Abstract
One-pot condensation of 4-hydroxy coumarins, aldehydes and urea/thiourea to build C–C and C–N bonds is described. Fused pyrimidines have been synthesized under mild reaction conditions using l-proline. The protocol has been performed rapidly and efficiently in water under metal free conditions. Heterocyclic derivatives have been synthesized using the present methodology and avoid the use of hazardous solvents over conventional organic solvents. A proposed mechanism could be established for three component reactions. The present study reveals the first case in which l-proline has been explored as a homogeneous catalyst in the synthesis of fused pyrimidines in water under microwave irradiation. This synthesis involves simple workup and acceptable efficiency. The most notable feature of this protocol is the ability of the catalyst to influence asymmetric induction in the reaction.
One-pot condensation of 4-hydroxy coumarins, aldehydes and urea/thiourea to build C–C and C–N bonds is described.
1. Introduction
Multicomponent reactions (MCRs) have high efficiency and are a tool for development of different scaffolds for synthesis of many active drugs.1,2 In modern organic chemistry, the development of environmentally benign procedures in chemical and pharmaceutical industries has become a crucial and demanding research area. MRCs offer several advantages such as one-pot rather than multi-step synthesis of target compounds, and avoiding unnecessary expensive purification, toxic reagents and solvents.3 Proline is a chiral organo-catalyst having advantages over other catalyst such as being inexpensive, efficient and readily available.4 Proline act as an acid or a base which catalyzes chemical transformations similar to enzymatic catalysis.5l-Proline has been effectively used in various organic transformations,6,7 direct catalytic asymmetric aldol, Mannish and Michael.8–10 Polysubstituted heterocyclic ortho-quinones,11 pyridines,12 acridine derivatives,13 pyrans and thiopyrans,14 and quinolines.15
Coumarin moieties are involved in plants16 and showed anticoagulation, antiviral,17 anti-inflammatory,18 antibacterial19 and anticancer20 activities. Fused pyrimidines,21 chomenopyrimidine,22 and pyrimidines have also been reported as having anti-viral, anti-tumor, anti-inflammatory, and antihypertensive activities,23–25 as well as being calcium channel modulators26 and antimicrobial agents.27–29 Coumarin derivatives (Fig. 1A) have become drugs such as the anticoagulants warfarin,30a acenocoumarin,30b and phenprocoumon,30c all acting as vitamin K antagonists, the choleretics armillarisin A,31a hymecromone (umbelliferone),31b and the antibiotic novobiocin32 which is a potent inhibitor of bacterial DNA gyrase (GyrB). Some drugs such as Lamivudine,33a Raltegravir,33b Imatinib,34a,b Erlotinib34c,d and Lapatinib35 are types of drugs with pyrimidine core (Fig. 1B).
Fig. 1. Some drugs with coumarin and pyrimidine core.
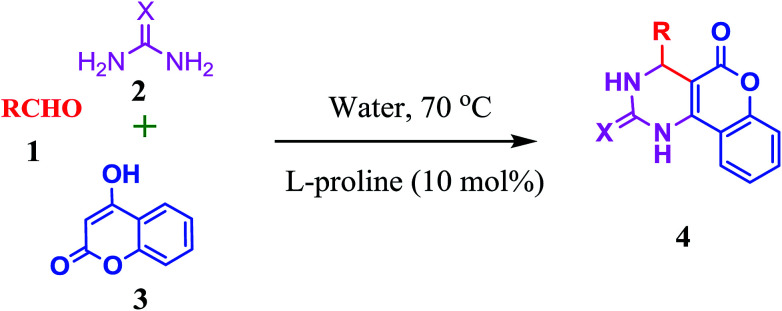
Thus as part of our research aimed at development of synthetic methodologies using environmentally benign catalysts through MCRs,36 we wish to report herein a metal free efficient and facile protocol for the three-component synthesis of fused pyrimidines in the presence of l-proline as an organo-catalyst in water at 70 °C, accompanied by moderate to good enantioselectivity (Scheme 1).
Scheme 1. Synthesis of fused pyrimidines.
2. Materials and methods
2.1. Experimental
All reagents such as l-proline, 4-hydroxy coumarin, aldehydes etc. were analytical grade and have more than 98% purity. 1H and 13C NMR spectra were recorded on BRUKER AVANCE II 500 NMR spectrometer using CDCl3 and DMSO-d6 as solvent. Purity of the compound was checked by TLC. MS 1927 microwave starter kit was used for microwave reactions. Reaction was carried out under microwave conditions at 300 W in open to air conditions. E-Merck precoated TLC plates, RANKEM silica gel G for preparative thin-layer chromatography were used. Melting points were determined in open capillary and are uncorrected.
2.2. Typical procedure for synthesis
In a 50 mL, 3 necks round bottom flask, charged appropriate aldehydes (5 mmol), 4-hydroxy coumarin (5 mmol) and urea/thiourea (5 mmol), water (10 mL) and l-proline (10 mol%). Stir the reaction mass and reflux at 70 °C. Reaction completion has monitored by TLC analysis. After reaction completion (monitoring by TLC), filtered the solid mass under vacuum then suck dried the solid and solid was recrystallized in ethanol.
3. Results & discussion
Initially study has been started with screening of solvents in one-pot reaction 4-hydroxy coumarin (5 mmol), benzaldehyde (5 mmol) and urea (5 mmol). Reaction was efficiently promoted in water according to screening results as compared to other catalysts (Table 1, entry 1).
Optimization of solventsa.
| Entry | Solvents | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | Water | 3.0 | 90 |
| 2 | Toluene | 5.0 | 40 |
| 3 | DMF | 7.5 | 41 |
| 4 | Ethanol | 4.0 | 35 |
| 5 | Acetonitrile | 5.5 | 40 |
| 6 | THF | 5.0 | 53 |
Reaction conditions: 4-hydroxy coumarin (5 mmol), benzaldehyde (5 mmol) and urea (5 mmol) using l-proline (10 mol%).
Above screening results revealed that the solvent plays a key role in this transformation. For instance, a best yield was obtained when water was utilized as medium (Table 1, entry 1). Nevertheless, when other solvents, such as toluene, DMF, ethanol, acetonitrile and THF were employed, we observed average yield of 4a even after 7.5 h at 70 °C (Table 1, entries 2–6). Additionally, water is an eco-friendly, cheaper, safe solvent and preferred as medium for clean synthesis. In respect of solvent selection, water has been selected as solvent or aqueous medium. Subsequently, same reaction has been done with different catalysts and results are shown in Table 2. As indicated in Table 2, good yield was obtained in the presence of l-proline (Table 2, entry 3). However, other catalysts (such as p-TSA, TEA, CaCl2, H2SO4, sulphamic acid) have been afforded moderate yield with higher reaction time (Table 2, entries 5–9). In the screening part, we have examined acids, amine and metal salt as catalysts. However, all catalysts showed some activity but were not efficient. l-Proline has dual functionality and both free NH and COOH groups of l-proline are essential for efficient transformation. l-Proline easily form iminium complex, this may be due the fact that protonation of amine moiety of catalyst which subsequent easily react with aldehyde. Protonation of amine may be easily achieved after dipolar structure of acid and amine resultant high yield was obtained due to combine effect of acid and amine moiety. Rest of catalysts have not this dual functionality or nature to catalyze the reaction efficiently results low yield was achieved.
Screening of catalystsa.
| Entry | Catalysts | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | l-Proline (2 mol%) | 8.0 | 61 |
| 2 | l-Proline (5 mol%) | 4.0 | 77 |
| 3 | l-Proline (10 mol%) | 3.0 | 90 |
| 4 | l-Proline (15 mol%) | 3.0 | 90 |
| 5 | p-TSA (10 mol%) | 10.0 | 49 |
| 6 | TEA (10 mol%) | 8.0 | 59 |
| 7 | CaCl2 (10 mol%) | 10.0 | 60 |
| 8 | H2SO4 (10 mol%) | 160 | 70 |
| 9 | Sulphamic acid (10 mol%) | 180 | 42 |
Reaction conditions: 4-hydroxy coumarin (5 mmol), benzaldehyde (5 mmol) and urea (5 mmol) using water.
After screening of solvents and catalysts, loading of catalyst has been evaluated in one pot condensation (Table 2, entries 1–4). Screening results have shown that catalyst amount play a crucial role in completion of reaction. Excellent yield was obtained with 10 mol% of l-proline which could not be raised by increasing the catalyst loading. Accordingly, 10 mol% of catalyst loading was acceptable for this transformation. The reaction was then conducted at different time interval, such as 0.5 h, 1.0 h, 1.5 h, 2.0 h, 2.5 h, 3.0 h, 3.5 h and 4.0 h, to determine the optimum time for this transformation. It can be concluded that after 3 h time interval, highest yield (91%) was obtained (Fig. 2).
Fig. 2. Comparison of reaction time with respect to yield.
There are few reports in literature for synthesis of 3,4-dihydro-1H-chromeno[4,3-d]pyrimidine-2,5-dione/thione derivatives using 4-hydroxy coumarin, aldehydes and urea/thiourea in the presence of homogeneous and heterogeneous catalysts. Reported methods show that researchers has used acids such as HCl37–39,41,42 and chloro sulphonic acid37,40 which has drawbacks such as longer reaction time,37–39,41 harsh reaction conditions such as ultrasonication used,38,40,42,43 higher temperature (60 °C, 110 °C and reflux), hazardous solvent (MeOH, EtOH and ACN)37–39,41–44 and often lower yield.39–43 Although the ultrasonication technology has been shown feasible on a small scale, the commercialization of sonolysis is still a challenge due to its high energy requirement.45 On the other hand, vanadium chloride has been used as catalyst44 with lower yield, hazardous solvent (ACN) and higher temperature. Many studies have been revealed that exposure of vanadium may cause respiratory dysfunction,46 hematological and biochemical alterations, and renal toxicity47 reproductive and developmental toxicity immunotoxicity, mutagenicity48 and neurotoxicity may also occur.49 All above reported methods have at least one mentioned drawback resultant there is need to develop a methodology which remove all drawback in a single procedure. It is important to note that the previous above methods reported in the literature do not show any asymmetric induction, however target compound has a chiral centre. To solve this problem, l-proline was used as enantioselective organocatalyst in water as environmental benign solvent under microwave conditions and shown good enantioselectivity. Several advantages offered by this method such as its generality, simplicity, high yields and environmental friendly solvent used (Table 3).
Comparison of present methodology with reported catalysts.
| Entry | Catalyst | Solvent | Conditions | Time (h/min) | Yield (%) | eec | Reference |
|---|---|---|---|---|---|---|---|
| 1 | HCl/chloro sulphonic acid | MeOH | 60 °C | 8.0 h | 96 | — | 37 |
| 2 | HCl | EtOH | Reflux/MWa | 12 h | 94 | — | 38 |
| 3 | HCl | MeOH | Reflux | Overnight | 59 | — | 39 |
| 4 | Chloro sulphonic acid | — | 60 °C/USb | 30 min | 92 | — | 40 |
| 5 | HCl | EtOH | Reflux | 12 h | 74 | — | 41 |
| 6 | HCl/silica gel/acidic alumina/montmorillonite-K10 clay | MeOH | 110 °C/MWa | 4–6 min | 60/83/90/85 | — | 42 |
| 7 | K2CO3 | EtOH/H2O | Reflux/MWa | 7 h | 53 | — | 43 |
| 8 | VCl3 | Acetonitrile | Reflux | 2 h | 82 | — | 44 |
| 9 | l-Proline | Water | MWa | 10 min | 90 | 98 | Present work |
Microwave conditions.
Ultrasonication.
ee = enantiomeric excess.
To explore the catalytic activity of l-proline, the scope the present methodology has been applied in the synthesis of various substituted fused pyrimidines. Different electron donating and electron withdrawing substituents have been investigated and results are incorporated in Table 4. From Table 4, the reaction was performed smoothly with para substituents and synthesized compounds have been characterized by spectroscopic analysis. Copy of all 1H and 13C NMR spectra is placed in the ESI† and confirmed the proposed structure of heterocycles.
Synthesis of library of fused pyrimidines under conventional method and microwave irradiation.
| Entry | Product | Time (h/min) | Yield (%) | ee% | ||
|---|---|---|---|---|---|---|
| CHa | MWb | CH | MW | |||
| 1 |

|
3.0 h | 10 min | 90 | 92 | 98 |
| 2 |

|
4.5 h | 8.0 min | 88 | 86 | 89 |
| 3 |

|
5.0 h | 10 min | 83 | 86 | 91 |
| 4 |

|
4.5 h | 10 min | 83 | 85 | 86 |
| 5 |

|
4.5 h | 8.0 min | 82 | 81 | 83 |
| 6 |

|
5.0 h | 5.0 min | 89 | 87 | 89 |
| 7 |

|
5.0 h | 10 min | 89 | 88 | 90 |
| 8 |

|
5.0 h | 10 min | 92 | 91 | 93 |
| 9 |

|
5.0 | 8.0 min | 93 | 92 | 96 |
| 10 |

|
5.0 h | 10 min | 92 | 90 | 92 |
| 11 |

|
5.0 h | 10 min | 91 | 92 | 97 |
| 12 |

|
5.0 h | 8.0 min | 87 | 85 | 85 |
| 13 |

|
5.0 h | 5.0 min | 90 | 88 | 90 |
| 14 |

|
5.0 h | 5.0 min | 90 | 91 | 91 |
| 15 |

|
5.0 h | 8.0 min | 79 | 83 | 84 |
| 16 |

|
5.0 h | 5.0 min | 83 | 85 | 88 |
| 17 |

|
5.0 h | 10 min | 83 | 83 | 86 |
| 18 |

|
4.5 | 10 min | 80 | 83 | 84 |
| 19 |

|
4.5 | 10 min | 81 | 82 | 89 |
| 20 |

|
5.0 | 10 min | 77 | 81 | 85 |
CH = conventional heating.
MW = microwave conditions.
Energy transfer depends on the thermal conductivity which is relatively slow and insufficient upon conventional heating resultant higher reaction time is required for completion of reaction. In contrast, microwave conditions are required minimum time to complete the reaction. Apart from this, the advantages of numerous microwave (MW) induced reactions over conventional reactions, and their utility in organic synthesis, have been fully recognized in the last two decades.50 To minimize the reaction time, reaction has performed under microwave conditions. The results showed that reaction has completed within 5–10 minutes with good yield under microwave conditions as compared to conventional heating. Therefore, microwave irradiation reducing the reaction time with good enantioselectivity (Table 4). The enantiomeric excess of the compounds synthesized was determined by employing chiral HPLC using OD-H column. Excellent enantioselectivity upto 98% ee was obtained (Table 4, entry 1). For the rest of the compounds enantiomeric excess was found to be in the range of to 83% ee to 97% ee. It is important to note that the previous methods reported in the literature do not show any asymmetric induction (Table 3).
Synthesized compounds 4a–4t were confirmed by spectroscopic analysis. 1H NMR of compound 4a showed characteristic signal at 6.36δ as singlet due to 4-H, multiplet for nine hydrogens of aromatic rings in the downfield region between 7.09–7.39δ and two singlet has been arised at 7.60 and 7.90 for –NH. Likewise derivatives 4d, 4k and 4f demonstrated the singlet at 3.11δ for six hydrogens of N(CH3)2 and singlet at 3.73δ for OCH3 respectively. 13C NMR of compound 4a showed characteristic signal at 36δ for C-1 carbon, signal at 104 has been assigned for C-2 carbon, other characteristic signal for ketonic carbon (C-4 and C-11) exhibited at 164δ and 165δ. Derivatives 4d, 4k and 4f have been showed characteristic signal at 45 and 56δ for –N(CH3)2 and OCH3 group. Mass spectra as well as elemental analysis also confirmed the structure of final product.
A plausible mechanism for reaction of 4-hydroxy coumarin (1), aldehydes (2), and urea/thiourea (3) to synthesis of fused pyrimidines (4) is depicted in Scheme 2. Based on literature, l-proline having dual functionality as acid and base can catalyze aldol related reactions such as Knoevenagel condensation as well as Michael addition.51 Previous study has shown that Knoevenagel condensation reaction efficiently catalyzed by amino acid catalyst and supports present mechanistic pathway.52 The reaction presumably proceeds through initial activation of the aldehyde by l-proline to form an iminium complex53 which further facilitates the Knoevenagel condensation to produce intermediate which pursue by Michael addition of urea/thiourea (3) on double bond of intermediate (A) to form intermediate (B). Furthermore, carbonyl and amino corner of the Michael adduct B was condensed through intramolecular cyclization to give desire target (4).
Scheme 2. Plausible reaction mechanism.
To support the plausible mechanism, proposed reaction intermediate (A) has been isolated and characterized. First of all reaction of 4-hydroxy coumarin and 2-hydroxy benzaldehyde has been carried out in optimized reaction conditions and formed the intermediate (A). Further intermediate (A) has been isolated and characterized by 1H and 13C NMR. Characterization data and literature have also been supported the structure of intermediate (A).54 Then isolated intermediate (A) was react with third component (urea) and achieved the product (4b). Present investigation has confirmed proposed mechanistic pathway by which target compound was achieved.
4. Conclusions
In conclusion, an enantioselective and metal free l-proline catalyzed protocol for the synthesis of fused pyrimidines with good yield in water as a green solvent using urea/thiourea, aldehydes and 4-hydroxy coumarin. Environmental benign one pot strategy has been explored with l-proline successfully which generate a green platform in future for enantioselective synthesis of novel molecules in water. Operational simplicity, metal-free approach, compatibility with various aldehydes and 4-hydroxy coumarin, simple se of workup, neat and clean synthesis are notable advantages of this protocol. In term of green solvent, an environmental benign solvent i.e. water was used which is very inexpensive and having reactivity and selectivity toward reaction media. Most notable feature of this methodology is enantioselective synthesis with more than 98% ee.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
Authors are grateful thanks to the IPC Ghaziabad, India for performing 1H and 13C NMR spectra.
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07517d
References
- (a) Hajinasiri R. Hossaini Z. Sheikholeslami-Farahani F. Comb. Chem. High Throughput Screen. 2015;18:42. doi: 10.2174/1386207317666141203123133. [DOI] [PubMed] [Google Scholar]; (b) Hossaini Z. Soltani S. Sheikholeslami-Farahani F. Sayyed-Alangi S. Z. Sajjadi-Ghotabadi H. Chem. Heterocycl. Compd. 2015;51:26. doi: 10.1007/s10593-015-1654-0. [DOI] [Google Scholar]; (c) Hossaini Z. Rostami-Charati F. Ghasemian M. Afshari Sharif Abad S. Synlett. 2015;26:1222. doi: 10.1055/s-0034-1380504. [DOI] [Google Scholar]
- (a) Hossaini Z. Rostami-Charati F. Ghambarian M. Siadati S. A. Phosphorus, Sulfur Silicon Relat. Elem. 2015;190:1177. doi: 10.1080/10426507.2014.978329. [DOI] [Google Scholar]; (b) Slobbe P. Ruijter E. Orru R. V. A. Med. Chem. Commun. 2012;3:1189. doi: 10.1039/C2MD20089A. [DOI] [Google Scholar]
- (a) Heijden V. D. G. Ruijter E. Orru R. V. A. Synlett. 2013:666. [Google Scholar]; (b) Hulme C., Ayaz M., Martinez-Ariza G., Medda F. and Shaw A., in Small molecule medicinal chemistry: strategies and technologies, ed. W. Czechtizky and P. Hamley, Wiley-VCH, Weinheim, 2015, ch. 6 [Google Scholar]; (c) Gollner A. Synlett. 2015:426. doi: 10.1055/s-0034-1379947. [DOI] [Google Scholar]
- (a) List B. Lerner R. A. Barbas C. F. J. Am. Chem. Soc. 2000;122:2395. doi: 10.1021/ja994280y. [DOI] [Google Scholar]; (b) Cordova A. Notz W. Barbas C. F. Chem. Commun. 2002:3024. doi: 10.1039/B207664K. [DOI] [PubMed] [Google Scholar]; (c) Chowdari N. S. Ramachary D. B. Barbas C. F. Synlett. 2003:1906. [Google Scholar]
- (a) Balalaie S. Bararjanian M. Amani A. M. Movassagh B. Synlett. 2006:263. doi: 10.1055/s-2006-926227. [DOI] [Google Scholar]; (b) Kotrusz P. Toma S. ARKIVOC. 2006;v:100. [Google Scholar]; (c) Mabry J. Ganem B. Tetrahedron Lett. 2006;47:55. doi: 10.1016/j.tetlet.2005.10.124. [DOI] [Google Scholar]; (d) Chandrasekher S. Vijeender K. Reddy V. K. Tetrahedron Lett. 2005;46:6991. doi: 10.1016/j.tetlet.2005.08.066. [DOI] [Google Scholar]; (e) Chandrasekhar S. Narsihmulu C. Reddy N. R. K. Sultana S. S. Tetrahedron Lett. 2004;45:4581. doi: 10.1016/j.tetlet.2004.03.116. [DOI] [Google Scholar]; (f) Darbre T. Machuqueiro M. Chem. Commun. 2003:1090. doi: 10.1039/B301117H. [DOI] [PubMed] [Google Scholar]
- (a) Wang Y. Shang Z. C. Wu T. X. Fan J. C. Chen X. J. Mol. Catal. A: Chem. 2006;253:212. doi: 10.1016/j.molcata.2006.03.035. [DOI] [Google Scholar]; (b) Srinivasan M. Perumal S. Selvaraj S. ARKIVOC. 2005;xi:201. [Google Scholar]; (c) Sabitha G. Fatima N. Reddy E. V. Yadav J. S. Adv. Synth. Catal. 2005;347:1353. doi: 10.1002/adsc.200505144. [DOI] [Google Scholar]; (d) Dodda R. Zhao C. G. Synthesis. 2006;19:3238. [Google Scholar]
- (a) Varala R. Ramu E. Sreelatha N. Adapa S. R. Tetrahedron Lett. 2006;476:877. doi: 10.1016/j.tetlet.2005.12.005. [DOI] [Google Scholar]; (b) Varala R. Adapa S. R. Org. Process Res. Dev. 2005;9:853. doi: 10.1021/op050129z. [DOI] [Google Scholar]; (c) An Z. Zhang W. Shi H. He J. J. Catal. 2006;241:319. doi: 10.1016/j.jcat.2006.04.035. [DOI] [Google Scholar]; (d) Karade N. N. Budhewar V. H. Shinde S. V. Jadhav W. N. Lett. Org. Chem. 2007;4:16. doi: 10.2174/157017807780037405. [DOI] [Google Scholar]
- Alcaide B. Almendros P. Luna A. Torres M. R. J. Org. Chem. 2006;71:4818. doi: 10.1021/jo0604235. [DOI] [PubMed] [Google Scholar]
- (a) Janey J. M. Hsiao Y. Armstrong J. D. J. Org. Chem. 2006;71:390. doi: 10.1021/jo0519458. [DOI] [PubMed] [Google Scholar]; (b) List B. Pojarliev P. Biller W. T. Martin H. J. J. Am. Chem. Soc. 2002;124:827. doi: 10.1021/ja0174231. [DOI] [PubMed] [Google Scholar]
- (a) Rasalkar M. S. Potdar M. K. Mohile S. S. Salunkhe M. M. J. Mol. Catal. A: Chem. 2005;235:267. doi: 10.1016/j.molcata.2005.03.024. [DOI] [Google Scholar]; (b) Kotrusz P. Toma S. Molecules. 2006;11:197. doi: 10.3390/11020197. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kotrusz P. Toma S. ARKIVOC. 2006:100. [Google Scholar]
- Rajesh S. M. Bala B. D. Perumal S. Menéndez J. M. Green Chem. 2011;13:3248. doi: 10.1039/C1GC15794A. [DOI] [Google Scholar]
- (a) Janardhan B. Ravibabu V. Crooks P. A. Rajitha B. Org. Commun. 2012;5:186. [Google Scholar]; (b) Mukhopadhyay C. Tapaswi P. K. Butcher R. J. Tetrahedron Lett. 2010;51:1797. doi: 10.1016/j.tetlet.2010.01.106. [DOI] [Google Scholar]
- Heravi M. R. P. Aghamohammadi P. C. R. Chim. 2012;15:448. doi: 10.1016/j.crci.2011.12.001. [DOI] [Google Scholar]
- (a) Elnagdi N. M. H. Al-Hokbany N. S. Molecules. 2012;17:4300. doi: 10.3390/molecules17044300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bora P. P. Bihani M. Bez G. RSC Adv. 2015;5:50597. doi: 10.1039/C5RA08785F. [DOI] [Google Scholar]
- Karamthulla S. Pal S. Parvin T. Choudhury L. H. RSC Adv. 2014;4:15319. doi: 10.1039/C4RA00876F. [DOI] [Google Scholar]
- (a) Murray R. D. H., Mendez J. and Brown S. A., Chemistry and Biochemistry, John Wiley & Sons Ltd, New York, 1982, p. 21 [Google Scholar]; (b) Garazd M. M. Garadz Y. L. Khilya V. P. Chem. Nat. Compd. 2003;39:54. doi: 10.1023/A:1024140915526. [DOI] [Google Scholar]; (c) Murray R. D. H. Nat. Prod. Rep. 1995;12:477. doi: 10.1039/NP9951200477. [DOI] [Google Scholar]; (d) Estvez-Braun A. Gonzalez A. G. Nat. Prod. Rep. 1997;14:465. doi: 10.1039/NP9971400465. [DOI] [PubMed] [Google Scholar]
- (a) Hwu J. R. Singha R. Hong S. C. Chang Y. H. Das A. R. Vliegen I. Clercq E. D. Neyts J. Antiviral Res. 2008;77:157. doi: 10.1016/j.antiviral.2007.09.003. [DOI] [PubMed] [Google Scholar]; (b) Neyts J. Clercq E. D. Singha R. Chang Y. H. Das A. R. Chakraborty S. K. Hong S. C. Tsay S. C. Hsu M. H. Hwu J. R. J. Med. Chem. 2009;52:1486. doi: 10.1021/jm801240d. [DOI] [PubMed] [Google Scholar]; (c) Hwu J. R. Lin S. Y. Tsay S. C. Clercq E. D. Leyssen P. Neyts J. J. Med. Chem. 2011;54:2114. doi: 10.1021/jm101337v. [DOI] [PubMed] [Google Scholar]
- (a) Li Z. P. Hu J. F. Sun M. N. Ji H. J. Chu S. F. Liu G. Chen N. H. Int. Immunopharmacol. 2012;14:145. doi: 10.1016/j.intimp.2012.06.004. [DOI] [PubMed] [Google Scholar]; (b) Li Z. P. Hu J. F. Sun M. N. Ji H. J. Zhao M. Wu D. H. Li G. Y. Liu G. Chen N. H. Eur. J. Pharmacol. 2011;661:118. doi: 10.1016/j.ejphar.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Shi Y. Zhou C. H. Bioorg. Med. Chem. Lett. 2011;21:956. doi: 10.1016/j.bmcl.2010.12.059. [DOI] [PubMed] [Google Scholar]
- (a) Meggio F. Pagano M. A. Moro S. Zagotto G. Ruzzene M. Sarno S. Cozza G. Bain J. Elliott M. Deana A. D. Brunati A. M. Pinna L. A. Biochemistry. 2004;43:12931. doi: 10.1021/bi048999g. [DOI] [PubMed] [Google Scholar]; (b) Chilin A. Battistutta R. Bortolato A. Cozza G. Zanatta S. Poletto G. Mazzorana M. Zagotto G. Uriarte E. Guiotto A. Pinna L. A. Meggio F. Moro S. J. Med. Chem. 2008;51:752. doi: 10.1021/jm070909t. [DOI] [PubMed] [Google Scholar]; (c) Bras G. L. Radanyi C. Peyrat J. F. Brion J. D. Alami M. Marsaud V. Stella B. Renoir J. M. J. Med. Chem. 2007;50:6189. doi: 10.1021/jm0707774. [DOI] [PubMed] [Google Scholar]; (d) Zhao H. P. Donnelly A. C. Kusuma B. R. Brandt G. E. L. Brown D. Rajewski R. A. Vielhauer G. Holzbeierlein J. Cohen M. S. Blagg B. S. J. J. Med. Chem. 2011;54:3839. doi: 10.1021/jm200148p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhao H. P. Yan B. Peterson L. B. Blagg B. S. J. ACS Med. Chem. Lett. 2012;3:327. doi: 10.1021/ml300018e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Donnelly A. C. Mays J. R. Burlison J. A. Nelson J. T. Vielhauer G. Holzbeierlein J. Blagg B. S. J. J. Org. Chem. 2008;73:8901. doi: 10.1021/jo801312r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Purohit A. Woo L. W. L. Potter B. V. L. Reed M. J. Cancer Res. 2000;60:3394. [PubMed] [Google Scholar]; (h) Woo L. W. L. Purohit A. Malini B. Reed M. J. Potter B. V. L. Chem. Biol. 2000;7:773. doi: 10.1016/S1074-5521(00)00023-5. [DOI] [PubMed] [Google Scholar]; (i) Malini B. Purohit A. Ganeshapillai D. Woo L. W. L. Potter B. V. L. Reed M. J. J. Steroid Biochem. Mol. Biol. 2000;75:253. doi: 10.1016/S0960-0760(00)00178-3. [DOI] [PubMed] [Google Scholar]
- Kempson J. Pitts W. J. Barbosa J. Guo J. Omotoso O. Watson A. Stebbins K. Starling G. C. Dodd J. H. Barrish J. C. Felix R. Fischer K. Bioorg. Med. Chem. Lett. 2005;15:1829. doi: 10.1016/j.bmcl.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Bruno O. Brullo C. Schenone S. Bondavalli F. Ranise A. Tognolini M. Impicciatore M. Ballabeni V. Barocelli E. Bioorg. Med. Chem. 2006;14:121. doi: 10.1016/j.bmc.2005.07.066. [DOI] [PubMed] [Google Scholar]
- (a) Al-Harbi N. O. Bahashwan S. A. Fayed A. A. Aboonq M. S. Amr A. E. E. Int. J. Biol. Macromol. 2013;57:165. doi: 10.1016/j.ijbiomac.2013.03.019. [DOI] [PubMed] [Google Scholar]; (b) Petersen E. Schmidt D. R. Expert Rev. Anti-Infect. Ther. 2003;1:175. doi: 10.1586/14787210.1.1.175. [DOI] [PubMed] [Google Scholar]
- (a) Nadal E. Olavarria E. Int. J. Clin. Pract. 2004;58:511. doi: 10.1111/j.1368-5031.2004.00173.x. [DOI] [PubMed] [Google Scholar]; (b) Dixon B. S. Beck G. J. Vazquez M. A. Greenberg A. Delmez J. A. Allon M. Dember L. M. Himmelfarb J. Gassman J. J. Greene T. Radeva M. K. Davidson I. J. Ikizler T. A. Braden G. L. Fenves A. Z. Kaufman J. S. Cotton Jr J. R. Martin K. J. McNeil J. W. Rahman A. Lawson J. H. Whiting J. F. Hu B. Meyers C. M. Kusek J. W. Feldman H. I. N. Engl. J. Med. 2009;360:2191. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran S. V. Bhargavi B. Srinivasan K. K. Der Pharma Chemica. 2012;4:255. [Google Scholar]
- (a) Rovnyak G. C. Kimbal S. D. Beyer B. Cucinotta G. Dimarco J. D. Gougoutas J. Hedberg A. Malley M. MaCaethy J. P. Zhang R. Mereland S. J. Med. Chem. 1995;38:119. doi: 10.1021/jm00001a017. [DOI] [PubMed] [Google Scholar]; (b) Kappe C. O. Fabian W. M. F. Semons M. A. Tetrahedron. 1997;53:2803. doi: 10.1016/S0040-4020(97)00022-7. [DOI] [Google Scholar]
- Lanjewar K. R. Rahatgaonkar A. M. Chorghade M. S. Saraf B. D. Indian J. Chem. 2009;48B:1732. [Google Scholar]
- (a) Heravi M. M. Ranjwar L. Deriknand F. Alimadadi B. Oskooie H. A. Mol. Diversity. 2008;12:181. doi: 10.1007/s11030-008-9086-8. [DOI] [PubMed] [Google Scholar]; (b) Chang S. Ji J. S. Yu L. J. Chin. Chem. Soc. 2008;55:292. doi: 10.1002/jccs.200800043. [DOI] [Google Scholar]; (c) Shah N. K. Patel M. P. Patel R. G. Phosphorus, Sulfur Silicon Relat. Elem. 2009;184:2704. doi: 10.1080/10426500802583504. [DOI] [Google Scholar]
- Kumar R. Malik S. Chamdra R. Indian J. Chem. 2009;48B:718. [Google Scholar]
- (a) Liu Y. Liu S. Shi Y. Qin M. Sun Z. Liu G. Xenobiotica. 2017;48:818. doi: 10.1080/00498254.2017.1361051. [DOI] [PubMed] [Google Scholar]; (b) Rathore S. S. Agarwal S. K. Pande S. Singh S. K. Mittal T. Mittal B. PLoS One. 2012;7:e37844. doi: 10.1371/journal.pone.0037844. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Prochaska J. H. Göbel S. Keller K. Coldewey M. Ullmann A. Lamparter H. Jünger C. Al-Bayati Z. Baer C. Walter U. Bickel C. Cate H. t. Münzel T. Wild P. S. BMC Med. 2015;13:14. doi: 10.1186/s12916-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Zhu J. B. Luan L. B. Shi Q. C. Yaoxue Xuebao. 1992;27:231. [PubMed] [Google Scholar]; (b) Abate A. Dimartino V. Spina P. Costa P. L. Lombardo C. Santini A. Del Piano M. Alimonti P. Drugs Exp. Clin. Res. 2001;27:223. [PubMed] [Google Scholar]
- Rodríguez-Cerrato V. Del Prado G. Huelves L. Naves P. Ruiz V. García E. Ponte C. Soriano F. Int. J. Antimicrob. Agents. 2010;35:544. doi: 10.1016/j.ijantimicag.2010.02.007. [DOI] [PubMed] [Google Scholar]
- (a) Gaonkar S. L. Shimizu H. Tetrahedron. 2010;66:3314. doi: 10.1016/j.tet.2010.03.006. [DOI] [Google Scholar]; (b) Gilchrist T. L., Heterocyclic Chemistry, John Wiley & Sons, 1997 [Google Scholar]
- (a) Parker R. H. Jones W. M. J. Org. Chem. 1978;43:2548. doi: 10.1021/jo00406a058. [DOI] [Google Scholar]; (b) Wiley R. H. and Smith N. R., Organic Syntheses; Collect, John Wiley & Sons, New York, 1963, vol. 4, p. 201 [Google Scholar]; (c) von Pechmann H. Justus Liebigs Ann. Chem. 1891;264:261. doi: 10.1002/jlac.18912640202. [DOI] [Google Scholar]; (d) Ashworth I. W. Bowden M. C. Dembofsky B. Levin D. Moss W. Robinson E. Szczur N. Virica J. Org. Process Res. Dev. 2003;7:74. doi: 10.1021/op025571l. [DOI] [Google Scholar]
- Li X. Abell C. Warrington B. H. Ladlow M. Org. Biomol. Chem. 2003;1:4392. doi: 10.1039/B307549D. [DOI] [PubMed] [Google Scholar]
- (a) Sahu P. K. Sahu P. K. Gupta S. K. Thavaselvam D. Agarwal D. D. Eur. J. Med. Chem. 2012;54:366. doi: 10.1016/j.ejmech.2012.05.020. [DOI] [PubMed] [Google Scholar]; (b) Sahu P. K. Sahu P. K. Thavaselvam D. Alafeefy A. M. Agarwal D. D. Med. Chem. Res. 2015;24:725. doi: 10.1007/s00044-014-1150-6. [DOI] [Google Scholar]; (c) Sahu P. K. Sahu P. K. Agarwal D. D. RSC Adv. 2013;3:9854. doi: 10.1039/C3RA40993G. [DOI] [Google Scholar]; (d) Sahu P. K. Sahu P. K. Gupta S. K. Agarwal D. D. Ind. Eng. Chem. Res. 2014;53:2085. doi: 10.1021/ie402037d. [DOI] [Google Scholar]; (e) Sahu P. K. Sahu P. K. Gupta S. K. Agarwal D. D. Catal. Sci. Technol. 2013;3:1520. doi: 10.1039/C3CY20807A. [DOI] [Google Scholar]; (f) Sahu P. K. Sahu P. K. Agarwal D. D. RSC Adv. 2014;4:40414. doi: 10.1039/C4RA03847A. [DOI] [Google Scholar]; (g) Sahu P. K. Sahu P. K. Sharma Y. Agarwal D. D. J. Heterocycl. Chem. 2014;51:1193. doi: 10.1002/jhet.1572. [DOI] [Google Scholar]; (h) Sahu P. K. Sahu P. K. Kaurav M. S. Messali M. Almutairi S. M. Sahu P. L. Agarwal D. D. RSC Adv. 2018;8:33952. doi: 10.1039/C8RA04363A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Sahu P. K. Sahu P. K. Kaurav M. S. Messali M. Almutairi S. M. Sahu P. L. Agarwal D. D. ACS Omega. 2018;3:15035. doi: 10.1021/acsomega.8b01993. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Sahu P. K. Sahu P. K. Agarwal D. D. J. Indian Chem. Soc. 2015;92:169. [Google Scholar]
- Bhut D. Gami R. Parikh A. Sharma C. Patel P. Pharma Sci. Monit. 2015;6:149. [Google Scholar]
- Kidwai M. Saxena S. Mohan R. Russ. J. Org. Chem. 2006;42:52. doi: 10.1134/S107042800601009X. [DOI] [Google Scholar]
- Brahmbhatt D. I. Raolji G. B. Pandya S. U. Pandya U. R. Indian J. Chem. 1999;38(B):839. [Google Scholar]
- Abdulkarim Al-Kadasi M. A. Nazeruddin G. M. J. Chem. Pharm. Res. 2013;5:204. [Google Scholar]
- Ambre P. K. Pissurlenkar R. R. S. Wavhale R. D. Shaikh M. S. Khedkar V. M. Wan B. Franzblau S. G. Coutinho E. C. Med. Chem. Res. 2014;23:2564. doi: 10.1007/s00044-013-0850-7. [DOI] [Google Scholar]
- Kidwai M. Sapra P. Synth. Commun. 2002;32:1639. doi: 10.1081/SCC-120004253. [DOI] [Google Scholar]
- Kidwai M. Priya Rastogi S. Z. Naturforsch. 2008;63b:71. [Google Scholar]
- Sabitha G. Reddy G. S. K. K. Reddy K. B. Yadav J. S. Tetrahedron Lett. 2003;44:6497. doi: 10.1016/S0040-4039(03)01564-8. [DOI] [Google Scholar]
- Crittenden J. C., Trussell R. R., Hand D. W. and Tchobanglouse G., Water treatment principle and design, John Wily and Sons, 2nd edn, 2004 [Google Scholar]
- Woodin M. A. Liu Y. Neuberg D. Hauser R. Smith T. J. et al. . Am. J. Ind. Med. 2000;37:353. doi: 10.1002/(SICI)1097-0274(200004)37:4<353::AID-AJIM5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Zaporowska H. Wasilewski W. Sŀotwińska M. BioMetals. 1993;6:3. doi: 10.1007/BF00154226. [DOI] [PubMed] [Google Scholar]
- Avila-Costa M. R. Montiel Flores E. Colin-Barenque L. Ordoñez J. L. Gutiérrez A. L. et al. . Neurochem. Res. 2004;29:1365. doi: 10.1023/B:NERE.0000026398.86113.7d. [DOI] [PubMed] [Google Scholar]
- Li H. Zhou D. Zhang Q. Feng C. Zheng W. et al. . NeuroToxicology. 2013;36:49. doi: 10.1016/j.neuro.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Perreux L. Loupy A. Tetrahedron. 2001;57:9199. doi: 10.1016/S0040-4020(01)00905-X. [DOI] [Google Scholar]; (b) Lindström P. Tierney J. Wathey B. Westman J. Tetrahedron. 2001;57:9225. doi: 10.1016/S0040-4020(01)00906-1. [DOI] [Google Scholar]; (c) Loupy A. Petit A. Hamelin J. Boullet F. T. Jacquault P. Mathe D. Synthesis. 1998:1213. doi: 10.1055/s-1998-6083. [DOI] [Google Scholar]
- (a) Chandrasekhar S. Narsihmulu C. Reddy N. R. K. Sultana S. S. Tetrahedron Lett. 2004;45:4581. doi: 10.1016/j.tetlet.2004.03.116. [DOI] [Google Scholar]; (b) Kotrusz P. Toma S. ARKIVOC. 2006;v:100. [Google Scholar]; (c) Darbre T. Machuqueiro M. Chem. Commun. 2003;7:1090. doi: 10.1039/B301117H. [DOI] [PubMed] [Google Scholar]
- (a) Ramachary D. B. Kishor M. Org. Biomol. Chem. 2010;8:2859. doi: 10.1039/C003588B. [DOI] [PubMed] [Google Scholar]; (b) Ramachary D. B. Pasha M. A. Thirupathi G. Angew. Chem., Int. Ed. 2017;56:12930. doi: 10.1002/anie.201706557. [DOI] [PubMed] [Google Scholar]; (c) Madhavachary R. Ramachary D. B. Eur. J. Org. Chem. 2014:7317. doi: 10.1002/ejoc.201403128. [DOI] [Google Scholar]; (d) Ramachary D. B. Jain S. Org. Biomol. Chem. 2011;9:1277. doi: 10.1039/C0OB00611D. [DOI] [PubMed] [Google Scholar]; (e) Ramachary D. B. Kishor M. Reddy Y. V. Eur. J. Org. Chem. 2008:975. doi: 10.1002/ejoc.200701014. [DOI] [PubMed] [Google Scholar]; (f) Ramachary D. B. Reddy Y. V. A. J. Org. Chem. 2010;75:74. doi: 10.1021/jo901799n. [DOI] [PubMed] [Google Scholar]; (g) Ramachary D. B. Kishor M. J. Org. Chem. 2007;72:5056. doi: 10.1021/jo070277i. [DOI] [PubMed] [Google Scholar]; (h) Ramachary D. B. Kishor M. Babul R. G. Org. Biomol. Chem. 2006;4:1641. doi: 10.1039/B602696F. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay C. Tapaswi P. K. Butcher R. J. Tetrahedron Lett. 2010;51:1797. doi: 10.1016/j.tetlet.2010.01.106. [DOI] [Google Scholar]
- Khoobi M. Foroumadi A. Emami S. Safavi M. Dehghan G. Alizadeh B. H. Ramazani A. Ardestani S. K. Shafiee A. Chem. Biol. Drug Des. 2011;78:580. doi: 10.1111/j.1747-0285.2011.01175.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.