use of various models in the study of many diseases, including AGA and the discovery of effective targeted therapies. AGA models to date have helped in the understanding of this disorder. Howbeit, most of these models are not ideal representatives of AGA in humans. Only four studies used whole human skin biopsies from patients (diseased and healthy), a closer approximation of AGA in humans. However, none of the studies used 3‐D organoids or organotypic skin culture models. Therefore, there is a need to improve these models with a focus on the use of diseased and healthy biopsies or cell lines isolated from these biopsies. These 3‐D models allow experimentation closer to humans, which may prove difficult or impossible with in vivo models while minimizing harm to animals. It is also vital to validate experiments with a minimum of three techniques. Finally, 3‐D experimental data should ultimately be tested in human clinical trials to achieve formidable progress towards fast and effective treatment solutions for AGA. A good translation of AGA preclinical research into human trials will ensure the discovery of new and more effective drugs.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Hair and Skin Research Laboratory, University of Cape Town, South Africa and Dr Ogheneochuko Oputu. Afolake Arowolo thanks the South African National Research Foundation (NRF) Thuthuka Funding Instrument (NRF Rating Track) (grant number: TTK170413227114).
AUTHOR CONTRIBUTION
All authors contributed equally to the drafting of this article. SN and AA made the searched and wrote the article. NK, ME and AA corrected drafts and organised the content.
REFERENCES
- 1. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001;145:95–9. [DOI] [PubMed] [Google Scholar]
- 2. Kabir Y, Goh C. Androgenetic alopecia: update on epidemiology, pathophysiology, and treatment. J Egypt Women's Dermatologic Soc. 2013;10:107–16. [Google Scholar]
- 3. Hamilton, JB . Male hormone stimulation is prerequisite and an incitant in common baldness. Am J Anat. 1942;71:451–80. [Google Scholar]
- 4. Bruchovsky N, Wilson JD. The conversion of testosterone to 5α‐androstan‐17β‐ol‐3‐one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–21. [PubMed] [Google Scholar]
- 5. Sharp F, Hay JB, Hodgins MB. Metabolism of androgens in vitro by human foetal skin. J Endocrinol. 1976;70:491–9. [DOI] [PubMed] [Google Scholar]
- 6. Wilson JD, Walker JD. The conversion of testosterone to 5α‐androstan‐17β‐ol‐3‐one (dihydrotestosterone) by skin slices of man. J Clin Invest. 1969;48:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hay JB. A study of the in vitro metabolism of androgens by human scalp and pubic skin. Br J Dermatol. 1977;97:237–46. [DOI] [PubMed] [Google Scholar]
- 8. Yang YC, Fu HC, Wu CY, Wei KT, Huang KE. Androgen Receptor Accelerates Premature senescence of human dermal papilla cells in association with DNA damage. PLoS ONE. 2013;8(11):e79434. 10.1371/journal.pone.0079434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim AR, Kim SN, Jung IK, Kim HH, Park YH. et al. The inhibitory effect of scutellaria baicalensis extract and its active compound, baicalin, on the translocation of the androgen receptor with implications for preventing androgenetic alopecia. Planta Med. 2014;80(02/03):153–8. 10.1055/s-0033-1360300. [DOI] [PubMed] [Google Scholar]
- 10. Takashima I, Adachi K, Montagna W. Studies of common baldness in the stumptailed macaque. IV. In vitro metabolism of testosterone in the hair follicles. J Invest Dermatol. 1970;55:329–34. [DOI] [PubMed] [Google Scholar]
- 11. Bingham KD, Shaw DA. The metabolism of testosterone by human male scalp skin. J Endocrinol. 1973;57:111–21. [DOI] [PubMed] [Google Scholar]
- 12. Thigpen AE, Davis DL, Milatovich A, Mendonca BB, Imperato‐McGinley J, Griffin JE, et al. Molecular genetics of steroid 5 alpha‐reductase 2 deficiency. J Clin Invest. 1992;90(3):799–809. 10.1172/jci115954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hay JB, Hodgins MB. Distribution of androgen metabolizing enzymes in isolated tissues of human forehead and axillary skin. J Endocrinol. 1978;79:29–39. [DOI] [PubMed] [Google Scholar]
- 14. Horton R. Markers of peripheral androgen action in vivo and in vitro. Clin Dermatol. 1988;6:46–51. [DOI] [PubMed] [Google Scholar]
- 15. Thornton M, Laing I, Hamada K, Messenger A, Randall V. Differences in testosterone metabolism by beard and scalp hair follicle dermal papilla cells. Clin Endocrinol (Oxf). 1993;39:633–9. [DOI] [PubMed] [Google Scholar]
- 16. Randall VA, Thornton MJ, Hamada K, Messenger AG. Androgen action in cultured dermal papilla cells from human hair follicles. Skin Pharmacol Physiol. 1994;7:20–6. [DOI] [PubMed] [Google Scholar]
- 17. Zouboulis CC, Akamatsu H, Stephanek K, Orfanos CE. Androgens affect the activity of human sebocytes in culture in a manner dependent on the localization of the sebaceous glands and their effect is antagonized by spironolactone. Skin Pharmacol Physiol. 1994;7:33–40. [DOI] [PubMed] [Google Scholar]
- 18. Weinberg WC, Brown PD, Stetler‐Stevenson WG, Yuspa SH. Growth factors specifically alter hair follicle cell proliferation and collagenolytic activity alone or in combination. Differentiation. 1990;45:168–78. [DOI] [PubMed] [Google Scholar]
- 19. Midorikawa T, Chikazawa T, Yoshino T, Takada K, Arase S. Different gene expression profile observed in dermal papilla cells related to androgenic alopecia by DNA macroarray analysis. J Dermatol Sci. 2004;36:25–32. [DOI] [PubMed] [Google Scholar]
- 20. El‐Domyati M, Attia S, Saleh F, Bassyouni MI, El‐Fakahany H, et al. Proliferation, DNA repair and apoptosis in androgenetic alopecia. J Eur Acad Dermatol Venereol: JEADV. 2009;23(1):7–12. 10.1111/j.1468-3083.2008.02937.x. [DOI] [PubMed] [Google Scholar]
- 21. Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone‐inducible IL‐6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132:43–9. [DOI] [PubMed] [Google Scholar]
- 22. Tochio T, Tanaka H, Nakata S, Hosoya H. Fructose‐1, 6‐bisphosphate aldolase A levels decrease in hair keratinocytes during androgenetic alopecia. Int J Dermatol. 2013;52:1439–41. 10.1111/j.1365-4632.2011.05242.x. [DOI] [PubMed] [Google Scholar]
- 23. Chew EG, Tan Joanna HJ, Bahta AW, Ho BSY, Liu X, Lim TC, et al. Differential expression between human dermal papilla Cells from balding and non‐balding scalps reveals new candidate genes for androgenetic alopecia. J Invest Dermatol. 2016;136: 8:1559–67. 10.1016/j.jid.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 24. Izgi A, Türkoğlu M, Akyıldırım B, Çelen S, Çelik N. Investigation of the gene expression levels of 5 α‐reductase, VEGF and IL‐1α in HaCaT cells after the application of a botanical extract. Am J Dermatol Venereol. 2014;3:23–9. [Google Scholar]
- 25. Bao L, Yu A, Luo Y, Tian T, Dong Y, Zong H, et al. Genomewide differential expression profiling of long non‐coding RNAs in androgenetic alopecia in a Chinese male population. J Eur Acad Dermatol Venereol. 2017;31(8):1360–71. 10.1111/jdv.14278. [DOI] [PubMed] [Google Scholar]
- 26. Dey‐Rao R, Sinha AA. A genomic approach to susceptibility and pathogenesis leads to identifying potential novel therapeutic targets in androgenetic alopecia. Genomics. 2017;109:165–76. 10.1016/j.ygeno.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 27. Dey‐Rao R, Sinha A. Genome‐wide gene expression dataset used to identify potential therapeutic targets in androgenetic alopecia. Data Brief. 2017;13:85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hochfeld LM, Keller A, Anhalt T, Fricker N, Nöthen MM, Heilmann HS. et al. Insights into Male Androgenetic Alopecia: differential gene expression profiling of plucked hair follicles and integration with genetic data. J Invest Dermatol. 2019;139(1):235–238. 10.1016/j.jid.2018.06.182. [DOI] [PubMed] [Google Scholar]
- 29. Randall VA, Hibberts NA, Thornton MJ, Merrick AE, Hamada K, Kato S, et al. Do androgens influence hair growth by altering the paracrine factors secreted by dermal papilla cells? Eur J Dermatol. 2001;11:315–320. https://pubmed.ncbi.nlm.nih.gov/11399537/. [PubMed] [Google Scholar]
- 30. Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen‐inducible TGF‐beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–9. 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- 31. Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Identification of androgen‐inducible TGF‐beta1 derived from dermal papilla cells as a key mediator in androgenetic alopecia. J Invest Dermatol Symp Proc. 2003;8:69–71. 10.1046/j.1523-1747.2003.12174.x. [DOI] [PubMed] [Google Scholar]
- 32. Itami S, Inui S. Role of androgen in mesenchymal epithelial interactions in human hair follicle. J. Investig. Dermatol. Symp. Proc. 2005;10(3):209–211. 10.1111/j.1087-0024.2005.10107.x. [DOI] [PubMed] [Google Scholar]
- 33. Tang L, Bernardo O, Bolduc C, Lui H, Madani S, Shapiro J. The expression of insulin‐like growth factor 1 in follicular dermal papillae correlates with therapeutic efficacy of finasteride in androgenetic alopecia. J Am Acad Dermatol. 2003;49(2):229–233. 10.1067/s0190-9622(03)00777-1. [DOI] [PubMed] [Google Scholar]
- 34. Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme‐derived hair follicle dermal papilla may be a key to male pattern baldness. Br J Dermatol. 2006;154:609–18. 10.1111/j.1365-2133.2006.07144.x. [DOI] [PubMed] [Google Scholar]
- 35. Yoo HG, Kim JS, Lee SR, Pyo HK, Moon HI, Lee JH, et al. Perifollicular fibrosis: pathogenetic role in androgenetic alopecia. Biol Pharm Bull. 2006;29(6):1246–1250. 10.1248/bpb.29.1246. [DOI] [PubMed] [Google Scholar]
- 36. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross‐talk between Wnt/β‐catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166(5):1035–1042. 10.1111/j.1365-2133.2012.10856.x. [DOI] [PubMed] [Google Scholar]
- 37. Leirós GJ, Ceruti JM, Castellanos ML, Kusinsky AG, Balañá ME. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol Cell Endocrinol. 2017;439:26–34. 10.1016/j.mce.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 38. Moon PG, Kwack MH, Lee JE, Cho YE, Park JH, Hwang D, Baek MC. et al. Proteomic analysis of balding and non‐balding mesenchyme‐derived dermal papilla cells from androgenetic alopecia patients using on‐line two‐dimensional reversed phase‐reversed phase LC–MS/MS. J Proteomics. 2013;85:174–191. 10.1016/j.jprot.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 39. Kretzschmar K, Cottle DL, Schweiger PJ, Watt FM. The androgen receptor antagonizes Wnt/β‐catenin signaling in epidermal stem cells. J Invest Dermatol. 2015;135:2753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Upton JH, Hannen RF, Bahta AW, Farjo N, Farjo B, Philpott MP. Oxidative stress–associated senescence in dermal papilla cells of men with androgenetic alopecia. J Invest Dermatol. 2015;135(5):1244–1252. 10.1038/jid.2015.28. [DOI] [PubMed] [Google Scholar]
- 41. de Rivero Vaccari JP, Sawaya ME, Brand F, Nusbaum BP, Bauman AJ, Bramlett HM, et al. Caspase‐1 level is higher in the scalp in androgenetic alopecia. Dermatol Surg. 2012;38(7pt1):1033–1039. 10.1111/j.1524-4725.2012.02378.x. [DOI] [PubMed] [Google Scholar]
- 42. Pan HJ, Wilding G, Uno H, Inui S, Goldsmith L, Messing E, et al. Evaluation of RU58841 as an anti‐androgen in prostate PC3 cells and a topical anti‐alopecia agent in the bald scalp of stumptailed macaques. Endocrine. 1998;9(1):39–44. 10.1385/endo:9:1:39. [DOI] [PubMed] [Google Scholar]
- 43. Pérez-Ornelas V, Cabeza M, Bratoeff E, Heuze I, Sánchez M, Ramírez E, et al. New 5α‐reductase inhibitors: In vitro and in vivo effects. Steroids. 2005;70(3):217–224. 10.1016/j.steroids.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 44. Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Preventable effect of L‐threonate, an ascorbate metabolite, on androgen‐driven balding via repression of dihydrotestosterone‐induced dickkopf‐1 expression in human hair dermal papilla cells. BMB Reports. 2010;43:688–92. 10.5483/BMBRep.2010.43.10.688. [DOI] [PubMed] [Google Scholar]
- 45. Chen L, Wang J, Mouser G, Li YC, Marcovici G. Blockade of androgen markers using a novel betasitosterol, thioctic acid and carnitine‐containing compound in prostate and hair follicle cell‐based assays. Phytother Res. 2016;30:1016–20. 10.1002/ptr.5611. [DOI] [PubMed] [Google Scholar]
- 46. Sasaki M, Shinozaki S, Shimokado K. Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone. Biochemical and biophysical research communications. 2016;472:250–4. 10.1016/j.bbrc.2016.02.099. [DOI] [PubMed] [Google Scholar]
- 47. Srivilai J, Rabgay K, Khorana N, Waranuch N, Nuengchamnong N, Wisuitiprot W, et al. Anti‐androgenic curcumin analogues as steroid 5‐alpha reductase inhibitors. Medicinal Chemistry Research. 2017;26(7):1550–6. 10.1007/s00044-017-1869-y. [DOI] [Google Scholar]
- 48. Tan JJ, Pan J, Sun L, Zhang J, Wu C, Kang L. Bioactives in Chinese proprietary medicine modulates 5α‐reductase activity and gene expression associated with androgenetic alopecia. Front Pharmacol. 2017;8:194. 10.3389/fphar.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pekmezci E, Türkoğlu M. Minoxidil acts as an antiandrogen: a study of 5α‐reductase zype 2 gene expression in a human keratinocyte cell line. Acta Dermatovenerol Croat. 2018;25:271. [PubMed] [Google Scholar]
- 50. Uno H, Cappas A, Brigham P. Action of topical minoxidil in the bald stump‐tailed macaque. J Am Acad Dermatol. 1987;16:657–68. [DOI] [PubMed] [Google Scholar]
- 51. Sintov A, Serafimovich S, Gilhar A. New topical antiandrogenic formulations can stimulate hair growth in human bald scalp grafted onto mice. Int J Pharm. 2000;194:125–34. [DOI] [PubMed] [Google Scholar]
- 52. Chen CH, Sheu MT, Wu AB, Lin KP, Ho HO. Simultaneous effects of tocopheryl polyethylene glycol succinate (TPGS) on local hair growth promotion and systemic absorption of topically applied minoxidil in a mouse model. Int J Pharm. 2005;306:91–8. 10.1016/j.ijpharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 53. Jang S, Lee Y, Hwang SL, Lee MH, Park SJ, Lee IH, et al. Establishment of type II 5α‐reductase over‐expressing cell line as an inhibitor screening model. J Steroid Biochem Mol Biol. 2007;107(3‐5):245–52. 10.1016/j.jsbmb.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 54. Park SY, Kwack MH, Chung EJ, Im SU, Han IS, Kim MK, et al. Establishment of SV40T‐transformed human dermal papilla cells and identification of dihydrotestosterone‐regulated genes by cDNA microarray. J Dermatol Sci. 2007;47(3):201–208. 10.1016/j.jdermsci.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 55. Wang JL, Liu HL, Zhou ZL, Chen WH, Ho Y. Discovery of novel 5α‐reductase type II inhibitors by pharmacophore modelling, virtual screening, molecular docking and molecular dynamics simulations. Mol Simul. 2015;41(4):287–97. 10.1080/08927022.2013.878865. [DOI] [Google Scholar]
- 56. Diani AR, Mulholland MJ, Shull KL, Kubicek MF, Johnson GA, Schostarez HJ, et al. Hair growth effects of oral administration of finasteride, a steroid 5 alpha‐reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J Clin Endocrinol Metab. 1992;74(2):345–50. 10.1210/jcem.74.2.1309834. [DOI] [PubMed] [Google Scholar]
- 57. Harris G, Azzolina B, Baginsky W, Cimis G, Rasmusson GH, Tolman RL, et al. Identification and selective inhibition of an isozyme of steroid 5 alpha‐reductase in human scalp. Proc. Natl. Acad. Sci. 1992;89(22):10787–10791. 10.1073/pnas.89.22.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen W, Zouboulis CC, Fritsch M, Kodelja V, Orfanos C. Heterogeneity and quantitative differences of type 1 5α‐reductase expression in cultured skin epithelial cells. Dermatology (Basel, Switzerland). 1998;196:51–2. [DOI] [PubMed] [Google Scholar]
- 59. Eicheler W, Happle R, Hoffmann R. 5α‐Reductase activity in the human hair follicle concentrates in the dermal papilla. Arch Dermatol Res. 1998;290:126–32. [DOI] [PubMed] [Google Scholar]
- 60. Hibberts N, Howell A, Randall V. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non‐balding scalp. J Endocrinol. 1998;156:59–65. [DOI] [PubMed] [Google Scholar]
- 61. Hoffmann R, Happle R. Finasteride is the main inhibitor of 5α‐reductase activity in microdissected dermal papillae of human hair follicles. Arch Dermatol Res. 1999;291:100–3. [DOI] [PubMed] [Google Scholar]
- 62. Kang JI, Kim SC, Kim MK, Boo HJ, Kim EJ, Im GJ, et al. Effects of dihydrotestosterone on rat dermal papilla cells in vitro. Eur J Pharmacol. 2015;757:74–83. 10.1016/j.ejphar.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 63. Asada Y, Sonoda T, Ojiro M, Kurata S, Sato T, Ezaki T, et al. 5α‐reductase type 2 is constitutively expressed in the dermal papilla and connective tissue sheath of the hair follicle in vivo but not during culture in vitro. J Clin Endocrinol Metab. 2001;86(6):2875–80. 10.1210/jcem.86.6.7545. [DOI] [PubMed] [Google Scholar]
- 64. Zhao J, Harada N, Okajima K. Dihydrotestosterone inhibits hair growth in mice by inhibiting insulin‐like growth factor‐I production in dermal papillae. Growth Hormone IGF Res. 2011;21:260–7. 10.1016/j.ghir.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 65. Sonoda T, Asada Y, Kurata S, Takayasu S. The mRNA for protease nexin‐1 is expressed in human dermal papilla cells and its level is affected by androgen. J Invest Dermatol. 1999;113:308–13. 10.1046/j.1523-1747.1999.00707.x. [DOI] [PubMed] [Google Scholar]
- 66. Chaudhri, N , Soni, GC , Prajapati, SK . Nanotechnology: an advance tool for nano‐cosmetics preparation. Int J Pharma Res Rev. 2015;4:28–40. [Google Scholar]
- 67. Hamishehkar H, Ghanbarzadeh S, Sepehran S, Javadzadeh Y, Adib ZM, Kouhsoltani M. Histological assessment of follicular delivery of flutamide by solid lipid nanoparticles: potential tool for the treatment of androgenic alopecia. Drug Dev Ind Pharm. 2016;42(6):846–53. 10.3109/03639045.2015.1062896. [DOI] [PubMed] [Google Scholar]
- 68. Roque LV, Dias IS, Cruz N, Rebelo A, Roberto A, Rijo P, et al. Design of finasteride‐loaded nanoparticles for potential treatment of alopecia. Skin Pharmacol Physiol. 2017;30(4):197–204. 10.1159/000475473. [DOI] [PubMed] [Google Scholar]
- 69. Zhu HL, Gao YH, Yang JQ, Li JB, Gao J. Serenoa repens extracts promote hair regeneration and repair of hair loss mouse models by activating TGF‐beta and mitochondrial signaling pathway. Eur Rev Med Pharmacol Sci. 2018;22:4000–8. [DOI] [PubMed] [Google Scholar]
- 70. Tricarico D, Maqoud F, Curci A, Camerino G, Zizzo N, Denora N, et al. Characterization of minoxidil/hydroxypropyl‐β‐cyclodextrin inclusion complex in aqueous alginate gel useful for alopecia management: efficacy evaluation in male rat. Eur J Pharm Biopharm. 2018;122:146–57. 10.1016/j.ejpb.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 71. Li JJ, Li Z, Gu LJ, Choi KJ, Kim DS, Kim HK, et al. The promotion of hair regrowth by topical application of a Perilla frutescens extract through increased cell viability and antagonism of testosterone and dihydrotestosterone. J Nat Med. 2018;72(1):96–105. 10.1007/s11418-017-1116-3. [DOI] [PubMed] [Google Scholar]
- 72. Feng Y, Ma LY, Li X, Ding WF, Chen XM. Hair growth promoting effect of white wax and policosanol from white wax on the mouse model of testosterone‐induced hair loss. Biomed Pharmacother. 2017;89:438–46. 10.1016/j.biopha.2017.02.036. [DOI] [PubMed] [Google Scholar]
- 73. Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non‐scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res. 2017;309(9):729–38. 10.1007/s00403-017-1777-5. [DOI] [PubMed] [Google Scholar]
- 74. Park S, Erdogan S, Hwang D, Hwang S, Han EH, Lim YH. Bee Venom Promotes Hair Growth in Association with Inhibiting 5α‐Reductase Expression. Biol Pharm Bull. 2016;39(6):1060–8. 10.1248/bpb.b16-00158. [DOI] [PubMed] [Google Scholar]
- 75. Zhang B, Zhang RW, Yin XQ, Lao ZZ, Zhang Z, Wu QG, et al. Inhibitory activities of some traditional Chinese herbs against testosterone 5α‐reductase and effects of Cacumen platycladi on hair re‐growth in testosterone‐treated mice. J Ethnopharmacol. 2016;177:1–9. 10.1016/j.jep.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 76. Shin HS, Park SY, Song HG, Hwang E, Lee DG, Yi TH. The androgenic alopecia protective effects of forsythiaside‐A and the molecular regulation in a mouse model. Phytother Res. 2015;29(6):870–6. 10.1002/ptr.5324. [DOI] [PubMed] [Google Scholar]
- 77. Huang B, Kang BG, Wang Z, Lim SS. Effect of ethanol extract of plant mixture on hair regeneration in human dermal papilla cells and C57BL/6J mice. J Med Plants Res. 2015;9(45):1103–10. 10.5897/jmpr2014.5355. [DOI] [Google Scholar]
- 78. Kang JI, Kim EJ, Kim MK, Jeon YJ, Kang SM, Koh YS, et al. The promoting effect of Ishige sinicola on hair growth. Mar Drugs. 2013;11(6):1783–99. 10.3390/md11061783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Murata K, Noguchi K, Kondo M, Onishi M, Watanabe N, Okamura K, et al. Inhibitory activities of Puerariae Flos against testosterone 5α‐reductase and its hair growth promotion activities. J Nat Med. 2012;66(1):158–65. 10.1007/s11418-011-0570-6. [DOI] [PubMed] [Google Scholar]
- 80. Murata K, Takeshita F, Samukawa K, Tani T, Matsuda H. Effects of ginseng rhizome and ginsenoside Ro on testosterone 5α‐reductase and hair re‐growth in testosterone‐treated mice. Phytother Res. 2012;26(1):48–53. 10.1002/ptr.3511. [DOI] [PubMed] [Google Scholar]
- 81. Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. J Sci Transl Med. 2012;4(126):126ra34. 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim YY, up No S, Kim MH, Kim HS, Kang H, Kim HO, et al. Effects of topical application of EGCG on testosterone‐induced hair loss in a mouse model. Exp Dermatol. 2011;20(12):1015–7. 10.1111/j.1600-0625.2011.01353.x. [DOI] [PubMed] [Google Scholar]
- 83. Dhanotia R, Chauhan NS, Saraf DK, Dixit VK. Effect of Citrullus colocynthis Schrad fruits on testosterone‐induced alopecia. Nat Prod Res. 2011;25:1432–43. 10.1080/14786410802632820. [DOI] [PubMed] [Google Scholar]
- 84. Pandit S, Chauhan NS, Dixit VK. Effect of Cuscuta reflexa roxb on androgen‐induced alopecia. J Cosmet Dermatol. 2008;7:199–204. 10.1111/j.1473-2165.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 85. Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC, et al. Human hair growth enhancement in vitro by green tea epigallocatechin‐3‐gallate (EGCG). Phytomedicine. 2007;14(7‐8):551–5. 10.1016/j.phymed.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 86. Park W‐s, Lee C‐h, Lee B‐g, Chang I‐s. The extract of Thujae occidentalis semen inhibited 5α‐reductase and androchronogenetic alopecia of B6CBAF1/j hybrid mouse. J Dermatol Sci. 2003;31:91–8. 10.1016/S0923-1811(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 87. Roh SS, Kim CD, Lee MH, Hwang SL, Rang MJ, Yoon YK. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J Dermatol Sci. 2002;30(1):43–9. 10.1016/s0923-1811(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 88. Brigham PA, Cappas A, Uno H. The stumptailed macaque as a model for androgenetic alopecia: effects of topical minoxidil analyzed by use of the folliculogram. Clin Dermatol. 1988;6:177–87. 10.1016/0738-081X(88)90084-3. [DOI] [PubMed] [Google Scholar]
- 89. Cho CH, Bae JS, Kim YU. 5alpha‐reductase inhibitory components as antiandrogens from herbal medicine. J Acupunct Meridian Stud. 2010;3:116–8. 10.1016/s2005-2901(10)60021-0. [DOI] [PubMed] [Google Scholar]
- 90. Obana N, Chang C, Uno H. Inhibition of hair growth by testosterone in the presence of dermal papilla cells from the frontal bald scalp of the postpubertal stumptailed macaque. Endocrinology. 1997;138:356–61. 10.1210/endo.138.1.4890. [DOI] [PubMed] [Google Scholar]
- 91. Ganesan P, Choi D‐K. Current application of phytocompound‐based nanocosmeceuticals for beauty and skin therapy. Int J Nanomed. 2016;11:1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gilhar A, Pillar T, Etzioni A. The effect of topical cyclosporin on the immediate shedding of human scalp hair grafted onto nude mice. Br J Dermatol. 1988;119:767–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


