Abstract
Pulmonary hypertension (PH) describes heterogeneous population of patients with a mean pulmonary arterial pressure greater than 20 mmHg. Rarely, PH presents as a primary disorder but is more commonly part of a complex phenotype associated with co-morbidities. Regardless of cause, PH reduces life expectancy and impacts quality of life. The current clinical classification divides PH into one of 5 diagnostic groups to assign treatment. There are currently no pharmacological cures for any form of PH. Animal models are essential to help decipher the molecular mechanisms underlying the disease, to assign genotype-phenotype relationships to help identify new therapeutic targets, and for clinical translation to assess the mechanism of action and putative efficacy of new therapies. However, limitations inherent of all animal models of disease limit the ability of any single model to fully recapitulate complex human disease. Within the PH community, we are often critical of animal models due to the perceived low success upon clinical translation of new drugs. In this review, we describe the characteristics, advantages and disadvantages of existing animal models developed to gain insight into the molecular and pathological mechanisms and test new therapeutics, focusing on adult forms of PH from groups 1 to 3. We also discuss areas of improvement for animal models with approaches combining several hits in order to better reflect the clinical situation and elevate their translational value.
Keywords: Animal Models of Human Disease, Pulmonary Hypertension, Pulmonary arterial Hypertension, Vascular remodeling, Vasoconstriction, Preclinical models, Translational research
Pulmonary hypertension (PH) encompasses a heterogenous group of disorders collectively defined by a mean pulmonary artery (PA) pressure above 20 mmHg at rest1. PH is classified into five groups by the World Health Organization (WHO) according to etiology. Group 1 refers to pulmonary arterial hypertension (PAH), which is characterized by a progressive and severe obliteration of PAs due to excessive vasoconstriction and vascular cell proliferation. The steady rise in pulmonary vascular resistances (PVR) increases right ventricular (RV) afterload pushing the RV to undergo adaptive remodeling events. Almost inevitably, these coping mechanisms become insufficient, and patients rapidly succumb to RV failure. PAH can be heritable, idiopathic, induced by drugs or associated with various conditions such as scleroderma and HIV infection. Group 2 (due to left-sided heart disease) and group 3 (secondary to chronic lung disease and/or alveolar hypoxia) are the most prevalent forms of PH2. Group 4 PH is related to obstruction of the pulmonary vasculature from thromboembolism. Lastly, group 5 consists of several forms of PH with multifactorial or unclear mechanisms. Regardless of the underlying etiology, PH is characterized by varying degrees of PA remodeling with restricted blood flow in the lung. Despite significant progress in basic research and improvements of patient management, PH still remains a life-threatening-disease with a poor prognosis.
Animal models are essential to help decipher the molecular mechanisms underlying the disease to identify new actionable therapeutic targets, and for clinical translation to assess the impact of putative therapies. However, because animals are not humans and PH is a complex and multifactorial disease, no single animal model faithfully reproduces the full clinical spectrum of PH, or indeed each PH subgroup. The limitation of PH animal models is further indirectly stressed by the fact that many drugs that were promising during preclinical evaluation have low success during subsequent clinical testing; a translational gap often referred to as the “Valley of Death”. In the present review, we detail the characteristics, advantages and drawbacks of different animal models developed to gain insight into the mechanisms and mimic aspects of the disease, focusing on adult forms of PH from groups 1 to 3 (Table 1).
Table 1.
Animal models of group 1 to 3 pulmonary hypertension
| EXPERIMENTAL MODELS | ANIMAL SPECIES | PH Group | PULMONARY VASCULAR REMODLEING | RVSP | RV DYSFUNCTION | COMMENTS | REFERENCES |
|---|---|---|---|---|---|---|---|
|
| |||||||
| MCT (single injection 60mg.kg−1) | RAT | 1 | +++ | ++++ | ++++ | Simple implementation Fast, Low cost Causes pronounced vascular remodeling Allow to interrogate the adaptive and maladaptive changes of the RV Myocarditis Reversible at lowest concentrations | 5–7 |
| MCT + Hypoxia | RAT | 1 | ++++ | ++++ | ++++ | Myocarditis | 19 |
| MCT + Pneumonectomy | RAT | 1 | ++++ | ++++ | ++++ | Requires technical skills Causes pronounced vascular remodeling and RV dysfunction | 20 |
| MCT + aortocaval shunt | RAT | 1 | ++++ | ++++ | ++++ | Requires technical skills Causes pronounced vascular remodeling and RV dysfunction | 21, 22 |
| Sugen-Hypoxia | RAT | 1 (3) | +++ | ++++ | +++ | Relatively inexpensive – Lack of harmonization among laboratories Causes pronounced vascular remodeling with development of plexiform-like lesions Strain variability | 25–28 |
| Sugen-Hypoxia | MOUSE | 1 (3) | ++ | +++ | + | Partially reversible | 33, 34 |
| Inducible overexpression dominant-negative BMPR2 targeted to SMCs (Tg+/Tagln-rtTA; Tg+/tetO-Bmpr2Δex4) | MOUSE | 1 | ++ | + | 42 | ||
| Inducible overexpression dominant-negative BMPR2 (Tg+/ROSA26-rtTA; Tg+/ tetO-Bmpr2R899X) | MOUSE | 1 | + | ++ | ++ | Variable penetrance PH reversed by rhACE treatment Lipid deposition in CMs | 44, 45 |
| Inducible overexpression mutated BMPR2R899X targeted to SMCs (Tg+/Tagln-rtTA; Tg+/tetO-Bmpr2R899X) | MOUSE | 1 | +++ | ++ | 43 | ||
| Bmpr2+/− | MOUSE | 1 | +/− | +/− | +/− | More susceptible to develop PH under stress conditions | 47–51 |
| Bmpr2+/R899X | MOUSE | 1 | + | + | − | 46 | |
| Bmpr2+/R899X; Smad1+/− | MOUSE | 1 | ++ | + | 46 | ||
| Bmpr2flox/flox; Tg+/ALK1-Cre | MOUSE | 1 | ++ | + | Incomplete penetrance | 53 | |
| Bmpr2+/Δ71Ex1 | RAT | 1 | + | + | + | Incomplete penetrance | 54 |
| Bmpr2+/Δ527Ex1 or Bmpr2+/Δ16Ex1 | RAT | 1 | − | − | − | Increased responsiveness to 5-LO-mediated inflammation | 55 |
| Alk1+/− | MOUSE | + | + | + | 57 | ||
| Eng+/− | MOUSE | + | + | 58 | |||
| Cav-1−/− | MOUSE | ++ | +++ | 59 | |||
| Kcnk3Δ94ex1/Δ94ex1 | RAT | + | + | Incomplete penetrance Low expressivity | 63 | ||
| Ucp2−/− | MOUSE | + | +/− | + | 93 | ||
| Sirt3−/− | MOUSE | ++ | ++ | + | 94 | ||
| Ucp2−/−; Sirt3−/− | MOUSE | ++++ | +++ | ++ | Development of plexiform-like lesions Insulin resistance | 95 | |
| Irp1−/− | MOUSE | + | 99 | ||||
| Nfu1G206C/G206C | RAT | ++ | + | 100 | |||
| Iron-deficient diet | RAT | ++ | ++ | 105 | |||
| Overexpression of IL-6 by airway epithelial cells (Tg+/Scgb1a1-Il6) | MOUSE | + | + | Increased responsiveness to chronic hypoxia | 68 | ||
| Overexpression of TNFα by airway/alveolar epithelial cells (Tg+/Sftpc-Tnfa) | MOUSE | ++ | + | Causes airspace enlargement | 70 | ||
| Tet2flox/flox; Tg+/Vav1-Cre | MOUSE | + | + | + | 75 | ||
| Hif2aG536W/G536W | MOUSE | + | ++ | + | 78 | ||
| Egln1flox/flox;Tg+/Cdh5-Cre | MOUSE | ++ | +++ | ++ | Robust PH prevented by loss of Hif2a function in ECs | 79, 81 | |
| Egln1flox/flox;Tg+/Tie2-Cre | MOUSE | +++ | +++ | ++ | Robust PH prevented by loss of Hif2a function in ECs Development of plexiform-like lesions | 80 | |
| VhlR200W/R200W | MOUSE | ++ | ++ | Partial rescue of the phenotype by reduced gene dosage of Hif2a | 82 | ||
| Ppargflox/flox;Tg+/Tagln-Cre | MOUSE | + | + | + | 84 | ||
| Overexpression of Fra2 (Tg+/H2k-Fra2) | MOUSE | 1 (3) | +++ | + | 88–90 | ||
| Overexpression of S100A4/Mts1 (Tg+/Hmgcr-s100A4) | MICE | +++ | + | Development of plexiform-like lesions Specific to females | 114, 115 | ||
| Dexfenfluramine | MOUSE | + | + | − | Prevented by loss of Tph1 function | 116 | |
| overexpression SERT targeted to SMCs (Tg+/Tagln-SERT) | MOUSE | ++ | + | + | 117 | ||
| cyclophosphamide | MOUSE RAT RABBIT | PVOD | ++ | + (mice) ++ (rat) | ++ | Low penetrance in mice | 121 |
| Mitomycin-C | RAT | PVOD | ++ | + | ++ | Female more sensitive | 122 |
| SIV or SHIV-nef infection | MACAQUE | 1 | +++ | ++ | High maintenance costs Variability among animals | 127–131 | |
| Overexpression of a gag−pol−deleted HIV-1 provirus regulated by the viral promoter | RAT | 1 | + | +/− | Increase responsiveness to PH induced by chronic hypoxia exposure or chronic administration of cocaine | 132–134 | |
| Chronic Hypoxia | MOUSE RAT | 3 | + | + | Simple implementation Low cost Helpful to determine whether genetically modified animals are protected or more susceptible to the disease | 139, 140 | |
| Cigarette smoke exposure | MOUSE RAT HAMSTER GUINEA-PIG | 3 | ++ | ++ | Vascular remodeling often precedes airspace enlargement | 147–152 | |
| Bleomycin | MOUSE RAT | 3 | + | Simple implementation Low cost Limited vascular remodeling | 154–157 | ||
| Bleomycin + MCT | RAT | 3 | +++ | +++ | Simple implementation Low cost Marked pulmonary vascular remodeling | 156, 157 | |
| Intra-tracheal adenovirus−mediated active TGF-β1 gene transfer | RAT | 3 | +++ | +++ | 159, 160 | ||
| Fawn-Hooded Rat | RAT | 3 (1) | ++ | + | Spontaneous development of PH at sea level. Increased severity upon exposure to mild hypoxia PH associated with defects in lung development | 174–178 | |
| Transverse Aortic Constriction | MOUSE RAT PIG | 2 | ++ | ++ | +/− | Robust model of load stress Doesn’t model human disease well Surgical expertise required | 182–185 |
| ApoE −/− | MOUSE | 2 | + | + | − | 189 | |
| AKR | MOUSE | 2 | + | + | + | Easy to implement Mild phenotype | 191, 192 |
| Sugen + HFD | ZSF1 RAT | 2 | ++ | ++ | + | Robust phenotype Doesn’t model human disease well (Sugen) | 195 |
| Supracoronary Banding + HFD or Olanzapine | RAT | 2 | ++ | +++ | ++ | Robust model Surgical expertise required | 196 |
1. Animal models used in the investigation of PAH
Monocrotaline
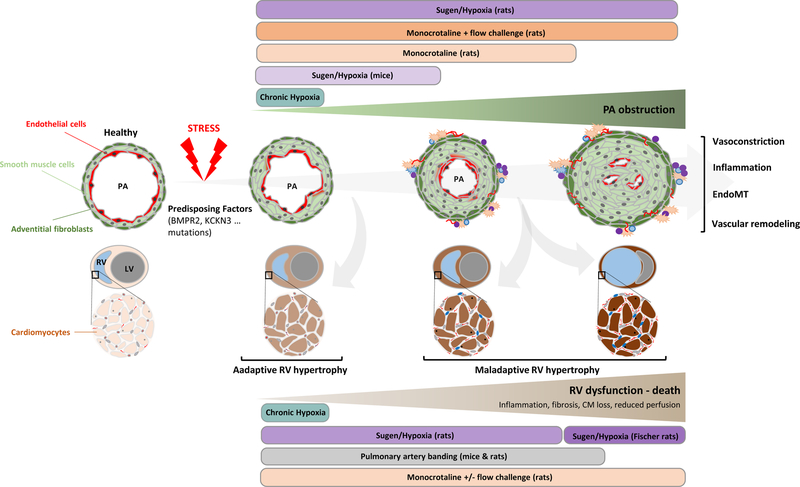
Although tested in large animals such as minipigs and dogs3, 4, injection of monocrotaline (MCT) in rat is the more classical and widely used in vivo PAH model for over half a century. Primarily carried out in rats due to the inability of mice to convert MCT to its active pneumotoxic metabolite (monocrotaline pyrrole) by hepatic cells, a single subcutaneous or intraperitoneal injection of MCT (60mg/kg in most cases) is known to produce robust pulmonary vascular remodeling5, 6. This in turn causes marked elevation of the mean PA pressure, RV dysfunction and death within 4–6 weeks7. Aside from the its direct pulmonary effects, MCT also causes significant toxicity in multiple organs, which can add confounding effects. The response to MCT is dose sensitive, with lower doses (30–40 mg/kg) causing acute muscularization of distal PAs, increased PVR and decreased cardiac output (CO) at 4 weeks after MCT administration with almost complete recovery at 8- and 12-weeks post MCT injection8, 9. Mechanistically, MCT initially induces PA endothelial cell dysfunction, interstitial oedema, vasoconstriction, perivascular inflammatory infiltrate and cytokine secretion followed by progressive medial hypertrophy and adventitial fibrosis10. As observed in PAH patients11, these histological and structural alterations are accompanied by a glycolytic switch in vascular cells with enhanced expression of pyruvate dehydrogenase kinase12, contributing to their enhanced capacity to survive and proliferate11, 13. Reduced BMPR2 expression14, increased growth factor signaling (PDGF, TGF-β, etc.)15, 16 as well as sustained activation of the DNA damage response17, 18, to name a few, are also key molecular traits shared by the remodeling vasculature of MCT rats and PAH patients. However, the typical manifestation of severe PAH, the so-called plexiform lesions, are not found in MCT rats (Figure 1). To create a model presenting this characteristic, monocrotaline has been combined with either chronic hypoxia exposure19 or left pneumonectomy20. In these two-hit models, a neointimal pattern of remodeling and severe RV hypertrophy occur providing a model that closely mimics the advanced stage of the disease. Similarly, increased pulmonary blood flow generated by abdominal aortocaval shunt coupled with MCT administration was implemented in rat21, 22. At similar PA pressures, MCT rats with shunt exhibited pronounced RV failure, increased morbidity and mortality but with a similar neo-intimal hyperplastic response when compared with non-shunted MCT-treated animals21, 22. Although these animal models produce more severe hemodynamic changes and histological alterations (Figure 1), they require surgical skills that significantly limit their use. It should be stressed that, besides its low cost and technical simplicity, interest of the MCT model lies in its ability to sequentially reproduce the adaptive and maladaptive responses (fibrotic, metabolic, inflammatory and hemodynamic) of the RV7, 23 driven by progressive and severe pulmonary vascular remodelling. Despite some caveats, the MCT rat remains a valuable model to explore the therapeutic benefit of anti-remodeling approaches10 (Figure 1). In this regard, it is often qualified as “easy to reverse”; a consideration perhaps biased by its popular use and a tendency to preferentially publish positive results.
Figure 1. Schematic progression of pulmonary arterial hypertension (PAH) with animal models commonly used to address different aspects of the pathophysiology and test candidate treatments or preventive interventions.
CM: cardiomyocyte; EndoMT: endothelial-to-mesenchymal transition; PA: pulmonary artery; LV; left ventricle; RV: right ventricle.
Sugen/Hypoxia (Su/Hx)
Although exposure to chronic hypoxia is long recognized to produce PH in rodents, the model doesn’t match the severity seen in PAH patients (see corresponding section below). As a result, the search of a more robust animal model has become a requirement. Taking benefit from findings showing that apoptosis of pulmonary endothelial cells and pruning of the pulmonary artery tree occurs in adult Sprague Dawley (SD) rats chronically treated with the VEGF receptor antagonist Sugen (Su) 541624, the same group demonstrated that combination of Su (weekly injection) and chronic hypoxia for 3 weeks markedly worsens pulmonary hypertension as witnessed by hemodynamic measurements and the appearance of plexiform-like lesions25. Similar obliterative lesions were noted in SD rats receiving a single injection of Su (20mg/kg) before exposure to hypoxia for 3 weeks followed by a return to normoxia for an additional 10 to 11 weeks26. Temporal examination of hemodynamic and histological changes revealed that RVSP gradually increases to reach a plateau (100 mmHg) at 3–5 weeks after SU exposure, a deterioration correlating with progression of PA vascular remodeling27. Not surprisingly, strain-dependent phenotypic variabilities were documented. This is exemplified by Fisher rats that display an increased susceptibility to develop RV failure and death as compared to SD rats despite comparable hemodynamic afterload and pulmonary vascular remodeling28. In addition, in support of the prevailing model of PAH development; i.e. initial apoptosis of PA endothelial cells (PAECs) followed by extensive proliferation of adjacent PA smooth muscle cells (PASMCs) and apoptosis-resistant PAECs29, the development of the rat Sugen-hypoxic model represents a significant advance providing a complementary alternative to the MCT rat model. Indeed, due to its ability to cause severe and irreversible PH with the appearance of angio-obliterative lesions, the Su/Hx rat model (while certainly not without criticism) has become one of the most used preclinical models of PAH with therapeutic intervention initiated at either 3 weeks (immediately after the end of hypoxia exposure) or 5–8 weeks (after 2–5 weeks of normoxic recovery) post Su injection26, 30–32 (Figure 1). More recently, the rat Su/Hx model was adapted to mice33 in order to take benefit of existing genetically modified mice and thus gain mechanistic insights into the pathogenesis of PAH. Mice subcutaneously injected with Su (20mg/kg once weekly) over a 3-week period of hypoxia were found to develop higher RV pressure (~50mmHg), RV hypertrophy and vascular remodeling compared with hypoxic control mice33. Contrary to rats, all these aforementioned parameters were found to be decreased over 10 weeks of normoxic follow-up33, 34. Thus, it is clear that rats are not simply huge mice, and that each species possesses advantages and disadvantages. The smaller size of mice makes them more cost-effective as a lower amount of drug is needed. On the other hand, assessment of RV function by echocardiography and closed-chest catheterization is more technically challenging. Finally, although largely assumed to be representative of group 1 PH, exposure to Su with or without chronic hypoxia, was reported by some groups to cause an airspace enlargement reminiscent of emphysema24, 35 36. This suggests that this model may also be helpful for studying group 3 PH. Be that as it may, the rat Su/Hx model should be given priority when the primary purpose is to evaluate the potential of novel new anti-remodeling agents (Figure 1), with mice more useful for assessing genotype-phenotype consequences in transgenics.
BMPR2 transgenic/knockout animals
The first evidence of genetic contributions to PAH was discovered following linkage studies in which heterozygous germline mutations in bone morphogenetic protein receptor 2 (BMPR2) were detected37–39. Although BMPR2 loss-of-function mutations are the underlying cause in approximatively 75% of patients with hereditary PAH and 10–40% of idiopathic PAH patients, only 20–30% of carriers develop the disease40. This low penetrance indicates that additional environmental or genetic factors are required for its clinical manifestation. Based on these findings, multiple genetically engineered mouse lines have been created to address the impact of BMPR2 signaling deficiency on the development of PH. To circumvent the embryonic lethality of Bmpr2−/− mice41, a transgenic mouse expressing an inducible and smooth muscle specific dominant-negative form of BMPR2 was generated42. Examination of these mice revealed that postnatal activation of the transgene induces a modest increase in PA medial thickness and a mild elevation of RVSP by 8 weeks of age, a phenotype slightly aggravated under hypoxia42. Similarly, a significant proportion of mice expressing postnatally a cytoplasmic tail mutation (R899X) of the Bmpr2 gene restricted or not to smooth muscle cells develop PH, as evidenced by an elevated RVSP and marked pulmonary vascular structural changes43–45. Notably, mice with universal expression of the Bmpr2R899X transgene demonstrated an impaired RV hypertrophic response associated with an accumulation of lipid within cardiomyocytes (CMs), a feature not seen in mice harboring the SMC-restricted conditional, inducible Bmpr2R899X transgene45.
Using a knock-in approach that fully preserves the native expression and splicing pattern, Long and colleagues found that Bmpr2+/R899X mice develop age-related PH with substantial worsening after loss of one copy of SMAD1, an important downstream mediator of BMPR2 signaling46. In parallel, different studies were undertaken using Bmpr2+/− mice. Although contradictory results have been found regarding whether or not heterozygous mice spontaneously develop mild PH under basal conditions47–51, most of these works demonstrated that Bmpr2+/− mice are more prone to develop signs of PH under stressed conditions such as prolonged hypoxia47, 48, 5-lipoxygenase- or lipopolysaccharide-mediated inflammation49, 50, IL-152 and chronic serotonin infusion51. In addition, Hong and coworkers found that a subset of mice with endothelial cells-specific heterozygous or homozygous Bmpr2 inactivation display sustained elevation of RV pressure associated with perivascular accumulation of inflammatory cells and conspicuous occlusion of distal PAs53.
By taking benefit of major advances in rat genome editing, including the CRISPR/Cas9 system, and advantages of rat as a model organism more sensitive to PH development than mice, several Bmpr2-mutated rats were recently generated and characterized54. In a first study, zinc finger nuclease technology was employed to target the first exon of the Bmpr2 gene leading to deletion of 71 bp associated with the loss of the translation initial codon. Heterozygous Bmpr2(Δ71) mutant rats showed age-dependent spontaneous mild PH with low penetrance (~20%). Interestingly, interleukin-6 overexpression was identified as a discriminating factor between rats that spontaneously develop PH to those that did not54. In a second study, the same methodology was used to create two strains of monoallelic mutant rats with frameshift mutations within exon 1 (Bmpr2+/Δ527Ex1 and Bmpr2+/Δ16Ex1) The follow up of these rats over one year revealed no sign of PH55. These mutant strains were then assessed for increased responsiveness to several putative PAH triggers, namely, 5-lipoxygenase exposure, MCT, Sugen, chronic hypoxia and Su/Hx. Among these, only the combined presence of Bmpr2 haploinsufficiency and overexpression of 5-lipoxygenase was shown to produce additive/synergistic effects on PAH development55. Collectively, these findings somewhat disappointing in view of the incomplete penetrance and variable expressivity of rodent Bmpr2 mutant phenotypes support a “two-hit” model in which two independent insults (BMPR2 deficiency and stressful experience) are assumed to be necessary for the development of PAH.
Rodent models related to other genes involved in heritable PAH
Since 2000, mutations in other genes related to BMPR2 signaling, including mutations in Activin receptor-like kinase 1 (ALK1), Endoglin (ENG), Caveolin-1 (CAV-1), and SMAD8 have been identified in PAH patients56. To examine the mechanisms linking mutations of these genes to the development of PAH, genetically modified mice have been generated. Most but not all Alk1 heterozygous mice were shown to develop signs of PH by 9 weeks of age, including a significant elevation of RVSP associated with RV hypertrophy and remodeling of peripheral arteries57. A similar phenotype was observed in Eng+/− 58 and Cav1−/− mice59. More recently, screening of patients with familial or idiopathic PAH by whole exome-sequencing identified mutations in the potassium (K+) channel subfamily K member 3 (KCNK3, also called TASK1)60. Decreased expression and activity of K+ channels in PASMCs is known to cause membrane depolarization, enhanced calcium (Ca2+) influx and increased cytosolic Ca2+ concentration which in turn, stimulates vasoconstriction and proliferation61. However, neither male nor female Kcnk3−/− mice manifested apparent signs of PH62 and only 45% (3/8) of homozygous mutant rats (Kcnk3Δ94ex1/Δ94ex1) exhibited RVSP above 40mmHg at 1 year63.
Animal models related to inflammation
Pulmonary inflammation is recognized as a key pathogenic driver of PH. This is underscored by the recruitment of inflammatory cells, such as macrophages, T and B-lymphocytes and mast cells around and within the remodeled vessels and the presence of high circulating levels of pro-inflammatory mediators in PAH patients that correlate with clinical outcomes64–66. Among these, the pro-inflammatory cytokine IL-6 has gained considerable attention with several studies demonstrating augmented serum concentration in severe PAH and up-regulation by smooth muscle cells as a result of deficient BMPR2 signaling64, 67. To test the hypothesis that increased expression of IL-6 actively participates in the remodeling process, transgenic mice that overexpress IL-6 in the airway epithelium were generated68. Detailed phenotypic characterization of these mice revealed a subtle elevation of the RVSP under normoxic conditions compared to littermate controls (~30 vs 20mmHg). However, transgenic mice exposed to 3-week hypoxia developed significantly worse PH (RVSP ~65mmHg) compared to hypoxic control mice (RVSP ~25mmHg) with extensive pulmonary vessel occlusion68. In support of the pathological role of IL-6 in PAH, subsequent studies shown that IL-6 directly stimulates survival of pulmonary vascular cells and that genetic or pharmacological inhibition of IL-6 signaling attenuates PH in multiple preclinical models69. Taken together, these findings indicate that IL-6 overexpression is necessary but not sufficient to cause PAH. Aside from IL-6, overexpression of tumor necrosis factor (TNF)-α by lung epithelial cells was documented to produce a marked elevation of RV systolic pressures in mice reared at moderate altitude (Denver, CO, 5280 feet). Although histological and morphometric analysis of pulmonary vessels were not conducted, a noticeable airspace enlargement (probably due to impaired alveolarization ± emphysema) was noted in transgenic mice70, potentially making them a more appropriate model for group 3 PH. The complex interplay between lung and vascular inflammation, perhaps highlighted by the overlap of patients with Group 1 PAH and Group 3 PH associated with lung disease is exemplified by work demonstrating that the TNF-related apoptosis-inducing ligand (TRAIL) can regulate neutrophil apoptosis in lung injury models to resolve inflammation 71 but while delivery of recombinant TRAIL reduces lung injury and fibrosis72, deficiency of TRAIL is protective to development of PAH73, 74.
Importance of inflammation in PAH onset and progression is further highlighted by a recent study examining PAH patients harboring rare and predicted deleterious variants in the Tet methylcytosine dioxygenase 2 (TET2) gene75, encoding for an enzyme known to promote DNA demethylation and repress cytokine gene expression in inflammatory cells76. The authors demonstrated that TET2 variant carriers exhibit increased overall inflammation compared to age/sex-matched healthy and PAH non-mutated patients. Similarly, they found that conditional inactivation of Tet2 in hematopoietic cells resulted in persistent lung inflammation associated with increased muscularization of distal PAs and mild elevation of the RVSP in 7–10-month-old mice; a phenotype reversed by administration of IL-1β neutralizing antibodies75.
Animal models related to abnormal activity of transcription factors
As the name implies, Hypoxia-inducible factors alpha (HIFα) family members are pivotal transcription factors that orchestrate adaptive responses under low oxygen conditions. Under normoxia, the HIFα protein levels are low due to constant degradation via the collaborative action of prolyl hydroxylases and the von Hippel-Lindau (VHL)-containing E3 ubiquitin ligase complex. Not surprisingly, both HIF1α and HIF2α isoforms have been extensively studies in PH77. Increased activation of the different isoforms was reported to regulate a large array of genes that promote a metabolic switch to glycolysis, cell survival and proliferation as well as endothelial to mesenchymal transition77. Mice bearing a missense gain-of-function mutation in the Hif2a gene were shown to develop PH with a significant increase in RVSP in heterozygotes (46mmHg) and an even more robust increase in homozygotes (65mmHg)78. In direct connection with this, consistent in vivo results were obtained by different groups regarding the implication of prolyl hydroxylases and their negative regulation of HIF abundance. Published data demonstrated that loss of prolyl hydroxylase domain protein 2 (PHD2, encoded by the Egln1 gene) targeted to endothelial cells induces severe PH in mice (RVSP reaching 60 to 75 mmHg in 3.5-month-old animals)79–81. Occlusive pulmonary vascular remodeling, severe RV hypertrophy and failure and progressive mortality were seen in mutant mice. Consistent with prior findings showing that PHD2 deficiency induces stabilization of HIFα transcription factors, both HIF-1α and HIF-2α were found elevated in lungs of Egln1 conditional knockout mice. To determine whether HIF-1α or HIF-2α derepression causes the phenotype, endothelial specific Egln1 inactivated mice were bred with Hif1aflox/flox or Hif2aflox/flox mice. Using this elegant approach, the authors demonstrated that activation of HIF-2α (not HIF-1α) is responsible for the obvious alterations seen in Egln1 conditional knockout mice79, 80. Similarly, mice homozygous for the R200W mutation in the tumor suppressor gene Vhl developed pulmonary hypertension independently of polycythemia similar to Chuvash patients. Lungs from homozygous carriers displayed pulmonary vascular remodeling, hemorrhage, edema, and macrophage infiltration, aggravated by fibrosis in older mice. As depicted in Egln1 conditional knockout mice, loss of 1 allele of Hif2α partially rescued the lung phenotype of VhlR200W/R200W mice82, providing genetic evidence that dysregulated HIFα signaling contributes to aberrant pulmonary vascular remodeling in PH. Besides HIFα signaling, a large body of evidence implicate peroxisome proliferator-activated receptor gamma (PPARγ) as a master regulator of vascular homeostasis in the lung, with diminished expression in remodeled PAs of PH patients83. Activation of PPARγ secondary to BMPR2 signaling was shown to exert anti-proliferative effects on PASMCs84. Additional protective effects of PPARγ also include anti-inflammatory and anti-vasoconstrictive effects. Accordingly, mice deficient for Pparg in smooth muscle cells presented a slight elevation of the RVSP as compared to controls (29 vs 21mmHg)84. Although transcription factors are usually regarded as difficult to target, several preclinical studies have shown that PPARγ agonists (rosiglitazone or pioglitazone) significantly prevent or reverse the pathological hallmarks of PAH85, adding further evidence that small-molecule modulation of transcription factor activity has substantial potential for PAH.
Systemic sclerosis (SSc) is a multisystem autoimmune disease characterized by chronic inflammation, proliferative vasculopathy and fibrosis of the skin and various organs. The autoimmune process can affect the lung resulting in scarring and vascular injury contributing to the development of PAH with significant negative impact on survival86. Although several animal models of SSc have been generated to dissect the cellular and molecular mechanisms of SSc, rare are those reproducing the pulmonary manifestations of the disease, pushing researchers to borrow animal models from idiopathic pulmonary fibrosis (IPF) to get valuable information about the pathogenesis of the human disease. This is illustrated by a recent randomized trial showing that Nindenadib (approved for the treatment of IPF) is effective in slowing progression of SSc-associated pulmonary fibrosis 87. Overexpression in mice of Fos-related protein 2 (FRA2), a subunit of the transcription factor AP-1, was shown to replicate many features of SSc with predominant alterations in the lung88, 89. Comprehensive characterization of the pulmonary phenotype of these mice at 12–17 weeks of age demonstrated that perivascular inflammation associated with concentric laminar lesions are the first apparent pathological alterations followed by prominent interstitial fibrosis88, 89. Accordingly, mutant mice exhibited a modest but significant elevation of RVSP (~30mmHg)90. Although enhanced PDGF-BB-mediated PDGFRβ signaling was proposed as a molecular link of adverse lung remodeling in Fra-2 transgenic mice89, how FRA2 mechanistically contributes to lung scaring and pulmonary vascular remodeling remains largely unknown. Nevertheless, this animal model may provide invaluable clues in better understanding SSc-related PAH and a promising entry point to the development of much-needed new therapies.
Animal models related to mitochondria dysfunction
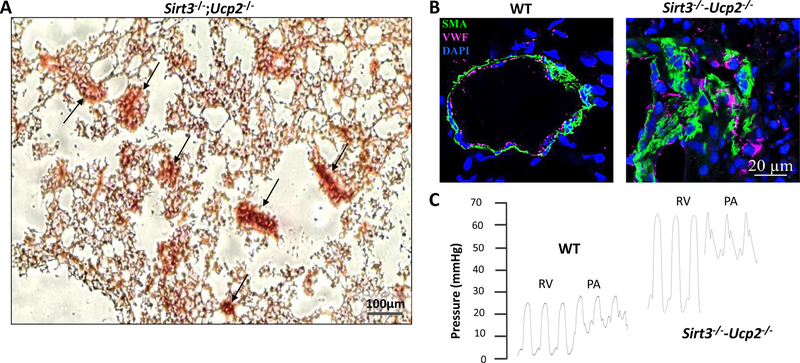
Metabolic dysfunctions are well-established critical components of PAH91, 92. Like cancer cells, vascular cells in PAH exhibit multiple metabolic alterations including a shift from oxidative phosphorylation to glycolysis92. This metabolic rewiring confers growth and survival advantages, in part, through hyperpolarization of the mitochondrial membrane and epigenetic regulation of gene expression programs92. Guided by accumulating evidence pinpointing occurrence of metabolic dysfunction in PAH patients, signs of PH were researched in mice deficient for some factors critically implicated in the regulation of mitochondrial function. Mice lacking mitochondrial uncoupling protein 2 (UCP2) or Sirtuin 3 (SIRT3, a mitochondrial deacetylase) were reported to spontaneously develop pulmonary vascular remodeling and mild PH93, 94. Interestingly, loss-of-function UCP2 and SIRT3 polymorphisms were found often both in the same patient in a homozygous or heterozygous manner and their presence correlated positively with the degree of PAH upon referral and outcomes (death, transplantation). Accordingly, Sirt3/Ucp2 double knockout mice displayed an increased severity of PH associated with RV dysfunction and dilatation, along with insulin resistance. Histological examination of lungs from double-mutant mice revealed the presence of multifocal inflammatory plexiform-like lesions occurring before the elevation of the RVSP (Figure 2), making these mutant mice one of the rare mouse models recapitulating many features of human PAH and thus a significant tool in the armamentarium of the PAH researcher95.
Figure 2. Plexogenic arteriopathy and severe PAH in mice lacking both Sirt3 and Ucp2.
(A) Representative histology (Hematoxylin-Eosin staining) of a lung from a Sirt3−/−;Ucp2−/− mouse shows numerous plexogenic lesions (back arrows). (B) Representative photomicrograph of confocal fluorescence immunohistochemistry of small pulmonary arteries from wild-type WT and Sirt3−/−;Ucp2−/− mice shows smooth muscle cells (green) and endothelial cells (magenta) in plexogenic lesions. (C) Representative tracings of RV and PA pressures from WT and Sirt3−/−;Ucp2−/− mice by close-chest right heart catheterization through the jugular vein shows that Sirt3−/−;Ucp2−/− mice have a significant increase in RV and PA pressure. SMA: smooth muscle actin, vWF: von Willebrand Factor (marking endothelial cells), DAPI (marking nuclei). (Images courtesy of Dr Gopinath Sutendra.)
The metabolic theory of PH is also supported by clinical reports describing the development of a metabolic syndrome associated with the occurrence of PAH in individuals carrying mutations in genes involved in iron-sulfur cluster biogenesis96, 97. Mainly assembled by the mitochondria, iron-sulfur clusters are essential cofactors for many proteins involved in immune response and various core cellular functions such as mitochondrial respiration, DNA replication and repair, and gene expression98. To examine the functional relevance of mitochondrial iron homeostasis in the onset of PH, different animal models have been established. Deletion, mutation, or knockdown of multiple iron metabolism-related genes such as Iron regulatory protein 199, NFU1100, BolA family member 3101 and Frataxin102 were documented to promote hemodynamic manifestations of PH in rodent by decreasing oxidative phosphorylation. Along with defects in mitochondrial iron metabolism, iron deficiency was found prevalent in PAH patients and correlated with worse clinical conditions103, 104. Importance of iron homeostasis in the cardio-pulmonary system was further supported by experimental animal studies showing that SD rats fed an iron-deficient diet for 4 weeks display an abnormal muscularization of PAs associated with up-regulation of HIFα and acquisition of a glycolytic state as well as increased PA pressure and RV hypertrophy105.
Animal models related to drug-induced PH and PVOD
According to the current classification, PAH can be associated with drug use1. Indeed, several types of medications such as anorexigens or anti-cancer agents as well as abuse consumption of illicit drugs were confirmed to be risk factors for PAH. One of the best-documented causes is the appetite-suppressants fenfluramine and aminorex. This association was derived from epidemiological studies conducted in the 1990s showing that the risk of developing PAH was 10 to 30 times higher in people who took certain appetite suppressants than in the general population106, eventually leading to their withdrawal from the world market. Since fenfluramide and aminorex are serotonin transporter substrates and indirect serotonergic agonists, many experimental studies have concentrated on further linking serotonin signaling to PAH development. In PAH patients, endothelial-derived serotonin was shown to activate serotonin receptors and enter the cell via the serotonin transporter (SERT) to induce contraction and proliferation of adjacent PASMCs107–109. The molecular mechanisms implicated in this response are complex and involve the modulation of voltage-gated K+/voltage-operated Ca2+ channels110, 111 and the serotonylation (covalent linkage of serotonin to proteins) of several intracellular targets112, 113. Not surprisingly, serotonin was reported to stimulate expression and release of various factors contributing to the pro-proliferative phenotype of PAH-PASMCs, including Ca2+-dependent S100A4 (Mts1) protein109, for which overexpression promotes the development of plexiform-like lesions with minimal elevation of the RVSP in female mice selectively114, 115. The instrumental role of serotonin signaling in the development and maintenance of PAH is strongly suggested by published data showing that pulmonary vascular remodeling and elevated RVSP were seen in mice chronically administered with dexfenfluramine116 or displaying increased internalization of serotonin secondary to SERT overexpression116–118.
In addition to appetite suppressants, tyrosine kinases inhibitors (TKI) such as Dasatinib and Xalkori have been associated with PAH119. Results gained from experimental studies demonstrated that PAECs exposed to these anti-cancer-agents present cell damage, as reflected by initiation of a global endoplasmic reticulum stress response, mitochondrial dysfunction and induction of apoptosis. However, the insults appear insufficient to cause PH, as rats chronically treated with these TKIs do not experience any major changes in pulmonary hemodynamic parameters and vascular remodeling9, 120. However, when given prior to PH inducers (MCT or hypoxia), these anti-cancer agents were shown to potentiate the development of the disease by exacerbating hemodynamical and structural changes9, 120, providing strong support to the two-hit theory of the pathophysiology of TKI-PAH.
Pulmonary veno-occlusive disease (PVOD), an uncommon form of PAH characterized by preferential remodeling of pulmonary veins, has long suffered from the lack of an animal model. Following repetitive case reports mentioning the occurrence of PVOD in patients receiving chemotherapy, the ability of alkylating agents to cause PVOD was tested in different animal species. In a first study, cyclophosphamide was reported to induce venular remodeling and PH in a dose-dependent manner preferentially in female rats and rabbits, whereas mice were less responsive121. In a second study, the same group demonstrated that a single injection of mitomycin-C produced a slight elevation of mPAP accompanied by a remodeling of pulmonary venous and capillary compartments122. Together, these findings highlight a probable cause-effect relationship between alkylating class chemotherapeutic drugs and PVOD. Of note, although mutations in the EIF2AK4 (also called GCN2) gene were identified as the major genetic cause of PVOD123, Eif2ak4Δ152Ex1/ Δ152Ex1 knockout mutant rats failed to present elevation of RVSP124.
Human immunodeficiency virus (HIV)-associated PAH (group 1.4.2)
PAH is overrepresented in individuals diagnosed as HIV-positive and again more prevalent in HIV-infected intravenous drug-abusing users125, 126. Nonhuman primates infected with simian immunodeficiency virus (SIV) or SHIV-nef (a chimeric viral construct containing the HIV nef gene in a SIV backbone) have been used to mimic the salient features of HIV-1 infection in humans and acquire scientific knowledge on the occurrence of pulmonary vasculopathy. Consistent with that seen in human, pulmonary vascular lesions characterized by intimal and medial hyperplasia, adventitial fibrosis and recruitment of inflammatory cells were commonly encountered in SIV or SHIV-infected animals127–130. Longitudinal evaluation of cardiopulmonary function in rhesus macaques before and after SIV infection demonstrated that around 50% of animals had elevated mean PA pressure greater than 25mmHg 6-month post infection, among these 50% exhibiting worsening at 1 year131. All animals presented evidence of pulmonary inflammation. Histological and metabolic analyses revealed an increased collagen deposition and glucose uptake in RV from SIV-infected macaques with hemodynamic evidence of PAH. However, no RV hypertrophy was observed and RV function was preserved131. Due to complex infrastructure to house nonhuman primates and high maintenance costs, efforts have focused on the development of small animals for investigating the pathogenesis of HIV. Signs of PH were researched in HIV-1 transgenic rats exposed or not to chronic stress. Different results were obtained with some showing a significant elevation of the RVSP in HIV-1 transgenic rats132, whereas other found no difference133. However, expression of the HIV-1 transgene was consistently shown to increase responsiveness to PH induced by either chronic hypoxia exposure133 or chronic administration of cocaine134. Although findings gained from this model can be difficult to extrapolate to human, it offers significant advantages as a noninfectious small-animal model of HIV-PAH.
2. Animal models of pulmonary hypertension due to hypoxia and/or lung diseases (Group 3 PH)
Hypoxic PH often complicates the course of patients with advanced lung diseases including but not limited to: sleep apnea, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and developmental lung disorders135.
Animal models related to chronic hypoxia
Although often employed to draw conclusions in group 1 PH, exposure to animals to chronic hypoxia is largely considered as a model recapitulating the pulmonary vascular changes observed during exposure to prolonged high altitude. It is well established that exposure to rodents to chronic alveolar hypoxia (usually 3 weeks) primary causes constriction of resistant vessels in the pulmonary vascular bed. When hypoxia is sustained, a moderate increase in the muscularization of proximal and distal pulmonary vessels occurs contributing to elevation of pulmonary vascular resistance. Several non-exclusive mechanisms for the de novo muscularization of small PAs have been proposed including distal expansion of pre-existing vascular smooth muscle cells and endothelial to mesenchymal transdifferentiation136–138. The magnitude of the hypoxic response varies across species with further intra-species differences relating to the strain, age and gender139. For instance, for the same hypoxic challenge, SD rats are more prone to develop pulmonary vascular remodeling than C57BL/6 mice140. Furthermore, younger animals are more susceptible to develop severe PH. Indeed, newborn calves placed under hypoxic conditions develop a more severe PH accompanied by a pronounced medial wall and adventitial thickening of PAs, as compared to rodent kept under chronic hypoxia141. The major shortcomings of this model are the moderate severity of PH, as determined in part, by the limited extent of pathological remodeling of the pulmonary vasculature and inability to replicate the maladaptive events leading to RV failure as well as the reversibility of structural and functional alterations in the pulmonary vasculature upon return to hypoxia142 (Figure 1). Despite these limitations, the chronic hypoxia model has and continues to provide us valuable insights into the vasoconstrictor component of PH. Past results obtained with this model are the groundwork for currently available PAH-based therapies Moreover, it offers the advantage to be relatively inexpensive, easy to implement, highly reproducible and remains an essential tool to appreciate the susceptibility of genetically modified mice to PH development.
Exposure for rodents to chronic intermittent hypoxia (CIH) is also used to mimic the cyclic pattern of hypoxemia observed in patients experiencing obstructive sleep apnea (OSA)143, 144; a condition complicated by mild or moderate PH if untreated145. Indeed, exposure to CIH for several weeks were shown to induce signs of PH, as illustrated by a thickening of small pulmonary vessels, a mild elevation in RV systolic pressure and a slight increase in RV mass144, 146.
Mild-to-moderate PH is frequently encountered in COPD, an irreversible lung condition due to chronic and persistent inflammatory response and leading to chronic bronchitis and emphysema. Because the disease most commonly results from long-standing inhalation of irritants, in particular cigarette smoke, cigarette smoke exposure has become one of the most utilized animal models. Findings from mice, rats, hamsters or guinea pigs revealed that chronic exposure to cigarette smoke, usually over a 4–6-month period, is accompanied by an airspace enlargement, thickening of the PA wall and elevated RVSP147–152. Analysis of the time course of lung injury in rodent models repeatedly demonstrated that pulmonary vascular remodeling and PH may precede the development of emphysema148, 149, providing evidence that cigarette smoke has a direct impact on the pulmonary vasculature and that PH in COPD is not necessarily driven by loss of capillaries in emphysema and chronic alveolar hypoxia.
Idiopathic pulmonary fibrosis (IPF)
Different animal models have been generated displaying parenchymal lung fibrosis with varying degrees of pulmonary vascular remodeling and PH. Although decried due to its tendency to spontaneously resolve in young mice or after a single challenge, administration of the chemotherapeutic agent bleomycin (BLM) in rodents is the best-characterized animal model of pulmonary fibrosis, and as such, is considered as the greatest model for preclinical testing153. In this relatively easy to handle animal model with many variations in terms of dosage and route of delivery, muscularization of pulmonary vessels accompanied with or without the presence of mild PH have been repeatedly described154–157. Since elevation of RVSP in BLM-challenged rodents is most often modest and occurs concomitantly with the appearance of fibrotic lesions, a two-hit rat model by sequential exposure to BLM and MCT was recently developed to more adequately recapitulate group 3 PH156, 157. As revealed by histological analyses, hydroxyproline assay, lung function testing and right heart catheterization, the combination of these insults results in pronounced fibrosis associated with altered mechanical properties of the respiratory system and a robust PH phenotype156, 157, making this model more appropriate to assess the impact of drugs on the development of PH in pre-existing pulmonary fibrosis. Besides these drug-induced animal models, intratracheal delivery of an adenoviral gene vector encoding biologically active transforming growth factor (TGF)-β1 has been shown to produce prolonged severe fibrosis in rat lung leading to marked pulmonary vascular remodeling and PH158, 159. Using this animal model, Bellaye and coworkers found that Macitentan (a dual endothelin receptor antagonist approved for the treatment of PAH) given two weeks after instillation of AdTGF-β1 for two weeks significantly prevents progression of lung fibrosis and PH160.
Lung immaturity
In the early 1990s, the British epidemiologist David Barker proposed that adverse influences early in development could elicit permanent structural changes thereby predisposing individuals to an increased risk of disease in adulthood 161. This concept, commonly referred to as “the Fetal Origins of Adult Disease” or “Fetal Programming of Adult Disease” expanded greatly during the past decades. It is now clearly established that extremely preterm-born infants and survivors of bronchopulmonary dysplasia (BPD, an important complication of prematurity) have a higher risk to develop COPD later in life162–164. Although less documented, the long-term effects of in utero and early life factors on pulmonary vascular dysfunction in adulthood have been highlighted by several longitudinal cohort studies. Jayet and coworkers found that preeclampsia, a complex placental dysfunction disorder and prenatal risk factor for chronic lung disease in very low birth weight infants, is associated with a persistent dysfunction in the pulmonary circulation of the offspring at age 13–14 years with a roughly 30% higher PA pressure than in control subjects165. In a direct link with this concept, premature birth was associated with an increased risk of adult PH in a Swedish population-based study166. Similarly, in a prospectively followed US cohort, young adults born preterm demonstrated early vascular disease characterized by elevated mean PA pressure (with 45% > 19mmHg) and RV dysfunction167, 168. These studies provide compelling evidence that interruption of normal pulmonary vascular development due to prematurity or early lung injury causes short-term and long-term cardiopulmonary sequelae and increases the risk of developing PH later in life. The predisposition of perinatal insults to pulmonary vascular dysfunction in adulthood is also exemplified by a series of studies conducted in rodents. Treatment of newborn rats with Sugen (Su) is known to result in reduced pulmonary angiogenesis, increased PA wall thickness, and decreased alveolarization, mimicking anomalies encountered in premature newborns who develop BPD169, 170. Despite catch-up growth, these anomalies persist into adulthood since diminished PA density, increased PA wall thickness, airspace enlargement and RV hypertrophy were also detected in adult rats170. The durable impairment of postnatal hyperoxia on the adult pulmonary vascular bed and RV function was also underscored by Goss and coworkers 171. In this study, newborn rats were exposed to hyperoxia for 2 weeks to mimic some aspects of BPD. At the end of the 2-week exposure period, animals were then allowed to age out 1 year in standard animal housing. Aged rats that had experienced hyperoxia during their first days of life demonstrated a decreased pulmonary vascular surface area and a significant increase in RV systolic pressure and hypertrophy 171. Additionally, brief perinatal hypoxia was documented in rats to increase severity after re-exposure to hypoxia at 2 weeks of age 172 and hypoxia exposure of adult rats treated with SU as newborns led to a more severe RV hypertrophy compared with those exposed only as adults, indicating that early lung injuries are accompanied with long-term pulmonary sequelae predisposing to the development of PH. In a similar fashion, postnatal treatment of newborn rats with glucocorticoids decreased alveolarization and PA density, leading to long-term alterations including increased PA pressure 173.
The Fawn-Hooded rat (FHR) strain, characterized by a chromosome 1 abnormality and increased serotonin blood levels due to a platelet storage pool deficiency, is reported to spontaneously develops pulmonary hypertension at sea level, a phenotype amplified with exposure to mild hypoxia174, 175. Mechanistic studies conducted in adult rats have revealed that the chromosome 1 abnormality is responsible for mitochondrial defects. Indeed, as observed in PAH-PASMCs, PASMCs from FHR rats exhibit fragmented and hyperpolarized mitochondria along with diminished expression of superoxide dismutase 2 (SOD2) leading to normoxic activation of hypoxia-inducible factor 1 alpha (HIF-1α) and subsequent downregulation of potassium voltage-gated channel subfamily A member 5 (Kv1.5)176, 177, all contributing to their abnormal pro-proliferative and apoptosis-resistant phenotype. As such, this genetic strain is classically used as a model of pulmonary hypertension in adult rats. Interestingly, when compared to the Sprague Dawley rat strain, lung architecture of FHR pups exhibits a marked airspace enlargement secondary to impaired alveolarization178. Supplementation in oxygen during the combined prenatal and early postnatal period significantly improved alveolarization and reduced the development of PH as reflected by measurements of right ventricular hypertrophy and wall thickness of small PAs, reinforcing the notion that pulmonary hypertension detected in adult life originates at least in part from developmental defects178.
3. Animal Models of Group 2 Pulmonary Hypertension
Group 2 PH is thought to occur as a result of increased back pressure due to left atrial, left ventricular, or valvular (mitral or aortic) pathology. While many models that have been proposed to study the pathogenesis of valvular disease179, systolic heart failure180, and diastolic heart failure181, very few reports have directly assessed the degree of pulmonary vascular remodeling in these models. While no models perfectly recapitulate the heterogeneity of Group 2 PH in humans, a number of models have been proposed that provide mechanistic insight into how comorbidities known to associate with Group 2 PH may cause or exacerbate pulmonary vascular remodeling.
Pressure overload
Elevated left sided filling pressures are a key hemodynamic finding in the diagnosis of Group 2. A robust model for causing pressure overload in animals is the transverse aortic banding model182, 183. In addition to the development significant fibrosis and upregulation of fibrotic gene programs in the lungs of mice with heart failure, pressure overload results in a large leukocytic infiltrate with concomitant upregulation of pro-inflammatory cytokines, injury, muscularization of small vessels, and increase in endothelin-1 and nitric oxide mediated signals. While a shortcoming of this model is the lack of clinical correlation with an acute load imposed by aortic banding, it remains a robust model for studying the load-dependent changes in pulmonary vascular remodeling. Notably, this model has been replicated in larger animal models that more closely approximate human pulmonary vascular pathology184, 185.
Obesity and Metabolic Syndrome
Given the known risk factors of diabetes and obesity in exacerbating Group 2 PH186, 187, a number of models have utilized mice prone to the development of metabolic syndrome to identify mechanisms by which obesity and diabetes contribute to Group 2PH. Hansmann et al utilized the ApoE−/− knockout mouse, which is prone to development of atherosclerotic heart failure, to show that the development of insulin resistance was associated with histologic and hemodynamic features of pulmonary hypertension that could be reversed with rosiglitazone, an activator of PPARγ188, 189. The AKR mouse, a mouse strain susceptible to development of hypertension and metabolic syndrome190, has been used to study the mechanisms underlying obesity-related pulmonary vascular remodeling in Group 2 PH191, 192. Strengths of these models are that they are relatively simple models to implement for the studying the effect of metabolic syndrome upon pulmonary vascular remodeling, but they each have the notable limitation of producing only a mild pulmonary vascular phenotype.
Multi-comorbidity Interactions
A number of studies have now shown that a combination of comorbid conditions induced by hemodynamic stressors, metabolic stressors, vascular injury, and/or inflammatory stressors are more robust models of Group 2 PH. Inflammation has been postulated to play an important role in the pathogenesis of Group 2 PH193. Lawrie et al expanded on previous work by Hansmann et al to show that pulmonary vascular remodeling in ApoE−/− mice was at least partially dependent on inflammation mediated by IL-1194. Treatment with an IL-1 receptor antagonist prevented high-cholesterol diet-mediated pulmonary vascular remodeling. Lai et al utilized a combination of vascular injury induced by Su and a metabolic stress induced by high fat diet in obese, ZSF1 (leptin receptor deficient) rats to cause pulmonary vascular remodeling with concomitant cardiac hypertrophy195. They further demonstrated that chronic treatment with nitrite and metformin prevented pulmonary vascular remodeling via activation of SIRT3 and AMP-activated protein kinase (AMPK). Finally, a recent model by Ranchoux et al utilized a combination of hemodynamic stress induced by supracoronary banding and metabolic stress induced by either high fat diet or olanzapine treatment (an anti-psychotic drug with known side effects of metabolic syndrome) to demonstrate an important role for IL-6 mediated STAT3 activation in the pathogenesis of Group 2 PH196. These models have the distinct advantage of likely recapitulating the mechanisms by which multiple comorbidities promote pulmonary vascular remodeling in Group 2 PH, but future translational studies are needed to determine whether the identified pathways are indeed actionable for the prevention or reversal of Group 2 PH.
4. Animal Models of RV Failure
Although the pulmonary vasculature is the locus of the initial insult, survival of PAH patients is intimately related to RV function197. Generally considered as a feature to combat, RV hypertrophy is classically divided into 2 patterns on the basis of morphological and functional changes: adaptive remodeling (compensated state) characterized by more concentric remodeling, minimal dilatation and fibrosis, normal ejection fraction, and CO, and maladaptive remodeling (decompensated state) associated with increased infiltration of inflammatory cells, accumulation of extracellular matrix due to fibroblast activation and proliferation, cardiomyocyte loss and capillary rarefaction resulting in altered ejection fraction, low CO, and diminish exercise capacity198, 199. While there is broad consensus on the fact that hypertrophy can be either beneficial or detrimental, the molecular mechanisms and pathways governing the process from adaptive to maladaptive hypertrophy remain largely unknown, and as a consequence, there are no clinically established treatments for RV failure. Along with the MCT model mentioned above, the constriction of the pulmonary artery with a clip or a ligature represents a suitable model to study RV remodeling and dysfunction in the setting of PAH. This surgical procedure, called pulmonary artery banding (PAB), is commonly used in various species, including rodents and larger animals such as sheep, to generate RV afterload200, 201. In this model, different degrees of constriction can be applied leading to either long-term compensatory hypertrophy (mild constriction) or RV failure (tight constriction)202, 203 (Figure 1). There are a number of benefits to using this model, first of all to replicate the adaptive and maladaptive continuum of the RV stress response and thus capture the cellular and molecular events implicated in the stress coping response, or inversely, disruption of the latter. To this end, a comprehensive hemodynamic characterization of PAB or MCT-challenged animals determined by right heart catheterization (mPAP, CO, RVEDP…) followed by their subsequent categorization into compensated or decompensated state are prerequisite7, 204, 205. In addition, the PAB model serves as a complementary tool to conventional PAH models in determining whether a drug that shows anti-remodeling effects on the pulmonary vasculature has a beneficial, direct damaging or no effect on the remodeled RV22, 206. This is all the more important given the increasing interest in “anti-remodeling“ approaches and that, paradoxically, the main purpose of such approaches is to stop proliferation and induce death of unwanted PA cells, while preserving survival and function of cardiomyocytes is desired. In direct connection with this, it can be hypothesized that the beneficial effects of the drug on the pulmonary vasculature masks potential deleterious effects on the stressed RV.
Conclusion and concluding remarks
Given the complex multifactorial nature of PH, no single animal model can per se completely mimic the human condition. Ideally, a model of PH would be more prevalent in female but more severe in male; progressive and irreversible in nature (culminating in RV failure and death); should mimic the pathophysiology of the disease (i.e. similar genetic, epigenetic, metabolic and inflammatory profiles leading to profound vascular lesions). Finally respond to standard of care should be limited as seen in patients. Such model can probably be generated by combining the existing ones. So far, multiple animal models have been developed, each of which shed light on different processes that appear to play a role (Table 1). Animal models have proven to be a great value in deciphering the molecular physiopathology of PH as well as key information on therapeutic strategies that cannot be obtained by using only alternative methods. Rather than an alternative, ex-vivo studies, such as human precision cut lung slice, can be considered as helpful complementary approaches allowing the researcher to bridge the gap between animal models and human patients, thus reducing the number of animals used to a minimum. It must keep in mind that PH animal models provide only clues, not final answers, for human disease. It is crucial to select the most appropriate animal model based on aspects they can reproduce. For instance, if the primarily goal is to assess the effects of a molecule on pulmonary vascular remodeling, the MCT and Su/Hx models should be privileged. Similarly, the MCT and PAB models should be prioritized to study the molecular mechanisms responsible for RV failure and evaluate the cardioprotective effects of new approaches. In parallel, improvement of animal models of PH by combining different risk factors or hits is needed. In that respect, the comparison of “one” versus multi-hit models that mix genetics with environmental interactions, various mechanisms and pathways, comorbidities and other diseases that lead to PH is encouraged to better capture the multifactorial nature of PH. Recent studies showing that (i) Sirt3;Ucp2 double knockout mice developed several features of the human disease that are typically absent in other PAH mouse models95 and (ii) phenotypically silent BMPR2 mutations predispose rats to inflammation-induced PAH55 echoes this view. Lastly, due to inherent limitations of currently available animal models, it is recommended that, where possible, the effects of an intervention be tested in multiple animal models, thereby maximizing the chance of translational success.
Acknowledgments
Sources of Funding
AL is supported by a BHF Senior Basic Science Research Fellowship (FS/18/52/33808). VA is supported by Team Phenomenal Hope Foundation Grant, Pulmonary Hypertension Association Young Investigator Award, and NIH (1K08-HL153956-01A1). OB is supported by the Canadian Institute of Health Research (CIHR, #IC121617). OB hold a junior scholar award from the Fonds de Recherche du Québec: Santé (FRQS). SB hold a distinguished research scholar from FRQS.
Nonstandard Abbreviations and Acronyms
- BPD
bronchopulmonary dysplasia
- BMPR2
bone morphogenetic protein receptor 2
- COPD
chronic obstructive pulmonary disease
- FHR
Fawn-Hooded rat
- HIV
Human immunodeficiency virus
- IPF
idiopathic pulmonary fibrosis
- MCT
monocrotaline
- mPAP
mean pulmonary artery pressure
- PA
pulmonary artery
- PAB
pulmonary artery banding
- PAEC
pulmonary artery endothelial cell
- PAH
pulmonary arterial hypertension
- PASMC
pulmonary artery smooth muscle cell
- PH
pulmonary hypertension
- PVOD
Pulmonary veno-occlusive disease
- PVR
pulmonary vascular resistance
- RV
right ventricle/ventricular
- RVSP
right ventricular systolic pressure
- SD
sprague-dawley
- SSc
Systemic sclerosis
- Su/Hx
Sugen/Hypoxia
Footnotes
Disclosures
The authors have nothing to disclose.
REFERENCES
- 1.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG and Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. The European respiratory journal. 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijeratne DT, Lajkosz K, Brogly SB, Lougheed MD, Jiang L, Housin A, Barber D, Johnson A, Doliszny KM and Archer SL. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ Cardiovasc Qual Outcomes. 2018;11:e003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada M, Yamashita C, Okada M and Okada K. Establishment of canine pulmonary hypertension with dehydromonocrotaline. Importance of larger animal model for lung transplantation. Transplantation. 1995;60:9–13. [DOI] [PubMed] [Google Scholar]
- 4.Zeng GQ, Liu R, Liao HX, Zhang XF, Qian YX, Liu BH, Wu QH, Zhao J, Gu WW and Li HT. Single intraperitoneal injection of monocrotaline as a novel large animal model of chronic pulmonary hypertension in Tibet minipigs. PLoS One. 2013;8:e78965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath D and Kay JM. Medical thickness of pulmonary trunk in rats with cor pulmonale induced by ingestion of Crotalaria spectabilis seeds. Cardiovasc Res. 1967;1:74–9. [DOI] [PubMed] [Google Scholar]
- 6.Smith P, Kay JM and Heath D. Hypertensive pulmonary vascular disease in rats after prolonged feeding with Crotalaria spectabilis seeds. J Pathol. 1970;102:97–106. [DOI] [PubMed] [Google Scholar]
- 7.Omura J, Habbout K, Shimauchi T, Wu WH, Breuils-Bonnet S, Tremblay E, Martineau S, Nadeau V, Gagnon K, Mazoyer F, Perron J, Potus F, Lin JH, Zafar H, Kiely DG, Lawrie A, Archer SL, Paulin R, Provencher S, Boucherat O and Bonnet S. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation. 2020;142:1464–1484. [DOI] [PubMed] [Google Scholar]
- 8.Ruiter G, de Man FS, Schalij I, Sairras S, Grunberg K, Westerhof N, van der Laarse WJ and Vonk-Noordegraaf A. Reversibility of the monocrotaline pulmonary hypertension rat model. The European respiratory journal. 2013;42:553–6. [DOI] [PubMed] [Google Scholar]
- 9.Awada C, Grobs Y, Wu WH, Habbout K, Romanet C, Breuils-Bonnet S, Tremblay E, Martineau S, Paulin R, Bonnet S, Provencher S, Potus F and Boucherat O. R-Crizotinib predisposes to and exacerbates pulmonary arterial hypertension in animal models. The European respiratory journal. 2021;57. [DOI] [PubMed] [Google Scholar]
- 10.Hill NS, Gillespie MN and McMurtry IF. Fifty Years of Monocrotaline-Induced Pulmonary Hypertension: What Has It Meant to the Field? Chest. 2017;152:1106–1108. [DOI] [PubMed] [Google Scholar]
- 11.Sutendra G and Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell metabolism. 2014;19:558–73. [DOI] [PubMed] [Google Scholar]
- 12.McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K and Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830–40. [DOI] [PubMed] [Google Scholar]
- 13.Rafikova O, Meadows ML, Kinchen JM, Mohney RP, Maltepe E, Desai AA, Yuan JX, Garcia JG, Fineman JR, Rafikov R and Black SM. Metabolic Changes Precede the Development of Pulmonary Hypertension in the Monocrotaline Exposed Rat Lung. PLoS One. 2016;11:e0150480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, Kouri FM, Peters DM, Dumitrascu R, Seeger W, Knaus P, Schermuly RT and Eickelberg O. Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2007;27:1072–8. [DOI] [PubMed] [Google Scholar]
- 15.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W and Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yung LM, Nikolic I, Paskin-Flerlage SD, Pearsall RS, Kumar R and Yu PB. A Selective Transforming Growth Factor-beta Ligand Trap Attenuates Pulmonary Hypertension. Am J Respir Crit Care Med. 2016;194:1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S and Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–97. [DOI] [PubMed] [Google Scholar]
- 18.Vitry G, Paulin R, Grobs Y, Lampron MC, Shimauchi T, Lemay SE, Tremblay E, Habbout K, Awada C, Bourgeois A, Nadeau V, Paradis R, Breuils-Bonnet S, Roux-Dalvai F, Orcholski M, Potus F, Provencher S, Boucherat O and Bonnet S. Oxidized DNA Precursors Cleanup by NUDT1 Contributes to Vascular Remodeling in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2021;203:614–627. [DOI] [PubMed] [Google Scholar]
- 19.Morimatsu Y, Sakashita N, Komohara Y, Ohnishi K, Masuda H, Dahan D, Takeya M, Guibert C and Marthan R. Development and characterization of an animal model of severe pulmonary arterial hypertension. J Vasc Res. 2012;49:33–42. [DOI] [PubMed] [Google Scholar]
- 20.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA and Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol. 1997;151:1019–25. [PMC free article] [PubMed] [Google Scholar]
- 21.van Albada ME, Schoemaker RG, Kemna MS, Cromme-Dijkhuis AH, van Veghel R and Berger RM. The role of increased pulmonary blood flow in pulmonary arterial hypertension. The European respiratory journal. 2005;26:487–93. [DOI] [PubMed] [Google Scholar]
- 22.Van der Feen DE, Kurakula K, Tremblay E, Boucherat O, Bossers GPL, Szulcek R, Bourgeois A, Lampron MC, Habbout K, Martineau S, Paulin R, Kulikowski E, Jahagirdar R, Schalij I, Bogaard HJ, Bartelds B, Provencher S, Berger RMF, Bonnet S and Goumans MJ. Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2019;200:910–920. [DOI] [PubMed] [Google Scholar]
- 23.Park JF, Clark VR, Banerjee S, Hong J, Razee A, Williams T, Fishbein G, Saddic L and Umar S. Transcriptomic Analysis of Right Ventricular Remodeling in Two Rat Models of Pulmonary Hypertension: Identification and Validation of Epithelial-to-Mesenchymal Transition in Human Right Ventricular Failure. Circ Heart Fail. 2021;14:e007058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J and Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF and Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:427–38. [DOI] [PubMed] [Google Scholar]
- 26.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF and Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–54. [DOI] [PubMed] [Google Scholar]
- 27.Toba M, Alzoubi A, O’Neill KD, Gairhe S, Matsumoto Y, Oshima K, Abe K, Oka M and McMurtry IF. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. American journal of physiology Heart and circulatory physiology. 2014;306:H243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suen CM, Chaudhary KR, Deng Y, Jiang B and Stewart DJ. Fischer rats exhibit maladaptive structural and molecular right ventricular remodelling in severe pulmonary hypertension: a genetically prone model for right heart failure. Cardiovasc Res. 2019;115:788–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelakis ED. Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: heterogeneous BMP signaling may have therapeutic implications. Circ Res. 2006;98:172–5. [DOI] [PubMed] [Google Scholar]
- 30.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, Francis SE, Rowlands DJ and Lawrie A. MicroRNA-140–5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold ND, Pickworth JA, West LE, Dawson S, Carvalho JA, Casbolt H, Braithwaite AT, Iremonger J, Renshall L, Germaschewski V, McCourt M, Bland-Ward P, Kowash H, Hameed AG, Rothman AMK, Frid MG, Roger Thompson AA, Evans HR, Southwood M, Morrell NW, Crossman DC, Whyte MKB, Stenmark KR, Newman CM, Kiely DG, Francis SE and Lawrie A. A therapeutic antibody targeting osteoprotegerin attenuates severe experimental pulmonary arterial hypertension. Nat Commun. 2019;10:5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grobs Y, Awada C, Lemay SE, Romanet C, Bourgeois A, Toro V, Nadeau V, Shimauchi K, Orcholski M, Breuils-Bonnet S, Tremblay E, Provencher S, Paulin R, Boucherat O and Bonnet S. Preclinical Investigation of Trifluoperazine as a Novel Therapeutic Agent for the Treatment of Pulmonary Arterial Hypertension. Int J Mol Sci. 2021;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, Stringer R, Jones P, Morrell NW, Jarai G, Walker C, Westwick J and Thomas M. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–82. [DOI] [PubMed] [Google Scholar]
- 34.Vitali SH, Hansmann G, Rose C, Fernandez-Gonzalez A, Scheid A, Mitsialis SA and Kourembanas S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ. 2014;4:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P and Rabinovitch M. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojonazarov B, Hadzic S, Ghofrani HA, Grimminger F, Seeger W, Weissmann N and Schermuly RT. Severe Emphysema in the SU5416/Hypoxia Rat Model of Pulmonary Hypertension. Am J Respir Crit Care Med. 2019;200:515–518. [DOI] [PubMed] [Google Scholar]
- 37.Nichols WC, Koller DL, Slovis B, Foroud T, Terry VH, Arnold ND, Siemieniak DR, Wheeler L, Phillips JA 3rd, Newman JH, Conneally PM, Ginsburg D and Loyd JE. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet. 1997;15:277–80. [DOI] [PubMed] [Google Scholar]
- 38.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE and Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.International PPHC, Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC and Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–4. [DOI] [PubMed] [Google Scholar]
- 40.Evans JD, Girerd B, Montani D, Wang XJ, Galie N, Austin ED, Elliott G, Asano K, Grunig E, Yan Y, Jing ZC, Manes A, Palazzini M, Wheeler LA, Nakayama I, Satoh T, Eichstaedt C, Hinderhofer K, Wolf M, Rosenzweig EB, Chung WK, Soubrier F, Simonneau G, Sitbon O, Graf S, Kaptoge S, Di Angelantonio E, Humbert M and Morrell NW. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. The Lancet Respiratory medicine. 2016;4:129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T and Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Developmental biology. 2000;221:249–58. [DOI] [PubMed] [Google Scholar]
- 42.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R and Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94:1109–14. [DOI] [PubMed] [Google Scholar]
- 43.West J, Harral J, Lane K, Deng Y, Ickes B, Crona D, Albu S, Stewart D and Fagan K. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. American journal of physiology Lung cellular and molecular physiology. 2008;295:L744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson JA, Hemnes AR, Perrien DS, Schuster M, Robinson LJ, Gladson S, Loibner H, Bai S, Blackwell TR, Tada Y, Harral JW, Talati M, Lane KB, Fagan KA and West J. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. American journal of physiology Lung cellular and molecular physiology. 2012;302:L474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, Maynard KB, Gleaves L, Talati M, Absi T, Disalvo T and West J. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD and Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E and Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. American journal of physiology Lung cellular and molecular physiology. 2004;287:L1241–7. [DOI] [PubMed] [Google Scholar]
- 48.Frank DB, Lowery J, Anderson L, Brink M, Reese J and de Caestecker M. Increased susceptibility to hypoxic pulmonary hypertension in Bmpr2 mutant mice is associated with endothelial dysfunction in the pulmonary vasculature. American journal of physiology Lung cellular and molecular physiology. 2008;294:L98–109. [DOI] [PubMed] [Google Scholar]
- 49.Song Y, Jones JE, Beppu H, Keaney JF Jr., Loscalzo J and Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Appleby S, Shanahan CM, Bloch KD, Pepke-Zaba J, Upton P and Morrell NW. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC and Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–27. [DOI] [PubMed] [Google Scholar]
- 52.Pickworth J, Rothman A, Iremonger J, Casbolt H, Hopkinson K, Hickey PM, Gladson S, Shay S, Morrell NW, Francis SE, West JD and Lawrie A. Differential IL-1 signaling induced by BMPR2 deficiency drives pulmonary vascular remodeling. Pulm Circ. 2017;7:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD and Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation. 2008;118:722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hautefort A, Mendes-Ferreira P, Sabourin J, Manaud G, Bertero T, Rucker-Martin C, Riou M, Adao R, Manoury B, Lambert M, Boet A, Lecerf F, Domergue V, Bras-Silva C, Gomez AM, Montani D, Girerd B, Humbert M, Antigny F and Perros F. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation. 2019;139:932–948. [DOI] [PubMed] [Google Scholar]
- 55.Tian W, Jiang X, Sung YK, Shuffle E, Wu TH, Kao PN, Tu AB, Dorfmuller P, Cao A, Wang L, Peng G, Kim Y, Zhang P, Chappell J, Pasupneti S, Dahms P, Maguire P, Chaib H, Zamanian R, Peters-Golden M, Snyder MP, Voelkel NF, Humbert M, Rabinovitch M and Nicolls MR. Phenotypically Silent Bone Morphogenetic Protein Receptor 2 Mutations Predispose Rats to Inflammation-Induced Pulmonary Arterial Hypertension by Enhancing the Risk for Neointimal Transformation. Circulation. 2019;140:1409–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, Trembath RC and Loyd JE. Genetics and genomics of pulmonary arterial hypertension. The European respiratory journal. 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jerkic M, Kabir MG, Davies A, Yu LX, McIntyre BA, Husain NW, Enomoto M, Sotov V, Husain M, Henkelman M, Belik J and Letarte M. Pulmonary hypertension in adult Alk1 heterozygous mice due to oxidative stress. Cardiovasc Res. 2011;92:375–84. [DOI] [PubMed] [Google Scholar]
- 58.Toporsian M, Jerkic M, Zhou YQ, Kabir MG, Yu LX, McIntyre BA, Davis A, Wang YJ, Stewart DJ, Belik J, Husain M, Henkelman M and Letarte M. Spontaneous adult-onset pulmonary arterial hypertension attributable to increased endothelial oxidative stress in a murine model of hereditary hemorrhagic telangiectasia. Arterioscler Thromb Vasc Biol. 2010;30:509–17. [DOI] [PubMed] [Google Scholar]
- 59.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J Jr. and Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A. 2002;99:11375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, Germain M, Tregouet DA, Borczuk A, Rosenzweig EB, Girerd B, Montani D, Humbert M, Loyd JE, Kass RS and Chung WK. A novel channelopathy in pulmonary arterial hypertension. The New England journal of medicine. 2013;369:351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhr FK, Smith KA, Song MY, Levitan I and Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca(2)(+) signaling. American journal of physiology Heart and circulatory physiology. 2012;302:H1546–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitagawa MG, Reynolds JO, Wehrens XHT, Bryan RM Jr. and Pandit LM. Hemodynamic and Pathologic Characterization of the TASK-1(−/−) Mouse Does Not Demonstrate Pulmonary Hypertension. Front Med (Lausanne). 2017;4:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lambert M, Capuano V, Boet A, Tesson L, Bertero T, Nakhleh MK, Remy S, Anegon I, Pechoux C, Hautefort A, Rucker-Martin C, Manoury B, Domergue V, Mercier O, Girerd B, Montani D, Perros F, Humbert M and Antigny F. Characterization of Kcnk3-Mutated Rat, a Novel Model of Pulmonary Hypertension. Circ Res. 2019;125:678–695. [DOI] [PubMed] [Google Scholar]
- 64.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G and Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–31. [DOI] [PubMed] [Google Scholar]
- 65.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J and Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–7. [DOI] [PubMed] [Google Scholar]
- 66.Rabinovitch M, Guignabert C, Humbert M and Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hagen M, Fagan K, Steudel W, Carr M, Lane K, Rodman DM and West J. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. American journal of physiology Lung cellular and molecular physiology. 2007;292:L1473–9. [DOI] [PubMed] [Google Scholar]
- 68.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA and Waxman AB. Interleukin-6 Overexpression Induces Pulmonary Hypertension. Circulation Research. 2009;104:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamura Y, Phan C, Tu L, Le Hiress M, Thuillet R, Jutant EM, Fadel E, Savale L, Huertas A, Humbert M and Guignabert C. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J Clin Invest. 2018;128:1956–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujita M, Shannon JM, Irvin CG, Fagan KA, Cool C, Augustin A and Mason RJ. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2001;280:L39–49. [DOI] [PubMed] [Google Scholar]
- 71.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, Dockrell DH and Whyte MK. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011;90:855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGrath EE, Lawrie A, Marriott HM, Mercer P, Cross SS, Arnold N, Singleton V, Thompson AA, Walmsley SR, Renshaw SA, Sabroe I, Chambers RC, Dockrell DH and Whyte MK. Deficiency of tumour necrosis factor-related apoptosis-inducing ligand exacerbates lung injury and fibrosis. Thorax. 2012;67:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hameed AG, Arnold ND, Chamberlain J, Pickworth JA, Paiva C, Dawson S, Cross S, Long L, Zhao L, Morrell NW, Crossman DC, Newman CM, Kiely DG, Francis SE and Lawrie A. Inhibition of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) reverses experimental pulmonary hypertension. J Exp Med. 2012;209:1919–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braithwaite AT, Marriott HM and Lawrie A. Divergent Roles for TRAIL in Lung Diseases. Front Med (Lausanne). 2018;5:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Potus F, Pauciulo MW, Cook EK, Zhu N, Hsieh A, Welch CL, Shen Y, Tian L, Lima P, Mewburn J, D’Arsigny CL, Lutz KA, Coleman AW, Damico R, Snetsinger B, Martin AY, Hassoun PM, Nichols WC, Chung WK, Rauh MJ and Archer SL. Novel Mutations and Decreased Expression of the Epigenetic Regulator TET2 in Pulmonary Arterial Hypertension. Circulation. 2020;141:1986–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q, Zhao K, Shen Q, Han Y, Gu Y, Li X, Zhao D, Liu Y, Wang C, Zhang X, Su X, Liu J, Ge W, Levine RL, Li N and Cao X. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pullamsetti SS, Mamazhakypov A, Weissmann N, Seeger W and Savai R. Hypoxia-inducible factor signaling in pulmonary hypertension. J Clin Invest. 2020;130:5638–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan Q, Kerestes H, Percy MJ, Pietrofesa R, Chen L, Khurana TS, Christofidou-Solomidou M, Lappin TR and Lee FS. Erythrocytosis and pulmonary hypertension in a mouse model of human HIF2A gain of function mutation. J Biol Chem. 2013;288:17134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kapitsinou PP, Rajendran G, Astleford L, Michael M, Schonfeld MP, Fields T, Shay S, French JL, West J and Haase VH. The Endothelial Prolyl-4-Hydroxylase Domain 2/Hypoxia-Inducible Factor 2 Axis Regulates Pulmonary Artery Pressure in Mice. Mol Cell Biol. 2016;36:1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai Z, Li M, Wharton J, Zhu MM and Zhao YY. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2alpha. Circulation. 2016;133:2447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S, Zeng H, Xie XJ, Tao YK, He X, Roman RJ, Aschner JL and Chen JX. Loss of prolyl hydroxylase domain protein 2 in vascular endothelium increases pericyte coverage and promotes pulmonary arterial remodeling. Oncotarget. 2016;7:58848–58861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, Christofidou-Solomidou M and Simon MC. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest. 2010;120:827–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW and Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92:1162–9. [DOI] [PubMed] [Google Scholar]
- 84.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW and Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hansmann G, Calvier L, Risbano MG and Chan SY. Activation of the Metabolic Master Regulator PPARgamma: A Potential PIOneering Therapy for Pulmonary Arterial Hypertension. American journal of respiratory cell and molecular biology. 2020;62:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mathai SC and Hassoun PM. Pulmonary arterial hypertension associated with systemic sclerosis. Expert Rev Respir Med. 2011;5:267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, Raghu G, Sauter W, Girard M, Alves M, Clerisme-Beaty E, Stowasser S, Tetzlaff K, Kuwana M, Maher TM and Investigators ST. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. The New England journal of medicine. 2019;380:2518–2528. [DOI] [PubMed] [Google Scholar]
- 88.Eferl R, Hasselblatt P, Rath M, Popper H, Zenz R, Komnenovic V, Idarraga MH, Kenner L and Wagner EF. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci U S A. 2008;105:10525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maurer B, Reich N, Juengel A, Kriegsmann J, Gay RE, Schett G, Michel BA, Gay S, Distler JH and Distler O. Fra-2 transgenic mice as a novel model of pulmonary hypertension associated with systemic sclerosis. Ann Rheum Dis. 2012;71:1382–7. [DOI] [PubMed] [Google Scholar]
- 90.Biasin V, Marsh LM, Egemnazarov B, Wilhelm J, Ghanim B, Klepetko W, Wygrecka M, Olschewski H, Eferl R, Olschewski A and Kwapiszewska G. Meprin beta, a novel mediator of vascular remodelling underlying pulmonary hypertension. J Pathol. 2014;233:7–17. [DOI] [PubMed] [Google Scholar]
- 91.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O’Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JSR and Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 2017;9. [DOI] [PubMed] [Google Scholar]
- 92.Xu W, Janocha AJ and Erzurum SC. Metabolism in Pulmonary Hypertension. Annu Rev Physiol. 2021;83:551–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pak O, Sommer N, Hoeres T, Bakr A, Waisbrod S, Sydykov A, Haag D, Esfandiary A, Kojonazarov B, Veit F, Fuchs B, Weisel FC, Hecker M, Schermuly RT, Grimminger F, Ghofrani HA, Seeger W and Weissmann N. Mitochondrial hyperpolarization in pulmonary vascular remodeling. Mitochondrial uncoupling protein deficiency as disease model. American journal of respiratory cell and molecular biology. 2013;49:358–67. [DOI] [PubMed] [Google Scholar]
- 94.Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, Haromy A, Webster L, Provencher S, Bonnet S and Michelakis ED. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell metabolism. 2014;20:827–39. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Y, Zervopoulos SD, Boukouris AE, Lorenzana-Carrillo MA, Saleme B, Webster L, Liu Y, Haromy A, Tabatabaei Dakhili SA, Ussher JR, Sutendra G and Michelakis ED. SNPs for Genes Encoding the Mitochondrial Proteins Sirtuin3 and Uncoupling Protein 2 Are Associated With Disease Severity, Type 2 Diabetes, and Outcomes in Patients With Pulmonary Arterial Hypertension and This Is Recapitulated in a New Mouse Model Lacking Both Genes. J Am Heart Assoc. 2021:e020451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navarro-Sastre A, Tort F, Stehling O, Uzarska MA, Arranz JA, Del Toro M, Labayru MT, Landa J, Font A, Garcia-Villoria J, Merinero B, Ugarte M, Gutierrez-Solana LG, Campistol J, Garcia-Cazorla A, Vaquerizo J, Riudor E, Briones P, Elpeleg O, Ribes A and Lill R. A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins. Am J Hum Genet. 2011;89:656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ahting U, Mayr JA, Vanlander AV, Hardy SA, Santra S, Makowski C, Alston CL, Zimmermann FA, Abela L, Plecko B, Rohrbach M, Spranger S, Seneca S, Rolinski B, Hagendorff A, Hempel M, Sperl W, Meitinger T, Smet J, Taylor RW, Van Coster R, Freisinger P, Prokisch H and Haack TB. Clinical, biochemical, and genetic spectrum of seven patients with NFU1 deficiency. Front Genet. 2015;6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheftel A, Stehling O and Lill R. Iron-sulfur proteins in health and disease. Trends Endocrinol Metab. 2010;21:302–14. [DOI] [PubMed] [Google Scholar]
- 99.Ghosh MC, Zhang DL, Jeong SY, Kovtunovych G, Ollivierre-Wilson H, Noguchi A, Tu T, Senecal T, Robinson G, Crooks DR, Tong WH, Ramaswamy K, Singh A, Graham BB, Tuder RM, Yu ZX, Eckhaus M, Lee J, Springer DA and Rouault TA. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2alpha. Cell metabolism. 2013;17:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Niihori M, Eccles CA, Kurdyukov S, Zemskova M, Varghese MV, Stepanova AA, Galkin A, Rafikov R and Rafikova O. Rats with a Human Mutation of NFU1 Develop Pulmonary Hypertension. American journal of respiratory cell and molecular biology. 2020;62:231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu Q, Tai YY, Tang Y, Zhao J, Negi V, Culley MK, Pilli J, Sun W, Brugger K, Mayr J, Saggar R, Saggar R, Wallace WD, Ross DJ, Waxman AB, Wendell SG, Mullett SJ, Sembrat J, Rojas M, Khan OF, Dahlman JE, Sugahara M, Kagiyama N, Satoh T, Zhang M, Feng N, Gorcsan J 3rd, Vargas SO, Haley KJ, Kumar R, Graham BB, Langer R, Anderson DG, Wang B, Shiva S, Bertero T and Chan SY. BOLA (BolA Family Member 3) Deficiency Controls Endothelial Metabolism and Glycine Homeostasis in Pulmonary Hypertension. Circulation. 2019;139:2238–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Culley MK, Zhao J, Tai YY, Tang Y, Perk D, Negi V, Yu Q, Woodcock CC, Handen A, Speyer G, Kim S, Lai YC, Satoh T, Watson AM, Aaraj YA, Sembrat J, Rojas M, Goncharov D, Goncharova EA, Khan OF, Anderson DG, Dahlman JE, Gurkar AU, Lafyatis R, Fayyaz AU, Redfield MM, Gladwin MT, Rabinovitch M, Gu M, Bertero T and Chan SY. Frataxin deficiency promotes endothelial senescence in pulmonary hypertension. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, van der Laarse WJ and Vonk-Noordegraaf A. Iron deficiency is common in idiopathic pulmonary arterial hypertension. The European respiratory journal. 2011;37:1386–91. [DOI] [PubMed] [Google Scholar]
- 104.Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili E, Gibbs JS, Wharton J and Wilkins MR. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58:300–9. [DOI] [PubMed] [Google Scholar]
- 105.Cotroneo E, Ashek A, Wang L, Wharton J, Dubois O, Bozorgi S, Busbridge M, Alavian KN, Wilkins MR and Zhao L. Iron homeostasis and pulmonary hypertension: iron deficiency leads to pulmonary vascular remodeling in the rat. Circ Res. 2015;116:1680–90. [DOI] [PubMed] [Google Scholar]
- 106.Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, Higenbottam T, Oakley C, Wouters E, Aubier M, Simonneau G and Begaud B. Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group. The New England journal of medicine. 1996;335:609–16. [DOI] [PubMed] [Google Scholar]
- 107.Morecroft I, Heeley RP, Prentice HM, Kirk A and MacLean MR. 5-hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol. 1999;128:730–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S and Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94:1263–70. [DOI] [PubMed] [Google Scholar]
- 109.Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt AM, Lukanidin E and Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97:227–35. [DOI] [PubMed] [Google Scholar]
- 110.Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, Tamargo J and Perez-Vizcaino F. Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res. 2006;98:931–8. [DOI] [PubMed] [Google Scholar]
- 111.Rodat-Despoix L, Aires V, Ducret T, Marthan R, Savineau JP, Rousseau E and Guibert C. Signalling pathways involved in the contractile response to 5-HT in the human pulmonary artery. The European respiratory journal. 2009;34:1338–47. [DOI] [PubMed] [Google Scholar]
- 112.Guilluy C, Eddahibi S, Agard C, Guignabert C, Izikki M, Tu L, Savale L, Humbert M, Fadel E, Adnot S, Loirand G and Pacaud P. RhoA and Rho kinase activation in human pulmonary hypertension: role of 5-HT signaling. Am J Respir Crit Care Med. 2009;179:1151–8. [DOI] [PubMed] [Google Scholar]
- 113.Penumatsa K, Abualkhair S, Wei L, Warburton R, Preston I, Hill NS, Watts SW, Fanburg BL and Toksoz D. Tissue transglutaminase promotes serotonin-induced AKT signaling and mitogenesis in pulmonary vascular smooth muscle cells. Cell Signal. 2014;26:2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greenway S, van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, Lukanidin E and Rabinovitch M. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol. 2004;164:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, Rabinovitch M and Maclean MR. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res. 2011;12:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dempsie Y, Morecroft I, Welsh DJ, MacRitchie NA, Herold N, Loughlin L, Nilsen M, Peacock AJ, Harmar A, Bader M and MacLean MR. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation. 2008;117:2928–37. [DOI] [PubMed] [Google Scholar]
- 117.Guignabert C, Izikki M, Tu LI, Li Z, Zadigue P, Barlier-Mur AM, Hanoun N, Rodman D, Hamon M, Adnot S and Eddahibi S. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res. 2006;98:1323–30. [DOI] [PubMed] [Google Scholar]
- 118.MacLean MR, Deuchar GA, Hicks MN, Morecroft I, Shen S, Sheward J, Colston J, Loughlin L, Nilsen M, Dempsie Y and Harmar A. Overexpression of the 5-hydroxytryptamine transporter gene: effect on pulmonary hemodynamics and hypoxia-induced pulmonary hypertension. Circulation. 2004;109:2150–5. [DOI] [PubMed] [Google Scholar]
- 119.Montani D, Bergot E, Gunther S, Savale L, Bergeron A, Bourdin A, Bouvaist H, Canuet M, Pison C, Macro M, Poubeau P, Girerd B, Natali D, Guignabert C, Perros F, O’Callaghan DS, Jais X, Tubert-Bitter P, Zalcman G, Sitbon O, Simonneau G and Humbert M. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125:2128–37. [DOI] [PubMed] [Google Scholar]
- 120.Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM, Chaumais MC, Bouchet S, Maneglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D and Humbert M. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest. 2016;126:3207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ranchoux B, Gunther S, Quarck R, Chaumais MC, Dorfmuller P, Antigny F, Dumas SJ, Raymond N, Lau E, Savale L, Jais X, Sitbon O, Simonneau G, Stenmark K, Cohen-Kaminsky S, Humbert M, Montani D and Perros F. Chemotherapy-induced pulmonary hypertension: role of alkylating agents. Am J Pathol. 2015;185:356–71. [DOI] [PubMed] [Google Scholar]
- 122.Perros F, Gunther S, Ranchoux B, Godinas L, Antigny F, Chaumais MC, Dorfmuller P, Hautefort A, Raymond N, Savale L, Jais X, Girerd B, Cottin V, Sitbon O, Simonneau G, Humbert M and Montani D. Mitomycin-Induced Pulmonary Veno-Occlusive Disease: Evidence From Human Disease and Animal Models. Circulation. 2015;132:834–47. [DOI] [PubMed] [Google Scholar]
- 123.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, Chelghoum N, Coulet F, Bonnet D, Dorfmuller P, Fadel E, Sitbon O, Simonneau G, Tregouet DA, Humbert M and Soubrier F. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–9. [DOI] [PubMed] [Google Scholar]
- 124.Manaud G, Nossent EJ, Lambert M, Ghigna MR, Boet A, Vinhas MC, Ranchoux B, Dumas SJ, Courboulin A, Girerd B, Soubrier F, Bignard J, Claude O, Lecerf F, Hautefort A, Florio M, Sun B, Nadaud S, Verleden SE, Remy S, Anegon I, Bogaard HJ, Mercier O, Fadel E, Simonneau G, Vonk Noordegraaf A, Grunberg K, Humbert M, Montani D, Dorfmuller P, Antigny F and Perros F. Comparison of Human and Experimental Pulmonary Veno-Occlusive Disease. American journal of respiratory cell and molecular biology. 2020;63:118–131. [DOI] [PubMed] [Google Scholar]
- 125.Butrous G Human immunodeficiency virus-associated pulmonary arterial hypertension: considerations for pulmonary vascular diseases in the developing world. Circulation. 2015;131:1361–70. [DOI] [PubMed] [Google Scholar]
- 126.Orcholski ME, Yuan K, Rajasingh C, Tsai H, Shamskhou EA, Dhillon NK, Voelkel NF, Zamanian RT and de Jesus Perez VA. Drug-induced pulmonary arterial hypertension: a primer for clinicians and scientists. American journal of physiology Lung cellular and molecular physiology. 2018;314:L967–L983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS and Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992;67:338–49. [PubMed] [Google Scholar]
- 128.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, Carville A, Shannon RP, Cota-Gomez A, Tuder RM, Voelkel NF and Flores SC. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med. 2006;174:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.George MP, Brower A, Kling H, Shipley T, Kristoff J, Reinhart TA, Murphey-Corb M, Gladwin MT, Champion HC, Morris A and Norris KA. Pulmonary vascular lesions are common in SIV- and SHIV-env-infected macaques. AIDS Res Hum Retroviruses. 2011;27:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.George MP, Champion HC, Simon M, Guyach S, Tarantelli R, Kling HM, Brower A, Janssen C, Murphy J, Carney JP, Morris A, Gladwin MT and Norris KA. Physiologic changes in a nonhuman primate model of HIV-associated pulmonary arterial hypertension. American journal of respiratory cell and molecular biology. 2013;48:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tarantelli RA, Schweitzer F, Simon MA, Vanderpool RR, Christman I, Rayens E, Kling HM, Zullo T, Carney JP, Lopresti BJ, Bertero T, Chan SY and Norris KA. Longitudinal Evaluation of Pulmonary Arterial Hypertension in a Rhesus Macaque (Macaca mulatta) Model of HIV Infection. Comp Med. 2018;68:461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lund AK, Lucero J, Herbert L, Liu Y and Naik JS. Human immunodeficiency virus transgenic rats exhibit pulmonary hypertension. American journal of physiology Lung cellular and molecular physiology. 2011;301:L315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Porter KM, Walp ER, Elms SC, Raynor R, Mitchell PO, Guidot DM and Sutliff RL. Human immunodeficiency virus-1 transgene expression increases pulmonary vascular resistance and exacerbates hypoxia-induced pulmonary hypertension development. Pulm Circ. 2013;3:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dalvi P, Spikes L, Allen J, Gupta VG, Sharma H, Gillcrist M, Montes de Oca J, O’Brien-Ladner A and Dhillon NK. Effect of Cocaine on Pulmonary Vascular Remodeling and Hemodynamics in Human Immunodeficiency Virus-Transgenic Rats. American journal of respiratory cell and molecular biology. 2016;55:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, Olsson KM, Peacock AJ, Pepke-Zaba J, Provencher S, Weissmann N and Seeger W. Pulmonary hypertension in chronic lung disease and hypoxia. The European respiratory journal. 2019;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crnkovic S, Marsh LM, El Agha E, Voswinckel R, Ghanim B, Klepetko W, Stacher-Priehse E, Olschewski H, Bloch W, Bellusci S, Olschewski A and Kwapiszewska G. Resident cell lineages are preserved in pulmonary vascular remodeling. J Pathol. 2018;244:485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qiao L, Nishimura T, Shi L, Sessions D, Thrasher A, Trudell JR, Berry GJ, Pearl RG and Kao PN. Endothelial fate mapping in mice with pulmonary hypertension. Circulation. 2014;129:692–703. [DOI] [PubMed] [Google Scholar]
- 138.Suzuki T, Carrier EJ, Talati MH, Rathinasabapathy A, Chen X, Nishimura R, Tada Y, Tatsumi K and West J. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. American journal of physiology Lung cellular and molecular physiology. 2018;314:L118–L126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rhodes J Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol (1985). 2005;98:1092–100. [DOI] [PubMed] [Google Scholar]
- 140.Hoshikawa Y, Nana-Sinkam P, Moore MD, Sotto-Santiago S, Phang T, Keith RL, Morris KG, Kondo T, Tuder RM, Voelkel NF and Geraci MW. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genomics. 2003;12:209–19. [DOI] [PubMed] [Google Scholar]
- 141.Stenmark KR, Fasules J, Hyde DM, Voelkel NF, Henson J, Tucker A, Wilson H and Reeves JT. Severe pulmonary hypertension and arterial adventitial changes in newborn calves at 4,300 m. J Appl Physiol (1985). 1987;62:821–30. [DOI] [PubMed] [Google Scholar]
- 142.Stenmark KR, Meyrick B, Galie N, Mooi WJ and McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2009;297:L1013–L1032. [DOI] [PubMed] [Google Scholar]
- 143.Campen MJ, Shimoda LA and O’Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol (1985). 2005;99:2028–35. [DOI] [PubMed] [Google Scholar]
- 144.Nisbet RE, Graves AS, Kleinhenz DJ, Rupnow HL, Reed AL, Fan TH, Mitchell PO, Sutliff RL and Hart CM. The role of NADPH oxidase in chronic intermittent hypoxia-induced pulmonary hypertension in mice. American journal of respiratory cell and molecular biology. 2009;40:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sajkov D, Wang T, Saunders NA, Bune AJ and McEvoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:152–8. [DOI] [PubMed] [Google Scholar]
- 146.Fagan KA. Selected Contribution: Pulmonary hypertension in mice following intermittent hypoxia. J Appl Physiol (1985). 2001;90:2502–7. [DOI] [PubMed] [Google Scholar]
- 147.Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, Kim SE, Lee YS and Lee SD. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005;172:987–93. [DOI] [PubMed] [Google Scholar]
- 148.Wright JL and Churg A. Effect of long-term cigarette smoke exposure on pulmonary vascular structure and function in the guinea pig. Exp Lung Res. 1991;17:997–1009. [DOI] [PubMed] [Google Scholar]
- 149.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, Milger K, Egemnazarov B, Turowska A, Fuchs B, Nikam S, Roth M, Sydykov A, Medebach T, Klepetko W, Jaksch P, Dumitrascu R, Garn H, Voswinckel R, Kostin S, Seeger W, Schermuly RT, Grimminger F, Ghofrani HA and Weissmann N. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147:293–305. [DOI] [PubMed] [Google Scholar]
- 150.Weissmann N, Lobo B, Pichl A, Parajuli N, Seimetz M, Puig-Pey R, Ferrer E, Peinado VI, Dominguez-Fandos D, Fysikopoulos A, Stasch JP, Ghofrani HA, Coll-Bonfill N, Frey R, Schermuly RT, Garcia-Lucio J, Blanco I, Bednorz M, Tura-Ceide O, Tadele E, Brandes RP, Grimminger J, Klepetko W, Jaksch P, Rodriguez-Roisin R, Seeger W, Grimminger F and Barbera JA. Stimulation of soluble guanylate cyclase prevents cigarette smoke-induced pulmonary hypertension and emphysema. Am J Respir Crit Care Med. 2014;189:1359–73. [DOI] [PubMed] [Google Scholar]
- 151.Wang T, Han SX, Zhang SF, Ning YY, Chen L, Chen YJ, He GM, Xu D, An J, Yang T, Zhang XH and Wen FQ. Role of chymase in cigarette smoke-induced pulmonary artery remodeling and pulmonary hypertension in hamsters. Respir Res. 2010;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han SX, He GM, Wang T, Chen L, Ning YY, Luo F, An J, Yang T, Dong JJ, Liao ZL, Xu D and Wen FQ. Losartan attenuates chronic cigarette smoke exposure-induced pulmonary arterial hypertension in rats: possible involvement of angiotensin-converting enzyme-2. Toxicol Appl Pharmacol. 2010;245:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jenkins RG, Moore BB, Chambers RC, Eickelberg O, Konigshoff M, Kolb M, Laurent GJ, Nanthakumar CB, Olman MA, Pardo A, Selman M, Sheppard D, Sime PJ, Tager AM, Tatler AL, Thannickal VJ, White ES, Cell ATSAoR and Molecular B. An Official American Thoracic Society Workshop Report: Use of Animal Models for the Preclinical Assessment of Potential Therapies for Pulmonary Fibrosis. American journal of respiratory cell and molecular biology. 2017;56:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hemnes AR, Zaiman A and Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. American journal of physiology Lung cellular and molecular physiology. 2008;294:L24–33. [DOI] [PubMed] [Google Scholar]
- 155.Almudever P, Milara J, De Diego A, Serrano-Mollar A, Xaubet A, Perez-Vizcaino F, Cogolludo A and Cortijo J. Role of tetrahydrobiopterin in pulmonary vascular remodelling associated with pulmonary fibrosis. Thorax. 2013;68:938–48. [DOI] [PubMed] [Google Scholar]
- 156.Ruffenach G, Umar S, Vaillancourt M, Hong J, Cao N, Sarji S, Moazeni S, Cunningham CM, Ardehali A, Reddy ST, Saggar R, Fishbein G and Eghbali M. Histological hallmarks and role of Slug/PIP axis in pulmonary hypertension secondary to pulmonary fibrosis. EMBO Mol Med. 2019;11:e10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu WH, Bonnet S, Shimauchi T, Toro V, Grobs Y, Romanet C, Bourgeois A, Vitry G, Omura J, Tremblay E, Nadeau V, Orcholski M, Breuils-Bonnet S, Martineau S, Ferraro P, Potus F, Paulin R, Provencher S and Boucherat O. Potential for inhibition of checkpoint kinases ½ in pulmonary fibrosis and secondary pulmonary hypertension. Thorax. 2021. [DOI] [PubMed] [Google Scholar]
- 158.Sime PJ, Xing Z, Graham FL, Csaky KG and Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M and Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119:1298–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bellaye PS, Yanagihara T, Granton E, Sato S, Shimbori C, Upagupta C, Imani J, Hambly N, Ask K, Gauldie J, Iglarz M and Kolb M. Macitentan reduces progression of TGF-beta1-induced pulmonary fibrosis and pulmonary hypertension. The European respiratory journal. 2018;52. [DOI] [PubMed] [Google Scholar]
- 161.Barker DJ and Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. [DOI] [PubMed] [Google Scholar]
- 162.Boucherat O, Morissette MC, Provencher S, Bonnet S and Maltais F. Bridging Lung Development with Chronic Obstructive Pulmonary Disease. Relevance of Developmental Pathways in Chronic Obstructive Pulmonary Disease Pathogenesis. Am J Respir Crit Care Med. 2016;193:362–75. [DOI] [PubMed] [Google Scholar]
- 163.Doyle LW, Andersson S, Bush A, Cheong JLY, Clemm H, Evensen KAI, Gough A, Halvorsen T, Hovi P, Kajantie E, Lee KJ, McGarvey L, Narang I, Nasanen-Gilmore P, Steinshamn S, Vollsaeter M, Vrijlandt E and Adults born Preterm International C. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. The Lancet Respiratory medicine. 2019;7:677–686. [DOI] [PubMed] [Google Scholar]
- 164.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, Guerra S, Marott JL, Martinez FD, Martinez-Camblor P, Meek P, Owen CA, Petersen H, Pinto-Plata V, Schnohr P, Sood A, Soriano JB, Tesfaigzi Y and Vestbo J. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. The New England journal of medicine. 2015;373:111–22. [DOI] [PubMed] [Google Scholar]
- 165.Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U and Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–94. [DOI] [PubMed] [Google Scholar]
- 166.Naumburg E and Soderstrom L. Increased risk of pulmonary hypertension following premature birth. BMC Pediatr. 2019;19:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lewandowski AJ, Bradlow WM, Augustine D, Davis EF, Francis J, Singhal A, Lucas A, Neubauer S, McCormick K and Leeson P. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013;128:713–20. [DOI] [PubMed] [Google Scholar]
- 168.Goss KN, Beshish AG, Barton GP, Haraldsdottir K, Levin TS, Tetri LH, Battiola TJ, Mulchrone AM, Pegelow DF, Palta M, Lamers LJ, Watson AM, Chesler NC and Eldridge MW. Early Pulmonary Vascular Disease in Young Adults Born Preterm. Am J Respir Crit Care Med. 2018;198:1549–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF and Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. American journal of physiology Lung cellular and molecular physiology. 2000;279:L600–7. [DOI] [PubMed] [Google Scholar]
- 170.Le Cras TD, Markham NE, Tuder RM, Voelkel NF and Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. American journal of physiology Lung cellular and molecular physiology. 2002;283:L555–62. [DOI] [PubMed] [Google Scholar]
- 171.Goss KN, Kumari S, Tetri LH, Barton G, Braun RK, Hacker TA and Eldridge MW. Postnatal Hyperoxia Exposure Durably Impairs Right Ventricular Function and Mitochondrial Biogenesis. American journal of respiratory cell and molecular biology. 2017;56:609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tang JR, Le Cras TD, Morris KG Jr. and Abman SH. Brief perinatal hypoxia increases severity of pulmonary hypertension after reexposure to hypoxia in infant rats. American journal of physiology Lung cellular and molecular physiology. 2000;278:L356–64. [DOI] [PubMed] [Google Scholar]
- 173.le Cras TD, Markham NE, Morris KG, Ahrens CR, McMurtry IF and Abman SH. Neonatal dexamethasone treatment increases the risk for pulmonary hypertension in adult rats. American journal of physiology Lung cellular and molecular physiology. 2000;278:L822–9. [DOI] [PubMed] [Google Scholar]
- 174.Sato K, Webb S, Tucker A, Rabinovitch M, O’Brien RF, McMurtry IF and Stelzner TJ. Factors influencing the idiopathic development of pulmonary hypertension in the fawn hooded rat. The American review of respiratory disease. 1992;145:793–7. [DOI] [PubMed] [Google Scholar]
- 175.Muramatsu M. [Fawn-Hooded Rat; an animal model of development of pulmonary hypertension]. Nihon Rinsho. 2001;59:1070–5. [PubMed] [Google Scholar]
- 176.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Bonnet S, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK and Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–41. [DOI] [PubMed] [Google Scholar]
- 177.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N and Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Le Cras TD, Kim DH, Gebb S, Markham NE, Shannon JM, Tuder RM and Abman SH. Abnormal lung growth and the development of pulmonary hypertension in the Fawn-Hooded rat. The American journal of physiology. 1999;277:L709–18. [DOI] [PubMed] [Google Scholar]
- 179.Sider KL, Blaser MC and Simmons CA. Animal models of calcific aortic valve disease. Int J Inflam. 2011;2011:364310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch WJ, American Heart Association Council on Basic Cardiovascular Sciences CoCC, Council on Functional G and Translational B. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–50. [DOI] [PubMed] [Google Scholar]
- 181.Valero-Munoz M, Backman W and Sam F. Murine Models of Heart Failure with Preserved Ejection Fraction: a “Fishing Expedition”. JACC Basic Transl Sci. 2017;2:770–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Y, Guo H, Xu D, Xu X, Wang H, Hu X, Lu Z, Kwak D, Xu Y, Gunther R, Huo Y and Weir EK. Left ventricular failure produces profound lung remodeling and pulmonary hypertension in mice: heart failure causes severe lung disease. Hypertension (Dallas, Tex : 1979). 2012;59:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dai ZK, Tan MS, Chai CY, Yeh JL, Chou SH, Chiu CC, Jeng AY, Chen IJ and Wu JR. Upregulation of endothelial nitric oxide synthase and endothelin-1 in pulmonary hypertension secondary to heart failure in aorta-banded rats. Pediatr Pulmonol. 2004;37:249–56. [DOI] [PubMed] [Google Scholar]
- 184.Pereda D, Garcia-Alvarez A, Sanchez-Quintana D, Nuno M, Fernandez-Friera L, Fernandez-Jimenez R, Garcia-Ruiz JM, Sandoval E, Aguero J, Castella M, Hajjar RJ, Fuster V and Ibanez B. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res. 2014;7:494–506. [DOI] [PubMed] [Google Scholar]
- 185.Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, Garcia-Alvarez A, Fuster V, Sengupta PP, Leopold JA and Hajjar RJ. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. American journal of physiology Heart and circulatory physiology. 2014;307:H1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guazzi M, Ghio S and Adir Y. Pulmonary Hypertension in HFpEF and HFrEF: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1102–1111. [DOI] [PubMed] [Google Scholar]
- 187.Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, Fong PP and Newman JH. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marino A, Zhang Y, Rubinelli L, Riemma MA, Ip JE and Di Lorenzo A. Pressure overload leads to coronary plaque formation, progression, and myocardial events in ApoE−/− mice. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, Sheikh AY, Suen RS, Stewart DJ and Rabinovitch M. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–84. [DOI] [PubMed] [Google Scholar]
- 190.Feng M, Deerhake ME, Keating R, Thaisz J, Xu L, Tsaih SW, Smith R, Ishige T, Sugiyama F, Churchill GA and DiPetrillo K. Genetic analysis of blood pressure in 8 mouse intercross populations. Hypertension (Dallas, Tex : 1979). 2009;54:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Meng Q, Lai YC, Kelly NJ, Bueno M, Baust JJ, Bachman TN, Goncharov D, Vanderpool RR, Radder JE, Hu J, Goncharova E, Morris AM, Mora AL, Shapiro SD and Gladwin MT. Development of a Mouse Model of Metabolic Syndrome, Pulmonary Hypertension, and Heart Failure with Preserved Ejection Fraction. American journal of respiratory cell and molecular biology. 2017;56:497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Agrawal V, Fortune N, Yu S, Fuentes J, Shi F, Nichols D, Gleaves L, Poovey E, Wang TJ, Brittain EL, Collins S, West JD and Hemnes AR. Natriuretic peptide receptor C contributes to disproportionate right ventricular hypertrophy in a rodent model of obesity-induced heart failure with preserved ejection fraction with pulmonary hypertension. Pulm Circ. 2019;9:2045894019878599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mirna M, Rohm I, Jirak P, Wernly B, Baz L, Paar V, Kretzschmar D, Hoppe UC, Schulze PC, Lichtenauer M, Jung C and Franz M. Analysis of Novel Cardiovascular Biomarkers in Patients With Pulmonary Hypertension (PH). Heart Lung Circ. 2020;29:337–344. [DOI] [PubMed] [Google Scholar]
- 194.Lawrie A, Hameed AG, Chamberlain J, Arnold N, Kennerley A, Hopkinson K, Pickworth J, Kiely DG, Crossman DC and Francis SE. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol. 2011;179:1693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, St Croix CM, Garcia-Ocana A, Goncharova EA, Tofovic SP, Mora AL and Gladwin MT. SIRT3-AMP-Activated Protein Kinase Activation by Nitrite and Metformin Improves Hyperglycemia and Normalizes Pulmonary Hypertension Associated With Heart Failure With Preserved Ejection Fraction. Circulation. 2016;133:717–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ranchoux B, Nadeau V, Bourgeois A, Provencher S, Tremblay E, Omura J, Cote N, Abu-Alhayja’a R, Dumais V, Nachbar RT, Tastet L, Dahou A, Breuils-Bonnet S, Marette A, Pibarot P, Dupuis J, Paulin R, Boucherat O, Archer SL, Bonnet S and Potus F. Metabolic Syndrome Exacerbates Pulmonary Hypertension due to Left Heart Disease. Circ Res. 2019;125:449–466. [DOI] [PubMed] [Google Scholar]
- 197.van de Veerdonk MC, Bogaard HJ and Voelkel NF. The right ventricle and pulmonary hypertension. Heart Fail Rev. 2016;21:259–71. [DOI] [PubMed] [Google Scholar]
- 198.Lemay SE, Awada C, Shimauchi T, Wu WH, Bonnet S, Provencher S and Boucherat O. Fetal Gene Reactivation in Pulmonary Arterial Hypertension: GOOD, BAD, or BOTH? Cells. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bogaard HJ, Abe K, Vonk Noordegraaf A and Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. [DOI] [PubMed] [Google Scholar]
- 200.Ukita R, Stokes JW, Wu WK, Talackine J, Cardwell N, Patel Y, Benson C, Demarest CT, Rosenzweig EB, Cook K, Tsai EJ and Bacchetta M. A Large Animal Model for Pulmonary Hypertension and Right Ventricular Failure: Left Pulmonary Artery Ligation and Progressive Main Pulmonary Artery Banding in Sheep. J Vis Exp. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mamazhakypov A, Sommer N, Assmus B, Tello K, Schermuly RT, Kosanovic D, Sarybaev AS, Weissmann N and Pak O. Novel Therapeutic Targets for the Treatment of Right Ventricular Remodeling: Insights from the Pulmonary Artery Banding Model. Int J Environ Res Public Health. 2021;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM and Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–60. [DOI] [PubMed] [Google Scholar]
- 203.Borgdorff MA, Bartelds B, Dickinson MG, Steendijk P, de Vroomen M and Berger RM. Distinct loading conditions reveal various patterns of right ventricular adaptation. American journal of physiology Heart and circulatory physiology. 2013;305:H354–64. [DOI] [PubMed] [Google Scholar]
- 204.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, Bonnet S and Michelakis ED. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116:56–69. [DOI] [PubMed] [Google Scholar]
- 205.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J and Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl). 2013;91:1315–27. [DOI] [PubMed] [Google Scholar]
- 206.Bogaard HJ, Mizuno S, Hussaini AA, Toldo S, Abbate A, Kraskauskas D, Kasper M, Natarajan R and Voelkel NF. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care Med. 2011;183:1402–10. [DOI] [PubMed] [Google Scholar]