Abstract
The Applying Wolbachia to Eliminate Dengue (AWED) trial was a parallel cluster randomised trial that demonstrated Wolbachia (wMel) introgression into Ae. aegypti populations reduced dengue incidence. In this predefined substudy, we compared between treatment arms, the relative abundance of Ae. aegypti and Ae. albopictus before, during and after wMel-introgression. Between March 2015 and March 2020, 60,084 BG trap collections yielded 478,254 Ae. aegypti and 17,623 Ae. albopictus. Between treatment arms there was no measurable difference in Ae. aegypti relative abundance before or after wMel-deployments, with a count ratio of 0.96 (95% CI 0.76, 1.21) and 1.00 (95% CI 0.85, 1.17) respectively. More Ae. aegypti were caught per trap per week in the wMel-intervention arm compared to the control arm during wMel deployments (count ratio 1.23 (95% CI 1.03, 1.46)). Between treatment arms there was no measurable difference in the Ae. albopictus population size before, during or after wMel-deployment (overall count ratio 1.10 (95% CI 0.89, 1.35)). We also compared insecticide resistance phenotypes of Ae. aegypti in the first and second years after wMel-deployments. Ae. aegypti field populations from wMel-treated and untreated arms were similarly resistant to malathion (0.8%), permethrin (1.25%) and cyfluthrin (0.15%) in year 1 and year 2 of the trial. In summary, we found no between-arm differences in the relative abundance of Ae. aegypti or Ae. albopictus prior to or after wMel introgression, and no between-arm difference in Ae. aegypti insecticide resistance phenotypes. These data suggest neither Aedes abundance, nor insecticide resistance, confounded the epidemiological outcomes of the AWED trial.
Author summary
Dengue is a mosquito-borne viral disease and a major public health problem in the tropical and subtropical world. It is caused by any of the four dengue virus serotypes. In a previously published randomised clinical trial, called the AWED trial, we demonstrated that releases of Aedes aegypti mosquitoes infected with the insect bacterium Wolbachia can reduce the case incidence of dengue by 77%. In this current study, we compared the abundance of Ae. aegypti mosquitoes in the neighbourhoods where Wolbachia-infected mosquitoes were released versus the untreated neighbourhoods. This was important to do so that scientists could understand the mechanism for how Wolbachia releases reduced dengue incidence. Between March 2015 and March 2020, we did not observe any differences in Ae. aegypti abundance before or after Wolbachia-deployments in the AWED trial area. There was also no difference in the abundance of the related mosquito, Ae. albopictus, before, during or after wMel-deployment. We also compared insecticide resistance characteristics amongst Ae. aegypti in the first and second years after Wolbachia -deployments and found no difference between mosquitoes from Wolbachia-treated and untreated neighbourhoods. These data suggest neither Aedes abundance, nor insecticide resistance, were confounding sources to the epidemiological outcomes of the AWED trial.
Introduction
Dengue is the most common mosquito-borne viral infection worldwide and is caused by any of the four serotypes of dengue virus (DENV). Aedes aegypti mosquitoes are the primary vectors of dengue viruses between humans. An estimated 4 billion people in 128 countries are at risk from local DENV transmission and the geographic range and disease burden have expanded substantially in the past 50 years [1].
Wolbachia bacteria are endosymbionts of insects that are present in several mosquito species including Aedes albopictus and Culex pipiens, but not in Ae. aegypti. Ae. aegypti stably transinfected with the Wolbachia strain wMel possess three traits that make them attractive as a public health intervention against dengue and other medically important arboviruses. First, wMel infection renders Ae. aegypti resistant to disseminated infection by dengue, Zika and chikungunya viruses [2–4]. Second, female mosquitoes vertically transmit wMel with high fidelity to their offspring, thus ensuring population-level Wolbachia establishment persists for many years [5–7]. Third, wMel manipulates mosquito reproductive outcomes, a process called cytoplasmic incompatibility (CI), to favour its own population introgression [5]. Combined, these traits enable the application of wMel as a public health intervention to reduce the vectorial capacity of mosquito populations to transmit DENV between humans.
The Applying Wolbachia to Eliminate Dengue (AWED) cluster randomised trial introgressed wMel into the Ae. aegypti population in 12 geographic clusters in Yogyakarta, Indonesia. Over 27 months, the incidence of virologically-confirmed dengue was reduced by 77% and the incidence of dengue hospitalisations by 86% when compared to untreated (control) clusters [8,9]. The most parsimonious explanation for the reduction in dengue incidence in the wMel introgressed clusters is the reduced vector competence of wMel-infected mosquito population. However, it’s plausible that wMel introgression had other effects on the Ae. aegypti population in Yogyakarta that contributed to these epidemiological outcomes. For example, wMel-infection decreases the survivorship of mosquito eggs [10–13] and this could plausibly result in a smaller Ae. aegypti population size in geographic clusters where wMel was introgressed. Periods of self-incompatibility represent another route to a smaller Ae. aegypti population size—this could occur if maternal transmission of wMel was periodically imperfect or CI transiently broke down [14,15]. Additionally, insecticide resistance characteristics in the wMel-infected mosquito population might change over time compared to wild-type mosquitoes in control clusters because of differences in selective pressure from insecticide use in the community.
Here we report longitudinal comparisons of Ae. aegypti relative abundance and insecticide resistance characteristics between wMel-intervention clusters and control clusters. We also report on the relative abundance and distribution of the secondary vector Ae. albopictus between intervention clusters and control clusters. These comparisons were prespecified secondary endpoints in the AWED protocol [9].
Methods
Study design
The AWED study was a parallel two-arm non-blinded cluster randomised controlled trial conducted in a single site in Yogyakarta City, Indonesia. The 26km2 study site with a population of 312,000 people was subdivided into twenty-four contiguous clusters approximately 1km2 in size (range 0.7km2-1.65km2). Twelve clusters were randomly selected to receive Wolbachia deployments and 12 were untreated. There are no buffer areas between clusters, but natural borders (roads, rivers, non-residential areas) were used to define cluster boundaries as much as possible, to limit the spatial spread of wMel from treated clusters into untreated areas, and of wild-type mosquitoes into wMel-treated clusters. No attempt was made to alter the routine dengue prevention and vector control activities conducted by public and private agencies throughout the study area (treated and untreated clusters).
Wolbachia deployment
wMel-infected Ae. aegypti were released as eggs using mosquito release containers (MRCs), as described previously [8]. Releases occurred between March and December 2017, with 9–14 rounds of releases in each intervention cluster. Releases stopped in each cluster when the prevalence of Wolbachia in field-caught mosquitoes was >60% for three consecutive release weeks. MRCs were reset every two weeks. An MRC was placed in 1–2 randomly selected locations within each 50x50m grid square across the intervention area. Permission was obtained from property owners to place MRCs on private property.
Mosquito surveillance
Weekly surveillance of Ae. aegypti in Yogyakarta commenced in March 2015, two years prior to the AWED trial releases, and continued until the trial’s conclusion in March 2020. Mosquitoes were collected using a network of BG Sentinel traps (Biogents, Germany) set indoors in residences throughout the city. Each trap was equipped with an accumulator to sustain power in case of a power outage. Written consent was obtained from heads of households hosting BG traps. The BG trap network had three temporal windows; the pre-release period, the release period during which wMel mosquitoes were released and the post-release period. For reasons related to budget and workforce capacity, it was not possible to keep the BG trap density constant between each phase but we did ensure balance between treatment arms for the great majority of the 5-year observation period. The median trapping density in the pre-release period March 2015—February 2017 was 3.5 traps/km2 (range 0.9 to 23.3) in the intervention clusters and 5.2 traps/km2 (range 0.6 to 6.8) in the untreated clusters. During the wMel release period March—December 2017, the median trapping density in the intervention clusters was increased to 15.7 traps/km2 (range 10.2 to 18.2). For the first seven weeks of releases the trapping density in the untreated clusters remained the same (median trapping density 5.2 traps/km2 [range 0.9 to 6.3]), followed by no trapping for two weeks. From May 2017 the number of traps in the untreated clusters was increased for the remainder of the release period to a median of 14.8 traps/km2 (range 5.9 to 16.8). In the post-intervention period January 2018—March 2020, a fixed spatial distribution of BG traps, with comparable density between study arms, was maintained. The median was 15.9 traps/km2 (range 12.3 to 18.1) in the intervention clusters and 14.8 traps/km2 (range 8.0 to 16.8) in the untreated clusters; the median (range) distance of traps from the cluster boundary was 68 (42–102) metres in the intervention clusters and 68 (32–84) metres in untreated clusters; and the median (range) of the average distance to the nearest trap was 211 (185–239) metres in intervention clusters and 225 (203–280) metres in untreated clusters. The median (range) nearest neighbour index was 1.6 (1.4–1.9) in the intervention clusters and 1.7 (1.5–1.9) in the untreated clusters, where a value of <1 indicates a clustered pattern and >1 indicates a dispersed pattern. The post-intervention BG trap distribution per cluster is shown in S1 Fig and tabulated in S1 Table.
Mosquitoes captured in BG traps were demobilised at -20°C for ≥1 hour, then identified by morphological features. The number of mosquitoes caught in each BG trap was recorded by species and sex. Ae. aegypti were stored at -20°C in 80% ethanol until testing for wMel infection. Individual whole mosquitoes were homogenised in 96 well plates using 1mm glass beads and a bead beater. After centrifugation, an aliquot of the homogenate supernatant was used for detection of wMel Wolbachia by qualitative PCR Taqman assay on a Roche LightCycler 480. The qPCR conditions consisted of a denaturation step at 95°C for 5 minutes followed by 45 cycles of PCR (denaturation at 95°C for 10 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 1 second with the single acquisition) followed by a cooling down step at 40°C for 10 seconds. Specific primers targeting the gene encoding Ae. aegypti Rps17 and wMel WD0513 were used as previously described [16], but with replacement of the Cy5-BHQ3 fluorophore-quencher pair in the wMel probe with the fluorophore-quencher LC640-IowaBlack (Integrated DNA technologies) [17]. Testing was at weekly intervals when Wolbachia prevalence was <80% and 4-week intervals when establishment was achieved (≥80% cluster level prevalence for two consecutive testing weeks).
Insecticide resistance testing
Insecticide resistance testing by bioassay was performed in 2018 and 2019 on field-derived adults sourced from each of the 24 clusters. Each cluster generated a cohort of mosquitoes that was tested for insecticide resistance, thus there were 12 cohorts from wMel-treated clusters and 12 from untreated clusters each year, with the exception of 2018 when there were 11 cohorts from wMel-treated clusters. Cyfluthrin and malathion were chosen for testing on the basis of their use in fine aerosol (fogging) spraying by the Yogyakarta Department of Health in response to dengue cases in the community. Permethrin was included on the basis of it being representative of pyrethroids commonly found in household insecticide sprays. Mosquito eggs were collected using ovitraps from in and around residential properties that hosted BG traps for Wolbachia monitoring. To maximise the probability that ovitraps were attracting only the desired populations of mosquitoes (i.e. 100% wMel infected or 100% wild-type) they were placed in properties where recent BG trap surveillance indicated 0% wMel prevalence (in untreated clusters) or 100% wMel prevalence (in intervention clusters). In 2018, a total of 346 ovitraps were deployed in BG host houses for egg collection over three weeks. In 2019, a total of 345 ovitraps were deployed in BG host houses and 370 in a neighbouring house for egg collection over two weeks. Neighbouring houses were added in 2019 to boost egg collection as there was some difficulty in getting sufficient eggs from only BG host houses in 2018. In 2018 egg collections were performed in June-July and in 2019 collections were performed in September. Eggs were dried for 1 day, stored, and hatched when required. Eggs from the same cluster were pooled before hatching. Ae. aegypti mosquitoes were visually identified and selected during the larval stage. Female Ae. aegypti mosquitoes were selected at the pupal stage. In 2018, F0 mosquitoes (i.e. the adults derived from field collected eggs) were used directly for insecticide resistance testing. In 2019, F1 adults (i.e. the offspring of F0 adults) were used for insecticide resistance testing. The move to using F1 adults was to reduce the resource-intensive collection of sufficient eggs from the field. Insecticide type and concentrations used were in line with recommendations for Ae. aegypti mosquitoes and followed the WHO standard bioassay method, noting that this method is only semi-quantitative [18]. Insecticide impregnated papers were purchased from the WHO Collaborating Centre at the Universiti Sains Malaysia, Penang, Malaysia. The papers used were Malathion 0.8%, Permethrin 1.25%, and Cyfluthrin 0.15%. Pyrethroid control paper was used for Permethrin and Cyfluthrin, and OP-Carbamate control was used for Malathion. In each year, six rounds of insecticide resistance testing were conducted, with two intervention clusters and two untreated clusters randomly allocated to each round. The first three testing rounds in 2018 (covering 12 clusters) included four replication tubes for each insecticide and two negative control tubes, with 25 female Ae. aegypti mosquitoes per tube. The mosquitoes were three to five days old, fed with sugar only. Mosquitoes were kept in a paper-free tube for one hour to adapt, transferred to the tube containing insecticide-impregnated paper for one hour, then transferred back to the holding tube, with access to sugar solution, for 24 hours. Dead and live mosquitoes were counted after 24 hours. The Rockefeller Ae. aegypti strain was used as a susceptible control line in the 2019 experiments.
Statistical analysis
The number of mosquitoes caught over time was summarised as the mean count per BG trap per week, by species and study arm (S1 Data). The ratio of female to male Ae. aegypti and Ae. albopictus over time was calculated on the basis of summed weekly counts and stratified by study arm. The ratio of Ae. aegypti to Ae. albopictus mosquitoes over time was also calculated on the basis of summed weekly counts and stratified by study arm.
Mixed-effects negative binomial regression was used to compare the number of Ae. aegypti caught per trap per week in the intervention arm versus the untreated arm, with the inclusion of BG trap as a random effect to account for clustering at the trap level. Zero-inflated negative binomial regression was used to compare the number of Ae. albopictus caught per trap per week in the intervention arm compared to the untreated arm, with a clustered sandwich estimator to control for clustering at the trap level. A zero-inflated model was used as >80% of trap collections had zero Ae. albopictus. All analyses included a binary indicator for dry/wet season as a covariate (wet season = November—April). The count ratio produced by the negative binomial regression model is the ratio of the mean number of Ae. aegypti or Ae. albopictus mosquitoes caught per trap per week in the intervention clusters compared to the untreated clusters. Data was analysed for the total observation period as well as separated by release status: pre-wMel release, during release, and post-wMel release, as defined earlier. Analysis of the total observation period was done with the inclusion of an indicator for release status as a covariate. The first nine weeks of releases were excluded from all analyses due to unequal trapping density by study arm and concerns that lower trapping density in the untreated clusters could result in less precise estimates of mosquito abundance compared to the intervention clusters.
Insecticide resistance was expressed as percentage mortality of Ae. aegypti exposed to various insecticides, calculated as the number of dead mosquitoes divided by the total number of mosquitoes in each replicate tube. The cluster-level percentage mortality is then the mean percentage mortality of the 4 replicate tubes in each cluster. Wilcoxon rank-sum test was used to compare the distribution of percentage mortality among 12 intervention clusters and 12 untreated clusters. Analysis was done separately for 2018 and 2019. One cluster from the intervention arm was excluded from analysis in 2018 due to low mosquito hatching rates resulting in insufficient mosquitoes for analysis.
All analyses were done using Stata version 16.0 (StataCorp, College Station, TX).
Sensitivity analysis
To account for Wolbachia contamination into untreated clusters, a sensitivity analysis was performed comparing the number of Ae. aegypti and Ae. albopictus caught per trap per week in the intervention arm versus the untreated arm, with untreated clusters reclassified as ‘treated’ when the cluster level Wolbachia frequency is >50% for 2 monthly monitoring events within a 6-month rolling window and >50% of the BG traps in the cluster have detected Wolbachia during those monitoring events. Two untreated clusters were reclassified as intervention clusters due to Wolbachia contamination, one from September 2019 onwards and the other from February 2020 onwards.
Power calculations
Power to detect a difference in mosquito abundance between wMel intervention and untreated arms was calculated using a simulation with 1,000 repeats of Ae. aegypti monitoring data from the pre-wMel release period. For each simulation, 50% of all BG traps were randomly assigned to the intervention arm and 50% to the untreated arm. The number of Ae. aegypti caught in traps assigned to the intervention arm was reduced by 10–40% in 2% increments, after first inflating by a factor of 1,000 to avoid non-integer values. A mixed-effect negative binomial regression was used to calculate the test statistic and p-value. The estimated power to detect a given effect size was determined as the proportion of observations (out of 1,000) for which the p-value was <0.05. The simulation indicated that we have 80% power to detect a difference in the number of Ae. aegypti of ≥20% between study arms, and 90% power to detect a difference of ≥23%.
Results
wMel introgression in treated clusters
Between March 2017 and December 2017, wMel deployments were performed in 12 of the 24 clusters of the AWED trial area as previously described [8]. wMel introgressed rapidly and stably in each intervention cluster (S2A Fig). wMel introgression (effectively contamination) was evident in some of the untreated clusters in 2019 and 2020 (S2B Fig).
Aedes aegypti relative abundance before and after wMel-deployments
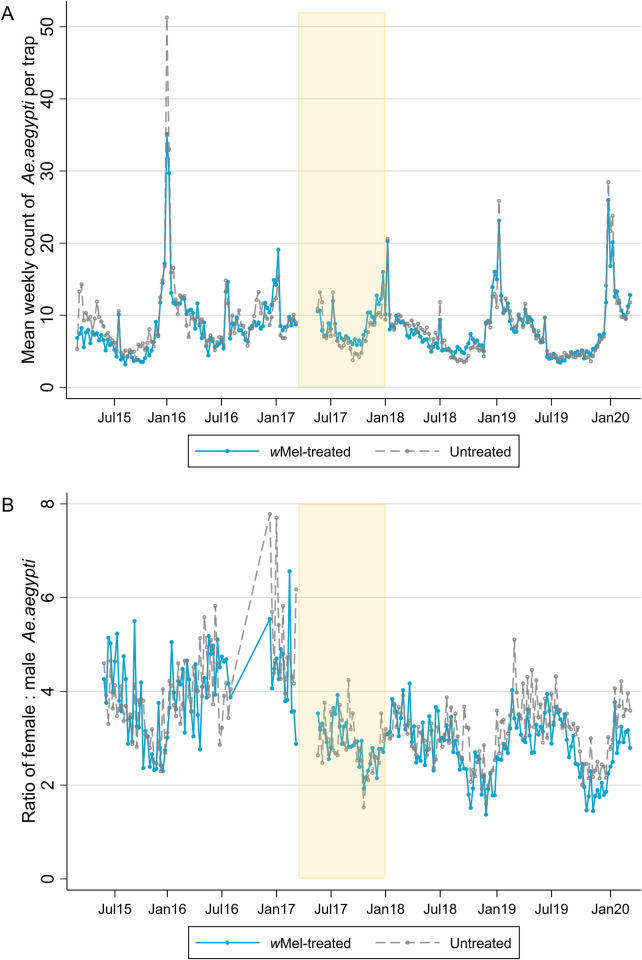
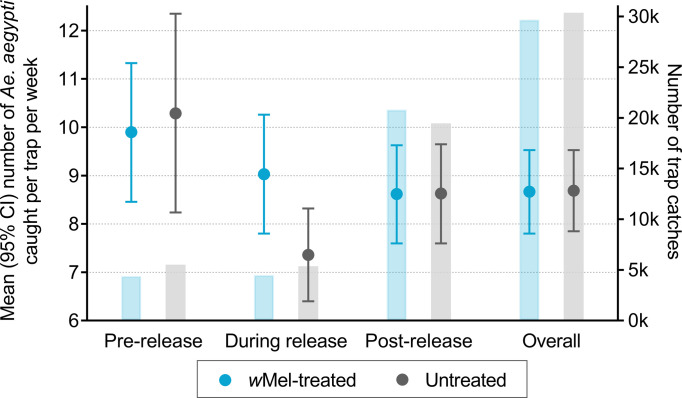
A total of 60,084 BG trap collections yielded 478,254 Ae. aegypti and 17,623 Ae. albopictus mosquitoes in the area of the AWED cluster randomised trial in Yogyakarta City between March 2015 and March 2020. Fig 1A illustrates that the mean number of Ae. aegypti caught per trap each week in wMel-treated versus untreated clusters was similar throughout this time period. The temporal trends in mean Ae. aegypti counts, peaking in January, are most likely due to climate factors, i.e. the warm rainy season producing abundant mosquito breeding sites in Yogyakarta between November to April. The ratio of female to male Ae. aegypti caught per week was also similar between wMel-treated and untreated arms during the observation period (Fig 1B). Unsurprisingly, slightly more Ae. aegypti were caught per trap per week in the wMel-treated arm compared to the untreated arm during deployments of wMel-infected mosquitoes (count ratio 1.23 (95% CI 1.03, 1.46)) (Fig 2).
Fig 1. Ae. aegypti mosquitoes caught per week by study arm.
Panel A is the mean number of Ae. aegypti mosquitoes caught per BG trap per week and panel B is the ratio of female to male Ae. aegypti caught. Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release. The warm, wet season in Yogyakarta is from November to April.
Fig 2. Mean number of Ae. aegypti mosquitoes caught per trap per week by study arm (circles) adjusted for season (wet/dry).
Analysis of the total study period was additionally adjusted for release status (pre-release [March 2015 –February 2017], during release [March 2017 –December 2017], post-release [January 2018 –March 2020]). Bars are the number of trap collections in each arm. The first nine weeks of releases were excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release. More Ae. aegypti were caught per trap per week in the wMel-treated arm compared to the untreated arm during wMel deployments (count ratio 1.23 (95% CI 1.03, 1.46)). When formally compared, Ae. aegypti abundance did not differ between the wMel-treated and untreated arm before (count ratio 0.96 (95% CI 0.76, 1.21), p = 0.74) or after wMel-deployments (count ratio 1.00 (95% CI 0.85, 1.17), p = 0.99). Similar results were found for the post-intervention period when wMel contamination was accounted for in a sensitivity analysis (count ratio 0.94 (95% CI 0.86, 1.03), p = 0.18).
Aedes albopictus relative abundance before, during and after wMel-deployments
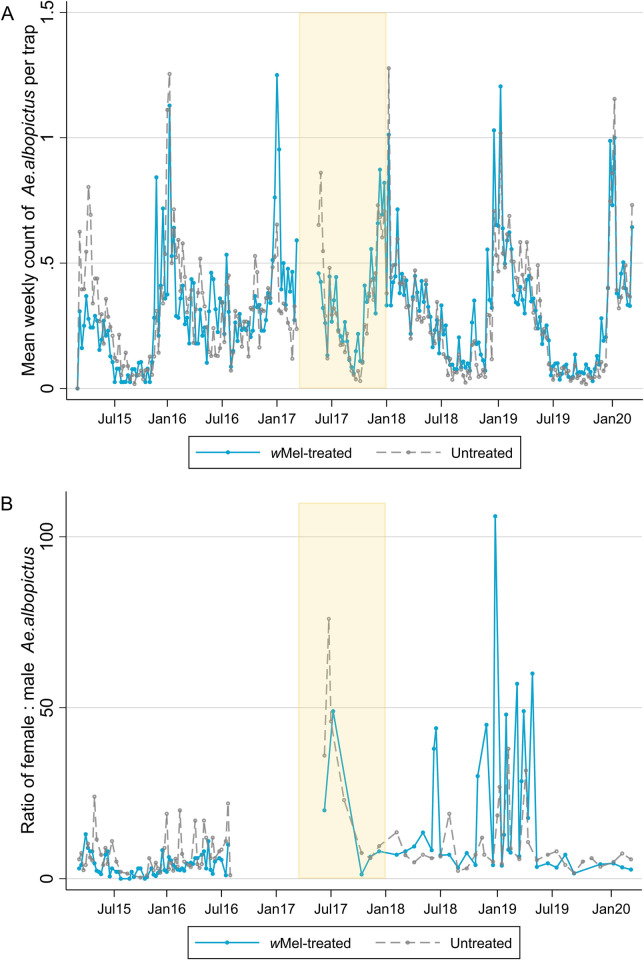
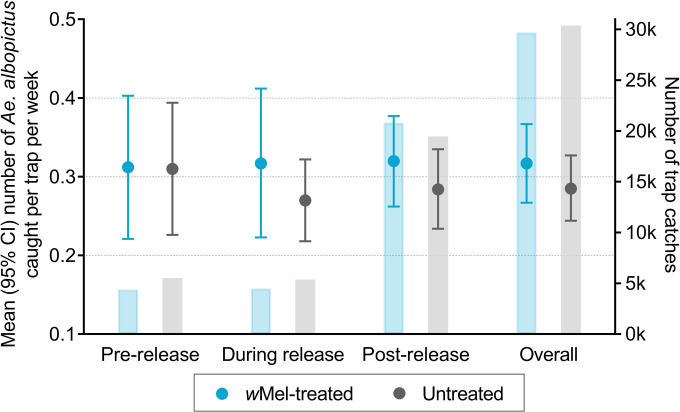
Fig 3A illustrates that the mean number of Ae. albopictus caught per trap each week in wMel treated versus untreated clusters was similar between March 2015 and March 2020. The ratio of female to male Ae. albopictus caught per week was also similar between treatment arms (Fig 3B). There were no male Ae. albopictus mosquitoes caught between mid-2016 and mid-2017 and very few female Ae. albopictus (median (interquartile range) 0 (0–0), range 0–97). When formally compared, Ae. albopictus abundance did not differ by study arm before, during or after wMel-deployment (Fig 4), with an overall average of 0.32 Ae. albopictus caught per trap per week (95% CI 0.27, 0.36) in the intervention arm and 0.29 (95% CI 0.25, 0.33) in the untreated arm (count ratio 1.11 (95% CI 0.90, 1.38), p = 0.33). Similar results were found when Wolbachia contamination in Ae. aegypti was accounted for, i.e. untreated clusters were reclassified as ‘treated’ as per the criteria described in the Methods section (count ratio 1.10 (95% CI 0.89, 1.36), p = 0.38).
Fig 3. Ae. albopictus mosquitoes caught per week by study arm.
Panel A is the mean number of Ae. albopictus mosquitoes caught per BG trap per week and panel B is the ratio of female to male Ae. albopictus caught. Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. No male and very few female Ae. albopictus mosquitoes (median (interquartile range) 0 (0–0), range 0–97) were caught between mid-2016 and mid-2017. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release.
Fig 4. Mean number of Ae. albopictus mosquitoes caught per trap per week by study arm (circles) adjusted for season (wet/dry).
Analysis of the total study period was additionally adjusted for release status (pre-release [March 2015 –February 2017], during release [March 2017 –December 2017], post-release [January 2018 –March 2020]). Bars are the number of trap catches in each arm. The first nine weeks of releases were excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release. There was no significant difference in Ae. albopictus abundance before (count ratio 1.00 (95% CI 0.68, 1.49)), during (1.08 (0.78, 1.51)) or after (1.12 (0.87, 1.45)) wMel-deployments.
Aedes aegypti abundance relative to Aedes albopictus
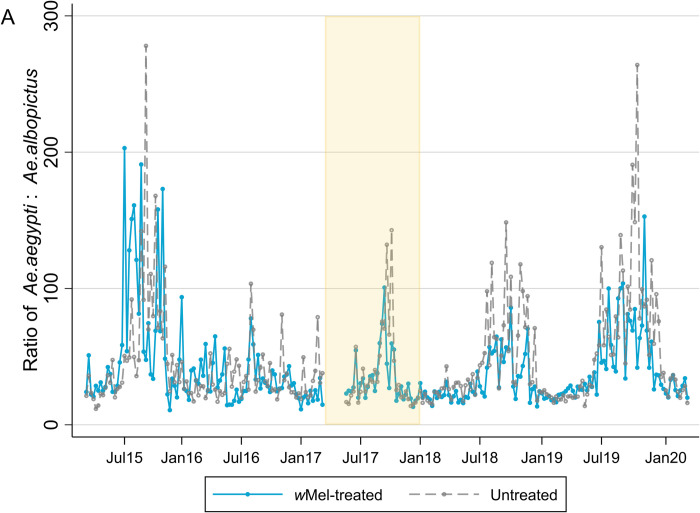
The Ae. aegypti to Ae. albopictus ratio in each study arm over time is shown in Fig 5 and demonstrates not only the much greater relative prevalence of Ae. aegypti but also the seasonal “dry season” peaks in this ratio. The ratio of Ae. aegypti to Ae. albopictus did not measurably differ between wMel-treated or untreated arms of the study (p = 0.06). The weekly abundance of Ae. aegypti and Ae. albopictus in each of the 24 clusters between March 2015 and March 2020 is plotted in S3 and S4 Figs.
Fig 5. Ratio of Ae. aegypti to Ae. albopictus mosquitoes caught per week by study arm.
Weekly counts of Ae. aegypti and Ae. albopictus were aggregated across 12 wMel-treated clusters and 12 untreated clusters. Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release.
Insecticide resistance profile of mosquito populations in the AWED study area
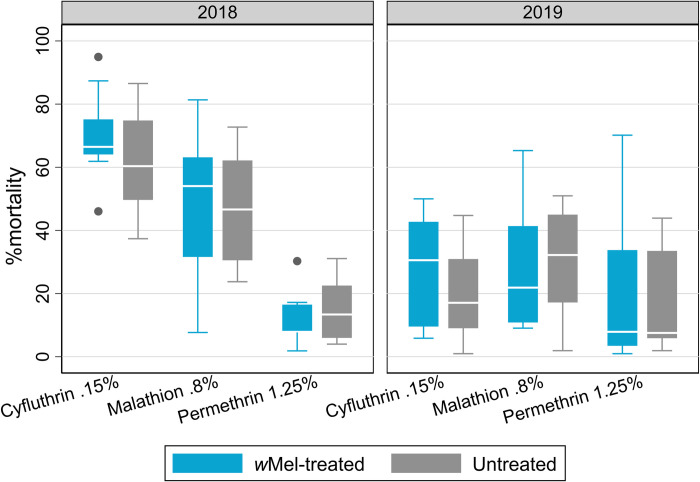
Insecticide resistance testing by bioassay was performed in 2018 and 2019 on field-derived adults sourced from each of the 24 clusters. There was no measurable difference in susceptibility between Ae. aegypti from the wMel-treated clusters compared to the untreated clusters in either 2018 or 2019, for any of cyfluthrin (0.15%) (p = 0.16 and 0.39, respectively), malathion (0.8%) (p = 1.00 and 0.77) or permethrin (1.25%) (p = 0.71 and 0.73) (Fig 6).
Fig 6. Percentage mortality of Ae. aegypti in the WHO bioassay for various insecticides by study arm in 2018 and 2019.
Boxes are median and interquartile range for percentage mortality in cohorts of mosquitoes derived from 12 wMel-treated clusters and 12 untreated clusters. Whiskers indicate the range, and circles indicate outliers. Insecticide type and concentrations used were in line with recommendations for Ae. aegypti mosquitoes and followed the WHO standard method [18]. The median (25th– 75th percentile) percentage mortality for the susceptible strain (Rockefeller) in 2019 is as follows: 100 (100–100) for cyfluthrin 0.15%; 91 (76–99) for malathion 0.8%; and 100 (97–100) for permethrin 1.25%.
Discussion
The AWED trial previously reported a 77% (95% CI 65, 85%) reduction in the incidence of virologically-confirmed dengue, and an 86% (95% CI, 66.2 to 94.3) reduction in dengue hospitalisations, in wMel-intervention versus control clusters [8]. The reduced vector competence of Ae. aegypti in wMel intervention clusters is the simplest explanation for the epidemiological findings in the AWED trial but it was important to consider other possibilities. The results reported here provide evidence that the trial’s epidemiological findings were likely not confounded by differences in Ae. aegypti adult abundance or insecticide resistance characteristics between the two study arms. Additionally, the relatively low abundance of Ae. albopictus, and the balance in numbers between study arms, suggests the trial outcomes were also not confounded by this secondary vector.
A body of literature, albeit mostly from laboratory studies, has described wMel-infection as having negligible or small fitness costs in one or more mosquito life history traits. For example, in laboratory conditions Walker et al described no impact of wMel on adult fecundity, egg survivorship or larval development time, but did report reduced (~10%) adult female survivorship times compared to controls [5]. Conversely, Fraser et al [12] described no impact of wMel on adult longevity but did find egg survival times were slightly reduced compared to wild-type counterparts. Ross et al reported that wMel-infection reduced larval survival by ~15% under strict starvation conditions that pressure tested the nutritional reserves of instar first and second larval instars [19]. Using field-derived adult mosquitoes, Hoffmann et al measured ~25% lower fecundity and larvae production from wMel-infected females when compared to wild-type counterparts [20]. In Brazil, Farnesi et al [11] found that wMel infection delayed embryogenesis, decreased desiccation resistance through delayed eggshell formation and eggs had substantially lower survivorship after 6 weeks storage compared to uninfected control mosquitoes. Why then did we find no differences in the abundance of Ae. aegypti adults in intervention clusters versus control clusters? One possible explanation is that the collective fitness costs of wMel in the field are too small to manifest as a measurable difference in Ae. aegypti counts between treatment arms, despite 60,084 BG trap collections and 478,254 Ae. aegypti caught. Our power estimates suggested 80% power to detect a 20% difference or greater in the number of Ae. aegypti between wMel intervention and control clusters, suggesting that if there is a true difference in relative abundance it is probably smaller than 20%.
Globally, Ae. albopictus is considered a secondary vector of dengue relative to Ae. aegypti. In Yogyakarta, Ae. aegypti was far more prevalent than Ae. albopictus in the BG trap network in all 24 trial clusters and there was no difference in Ae. albopictus abundance between study arms. This is encouraging from the perspective that wMel introgression into Ae. aegypti did not lead to any measurable alteration in the ecological balance between the two species that might inadvertently allow Ae. albopictus to expand its population size in Yogyakarta. The much lower abundance of Ae. albopictus relative to Ae. aegypti is partly explained by Yogyakarta being mostly a highly urbanised environment that favours Ae. aegypti’s domesticated lifestyle. The role of Ae. albopictus in the epidemiology of dengue in Yogyakarta is unclear. Going forward, it will be important to explore the relative contributions of Ae. albopictus versus wMel-infected Ae. aegypti in any ongoing dengue transmission that occurs in Yogyakarta after city-wide wMel introgression is completed in 2021.
In Indonesia, organophosphates (temephos and malathion) and pyrethroids are the two major classes of insecticide utilized for Aedes control. Cyfluthrin (a pyrethroid) and malathion are used by Yogyakarta authorities in fogging activities in response to dengue cases in the community. Resistance to pyrethroids was documented in Ae. aegypti from Yogyakarta City in 2015 [21]. To help ensure the competitiveness of the wMel release material deployed in the AWED trial the insecticide resistance profile of the mosquito line was matched to the wild-type field population by repeated outcrossing with wild-type mosquitoes from Yogyakarta City [8]. It was reassuring therefore that we found equivalent insecticide resistance profiles between cohorts of mosquitoes from the wMel treated clusters versus the untreated clusters in 2018 and again in 2019. This provides evidence that the release material used in 2017 was suitably matched to the local insecticide resistance profile. It also provides further reassurance that the epidemiological effect measured in the AWED trial was not confounded by a difference in the sensitivity of mosquitoes to insecticides between the two study arms. We note however that WHO insecticide resistance bioassays are semi-quantitative in nature and that small differences in absolute insecticide resistance are difficult to measure. It will be of great interest to track the insecticide resistance profile of the Ae. aegypti in Yogyakarta over coming years. One hypothesis is that dengue case incidence will be very low in Yogyakarta after city-wide establishment of wMel and that this will significantly decrease insecticide fogging episodes carried out by local authorities. In turn, this diminished selective pressure might lead to a degree of restoration of insecticide susceptibility in the mosquito population.
Our study had some limitations. We did not measure variables such as household-level insecticide usage or local community behaviours that might have been confounding to the trial results, though we think that randomisation will have balanced these parameters. Our methods of mosquito surveillance (BG traps located predominantly indoors) were plausibly biased to detecting Ae. aegypti rather than Ae. albopictus. Indeed, Ae. albopictus should be taken into consideration when interpreting dengue epidemiology in Yogyakarta from 2021 onwards.
Collectively, the results here suggest wMel introgression to a high and stable prevalence had no measurable impact on adult Ae. aegypti population size or insecticide resistance phenotype. The results will be reassuring to audiences concerned about ecological impacts of wMel introgression. More generally, we’d expect the wMel introgression method to deliver similar positive public health benefits and the same unremarkable entomological outcomes in other dengue-endemic urban settings in Indonesia.
Supporting information
(DOCX)
Each point on the map shows the location of a BG trap in the post-intervention time period of January 2018—March 2020. This map was created using ArcGIS software by ESRI. The vector map was sourced from the local government (Regional body for planning and development) and ground-truthed by the study team. The shapefile for the map can be found at https://doi.org/10.6084/m9.figshare.19450415.v1.
(TIF)
In panel A, the yellow shading represents the wMel-infected mosquito release period for each cluster.
(TIF)
Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release.
(TIF)
Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release.
(TIF)
(XLSX)
Acknowledgments
We are grateful to all the World Mosquito Program staff in Yogyakarta who contributed to this study and the people of Yogyakarta for their cooperation and support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
SLO and AU received funding from the Tahija Foundation (Indonesia) for this work (no website for this funder). CPS received funding from Australia's National Health and Medical Research Council (GNT1042782)(https://www.nhmrc.gov.au). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 2012;6:e1760. doi: 10.1371/journal.pntd.0001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aliota MT, Peinado SA, Velez ID, Osorio JE. The wMel strain of Wolbachia Reduces Transmission of Zika virus by Aedes aegypti. Sci Rep 2016;6:28792. doi: 10.1038/srep28792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington LB, Tran BCN, Le NTH, Luong TTH, Nguyen TT, Nguyen PT, et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 2018;115:361–6. doi: 10.1073/pnas.1715788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis 2014;8:e2688. doi: 10.1371/journal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011;476:450–3. doi: 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 6.O’Neill SL, Ryan PA, Turley AP, Wilson G, Retzki K, Iturbe-Ormaetxe I, et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res 2018;2:36. doi: 10.12688/gatesopenres.12844.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan PA, Turley AP, Wilson G, Hurst TP, Retzki K, Brown-Kenyon J, et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland, Australia. Gates Open Res 2019;3:1547. doi: 10.12688/gatesopenres.13061.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utarini A, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Ansari MR, et al. Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. New England Journal of Medicine 2021. Jun 10; 384(23):2177–2186. doi: 10.1056/NEJMoa2030243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anders KL, Indriani C, Ahmad RA, Tantowijoyo W, Arguni E, Andari B, et al. The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomised controlled trial. Trials 2018;19:302. doi: 10.1186/s13063-018-2670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA. Fitness of wAlbB Wolbachia Infection in Aedes aegypti: Parameter Estimates in an Outcrossed Background and Potential for Population Invasion. Am J Trop Med Hyg 2016;94:507–16. doi: 10.4269/ajtmh.15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farnesi LC, Belinato TA, Gesto JSM, Martins AJ, Bruno RV, Moreira LA. Embryonic development and egg viability of wMel-infected Aedes aegypti. Parasit Vectors 2019;12:211. doi: 10.1186/s13071-019-3474-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, Flores HA, et al. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog 2017;13:e1006751. doi: 10.1371/journal.ppat.1006751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joubert DA, Walker T, Carrington LB, De Bruyne JT, Kien DH, Hoang Nle T, et al. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ross PA, Ritchie SA, Axford JK, Hoffmann AA. Loss of cytoplasmic incompatibility in Wolbachia-infected Aedes aegypti under field conditions. PLoS Negl Trop Dis 2019;13:e0007357. doi: 10.1371/journal.pntd.0007357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA. Wolbachia Infections in Aedes aegypti Differ Markedly in Their Response to Cyclical Heat Stress. PLoS Pathog 2017;13:e1006006. doi: 10.1371/journal.ppat.1006006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeap HL, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, Ritchie SA, et al. Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors 2014;7:58. doi: 10.1186/1756-3305-7-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dar M, Giesler T, Richardson R, Cai C, Cooper M, Lavasani S, et al. Development of a novel ozone- and photo-stable HyPer5 red fluorescent dye for array CGH and microarray gene expression analysis with consistent performance irrespective of environmental conditions. BMC Biotechnol 2008;8:86. doi: 10.1186/1472-6750-8-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organisation. Monitoring and managing insecticide resistance in Aedes mosquito populations. Interim guidance for entomologists. Geneva: WHO; 2016. Report No.: WHO/ZIKV/VC/16.1. [Google Scholar]
- 19.Ross PA, Endersby NM, Hoffmann AA. Costs of Three Wolbachia Infections on the Survival of Aedes aegypti Larvae under Starvation Conditions. PLoS Negl Trop Dis 2016;10:e0004320. doi: 10.1371/journal.pntd.0004320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, et al. Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 2014;8:e3115. doi: 10.1371/journal.pntd.0003115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuliandari JR, Hoffmann AA, Tantowijoyo W, Endersby-Harshman NM. Frequency of kdr mutations in the voltage-sensitive sodium channel (VSSC) gene in Aedes aegypti from Yogyakarta and implications for Wolbachia-infected mosquito trials. Parasit Vectors 2020;13:429. doi: 10.1186/s13071-020-04304-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Each point on the map shows the location of a BG trap in the post-intervention time period of January 2018—March 2020. This map was created using ArcGIS software by ESRI. The vector map was sourced from the local government (Regional body for planning and development) and ground-truthed by the study team. The shapefile for the map can be found at https://doi.org/10.6084/m9.figshare.19450415.v1.
(TIF)
In panel A, the yellow shading represents the wMel-infected mosquito release period for each cluster.
(TIF)
Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release.
(TIF)
Releases of wMel-infected Ae. aegypti mosquitoes occurred from March 2017 –December 2017 (yellow shading). The first nine weeks of releases are excluded from analyses due to unequal trapping density in the treated and untreated clusters. There was an average of 4 BG traps/km2 in wMel-treated clusters and 5 BG traps/km2 in untreated clusters in the pre-release period, and 16 BG traps/km2 and 15 BG traps/km2 during and post-release.
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.