INTRODUCTION
Hyponatremia, arbitrarily defined as a serum sodium concentration (SNa) <130 mmol/L, is the most common electrolyte disorder and a well-known complication of cirrhosis1 Hyponatremia in cirrhosis is a predictor of poor outcomes and its treatment remains challenging. In this review, we summarize the current understanding of the epidemiology, clinical outcomes, pathogenesis, etiology, evaluation, and management of hyponatremia in cirrhosis.
EPIDEMIOLOGY AND CLINICAL OUTCOMES
The prevalence of hyponatremia in cirrhosis varies depending on the definition used and the population studied, ranging between 20–60%.2–5 Hyponatremia is more likely to be present in more advanced stages of cirrhosis. As a result, the incidence of hyponatremia among patients hospitalized with decompensated cirrhosis is much higher compared to what is observed in the outpatient setting. Hyponatremia has been found in 15–20% and 30–40% of those with Child-Pugh Class B and Class C, respectively.2,3 Similarly, hyponatremia is strongly associated with severity of liver disease (Odds ratio for Model for End stage Liver Disease (MELD) >16=7.84).5 Furthermore, among those affected with hepatorenal syndrome type 1 (HRS-1), an ominous form of acute kidney injury in advanced cirrhosis, hyponatremia is virtually universal and may be used a clue to point toward HRS-1 diagnosis.6
Hyponatremia in cirrhosis has been associated with important clinical outcomes including increased mortality and increase risk of cirrhotic complications as well as reduced quality of life and increased healthcare burden (Figure 1).
Figure 1. Clinical Outcomes of Hyponatremia of Cirrhosis.
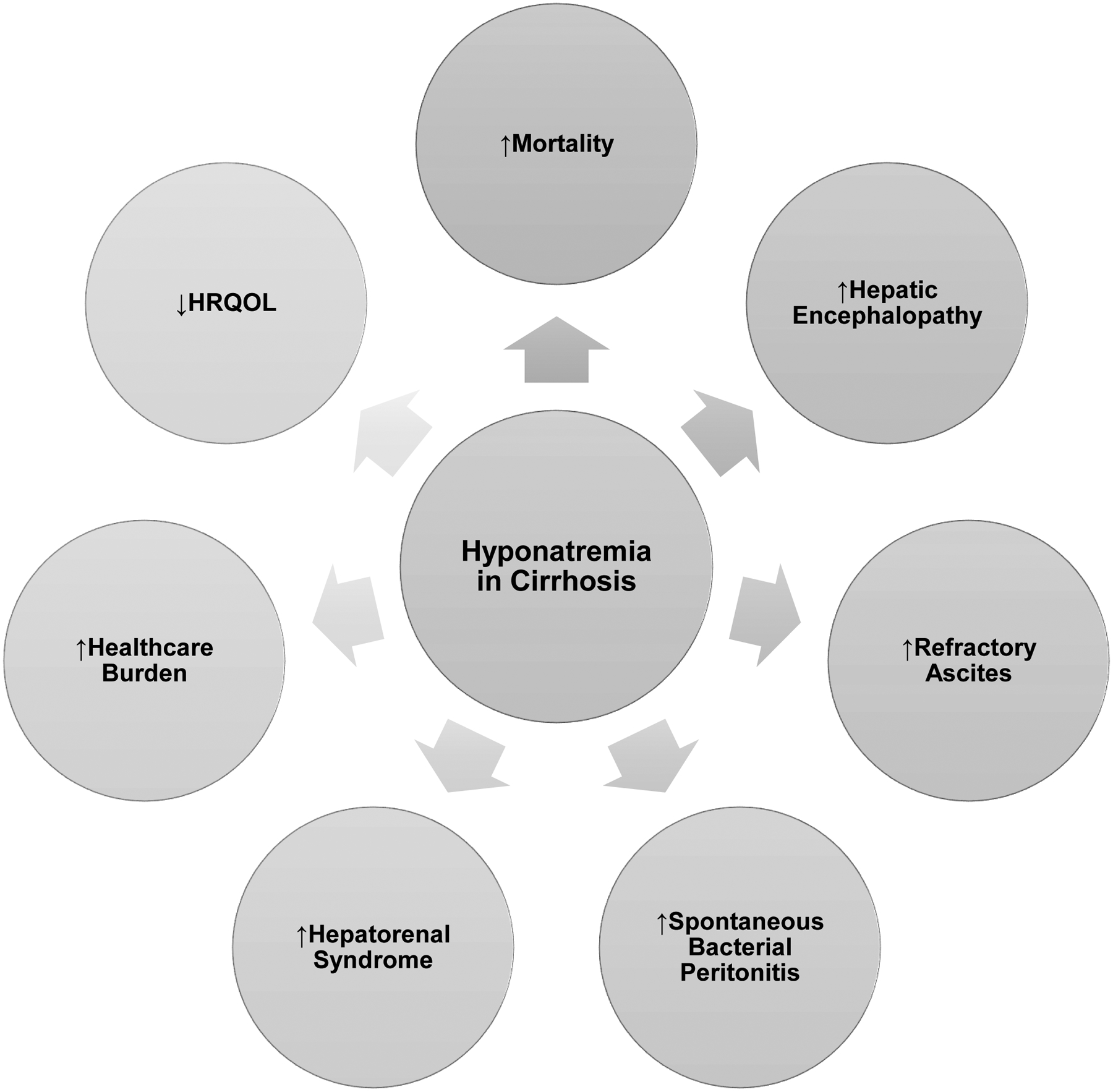
HRQOL=health-related quality of life
Mortality
It has long been recognized that hyponatremia in cirrhosis is a predictor of mortality.7 An analysis of a registry of 6796 patients awaiting liver transplant estimated an increase in the risk of death of 5% (HR=1.05; 95%CI, 1.03–1.08; p<0.001) per unit decrease in SNa for SNa between 125 and 140 mmol/L.8 In 2002, allocation for deceased donor livers in the United States changed to a “sickest first” policy, with priority based on a MELD score.9 A number of single-center retrospective studies have shown that the addition of SNa to MELD score (MELD-Na), significantly increases the accuracy to predict mortality.10 It is estimated that the use of MELD-Na scores could have averted 7% of deaths in patients awaiting liver transplant.8 These findings led to the United Network for Organ Sharing (UNOS) to incorporate SNa in the calculation of the MELD score for organ allocation in 2016.11
Cirrhosis complications
Hyponatremia has been implicated in the pathogenesis of hepatic encephalopathy (HE). A study of 997 patients demonstrated a higher frequency of HE episodes within a 4-week period with decreasing baseline SNa levels (HE in 15, 24 and 38% in patients with baseline SNa >135, 131–135 and ≤130 mmol/L, respectively).2 In another study of 61 patients with cirrhosis, hyponatremia was found to be an independent predictor of overt HE (OR=10.5, 95%CI 5.44–20.30, p<0.001).12 The relationship between hyponatremia and HE could be explained by brain adaptation to hyponatremia. Reduction in plasma tonicity causes water movement into brain cells, specifically astrocytes, which adapt to acute swelling by releasing solutes to the extracellular space. Initially, electrolytes (potassium and chloride ions) are lost but further cell volume reduction is achieved by loss of organic osmolytes such as glutamate, glutamine, and myo-inositol (MI).13
HE similarly reflects the manifestations of a low-grade cerebral edema without clinically overt increase in intracranial pressure.14 Ammonia is exclusively metabolized in astrocytes by glutamine synthase, which converts ammonia and glutamate into glutamine. It has been hypothesized that glutamine contributes to the pathogenesis of HE, at least in part, by acting as an osmolyte causing water translocation and astrocyte swelling.15 Consistent with this, proton magnetic resonance spectroscopy has noted a reduction of MI signal in the brain of cirrhotic patients confirming an osmotic adaptation.16 Under conditions of low grade cerebral edema associated with HE where MI stores for counteraction of cell swelling are largely depleted, hyponatremia may act as a second osmotic hit resulting in overt HE.12
Hyponatremia has been associated with refractory ascites (RA) with higher incidences observed at more severe degrees of hyponatremia.2 In a retrospective study of 188 hospitalized patients with cirrhosis, patients with hyponatremia had a significant risk of developing ascites (OR=2.708, 95%CI 1.034–7.092, p=0.043).3 In addition, hyponatremia has also been linked to greater ascitic fluid accumulation, greater likelihood of undergoing paracentesis, greater requirement for large volume paracentesis, and shorter time interval between paracentesis2 as well as decreased sensitivity to diuretics.17 Hyponatremia is also an important predictor for SBP.2, 3
HRS-1 is more common in patients with hyponatremia and ascites (OR=3.45, 95%CI 2.04–5.82 for patients with SNa≤130 mmol/L).2 A prospective study of 234 cirrhotic patients with ascites followed over 5 years demonstrated that SNa≤133 mmol/L was strongly predictive of HRS-1.18
In addition, hyponatremia may also have an impact on health-related quality of life (HRQOL) and healthcare burden in patients with cirrhosis.19, 20
Post liver transplant outcomes
Early European studies demonstrated an association between pre liver transplant (pre-LT) hyponatremia and increased post-LT mortality and complications.4, 21 A single-center retrospective study of 241 patients with cirrhosis in Spain showed that patients with pre-LT hyponatremia had a higher post-LT 90-day mortality compared to patients with pre-LT normonatremia after adjusting for cofounders (84% vs 95%, respectively, p<0.05).4 In addition, patients with pre-LT hyponatremia had a greater risk of postoperative complications. In a multicenter study based on a registry of 5,152 liver transplant recipients from the UK and Ireland, patients with pre-LT hyponatremia had a greater adjusted risk of post-LT 90-day mortality compared to pre-LT normonatremic patients (HR=1.55, 95% CI: 1.18–2.04; p<0.002).21 However, cofounders could have affected the findings of the above studies.22 More recent and larger studies have not confirmed these initial findings.23
PATHOGENESIS
The development of portal hypertension is the cornerstone for the pathogenesis of hyponatremia in individuals with cirrhosis. Increase in hydraulic pressure in the hepatic sinusoids resulting from liver fibrosis translates into increases in portal vein pressure. This phenomenon results in the development of ascites.24 As a consequence of the increased intrahepatic sinusoidal pressure, vasodilators are released, specifically nitric oxide and prostaglandins.25 These vasodilators exert their effects in peripheral vascular beds. Thus, splanchnic arterial vasodilatation ensues leading to pooling of blood, decrease in systemic vascular resistance and compensatory increase in cardiac output, i.e., hyperdynamic circulation. Early on, this adaptation maintains systemic arterial blood pressure. But at advanced stages, this adaptive mechanism becomes insufficient, and the mean arterial pressure (MAP) starts to fall. This cascade of events leads to a reduction in effective arterial blood volume (EABV).26 Baroreceptors localized in the carotid sinus, aortic arch and other areas sense the fall in EABV and activate the SNS. The RAAS is also activated by 2 related mechanisms: 1) β-adrenergic receptor stimulation originated by the activated SNS leads to renin release; 2) fall in EABV leads to decrease in kidney perfusion, fall in glomerular filtration rate (GFR), decrease in delivery of filtered chloride to the macula densa, activation of prostaglandins and renin release. Renin is responsible for cleavage of angiotensinogen into angiotensin I which is further converted into angiotensin II. In turn, activation of the SNS and RAAS induce an increase in sodium and water retention in the kidney (Figure 2).
Figure 2. Pathogenesis of Hyponatremia of Cirrhosis.

NO=nitric oxide, EABV=effective arterial blood volume, RAAS=renin-angiotensin-aldosterone system, SNS=sympathetic nervous system, AVP=arginine vasopressin, V2R=vasopressin 2 receptor, AQP2=aquaporin 2, Na=sodium, H20=water, ↑=increased, ↓=decreased
Independently of the activation of the SNS and the RAAS, baroreceptor stimulation by low EABV signals to the hypothalamus via afferent sympathetic nerves to induce non-osmotic synthesis and release of AVP.27 The net result of avid reabsorption of water mediated by AVP is hyponatremia.
Although the hyponatremia of cirrhosis is primarily dilutional, there is data suggesting it might also be a function of potassium deficiency.28 Edelman empirically showed that the SNa is determined by the ratio between total body exchangeable sodium and potassium and total body water29 with changes in potassium mass balance leading to hyponatremia or its correction.30 Potassium deficiency could be originated from renal or gastrointestinal losses (see etiology).
ETIOLOGY
Cirrhosis itself
Most cases of hyponatremia in cirrhosis encountered in clinical grounds are AVP-dependent (Figure 3). As described above, sequential development of cirrhosis, portal hypertension, hyperdynamic circulation and fall in EABV lead to adaptive AVP release. Thus, progressive worsening of cirrhosis and portal hypertension is a sufficient factor on its own to cause hyponatremia.
Figure 3. Etiology of Hyponatremia in Cirrhosis.

AVP=arginine vasopressin, SVR=systemic vascular resistance, ETOH=ethanol, ↓=decreased
Gastrointestinal fluid losses
Another etiology of hyponatremia relates to causes of hypovolemic hyponatremia. This category includes scenarios triggered by fluid losses as the primary insult. However, it is the adaptive release of AVP secondary to the fall in EABV that ultimately drives the hyponatremia, rather than the sodium depletion itself. Prophylaxis for hepatic encephalopathy with oral lactulose is commonly used in cirrhosis. Lactulose can cause profuse diarrhea, volume depletion and lead to clinical presentation with hyponatremia.
Renal sodium losses
Diuretic therapy is commonly utilized to manage ascites in patients with cirrhosis. Loop diuretics are unlikely to cause hyponatremia because blockade of the Na+-K+-2Cl− cotransporter by them leads to reduction in the renal medullary interstitial tonicity necessary for water reabsorption. On the other hand, aldosterone receptor antagonists, such as spironolactone, do not perturb the renal medullary tonicity. Furthermore, they block Na reabsorption in the collecting duct and can potentially lead to significant urinary loss of Na, hypovolemia, and exacerbation in AVP release and water retention.31
Adrenal insufficiency
The prevalence of adrenal insufficiency in cirrhosis has been reported to be increased, with a prevalence of up to 49%.32 Thus, the term hepatoadrenal insufficiency has been proposed.33 Under physiological conditions, cortisol inhibits AVP release in the hypothalamus. Thus, cortisol deficiency, as seen in adrenal insufficiency, leads to unopposed AVP release. Because AVP release is already augmented in the context of cirrhosis, it might be difficult to ascertain clinically to what extent adrenal insufficiency may contribute to hyponatremia. Supporting a contributing role of adrenal insufficiency, hyponatremia was found to be present in 42% of patients with cirrhosis and adrenal insufficiency compared to only 17% in those without adrenal insufficiency.32
Terlipressin
Terlipressin is a vasopressin analog with greater affinity for the V2 receptor compared to that for the V1a receptor. Thus, it can mimic the actions of AVP in the renal collecting duct. Terlipressin is currently used as a vasoconstrictor for the treatment of HRS-1 and acute variceal bleeding in Europe, Asia, and some countries in Latin America, but it is not approved in North America. In a retrospective study of 44 patients treated with terlipressin for acute variceal bleeding, a mean decrease in SNa of 11 mmol/L was observed.34 In a larger multicenter cohort of patients treated with terlipressin for acute gastrointestinal bleeding, hyponatremia and severe hyponatremia (< 125 mmol/L) were present in 26% and 13%, respectively.35 Notably, in a recent randomized placebo-controlled trial testing the use of terlipressin for HRS-1 in North America (CONFIRM trial), hyponatremia was not reported to be more common in terlipressin-treated subjects.36
Low solute intake
Individuals with alcoholic cirrhosis may also present with hyponatremia after a period of heavy intake of alcoholic beverages coupled with low solute intake, as seen in beer potomania.37 In these cases, AVP release is not stimulated. Importantly, complex cases of chronic high AVP state combined with low solute intake may be encountered and may represent a therapeutic challenge.
Pseudohyponatremia
Obstructive cholestasis can be associated with substantial increase in total cholesterol and lipoprotein X. This rare phenomenon has also been described in primary biliary cirrhosis and should considered in cases of unexplained hyponatremia.38 The lipid layer artificially increases the denominator in SNa estimate. Sodium determination by direct potentiometry overcomes this problem.
DIAGNOSTIC APPROACH
The diagnostic evaluation of hyponatremia in patients with cirrhosis is similar to the one in the general population (Figure 4). The main goals are to determine whether hyponatremia is hypotonic; and if so, whether hypotonic hyponatremia is mediated by AVP; and in this latter case, whether AVP secretion is physiologically appropriate.39
Figure 4. Diagnostic Approach to Hyponatremia in Cirrhosis.

SNa=serum sodium, SOsm=serum osmolality, UOsm=urine osmolality, AVP=arginine vasopressin, UNa=urine sodium, POCUS=point-of-care ultrasound, LVP=large volume paracentesis, GI=gastrointestinal. POCUS (+): presence of features of intravascular volume depletion. POCUS (−): absence of features of intravascular volume depletion.
Is the hyponatremia hypotonic?
A serum osmolality (SOsm) <275 mOsm/kg confirms hypotonicity. A SOsm ≥ 275 mOsm/kg can represent isotonicity or hypertonicity, but in some instances also hypotonicity when ineffective osmoles (e.g., urea and ethanol) are present in large concentrations. Therefore, SOsm should be interpreted carefully to prevent misclassification of hyponatremia. Having rule out the presence of ineffective osmoles, a SOsm ≥275 mOsm/kg indicates either hypertonicity usually from hyperglycemia or isotonicity due to pseudohyponatremia.
Is the hypotonic hyponatremia mediated by AVP?
Elevated AVP is the most common mechanism of hypotonic hyponatremia. This is characterized by the presence of urine that is not maximally diluted (UOsm ≥100mOsm/kg), whereas a urine that is maximally diluted (UOsm<100 mOsm/kg) suggests AVP-independent causes such as low solute intake (e.g., beer potomania). Renal insufficiency is also a cause of AVP-independent hyponatremia, and UOsm is typically less than SOsm but not <100 mOsm/kg.
Is AVP secretion in this hypotonic hyponatremia physiologically appropriate?
The presence of elevated AVP in hyponatremia is physiologically appropriate when the EABV is reduced. States of reduced EABV are characterized by renal sodium avidity manifested by UNa <20 mmol/L as a result of RAAS activation.40 The two main considerations include cirrhosis itself and hypovolemia.41 Distinguishing between these two possibilities is difficult as clinical assessment of volume status in hyponatremia has poor sensitivity and specificity.42 Point-of-care Ultrasound (POCUS) has emerged as an effective tool to assess volume status in hyponatremia43 and may distinguish among patients with cirrhosis who are volume depleted.44 If the EABV is not reduced, then AVP release is physiologically inappropriate (i.e., syndrome of inappropriate antidiuresis or SIAD).The latter is manifested by UNa>30 mmol/L.
THERAPY
Overview of the management of hyponatremia in cirrhosis
The treatment of hyponatremia in cirrhosis follows the same principles of management in the general population with some caveats. The choice and timing of therapy are guided by the duration of hyponatremia and the severity of symptoms. Hyponatremia can be classified based on its duration as acute (<48 hours) or chronic (≥48 hours) and based on the presence and severity of symptoms as apparently asymptomatic and symptomatic (with mild, moderate, or severe symptoms). The American Expert Panel and European Clinical Practice Guidelines agree that severe symptoms include seizures and coma while there is some disagreement of what constitutes moderate symptoms.45, 46
The above guidelines recommend that patients with moderately or severely symptomatic hyponatremia, or patients with acute hyponatremia with SNa<130 mmol/L with headache, vomiting or confusion who are at risk of brain herniation require emergent therapy with hypertonic saline 3% intravenous (IV) bolus injection 100 or 150 mL, up to 3 times.45, 46 The goal of correction is to increase SNa by 4–6 mmol/L within the first hour. Once symptoms subside, further correction should be postponed for the next day with a limit of no more than 7 mmol/L per day.47 The American Expert panel alternatively recommend the use of hypertonic saline 3% continuous infusion in patients with moderate symptoms.46 It is unclear whether bolus injection is superior to continuous infusion. The recent SALSA trial, a randomized controlled trial that recruited 178 patients with hyponatremia with moderate or severe symptoms, demonstrated that rates of overcorrection of hyponatremia with hypertonic saline 3% were similar among patients treated with bolus injection vs. continuous infusion (17.4% vs 24.2%, absolute risk difference, −6.9%, 95% CI −18.8% − 4.9%; p=0.26).48
Hyponatremia in a patient with cirrhosis that is chronic and apparently asymptomatic or mildly symptomatic does not require emergent therapy. In contrast to acute or symptomatic hyponatremia, a SNa correction goal of 4 mmol/L per day should be attained gradually over 24 hours; and similarly, a limit of no more than 7 mmol/L per any 24-hour period should be established to prevent the development of complications.47
Hypovolemic hyponatremia
Patients with cirrhosis with hypovolemic hyponatremia need volume expansion with isotonic crystalloids aiming to remove the stimuli for ongoing AVP secretion along with the removal of the precipitating factor (e.g., discontinuation of diuretics).
Hyponatremia of cirrhosis
The treatment of chronic hyponatremia of cirrhosis constitutes a challenge as most treatment modalities have not been extensively studied in this population and/or suffer from limited efficacy or tolerability.
Discontinuation of diuretics
A temporary discontinuation of diuretics is usually recommended in patients with hyponatremia of cirrhosis although this can be challenging as can lead to worsening ascites requiring repeated paracentesis.
Correction of hypokalemia
As mentioned before hypokalemia is involved in the pathogenesis of hyponatremia of cirrhosis. In addition, hypokalemia can precipitate hepatic encephalopathy, which underscores the importance of correcting potassium deficits in these patients. Correction of hypokalemia should be done carefully as it can result in (rapid) hyponatremia correction.30
Fluid restriction
The goal of fluid restriction (FR) is to create a state of negative free water balance. In several randomized controlled trials comparing vaptans to placebo, the efficacy of FR in the placebo group in improving SNa >5 mmol/L ranged from 0% to 26%.49, 50 A hyponatremia registry which included 595 patients with cirrhosis showed that only 27% and 36% of patients with moderate (120–125 mmol/L) and severe (<120 mmol/L) hyponatremia treated with FR increased their SNa by ≥5 mmol/L at days 2 and 3, respectively.51 Nevertheless, FR of ≤ 1.0–1.5 L/day is still recommended although efficacy is limited and patient adherence is difficult.
Albumin
The first clues about the potential effects of albumin on the hyponatremia of cirrhosis came from old studies demonstrating that acute expansion of plasma volume with IV albumin and saline in cirrhosis increases renal free water clearance.52 Further evidence was provided by a small case series.53
In a prospective cohort of 2435 hospitalized patients with cirrhosis, there was significant difference in hyponatremia resolution between those who did and those did not receive albumin (85.41% vs. 44.78%, p=0.0057; OR=1.50, 95% CI: 1.13–2.00).54
More recently, the human albumin for the treatment of ascites in patients with hepatic cirrhosis (ANSWER) trial randomized 431 patients with cirrhosis and uncomplicated ascites to standard medical treatment (SMT) vs. SMT and albumin (40 g twice weekly for 2 weeks, and then 40 g weekly for up to 18 months).55 The incidence of hyponatremia was significantly lower in the SMT and albumin group compared to SMT alone (incidence rate ratio = 0.51, 95%CI: 0.40–0.67, p<0.001). Overall, existing evidence suggest a beneficial effect of albumin in the treatment of hyponatremia of cirrhosis but further evidence from prospective studies is needed.
Urea
Urea is an endogenous product of amino acid metabolism and has been used to treat hyponatremia since the early 1980s because of its osmotic diuretic properties causing free water excretion without sodium.56 Only a few case reports document its efficacy for the hyponatremia of cirrhosis.57, 58 There is a theoretical concern of HE as a small amount of urea may reach the colon where it may be metabolized by urease-containing bacteria leading to increased ammonia production.59 Given the limited evidence, the role of urea in the treatment of hyponatremia of cirrhosis remains unclear.
Vasopressin antagonists
AVP binds to the V2 receptor (V2R) on the basolateral membrane of principal cells of the collecting duct activating adenylyl cyclase and generating cyclic AMP (cAMP). In turn, cAMP activates protein kinase A, which phosphorylates aquaporin 2 water channels inducing their relocation to the apical membrane, promoting net free water reabsorption. Vasopressin antagonists (vaptans) work in hyponatremia by penetrating deep into the V2R and altering the affinity of AVP to its receptor. Therefore, water is not reabsorbed, causing the excretion of diluted urine and thereby increasing SNa.60 There is significant evidence on the efficacy of vaptans in various forms of AVP-dependent hyponatremia, however the data in cirrhosis is less convincing.61
The SALT trials enrolled 448 adult patients with euvolemic and hypervolemic hyponatremia with the purpose of investigating the efficacy of tolvaptan to raise SNa.62 The main causes of euvolemic and hypervolemic hyponatremia were SIAD (42.4%), heart failure (30.8%) and cirrhosis (26.7%). Compared to placebo, patients in the tolvaptan group had a significantly higher mean SNa at day 4 (133.9±4.8 vs. 128.7±4.1 mmol/L, respectively) and at day 30 (135.7±5 vs. 131±6.2 mmol/L, respectively). Adverse events were similar in both groups with dry mouth and thirst being the most common. A sub-analysis of the SALT trials that examined only patients with cirrhosis found similar results.63
The SALTWATER trial was an extension of the SALT trials enrolling 111 patients at least 7 days after the final tolvaptan dose was received and followed for a mean duration of 1.9 years.64 The main etiologies of hyponatremia were SIAD (52.3%), heart failure (29.7%) and cirrhosis (18%). Normonatremia was achieved and maintained throughout the duration of the study in 57% of patients.
Overall, the above trials enrolled small numbers of patients with cirrhosis. In addition, a less robust response to tolvaptan was observed in these trials and some metanalyses.62, 64–66 States associated with low GFR and/or enhanced proximal tubular reabsorption of water such as heart failure and cirrhosis display a reduced amount of tubular fluid delivered to distal nephron segments, the site of vaptan action, limiting their efficacy.67 Additionally, “real-life” experience with tolvaptan in a small case series of patients with cirrhosis suggest limited efficacy.61
At present, only tolvaptan and conivaptan have been approved in the US by the Food and Drug Administration (FDA), while only tolvaptan in Europe has been approved for the management of severe hypervolemic hyponatremia. Conivaptan has significant V1A receptor affinity and its use in cirrhosis is not recommended because V1 blockade could exacerbate splanchnic vasodilatation and interfere with platelet aggregation, thus promoting hypotension and variceal bleeding.68
A concern with the use of vaptans in cirrhosis is the potential to exacerbate liver injury. In the SALT or SALTWATER trials, no significant elevation of hepatic enzymes was observed.62, 64 However, the use of tolvaptan in the TEMPO 3:4 study,69 aimed to determine the efficacy and safety of tolvaptan in autosomal dominant polycystic kidney disease, was associated with a significant increase in liver function tests. Based on these concerns, the FDA issued a warning limiting the use of tolvaptan to 30 days and recommending against the use of vaptans in patients with liver disease.70 Notably, the dose of tolvaptan used in this study was 4 times the dose commonly used to treat hyponatremia. Thus, it is recommended to limit the use of tolvaptan in cirrhosis when the clinical benefit outweighs the risks, such as in patients who are imminently awaiting liver transplantation (see perioperative management in patients awaiting liver transplant).).
PERIOPERATIVE MANAGEMENT IN PATIENTS AWAITING LIVER TRANSPLANT
Correction of SNa ≥8 mmol/L in any 24 hour period in patients with chronic hyponatremia can lead to the osmotic demyelination syndrome (ODS)47, a potentially devastating neurological complication. In this setting, cirrhosis and other comorbid conditions (SNa ≤105 mmol/L, alcohol use disorder, hypokalemia, and malnutrition) increase the susceptibility for ODS.46 Patients undergoing liver transplant are particularly at risk for rapid SNa correction due to fluid shifts that occur during surgery as a consequence of intraoperative administration of intravenous crystalloids, blood products, and sodium bicarbonate.22, 71, 72 The risk of ODS post-LT inversely correlates with the baseline SNa.73
The incidence of ODS post-LT ranges from 0.5% to 1.5%.73–75 Symptom onset usually occur within 1–2 weeks after surgery and it is commonly manifested as encephalopathy (36–45%), quadriparesis (up to 45%), and seizures (27–36%).74, 76, 77 Diagnosis is clinical although MRI brain demonstrating the characteristic T2/FLAIR hyperintensity in the central pons (“trident” shaped appearance) supports the diagnosis.78 MRI changes may not manifest until 4 weeks after symptom onset; hence serial imaging should be considered.
ODS outcomes post-LT are variable. While some series showed no difference in mortality74, 76, two of the largest series demonstrated mortality of 40% at 3 months77 and 63% at 1 year.73 In addition, up to 84% of patients that survived remained with permanent sequalae.76, 77
This heightened risk of ODS in patients with cirrhosis constitutes a real concern for many transplant surgeons who might delay a life-saving surgery until SNa is corrected to an acceptable level but there is no standardized protocol or SNa threshold across all transplant programs22. Some have advocated the short-term use of vaptans to expedite liver transplant surgery. Tolvaptan can be used because the potential for hepatotoxicity is less of a concern in this setting.79 Intraoperative CRRT with customized low-sodium dialysate has also been used.80
Management of established ODS in liver transplant recipients is mainly supportive with the help of a multidisciplinary team. We suggest correcting SNa to at least 125 mmol/L when patient can proceed with surgery as the risk of ODS above this SNa becomes negligible. Compulsory frequent SNa checks are recommended. A suggested approach to the perioperative management of hyponatremia in patients awaiting liver transplant is outlined in Figure 5.
Figure 5. Perioperative Management of Hyponatremia in Patients Awaiting Liver Transplant.

For patients with enough time to correct serum sodium (SNa) slowly (e.g., 7-day window before surgery), discontinuation of diuretics, potassium repletion, fluid restriction, and albumin should be attempted for the first 48 hours before moving to tolvaptan. For patients who are immediately going to the operating room where there is no time to correct SNa slowly, intraoperative continuous renal replacement therapy (CRRT) with low sodium dialysate or post filter fluid replacement with dextrose 5% in water (D5W) could be considered.
SUMMARY
Hyponatremia in cirrhosis is common and its pathogenesis principally involves non-osmotic AVP release resulting from the circulatory dysfunction associated with later stages of liver disease. Hyponatremia of cirrhosis is associated with poor clinical outcomes including increased mortality and increased risk of cirrhotic complications. The major differential diagnosis for hyponatremia of cirrhosis is hypovolemia and POCUS can help distinguish them. The treatment of hyponatremia of cirrhosis is challenging and involves fluid restriction, discontinuation of diuretics, hypokalemia correction, and possibly albumin. The clinical benefits and risk profile of vaptans is unclear and its use should be restricted to patients who are imminently awaiting liver transplantation. Patients undergoing liver transplant are at higher risk for ODS and slow SNa correction is recommended.
CLINIC CARE POINTS.
POCUS can help distinguish between hyponatremia of cirrhosis and hypovolemia.
Preoperative management of hyponatremia prior to liver transplantation surgery should aim to correct SNa by less than 8 mmol/L in any 24-h period to avoid ODS utilizing tools that include tolvaptan and CRRT.
KEY POINTS.
Hyponatremia is the most common electrolyte abnormality in cirrhosis, and it is associated with ominous outcomes.
The most common type of hyponatremia in cirrhosis is mediated by an arginine vasopressin-dependent mechanism of water retention.
Treatment of hyponatremia of cirrhosis should start with conservative measures including fluid restriction, correction of hypokalemia and discontinuation of diuretics.
SYNOPSIS.
Hyponatremia is the most common electrolyte disorder encountered in clinical practice and a common complication of cirrhosis reflecting an increase in non-osmotic secretion of arginine vasopressin as a result of the circulatory dysfunction that is characteristic of advanced liver disease. Hyponatremia in cirrhosis has been associated with poor clinical outcomes including increased risk of morbidity and mortality, poor quality of life, and heightened healthcare utilization. Despite this, the treatment of hyponatremia in cirrhosis remains challenging as conventional therapies such as fluid restriction are frequently ineffective. In this review we discuss the epidemiology, clinical outcomes, pathogenesis, etiology, evaluation, and management of hyponatremia in cirrhosis.
DISCLOSURE STATEMENT
Helbert Rondon-Berrios is funded by exploratory/developmental research grant R21DK122023 from National Institutes of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Juan Carlos Q. Velez has participated in consulting for Mallinckrodt Pharmaceuticals and Bayer, advisory board for Mallinckrodt Pharmaceuticals, Travere, and speaker bureau for Otsuka Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gines P, Berl T, Bernardi M, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. Sep 1998;28(3):851–64. doi: 10.1002/hep.510280337 [DOI] [PubMed] [Google Scholar]
- 2.Angeli P, Wong F, Watson H, Gines P, Investigators C. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. Dec 2006;44(6):1535–42. doi: 10.1002/hep.21412 [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Lee JS, Lee SH, et al. The association between the serum sodium level and the severity of complications in liver cirrhosis. Korean J Intern Med. Jun 2009;24(2):106–12. doi: 10.3904/kjim.2009.24.2.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Londono MC, Guevara M, Rimola A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. Apr 2006;130(4):1135–43. doi: 10.1053/j.gastro.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 5.Ennaifer R, Cheikh M, Romdhane H, et al. Hyponatremia in cirrhosis: Risk factors and prognostic value. Tunis Med. May 2016;94(5):401–405. [PubMed] [Google Scholar]
- 6.Velez JCQ, Therapondos G, Juncos LA. Reappraising the spectrum of AKI and hepatorenal syndrome in patients with cirrhosis. Nat Rev Nephrol. Mar 2020;16(3):137–155. doi: 10.1038/s41581-019-0218-4 [DOI] [PubMed] [Google Scholar]
- 7.Arroyo V, Rodes J, Gutierrez-Lizarraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis. Mar 1976;21(3):249–56. doi: 10.1007/BF01095898 [DOI] [PubMed] [Google Scholar]
- 8.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. Sep 4 2008;359(10):1018–26. doi: 10.1056/NEJMoa0801209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman RB Jr., Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. Sep 2002;8(9):851–8. doi: 10.1053/jlts.2002.35927 [DOI] [PubMed] [Google Scholar]
- 10.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. Mar 2005;11(3):336–43. doi: 10.1002/lt.20329 [DOI] [PubMed] [Google Scholar]
- 11.Sharing UNfO. Changes to OPTN Bylaws and Policies from actions at OPTN/UNOS Exectuive Committee Meetings July 2015-November 2015. Accessed May 3, 2021. https://optn.transplant.hrsa.gov/media/1575/policynotice_20151101.pdf
- 12.Guevara M, Baccaro ME, Torre A, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. Jun 2009;104(6):1382–9. doi: 10.1038/ajg.2009.293 [DOI] [PubMed] [Google Scholar]
- 13.Verbalis JG. Brain volume regulation in response to changes in osmolality. Neuroscience. Jul 28 2010;168(4):862–70. doi: 10.1016/j.neuroscience.2010.03.042 [DOI] [PubMed] [Google Scholar]
- 14.Cordoba J, Alonso J, Rovira A, et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)H-magnetic resonance abnormalities after liver transplantation. J Hepatol. Nov 2001;35(5):598–604. doi: 10.1016/s0168-8278(01)00181-7 [DOI] [PubMed] [Google Scholar]
- 15.Haussinger D, Schliess F. Pathogenetic mechanisms of hepatic encephalopathy. Gut. Aug 2008;57(8):1156–65. doi: 10.1136/gut.2007.122176 [DOI] [PubMed] [Google Scholar]
- 16.Haussinger D, Laubenberger J, vom Dahl S, et al. Proton magnetic resonance spectroscopy studies on human brain myo-inositol in hypo-osmolarity and hepatic encephalopathy. Gastroenterology. Nov 1994;107(5):1475–80. doi: 10.1016/0016-5085(94)90552-5 [DOI] [PubMed] [Google Scholar]
- 17.Angeli P, Dalla Pria M, De Bei E, et al. Randomized clinical study of the efficacy of amiloride and potassium canrenoate in nonazotemic cirrhotic patients with ascites. Hepatology. Jan 1994;19(1):72–9. [PubMed] [Google Scholar]
- 18.Gines A, Escorsell A, Gines P, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. Jul 1993;105(1):229–36. doi: 10.1016/0016-5085(93)90031-7 [DOI] [PubMed] [Google Scholar]
- 19.Deitelzweig S, Amin A, Christian R, Friend K, Lin J, Lowe TJ. Hyponatremia-associated healthcare burden among US patients hospitalized for cirrhosis. Adv Ther. Jan 2013;30(1):71–80. doi: 10.1007/s12325-012-0073-1 [DOI] [PubMed] [Google Scholar]
- 20.Sola E, Watson H, Graupera I, et al. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol. Dec 2012;57(6):1199–206. doi: 10.1016/j.jhep.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 21.Dawwas MF, Lewsey JD, Neuberger JM, Gimson AE. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl. Aug 2007;13(8):1115–24. doi: 10.1002/lt.21154 [DOI] [PubMed] [Google Scholar]
- 22.Leise M, Cardenas A. Hyponatremia in Cirrhosis: Implications for Liver Transplantation. Liver Transpl. Nov 2018;24(11):1612–1621. doi: 10.1002/lt.25327 [DOI] [PubMed] [Google Scholar]
- 23.Leise MD, Yun BC, Larson JJ, et al. Effect of the pretransplant serum sodium concentration on outcomes following liver transplantation. Liver Transpl. Jun 2014;20(6):687–97. doi: 10.1002/lt.23860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gines P, Fernandez-Esparrach G, Arroyo V, Rodes J. Pathogenesis of ascites in cirrhosis. Semin Liver Dis. 1997;17(3):175–89. doi: 10.1055/s-2007-1007196 [DOI] [PubMed] [Google Scholar]
- 25.Rizvi MR, Tauseef M, Shahid M, et al. Nitric oxide and prostaglandin as mediators in the pathogenesis of hyperkinetic circulatory state in a model of endotoxemia-induced portal hypertension. Hepatol Int. Jun 2013;7(2):622–35. doi: 10.1007/s12072-012-9397-9 [DOI] [PubMed] [Google Scholar]
- 26.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. Sep-Oct 1988;8(5):1151–7. doi: 10.1002/hep.1840080532 [DOI] [PubMed] [Google Scholar]
- 27.Schrier RW. Water and sodium retention in edematous disorders: role of vasopressin and aldosterone. Am J Med. Jul 2006;119(7 Suppl 1):S47–53. doi: 10.1016/j.amjmed.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 28.Birkenfeld LW, Leibman J, O’Meara MP, Edelman IS. Total exchangeable sodium, total exchangeable potassium, and total body water in edematous patients with cirrhosis of the liver and congestive heart failure. J Clin Invest. May 1958;37(5):687–98. doi: 10.1172/JCI103655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. Sep 1958;37(9):1236–56. doi: 10.1172/JCI103712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berl T, Rastegar A. A patient with severe hyponatremia and hypokalemia: osmotic demyelination following potassium repletion. Am J Kidney Dis. Apr 2010;55(4):742–8. doi: 10.1053/j.ajkd.2009.12.024 [DOI] [PubMed] [Google Scholar]
- 31.Handler J Well tolerated spironolactone-related hyponatremia. J Clin Hypertens (Greenwich). Apr 2008;10(4):317–21. doi: 10.1111/j.1751-7176.2008.08063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh RR, Walia R, Sachdeva N, Bhalla A, Singh A, Singh V. Relative adrenal insufficiency in cirrhotic patients with ascites (hepatoadrenal syndrome). Dig Liver Dis. Nov 2018;50(11):1232–1237. doi: 10.1016/j.dld.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 33.Marik PE, Gayowski T, Starzl TE, Hepatic Cortisol R, Adrenal Pathophysiology Study G. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit Care Med. Jun 2005;33(6):1254–9. doi: 10.1097/01.ccm.0000164541.12106.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han X, Li J, Yang JM, Gao M, Wang L. A retrospective analysis of hyponatremia during terlipressin treatment in patients with esophageal or gastric variceal bleeding due to portal hypertension. JGH Open. Jun 2020;4(3):368–370. doi: 10.1002/jgh3.12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Lin S, Yang Y, et al. Development of hyponatremia after terlipressin in cirrhotic patients with acute gastrointestinal bleeding: a retrospective multicenter observational study. Expert Opin Drug Saf. May 2020;19(5):641–647. doi: 10.1080/14740338.2020.1734558 [DOI] [PubMed] [Google Scholar]
- 36.Wong F, Pappas SC, Curry MP, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. Mar 4 2021;384(9):818–828. doi: 10.1056/NEJMoa2008290 [DOI] [PubMed] [Google Scholar]
- 37.Ouellette L, Michel K, Riley B, Jones J. Beer potomania: Atypical cause of severe hyponatremia in older alcoholics. Am J Emerg Med. Jul 2018;36(7):1303. doi: 10.1016/j.ajem.2017.10.065 [DOI] [PubMed] [Google Scholar]
- 38.Hickman PE, Dwyer KP, Masarei JR. Pseudohyponatraemia, hypercholesterolaemia, and primary biliary cirrhosis. J Clin Pathol. Feb 1989;42(2):167–71. doi: 10.1136/jcp.42.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Workeneh BT, Jhaveri KD, Rondon-Berrios H. Hyponatremia in the cancer patient. Kidney Int. Oct 2020;98(4):870–882. doi: 10.1016/j.kint.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 40.Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol. Jul 2007;18(7):2028–31. doi: 10.1681/ASN.2006111302 [DOI] [PubMed] [Google Scholar]
- 41.Gines P, Guevara M. Hyponatremia in cirrhosis: pathogenesis, clinical significance, and management. Hepatology. Sep 2008;48(3):1002–10. doi: 10.1002/hep.22418 [DOI] [PubMed] [Google Scholar]
- 42.Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. Nov 1987;83(5):905–8. doi: 10.1016/0002-9343(87)90649-8 [DOI] [PubMed] [Google Scholar]
- 43.Evins C, Rao A. Point-of-care ultrasound to evaluate volume status in severe hyponatremia. BMJ Case Rep. Jun 28 2020;13(6)doi: 10.1136/bcr-2020-235304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velez JCQ, Petkovich B, Karakala N, Huggins JT. Point-of-Care Echocardiography Unveils Misclassification of Acute Kidney Injury as Hepatorenal Syndrome. Am J Nephrol. 2019;50(3):204–211. doi: 10.1159/000501299 [DOI] [PubMed] [Google Scholar]
- 45.Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant. Apr 2014;29 Suppl 2:i1–i39. doi: 10.1093/ndt/gfu040 [DOI] [PubMed] [Google Scholar]
- 46.Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. Oct 2013;126(10 Suppl 1):S1–42. doi: 10.1016/j.amjmed.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 47.Tandukar S, Sterns RH, Rondon-Berrios H. Osmotic Demyelination Syndrome Following Correction of Hyponatremia by ≤10 mEq/L per day. Kidney360. 2021:10.34067/KID.0004402021. doi: 10.34067/kid.0004402021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baek SH, Jo YH, Ahn S, et al. Risk of Overcorrection in Rapid Intermittent Bolus vs Slow Continuous Infusion Therapies of Hypertonic Saline for Patients With Symptomatic Hyponatremia: The SALSA Randomized Clinical Trial. JAMA Intern Med. Jan 1 2021;181(1):81–92. doi: 10.1001/jamainternmed.2020.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerbes AL, Gulberg V, Gines P, et al. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. Apr 2003;124(4):933–9. doi: 10.1053/gast.2003.50143 [DOI] [PubMed] [Google Scholar]
- 50.Gines P, Wong F, Watson H, et al. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. Jul 2008;48(1):204–13. doi: 10.1002/hep.22293 [DOI] [PubMed] [Google Scholar]
- 51.Sigal SH, Amin A, Chiodo JA 3rd, Sanyal A. Management Strategies and Outcomes for Hyponatremia in Cirrhosis in the Hyponatremia Registry. Can J Gastroenterol Hepatol. 2018;2018:1579508. doi: 10.1155/2018/1579508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vlahcevic ZR, Adham NF, Jick H, Moore EW, Chalmers TC. Renal Effects of Acute Expansion of Plasma Volume in Cirrhosis. N Engl J Med. Feb 25 1965;272:387–90. doi: 10.1056/NEJM196502252720802 [DOI] [PubMed] [Google Scholar]
- 53.McCormick PA, Mistry P, Kaye G, Burroughs AK, McIntyre N. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut. Feb 1990;31(2):204–7. doi: 10.1136/gut.31.2.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bajaj JS, Tandon P, O’Leary JG, et al. The Impact of Albumin Use on Resolution of Hyponatremia in Hospitalized Patients With Cirrhosis. Am J Gastroenterol. Sep 2018;113(9):1339. doi: 10.1038/s41395-018-0119-3 [DOI] [PubMed] [Google Scholar]
- 55.Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. Jun 16 2018;391(10138):2417–2429. doi: 10.1016/S0140-6736(18)30840-7 [DOI] [PubMed] [Google Scholar]
- 56.Decaux G, Brimioulle S, Genette F, Mockel J. Treatment of the syndrome of inappropriate secretion of antidiuretic hormone by urea. Am J Med. Jul 1980;69(1):99–106. doi: 10.1016/0002-9343(80)90506-9 [DOI] [PubMed] [Google Scholar]
- 57.Decaux G, Mols P, Cauchi P, Delwiche F. Use of urea for treatment of water retention in hyponatraemic cirrhosis with ascites resistant to diuretics. Br Med J (Clin Res Ed). Jun 15 1985;290(6484):1782–3. doi: 10.1136/bmj.290.6484.1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Decaux G, Mols P, Cauchie P, Flamion B, Delwiche F. Treatment of hyponatremic cirrhosis with ascites resistant to diuretics by urea. Nephron. 1986;44(4):337–43. doi: 10.1159/000184016 [DOI] [PubMed] [Google Scholar]
- 59.Rondon-Berrios H Urea for Chronic Hyponatremia. Blood Purif. 2020;49(1–2):212–218. doi: 10.1159/000503773 [DOI] [PubMed] [Google Scholar]
- 60.Berl T Vasopressin antagonists. N Engl J Med. Jun 4 2015;372(23):2207–16. doi: 10.1056/NEJMra1403672 [DOI] [PubMed] [Google Scholar]
- 61.Pose E, Sola E, Piano S, et al. Limited Efficacy of Tolvaptan in Patients with Cirrhosis and Severe Hyponatremia: Real-Life Experience. Am J Med. Mar 2017;130(3):372–375. doi: 10.1016/j.amjmed.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 62.Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. Nov 16 2006;355(20):2099–112. doi: 10.1056/NEJMoa065181 [DOI] [PubMed] [Google Scholar]
- 63.Cardenas A, Gines P, Marotta P, et al. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. Mar 2012;56(3):571–8. doi: 10.1016/j.jhep.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 64.Berl T, Quittnat-Pelletier F, Verbalis JG, et al. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. Apr 2010;21(4):705–12. doi: 10.1681/ASN.2009080857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jaber BL, Almarzouqi L, Borgi L, Seabra VF, Balk EM, Madias NE. Short-term efficacy and safety of vasopressin receptor antagonists for treatment of hyponatremia. Am J Med. Oct 2011;124(10):977 e1–9. doi: 10.1016/j.amjmed.2011.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rozen-Zvi B, Yahav D, Gheorghiade M, Korzets A, Leibovici L, Gafter U. Vasopressin receptor antagonists for the treatment of hyponatremia: systematic review and metaanalysis. Am J Kidney Dis. Aug 2010;56(2):325–37. doi: 10.1053/j.ajkd.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 67.Rondon-Berrios H, Berl T. Vasopressin receptor antagonists: Characteristics and clinical role. Best Pract Res Clin Endocrinol Metab. Mar 2016;30(2):289–303. doi: 10.1016/j.beem.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 68.Hline SS, Pham PT, Pham PT, Aung MH, Pham PM, Pham PC. Conivaptan: a step forward in the treatment of hyponatremia? Ther Clin Risk Manag. Apr 2008;4(2):315–26. doi: 10.2147/tcrm.s340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. Dec 20 2012;367(25):2407–18. doi: 10.1056/NEJMoa1205511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.US Food and Drug Administration. FDA Drug Safety Communication: FDA limits duration and usage of Samsca (tolvaptan) due to possible liver injury leading to organ transplant or death. Accessed April 16, 2021. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-limits-duration-and-usage-samsca-tolvaptan-due-possible-liver
- 71.Crismale JF, Meliambro KA, DeMaria S Jr., Bronster DB, Florman S, Schiano TD. Prevention of the Osmotic Demyelination Syndrome After Liver Transplantation: A Multidisciplinary Perspective. Am J Transplant. Oct 2017;17(10):2537–2545. doi: 10.1111/ajt.14317 [DOI] [PubMed] [Google Scholar]
- 72.Romanovsky A, Azevedo LC, Meeberg G, Zibdawi R, Bigam D, Bagshaw SM. Serum sodium shift in hyponatremic patients undergoing liver transplantation: a retrospective cohort study. Ren Fail. Feb 2015;37(1):37–44. doi: 10.3109/0886022X.2014.975102 [DOI] [PubMed] [Google Scholar]
- 73.Yun BC, Kim WR, Benson JT, et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology. May 2009;49(5):1610–5. doi: 10.1002/hep.22846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crivellin C, Cagnin A, Manara R, et al. Risk factors for central pontine and extrapontine myelinolysis after liver transplantation: a single-center study. Transplantation. Jun 2015;99(6):1257–64. doi: 10.1097/TP.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 75.Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: a systematic review. Eur J Neurol. Dec 2014;21(12):1443–50. doi: 10.1111/ene.12571 [DOI] [PubMed] [Google Scholar]
- 76.Lee EM, Kang JK, Yun SC, et al. Risk factors for central pontine and extrapontine myelinolysis following orthotopic liver transplantation. Eur Neurol. 2009;62(6):362–8. doi: 10.1159/000242426 [DOI] [PubMed] [Google Scholar]
- 77.Morard I, Gasche Y, Kneteman M, et al. Identifying risk factors for central pontine and extrapontine myelinolysis after liver transplantation: a case-control study. Neurocrit Care. Apr 2014;20(2):287–95. doi: 10.1007/s12028-013-9928-9 [DOI] [PubMed] [Google Scholar]
- 78.Alleman AM. Osmotic demyelination syndrome: central pontine myelinolysis and extrapontine myelinolysis. Semin Ultrasound CT MR. Apr 2014;35(2):153–9. doi: 10.1053/j.sult.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 79.Lenci I, Milana M, Angelico M, Baiocchi L. Short-Term, Low-Dose Use of Tolvaptan as a Bridge Therapy to Expedite Liver Transplant for Severe Hyponatremic, Cirrhotic Patients With High Model for End-Stage Liver Disease Scores. Exp Clin Transplant. Dec 2017;15(6):689–692. doi: 10.6002/ect.2015.0209 [DOI] [PubMed] [Google Scholar]
- 80.Nagai S, Moonka D, Patel A. Novel intraoperative management in the model for endstage liver disease-sodium era: Continuous venovenous hemofiltration for severe hyponatremia in liver transplantation. Liver Transpl. Feb 2018;24(2):304–307. doi: 10.1002/lt.24982 [DOI] [PubMed] [Google Scholar]


