Abstract
Cross-presentation was first observed serendipitously in the 1970s. The importance of it was quickly realized and subsequently attracted great attention from immunologists. Since then, our knowledge of the ability of certain antigen presenting cells to internalize, process, and load exogenous antigens onto MHC-I molecules to cross-prime CD8+ T cells has increased significantly. Dendritic cells (DCs) are exceptional cross-presenters, thus making them a great tool to study cross-presentation but the relative rarity of DCs in circulation and in tissues makes it challenging to isolate sufficient numbers of cells to study this process in vitro. In this paper, we describe in detail two methods to culture DCs from bone-marrow progenitors and a method to expand the numbers of DCs present in vivo as a source of endogenous bona-fide cross-presenting DCs. We also describe methods to assess cross-presentation by DCs using the activation of primary CD8+ T cells as a readout.
Basic Protocol 1:
Isolation of bone marrow progenitor cells
Basic Protocol 2:
In vitro differentiation of dendritic cells with GM-CSF
Support Protocol 1:
Preparation of conditioned medium from GM-CSF producing J558L cells
Basic Protocol 3:
In vitro differentiation of dendritic cells with Flt3L
Support Protocol 2:
Preparation of Flt3L containing medium from B16-Flt3L cells
Basic Protocol 4:
Expansion of cDC1s in vivo for use in ex vivo experiments
Basic Protocol 5:
Characterizing resting and activated dendritic cells
Basic Protocol 6:
Dendritic cell stimulation, antigenic cargo, and fixation
Support Protocol 3:
Preparation of model antigen coated microbeads
Support Protocol 4:
Preparation of apoptotic cells
Support Protocol 5:
Preparation of recombinant bacteria
Basic Protocol 7:
Immunocytochemistry immunofluorescence (ICC/IF)
Support Protocol 6:
Preparation of Alcian blue-coated coverslips
Basic Protocol 8:
CD8+ T cell activation to assess cross-presentation
Support Protocol 7:
Isolation and labeling of CD8+ T cells with CFSE
Keywords: cross-presentation, cross-priming, DC, CD8+ T cell, dendritic cells, MHC-I
INTRODUCTION
Antigen presentation is an essential step in the process of mounting an adaptive immune response against harmful non-self or altered-self entities, such as during viral infection or cancer. Antigen presenting cells (APCs) process and load antigenic peptides onto major histocompatibility complex (MHC) class I or class II molecules and display them on their surfaces to be recognized by CD8+ and CD4+ T cells, respectively (Blum, Wearsch, & Cresswell, 2013; Cresswell, 2005). T cells recognize cognate peptides complexed with MHC molecules via T-cell receptors (TCRs). This interaction is complemented by T cell costimulatory signals induced by pattern recognition receptor (PRR) signaling, such as Toll-like receptors (TLRs), which detect microbial components, in order to specify the origin of the peptide as being non-self (Akira, Uematsu, & Takeuchi, 2006; Blander, 2018).
Virtually all nucleated cells can process intracellular antigens and load antigen-derived peptides onto MHC class I molecules to display on their surfaces, whereas only professional APCs, namely dendritic cells (DCs), macrophages, and B cells, can process endocytosed or phagocytosed extracellular antigens and load antigen-derived peptides onto MHC class II molecules (Blander, 2018). However, some professional APCs have been demonstrated to cross-present extracellular antigen-derived peptides onto MHC class I molecules to cross-prime and activate CD8+ T cells. This process is termed cross-presentation and it is critical for the instigation of an antigen-specific CD8+ T cell response (Blander, 2018; Grotzke, Sengupta, Lu, & Cresswell, 2017). In vivo and in vitro studies in both murine models and humans have shown that DCs are the best cross-presenting cell type (Embgenbroich & Burgdorf, 2018). DC cross-presentation provides a vital defense mechanism against many pathogens and malignancies that are able to evade or dampen the immune response of the infected/affected cell, thus rendering the process an important therapeutic strategy in creating anti-cancer vaccines. Additionally, DC cross-presentation is implicated in promoting central and peripheral immune tolerance (Joffre, Segura, Savina, & Amigorena, 2012).
DC cross-presentation occurs via one of the two currently accepted pathways, pertaining mainly to the kinetics and processing of the exogenous antigens and called the vacuolar pathway or the cytosolic pathway (Fig. 1; Blander, 2018). In the vacuolar pathway, antigens are taken up for processing in the endocytic compartment, and degraded primarily by the lysosomal protease Cathepsin S, whereas in the cytosolic pathway, antigens are internalized and translocated into the cytosolic space for processing and degradation by proteasomes (Shen, Sigal, Boes, & Rock, 2004). The resultant peptides, from the latter pathway, are subsequently transported by the protein transporter associated with antigen processing (TAP) either to transport the peptides into the endoplasmic reticulum or into the nascent endosome for loading onto MHC class I (MHC-I) molecules (Ackerman, Giodini, & Cresswell, 2006; Kovacsovics-Bankowski & Rock, 1995; Palmowski et al., 2006). A single mouse DC can cross-present exogenous peptides via both pathways simultaneously, though it has been demonstrated that a CD8α+ subpopulation of classical or conventional DCs (cDCs), known as class 1 DCs (cDC1), is only able to utilize the cytosolic pathway (Segura, Albiston, Wicks, Chai, & Villadangos, 2009).
Figure 1.
An illustration of the two pathways by which cross-presentation is currently understood to occur in DCs.Exogenous peptides are endocytosed or phagocytosed into the cell. In the cytosolic pathway, the cargo is transported into the cytosolic space for processing via cellular proteasomes. The resultant peptides are either translocated back into a nascent endosome for loading of MHC-I molecules or conceivably, as less evidence exists in support of this, they are chaperoned to the ER for loading onto MHC-I molecules and cross-presented on the surface of the cell. In the vacuolar pathway, the cargo is processed in the endosome/phagosome and the resultant peptides are loaded onto MHC-I molecules there, then presented on the surface of the cell. The endocytic recycling center (ERC) is an important source of MHC-I for cross-presentation of microbial antigens. The PLC and TAP molecules are provided by the ERGIC. DCs, dendritic cells; ER, endoplasmic reticulum; ERGIC, ER-Golgi intermediate compartment;PLC, peptide-loading complex; TAP, transporter associated with antigen processing.
Over the years, we have extensively studied DC cross-presentation in mice. We have carefully optimized the conditions to study this process in murine cells, including the isolation and differentiation of murine DCs, the isolation of antigen-specific transgenic murine CD8+ T cells, stimulating DCs for cross-presentation with soluble or particulate cargoes, and cross-priming CD8+ T cells by DCs. We have optimized the necessary readouts, in order to delineate the cell biology of cross-presentation and the complex cross-talk between vesicular traffic and TLR signaling pathways (Blander, 2008, 2016, 2018; Nair-Gupta & Blander, 2013; Nair-Gupta et al., 2014). This paper details such protocols with clear instructions to set up cross-presentation assays in vitro along with the appropriate experimental readouts that need to be conducted.
STRATEGIC PLANNING
An important consideration for in vitro cross-presentation assays is the method by which the cross-presenting DCs are prepared. In mice and in humans, cDCs that reside in lymphoid tissues can be broadly divided into two subsets, class 1 and class 2 cDCs. These subsets have distinct phenotypes, developmental requirements, and functions. CD11b+SIRPα+ cDC2s present exogenous antigen to CD4+ T cells, while CD8α+CD103+ cDC1s are the most efficient at cross-priming CD8+ T cells in vivo (Mildner & Jung, 2014). For decades, the standard method of culturing DCs in vitro has been to differentiate bone marrow (BM) progenitor cells in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) for 5 days, which may require the addition of interleukin-4 (IL-4; Inaba et al., 1992). While these so-called granulocyte-macrophage dendritic cells (GM-DCs) have been used in cross-presentation assays for decades and have been indispensable in research elucidating the machinery required for cross-presentation, it has recently become apparent that the output of this culture system does not phenotypically resemble the bona fide cross-presenting cDC1s (Helft et al., 2015). This is not to say that all knowledge gained by work in GM-DCs is to no avail, as our fundamental understanding of DC maturation and antigen presentation including cross-presentation comes from GM-CSF DCs. In the past couple of decades, a culture system which makes use of Fms-like tyrosine kinase 3 ligand (Flt3L) allows for the production of DCs which more closely resemble cDC1s found in vivo, as well as their CD103+ migratory counterparts (Brasel, De Smedt, Smith, & Maliszewski, 2000; Liu & Nussenzweig, 2010; Maraskovsky et al., 1996). Flt3L may give rise to some plasmacytoid DC (pDC)-like cells as well (Naik et al., 2005), and recently, Kirkling and coworkers described an in vitro culture system whereby feeder cells expressing Notch ligand Deltalike 1 drive optimal Flt3L-dependent development of murine cDC1s from bone marrow progenitor cells (Kirkling et al., 2018). These cDC1s express surface markers and exhibit migratory properties that closely resemble the surface markers and migratory phenotype of cDC1s seen in vivo (Kirkling et al., 2018). Despite these encouraging properties, the aforementioned Flt3L-dependent cDC1 culture system has mainly been utilized to study cDC1 development, with only a few groups choosing to use it for functional studies of cross-presentation (Beshara et al., 2018; Brawand et al., 2002; Lau et al., 2018; Masten, Olson, Kusewitt, & Lipscomb, 2004; Ou et al., 2019; Pulendran et al., 1997; Waskow et al., 2008). Importantly, many of the fundamental principles of cross-presentation have been validated in Flt3L-dependent cDC1 as well. This includes the capacity of these cells to stimulate and cross-prime CD8+ T cells (Masten et al., 2004).
In addition to the above described in vitro differentiation of cDC1s from bone marrow-derived progenitor cells, cross-presenting cDC1s can also be directly obtained from the spleen of a mouse that has been implanted with a tumor of B16-melanoma cells secreting Flt-3L (B16-Flt3L; Arora & Porcelli, 2016). These tumor cells provide a sustained systemic level of Flt3L in vivo which stimulates the propagation of all subsets of DCs in the mouse spleen. This generally results in mice developing splenomegaly, in which DCs comprise up to 60% of the total spleen cells after ∼10 days. Commercial kits are subsequently used to isolate the cDC1 subtype using phenotypic markers. This method yields high numbers of immature cDC1s for use in downstream in vitro cross-presentation experiments.
Below we have outlined the protocols most commonly used to isolate murine DC progenitor cells and differentiated them into GM-DCs and cDC1-like cells in vitro, in Basic Protocol 1, 2, and 3, respectively. In Basic Protocol 4, we outline a protocol to culture B16-Flt3L cells for subsequent tumor implantation in mice to expand cDC1s in vivo and isolation from the mouse spleen. The resultant non-activated and immature DCs will be used in downstream in vitro cross-presentation experiments using CD8+ T cells as a readout. An overview of the workflow is schematized in Figure 2.
Figure 2.
A schematic workflow showing the outline for the preparation of dendritic cells (DCs) and CD8+ T cells and the co-culture of the two to study cross-presentation in vitro. In the cell preparation phase, DC progenitor cells are isolated from femur and tibia bone marrow of 7–11 week-old mice and are differentiated in vitro into GM-DCs or cDC1-like cells, by culturing them for 5 days or 7–10 days in the presence of GM-CSF or Flt3L, respectively. In vivo differentiated cDC1 cells are isolated from the spleen of similar aged mice implanted with B16-Flt3L tumor cells for 7 days.Similar aged mice specific for the model antigen are used to obtain CD8+ T cells from the spleen or lymph nodes. In the cross-presentation phase, DCs are given a model antigen cargo for cross-presentation (exogenous antigen) along with an appropriate stimulant, e.g., lipopolysaccharides (LPS) as shown here. Isolated CD8+ T cells are co-cultured with stimulated, fixed DCs to evaluate cross-presentation. In the analyses phase, DCs can be analyzed with immunofluorescence microscopy to visualize cargo internalization and subcellular localization and the cellular distribution of MHC-I molecules. ELISA tests can be used to detect the expression levels of appropriate cytokines which may indicate the activation of TLR signaling in DCs, while flow cytometry analysis can indicate the activation/maturation state of DCs by assessing surface expression markers. T cells that had been co-cultured with DCs are analyzed by flow cytometry to evaluate their activation state and later to assess proliferation after cross-priming by DCs. ELISA tests can also be utilized to measure cytokine expression in activated CD8+ T cells. TLR, Toll-like receptor.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
BASIC PROTOCOL 1
ISOLATION OF BONE MARROW PROGENITOR CELLS
The study of cross-presentation using an in vitro system necessitates a large number of DCs. In vivo, DCs comprise a relatively rare immune cell subset, meaning that the isolation of enough immature DCs from an animal for use in vitro is impractical. In addition to the issue of rarity, the isolation of differentiated DCs from different tissues has the potential to induce activation and phenotypic changes which may preclude proper assessment of some of their functions (O’Connell, Morelli, Logar, & Thomson, 2000). However, in the past few decades, it has been possible to differentiate DCs from bone marrow progenitors in vitro (Inaba et al., 1992) and utilize them for controlled cross-presentation experiments. The protocol below outlines how to extract bone marrow from the femur and tibia of a mouse. The progenitor cells obtained from the bone marrow can then be differentiated into DCs in vitro for downstream experiments. Typically, the bone marrow recovered from the femur and tibia bones of a single mouse yield ∼50 to 80 million DC progenitor cells.
Materials
7- to 11-week-old C57BL/6J mice (The Jackson Laboratory, cat. no. 000664) or mouse of desired genotype
PBS, pH 7.4 (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Endotoxin-free red blood cell (RBC) lysis buffer (MilliporeSigma, cat. no. R7757)
Heat-inactivated FCS with endotoxin levels of ≤0.06 EU/ml (R&D Systems, cat. no. S11550H; see Critical Parameters)
Hank’s Balanced Salt Solution (HBSS; VWR, cat. no. L0121–0500)
RPMI 1640-supplemented medium (see recipe)
Dissecting scissors
Forceps
10-cm polystyrene petri dish
15- and 50-ml polypropylene conical tubes
Hemocytometer or cell counter
27-gauge needle
10-cc syringe
70-μm nylon mesh cell strainer
- Obtain a 7- to 11-week-old C57BL/6J mouse with the correct genetic modification.For instance, if the impact of TLR signaling on cross-presentation is being investigated, then one might consider using mice deficient for the receptor or the signaling adaptors for the receptor.
Sacrifice mouse according to your institute’s guidelines and minimize animal suffering.
- Place mouse in supine position with its forelimbs and hindlimbs pinned onto a dissecting board and spray mouse with ethanol before placing it into a tissue culture hood.All remaining steps are to be carried out under sterile conditions.
Using forceps, gently lift the abdominal wall and use dissecting scissors to make avertical cut from near the urethral orifice to the area just below the sternum. Take care not to cut or puncture any organs.
Make a diagonal cut alongside each of the hindlimbs to reveal leg bones and tissues.
Using two forceps, separate leg tissue from skin.
Using two forceps, carefully remove femur and tibia bones individually from the mouse.
Hold the tip of each bone with one set of forceps, while gently using another set to remove all associated muscle and tissue masses around bones.
Place bones in a clean 10-cm petri dish and wash with PBS to remove any debris.
Transfer bones to a clean 10-cm petri dish containing 2 ml HBSS medium with 2%FCS.
Carefully sever epiphyses to expose bone marrow cells, using sharp dissecting scissors.
Using a 27-gauge needle attached to a 10-cc syringe containing HBSS medium with2% FCS, flush bone marrow cells carefully from each bone into a 10-cm petri dish.
Isolate and suspend cells by gentle pipetting up and down several times and transfer to a 15-ml conical tube.
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
Resuspend cells in 1 ml RBC lysis buffer and incubate for exactly 1 min at room temperature.
Add 10 ml HBSS medium with 2% FCS to neutralize RBC lysis buffer.
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
Resuspend cells in 10 ml HBSS medium with 2% FCS.
Pass resuspended cells through a 70-μm cell strainer into a 50-ml conical tube.
Count the cells.
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
Resuspend cells in supplemented RPMI 1640 medium at a concentration of 1 × 106 per ml and immediately continue to the differentiation protocol in Basic Protocol 2 below.
BASIC PROTOCOL 2
IN VITRO DIFFERENTIATION OF DENDRITIC CELLS WITH GM-CSF
In vitro differentiation of bone marrow-derived DCs with GM-CSF, so-called GM-DCs, begins with the isolation of bone marrow progenitor cells, as described in Basic Protocol 1. The bone marrow progenitor cells are then cultured in the presence of GM-CSF to activate the downstream signaling pathways that are necessary in driving DC differentiation (van de Laar, Coffer, & Woltman, 2012). GM-CSF can either be obtained by filtering the conditioned medium of cultured J558L cells transfected with murine GMCSF cDNA or by purchasing recombinant GM-CSF from commercial vendors. The bone marrow progenitor cells are cultured with GM-CSF supplemented medium for 5 days, with an additional replenishment with the GM-CSF-containing medium on day 3. The DCs will appear in clusters that situate mainly around the edges of each well.
Materials
Bone marrow cells (as isolated in Basic Protocol 1)
RPMI 1640-supplemented medium (see recipe)
Recombinant murine GM-CSF (R&D Systems, cat. no. ML-415) or genetically engineered GM-CSF-expressing J558L cell conditioned medium as a source of GM-CSF (see Support Protocol 1)
24-well plates, polystyrene and tissue culture treated
Resuspend bone marrow-derived progenitor cells at a concentration of 1 × 106 cells per ml in RPMI 1640-supplemented medium.
Add 10 ng/ml recombinant murine GM-CSF or GM-CSF conditioned medium at the experimentally determined appropriate percentage (see Support Protocol 1) to the resuspended cells.
Plate 1 ml cell suspension in each well of a 24-well plate, i.e., 1 × 106 cells per well.
- Incubate cells for 5 days (total) at 37°C in a humidified atmosphere containing 5%CO2. Take extra care not to disturb the cells while they differentiate and grow to avoid spontaneous maturation.The cells do not need to be replated and replating prior to experiments should especially be avoided.
- On day 3, add 1 ml prewarmed fresh RPMI 1640-supplemented medium containing10 ng/ml recombinant murine GM-CSF or GM-CSF conditioned medium to each well.At the end of the incubation period, i.e., at day 5, clusters of DCs should be visible, particularly around the edges of the wells.J558L cell GM-CSF conditioned medium can be substituted for recombinant GM-CSF.
SUPPORT PROTOCOL 1
PREPARATION OF CONDITIONED MEDIUM FROM GM-CSF PRODUCING J558L CELLS
While recombinant murine GM-CSF is readily available commercially, many groups choose to generate their own in an effort to cut down on costs. J558L cells that have been transduced with a GM-CSF expression vector are cultured for 3 days in RPMI 1640-supplemented medium. The culture supernatant is then centrifuged and filter-sterilized. In order to determine the concentration of GM-CSF in the culture supernatant, an ELISA test is performed using the J558L medium supernatant. Alternatively, a better evaluation would involve culturing and differentiating bone marrow progenitor cells with a serial dilution of the J558L conditioned medium, then empirically calculating the best concentration guided by evaluating the resulting GM-DCs, from each J558L conditioned medium dilution and assessing their expression of DC markers, viability, and maturation state.
Materials
J558L cells transfected with murine GM-CSF cDNA (Qin, Noffz, Mohaupt, & Blankenstein, 1997)
IMDM-supplemented medium (see recipe)
Geneticin G418 (InvivoGen, cat. no. ant-gn-1)
Murine GM-CSF ELISA Development Kit (PeproTech, cat. no. 900-K55) or other suitable ELISA kit
15- and 50-ml polypropylene conical tube
T25 tissue culture flask, polystyrene and tissue culture treated
T175 tissue culture flask, polystyrene and tissue culture treated
500-ml 0.22-μm filter bottle, cellulose acetate membrane
Thaw a vial of GM-CSF producing J558L cells, containing 5 × 106 cells, slowly on ice and transfer cells into a 15-ml conical tube containing 10 ml prewarmed IMDM-supplemented medium.
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
Resuspend cells in 5 ml prewarmed IMDM-supplemented medium without geneticin G418, culture in a T25 flask, and incubate overnight at 37°C in a humidified atmosphere containing 5% CO2.
Transfer cells to a T175 flask and add 175 ml prewarmed IMDM-supplemented medium containing geneticin G418 at a concentration of 1 mg/ml. Incubate as before (see step 3).
- After ∼3 days, cells should have expanded sufficiently as indicated by the color of the phenol red in the medium turning orange.Phenol red is a pH sensitive dye which turns to orange and then yellow when the culture medium becomes increasingly acidic as the cells proliferate and release waste products.
- As soon as the medium becomes orange, propagate cells further by seeding into twoT175 flasks each with 175 ml IMDM-supplemented medium containing geneticin G418 and culture for an additional 3 days.If a larger batch of GM-CSF is desired, this can be scaled up as appropriate.
Carefully collect culture supernatant in each flask at day 3 and transfer to 50-mlconical tubes.
Centrifuge supernatant at 300 × g for 5 min at room temperature to pellet cellular debris. Collect supernatant carefully, leaving ∼1 ml medium at the bottom of the tube to avoid cellular contaminants from being collected.
Pool and filter supernatant using a 500-ml 0.22-μm filter bottle. Aliquot into 50-ml conical tubes and store at −80°C for later use.
- Calculate GM-CSF concentration in the supernatant by utilizing commercially available ELISA kits or empirically by using serially diluted GM-CSF-containing medium to differentiate bone marrow progenitor cells into GM-DCs, as described in Basic Protocol 2, then analyze the culture output as described in Basic Protocol 5.GM-CSF producing J558L cells are J558 cells that have been transfected with murine GM-CSF cDNA. The J558 cell line (ATCC, cat. no. TIB-6) does not endogenously express murine GM-CSF.J558L cells can be initially cultured in 20% FCS after thawing to aid establishing the cell culture (step 3) prior to the addition of geneticin G418 (step 4).
BASIC PROTOCOL 3
IN VITRO DIFFERENTIATION OF DENDRITIC CELLS WITH Flt3L
As previously discussed, it has recently been argued that the GM-DC culture system (see Basic Protocol 2) does not phenotypically share many features with cDC1s, which have been shown to be the bona fide cross-presenting subset of DCs in vivo (Helft et al., 2015). Subsequently, the protocol below describes the steps to ob tain cDC1-like cells; it starts with the bone marrow progenitor cells (isolated in Basic Protocol 1) and utilizes Flt3L in the culture medium to drive the differentiation of cDC1-like cells over 7 to 11 days (Brasel et al., 2000; Naik et al., 2005; van de Laar et al., 2012).
Materials
Murine bone marrow progenitor cells (see Basic Protocol 1)
RPMI 1640-supplemented medium (see recipe)
Recombinant Flt3L (PeproTech, cat. no. 250–31 L) or B16-Flt3L cell Flt3L conditioned m edium (see Support Protocol 2)
24-well plates, polystyrene and tissue culture treated
Resuspend bone marrow-derived progenitor cells at a concentration of 1 × 106 cells/ml in RPMI 1640-supplemented medium.
Add 300 ng/ml recombinant murine Flt3L or the equivalent in the form of B16-Flt3Lconditioned medium (v/v) to the resuspended cells (see Support Protocol 2).
Plate 2 ml cell suspension in each well of a 24-well plate, i.e., 2 × 106 cells per well.
- Incubate cells for 7 (total) days at 37°C in a humidified atmosphere containing 5% CO2. Take extra care not to disturb cells while they differentiate and grow to avoid spontaneous maturation.The cells do not need to be replated and replating prior to experiments should especially be avoided.
- On day 3, carefully pipet 1 ml medium out of each well, without disturbing the cells, and replace with 1 ml prewarmed fresh RPMI 1640-supplemented medium containing 300 ng/ml recombinant murine Flt3L or the equivalent in the form of B16-Flt3L conditioned medium (v/v).B16-Flt3L cell Flt3L conditioned medium can be substituted for recombinant Flt3L.
SUPPORT PROTOCOL 2
PREPARATION OF Flt3L CONTAINING MEDIUM FROM B16-Flt3L CELLS
Flt3L conditioned medium, from cultured B16-Flt3L cells, can be used to differentiate bone marrow-derived progenitor cells to cDC1-like cells, in lieu of commercially available recombinant murine Flt3L or as an economically viable alternative. The process is simple and the protocol below describes in detail the steps involved to generate Flt3L containing medium from B16-Flt3L cells. B16-Flt3L cells are a murine melanoma cell line that are transfected with the Flt3L cytokine gene and subsequently expresses high concentrations of Flt3L (Vargas, Cortes, Vargas, Rosemblatt, &Bono, 2006). These adherent cells are cultured in DMEM-supplemented medium until they reach ∼85% confluency. The culture supernatant is then collected, centrifuged, and filter-sterilized and stored at −80°C for later use. Prior to use in experiments, an ELISA test can be performed using a commercially available kit in order to determine the concentration of the Flt3L in the supernatant, which is to be diluted to a final working concentration of 300 ng/ml.
Materials
B16-Flt3L cells (RRID: CVCL_IJ12; Vargas et al., 2006)
DMEM-supplemented medium (see recipe)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Trypsin-EDTA (0.25%), phenol red (Gibco brand, Thermo Fisher Scientific, cat. no. 25200056)
Murine Flt3L ELISA Development Kit (R&D Systems, cat. no. MFK00) or other suitable ELISA kit
T25 tissue culture flask, polystyrene and tissue culture treated
T75 tissue culture flask, polystyrene and tissue culture treated
T175 tissue culture flask, polystyrene and tissue culture treated
15- and 50-ml polypropylene conical tube
0.22-μm Steriflip filter system (MilliporeSigma, cat. no. SE1M003M00) or other sterile 0.22-μm filter system
-
1
Thaw a vial of B16-Flt3L cells quickly by dipping the vial halfway in a 37°C water bath and agitating the vial, being careful not to allow water to touch the area near the lid.
-
2
Transfer cells into a 15-ml conical tube containing 10 ml prewarmed DMEM-supplemented medium.
-
3
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
4
Resuspend cells in 5 ml prewarmed DMEM-supplemented medium, culture in a T25 flask, and incubate at 37°C in a humidified atmosphere containing 5% CO2.
-
5
Once the culture reaches 80% to 90% confluency, aspirate culture medium then wash once with PBS before incubating cells with 3 ml trypsin for 5 min at 37°C.
-
6
Add 5 ml DMEM-supplemented medium to the flask to inactivate trypsin and stop digestion. Collect cell suspension in a 15-ml conical tube.
-
7
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
8
Resuspend cells in 10 ml prewarmed DMEM-supplemented medium and culture in a T75 flask and incubate at 37°C in a humidified atmosphere containing 5% CO2.
-
9
Split culture again, once it reaches 80% to 90% confluency, by trypsinizing cells with 5 ml trypsin as before and seed at 50% confluency in a T175 flask with 50 ml prewarmed DMEM-supplemented medium. Incubate at 37°C in a humidified atmosphere containing 5% CO2.
-
10Allow adherent cells to expand to a confluency of 85% or when the cell medium turns orange.At this point, conditioned medium can be collected or the cells can be split further in order to make more conditioned medium.
-
11
Carefully collect culture supernatant and transfer to a 50-ml conical tube.
-
12
Centrifuge supernatant at 300 × g for 10 min at 4°C to pellet cellular debris. Collect supernatant carefully, leaving ∼1 ml of medium at the bottom of the tube to avoid cellular contaminants from being collected.
-
13Pool, if there is more than one tube, and filter supernatant.It is recommended that a 0.22-μm Steriflip 50-ml tube filter system be used, though other 0.22-μm filter systems may be used as well.
Aliquot up to 50 ml conditioned medium into 50-ml conical tubes and store at −80°C for later use.
-
14Calculate Flt3L concentration in the supernatant by utilizing commercially available ELISA kits.Many laboratories simply use the conditioned medium at a concentration of 10% (v/v) in the culture system in Basic Protocol 3.
BASIC PROTOCOL 4
EXPANSION OF cDC1s IN VIVO FOR USE IN EX VIVO EXPERIMENTS
In order to expand the numbers of DCs which naturally occur in the spleen of mice, mice are injected subcutaneously with 1 × 107 B16-Flt3L cells. Within 7 to 11 days, the tumor will reach a size measuring ∼2 to 10 mm in diameter, which makes it readily visible (Arora & Porcelli, 2016). The lymphoid organs, in particular the spleen, are then harvested and digested, followed by depleting RBC with a lysis buffer. The resulting single cell suspension consists mainly of the different subsets of DCs, which are labeled with microbeads for separation with a magnetic cell separation apparatus. The subsequent cDC1s that are isolated should then immediately be placed into culture, stimulated, and given cross-presentation cargo. This is because removal and handling of the cDC1s from lymphoid organs causes them to spontaneously become activated and thus mature, which will render them useless for in vitro cross-presentation assays.
Additional Materials (also see Support Protocol 2)
7- to 11-week-old C57BL/6J mice (The Jackson Laboratory, cat. no. 000664) or mouse of desired genotype
B16-Flt3L cells (RRID, CVCL_IJ12)
RPMI 1640-supplemented medium (see recipe)
RPMI 1640 medium (Gibco brand, Thermo Fisher Scientific, cat. no. 11875093)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Trypsin-EDTA (0.25%), phenol red (Gibco brand, Thermo Fisher Scientific, cat. no. 25200056)
Endotoxin-free red blood cell (RBC) lysis buffer (MilliporeSigma, cat. no. R7757)
Heat-inactivated FCS (R&D Systems, cat. no. S11550H)
DNAse/collagenase solution:
DNAse I (Roche, cat. no. 11 284 932 001)
Collagenase D (Roche, cat. no. 11 088 858 001)
CD8α+ Dendritic Cell Isolation Kit, mouse (Miltenyi Biotec, cat. no. 130–091-169)
T175 flask, polystyrene and tissue culture treated
1.5-ml Eppendorf tube
1- and 10-cc syringes
25-gauge needle
Dissecting scissors
Forceps
70-μm nylon mesh cell strainer
6- and 24-well plates
15- and 50-ml polypropylene conical tube
10-cm polystyrene petri dish
Hemocytometer or cell counter
LS magnetic separation column (Miltenyi Biotec, cat. no. 130–042-401)
QuadroMACS™ Separator (Miltenyi Biotec, cat. no. 130–091-051)
MACS MultiStand (Miltenyi Biotec, cat. no. 130–042-303)
-
1
Seed B16-Flt3L cells in a T175 flask and grow to 90% confluency, following Support Protocol 2, steps 1 to 8.
-
2
Harvest B16-Flt3L cells. Begin by aspirating culture medium, then wash once with ice-cold PBS before incubating cells with 10 ml trypsin for 5 min at 37°C.
-
3
Add 10 ml DMEM-supplemented medium to the flask to inactivate trypsin and stop digestion. Collect cell suspension in a 50-ml conical tube.
-
4
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully. Wash once in ice-cold PBS and centrifuge as before.
-
5
Count cells with a hemocytometer or a cell counter.
-
6
Resuspend cells at a concentration of 1 × 108 cells per ml in PBS. Transfer cells to a 1.5-ml Eppendorf tube and keep on ice. Immediately bring cell suspension to the animal facility where the mice are kept.
-
7Work in a biosafety hood and use a 1-cc syringe fitted with a 25-gauge needleto draw 100 μl B16-Flt3L cells suspended in PBS for each mouse that is to be injected.Do not pass the B16-Flt3L cells through the needle more than once as this may cause excessive cellular stress resulting in shearing the cells, hence reducing the total number of viable cells and in turn reducing the rate of successful implantation.
-
8Immobilize mouse by holding it by the scruff of its neck, then turn it over so the abdomen faces you, and tuck its tail behind your pinky finger. Using your dominant hand, inject mouse subcutaneously with the 100 μl B16-Flt3L cells contained in the syringe into the side flank. To minimize animal suffering and to successfully implant the tumor, place the needle in nearly parallel to the skin of the mouse and push the needle into the skin slowly. A small “bubble” should form just under the skin while injecting, which indicates correct application.If immobilizing the mouse is proving difficult or direct injection not permitted by the institute, then the mouse can be anaesthetized using ketamine or isoflurane prior to the injection.
Place mouse back in its usual enclosure.
-
9
Wait for 7 to 11 days for the tumor to reach a noticeable size at which point the various DC subsets, and in particular cDC1s in the spleen, should have significantly increased in number. During the period following the tumor implantation, assess and monitor the animal’s health according to an accepted method, such as those published by Ullman-Culleré and Foltz (1999).
-
10
Sacrifice mouse once the tumor has reached a diameter of 2 to 10 mm across and is visibly palpable. Follow your institute’s guidelines on the humane criteria for determining at which point of tumor growth to sacrifice the animal and minimize its suffering.
-
11Place mouse in supine position with its forelimbs and hindlimbs pinned onto a dissecting board and spray it with ethanol before placing it into a tissue culture hood.All subsequent steps are to be carried out under sterile conditions.
-
12
Using a pair of forceps, gently lift the abdominal wall and use dissecting scissors to make a vertical cut from near the urethral orifice to the area just below the neck. Take care not to cut or puncture any organs.
-
13
Make two horizontal cuts on the left side of the mouse from top and bottom ends of the first cut to peel the abdominal wall back to reveal the internal abdominal organs. Using a pair of forceps, gently harvest the spleen.
-
14
Place spleen inside a 70-μm cell strainer that is sitting on a well of a 6-well plate containing RPMI 1640 medium in the presence of DNAse/collagenase solution (100 μg/ml DNAse I and 1 mg/ml collagenase D). Mash spleen through the strainer using the plunger portion of a 10-cc syringe.
-
15
Incubate mashed-up spleen including the cell strainer in the DNAse/collagenase solution for 30 min at 37°C.
-
16
Mix splenocyte suspension by gently pipetting up and down using a Pasteur pipet (pipet through the cell strainer also) and transfer cells to a 15-ml conical tube.
-
17
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
18
Resuspend cells in 1 ml RBC lysis buffer and incubate for exactly 1 min at room temperature.
-
19
Add 10 ml RPMI 1640-supplemented medium to neutralize the RBC lysis buffer.
-
20
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
-
21
Wash cells once in 10 ml PBS containing 5% FCS, then centrifuge as before.
-
22Isolate the cDC1 subset using MACS mouse CD8α+ DC isolation kit, following the manufacturer’s instructions (Miltenyi Biotec).All steps must be completed using pre-cooled buffers. All reagents and cell suspensions must be kept on ice to maintain a temperature of ∼4°C. This is to maximize cell viability and minimize spontaneous maturation and activation of the DCs.
-
23
Estimate the number of DCs using a hemocytometer or a cell counter and immediately resuspend cells at a concentration of 1 × 106 cells per ml in RPMI 1640-supplemented medium.
-
24
Add 1 ml cell suspension (i.e., 1 × 106 cells) to each well of a 24-well plate and immediately proceed to the addition of cross-presentation cargo and DC stimulation, as described in Basic Protocol 6.
BASIC PROTOCOL 5
CHARACTERIZING RESTING AND ACTIVATED DENDRITIC CELLS
DCs can become activated with very little stimuli, which begins the maturation process. It is, therefore, imperative to ensure that all reagents used in DC culture are completely free of endotoxins. Additionally, it has been shown that the simple disruption of E-cadherin mediated contacts, that help form the clusters which appear in DC cultures, is enough to induce DC maturation (Jiang et al., 2007). Consequently, it is highly recommended that these cells be handled with caution and any disruptions be minimized while in culture, including excessive movement and handling of the wells or culture flasks; or excessive vibrations caused by opening and closing the incubator door; or by unbalanced centrifuge operation that is in close proximity to the incubator. The cross-presentation capability of DCs is negatively affected by their maturation, a state in which the phagocytic capability of DCs decrease (Blander, 2018). For this reason, when establishing an in vitro cross-presentation system, it is important to ensure the baseline ‘resting’ state of DCs prior to their use in cross-presentation experiments.
The method below describes a simple way to do this by staining the cultured DCs for activation markers prior to any stimulation and performing flow cytometric analyses. This should be done regularly, particularly when an already established reagent has been replaced with a new brand or new lot. In particular, close attention must be paid to the lot of serum, as serum is notorious for containing endotoxins at levels which many manufacturers deem to be an insignificant amount but in fact is adequate to activate DCs in vitro. In addition to the aforementioned flow cytometric analyses, ELISA tests for the pro-inflammatory cytokines TNFα and IL-6 are also utilized to assess DC activation by probing for the expression of these pro-inflammatory cytokines that are typically secreted as a result of TLR signaling, such as lipopolysaccharides (LPS). Stimulating the TLR signaling pathways in DCs will serve as a positive control for the phenotypic markers and cytokine expression of activated and matured DCs in comparison to resting and immature DCs.
Materials
GM-CSF or Flt3L in vitro differentiated DCs (see Basic Protocol 2 and 3)
Lipopolysaccharides (LPS; MilliporeSigma, cat. no. L2880)
RPMI 1640-supplemented medium (see recipe)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
ELISA kits for detecting murine TNFα and IL-6 cytokines (e.g., R&D Systems)
Trypsin-EDTA (0.25%), phenol red (Gibco brand, Thermo Fisher Scientific, cat. no. 25200056)
FACS buffer (see recipe)
Aqua Live/Dead stain (Invitrogen brand, Thermo Fisher Scientific, cat. no. L34957)
Anti-mouse CD16/CD32 Fc-block antibody (clone 93; eBioscience, cat. no. 14–0161-85)
Antibody cocktail and isotype controls:
Anti-mouse CD11b antibody (clone M1/70; Invitrogen brand, Thermo Fisher Scientific, cat. no. 25–0112-81)
Anti-mouse CD11c antibody (clone N418; BioLegend, cat. no. 117320)
Anti-mouse MHC-II antibody (clone M5/114.15.2; BioLegend, cat. no. 107631)
Anti-mouse CD40 antibody (clone 1C10; eBioscience, cat. no. 12–0401-83)
Anti-mouse CD80 antibody (clone 16–10A1; BioLegend, cat. no. 104706)
Anti-mouse CD86 antibody (clone GL1; BioLegend, cat. no. 105028)
24-well plates
1.5-ml Eppendorf tube
15-ml polypropylene conical tube
Hemocytometer or cell counter
P1000 pipets
U-shaped 96-well plate, polystyrene and tissue culture treated
Aluminum foil
For bone marrow-derived DCs
1a. Culture bone marrow-derived DCs using either the GM-CSF protocol (see Basic Protocol 2) or the Flt3L protocol (see Basic Protocol 3).
2a. To evaluate the overall level of DC differentiation from the bone marrow-derived progenitor cells, as well as to assess the ability of these newly differentiated DCs to mature and become activated upon stimulation, add 100 ng/ml LPS directly to the wells housing the differentiated DCs in the 24-well plates and incubate 6 hr or overnight at 37°C in a humidified atmosphere containing 5% CO2. Make sure to include unstimulated DC control wells alongside the stimulated DC wells and conduct each condition in triplicate.
For non-adherent immature DCs
1b. Alternatively, use only non-adherent immature DCs to assess the ability of these newly differentiated DCs to mature and become activated upon stimulation. To harvest non-adherent immature DCs, flush medium contained in the wells up and down several times using a P1000 pipet. Pipet carefully and do not let the pipetted contents touch the bottom of the pipet itself to avoid contamination. If this is not possible, use filtered pipet tips to avoid contamination. Ensure that around the edges of the wells are flushed, as this is where much of the DC clusters are formed. Then collect RPMI 1640 medium-containing DCs in the wells and place into a 15-ml conical tube. Immediately add 1 ml ice-cold PBS to the well, flush, and collect the remainder of DCs.
2b. Count cells using a hemocytometer or a cell counter and centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
3b. Resuspend DCs at a concentration of 1 × 106 cells/ml in RPMI 1640-supplemted medium.
4b. Transfer 1 ml suspended DCs, i.e., 1 × 106, to a new 24-well plate well, immediately stimulate DCs with 100 ng/ml LPS, and incubate 6 hr or overnight at 37°C in a humidified atmosphere containing 5% CO2. Make sure to include unstimulated DC control wells alongside the stimulated DC wells and conduct each condition in triplicate.
5. Once the treatment incubation period has been completed, DCs are now ready for the assessment of maturation markers via cytokine expression and secretion. Centrifuge cells at 300 × g for 5 min at 4°C.
6. For cytokine secretion, carefully and slowly remove supernatants with a pipet and transfer to labeled 1.5-ml Eppendorf tubes for ELISA tests. Do not disturb DCs at the bottom of the wells. Continue with steps 7 to 8 below for ELISA tests, or for assessment of maturation markers on DCs by flow cytometry, continue from step 9 below.
- 7. Centrifuge supernatants at 300 × g for 10 min at 4°C to pellet cellular debris. Carefullycollectsupernatantswithoutdisturbingtheareawhereapelletmayhaveformed and aliquot into clean and labeled 1.5-ml Eppendorf tubes for immediate use or store in the refrigerator overnight for use the next day.Avoid repeat freeze-thaw cycles. It is highly recommended that the ELISA tests are performed immediately after collecting the supernatant. This is because freezing and thawing the supernatant even once may alter the cytokine concentration. Plan ahead of the experiment, for example, coat ELISA plates with the appropriate capture antibody overnight, if not using a kit or as and when required.
8. Use commercially available ELISA kits to detect the levels of the pro-inflammatory cytokines TNFα and IL-6 in the LPS-stimulated DCs compared to unstimulated DCs as indicators of DC activation. Follow ELISA manufacturers’ guidelines.
9. Harvest stimulated DCs (and negative control, unstimulated DCs) by adding 1 ml PBS to each well and flushing the medium/PBS contained in the well up and down several times using a P1000 pipet. Pipet carefully and do not let the pipetted contents touch the bottom of the pipet itself to avoid contamination. If this is not possible, use filtered pipet tips to avoid contamination. Ensure that around the edges of the wells are flushed, as this is where much of the DC clusters are formed. Collect pipetted contents from all the wells and place into labeled 15-ml conical tubes and place on ice. Immediately after medium is removed from the wells, add 1 ml ice-cold PBS to each well and flush wells as before to collect remaining cells, then pool them with the other harvested cells in the corresponding 15-ml conical tube.
10. Add 500 μl trypsin to each well and incubate for no longer than 5 min at 37°C in a humidified atmosphere containing 5% CO2. Lightly agitate plate manually to dislodge adherent cells.
11. Add 3 ml RPMI 1640-supplemted medium to each well to neutralize trypsin. Transfer contents to corresponding 15-ml conical tubes. Add 1 ml ice-cold PBS to each well and flush wells as before to collect remaining cells, then pool them with the other harvested cells in the corresponding 15-ml conical tube.
12. Count cells suspended in medium/PBS using a hemocytometer or a cell counter.
13. Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully. Wash once in ice-cold PBS and centrifuge as before.
14. Resuspend DCs at a concentration of 2 × 106 cells/ml in PBS and add 100 μl (2 × 105 cells) of each treatment of interest into wells of a 96-well plate.
15. Ensure adequate samples are included to satisfy all staining controls; include technical replicates, isotope controls, single stain controls, as well negative controls, i.e., unstained controls, for your experiment.
16. Wash again by adding another 100 μl PBS to each of the wells and centrifuge as before.
17. Remove and discard supernatant carefully using either an aspirator on a low setting or manually using a pipet.
- 18. Resuspend all DCs in the wells in 100 μl PBS containing anti-CD16/CD32 (Fc-block) diluted 1/100 and Aqua Live/Dead stain diluted 1/1,000. Control cells for these are resuspended in PBS only. Cover the 96-well plate with foil or another light impenetrable material and incubate on ice or at 4°C for at least 30 min.It is important to use PBS, not FACS buffer in this step, as the FCS in the FACS buffer will sequester the Aqua Live/Dead stain.
19. Wash cells by adding another 100 μl FACS buffer to each of the wells and centrifuge as before.
20. Wash cells once more using 100 μl FACS buffer and centrifuge as before.
- 21. Add 100 μl of the appropriate antibody cocktail or corresponding isotype control antibodies diluted in FACS buffer to the cells in each of the appropriate wells. Incubate 30 min on ice or at 4°C. The recommended surface marker controls are: CD11b, CD11c, MHC II, CD80, CD86, and CD40.For each individual laboratory, an antibody cocktail should be created based on available materials, being mindful of spectral overlap and the flow cytometer capabilities, with antibodies that have been titrated and validated.
22. Wash cells by adding 100 μl FACS buffer to each of the wells and centrifuge as before. Wash twice more by adding 200 μl FACS buffer to each of the wells and centrifuge as before.
- 23. Resuspend cells in 200 μl FACS and proceed to acquisition or fix cells in a preferred fixative to store for later analyses. Use single stain controls for compensation. Sample data is shown below in Figure 3.Immature differentiated DCs should be positive for CD11b and CD11c and have moderate expression of MHC II. In contrast, activated and matured DCs will upregulate surface MHC-II expression, as well as the induction of expression of the co-stimulatory markers CD80/CD86 and CD40, with the latter being considered as the clearest indicator of DC maturation.Compare and contrast the levels of expression of the aforementioned markers on cells that have not been stimulated with LPS to those that have been stimulated to ensure that your DC culture system generates DCs that respond appropriately to TLR stimulation and most importantly have intact phagocytic activity. Cultured DCs that express low levels of DC maturation markers are indicative of DCs in the resting state. If the levels are similar to those on LPS-treated DCs, then the cultured DCs should not be used as they are already activated and importantly will have compromised phagocytic activity.It is vital to utilize this protocol every single time a new reagent is introduced or an existing reagent is switched (e.g., new batch) in order to ensure they do not induce spontaneous maturation of DCs in culture.As a cost-effective measure, ELISA kits can be substituted for individually purchased ELISA components (such as the appropriate capture and detection antibodies, recombinant proteins used as standards, substrates, stop solution, blocking buffer) to assay cytokine concentrations, and following protocols published previously by the Blander Lab (Nair-Gupta et al., 2014)
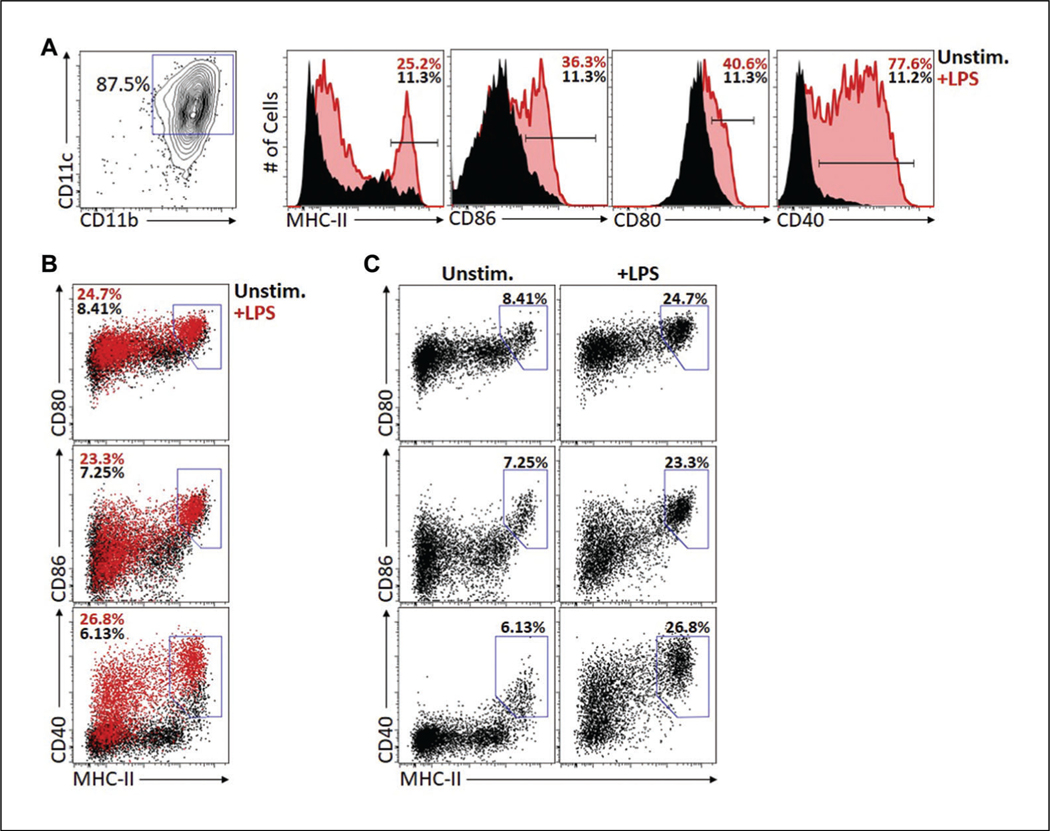
Figure 3.
Examples of dendritic cell (DC) maturation profiles as assessed by flow cytometry. DCs were cultured as described in Basic Protocol 2.At the end of the culture period (18 hr), cells were analyzed with flow cytometry to assess the expression level of CD11b, CD11c, MHC II, CD86, CD80, and CD40. (A) CD11c and CD11b expression on the DC showing 87.5% of the cells are positive for these DC markers. Histogram plots gated on the CD11b+CD11c+ DCs show expression of MHC II, CD86, CD80, and CD40 on unstimulated (black histograms) versus lipopolysaccharides (LPS) stimulated (red histograms) DCs. It is expected that resting DC will have some level of expression of these markers, however as the data show, their expression is markedly increased upon LPS stimulation. (B and C) The same data as in A but plotted as dot plots rather than histograms and either as an overlap of the resting versus LPS-stimulated DCs (B) or each DC condition separately (C). This type of plotting of the data enables one to simultaneously assess the expression of each costimulatory molecule versus MHC II. These are typical data that are consistent with GM-DCs being successfully cultured and activated in the presence of the TLR agonist LPS. TLR, Toll-like receptor; Unstim., unstimulated.
BASIC PROTOCOL 6
DENDRITIC CELL STIMULATION, ANTIGENIC CARGO, AND FIXATION
DCs are able to internalize a wide range of microbial antigens or whole microbes for cross-presentation in vivo. It has been widely demonstrated that DCs engage different mechanisms to internalize cargo by utilizing different surface receptors and process antigens from these cargoes for cross-presentation. The activation of various downstream signaling cascades during the process of internalization is in turn dictated by the engagement of different DC PRRs (Blander, 2018). Therefore, the choice of the model antigen is an important factor when designing in vitro cross-presentation assays because this dictates the method by which the antigen will be internalized and processed.
Ovalbumin (OVA), for example, is a mannosylated soluble protein and by engaging the mannose receptors on the DC surface is internalized into EEA+Rab5+ endosomes (Gazi & Martinez-Pomares, 2009). While soluble antigens of 0.5 μm in diameter or smaller in size, such as OVA and other proteins, are internalized by endocytosis, particles larger than 0.5 μm in diameter (Aderem & Underhill, 1999), such as apoptotic cells, bacteria, or beads coated by a model antigen, are phagocytosed and will engage a different set of receptors in the process. Such receptors include Clec9a, a C-type lectin-like receptor which engages filamentous actin on the surface of dying cells, and are essential for the cross-presentation of dead and dying cells (Ahrens et al., 2012). The protocol below outlines instructions to stimulate DCs and to prepare and deliver cross-presentation cargoes to these cells for internalization via the endocytic or phagocytic pathways, as well instructions to prepare cargo that contains TLR ligands, namely LPS.
Materials
GM-CSF or Flt3L in vitro differentiated DCs (see Basic Protocol 2 and 3) or in vivo derived splenic DCs (see Basic Protocol 4)
LPS (MilliporeSigma, cat. no. L2880)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Cross-presentation cargo (see Support Protocols 3, 4, and 5 and Table 1)
Table 1.
Different Cross-Presentation Cargoes Given to Dendritic Cellsa
| Cross-presentation cargo | Concentration | Incubation time | Notes |
|---|---|---|---|
|
| |||
| Apoptotic cells | 2 cells:1 DC | 16–18 hr | Model infected apoptotic cells can be prepared; see Support Protocol 4 |
| Antigen-coated microbeads | 2 beads:1 DC | ≥5 hr | LPS used as PRR agonist; see Support Protocol 3 |
| Endocytic cargo, e.g., OVA | 100 ng per ml (a titration may be required for optimum concentration of each endocytic model antigen) | 3–6 hr | An agonist such as LPS can be added, depending on the experimental conditions |
| Bacteria | 100 bacteria: 1 DC for cytokine measurements or 25 bacteria: 1 DC for T cell proliferation and activation readouts |
1–12 hr | See Support Protocol 5 |
Abbreviations: DC, dendritic cell; LPS, lipopolysaccharides; PRR, pattern recognition receptor; OVA, ovalbumin.
16% paraformaldehyde (PFA; Thermo Fisher Scientific, cat. no. 28906)
Glycine (Thermo Fisher Scientific, cat no. BP381–5)
RPMI 1640 medium (Gibco brand, Thermo Fisher Scientific, cat. no. 11875093)
RPMI 1640-supplemented medium (see recipe)
U-shaped 96-well plate, polystyrene and tissue culture treated
24-well plate, polystyrene and tissue culture treated
Hemocytometer or a cell counter
15-ml polypropylene conical tube
P200 and P1000 pipets
Start with fully differentiated DC culture in 24-well plates, as prepared in BasicProtocol 2, 3, or 4.
Approximate the number of DCs in each well by counting DCs in a representativewell using a hemocytometer or a cell counter.
Calculate the number of DCs required for your experiments (see Table 1) and harvest the required amount of DCs, accordingly. To harvest DCs, begin with flushing the medium contained in the wells up and down several times using a P1000 pipet. Pipet carefully and do not let the pipetted contents touch the bottom of the pipet itself to avoid contamination. If this is not possible, use filtered pipet tips to avoid contamination. Ensure that around the edges of the wells are flushed, as this is where much of the DC clusters are formed. Then, collect RPMI 1640 medium-containing DCs in the wells and place into a 15-ml conical tube. Immediately add 1 ml ice-cold PBS to the well, flush, and collect the remainder of DCs.
Count cells suspended in medium/PBS using a hemocytometer or a cell counter.
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
Resuspend DCs at a concentration of 2 × 106 cells/ml in RPMI 1640-supplemted medium.
Add 50 μl DC suspension (i.e., 1 × 105 DCs) to each well of a 96-well plate.
-
Stimulate DCs with a cross-presentation cargo by addition of 50 μl of a model antigen listed in Table 1, reconstituted in RPMI 1640-supplemted medium, at the indicated concentrations (and for the time indicated). Add the antigen model or cargo slowly and gently to the center of the wells; try not to disturb DC clusters to avoid spontaneous maturation.The concentrations of antigens and incubation times can be adjusted for the purpose and objective of the experiment conducted. An appropriate PRR agonist, such as LPS (100 ng per ml), must be included in the stimulation, if the model antigen lacks the ability to activate the appropriate PRR, in order to activate the signaling cascades required for cross-presentation. Model antigens and cargoes must be reconstituted in the same medium used to culture the DCs, i.e., RPMI 1640-supplemented medium.
Make sure the appropriate controls for your experiments are included in the plate, i.e., addition of model antigens in the presence or absence of a PRR agonist such as LPS. Make sure the appropriate controls are included for each condition tested.
- Centrifuge the 96-well plate at 300 × g for 2 min at room temperature and incubate at 37°C in a humidified atmosphere containing 5% CO2 for the duration indicated in Table 1 or according to the objectives of your study.Generally, the larger the cargo, the more time is required for the DCs to internalize and process the cargo.
At the end of the desired incubation period, centrifuge the stimulated DCs in the 96-well plate at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully using a P200 pipet. Avoid accidentally pipetting and discarding DCs at the bottom of the wells.
Wash DCs twice by adding 200 μl ice-cold PBS to each well and centrifuge and discard supernatants, as before.
Fix DCs by adding 100 μl of 0.05% PFA in PBS to each well and incubate cells 5 min at room temperature. Use fresh or recently thawed PFA. Frozen PFA must be thoroughly thawed in a water bath and if a white precipitate forms after thawing, then incubate at 60°C briefly.
Wash once by adding 100 μl PBS to each well containing PFA, centrifuge at room temperature, and discard supernatant carefully, as before. Wash again in 200 μl PBS, centrifuge, and discard supernatant, as before.
Quench PFA fixation by adding 100 μl of 0.5 M glycine dissolved in serum-free RPMI 1640 medium to each well and incubate 5 min at room temperature. Centrifuge at room temperature and discard supernatant, as before.
Wash four times in PBS, centrifuge, and discard supernatant, as before.
- Add 200 μl RPMI 1640-supplemted medium to each well and incubate 20 min at 37°C in a humidified atmosphere containing 5% CO2. This is to allow the release of excess PFA from the DCs. Centrifuge and discard supernatant as before, followed by two more washes in PBS.It is absolutely crucial to wash away any residual PFA from the fixed DCs before adding and co-culturing CD8+ T cells (see Basic Protocol 8). This is because PFA is highly toxic to living cells, and as such, any residual PFA would kill the CD8+ T cells.For most downstream applications, the fixed DCs are now ready to use, such as coculturing with CD8+ T cells to assess the cross-priming abilities of DCs as indicated by proliferation of CD8+ T cells (see Basic Protocol 8).
SUPPORT PROTOCOL 3
PREPARATION OF MODEL ANTIGEN COATED MICROBEADS
Microbead-coated antigens are utilized to mimic the phagocytic internalization of microbes and insoluble antigens by DCs in cross-presentation. Streptavidin coated microbeads are normally utilized due to the extraordinarily high affinity of streptavidin tetramers for biotin, which makes the coupling of biotinylated model antigens to the microbeads extremely reliable, durable, and convenient. Streptavidin coated microbeads are readily available to purchase from vendors. The protocol below outlines instructions to prepare microbeads coated with model antigens to use in DC cross-presentation experiments. Green fluorescent protein (GFP)-OT is a biotinylated recombinant fusion protein which is comprised of GFP fused to OVA-derived SIINFEKL peptide. The SIINFEKL peptide is internalized, processed, and loaded onto MHC-I molecules in DCs, which is then specifically recognized by CD8+ OT-I transgenic T cells. This recombinant GFPOT protein can be custom ordered from GenScript.
Materials
ProMag® 3 Series Streptavidin Microspheres (Polysciences, cat. no. 86056)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Biotinylated model antigens, e.g.,
Biotinylated GFP-OT (Drutman & Trombetta, 2010; custom order by GenScript or other commercial vendors)
Biotinylated LPS (Invivogen, cat. no. tlrl-lpsbiot)
Heat-inactivated FCS (R&D Systems, cat. no. S11550H)
1.5-ml Eppendorf tube
- Wash microbeads (streptavidin magnetic microspheres) by resuspending the desired amount in PBS and centrifuging at 2,000 × g for 5 min at room temperature. Carefully aspirate and discard PBS.This step is necessary to remove any EDTA, surfactant, or other reagents used in the preparation or storing of the microbeads but may interfere or contaminate the coupling of biotinylated antigens to the microbeads and their use in cross-presentation experiments.
- Incubate microbeads in the presence of biotinylated GFP-OT for 30 min at room temperature in PBS, with constant shaking on a vortex.The GFP-OT protein should only occupy a quarter of the streptavidin molecules on the surface of the microbeads in order to allow sufficient LPS binding as well. According to calculations, guided by the details provided by the manufacturer and the molecular weight of GFP-OT protein, 62 ng of GFP-OT protein would effectively saturate a quarter of 1 × 106 microbeads.
- Wash conjugated microbeads four times in PBS containing 0.5% FCS by centrifugingat 2,000 × g for 5 min at room temperature.The FCS is important to allow proper pelleting of the beads with each centrifugation.
- Depending on your experimental design, incubate conjugated microbeads in100 μg/ml of biotinylated LPS for 30 min at room temperature with constant shaking on a vortex.It is highly recommended that the cross-presentation outcome of phagocytic cargo derived OVA be compared in the presence or absence of LPS stimulation. Therefore, microbeads sequentially conjugated with GFP-OT then LPS would serve as TLR ligand positive antigenic cargo and those conjugated with GFP-OT alone would serve as TLR ligand negative antigenic cargo.
- Wash microbeads four times in PBS containing 0.5% FCS, as before.The microbead-coated antigens can now be resuspended in the appropriate medium for immediate use or stored at 4°C overnight for use in DC cross-presentation experiments the next day, as described in detail in Basic Protocol 6.
SUPPORT PROTOCOL 4
PREPARATION OF APOPTOTIC CELLS
Similar to microbead-coated antigens, apoptotic cells are used as a source of cell-associated antigen that would be internalized by phagocytosis in vivo, and then processed and cross-presented by DCs. Typically, murine CD19+ B cells are used as a model apoptotic cell, which has been isolated from the lymphoid organs, specifically the spleen, of a transgenic mouse that endogenously expresses a model antigen of interest, such as OVA-expressing transgenic mice, which express the OVA antigen on the surfaces of all cells in the animal, including B cells. We have bred our OVA-transgenic mice onto a BALB/c background(H2d) to avoid potential presentation of OVA by B cells to OVA-specific H2brestricted CD8+ T cells. The CD19+ H2d B cells are isolated and subjected to apoptosis ex vivo to be used as a model of an uninfected apoptotic cell or alternatively, if blasting of B cells is required, then this is done by incubating the B cells with endotoxin free anti-mouse immunoglobulin (H+L) prior to induction of apoptosis (Blander & Medzhitov, 2006). Additionally, to prepare a model of infected apoptotic cells, the CD19+ B cells are cultured ex vivo and stimulated with LPS to induce blasting of the B cells prior to induction of apoptosis. Flow cytometric analysis is used to confirm the induction of apoptosis and absence of secondary necrosis. Apoptotic B cells are resuspended in RPMI to prepare them for addition to DC cultures. The protocol below outlines instructions to prepare such cells to use in DC cross-presentation experiments.
Materials
7- to 11-week-old ACT-mOVA mice, transgenic for a membrane-bound form of ovalbumin (The Jackson Laboratory, cat. no. 005145)
RPMI 1640-supplemented medium with 2% heat-inactivated FCS (see recipe)
RPMI 1640-supplemented medium (see recipe)
Endotoxin-free RBC lysis buffer (MilliporeSigma, cat. no. R7757)
MACS buffer (see recipe)
CD19 MicroBeads, mouse (Miltenyi Biotec, cat. no.130–121-301)
Recombinant Murine IL-4 (PeproTech, cat. no. 214–14)
Rabbit Anti-Mouse IgG (H+L)-UNLB (SouthernBiotech, cat. no. 6170–01)
LPS (MilliporeSigma, cat. no. L2880)
FITC Annexin-V Apoptosis detection kit with 7-AAD (BioLegend, cat. no. 640922)
Heat-inactivated FCS (R&D Systems, cat. no. S11550H)
Dissecting scissors
Forceps
70-μm nylon-mesh cell strainer
6-well plate
10-cc syringe
15- and 50-ml polypropylene conical tube
LS magnetic separation column (Miltenyi Biotec, cat. no. 130–042-401)
QuadroMACS™ Separator (Miltenyi Biotec, cat. no. 130–091-051)
MACS MultiStand (Miltenyi Biotec, cat. no. 130–042-303)
Hemocytometer or a cell counter
UV irradiator
15- and 20-cm polystyrene petri dish
T175 flask, polystyrene and tissue culture treated
Isolate and magnetically separate antigen expressing splenic CD19+ B cells
-
1
Four days prior to adding the apoptotic B cells to the DCs for cross-presentation experiments, sacrifice the mouse which expresses the required antigen and harvest its spleen. Follow your institute’s sacrificing guidelines and minimize animal suffering.
-
2Place mouse in supine position with its forelimbs and hindlimbs pinned onto a dissecting board and spray it with ethanol before placing it into a tissue culture hood.All remaining steps are to be carried out under sterile conditions.
-
3
Using a pair of forceps, gently lift the abdominal wall and use dissecting scissors to make a vertical cut from near the urethral orifice to the area just below the neck. Take care not to cut or puncture any organs.
-
4
Make two horizontal cuts on the left side of the mouse from top and bottom ends of the first cut to peel the abdominal wall back and to reveal the internal abdominal organs. Using forceps, gently harvest spleen.
-
5
Place spleen inside a 70-μm cell strainer that is sitting on a well of a 6-well plate containing RPMI 1640-supplemented medium. Mash spleen through the strainer using the plunger portion of a 10-cc syringe.
-
6
Mix splenocyte suspension by gently pipetting up and down using a Pasteur pipet, pipet through the cell strainer as well, and transfer cells to a 15-ml conical tube.
-
7
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
8
Resuspend cells in 1 ml RBC lysis buffer and incubate for exactly 1 min at room temperature.
-
9
Add 10 ml MACS buffer to neutralize RBC lysis buffer.
-
10
Centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
-
11
Resuspend cells in 176 μl MACS buffer and 24 μl CD19 microbeads. Incubate at 4°C for 25 min.
-
12
Sterilize the MACS separation apparatus (MACS™ Separator and MACS MultiStand) with ethanol prior to placing it into the biosafety cabinet. Carefully place a LS magnetic separation column into position in the MACS separation apparatus. Activate column by adding 3 ml MACS buffer to it and allow it to completely pass through the column. Discard eluate.
-
13
Add 800 μl MACS buffer to the cells and add suspension to the column. Wash the 15-ml conical tube with 2 ml MACS buffer and add it to the column to recover the majority of the CD19+ B cells. Allow the volume to pass through the column and discard eluate.
-
14
Wash column three times by adding 3 ml MACS buffer each time (9 ml total) and allowing it to pass through the column. Discard eluate.
-
15Remove column from the MACS separation apparatus and add 5 ml MACS buffer to the column. Use the column plunger to swiftly expel and elute MACS buffer from the column into a clean 15-ml conical tube.Contained in this fraction are the CD19+ OVA expressing B cells.
-
16
Count the CD19+ B cells and centrifuge at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
-
17
The CD19+ B cells can now be subjected to apoptosis ex vivo to be used as a model of uninfected apoptotic cells by following the steps in the next section of this protocol, starting from step 20. However, continue with the next steps if blasting of B cells is required to prepare a model of either uninfected or infected apoptotic B cells.
-
18
Resuspend cells at a concentration of 3 × 106 cells per ml in RPMI 1640-supplemented medium and plate 3 ml per well in a 6-well plate.
-
19
Induce blasting in the CD19+ B cells by stimulating the cells with either 100 μg/ml anti-mouse IgG (H+L) (and 50 ng/ml of recombinant murine IL-4) or add 25 μg/ml LPS (dissolved in PBS) to each well; IL-4 can be added to aid B cell viability. Incubate cells at 37°C in a humidified atmosphere containing 5% CO2 for 3 days.
Induce and assess apoptosis in antigen expressing B cells
-
20
The night before the final day of culturing the CD19+ B cells, add 10 ml FCS to a 20-cm petri dish and incubate at 37°C in a humidified atmosphere containing 5% CO2 overnight.
-
21
Harvest CD19+ B cells from the 6-well plate. Count cells and resuspend in PBS at 1 × 106 cells per ml.
-
22
Remove FCS in the petri dish and add up to 30 × 106 cells to the FCS-coated 15-cm petri dish. Induce cells to undergo apoptosis by UV irradiation at 2.5 mJ/cm2.
-
23
Pipet cells up and down several times in the petri dish to dislodge any cells that may have adhered to the dish and transfer cells to a 50-ml conical tube. Count the cells.
-
24
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
25
Resuspend cells in RPMI 1640-supplemented medium (containing serum). Incubate for different times (4, 6, 10, and 18 hr).
-
26At each time point, take an aliquot of 1 × 106 of the cells to assess for apoptosis by flow cytometry.A time course must be conducted to determine the earliest time point at which annexin-V positive cells appear.
-
27
Wash that portion of cells twice with 10 ml ice-cold PBS, then resuspend in 1× annexin-V binding buffer (contained in the kit) at a concentration of 1 × 106 cells per ml. Transfer 100 μl (i.e., 1 × 105) of cell suspension to a new 15-ml conical tube for use as an unstained control.
-
28
Add 5 μl FITC annexin-V dye and 5 μl 7-AAD dye (contained in the kit) to the remainder of the cells to stain for apoptosis and secondary necrosis. Gently vortex cell suspension and incubate 15 min at room temperature in the dark.
-
29Analyze cells by flow cytometry immediately or within an hour; annexin-V binding buffer must be used to flush the flow cytometer prior to sample analysis.Annexin-V positive but 7-AAD negative cells indicate the correct induction of apoptosis, because expression of 7-AAD is indicative of secondary necrosis, a more inflammatory form of cell death which could alter the results of downstream cross-presentation experiments.The apoptotic CD19+ B cells can now be resuspended in the appropriate medium (i.e., RPMI 1640-supplemented medium) for immediate use in DC cross-presentation experiments, as described in detail in Basic Protocol 6.It is recommended that the apoptotic CD19+ B cells be added as soon as a percentage of them begins to label with annexin V. This is because the apoptosis will continue in culture after the CD19+ B cells are added to the DCs in culture. This is best practice because the DCs will internalize the apoptotic CD19+ B cells as soon as the phosphatidylserine (target of the annexin V protein) is exposed on the outer leaflet of the plasma membrane. Waiting too long to increase the percentages of apoptotic cells comes at the cost of increasing the percentages of cells undergoing secondary necrosis which is inflammatory to the DCs and should be avoided when one is studying the non-inflammatory phagocytosis of apoptotic cells.
SUPPORT PROTOCOL 5
PREPARATION OF RECOMBINANT BACTERIA
Bacteria are usually internalized by DCs through phagocytosis and the cargo processed for cross-presentation in these cells. This process involves DCs utilizing and engaging different surface receptors to capture and phagocytose bacteria. Subsequent processing of the microbe and the generation of peptides, as well as loading onto MHC-I molecules, may engage signaling cascades differing from those of endocytic cargoes or the phagocytosis of apoptotic cells. The protocol below outlines instructions for the preparation of recombinant bacteria for use in DC cross-presentation experiments.
Materials
Log-phase glycerol stock of recombinant OVA-expressing Escherichia coli (see Blander & Medzhitov, 2006)
LB Broth Base (Invitrogen brand, Thermo Fisher Scientific, cat. no. 12780052)
Ampicillin (MilliporeSigma, cat. no. A5354)
IPTG (MilliporeSigma, cat. no. 367–93-1)
Glycerol
RPMI 1640-supplemented medium (see recipe)
Spectrophotometer capable of reading absorbance at 600 nm
50-ml polypropylene conical tube
2-ml cryovials
Inoculate 1 ml of the log-phase of recombinant E. coli, which expresses your model antigen of interest, to 50 ml LB broth in an Erlenmeyer flask and supplement with 100 μg/ml ampicillin or other appropriate selective antibiotic. To prepare a log-phase E. coli working stock, grow a flask of E. coli to an OD600 reading of ∼1, then aliquot into 1 ml cryovials containing 25% glycerol (diluted in distilled H2O) and store at −80°C.
- Grow bacteria in a shaker at 37°C to an OD600 reading of ∼0.6 to ensure they are in log-phase.The amount of time to reach 0.6 will vary depending on the batch and strain of bacteria, as well incubation temperature, and this will need to be determined in a separate experiment.
- Induce expression of the model antigen of interest by incubating bacteria with fresh2 mM IPTG and incubate in a shaker at 37°C for 5 to 6 hr.It is important to always use freshly prepared/freshly thawed IPTG for induction of expression of the model antigen of interest.
- Heat-inactivate bacteria in 60°C water for 45 min. To do this, dilute the induced culture to an OD600 reading of 0.6 in a 50-ml conical tube. Screw the tube cap very tightly, wrap it with Parafilm, then submerge and incubate the conical tube in a 60°C water bath for 45 min.The dilution to an OD600 reading of 0.6 is to ensure that the heat inactivation happens in the amount of time the bacteria are incubated at 60°C.
Store bacteria for up to 18 hr at 4°C prior to being added to DCs or proceed immediately.
Count the number of heat-inactivated bacteria in the 50-ml conical tube with a spectrophotometer. Centrifuge bacteria at 3,000 × g for 5 min. Remove and discard supernatant carefully.
Wash heat-inactivated bacteria once in RPMI 1640-supplemented medium to get rid of any remaining salts from the LB broth. Centrifuge and discard supernatant as before.
Resuspend heat-inactivated bacteria in RPMI 1640-supplemented medium to the required concentration for immediate use in DC cross-presentation experiments, as described in detail in Basic Protocol 6.
BASIC PROTOCOL 7
IMMUNOCYTOCHEMISTRY IMMUNOFLUORESCENCE (ICC/IF)
Immunofluorescence microscopy, specifically confocal microscopy, is a powerful tool that has been utilized to gain an insight into the complex mechanisms which orchestrate cross-presentation in DCs. The protocol below has been optimized to be used to probe, visualize, and unravel the signaling molecules, including proteins, lipids, structures, and organelles in DCs that are responsible for the capturing, internalization, and processing of endocytic and phagocytic cargoes, organelle fusion, the loading of peptides on MHC-I molecules, and cross-presenting them on the cell surface.
Materials
GM-CSF or Flt3L in vitro differentiated DCs (see Basic Protocol 2 and 3) or in vivo derived splenic DCs (see Basic Protocol 4)
RPMI 1640 medium (Gibco brand, Thermo Fisher Scientific, cat. no. 11875093)
RPMI 1640-supplemented medium (see recipe)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Heat-inactivated FCS (R&D Systems, cat. no. S11550H)
16% PFA (Thermo Fisher Scientific, cat. no. 28906)
Glycine (Thermo Fisher Scientific, cat no. BP381–5)
BSA (MilliporeSigma, cat. no. 9048–46-8)
Saponin (MilliporeSigma, cat. no. S4521)
VectaShield Hardset mounting medium (Vector Laboratories, cat. no H-1400)
Alcian blue-coated round 12-mm glass coverslips (see Support Protocol 6)
24-well plate, polystyrene and tissue culture treated
Hemocytometer or a cell counter
45° curved tapered fine point forceps
P1000 pipet
Place sterile Alcian blue-treated 12-mm glass coverslips into the wells of a sterile24-well plate using a set of curved fine point forceps.
- Collect fully differentiated DCs (as prepared in Basic Protocol 2, 3, or 4). Harvest DCs in the 24-well plate by flushing medium contained in the well up and down several times using a P1000 pipet. Pipet carefully and do not let the pipetted contents touch the bottom of the pipet itself to avoid contamination. If this is not possible, use filtered pipet tips to avoid contamination. Ensure that around the edges of the wells are flushed, as this is where much of the DC clusters are formed. Then collect medium in the wells and place into a 15-ml conical tube. Immediately add 1 ml ice-cold PBS to the well, flush, and collect the remainder of DCs.If DCs were isolated as in Basic Protocol 4, skip this step and proceed to counting the cells, as described in step 3 below.
Count cells suspended in medium/PBS using a hemocytometer or a cell counter.
Plate 2 × 105 DCs per well in 1 ml RPMI 1640-supplemented medium into wells of a 24-well plate containing Alcian blue-coated coverslips.
Centrifuge cells at 300 × g for 2 min at room temperature to sediment the cells. Avoid excessive handling or shaking of the cells to prevent spontaneous DC maturation.
- Incubate cells for 30 to 60 min at 37°C in a humidified atmosphere containing 5%CO2.You should see that the cells are attached to the bottom of the plate and stretching out their dendrites, when viewed under a light microscope.
Stimulate DCs with the desired cross-presentation cargo and for the desired time, asdescribed in Basic Protocol 6.
Aspirate medium from the wells and wash coverslips once by adding 1 ml cold PBS to each well, then aspirate and discard PBS. Fix cells by adding 1 ml 1% PFA to each well and incubate 20 min at room temperature.
Carefully aspirate PFA and wash once with 2 ml PBS containing 2% BSA.
Quench PFA fixation by adding 1 ml serum-free RPMI 1640 medium containing 0.5 M glycine to each well and incubate 20 min at room temperature.
- Aspirate PFA/medium from each well and wash once with 2 ml PBS containing 2%BSA.The fixed DCs can now be temporarily stored in PBS at 4°C or processed immediately.
Permeabilize and block DCs by adding 1 ml RPMI 1640 medium containing 0.2%saponin and 10% FCS to each well. Incubate 30 min at room temperature. Aspirate and discard medium.
Dilute primary antibodies in RPMI 1640 medium containing 0.2% saponin and 10%FCS and incubate 1 hr at room temperature in the dark. Aspirate and discard medium containing the primary antibody. Minimize light exposure from this point forward.
Wash coverslips four times with 2 ml RPMI 1640 medium containing 0.2% saponinand 10% FCS. Leave coverslips for 1 min in each wash. Aspirate and discard wash buffer.
Dilute secondary antibody in RPMI 1640 medium containing 0.2% saponin and 10%FCS and incubate 30 min at room temperature in the dark. Aspirate and discard medium containing the secondary antibody.
Wash coverslips four times with 2 ml PBS. Leave coverslips for 1 min in each wash.Aspirate and discard wash buffer.
- Mount coverslips face down onto glass microscope slides using VectaShield or othersuitable mounting medium containing an appropriate nuclear dye, e.g., DAPI or Hoechst. Leave to air dry for 1 hr at room temperature or overnight at 4°C in the dark.The mounted coverslips can now be imaged immediately or stored at 4°C in the dark for later use.
- View slides using a confocal microscope with sections spanning 0.4 to 0.9 μm on the z axis. Representative data is shown in Figure 4.Labeling plasma membrane bound proteins or lipids, such as surface receptors or to compartmentalize the plasma membrane, must take place prior to the permeabilization step.The primary and secondary antibody concentrations and incubation times may need to be optimized empirically for best results or may need to be used according to the manufacturers’guidelines
Figure 4.
Confocal image of GM-DCs showing the recruitment of MHC I to phagosomes carrying LPS-conjugated beads. DCs were cultured and given LPS-coated microbeads, as described in Basic Protocol 6. The DCs were then prepared as described in Basic Protocol 7 and labeled with antibodies against: lysosomal-associated membrane protein (LAMP)-1 to probe the phagocytosed microbeads; and MHC I (labeled with H2-Kb antibody) to probe the recruitment and colocalization of MHC-I molecules to the phagosome. Images are of a single z-stack plane acquired using a confocal microscope with a 63×/1.4 NA oil immersion objective. Scale bars represent 10 μm. Image adapted from previously published data by the Blander Lab (Nair-Gupta et al., 2014). LPS, lipopolysaccharides; DCs, dendritic cells.
SUPPORT PROTOCOL 6
PREPARATION OF ALCIAN BLUE-COATED COVERSLIPS
Alcian blue is a polyvalent basic dye that is used to treat glass coverslips to form positively charged surfaces. This positively charged surface will allow the negatively charged plasma membrane of DCs to firmly adhere to the glass coverslip. This strong interaction is aided by the binding of multiple localized charges and Van der Waals forces (Fadeel & Xue, 2009). Precationization of glass coverslips is essential to ensure the DCs remain adhered to the glass coverslips while subjected to the multiple wash and incubation steps during the sample preparation.
Materials
Alcian blue 8 GX dye (MilliporeSigma, cat. no. A9186)
Distilled water
12-mm round glass coverslips, thickness 0.13–0.17 mm (Carolina, cat. no. 633029)
500-ml 0.22-μm filter bottle, cellulose acetate membrane
Microwave
Glass jar
45° curved tapered fine point forceps
Prepare 1% Alcian blue 8 GX dye in distilled water and filter using a 0.22-μm filterbottle. Store at 4°C.
Place coverslips in the Alcian blue solution in a glass jar. Ensure adequate coverage.
Heat by microwaving at High setting for 1 to 2 min. Take care not to boil the Alcianblue solution.
Let coverslips sit in the hot solution for 10 min. Ensure coverslips are well dispersed by occasionally swirling the glass jar every 2 to 3 min.
Wash coverslips with deionized distilled water to remove excess Alcian blue.
- Dry coverslips by placing them individually on paper towels, using a pair of forceps. Make sure water droplets do not dry on the coverslips.This is best avoided by not having a large droplet on the coverslip but rather a thin film that should dry quickly.
- Sterilize coverslips by autoclaving and store under sterile conditions for later use.Alcianblueisastronglycoloreddye.Handlewithcaretoavoidstainingofskinandclothes.
BASIC PROTOCOL 8
CD8+ T CELL ACTIVATION TO ASSESS CROSS-PRESENTATION
The in vitro co-culture system of transgenic CD8 T cells with stimulated and activated DCs is a robust simulation of the physiological interactions between those two cells in vivo. Cross-priming of antigen-specific CD8+ T cells by activated DCs causes stimulation, activation, and proliferation of the former, which in turn indicates successful cross-presentation of antigens in the latter. Therefore, assessing and monitoring the activation and proliferation of CD8+ T cells allows for several experimental opportunities to asses cross-presentation.
For instance, activated CD8+ T cells upregulate the expression of CD69 within several hours post stimulation that peaks at ∼24 hr, which makes it ideal to be detected by flow cytometric analysis and used as an early activation marker of CD8+ T cells (Cibrián & Sánchez-Madrid, 2017; Simms & Ellis, 1996). Another flow cytometric method that is readily utilized to assess activated CD8+ T cell proliferation is the dilution of carboxyfluorescein diacetate succinimidyl ester (CFSE). This method is a common, powerful, and sensitive technique to measure the cross-presentation capacity of APCs, including DCs. For instance, this detection technique is >104-fold more sensitive than detection by a conformation dependent antibody such as the 25D1.16 antibody, which is one of the few available antibodies specific to form peptide:MHC-I complexes (Nair-Gupta et al., 2014). T cells internalize the CFSE dye by diffusion, which is then cleaved by cellular esterases and subsequently reacts with amine and lysine resides in the cytosol of the cells. In order to induce activation and proliferation, a CFSE-labeled TCR-transgenic T cell only needs to encounter a very small number of cognate peptide:MHC-I complexes and the subsequent cell division will result in two daughter cells where each carries half of the CFSE dye molecules as the parent cell. Further successive cell divisions will result in further halving of the stain in daughter cells, thus establishing an inverse correlation between the number of cell divisions and the level of detected CFSE dye. This change in fluorescence can be readily measured using a flow cytometer and the resultant data provides a robust way to assay and evaluate the differences in cross-presentation under various conditions, including cross-priming abilities of APCs, the effect of antigens used, incubation time, among other factors and variables being studied (Quah & Parish, 2010).
The co-culture medium represents yet another opportunity to assess the activation and proliferation of the CD8+ T cells. The co-culture medium can be used in ELISA tests for several secreted cytokines indicative of T cell activation and proliferation. Upon activation, CD8+ T cells express cytokines for expression on the cell surface or to secrete into their environment either to directly engage in immune defense, as effector cytokines, or aid in their own proliferation (Charles, Janeway, Travers, Walport, & Shlomchik, 2001; Cox, Kahan, & Zajac, 2013). Three such cytokines, which are frequently used to assay for CD8+ T cell activation in vitro, are interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and IL-2. IFN-γ works by directly interfering and inhibiting viral replication and replication of other pathogens, while at the same time causing infected cells to increase the production of the necessary molecules, including MHC-I molecules, to present antigens on their surface (Charles et al., 2001; Wong & Pamer, 2003). TNF-α is a powerful pro-inflammatory cytokine and can synergize with IFN-γ to activate innate immune cells to kill infected or cancerous cells (Ye, Wei, Zhang, Niu, & Zhou, 2018). TNF-α is secreted within hours of CD8+ T cell stimulation (Brehm, Daniels, & Welsh, 2005). IL-2 is a vital cytokine for the proliferation and development of many immune cells, including CD8+ T cells (Boyman & Sprent, 2012; Zhang & Bevan, 2011).
CD8+ T cells bearing a TCR which corresponds to the model antigen, whose cross-presentation by DCs is being monitored, are first harvested from the lymphoid organs, specifically the spleen, of TCR-transgenic mice, such as the OT-I mouse specific for OVA antigen. The cells are then labeled with the CFSE dye and co-cultured with fixed DCs, which had been previously stimulated with model antigen or unstimulated for control experiments at a ratio of two CD8+ T cells for every DC. CD8+ T cells require costimulation to remain viable, which is provided on the surface of DCs that have been stimulated with a PRR ligand such as LPS. However, CD8+ T cells which are co-cultured with unstimulated DCs or DCs deficient for TLR or TLR signaling molecules require an exogenous source of CD28 costimulatory signals, such as anti-CD28 antibodies. The resulting CFSE-labeled CD8+ T cells are analyzed with a flow cytometer. The protocols below outline instructions to prepare and assay such cells to use in DC cross-presentation experiments.
Materials
Fixed DCs, both antigen treated and untreated (see Basic Protocol 6)
RPMI 1640-supplemented medium (see recipe)
CFSE-labeled TCR-transgenic CD8+ T cells (see Support Protocol 7)
Ultra-LEAF™ Purified anti-mouse CD3 Antibody (BioLegend, cat. no. 100238)
Ultra-LEAF™ Purified anti-mouse CD28 Antibody (BioLegend, cat. no.102115)
ELISA kits for detecting murine IFN-γ, TNF-α, and IL-2 cytokines (e.g., R&D Systems)
FACS buffer (see recipe)
Alexa Fluor® 647 anti-mouse CD8β Antibody (clone YTS156.7.7; Biolegend, cat. no. 126612)
Purified anti-mouse CD69 antibody (clone H1.2F3; Biolegend, cat. no. 104502)
Hemocytometer or cell counter
U-shaped 96-well plate, polystyrene and tissue culture treated
15-ml polypropylene conical tube
1.5-ml Eppendorf tube
P200 pipet
- Prepare fixed DCs stimulated with the desired cross-presentation antigens or cargoes as described in detail in Basic Protocol 6 in three separate 96-well plates, containing identically treated DCs, with each plate designated for the following experiments:
-
Adetecting CD8+ T cell activation cytokine markers IFN-γ, TNF-α, and IL-2 using ELISA tests;
-
Bdetecting expression of the early activation marker CD69 on the surface of CD8+ T cells by flow cytometric analysis; and
-
Cassessing the proliferation of CFSE-labeled CD8+ T cells (see Support Protocol 7) by flow cytometric analysis.
-
A
Label each 96-well plate as “Experiment A, B, or C”. Make sure the appropriate crosspresentation controls are included.
-
4Resuspend freshly isolated and CFSE-labeled or unlabeled CD8+ T cells, as described in Support Protocol 7, in RPMI 1640-supplemented medium, at a concentration of 1 × 106 cells per ml.Make sure the correct transgenic CD8+ T cells are isolated, which express the TCR specific for the model antigen used for cross-presentation by the DCs.
-
5
Add 200 μl (i.e., 2 × 105 CD8+ T cells) of unlabeled CD8+ T cells into the wells designated for experiments A and B, and CFSE-labeled CD8+ T cells into the wells designated for experiment C into the correct 96-well plates that already contain the fixed DCs. Make sure a positive control for CD8+ T cell proliferation is included in each plate by adding 2 μg/ml anti-mouse CD3 and 0.5 μg/ml anti-mouse CD28 antibodies (dissolved in RPMI 1640-supplemented medium) to CD8+ T cells that are co-cultured with unstimulated fixed DCs, i.e., DCs that were not stimulated with antigens or cargoes, or exposed to PRR engaging agents.
-
6Add 0.5 μg/ml anti-mouse CD28 antibodies to the wells which contain unstimulated control DCs, DCs deficient for TLR or TLR signaling adaptors, or DCs that were not stimulated in the presence of a PRR engaging agent, such as LPS, bacteria, or apoptotic cells.Without PRR stimulation, the DCs will not express co-stimulatory ligands on their own, thus no cross-priming will occur even if the peptide:MHC-I complex is expressed on the DC surface.
-
7Incubate cells at 37°C in a humidified atmosphere containing 5% CO2.In positive control wells, within 24 to 48 hr dark clusters of cells should be visible in the center of the wells to indicate that CD8+ T cells have begun proliferating.
-
8
At 20 hr post stimulation, collect the 96-well plate labeled “Experiment A,” whichhad been designated for use in ELISA tests, carefully and slowly remove supernatants with a P200 pipet, and transfer to labeled 1.5-ml Eppendorf tubes for ELISA tests. Do not disturb DCs at the bottom of the wells.
-
9Centrifuge supernatants at 300 × g for 10 min at 4°C to pellet cellular debris. Carefully aspirate supernatants for immediate use in ELISA tests or store at −80°C for later use. Measure the secretion of IFN-γ, TNF-α, and IL-2 cytokines as activation markers for CD8+ T cells.Follow the manufacturers’guidelines for commercially available ELISA kits.
-
10
At 24 hr post stimulation, collect the 96-well plate labeled “Experiment B” to usefor flow cytometric analysis to evaluate the expression of CD69 molecules on the surface of stimulated CD8+ T cells as an early activation marker of these cells.
-
11
Centrifuge cells at 300 × g for 5 min at 4°C and discard supernatants carefully using a P200 pipet. Avoid accidentally pipetting and discarding cells at the bottom of the wells.
-
12Wash cells twice by adding 200 μl cold FACS buffer to each well of the 96-well plate and centrifuge as before (step 9). While washing cells, prepare a staining cocktail by dilu ting mouse anti-CD8β and anti-CD69 antibodies in FACS buffer.The concentration of both of the mouse antibodies must be empirically calculated, guided by the manufacturer’s instructions.
-
13Resuspend cells in each well in 50 μl staining cocktail then incubate 15 min at 4°C in the dark.Remember to exclude some wells for use as negative controls for unstained and single stained cells for gating.
-
14
Centrifuge cells at 300 × g for 5 min at 4°C and wash twice in FACS buffer as before. Resuspend cells in 200 μl FACS buffer and analyze with a flow cytometer using the anti-CD8β antibody to gate for CD8+ T cells and the anti-CD69 antibody to gate for activated CD8+ T cells.
-
15
At 96 hr post stimulation, collect the final 96-well plate labeled “Experiment C” touse for flow cytometric analysis to evaluate the dilution of the CFSE dye as a marker of proliferation of the cross-primed or stimulated CD8+ T cells.
-
16Prepare samples for flow cytometric analysis by following steps 9 to 12 above with the following adjustments: omit the mouse anti-CD69 antibody from the staining cocktail prepared in step 10, use the CFSE dye to gate for CFSE-labeled CD8+ T cells, and plot the data as a histogram.On the flow cytometer, set the voltage such that the CFSE signal is positioned on the far right side of the histogram flow plot. This is important, as it will allow visualization of the separate peaks that correspond with each division of the CFSE-labeled CD8+ T cells. Sample data is shown below in Figure 5.As a cost-effective measure, ELISA kits can be substituted for individually purchased ELISA components (such as the appropriate capture and detection antibodies, recombinant proteins used as standards, substrates, stop solution, blocking buffer) to assay cytokine concentrations and following protocols published previously by the Blander Lab (Nair-Gupta et al., 2014).
Figure 5.
The representative results of a CFSE dilution assay to measure the proliferation of OT-I T cells in response to cross-presented antigen. CD8+ T cells were co-cultured with DCs that had been given (A) soluble OVA protein as a model of endocytic antigen or (B) E. coli or recombinant OVA-expressing E. coli (E. coli-OVA). DCs were incubated for a period of 4.5 hr then then fixed. (A) Panels from left to right: OT-I T cells alone; OT-I T cells co-cultured with DCs in absence of a cross-presentation cargo; OT-I T cells co-cultured with DCs that had been given the endocytic cargo OVA to cross-present but in absence of LPS; OT-I T cells co-cultured with DCs that had been given the endocytic cargo OVA to cross-present in presence of LPS. (B) The histogram plots show two different ways of depicting the CFSE dilution data, either as each histogram separately (left) or overlaid (right panel). All wells in A also contained anti-CD28 stimulating antibodies. As expected, TLR stimulation of DCs augments the cross-presentation of OVA but can only occur when the DCs are stimulated with LPS, thus allowing the DCs to cross-prime and activate the OT-I T cells causing them to proliferate, as seen by the dilution of the CFSE dye in panel 4 of A. E. coli naturally contain LPS in their cell walls and stimulate robust costimulatory expression by DCs, thus no exogenous provision of anti-CD28 antibodies is necessary. OVA, ovalbumin; CFSE, carboxyfluorescein diacetate succinimidyl ester; LPS, lipopolysaccharides; DCs, dendritic cells; TLR, Toll-like receptor.
SUPPORT PROTOCOL 7
ISOLATION AND LABELING OF CD8+ T CELLS WITH CFSE
Mouse transgenic CD8+ T cells are isolated from the spleen and the lymph nodes of 7- to 11-week-old mice. Upon recognition of cognate ligands by TCRs, presented on MHC-I molecules on DCs, these cells proliferate and multiply. CD8+ T cells are labeled with CFSE dye to assess proliferation by flow cytometric analysis. See the introduction section of Basic Protocol 8.
Materials
7- to 11-week-old TCR-transgenic mice:
OT-I mouse (Charles River Laboratories, C57BL/6-Tg (TcraTcrb) 1100Mjb/Crl)
CL4 mouse (The Jackson Laboratory, cat. no. 005308)
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
Endotoxin-free RBC lysis buffer (MilliporeSigma, cat. no. R7757)
Heat-inactivated FCS (R&D Systems, cat. no. S11550H)
MACS buffer (see recipe)
Mouse CD8α (Ly-2) MicroBeads (Miltenyi Biotec, cat. no. 130–117-044)
RPMI 1640-supplemented medium (see recipe)
CellTrace™ CFSE Cell Proliferation Kit (Invitrogen brand, Thermo Fisher Scientific, cat. no. C34554)
Dissecting scissors
Forceps
70-μm nylon-mesh cell strainer
6-well plate
10-cc syringe
15-ml polypropylene conical tube
LS magnetic separation column (Miltenyi Biotec, cat. no. 130–042-401)
QuadroMACS™ Separator (Miltenyi Biotec, cat. no. 130–091-051)
MACS MultiStand (Miltenyi Biotec, cat. no. 130–042-303)
Hemocytometer or a cell counter
Isolate transgenic CD8+ T cells specific to a model antigen
-
1
Obtain a 7- to 11-week-old transgenic mouse with the correct TCR specific for the model antigen of interest that is cross-presented by the DCs.
-
2
Sacrifice mouse and harvest spleen, brachial, and/or axillary lymph nodes. Follow your institute’s sacrificing guidelines to minimize animal suffering.
-
3Place mouse in supine position with its forelimbs and hindlimbs pinned onto a dissecting board and spray it with ethanol before placing it into a tissue culture hood.All remaining steps are to be carried out under sterile conditions.
-
4
Using a pair of forceps, gently lift the abdominal wall and use dissecting scissors to make a vertical cut from near the urethral orifice to the area just below the neck. Take care not to cut or puncture any organs.
-
5
Make two horizontal cuts on the left side of the mouse from top and bottom ends of the first cut to peel the abdominal wall back to reveal the internal abdominal organs. Using a pair of forceps, gently harvest the spleen.
-
6
Place spleen inside a 70-μm cell strainer that is sitting on a well of a 6-well plate containing RPMI 1640-supplemented medium. Mash spleen through the strainer using the plunger portion of a 10-cc syringe.
-
7
Mix the splenocyte suspension by gently pipetting up and down using a Pasteur pipet, pipet through the cell strainer as well, and transfer cells to a 15-ml conical tube.
-
8
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
9
Resuspend cells in 1 ml RBC lysis buffer and incubate for exactly 1 min at room temperature.
-
10
Add 10 ml MACS buffer to neutralize RBC lysis buffer.
-
11
Count and centrifuge cells at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
-
12
Resuspend cells in 90 μl MACS buffer and 10 μl mouse CD8α microbeads per 1 × 107 cells. Incubate at 4°C for 25 min.
-
13
Sterilize MACS separation apparatus (MACS™ Separator and MACS MultiStand) with ethanol prior to placing it into the biosafety cabinet. Carefully place a LS magnetic separation column into position in the MACS separation apparatus. Activate column by adding 3 ml MACS buffer to it and allow it to completely pass through the column. Discard eluate.
-
14
Add 800 μl MACS buffer to the cells and add it to the column. Wash the 15-ml conical tube with 2 ml MACS buffer and add it to the column to recover the majority of the CD8+ T cells. Allow the volume to pass through the column and discard eluate.
-
15
Wash column three times by adding 3 ml MACS buffer and allowing it to passthrough the column. Discard eluate.
-
16Remove column from the MACS separation apparatus and add 5 ml MACS bufferto the column. Use the column plunger to swiftly expel and elute MACS buffer from the column into a clean 15-ml conical tube.Contained in this fraction are the CD8+ T cells.
-
17
Count CD8+ T cells and centrifuge at 300 × g for 5 min at 4°C. Remove and discard supernatant carefully.
-
18
Wash CD8+ T cells with 10 ml PBS and centrifuge as before.
-
19
Proceed to CFSE labeling in PBS, if the cells are to be used in a cross-presentationexperiment where the readout is CFSE dilution. Otherwise, resuspend CD8+ T cells at a concentration of 1 × 106 cells per ml in RPMI 1640-supplemented medium (and continue with the protocol in Basic Protocol 8, step 2).
Label CD8+ T cells with CFSE dye
-
20
Resuspend CD8+ T cell fraction at a concentration of 2 × 107 cells per ml in PBS (without serum) in a 15-ml conical tube.
-
21
In a separate tube, dilute CFSE dye which has been resuspended in DMSO in PBS(without serum) to a concentration of 10 μM.
-
22
Add an equal volume of CFSE diluted in PBS to the CD8+ T cell suspension to create a final concentration of 5 μM of CFSE dye in 1 × 107 cells per ml. Incubate mixture for 8 to 10 min at 37°C in the dark. Lightly agitate tube manually every 2 min.
-
23Add 10 ml PBS containing 2% FCS (or at least five times the volume of CFSE/CD8+ T cell mixture from the previous step).This step is important to remove any free dye remaining in the solution.
-
24
Centrifuge cells at 300 × g for 5 min at room temperature. Remove and discard supernatant carefully.
-
25
Count and wash CFSE-labeled CD8+ T cells once more in 10 ml PBS containing 2% FCS and centrifuge as before (step 24).
-
26Resuspend CFSE-labeled CD8+ T cells at a concentration of 1 × 106 cells per ml in RPMI 1640-supplemented medium and proceed with Basic Protocol 8 to co-culture cells with DCs for cross-priming and cross-presentation experiments.Cervical, mesenteric, and inguinal lymph nodes should not be collected, as these often contain a high proportion of activated T cells which could alter the results of the assay.The CFSE dye is toxic to cells therefore labeling CD8+ T cells with the CFSE dye may result in losing some cells as a result.
REAGENTS AND SOLUTIONS
DMEM-supplemented medium
DMEM (Gibco brand, Thermo Fisher Scientific, cat. no.11965–092)
5% heat-inactivated FCS (R&D Systems, cat. no. S11550H)
2 mM L-glutamine (Gibco brand, Thermo Fisher Scientific, cat. no. 25030081)
100 Units/ml penicillin-streptomycin (Gibco brand, Thermo Fisher Scientific, cat. no. 15140122)
1 mM sodium pyruvate (Gibco brand, Thermo Fisher Scientific, cat. no. 11360070)
10 mM HEPES buffer (Gibco brand, Thermo Fisher Scientific, cat. no. 15630080)
1× non-essential amino acid solution (Gibco brand, Thermo Fisher Scientific, cat. no. 11140050)
0.055 mM 2-mercaptoethanol (Gibco brand, Thermo Fisher Scientific, cat. no. 21985023)
Store at 4°C for up to 3 months in sterile conditions.
FACS buffer
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
5% heat-inactivated FCS (R&D Systems, cat. no. S11550H)
0.1% sodium azide (NaN3; MilliporeSigma, cat. No. S2002)
Store at 4°C for up to 3 months in sterile conditions.
IMDM-supplemented medium
IMDM (Gibco brand, Thermo Fisher Scientific, cat. no. 12440053)
5% heat-inactivated FCS (R&D Systems, cat. no. S11550H)
2 mM L-glutamine (Gibco brand, Thermo Fisher Scientific, cat. no. 25030081)
100 Units/ml penicillin-streptomycin (Gibco brand, Thermo Fisher Scientific, cat. no. 15140122)
1 mM sodium pyruvate (Gibco brand, Thermo Fisher Scientific, cat. no. 11360070)
10 mM HEPES buffer (Gibco brand, Thermo Fisher Scientific, cat. no. 15630080)
1× non-essential amino acid solution (Gibco brand, Thermo Fisher Scientific, cat. no. 11140050)
0.055 mM 2-mercaptoethanol (Gibco brand, Thermo Fisher Scientific, cat. no. 21985023)
Store at 4°C for up to 3 months in sterile conditions.
MACS buffer
PBS (Gibco brand, Thermo Fisher Scientific, cat. no. 14190144)
2% heat-inactivated FCS (R&D Systems, cat. no. S11550H)
2 mM EDTA (Thermo Fisher Scientific, cat. no. 15575020)
Store at 4°C for up to 3 months in sterile conditions.
RPMI 1640-supplemented medium
RPMI 1640 medium (Gibco brand, Thermo Fisher Scientific, cat. no. 11875093)
5% heat-inactivated FCS (R&D Systems, cat. no. S11550H)
2 mM L-glutamine (Gibco brand, Thermo Fisher Scientific, cat. no. 25030081)
100 Units/ml penicillin-streptomycin (Gibco brand, Thermo Fisher Scientific, cat. no. 15140122)
1 mM sodium pyruvate (Gibco brand, Thermo Fisher Scientific, cat. no. 11360070)
10 mM HEPES buffer (Gibco brand, Thermo Fisher Scientific, cat. no. 15630080)
1× non-essential amino acid solution (Gibco brand, Thermo Fisher Scientific, cat. no. 11140050)
0.055 mM 2-mercaptoethanol (Gibco brand, Thermo Fisher Scientific, cat. no. 21985023)
Store at 4°C for up to 3 months in sterile conditions.
COMMENTARY
Background Information
The methods described here have been refined and utilized extensively over the last decades to study cross-presentation by DCs and they continue to be instrumental for this field. Our group and other researchers continue to improve and optimize these methods as we advance our understanding of the intricate biology in cross-presenting DCs. In recent years, we have witnessed an increasing consensus for using bona-fide cross-presenting DCs for in vitro experiments in order to replicate the in vivo biology as accurately as possible. Much of our current cross-presentation knowledge is derived from GM-CSF differentiated DCs. While many research groups continue to use GM-CSF differentiated DCs to study cross-presentation, others have advocated and proposed the use of Flt3L differentiated cDC1-like DCs as bona-fide cross-presenting DCs. However, many of the established principles of cross-presentation have yet to be demonstrated in the latter DCs. Nonetheless, future research and findings in the field will dictate the use of either or both DCs, or perhaps another type of DC more closely related to their physiological counterparts in vivo. For now: research conducted using these currently available methods have been paramount for advancing our knowledge of cross-presentation in DCs with important clinical implications, such as understanding viral infection and vaccine development.
Critical Parameters
Prior to starting any DC cross-presentation experiments, it is vitally important to test that all reagents are free from endotoxins. Particular attention must be paid to ensuring every batch of FCS that is used to supplement any culture media or buffers at any stage of the DC cross-presentation experiments is endotoxin free. This is because constituting components in the FCS solution, mainly endotoxins, may activate and mature DCs, making it difficult to plan controlled experiments, resulting in irreproducible and invalid results. The steps outlined in Basic Protocol 5 are to be followed to test each new reagent, including new batches, that is introduced at any stage of the protocols described throughout to ensure the said new reagent does not cause activation and maturation of DCs because of trace amounts of endotoxin that are not reported by manufacturers.
When performing GM-CSF or Flt3L DC differentiation for the first time in your laboratory, the DC cultures should be analyzed by flow cytometry (see Basic Protocol 5) to determine the desired phenotypic expression profile of the DCs that are required for your experimental needs.
Trypan blue exclusion test of cell viability can be incorporated into the protocol when counting cells to assess cell viability.
Troubleshooting
See Table 2 for common problems encountered when performing these protocols and suggested solutions.
Table 2.
Troubleshootinga
| Problem | Possible cause | Solution |
|---|---|---|
|
| ||
| No cross-presentation | No PRR stimulation | Include a PRR agonist, e.g., LPS |
| Spontaneous maturation of DCs | Take extra care while handling | |
| DCs and minimize vibrations | ||
| Antigen (particularly if this is cell-associated antigen from apoptotic cells) was not delivered to the DC | See Blander and Medzhitov, 2006 for a published protocol to verify phagocytosis of apoptotic cells by DCs | |
| Cross-presentation in control/non-stimulated DCs | Non-endotoxin-free reagents used and/or contamination | Test all reagents for endotoxins prior to use |
| Apoptotic B cell blasts not staining for annexin V | Did not use the proper buffer | Ensure all staining and acquisition is done in the annexin V staining buffer included in the kit |
| Low yields of DCs | Incubation with RBC lysis buffer is too long | Be very careful to incubate for exactly 1 min with the RBC lysis buffer |
| Cross-presentation is observed even in the absence of TLR ligands, for instance, when using the microbeads coated with model antigen | Concentration of model antigen is too high. In the presence of high amounts of model antigen, the need for TLR ligands can be bypassed. See Blander and Medzhitov, 2006 where titration with and without LPS was conducted | Carefully titrate the amount of model antigen given to the DCs by creating serial dilutions of the antigen, in whatever form it is being delivered, and evaluate cross-presentation |
| CD8+ T cells dying after CFSE labeling | CFSE at high concentrations can be toxic to the CD8+ T cells | Use less of the CFSE dye to label CD8+ T cells |
| CD8+ T cells dying after co-culturing with PFA fixed DCs | Residual PFA has not been adequately washed away from fixed DCs. PFA is highly toxic to living cells, and as such, any residual PFA would kill the CD8+ T cells | Follow protocol to adequately wash away any residual PFA from the fixed DCs. If necessary, include extra washes and longer incubations before co-culture |
| No cross-presentation occurring in DCs stimulated with E. coli expressing the model antigen of interest | IPTG used to induce expression of the model antigen of interest is old | Use freshly prepared or freshly thawed IPTG to induce expression of the model antigen of interest |
Abbreviations: DC, dendritic cell; LPS, lipopolysaccharides; PRR, pattern recognition receptor; OVA, ovalbumin; RBC, red blood cell; TLR, Toll-like receptor; CFSE, carboxyfluorescein diacetate sccinimidyl ester; PFA, paraformaldehyde
Understanding Results
Bone marrow (BM) derived and in vivo differentiated dendritic cells (DCs) should express the DC markers CD11b and CD11c on the cell surface. In resting and nonactivated DCs, MHC-II molecules and the costimulatory molecules C40, CD80, and CD86 are expressed at low levels on the cell surface. The expression of the latter four molecules increase significantly upon DC stimulation and activation (see Basic Protocol 5 and Figure 3). Additionally, stimulated and activated DCs secrete pro-inflammatory cytokines such as TNFα and IL-6, which can be detected with ELISA tests (see Basic Protocol 5). Resting DCs or DCs which have been stimulated with an endocytic or a phagocytic cargo can be fixed and visualized with immunofluorescence microscopy and probed for different molecules and markers involved in cross-presentation (see Basic Protocol 7 and Figure 4),
Transgenic CD8+ T cells, which are specific for their cognate peptide cross-presented on the MHC-I molecule on the surface of DCs, are utilized to assess cross-presentation. These CD8+ T cells are co-cultured with fixed DCs (unstimulated or stimulated) and the proliferation of the CD8+ T cells is measured by flow cytometry to assess if successful cross-priming and CD8+ T cell activation has taken place, and hence, a robust readout for cross-presentation of antigens by DCs (see Basic Protocol 8 and Figure 5). Co-cultured CD8+ T cells with unstimulated DCs or DCs deficient for TLR or TLR signaling molecules must be cultured with exogenous anti-CD28 costimulatory antibodies. Additionally, early CD8+ T cell activation markers can also be used to assess cross-presentation. The expression of CD69 molecules on the surface of CD8+ T cells increases significantly 24 hr post activation and can be measured by flow cytometry, while the secretion of cytokines such as IFN-γ, TNF-α, and IL-2 is also upregulated 20 hr post stimulation and can be detected in the supernatant as indicators of CD8+ T cell early activation markers with ELISA tests (see Basic Protocol 8).
Time Considerations
A summary of time requirements for all protocols in this article are provided in Table 3.
Table 3.
Time Considerations
| Protocol | Time required | Hands-on time |
|---|---|---|
|
| ||
| Basic Protocol 1 | 1–1.5 hr | 45 min |
| Basic Protocol 2 | 5 days | 1 hr |
| Support Protocol 1 | 7 days | 1 hr |
| Basic Protocol 3 | 7 days | 45 min |
| Support Protocol 2 | 7–10 days | 1–2 hr |
| Basic Protocol 4 | 7–11 days | 4–6 hr |
| Basic Protocol 5 | 8–10 hr | 3–5 hr |
| Basic Protocol 6 | 3–24 hr | 1–3 hr |
| Support Protocol 3 | 3 hr | 2 hr |
| Support Protocol 4 | 4 days | 3 hr |
| Support Protocol 5 | 1 day | 2 hr |
| Basic Protocol 7 | 5–24 hr | 3–4 hr |
| Support Protocol 6 | 1–2 hr | 0.5–1 hr |
| Basic Protocol 8 | 4 days | 4–6 hr |
| Support Protocol 7 | 1 day | 2–4 hr |
Acknowledgments
We are indebted to Gaetan Barbet for advice on experimental methodology and for the data in Figures 3 and 5B, and to Priyanka Nair-Gupta for the data in Figure 4. J.M.B. and her lab are supported by National Institutes of Health (NIH) grants AI154294, AI127658, AI123284, and DK111862. J.M.B is a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease.
Literature Cited
- Ackerman AL., Giodini A., & Cresswell P. (2006). A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity, 25(4), 607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Aderem A, & Underhill DM (1999). Mechanisms of phagocytosis in macrophages. Annual Review of Immunology, 17(1), 593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Ahrens S, Zelenay S, Sancho D, Hanc P, Kjær,ˇ S., Feest, C., … Schulz, O. (2012). F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity, 36(4), 635–645. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, & Takeuchi O. (2006). Pathogen recognition and innate immunity. Cell, 124(4), 783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Arora P, & Porcelli SA (2016). An efficient and high yield method for isolation of mouse dendritic cell subsets. Journal of Visualized Experiments, 110, e53824. doi: 10.3791/53824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshara R, Sencio V, Soulard D, Barthélémy A, Fontaine J, Pinteau T, … Faveeuw C. (2018). Alteration of Flt3-Ligand-dependent de novo generation of conventional dendritic cells during influenza infection contributes to respiratory bacterial superinfection. PLoS Pathogens, 14(10), e1007360. doi: 10.1371/journal.ppat.1007360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM (2008). Phagocytosis and antigen presentation: A partnership initiated by Toll-like receptors. Annals of the Rheumatic Diseases, 67(Suppl 3), iii44–iii49. doi: 10.1136/ard.2008.097964. [DOI] [PubMed] [Google Scholar]
- Blander JM (2016). The comings and goings of MHC class I molecules herald a new dawn in cross-presentation. Immunological Reviews, 272(1), 65–79. doi: 10.1111/imr.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM (2018). Regulation of the cell biology of antigen cross-presentation. Annual Review of Immunology, 36, 717–753. doi: 10.1146/annurev-immunol-041015-055523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander JM, & Medzhitov R. (2006). Tolldependent selection of microbial antigens for presentation by dendritic cells. Nature, 440(7085), 808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- Blum JS, Wearsch PA, & Cresswell P. (2013). Pathways of antigen processing. Annual Review of Immunology, 31(1), 443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyman O, & Sprent J. (2012). The role of interleukin-2 during homeostasis and activation of the immune system. Nature Reviews Immunology, 12(3), 180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- Brasel K, de Smedt T, Smith JL, & Maliszewski CR (2000). Generation of murine dendritic cells from flt3-ligand—supplemented bone marrow cultures. Blood, 96(9), 3029–3039. doi: 10.1182/blood.V96.9.3029. [DOI] [PubMed] [Google Scholar]
- Brawand P., Fitzpatrick DR., Greenfiel BW., Brasel K., Maliszewski CR., & Smedt TD. (2002). Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. The Journal of Immunology, 169(12), 6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Daniels KA, & Welsh RM (2005). Rapid production of TNF-α following TCR engagement of naive CD8 T cells. The Journal of Immunology, 175(8), 5043–5049. doi: 10.4049/jimmunol.175.8.5043. [DOI] [PubMed] [Google Scholar]
- Charles A, Janeway J, Travers P, Walport M, & Shlomchik MJ (2001). T cellmediated cytotoxicity. Immunobiology: The Immune System in Health and Disease. 5th Edition. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK27101/. [Google Scholar]
- Cibrián D, & Sánchez-Madrid F. (2017). CD69: From activation marker to metabolic gatekeeper. European Journal of Immunology, 47(6), 946–953. doi: 10.1002/eji.201646837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MA, Kahan SM, & Zajac AJ (2013). Anti-viral CD8 T cells and the cytokines that they love. Virology, 435(1), 157–169. doi: 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell P. (2005). Antigen processing and presentation. Immunological Reviews, 207(1), 5–7. doi: 10.1111/j.0105-2896.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- Drutman SB, & Trombetta ES (2010). Dendritic cells continue to capture and present antigens after maturation in vivo. Journal of Immunology, 185(4), 2140–2146. doi: 10.4049/jimmunol.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embgenbroich M, & Burgdorf S. (2018). Current Concepts of Antigen Cross-Presentation. Frontiers in Immunology, 9, 1643. doi: 10.3389/fimmu.2018.01643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, & Xue D. (2009). The ins and outs of phospholipid asymmetry in the plasma membrane: Roles in health and disease. Critical Reviews in Biochemistry and Molecular Biology, 44(5), 264–277. doi: 10.1080/10409230903193307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazi U, & Martinez-Pomares L. (2009). Influence of the mannose receptor in host immune responses. Immunobiology, 214(7), 554–561. doi: 10.1016/j.imbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Grotzke JE, Sengupta D, Lu Q, & Cresswell P. (2017). The ongoing saga of the mechanism(s) of MHC class I-restricted cross-presentation. CurrentOpinioninImmunology,46,89–96.doi: 10.1016/j.coi.2017.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, …. Reis e Sousa, C. (2015). GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+MHCII+ macrophages and dendritic cells. Immunity, 42(6), 1197–1211. doi: 10.1016/j.immuni.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, … Steinman RM (1992). Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colonystimulating factor. The Journal of Experimental Medicine, 176(6), 1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, … Mellman I. (2007). Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity, 27(4), 610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre OP, Segura E, Savina A, & Amigorena S. (2012). Cross-presentation by dendritic cells. Nature Reviews Immunology, 12(8), 557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- Kirkling ME, Cytlak U, Lau CM, Lewis KL, Resteu A, Khodadadi-Jamayran A, … Reizis B. (2018). Notch signaling facilitates in vitro generation of cross-presenting classical dendritic cells. Cell Reports, 23(12), 3658–3672.e6. doi: 10.1016/j.celrep.2018.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M, & Rock KL (1995). A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science, 267(5195), 243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Lau CM, Tiniakou I, Perez OA, Kirkling ME, Yap GS, Hock H, & Reizis B. (2018). Transcription factor Etv6 regulates functional differentiation of cross-presenting classical dendritic cells. The Journal of Experimental Medicine, 215(9), 2265–2278. doi: 10.1084/jem.20172323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, & Nussenzweig MC (2010). Origin and development of dendritic cells. Immunological Reviews, 234(1), 45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, & McKenna HJ (1996). Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: Multiple dendritic cell subpopulations identified. The Journal of Experimental Medicine, 184(5), 1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten BJ, Olson GK, Kusewitt DF, & Lipscomb MF (2004). Flt3 ligand preferentially increases the number of functionally active myeloid dendritic cells in the lungs of mice. The Journal of Immunology, 172(7), 4077–4083. doi: 10.4049/jimmunol.172.7.4077. [DOI] [PubMed] [Google Scholar]
- Mildner A, & Jung S. (2014). Development and function of dendritic cell subsets. Immunity, 40(5), 642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, … Wu L. (2005). Cutting edge: Generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. Journal of Immunology, 174(11), 6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, … Blander JM (2014). TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation. Cell, 158(3), 506– 521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Gupta P,&Blander JM.(2013).Anupdated view of the intracellular mechanisms regulating cross-presentation. Frontiers in Immunology, 4, 401. doi: 10.3389/fimmu.2013.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell PJ, Morelli AE, Logar AJ, & Thomson AW (2000). Phenotypic and functional characterization of mouse hepatic CD8α+ lymphoid-related dendritic cells. Journal of Immunology, 165(2), 795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- Ou P, Wen L, Liu X, Huang J, Huang X, Su C, … Yang CY (2019). Thioesterase PPT1 balances viral resistance and efficient T cell crosspriming in dendritic cells. The Journal of Experimental Medicine, 216(9), 2091–2112. doi: 10.1084/jem.20190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmowski MJ, Gileadi U, Salio M, Gallimore A, Millrain M, James E, … Cerundolo V. (2006). Role of immunoproteasomes in cross-presentation. Journal of Immunology (Baltimore, Md.: 1950), 177(2), 983–990. doi: 10.4049/jimmunol.177.2.983. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Lingappa J, Kennedy MK, Smith J, Teepe M, Rudensky A, … Maraskovsky E. (1997). Developmental pathways of dendritic cells in vivo: Distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. Journal of Immunology, 159(5), 2222–2231. [PubMed] [Google Scholar]
- Qin Z, Noffz G, Mohaupt M, & Blankenstein T. (1997). Interleukin-10 prevents dendritic cell accumulation and vaccination with granulocytem-acrophage colony-stimulating factor genemodified tumor cells. The Journal of Immunology, 159(2), 770–776. [PubMed] [Google Scholar]
- Quah BJC, & Parish CR (2010). the use of carboxy fluoresce indiacetate succinimidy lester (CFSE) to monitor lymphocyte proliferation. Journal of Visualized Experiments, 44, e2259. doi: 10.3791/2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Albiston AL, Wicks IP, Chai SY, & Villadangos JA (2009). Different cross-presentation pathways in steady-state and inflammatory dendritic cells. Proceedings of the National Academy of Sciences, 106(48), 20377–20381. doi: 10.1073/pnas.0910295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She L., Siga LJ., Boe M., & Roc KL. (2004). Important role of cathepsin S in generating peptides for TAP-independent MHC class I cross-presentation in vivo.Immunity,21(2),155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Simms PE, & Ellis TM (1996). Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clinical and Diagnostic Laboratory Immunology, 3(3), 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman-Culleré MH, & Foltz CJ (1999). Body condition scoring: A rapid and accurate method for assessing health status in mice. Laboratory Animal Science, 49(3), 319–323. [PubMed] [Google Scholar]
- van de Laar L, Coffer PJ, & Woltman AM (2012). Regulation of dendritic cell development by GM-CSF: Molecular control and implications for immune homeostasis and therapy. Blood, 119(15), 3383–3393. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- Vargas P, Cortés C, Vargas L, Rosemblatt M, & Bono MR (2006). Immunization with antigen-pulsed dendritic cells significantly improves the immune response to weak selfantigens. Immunobiology, 211(1), 29–36. doi: 10.1016/j.imbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Waskow C, Liu K, Darrasse-Jèze G, Guermonprez P, Ginhoux F, Merad M, … Nussenzweig M. (2008). The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nature Immunology, 9(6), 676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, & Pamer EG (2003). CD8 T cell responses to infectious pathogens. Annual Review of Immunology, 21, 29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- Ye L-L, Wei X-S, Zhang M, Niu Y-R, & Zhou Q. (2018). The significance of tumor necrosis factor receptor type II in CD8+ regulatory T cells and CD8+ effector T cells. Frontiers in Immunology, 9. doi: 10.3389/fimmu.2018.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, & Bevan MJ (2011). CD8+ T cells: Foot soldiers of the immune system. Immunity, 35(2), 161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]